The mouse homolog of Drosophila Vasa is required for the development of male germ cells (original) (raw)
. 2000 Apr 1;14(7):841–853.
Abstract
Restricted expression of a mouse Vasa homolog gene (Mvh) expression is first detected in primordial germ cells (PGCs) after colonization of the genital ridges. Subsequently, Mvh is maintained until postmeiotic germ cells are formed. Here, we demonstrate that male mice homozygous for a targeted mutation of Mvh exhibit a reproductive deficiency. Male homozygotes produce no sperm in the testes, where premeiotic germ cells cease differentiation by the zygotene stage and undergo apoptotic death. In addition, the proliferation of PGCs that colonize homozygous male gonads is significantly hampered, and OCT-3/4 expression appears to be reduced. These results indicate that the loss of Mvh function causes a deficiency in the proliferation and differentiation of mouse male germ cells.
Keywords: Vasa, primordial germ cell, spermatogenesis, meiosis, mouse
Germ cells, highly specialized for transmitting genetic information to the next generation, are segregated from somatic lineages at a very early stage of embryogenesis. In Drosophila, several maternal-effect mutations indicate that maternal determinants of germ cells are deposited in polar granules (germ plasm) at the posterior pole of the oocyte (Rongo and Lehmann 1996). Thus, the mechanism by which the germ line is predetermined by the inheritance of maternal factors is conserved throughout evolution in many animal species such as Caenorhabditis elegans and Xenopus laevis (Eddy 1975; Wylie 1999). However, maternal-effect germplasm has never been observed in mammals (Tam and Zhou 1996; Zernicka-Goetz 1998). Germ cell specification takes place during early gastrulation after the implantation of embryos and requires some inductive factors from extra-embryonic tissues (Lawson et al. 1999).
In the mouse, the germ-line precursor cells giving rise to primordial germ cells (PGCs) are located in the rim of the epiblast adjacent to the extra-embryonic ectoderm before gastrulation (Lawson and Hage 1994). The earliest identification of putative PGCs is feasible in the gastrulating embryo at 7.25 days postcoitum (dpc). A cluster of PGCs that are distinguishable by their high activity of tissue nonspecific alkaline phosphatase (TN-AP) is detected in the extra-embryonic mesoderm posterior to the primitive streak (Ginsburg et al. 1990). Subsequently, proliferating PGCs migrate into the genital ridges around 10.5–11.5 dpc and PGCs colonizing the genital ridge differentiate into precursor cells of either male or female gametes under the control of cell interactions in the developing gonad.
Genetic studies in Drosophila have identified numerous genes (i.e., Oskar, Vasa, Nanos, and Tudor) that are involved in the formation of germ cell precursors, pole cells (Rongo and Lehmann 1996). Among these genes, Vasa, the best characterized, is a member of the DEAD-box family of genes encoding an ATP-dependent RNA helicase. Vasa is required for the assembly and function of the pole plasm (Hay et al. 1988; Lasko and Ashburner 1988; Liang et al. 1994). In addition, on the basis of structural conservation, genes homologous to Vasa have been identified in many animal species, such as C. elegans, Xenopus, zebrafish, and mouse (Roussell and Bennett 1993; Komiya et al. 1994; Yoon et al. 1997; Olsen et al. 1997; Fujiwara et al. 1994). All of these Vasa homolog genes have been found to be expressed specifically in germ cell lineages. As is the case in Drosophila, the products of Vasa homolog genes are localized within P-granules in C. elegans eggs and germinal granules in Xenopus eggs (Gruidl et al. 1996; Komiya et al. 1994).
The mouse Vasa homolog (Mvh) gene also exhibits specific expression in developing germ cells (Fujiwara et al. 1994). The expression of MVH protein is first detected in PGCs that colonize 10.5–11.5 dpc embryonic gonads. It is at this time that PGCs begin to interact with gonadal supporting cells. The expression of MVH protein is maintained in both male and female germ cells ranging from PGCs of 12.5 dpc gonads to postmeiotic spermatids and primary oocytes. PGCs in mouse embryos have been distinguished by their characteristic AP activity (Hahnel et al. 1990; Ginsburg et al. 1990; MacGregor et al. 1995), Oct-3/4 expression (Okazawa et al. 1991; Yeom et al. 1996), and several cell-surface antigens recognized by monoclonal antibodies such as SSEA-1 (Fox et al. 1981), 4C9 (Yoshinaga et al. 1991), and EMA-1 (Hahnel and Eddy 1986). However, because all of these characteristics are common in undifferentiated embryonic cells at the blastocyst and epiblast stages, they are not specific enough to distinguish cells destined to a germ cell fate from pluripotent stem cells remaining in the undifferentiated state. With respect to this point, our previous finding that MVH expression is not detected in pluripotent cell lines such as embryonic germ (EG) and embryonic stem (ES) cells derived from PGCs and inner cell mass (ICM) cells shows precisely the developmental difference between PGCs in genital ridges from totipotent cells.
Mutation of Drosophila Vasa has shown that maternal expression of Vasa is required for the assembly and function of polar granules, and zygotic expression is required for the completion of oogenesis (Lasko and Ashburner 1988, 1990; Styhler et al. 1998; Tomancak et al. 1998). By analogy to Drosophila Vasa, we carried out targeted mutagenesis in ES cells to investigate the functional requirement for Mvh in the mouse. The resulting homozygous mutant mice showed reproductive defects in a sex-dependent manner. Male homozygotes produce no sperm in testes, as premeiotic germ cells could not complete the meiotic process and underwent apoptotic cell death. In addition, the proliferative activity of PGCs in the homozygous male gonad is remarkably decreased, suggesting a novel role for the cytoplasmic RNA helicase in regulating cell proliferation. Our findings demonstrate an essential role for Mvh in mouse germ cell development.
Results
Generation of mice with targeted mutagenesis of the Mvh gene
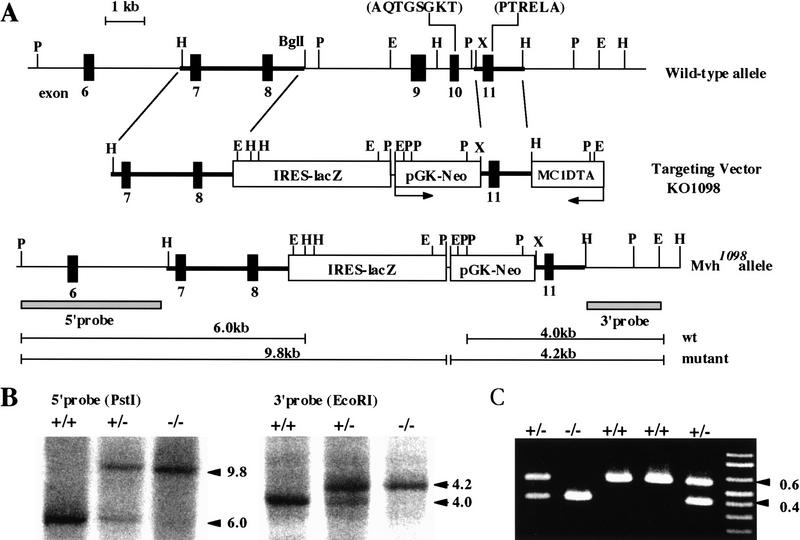
The gene-targeting scheme used to generate ES cells containing a modified Mvh gene is shown in Figure 1A. In the mutant allele, designated as Mvh1098, a 4.5-kb genomic fragment encompassing exons 9–10 is deleted. This region encodes amino acid sequences containing an ATPase motif (MACAQTGSGKT) conserved among VASA-like family proteins. Transcripts made from the Mvh1098 allele would contain a novel in-frame stop codon in the replacement cassette or in the 5′-junction of the new spliced form. Therefore, it is predicted that no functional MVH protein would be produced from the Mvh1098 allele. Screening for the desired targeting events by Southern blot analysis resulted in the isolation of two different targeted ES cell lines (V72 and V323). These two cell lines were injected into blastocysts, and both lines transmitted the Mvh1098 allele through the male germ line of the resulting chimeras. To generate homozygous mutant mice, heterozygotes were interbred and the offspring genotyped by Southern blot and PCR amplification (Fig. 1B,C). Homozygous mutant mice from both lines exhibited the same phenotype, and the mutant mice were maintained on a mixed genetic background (129/ola × C57BL/6Njcl) for this study.
Figure 1.
