TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity (original) (raw)
Non-technical summary
Detecting mechanical stimuli is vital to determining our responses to environmental challenges. The speed required for this process suggests ion channels are opened in response to mechanical forces. A specialised membrane protein called the TRPA1 ion channel mediates chemical based pain; however its role in mechanical pain remains unresolved. Here we show that TRPA1 contributes to the detection of mechanical stimuli in a select set of pain sensing neurons. Furthermore, we also show that acute activation of this channel enhances the mechanical responsiveness of these neurons. Finally, we also show that increasing the expression of TRPA1 causes a further enhancement in the mechanical response. These findings suggest that a drug designed to block TRPA1 would be beneficial for the treatment of numerous pathological conditions associated with mechanical pain.
Abstract
Abstract
The mechanosensory role of TRPA1 and its contribution to mechanical hypersensitivity in sensory neurons remains enigmatic. We elucidated this role by recording mechanically activated currents in conjunction with TRPA1 over- and under-expression and selective pharmacology. First, we established that TRPA1 transcript, protein and functional expression are more abundant in smaller-diameter neurons than larger-diameter neurons, allowing comparison of two different neuronal populations. Utilising whole cell patch clamping, we applied calibrated displacements to neurites of dorsal root ganglion (DRG) neurons in short-term culture and recorded mechanically activated currents termed intermediately (IAMCs), rapidly (RAMCs) or slowly adapting (SAMCs). Trpa1 deletion (–/–) significantly reduced maximum IAMC amplitude by 43% in small-diameter neurons compared with wild-type (+/+) neurons. All other mechanically activated currents in small- and large-diameter _Trpa1_−/− neurons were unaltered. Seventy-three per cent of Trpa1+/+ small-diameter neurons responding to the TRPA1 agonist allyl-isothiocyanate (AITC) displayed IAMCs to neurite displacement, which were significantly enhanced after AITC addition. The TRPA1 antagonist HC-030031 significantly decreased Trpa1+/+ IAMC amplitudes, but only in AITC responsive neurons. Using a transfection system we also showed TRPA1 over-expression in Trpa1+/+ small-diameter neurons increases IAMC amplitude, an effect reversed by HC-030031. Furthermore, TRPA1 introduction into _Trpa1_−/− small-diameter neurons restored IAMC amplitudes to Trpa1+/+ levels, which was subsequently reversed by HC-030031. In summary our data demonstrate TRPA1 makes a contribution to normal mechanosensation in a specific subset of DRG neurons. Furthermore, they also provide new evidence illustrating mechanisms by which sensitisation or over-expression of TRPA1 enhances nociceptor mechanosensitivity. Overall, these findings suggest TRPA1 has the capacity to tune neuronal mechanosensitivity depending on its degree of activation or expression.
Introduction
TRPA1 is a non-selective, calcium-permeable cation channel activated by several exogenous and endogenous compounds including mustard oil, acrolein, formalin, 4-hydroxynonenal and reactive oxygen species (Bandell et al. 2004; Bautista et al. 2005; Macpherson et al. 2007_a_,b; McNamara et al. 2007; Trevisani et al. 2007; Materazzi et al. 2008; Taylor-Clark et al. 2008). TRPA1 has also been implicated as a mechanosensor, but despite considerable study the contribution of TRPA1 to mechanosensitivity remains contentious. Interest in TRPA1 as a mechanosensor originated from its unique anykinin repeats, thought to act as a spring to gate TRPA1 in response to mechanical stimuli, and from studies in lower species (Corey, 2003; Tracey et al. 2003; Corey et al. 2004; Lin & Corey, 2005; Sotomayor et al. 2005). In higher species this observation initially appeared confirmed with TRPA1 messenger RNA expression in hair cell epithelia coinciding developmentally with the onset of mechanosensitivity (Corey et al. 2004), whilst inhibition of TRPA1 protein expression via siRNA decreased receptor cell function (Corey et al. 2004). However, these results were subsequently tempered by the observation that two different lines of _Trpa1_−/− mice failed to show deficits in auditory mechanosensation (Bautista et al. 2006; Kwan et al. 2006). The role of TRPA1 in mechanically induced pain was similarly equivocal, since only one of two lines of _Trpa1_−/− mice displayed deficits in sensing noxious punctate cutaneous mechanical stimuli. These mice had both higher mechanosensory pain thresholds than Trpa1+/+ mice and reduced responses to suprathreshold stimuli (Kwan et al. 2006). More recent data from isolated neurons (Bhattacharya et al. 2008; Vilceanu & Stucky, 2010), cell lines (Rugiero & Wood, 2009) and mechanosensitive sensory afferent fibres (Brierley et al. 2009; Kerstein et al. 2009; Kwan et