Targeted disruption of the Mvh gene. (A) Schematic representation of the wild-type and mutated Mvh1098 alleles and the structure of the targeting vector. Genomic DNA fragments used as 5′ and 3′ homology arms in the KO1098 targeting vector are indicated by thick lines. (Closed boxes) Coding exons. Coding exons 9 and 10 are replaced with IRES–lacZ and pGK-Neo cassettes (open boxes); the arrow indicates the direction of Neo transcription. The expression cassette MC1DTA was used for negative selection. The 5′ and 3′ probes (shaded boxes) used for Southern blots are shown below the Mvh1098 allele. (E) _Eco_RI; (H) _Hin_dIII; (P) _Pst_I; (X) _Xho_I. (B) Southern blots of progeny from interbreeding of Mvh1098 heterozygotes. Ten micrograms of genomic DNA was digested with _Pst_I and _Eco_RI. The 5′ external probe hybridizes to 6.0-kb (wild type) and 9.8-kb (targeted) _Pst_I fragments. The 3′ probe hybridizes to 4.0-kb (wild type) and 4.2-kb (targeted) _Eco_RI fragments. (C) PCR analysis of the Mvh mice. The wild-type and Mvh1098 alleles generate 0.6- and 0.4-kb fragments, respectively. (+/+) Wild type; (+/−) heterozygote; (−/−) homozygote.
Spermatogenic deficiency in Mvh mutant male mice
Interbreeding of heterozygous mice produced offspring without any significant decrease in litter size and yielded normal Mendelian segregation ratios for wild-type, heterozygous, and homozygous mutant offspring (data not shown). Both male and female homozygous mutant mice were viable, grew to adulthood normally and appeared to have normal sexual behavior. Female homozygous mice did not show any obvious reproductive defects, and histologically, their ovaries did not appear to be different from those of their heterozygous littermates (Fig. 2C,D). Indeed, homozygous female mice were fertile and offspring delivered after mating with heterozygous males showed no difference in number compared with wild-type mice. However, male mice homozygous for the Mvh mutation were infertile. As shown in Figure 2A, the homozygous adult testes atrophied to about one-fifth the size of heterozygous and wild-type adult testes. Histological examination revealed complete depletion of postmeiotic germ cells in mature homozygous testes, whereas the testes of the heterozygous male littermates exhibited apparently normal spermatogenesis (Fig. 2E,F). On day 15 after birth, mature spermatocytes appeared to differentiate in the innermost layer of the seminiferous tubules in heterozygous testes, but partial depletion of the spermatocyte layer was clearly observed in homozygous testes (Fig. 2G,H). Two days after birth, no remarkable difference was observed between heterozygous and homozygous testes, whereas the seminiferous tubules of homozygous testes seemed to be slightly smaller than those of heterozygous testes (Fig. 2I,J). Other reproductive organs such the vas deferens and epididymus cauda appeared to be normal in homozygous males.
Figure 2.
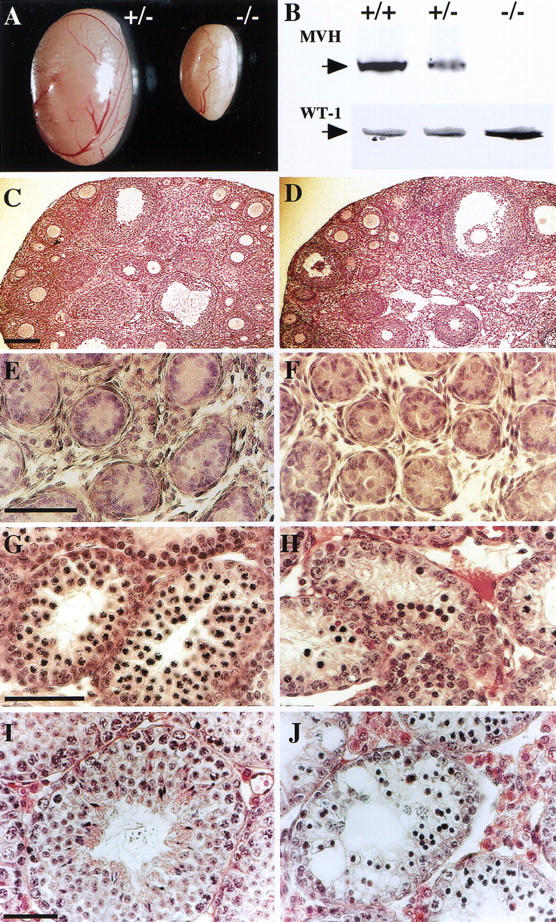
Comparison of Mvh1098 heterozygous and homozygous mouse testes at different ages. (A) Testes from a 1-year-old heterozygous (+/−) mouse and a homozygous (−/−) mutant littermate. (B) Immunoblot analysis of MVH (85 kD) and WT-1 (58 kD) expression in testes from a 5-week-old wild-type (+/+), heterozygous (+/−) and homozygous littermates (−/−). (C,D) Histology of adult ovaries from heterozygous (C) and homozygous (D) littermates. (E–J) Sections stained with hematoxylin and eosin of 2-day-old (E,F), 15-day-old (G,H), and 35-day-old testes (I,J) from heterozygous (E,G,I) and homozygote (F,H,J) littermates. Testes and ovaries of heterozygous mice apparently show no differences in size and histology from those of wild type (data not shown). Bar, 200 μm in C (for C and D); 50 μm in E, G, and I (for E–J).
On the basis of the structure of the Mvh1098 targeted allele, it is assumed that the Mvh1098 transcript is degraded before translation or translated into a non-functional truncated form of the MVH protein. As shown in Figure 2B, immunoblot analysis with anti-MVH antibody raised against an amino acid sequence encoded in exon 3 (Fujiwara et al. 1994) revealed that neither the wild-type nor any truncated form of MVH protein was detected in cell extracts from Mvh1098 homozygous testes (5 weeks old). Moreover, RT-PCR analysis with primers (VG2, Vas3) generated from sequences coded in exons 8 or 11 showed that no products were obtained with homozygous testis RNA as templates (Fig. 3). These results indicate that the introduced cassette completely prevents Mvh expression from the Mvh1098 allele. MVH protein (85 kD) was detected in wild-type and heterozygous testes, although the amount of MVH protein in heterozygous testes was reduced remarkably to less than half of that found in wild-type testes (Fig. 2B). On the other hand, the expression of WT1 protein (58 kD) detected by immunoblot, which is expressed in Sertoli cells, showed no differences between the testes of different genotypes (Fig. 2B).
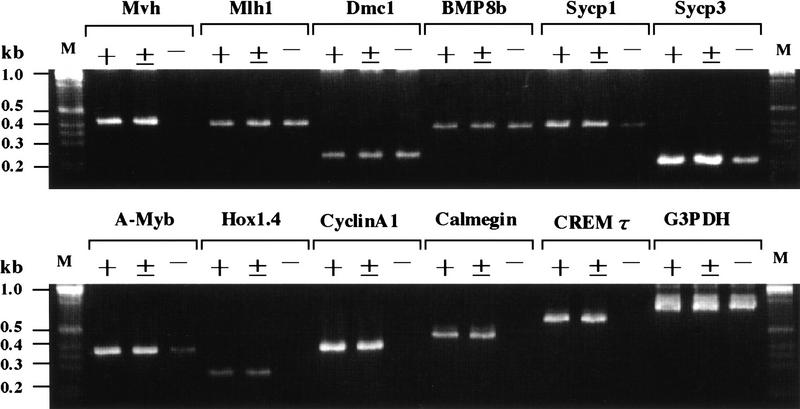
Figure 3.
Analysis of gene expression in _Mvh_-deficient mouse testes. Expression of stage-specific and germ-cell-specific genes in spermatogenesis was analyzed by RT–PCR. G3PDH was used as a standard to quantify the reaction. Total testis RNA was prepared from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) littermates 35 days after birth. Primers used in each reaction were selected to anneal to sequences in the coding exons of each gene (Table 1, Vg2 and Vas3 were used for Mvh). (M) DNA molecular marker.
The onset of meiotic impairment in Mvh1098 homozygous testes
The histological findings indicated that spermatogenic cells beyond the postmeiotic stage were completely absent in all seminiferous tubules of Mvh1098 homozygous testes, suggesting that abnormal meiosis and/or apoptosis must occur in the postmeiotic stage. RT–PCR analysis of genes expressed in germ cells of different spermatogenic stages was carried out to determine the stage at which spermatogenesis is blocked in homozygous testes. As shown in Figure 3, analysis of genes that appear in early spermatogenic cells before they reach the pachytene spermatocyte stage, the DNA mismatch repair gene Mlh-1 (Baker et al. 1996; Edelmann et al. 1996), the disrupted meiotic cDNA gene Dmc1 (Habu et al. 1996), and the bone morphogenetic protein (BMP) 8 gene (Zhao and Hogan 1996), showed no differences among genotypes. In contrast, the expression of genes that appear in pachytene spermatocytes, Hox1.4 (Rubin et al. 1986), Cyclin A1 (Sweeney et al. 1996), Calmegin (Watanabe et al. 1994), and the gene encoding the cyclic AMP-responsive element modulator isoform, _CREM_-τ (Foulkes et al. 1992), were not detected in homozygous testes, indicating the lack of pachytene spermatocytes in homozygous testes. Synaptosomal complex protein genes Sycp1 and Sycp3, which are restricted in zygotene to deplotene spermatocytes (Meuwissen et al. 1992; Sage et al. 1995; Klink et al. 1997) were detected in homozygous testes but the level of their expression was much lower than that in heterozygous and wild-type testes. Similarly, analysis of A-myb, which is expressed at a high level in type-B spermatogonia and spermatocytes ranging from the leptotene to pachytene stages (Mettus et al. 1994; Trauth et al. 1994), demonstrated a decrease in expression in homozygous testes. These results suggest that Mvh1098 homozygous male germ cells cease their differentiation program before reaching the pachytene spermatocyte stage.
Immunological analysis was performed with antibodies against stage-specific antigens of mouse spermatogenic germ cells to verify the spermatogenic stage at which Mvh1098 homozygous male germ cells showed a deficiency. First, immunoblotting with anti-calmegin (TRA369) antibody, which recognizes a calmegin protein of 93 kD expressed in cells ranging from pachytene spermatocytes to elongated spermatids (Watanabe et al. 1992), showed a specific band in wild-type and heterozygous testes but no signal in homozygous testes (Fig. 4A). The same result was obtained by immunohistochemical staining with anti-calmegin. Germ cells in and after the pachytene spermatocyte stage were stained in heterozygous testes but no positive cells were detected in homozygous testes (Fig. 4C,D). Second, TRA98 antibody, whose antigen is expressed in spermatogonia as well as in embryonic gonad PGCs (Tanaka et al. 1997), detected a band of 110-kD band in testicular extracts of all genotypes (Fig. 4B). Immunostaining with TRA98 also showed the presence of TRA98-positive cells in homozygous testes as well as in heterozygous testes (Fig. 4E,F). Third, BC7 monoclonal antibody, which recognizes early spermatocytes in the leptotene to early pachytene stages (Koshimizu et al. 1993), was used for immunostaining. BC7-positive cells certainly existed in homozygous testes; however, the positive cells were found to be much more scattered and decreased in number compared with those in heterozygous testes (Fig. 4G,H). These data, which are consistent with RT-PCR analysis, demonstrate clearly that loss of a functional Mvh gene suspends premeiotic differentiation of spermatogenic cells, most likely in the vicinity of the leptotene to zygotene stages.
Figure 4.
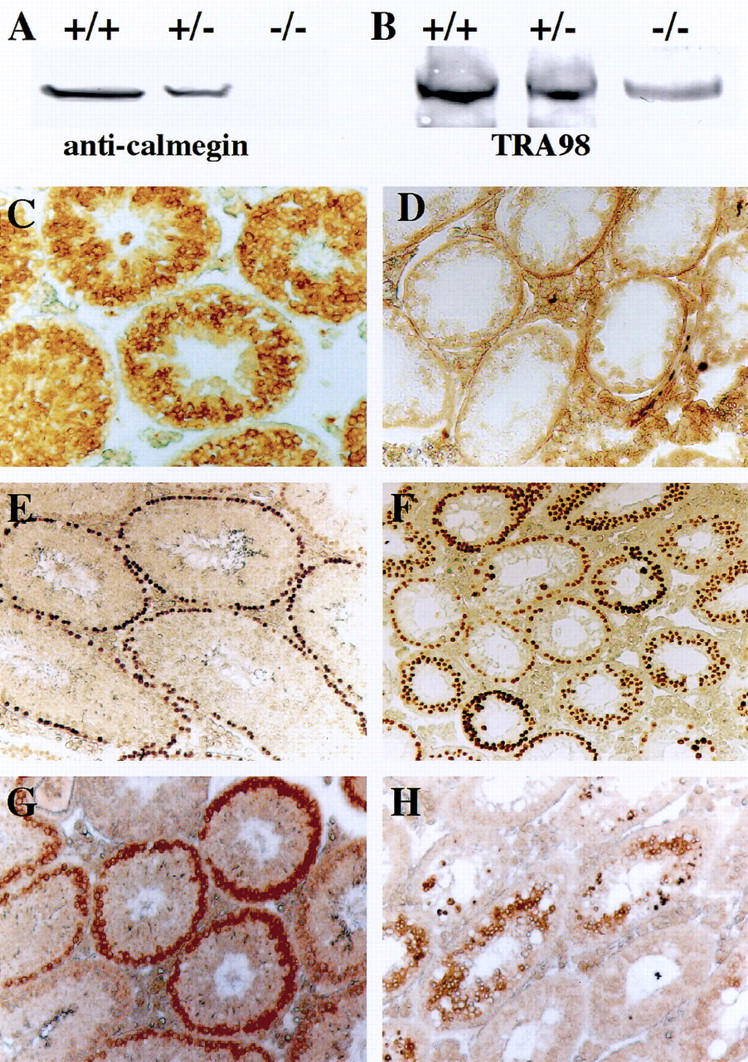
Immunohistochemical analysis of _Mvh_-deficient mouse testes. Testes from 35-day-old wild-type (+/+), heterozygous (+/−), and homozygous (−/−) littermates were analyzed using three kinds of monoclonal antibodies specific for spermatogenic cells at different stages. (A) Immunoblot analysis using anti-calmegin (TRA369) shows a 93-kD band in wild-type and heterozygous mice but not in homozygous. (B) Immunoblot analysis using TRA98 shows a 110-kD band in all genotypes. (C–H) Immunostaining with anti-calmegin (C,D), TRA98 antibody (E,F) and BC7 antibody (G,H). In a heterozygous testis (C,E,G), the seminiferous tubules contain TRA98-positive spermatogonia, BC7-positive spermatocytes, and anti-calmegin-positive spermatids. In contrast, tubules of a homozygous mouse (D,F,H) show TRA98-positive cells and a small number of BC7-positive cells but no anti-calmegin positive cells. In D, the staining in the basement membrane of seminiferous tubules and interstitial cells is nonspecific coloration due to overexposure to the reaction substrates.
Increased apoptosis and ectopic germ cells in Mvh1098 homozygous mouse testes
To investigate the continuous degeneration of early spermatocytes in homozygous mutant mice, TUNEL labeling was carried out to detect apoptotic cell death. As shown in Figure 5, in both homozygous and heterozygous neonatal testes, TUNEL-labeled cells were rarely detected with our detection procedure (Fig. 5A,B). However, in heterozygous and homozygous testes 15 and 35 days after birth, a significant number of TUNEL-labeled cells were detected in their spermatocyte layers. Furthermore, a much higher number (∼10-fold) of labeled cells was found in the homozygous testes compared with those in heterozygous testes (Fig. 5C–F). These results indicate that meiotic-arrested germ cells in homozygous mutant testes undergo apoptotic cell death and are subsequently eliminated from the seminiferous tubules, most likely by the phagocytic activity of Sertoli cells.
Figure 5.
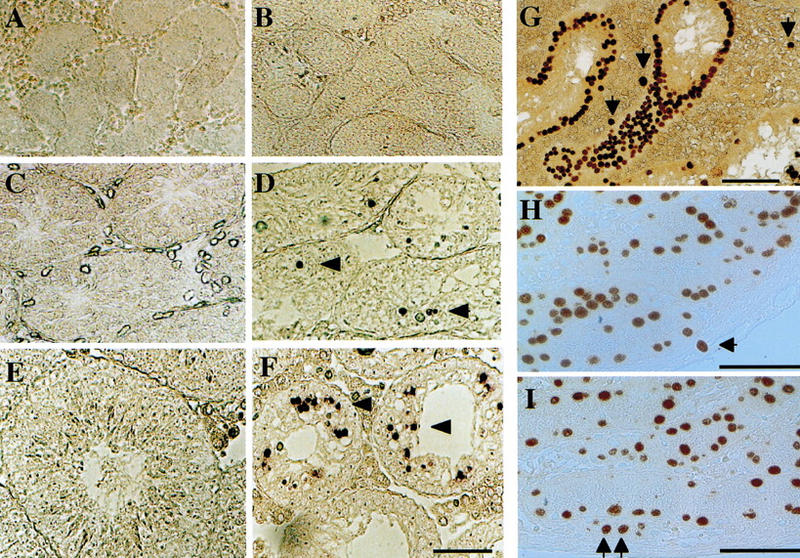
TUNEL labeling of _Mvh_-deficient mouse testes. Testes from heterozygous (A,C,E) and homozygous (B,D,F) littermates were stained with TUNEL labeling to detect in situ apoptotic cells. (A–F) Testis sections from 2-day-old (A,B), 15-day-old (C,D), and 35-day-old mice (E,F). Numerous apoptotic cells (arrowheads) are detected in seminiferous tubules of homozygous _Mvh_-mutant mice. (G–I) Ectopic germ cells in _Mvh_-deficient mouse testes. Testis sections from a 1-year-old homozygous mouse (G), a 4-day-old heterozygote (H) and a homozygous littermate mouse (I) were stained with TRA98 antibody. (Arrow) Ectopic germ cells localized outside of the seminiferous tubules. Bar in F for A–F and bars in G–I, 50 μm.