al. 2009) have been interpreted both for and against TRPA1 as a mechanonociceptor. Interpretation is further complicated by reports showing TRPA1 expression in different sized neurons, contained within varying sensory ganglia (Story et al. 2003; Nagata et al. 2005; Kwan et al. 2009) and within non-neuronal structures (Purhonen et al. 2008; Doihara et al. 2009; Kwan et al. 2009; Nozawa et al. 2009). As such a mechanosensory role of TRPA1 seems to be complicated by the setting of expression, the type of neurons or afferents studied and the methodology used. In particular conflicting mechanosensory studies from isolated neurons (Bhattacharya et al. 2008; Vilceanu & Stucky, 2010) have not specifically determined if the neurons tested explicitly express TRPA1, or if TRPA1 sensitisation or over-expression alters mechanosensitivity. Therefore critical pieces of information are missing: the relationship between the level of TRPA1 expression and activation and the mechanosensory responses of individual sensory neurons. This would provide conclusive evidence for or against a mechanonociceptive role. This information is crucial since we need to know how an organism perceives environmental challenges, in particular painful stimuli, and whether this changes in acute and chronic conditions of mechanical hypersensitivity (Lewin & Moshourab, 2004; Lumpkin & Caterina, 2007; Brierley, 2010). As such resolving the mechanosensory nature of TRPA1 and its contribution to acute and chronic mechanical hypersensitivity would determine whether or not we should focus on TRPA1 as a potential therapeutic target for the numerous pathological conditions associated with mechanical allodynia and hyperalgesia. Therefore, we determined which population of dorsal root ganglion (DRG) neurons express TRPA1 and then specifically investigated the mechanosensory role of TRPA1 in these neurons using patch clamping with concurrent mechanostimulation. This utilised native sensory neurons where mechanically activated currents were evoked within millisecond latency in response to probing neurites, rather than from the cell body, which is relatively less sensitive (Hu & Lewin, 2006; Wetzel et al. 2007). This neurite probing technique has been shown to correlate well with nerve fibre recordings and together have provided corresponding information regarding the role of the stomatin-domain protein SLP3 in cutaneous mechanosensation (Wetzel et al. 2007). By employing a combination of additional techniques including immunohistochemistry, in situ hybridisation, laser capture microdissection and quantitative RT-PCR to determine TRPA1 expression and in conjunction with knockout studies, TRPA1 pharmacology and over-expression using a neuronal transfection system we have identified a crucial role for TRPA1 in regulating nociceptor mechanosensitivity.
Methods
Ethical approval
All procedures were performed in accordance with the guidelines of the Animal Ethics Committees of the Institute for Medical and Veterinary Science and the University of Adelaide, Adelaide, Australia.
Targeted deletion of Trpa1
Mice with disruption to the Trpa1 gene were generated by homologous recombination on a C57/BL6 background as we have described previously in detail (Kwan et al. 2006). However, of particular note Trpa1 knockout (–/–) mice had the entire TRPA1 pore domain deleted and replaced with placental alkaline phosphatase (PLAP) reporter gene (Kwan et al. 2006). Subsequently, separate lines of _Trpa1_−/− and wild-type (+/+) mice were bred and the colonies maintained. Mice were grouped housed in cages, given free access to food and water, and maintained on a 12 h:12 h light–dark cycle. Animals of both sexes were used for experiments.
Neuronal culture
TRPA1+/+ and TRPA1−/− mice between 8 and 12 weeks of age were killed by CO2 inhalation and dorsal root ganglia (DRGs) from T10–L1 removed and digested with 4 mg ml−1 collagenase II (Gibco) and 4 mg ml−1 dispase (Gibco) for 30 min at 37°C, followed by 4 mg ml−1 collagenase II only for 10 min at 37°C. Neurons were mechanically dissociated into a single cell suspension via trituration through fire-polished Pasteur pipettes. Neurons were resuspended in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% fetal calf serum (FCS; Invitrogen), 2 mm l-glutamine (Gibco), 100 μm minimum essential medium (MEM) non-essential amino acids (Gibco) and 100 mg ml−1 penicillin–streptomycin (Invitrogen). Nerve growth factor (NGF) at 100 ng ml−1 (Sigma-Aldrich) was also included as it has been previously demonstrated to be critical in maintaining TRPA1 function (Akopian et al. 2007), although it should be noted that NGF has been shown to be effective in inducing IAMC (Lechner et al. 2009) and enhances the response of nociceptors to mechanical stimuli (Di Castro et al. 2006). Neurons were spot-plated on 8 mm HCl treated coverslips coated with poly d-lysine (800 μg ml−1) and laminin (20 μg ml−1) and maintained at 37°C in 5% CO2.