Histological examination of 1-year-old homozygous adult testes revealed some abnormally shaped cells in interstitial areas, a finding that is never observed in wild-type mice. Immunostaining with TRA98 antibody, which is specific for spermatogonia and PGCs as indicated above, revealed that these were TRA98-positive germ cells residing ectopically outside of the seminiferous tubules (Fig. 5G). Similarly, the ectopic germ cells were found in neonatal testes of heterozygous and homozygous mice (Fig. 5H,I). Because all of the ectopic cells are recognized by TRA98 but not by BC7 or anti-calmegin antibodies (Fig. 4), the ectopic germ cells seem to be maintained throughout their lifespan without forming tumors or differentiating into mature spermatocytes.
Abnormal development of male gonadal PGCs in Mvh1098 homozygous embryos
One of our previous studies indicated (Fujiwara et al. 1994) that onset of MVH protein expression takes place in PGCs after their entry into both male and female genital ridges. At that time, PGCs first interact with gonadal supporting cells such as pre-Sertoli and prefollicle cells. Therefore, it is of interest to ascertain whether the loss of Mvh function causes any aberrations in the development of embryonic germ cells. First, PGCs in mutant embryonic gonads were examined with an AP-staining method. In 11.5 dpc male and female gonads, AP-positive PGCs showed no differences in either the number or localization pattern among any of the genotypes (Fig. 6A–C), indicating that PGCs migrate and invade normally into embryonic gonads even in Mvh1098 homozygous mice. In contrast, in 12.5 dpc embryos, although PGCs in female gonads were comparable in all genotypes (Fig. 6H,I), PGCs in homozygous male gonads were observed to be remarkably decreased in number, and PGCs in heterozygous gonads also showed a significant decrease compared with those in wild-type gonads (Fig. 6D–F). In addition, it was found that some PGCs were located outside of developing testis cords in both homozygous and heterozygous male gonads, a finding that was seldom observed in wild-type gonads at 12.5 dpc (Fig. 6G).
Figure 6.
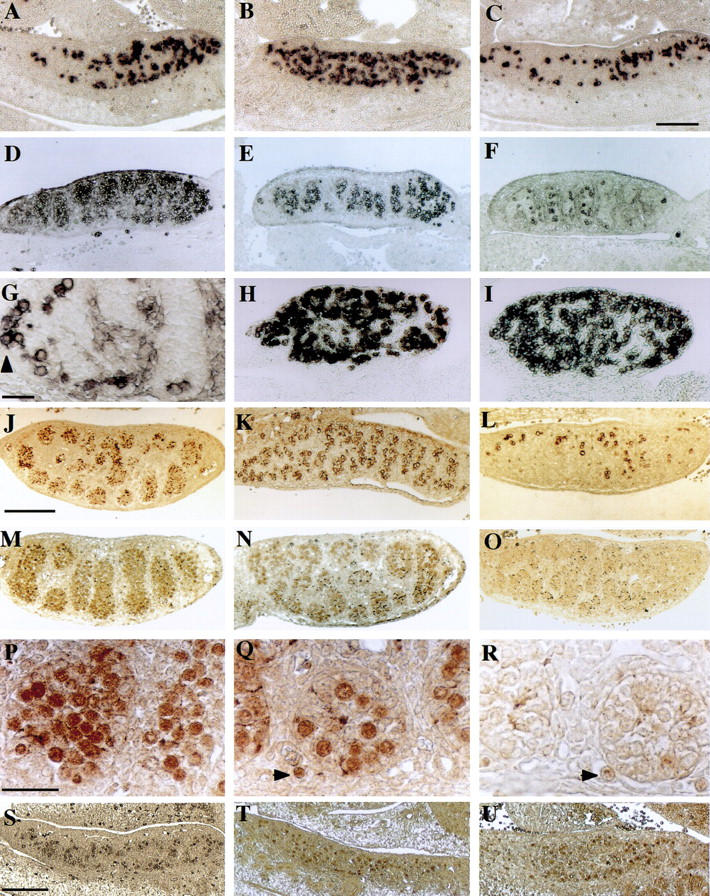
Sections of gonads from _Mvh_-deficient mouse embryos. (A–C) AP staining of male 11.5 dpc gonads from wild-type (A), heterozygous (B), and homozygous (C) littermate embryos. Serial sections revealed that numbers of AP-positive PGCs per gonad were 1960, 1796, and 1682 cells in wild-type, heterozygous, and homozygous embryos, respectively. (D–G) AP staining of male 12.5 dpc gonads from wild-type (D), heterozygous (E), and homozygous (F), embryos. (G) High power image of testicular cords of a homozygous male gonad. Some AP-positive cells are present outside of the cords (arrowhead). (H,I) AP staining of female 12.5 dpc gonads from heterozygous (H) and homozygous (I) littermates. (J–L) Sections of 12.5 dpc gonads from wild-type (J), heterozygous (K), and homozygous (L) littermate embryos were stained with 4C9. (M–R) Sections of 12.5 dpc gonads from wild-type (M), heterozygous (N), and homozygous (O) littermates were stained with anti-OCT3/4. High magnification images of the stained testicular cords are presented in wild type (P), heterozygote (Q), and homozygote (R). (Arrowheads) Ectopic PGCs located outside of the cords. (S–U) Anti-OCT3/4 staining of 11.5 dpc gonad sections from wild-type (S), heterozygous (T), and homozygous (U) littermates. Bar in C for A–F and H–I, 200 μm; bar in G, 20 μm; bar in J for J–O, 400 μm; bar in P for P–R, 50 μm; bar in S for S–U, 200 μm.
To ascertain whether Mvh deficiency causes loss of endogenous AP activity in some gonadal PGCs, 4C9 (Yoshinaga et al. 1991) and anti-OCT3/4 antibody (Saijoh et al. 1996) were used to verify the presence of PGCs. Antigens to these two antibodies have been well characterized as specific markers for migrating and gonadal PGCs as well as for totipotent stem cells. 4C9 staining showed almost the same pattern as AP staining (Fig. 6J–L), indicating that the decrease in AP-positive cells is due to a decrease in PGC number rather than to a loss of AP activity in some potential PGCs. Similarly, OCT-3/4-positive cells were found to be decreased in the heterozygous male gonad (Fig. 6M,N,P,Q). However, it is noteworthy that OCT-3/4-positive cells in homozygous male gonad were hardly detectable at 12.5 dpc (Fig. 6O,R), although no remarkable difference was found in OCT-3/4 staining in 11.5 dpc gonads between all genotypes (Fig. 6S–U).
Proliferation of gonadal PGCs is decreased in Mvh1098 homozygous embryos
Among dissociated cells from 11.5 dpc embryonic gonads, MVH expression was specifically detected in PGCs (Fig. 7A,B). Therefore, the decrease in the number of PGCs observed in Mvh1098 homozygous male 12.5 dpc gonads seems to be due to a cell-autonomous effect resulting from the loss of Mvh function. To test whether the decrease is due to an abnormality in the proliferation or survival of PGCs, BrdU incorporation assays were performed on 2-hr cultures of gonadal cells prepared from male 11.5 dpc littermate embryos. The results showed that the proportion of BrdU-positive PGCs in homozygous gonads was significantly lower than that in wild-type and heterozygous gonads (Fig. 7C–E). In contrast, no significant increase of TUNEL-labeled cells was detected in the homozygous male 12.5 dpc gonads compared with those in wild-type littermate gonads (Fig. 7F,G). These results suggest that the loss of Mvh function mainly reduces the proliferative activity of male germ cells after they reside in genital ridges.
Figure 7.
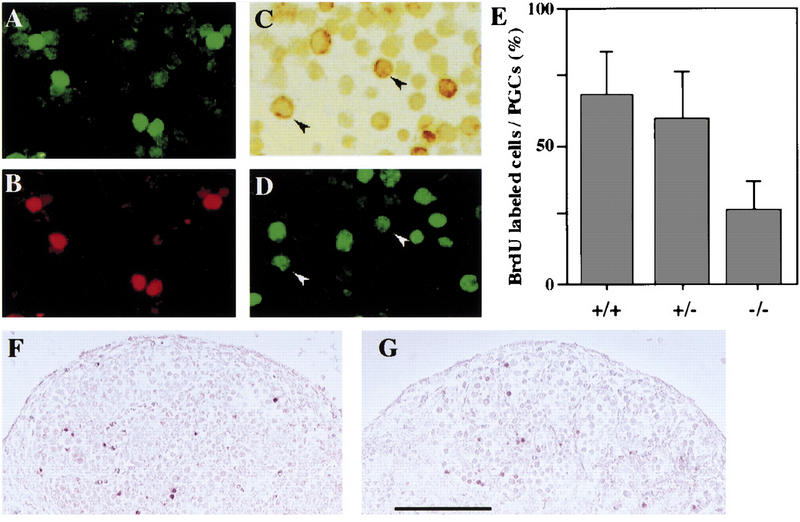
Proliferation of gonadal PGCs from _Mvh_-deficient mouse embryos. Cells dissociated from wild-type male 11.5 dpc gonads were double-stained with 4C9 using FITC-conjugated anti-rat IgG (A) and anti-MVH using Texas Red-conjugated anti-rabbit IgG (B), showing that 4C9-positive cells were the same as MVH-positive cells. (C,D) Dissociated cells of male 11.5 dpc gonads from wild-type, heterozygous, and homozygous littermate embryos were BrdU labeled and stained with 4C9 using the HRP-complex system. A typical image of double staining is shown in C (4C9) and D (BrdU). (Arrowheads) BrdU-positive PGCs. (E) Shaded bars represent the mean (±sd) proportion of 4C9-positive PGCs incorporating BrdU: wild-type (67.7 ± 15.6), heterozygous (52.3 ± 18.4), and homozygous (27.5 ± 10.6) gonads. (F,G) Sections of male 12.5 dpc gonads from wild-type (F) and homozygous (G) littermates were stained by TUNEL labeling. Bar in G for F, 200 μm.
Discussion
In this report, we generated a mutation of the mouse Vasa homolog Mvh gene, and its analysis demonstrated that Mvh function was required for male germ cell development but had no direct connection with the allocation of germ-line precursor cells. In Drosophila, zygotic expression of Vasa also initiates in PGCs around the time of their migration to the embryonic gonads following which it continues in gonadal germ cells proceeding through both oogenesis and spermatogenesis. The abrogation of zygotic Vasa results in deficiencies of multiple aspects of oogenesis such as cystocyte division and oocyte differentiation, whereas no obvious effect is found in spermatogenesis (Lasko and Ashburner 1990; Tomancak et al. 1998; Styhler et al. 1998). Therefore, it seems likely that although mammals do not exhibit maternal effects of Vasa expression nor a cell-fate determination system controlled by maternal inheriting factors, the zygotic expression and function of Vasa is essentially conserved throughout evolution with the mutation phenotypes becoming visible during the gametogenesis of different sexes between the fruit fly and mouse.
Mvh is required for premeiotic differentiation in spermatogenesis
Spermatogenesis in _Mvh_-deficient mouse is blocked at the premeiotic stage from leptotene to zygotene. The lack of Mvh function causes germ cells to cease differentiation into pachytene spermatocytes and to undergo apoptotic cell death. During normal spermatogenesis in mammals, germ cell deletion by apoptotic death is estimated to result in a 25%–75% decrease in the potential number of mature sperm, and spermatocytes are regarded as the major cell-type undergoing apoptosis (Allan et al. 1992; Billig et al. 1995). It has been suggested that the degeneration of germ cells may be a regulatory mechanism for eliminating cells with abnormal chromosomes (Oakberg 1956). In addition, several knock-out mouse strains have been generated that exhibit apoptotic cell death of spermatogenic cells. For instance, _CREM_-mutant mice experience postmeiotic arrest at the first stage of spermatogenesis, and the degraded germ cells undergo apoptosis (Blendy et al. 1996; Nantel et al. 1996). A-myb mutant mice show meiotic arrest of spermatogenesis at the pachytene stage and apoptotic cell death of the meiotic germ cells (Toscani et al. 1997). In general, it seems likely that cells lacking a gene that is essential for normal spermatogenesis are degenerated by apoptosis just after the arresting step. Therefore, the increase in apoptotic cells in _Mvh_-deficient testes is most likely due to the fact that Mvh function is essential for the differentiation rather than for the survival of male germ cells.
Similar to A-myb mutant mice, the loss-of-function of several genes involved in the meiotic machinery causes both meiotic arrest in spermatogenesis and male infertility. Targeted gene disruptions of the Hsp-70.2 gene, which is required for CDC2 kinase activity in meiosis (Zhu et al. 1997; Dix et al. 1996), and several DNA repair genes, MLH1 (Baker et al. 1996; Edelmann et al. 1996), HR6B (Roest et al. 1996), and Msh5 (de Vries et al. 1999; Edelmann et al. 1999), result in a meiotic deficiency with aberrant chromosomal synapsis, and postmeiotic cells undergo apoptosis. Inactivation of ATM, a gene encoding a protein kinase involved in DNA metabolism and the mouse homolog of the gene responsible for the inherited human disease ataxia–telangiectasia, leads to abnormal chromosomal synapsis at the zygotene/pachytene stage (Barlow et al. 1996; Elson et al. 1996; Xu et al. 1996). Compared with these meiotic genes, the Mvh mutation defect appears at an earlier spermatogenic stage. Detailed analyses of Mvh1098 mice testes revealed a complete lack of pachytene spermatocytes and postmeiotic cells (Figs. 3 and 4). Their spermatogenesis appeared to be arrested at a stage ranging from leptotene to zygotene, as cells recognized with the leptotene-specific antibody (BC7) were present in the homozygous testes at a much lower frequency than in heterozygous testes, and the expression of Sycp1 and Sycp3, predominantly transcribed in zygotene to deplotene spermatocytes (Meuwissen et al. 1992), was decreased significantly in the homozygous testes. These findings suggest that Mvh plays a crucial role in the normal progression of meiotic prophase leading to the onset of chromosomal rearrangement for meiotic cell division. In this connection, anti-MVH staining indicates that the highest expression of MVH protein is detected in the cytoplasm of early spermatocytes, and, subsequently, MVH becomes localized within the chromatoid bodies in postmeiotic spermatids in normal testes (Toyooka et al. 2000). The chromatoid body in spermatids is an electron-dense perinuclear granule whose structural composition appears to be identical to the polar granule in Drosophila oocytes (Russell and Frank 1978; for review, see Eddy 1975). The formation of a chromatoid body is first recognized in early spermatocytes as several small particles associated with a mitochondrial cluster, corresponding to the period during which the critical deficiency appears in the _Mvh_-mutant testes.
Effects of Mvh deficiency on the development of male gonadal PGCs
Some mutations of genes expressed in germ-line cells are known to reduce the population of PGCs before and after their homing to the genital ridges. Mutations of c-kit, which encodes a receptor tyrosine kinase (Besmer et al. 1993), and β1-integrins (Anderson et al. 1999) mainly affect the migratory process of PGCs. A deficiency in tiar, which encodes an RNA-binding protein, causes PGCs to die around 11.5 dpc (Beck et al. 1998).
In 11.5 dpc embryonic gonads of _Mvh_-deficient mice there were no differences in the number of PGCs residing in the gonads of wild-type and heterozygous littermates. This result indicates that the _Mvh_-mutation has no effect on the establishment and migration processes of PGCs. In contrast, in 12.5 dpc embryos, PGCs in the homozygous male gonads were remarkably decreased in number (Fig. 6A–F). BrdU incorporation assays demonstrated that PGC proliferation was significantly reduced in homozygous male gonads (Fig. 7). This finding suggests that the DEAD-box RNA helicase is involved in regulating germ cell proliferation.
After entering the genital ridge, PGCs undergo five to six division cycles from 10.5 to 12.5 dpc (Tam and Snow 1981). Subsequently, they stop dividing by 13.5 dpc, and those in the female enter meiotic prophase, whereas those in the male undergo mitotic arrest in the G1 stage of the cell cycle according to sexual differentiation. This proliferation and differentiation of PGCs is believed to proceed under control of surrounding gonadal somatic cells (McLaren 1994). Therefore, a close association between the Mvh deficiency and the germ/soma interaction is postulated as follows.
First, the Mvh expression profile that is initiated in PGCs just after the PGCs settle in the gonads indicates that the expression is dependent on the cross talk between the PGCs and surrounding supporting cells. Indeed, EG cells derived from migrating PGCs can be induced to express the MVH protein by co-culturing with gonadal somatic cells prepared from 12.5 dpc embryos (Toyooka et al. 2000), suggesting that MVH expression reflects a key transition induced by a signal(s) from supporting cells. Similarly, several nuclear proto-oncogenes such as c-myc, c-fos, and c-jun are known to change their expression in PGCs during these periods (Coucouvanis and Jones 1993). In relation to this, it has been shown that proliferating PGCs obtained from 11.5–12.5 dpc embryos rapidly undergo apoptotic death when cultured in vitro in the absence of somatic supporting cells (Donovan et al. 1986; Pesce et al. 1993).
Second, in this study we have shown that OCT-3/4 expression in male PGCs of 12.5 dpc Mvh1098 homozygous embryos decreased to an undetectable level (Fig. 6M–N). Oct-3/4 is a well-known gene specific for totipotent embryonic stem cells and germ-line cells in mammals, and it is believed to be involved in the maintenance of the totipotency. OCT-3/4 expression in normal germ cells is sustained with a gradual decrease until after birth in males, whereas in females it ceases to be expressed upon entry of germ cells into meiotic prophase around 13.5 dpc (Pesce et al. 1998). As an interpretation of our finding, we think it is more likely that it is an indirect effect due to a change in the developmental potency of _Mvh_-deficient PGCs, although we cannot exclude the possibility that Oct 3/4 expression is under the direct control of MVH function in a stage-specific manner.