Transfection of sensory neurons
Neurons were transiently transfected with the use of commercially available Nucleofector system (Amaxa Biosystems, Lonza Australia, Mt Waverley, Victoria, Australia) (Harty & Waxman, 2007; Wetzel et al. 2007). For the control green fluorescent protein (GFP) plasmid we used the pmaxGFP plasmid, contained in the mouse Neuronal Nucleofector kit (Amaxa Biosystems), whilst for TRPA1 we used TRPA1-IRES-GFP plasmid provided by Prof. David Corey (Harvard Medical School). Neurons were dissociated as above and suspended in 200 μl of mouse neuron nucleofector solution and 5 μg of either control GFP (Amaxa Biosystems) or mouse TRPA1-IRES-GFP plasmid DNA at room temperature. These mixtures were then transferred to a cuvette for electroporation. After electroporation the cell suspension was transferred to 250 μl of DMEM (Gibco) containing 10% FCS (Invitrogen), 2 mm l-glutamine (Gibco), 100 μm MEM non-essential amino acids (Gibco) and 100 ng ml−1 NGF, and cells were spot-plated onto glass coverslips for recording. We observed a transfection efficiency of 5–10%. Images of control-GFP or TRPA1-GFP neurons were obtained using an epifluorescence microscope (Olympus BX51) and confocal scanning microscope (Leica SP5).
Whole-cell patch-clamp recordings from isolated thoracolumbar DRG neurons
Whole-cell recordings were made from thoracolumbar (TL) DRG neurons 20–48 h after plating, using fire-polished glass electrodes with a resistance of 2–5 MΩ. All recordings were performed at room temperature (20–22°C). Signals were amplified by using an Axopatch 200A amplifier, digitised with a Digidata 1322A and recorded using pCLAMP 9 software (Molecular Devices, Sunnyvale, CA, USA). For all neurons holding potential was –70 mV. Intracellular solutions contained (mm): KCl, 135; MgCl2, 2; MgATP, 2; EGTA-Na, 5; Hepes-Na, 10; adjusted to pH 7.4. Extracellular solutions contained (mm): NaCl, 140; KCl, 4; MgCl2, 2; CaCl2, 2; Hepes-Na, 10; glucose, 5; adjusted to pH 7.4. In some experiments a reduced extracellular Ca2+ solution was used containing (mm): NaCl 140; KCl, 4; MgCl2, 4; CaCl2, 0.1; Hepes-Na, 10; glucose, 5; adjusted to pH 7.4. We recorded from and compared two different populations of neurons in Trpa1+/+ and _Trpa1_−/− mice. These were small-diameter neurons (maximum soma diameter <20 μm, cross sectional area <∼300 μm and larger diameter neurons (maximum soma diameter >30 μm, cross sectional area >1000 μm). The exact measurement of soma size was carried out post hoc for each recorded neuron. Control solutions and drugs were applied with a gravity driven multi-barrel perfusion system positioned within 1 mm of the neuron under investigation, as used previously (Duffield et al. 2005; Keating et al. 2005; Litjens et al. 2007).