Third, we found that a significant number of PGCs remain localized outside of the developing testicular cords in both homozygous and heterozygous 12.5 dpc male gonads (Fig. 6G,Q,R). Similarly, some ectopic germ cells remained outside of the seminiferous tubules in postnatal _Mvh_-deficient testes (Fig. 5). It has been found that some PGCs fail to become incorporated into the testicular cords and instead reside in the interstitial region of gonads or in extragonadal tissues such as the adrenal and mesonephric tissues (Zamboni and Upadhyay 1983). Some of these ectopic PGCs enter the meiotic prophase and some mitotically arrest (Francavilla and Zamboni 1985). Therefore, it is conceivable that _Mvh_-deficient male PGCs are under conditions that are similar to ectopic PGCs residing outside of the testicular cords and that the ectopic cells survive in the Mvh mutant gonads. Furthermore, together with the fact that no small numbers of germ cells were found in the homozygous neonatal testes (Fig. 5I), impairments resulting from the loss of Mvh function would not be fatal for the survival of PGCs and spermatogonia. For these reasons, it is conceivable that the loss of MVH function causes gonadal PGCs to interrupt their developmental pathway under the control of gonadal supporting cells.
Although the relationship with Mvh function is not clear at present, a similar reduction in the PGC population and subsequent meiotic impairment has also been reported in the case of gene disruption of Dazla, a homolog gene of human Deleted in Azoospermia (Ruggiu et al. 1997). In _Dazla_-deficient mouse, meiotic arrest occurs in the pachytene stage in both male and female gametogenesis. Homozygous gonads of 15 dpc embryos appear normal, however, by 19 dpc, male germ cells undergo apoptosis to a great degree than the wild-type.
Mvh is not essential for female germ cell development
Although MVH was specifically expressed in both male and female germ cells, disruption of the Mvh gene had no effect on the development of female germ cells. Interestingly, the zygotic function of the Vasa gene in Drosophila is essential for oogenesis but not for spermatogenesis. It is quite possible that another DEAD-box family gene(s) that is functionally redundant is expressed during spermatogenesis (Hay et al. 1988; Lasko and Ashburner 1990). A similar possibility may apply to the mouse. However, no duplicated gene highly homologous to Mvh has been found in the mouse genome. Genomic Southern blot analysis has revealed no additional signal other than Mvh derivatives even under relatively low-stringency conditions (Fujiwara et al. 1994). On the other hand, by use of cDNA prepared from Mvh1098 homozygous ovaries and 12.5 dpc gonads as templates, PCR amplification with degenerate primers for DEAD-family genes showed products containing a partial sequence of PL10 (data not shown), which had been reported to be a spermatogenic cell-specific DEAD-family gene (Leroy et al. 1989). Moreover, it has been shown that the embryonic RNA helicase gene ERH, with high sequence similarity to PL10, was expressed in oocytes as well as other tissues like kidney and brain (Sowden et al. 1995). Therefore, PL10 and ERH are regarded as the most likely candidates for the functional redundancy with Mvh.
Evolutionary conservation of Vasa family genes
In this study, we have demonstrated that a mouse homolog gene to Vasa plays a crucial role in germ cell development, indicating that the functional requirement is essentially conserved in the evolution of animal species between the fruit fly and the mouse. In Drosophila, Vasa is present in the germ-cell determining machinery and performs its function in a cascade with other molecules, i.e., Oskar, Tudor, and Nanos (Rongo and Lehmann 1996). It has been demonstrated that Vasa functions at least in part through translational regulation of several target mRNAs in germ-line cells of different stages, e.g., Oskar and Nanos mRNAs in pole cell formation and Gurken mRNA in oogenesis (Gavis et al. 1996; Dahanukar and Wharton 1996; Tomancak et al. 1998; Styhler et al. 1998). Assuming an analogous molecular function, it is likely that MVH acts as a translational regulator for the translation of different target mRNAs in spermatogenic cells and PGCs.
From the viewpoint of diversity of animal species, it is highly significant to investigate what is conserved or changed in the regulatory mechanisms of germ-line development, which forms the basis of phylogenic evolution. Among the genes involved in the germ-line determination system in Drosophila, Vasa is known as the only one for which homologous genes have been identified in many animals, including mammals. Therefore, further investigations into the mechanisms controlling the expression and function of MVH should provide important clues for understanding the features of mammalian germ cell development.
Materials and methods
Construction of the Mvh targeting vector
Genomic DNA clones of the Mvh locus were isolated from a mouse (129/Svj) FixII-phage genomic library (Stratagene). The genomic structure of a Mvh genomic clone covering five exons from 7 to 11 was analyzed by restriction mapping and sequencing (Fig. 1). A replacement targeting vector, KO1098, was constructed using a 3.0-kb Hin_dIII–_Bgl_II fragment and a 1.2-kb Xho_I–_Hin_dIII fragment for the 5′ and 3′ flanking homologous regions, respectively. A 4.5-kb segment containing exons 9 and 10 between both flanking regions was replaced by an IRES–_lacZ (MacGregor et al. 1995) and a pGK–_neo cassette (Boer et al. 1990), resulting in a deletion of exons encoding the ATPase domain of MVH. A cassette of MC1_DT-A_ (Yagi et al. 1990) was attached to the 3′ end of the 3′ flanking region for negative selection. The IRES–lacZ was used to trace cells expressing Mvh. However, the resulting mutant allele showed no expression of lacZ, probably because the splicing acceptor sequence was unexpectedly deleted when the cassette was inserted into the vector plasmid. The KO1098 targeting construct was cut out of the plasmid and linearized by _Not_I digestion.
Generation of recombinant ES cells and mouse chimeras
One hundred micrograms of KO1098 DNA was electroporated into a total of 2 × 107 E14TG2a ES cells with a single pulse at 1000 V, 3 μF in 800 μl of PBS. About 24 hr after electroporation, G418 (Sigma) was added at a final concentration of 175 μg/ml. The desired recombination events were verified by Southern blot analysis using a 3.1-kb _Pst_I–_Hin_dIII fragment and a 1.4-kb _Hin_dIII–_Eco_RI fragment external to the 5′ and 3′ ends of the targeting construct, respectively, as probes. Two targeted ES clones out of 213 G418-resistant clones were sorted out and used for host blastocyst (C57BL/6Njcl) injection according to a method described previously (Hogan et al. 1994). Mice were genotyped by PCR with three primers, two forward primers, Neo3 and Vas1 and one reverse primer, Vas3 (Table 1). All analyses were carried out with mice of a mixed genetic background of 129/sv and C57BL/6Njcl.
Table 1.
PCR primers used in this study
| Gene | PCR primers (5′ → 3′) |
|---|---|
| Mvh | |
| Vas1 | GCTCAAACAGGGTCTGGGAAG |
| Vg2 | CCAAAAGTGACATATATACCC |
| Vas3 | TTGGTTGATCAGTTCTCGAG |
| Neo | |
| Neo3 | CGCCTTCTATCGCCTTCTTGACGAGT |
| Dmc1 | TTCGTACTGGAAAAACTCAGCTGTATC |
| CTTGGCTGCGACATAATCAAGTAGCTCC | |
| Mlh1 | AGGAGCTGATGCTGAGGC |
| TTTCATCTTGTCACCCGATG | |
| CyclinA1 | ATGCATCGCCAGAGCTCCAAGAG |
| GGAAGTGGAGATCTGACTTGAGC | |
| A-Myb | AAGAAGTTGGTTGAACAACACGG |
| AGGAAGTAACTTAGCAATCTCGG | |
| Calmegin | ATATGCGTTTCCAGGGTGTTGGAC |
| GTATGCACCTCCACAATCAATACC | |
| Bmp-8b | CGCAACATGGTAGTCCAGGC |
| GGATACTGAAGAGCCTGAGC | |
| CREM-τ | GATTGAAGAAGAAAAATCAGA |
| CATGCTGTAATCAGTTCATAG | |
| HoxA4 | TGAGCGCTCTCGAACCGCCTATACC |
| GATGGTGGTGTGGGCTGTGAGTTTG | |
| Sycp-1 | ATGGAGAAGCAAAAGCCCTTC |
| TTTCTGCTTCAGTTCAGATTC | |
| Sycp-3 | GGTGGAAGAAAGCATTCTGG |
| CAGCTCCAAATTTTTCCAGC | |
| G3PDH | ACCACAGTCCATGCCATCAC |
| TCCACCACCCTGTTGCTGTA |
RT–PCR analysis
Total RNA was isolated from adult testes of the wild-type, heterozygous, and homozygous littermate mice using Trizol reagent (GIBCO BRL). Single-strand cDNAs were prepared by reverse transcription of 5 μg of testis RNA (Superscript; GIBCO BRL). Each PCR amplification was performed in a 50-μl reaction mixture containing 100 ng of cDNA and 5 ng/ml of each primer by use of the Ex-taq system (Takara-Shuzo, Kyoto, Japan). The sequences of the primers used in this study are listed in Table 1. The cycling conditions were as follows: 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C (25 cycles) followed by 5 min at 72°C (1 cycle).