We employed a system to mechanically probe neurons similar to those used previously (Drew et al. 2002; Di Castro et al. 2006; Hu & Lewin, 2006; McCarter & Levine, 2006; Wetzel et al. 2007; Lechner et al. 2009; Lechner & Lewin, 2009; Rugiero & Wood, 2009; Coste et al. 2010; Hu et al. 2010; Rugiero et al. 2010; Vilceanu & Stucky, 2010). However, we focused on the mechanical stimulation of neurites (Hu & Lewin, 2006; Wetzel et al. 2007; Lechner & Lewin, 2009; Hu et al. 2010) as opposed to soma probing (Drew et al. 2002; Di Castro et al. 2006; McCarter & Levine, 2006; Lechner et al. 2009; Rugiero & Wood, 2009; Rugiero et al. 2010; Vilceanu & Stucky, 2010; Coste et al. 2010) or radial stretch (Bhattacharya et al. 2008). Specifically, we applied mechanical stimuli using a heat-polished glass pipette with a sealed tip (diameter, 1–3 μm) and long taper. The glass pipette probe was positioned at an angle of 45 deg to the surface of the coverslip and was driven by a programmable piezo-micromanipulator in 1–5 μm steps at a stable speed of 2.5 μm ms−1. Neurites with the most bulbous ending were selected for mechanical stimulation. Initially the probe was moved towards the neurite and positioned as close as possible without touching it. Then it was retracted diagonally by a previously calibrated 1 μm step and was within ∼1 μm of the neurite, as observed through the microscope with calibration markers. All consequent stimuli were applied from this starting position. A 2 μm stimulus produced an observable deflection in the neurite, without displacing the neurite, and elicited a mechanically activated current. Therefore this 2 μm movement is referred to as a 1 μm probe. Similarly, returning the probe to its starting position ∼1 μm away from the neurite and applying a 3 μm movement produced an greater observable deflection in the neurite, again without displacing the neurite, and elicited a larger mechanically activated current. Therefore this 3 μm movement is referred to as a 2 μm probe and this was the primary stimulus used in this study. In some experiments 5 μm movements, called a 4 μm probe, caused noticeable neurite displacement, and therefore greater steps were not used to probe neurites. For each mechanical stimulus, the pipette was moved toward the neurite or in some cases the soma, paused for ≥250 ms, and then moved back to its original starting position. Repeated 2 μm probing at a speed of 2.5 μm ms−1 at 30–60 s intervals allowed full recovery of the mechanosensitive currents and produced highly reproducible mechanically activated currents with consistent amplitudes allowing us to test the effects of a TRPA1 agonist (allyl-isothiocyanate; AITC) or TRPA1 antagonist (HC-030031) on mechanically activated current amplitude within individual neurons. In some experiments probing of the cell soma with a 7 μm movement (6 μm probe) was used to determine the relative effects of neurite vs. soma probing on mechanically activated currents. Different types of mechanically activated current were classified based on their half-activation (τ1) and half-inactivation (τ2) times. Briefly, RAMCs were classified by half-inactivation times <10 ms, whereas IAMCs were between 20–50 ms and SAMCs were greater >100 ms. Neurons with mechanically activated currents that did not reach half-inactivation by the end of the 250 ms stimulus were given a half-inactivation τ2 of 250 ms. The maximum amplitude of the inward currents generated in response to mechanical stimulation from small- and large-diameter neurons were directly compared between Trpa1+/+ and _Trpa1_−/−. Statistical analysis was performed with GraphPad Prism 4 using two-way ANOVA, and Student's t test for paired or unpaired data as appropriate. Data are shown as means ± SEM, with significance considered at P < 0.05.
Drugs
The TRPA1 agonist allyl-isothiocyanate (AITC; 40–400 μm) was purchased from Sigma-Aldrich (St Loius, MO, USA), whilst the TRPA1 antagonist HC-030031 (10 μm) was purchased from Hydra Biosciences Inc., Cambridge, MA, USA. Fresh solutions were made each recording day.