Immunoblot analysis
Equal amounts of protein (20 μg) from adult testes of each genotype were analyzed by immunoblotting as described previously (Fujiwara et al. 1994). Rabbit polyclonal antibodies against MVH (Fujiwara et al. 1994) and Wilm's tumor suppressor (WT1) protein (Santa-Cruz) were diluted 1:1000, and TRA98 (Tanaka et al. 1997) and TRA369 (anti-calmegin: Watanabe et al. 1992) rat monoclonal antibodies were diluted 1:500 in PBS and 0.1% Tween-20. After incubation with a 1:1000 dilution of either alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG (Bio-Rad) or AP-conjugated anti-rat IgG (Boehringer Mannheim), AP activity was detected by use of NBT and BCIP as substrates.
Histological analyses of postnatal testes
Dissected testes and ovaries were fixed in Bouin's solution (Sigma) for 24 hr at 4°C, progressively dehydrated in a graded ethanol series, and embedded in paraffin (Paraplast; Oxford Labware). Mounted sections (5 μm thick) were used for terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) reactions, for immunohistochemical staining, and for hematoxylin/eosin staining. For the TUNEL reactions, sections were treated with proteinase-K (20 μg/ml) for 15 min at 25°C. After washing with PBS, specimens were incubated with TdT buffer containing 300 U/ml TdT (GIBCO) and 20 mm fluorescein-11-dUTP (Amersham) for 2 hr at 37°C, and then incubated in a 1:500 dilution of AP-conjugated anti-fluorescein (Amersham) for 30 min. End-labeled signals were detected by enzymatic colorization using NBT and BCIP. For the immunohistochemical analysis, sections were incubated with either a 1:1000 dilution of TRA98 or a 1:10 dilution of BC7 in PBS at 4°C overnight. For TRA369 staining, testes were fixed with 4% paraformaldehyde dissolved in PBS for 24 hr at 4°C, rapidly frozen in dry ice-ethanol and embedded in OTC compound (Tissue-Tek). After blocking treatment, frozen sections (10 μm thick) were incubated with a 1:1000 dilution of TRA369 at 4°C overnight. The specimens were then reacted with a 1:100 dilution of horseradish peroxidase (HRP)-conjugated anti-rat IgG (Cappel) for 1 hr at room temperature, and the detection was performed by HRP reaction with DAB as the substrate.
Histological analyses of embryonic gonads
Littermate embryos of 11.5 and 13.5 dpc were fixed in absolute ethanol-glacial acetic acid (7:1) at 4°C overnight, progressively dehydrated, and embedded in paraffin. Sexing of the 11.5 dpc embryos was performed by genomic PCR with primers complementary to the Sry gene (Toyooka et al. 1998). For AP staining, sections (5 μm) were reacted with NBT and BCIP as substrates. For immunostaining with anti-MVH and anti-OCT-3/4 rabbit antibodies and 4C9 rat monoclonal antibody (purchased from Funakoshi, LEX-2), specimens were reacted with a 1:300 dilution of each antibody overnight at 4°C as described previously (Yoshinaga et al. 1991; Fujiwara et al. 1994; Saijoh et al. 1996). Signals were detected with Texas Red-conjugated anti-rabbit IgG, FITC-conjugated anti-rat IgG antibodies (Amersham), or an avidin/biotin-conjugated HRP complex system (Vectastain ABC kit; Vector Lab.) with DAB as the substrate according to the manufacturer's instructions.
Bromodeoxyuridine (BrdU) incorporation was assayed using the in situ Cell Proliferation Kit (FLUOS, Roche). Gonads from 11.5 dpc embryos were dissociated with 0.25% trypsin-EDTA (GIBCO), and the resulting cell suspension was cultured in DMEM plus 10% FCS. After 2 hr labeling with BrdU (10 μm), cells were fixed and double-stained for 4C9 and for BrdU according to the manufacturer's instructions. The proportion of BrdU-labeled PGCs was assessed.
Acknowledgments
We are indebted to Dr. Yoshitake Nishimune for TRA98, BC7 and anti-calmegin monoclonal antibodies, Drs. Yukio Saijoh and Hiroshi Hamada for anti-OCT3/4, and Dr. Austin G. Smith for providing us E14TG2a ES cells. We are very grateful to Dr. Yuko Fujiwara and Dr. Colin Stewart for helpful cooperation in initiating on a course of this study.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL noce@libra.ls.m-kagaku.co.jp; FAX 81-427-24-6314.
References
- Allan DJ, Harmon BV, Roberts SA. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Prolif. 1992;25:241–250. doi: 10.1111/j.1365-2184.1992.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Anderson R, Fassler R, Georges-Labouesse E, Hynes RO, Bader BL, Kreidberg JA, Schaible K, Heasman J, Wylie CC. Mouse primordial germ cells lacking beta1 integrins enter the germline but fail to migrate normally to the gonads. Development. 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–342. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh A. Apoptosis in testis germ cells: Developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- Beck ARP, Miller IJ, Anderson P, Streuli M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc Natl Acad Sci. 1998;95:2331–2336. doi: 10.1073/pnas.95.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer, P., K. Manova, R. Duttlinger, E. J. Huang, A. Packer, C. Gyssler, and R.F. Bachvarova. 1993. The kit-ligand (steel factor) and its receptor c-kit/W: Pleiotropic roles in gametogenesis and melanogenesis. Development Suppl. 125–137. [PubMed]
- Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- Boer PH, Potten H, Adra CN, Jardine K, Mullhofer G, McBurney MW. Polymorphisms in the coding and noncoding regions of murine Pgk-1 alleles. Biochem Genet. 1990;28:299–308. doi: 10.1007/BF02401419. [DOI] [PubMed] [Google Scholar]
- Coucouvanis CE, Jones PP. Changes in protooncogene expression correlated with general and sex specific differentiation in murine primordial germ cells. Mech Dev. 1993;42:49–58. doi: 10.1016/0925-4773(93)90097-h. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Wharton RP. The Nanos gradient in Drosophila embryos is generated by translational regulation. Genes & Dev. 1996;10:2610–2620. doi: 10.1101/gad.10.20.2610. [DOI] [PubMed] [Google Scholar]
- de Vries SS, Baart EB, Dekker M, Siezen A, de Rooij DG, de Boer P, de Riele H. Mouse MutS-like protein Msh5 is required for proper chromosome synapsis in male and female meiosis. Genes & Dev. 1999;13:523–531. doi: 10.1101/gad.13.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan PJ, Stott D, Godin I, Heasman J, Wylie CC. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell. 1986;44:831–838. doi: 10.1016/0092-8674(86)90005-x. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kane M, Lau K, Morrow B, Bennett S, Umar A, Kunkel T, Cattoretti G, Chaganti R, et al. Meiotic pachytene arrest in MLH1-deficient mice. Cell. 1996;85:1125–1134. doi: 10.1016/s0092-8674(00)81312-4. [DOI] [PubMed] [Google Scholar]
- Edelmann W, Cohen PE, Kneitz B, Winand N, Lia M, Heyer J, Kolodner R, Pollard JW, Kucherlapati R. Mammalian MutS homologue 5 is required for chromosome pairing in meiosis. Nat Genet. 1999;21:123–127. doi: 10.1038/5075. [DOI] [PubMed] [Google Scholar]
- Elson A, Wang Y, Daugherty CJ, Morton CC, Zhou F, Campos-Torres J, Leder P. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci. 1996;93:13084–13089. doi: 10.1073/pnas.93.23.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes NS, Mellstrom B, Benusiglio E, Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: From antagonist to activator. Nature. 1992;355:80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Fox N, Damjanov I, Martinez-Hernandez A, Knowles BB, Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- Francavilla S, Zamboni L. Differentiation of mouse ectopic germinal cells in intra- and perigonadal locations. J Exp Zool. 1985;233:101–109. doi: 10.1002/jez.1402330114. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Komiya T, Kawabata H, Sato M, Fujimoto H, Furusawa M, Noce T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc Natl Acad Sci. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis ER, Lunsford L, Bergsten SE, Lehmann R. A conserved 90 nucleotide element mediates translational repression of nanos RNA. Development. 1996;122:2791–2800. doi: 10.1242/dev.122.9.2791. [DOI] [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gruidl ME, Smith PA, Kuznicki KA, McCrone JS, Kirchner J, Roussell DL, Strome S, Bennett KL. Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc Natl Acad Sci. 1996;93:13837–13842. doi: 10.1073/pnas.93.24.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu T, Taki T, West A, Nishimune Y, Morita T. The mouse and human homologs of DMC1, the yeast meiosis-specific homologous recombination gene, have a common unique form of exon- skipped transcript in meiosis. Nucleic Acids Res. 1996;24:470–477. doi: 10.1093/nar/24.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel AC, Eddy EM. Cell surface markers of mouse primordial germ cells defined by two monoclonal antibodies. Gamete Res. 1986;15:25–34. [Google Scholar]
- Hahnel AC, Rappolee DA, Millan JL, Manes T, Ziomek CA, Theodosiou NG, Werb Z, Pedersen RA, Schultz GA. Two alkaline phosphatase genes are expressed during early development in the mouse embryo. Development. 1990;110:555–564. doi: 10.1242/dev.110.2.555. [DOI] [PubMed] [Google Scholar]
- Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55:577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Klink A, Lee M, Cooke HJ. The mouse synaptosomal complex protein gene Sycp3 maps to band C of chromosome 10. Mamm Genome. 1997;8:376–377. doi: 10.1007/s003359900638. [DOI] [PubMed] [Google Scholar]
- Komiya T, Itoh K, Ikenishi K, Furusawa M. Isolation and characterization of a novel gene of the DEAD box protein family which is specifically expressed in germ cells of Xenopus laevis. Dev Biol. 1994;162:354–363. doi: 10.1006/dbio.1994.1093. [DOI] [PubMed] [Google Scholar]
- Koshimizu U, Watanabe D, Sawada K, Nishimune Y. A novel stage-specific differentiation antigen is expressed on mouse testicular germ cells during early meiotic prophase. Biol Reprod. 1993;49:875–884. doi: 10.1095/biolreprod49.5.875. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- ————— Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes & Dev. 1990;4:905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Hage WJ. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp. 1994;182:68–84. doi: 10.1002/9780470514573.ch5. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes & Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P, Alzari P, Sassoon D, Wolgemuth D, Fellous M. The protein encoded by a murine male germ cell-specific transcript is a putative ATP-dependent RNA helicase. Cell. 1989;57:549–559. doi: 10.1016/0092-8674(89)90125-6. [DOI] [PubMed] [Google Scholar]
- Liang L, Diehl-Jones W, Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- MacGregor RG, Zambrowicz PR, Soriano P. Tissue non-specific alkaline phosphatase is expressed in both embryonic and extraembryonic lineages during mouse embryogenesis but is not required for migration of primordial germ cells. Development. 1995;121:1487–1496. doi: 10.1242/dev.121.5.1487. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germline and soma: Interactions during early mouse development. Semin Dev Biol. 1994;5:43–49. [Google Scholar]
- Mettus RV, Litvin J, Wali A, Toscani A, Latham K, Hatton K, Reddy EP. Murine A-myb: Evidence for differential splicing and tissue-specific expression. Oncogene. 1994;9:3077–3086. [PubMed] [Google Scholar]
- Meuwissen RL, Offenberg HH, Dietrich AJ, Riesewijk A, van Iersel M, Heyting C. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992;11:5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantel F, Monaco L, Foulkes NS, Masquilier D, LeMeur M, Henriksen K, Dierich A, Parvinen M, Sassone-Corsi P. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- Oakberg EF. A description of spermatogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat. 1956;99:391–414. doi: 10.1002/aja.1000990303. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Okamoto K, Ishino F, Ishino-Kaneko T, Takeda S, Toyoda Y, Muramatsu M, Hamada H. The Oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 1991;10:2997–3005. doi: 10.1002/j.1460-2075.1991.tb07850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen LC, Aasland R, Fjose A. A vasa-like gene in zebrafish identifies putative primordial germ cells. Mech Dev. 1997;66:95–105. doi: 10.1016/s0925-4773(97)00099-3. [DOI] [PubMed] [Google Scholar]
- Pesce M, Farrace MG, Piacentini M, Dolci S, De Felici M. Stem cell factor and leukemia inhibitory factor promote primordial germ cell survival by suppressing programmed cell death (apoptosis) Development. 1993;118:1089–1094. doi: 10.1242/dev.118.4.1089. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Roest HP, van Klaveren J, de Wit J, van Gurp CG, Koken MH, Vermey M, van Roijen JH, Hoogerbrugge JW, Vreeburg JT, Baarends WM, et al. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell. 1996;86:799–810. doi: 10.1016/s0092-8674(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Rongo C, Lehmann R. Regulated synthesis, transport and assembly of the Drosophila germ plasm. Trends Genet. 1996;12:102–109. doi: 10.1016/0168-9525(96)81421-1. [DOI] [PubMed] [Google Scholar]
- Roussell DL, Bennett KL. glh-1, a germ-line putative RNA helicase from Caenorhabditis, has four zinc fingers. Proc Natl Acad Sci. 1993;90:9300–9304. doi: 10.1073/pnas.90.20.9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MR, Toth LE, Patel MD, D'Eustachio P, Nguyen-Huu MC. A mouse homeo-box gene is expressed in spermatocytes and embryos. Science. 1986;233:663–667. doi: 10.1126/science.3726554. [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Speed R, Taggart M, McKay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- Russell L, Frank B. Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat Rec. 1978;190:79–97. doi: 10.1002/ar.1091900108. [DOI] [PubMed] [Google Scholar]
- Sage J, Martin L, Cuzin F, Rassoulzadegan M. cDNA sequence of the murine synaptonemal complex protein 1 (SCP1) Biochim Biophys Acta. 1995;1263:258–260. doi: 10.1016/0167-4781(95)00126-2. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Fujii H, Meno C, Sato M, Hirota Y, Nagamatsu S, Ikeda M, Hamada H. Identification of putative downstream genes of Oct-3, a pluripotent cell-specific transcription factor. Genes Cells. 1996;1:239–252. doi: 10.1046/j.1365-2443.1996.d01-237.x. [DOI] [PubMed] [Google Scholar]
- Sowden J, Putt W, Morrison K, Beddington R, Edwards Y. The embryonic RNA helicase gene (ERH): A new member of the DEAD box family of RNA helicases. Biochem J. 1995;308:839–846. doi: 10.1042/bj3080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styhler S, Nakamura A, Swan A, Suter B, Lasko P. Vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development. 1998;125:1569–1578. doi: 10.1242/dev.125.9.1569. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, Wolgemuth DJ, Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- Tam PPL, Snow MHL. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morph. 1981;64:133–147. [PubMed] [Google Scholar]
- Tam PPL, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Pereira LA, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y. A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int J Androl. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Tomancak P, Guichet A, Zavorszky P, Ephrussi A. Oocyte polarity depends on regulation of gurken by Vasa. Development. 1998;125:1723–1732. doi: 10.1242/dev.125.9.1723. [DOI] [PubMed] [Google Scholar]
- Toscani A, Mettus RV, Coupland R, Simpkins H, Litvin J, Orth J, Hatton KS, Reddy EP. Arrest of spermatogenesis and defective breast development in mice lacking A-myb. Nature. 1997;386:713–717. doi: 10.1038/386713a0. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Tanaka SS, Hirota O, Tanaka S, Takagi N, Yamanouchi K, Tojo H, Tachi C. Wilms' tumor suppressor gene (WT1) as a target gene of SRY function in a mouse ES cell line transfected with SRY. Int J Dev Biol. 1998;42:1143–1151. [PubMed] [Google Scholar]
- Toyooka, Y., N. Tsunekawa, Y. Takahashi, Y. Matsui, M. Satoh, and T. Noce. 2000. Expression and intracellular localization of mouse Vasa-homolog protein during germ cell development. Mech. Dev. (in press). [DOI] [PubMed]
- Trauth K, Mutschler B, Jenkins NA, Gilbert DJ, Copeland NG, Klempnauer KH. Mouse A-myb encodes a trans-activator and is expressed in mitotically active cells of the developing central nervous system, adult testis and B lymphocytes. EMBO J. 1994;13:5994–6005. doi: 10.1002/j.1460-2075.1994.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Sawada K, Koshimizu U, Kagawa T, Nishimune Y. Characterization of male meiotic germ cell-specific antigen (Meg 1) by monoclonal antibody TRA 369 in mice. Mol Reprod Dev. 1992;33:307–312. doi: 10.1002/mrd.1080330312. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Yamada K, Nishina Y, Tajima Y, Koshimizu U, Nagata A, Nishimune Y. Molecular cloning of a novel Ca(2+)-binding protein (calmegin) specifically expressed during male meiotic germ cell development. J Biol Chem. 1994;269:7744–7749. [PubMed] [Google Scholar]
- Wylie C. Germ cells. Cell. 1999;96:165–174. doi: 10.1016/s0092-8674(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Yagi T, Ikawa Y, Yoshida K, Shigetani Y, Takeda N, Mabuchi I, Yamamoto T, Aizawa S. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin A-fragment gene in negative selection. Proc Natl Acad Sci. 1990;87:9918–9922. doi: 10.1073/pnas.87.24.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, Hubner K, Scholer HR. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122:881–894. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Muramatsu H, Muramatsu T. Immunohistochemical localization of the carbohydrate antigen 4C9 in the mouse embryo: A reliable marker of mouse primordial germ cells. Differentiation. 1991;48:75–82. doi: 10.1111/j.1432-0436.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M. Fertile offspring derived from mammalian eggs lacking either animal or vegetal poles. Development. 1998;125:4803–4808. doi: 10.1242/dev.125.23.4803. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Hogan BL. Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech Dev. 1996;57:159–168. doi: 10.1016/0925-4773(96)00543-6. [DOI] [PubMed] [Google Scholar]
- Zhu D, Dix DJ, Eddy E M. HSP70-2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development. 1997;124:3007–3014. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]