Dissociated ganglion cell culture, laser capture microdissection and quantitative RT-PCR
Trpa1+/+ mice were killed by CO2 inhalation and DRGs from T10–L1 removed. Neurons were mechanically dissociated as described above and resuspended in Hanks' buffered salt solution (HBSS) (Gibco) and spot-plated onto 50 mm Zeiss Duplex-Dishes (Carl Zeiss Australia, North Ryde, NSW, Australia), then maintained at 37°C in 5% CO2 for 2 h allowing optimal cell adhesion. Two different populations of neuron were captured, those with soma diameter <20 μm, termed small-diameter neurons, and those with soma diameters >30 μm, termed large-diameter neurons. These different populations were isolated using a PALM Microlaser Technologies microdissection system (Carl Zeiss, Germany) and catapulted directly into a lysis/stabilisation buffer containing carrier RNA (4 ng ul−1) (Qiagen, Valencia, CA, USA) (Page et al. 2005; Hughes et al. 2007). Neurons were pooled from multiple mice with equal numbers of neurons (200) between populations. RNA was isolated from these two groups of neurons and extracted using a Qiagen RNAse micro kit (Sydney, Australia). QRT-PCR was performed using a Chromo4 real-time instrument and Opticon Monitor software (MJ Research, Watertown, MA, USA) (Brierley et al. 2008, 2009). We used Qiagen (USA) QuantiTect SYBR Green RT-PCR one-step kits according to the manufacturer's specifications, with the following primers and conditions for TRPA1: forward, 5′–3′: ACAAGAAGTACCAAACATTGACACA; reverse, 5′–3′: TTAACTGCGTTTAAGACAAAATTCC; β-tubulin primers: forward 5′–3′: CCAAGTTCTGGGAGGTCATC, reverse 5′–3′: TGAGAGGAGGCCTCATTGTAG (GeneWorks, Hindmarsh, SA, Australia). Reverse transcription, 50°C (30 min); initial PCR activation, 95°C (15 min); PCR cycles, 94°C (15 s), 47°C (30 s), and 72°C (30 s) repeated for 44 cycles. Size of amplified products was confirmed by gel electrophoresis. Each assay was run in at least triplicate in separate experiments. Control PCRs were performed by substituting RNA template with distilled RNAse-free water or by omitting the RT step. The comparative cycle threshold method was used to quantify the abundance of TRPA1 transcript in small- and large-diameter neuron mice as previously described (Brierley et al. 2008, 2009). Quantitative data are expressed as means ± SD, and significant differences in transcript expression determined by a Mann–Whitney test.
In situ hybridisation
Trpa1+/+ mice were anaesthetised with Nembutal (100 mg kg−1, Virbac Animal Health, Reagents Park, NSW, Australia) and transcardial perfusion performed first with warm sterile heparinised saline, followed by ice-cold 20% sucrose in 4% paraformaldehyde (PFA)/0.1 m phosphate buffer (PB), pH 7.4. Following fixation, thoracolumbar DRGs from bilateral spinal levels T10–L1 were removed and sections (12 μm) cut and post-fixed as described previously (Hughes et al. 2007; Brierley et al. 2008, 2009). Digoxigenin-labelled oligonucleotide probe anti-sense to 1571–1618 of murine TRPA1 messenger RNA (mRNA) was used to target TRPA1 (Brierley et al. 2009). Complementary sense probe was used as a negative control revealing no labelling above background. Digoxigenin was detected using CARD amplification (Perkin-Elmer, Waltham, MA, USA) combined with streptavidin-conjugated AlexaFluor 546 (SAF546) (Invitrogen, Mt Waverly, VIC, Australia). Only cells with intact nuclei were included for analysis. To determine TRPA1 expression in small- vs. large-diameter neurons, data are expressed as the percentage of neurons expressing TRPA1 transcript in 5 μm bin sizes. Data were taken from DRG section in four to eight DRG sections per mouse averaged across five or six mice.
Immunohistochemistry
Trpa1+/+ or _Trpa1_−/− mice were killed by CO2 inhalation and DRGs from T10-L1 removed. Neurons were dissociated onto HCl treated coverslips and were fixed for 10 min at room temperature in 4% paraformaldehyde-phosphate buffered saline (PBS) after 24 h incubation. After fixation, coverslips were flushed three times with 0.1 m PBS and immediately incubated with blocking solution, 5% normal donkey serum diluted in 0.2% Triton TX-200 (Sigma-Aldrich, USA) in PBS for 30 min. Coverslips were then incubated with a rabbit anti-TRPA1 (1/1000; no. AB58844; Abcam) overnight at 4°C (Brierley et al. 2009). This was followed by three PBS washes and incubation with secondary antibody donkey anti-rabbit AF568 (1/400; Molecular Probes/Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. The coverslips were then flushed three times with PBS before being mounted on microscope slides with antifade mounting solution (Prolong antifade, Molecular Probes/Invitrogen). Negative controls were prepared as above with the primary antibody omitted or in tissue from _Trpa1_−/− mice. Furthermore, as these _Trpa1_−/− mice have the placental alkaline phosphatase gene (PLAP) substituted for Trpa1, we also used mouse anti-PLAP antibody (Sigma-Aldrich) at 1:100 (Kwan et al. 2009) in addition to the TRPA1 labelling, using 5% normal goat serum and mouse-IgG (1/10; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) in PBS to block non-specific labelling and visualised with goat anti-mouse AF488 secondary antibody (1/400; Molecular Probes/Invitrogen). Images were obtained using an epifluorescence microscope (Olympus BX51) and confocal scanning microscope (Leica SP5).
Results
TRPA1 is expressed predominantly in small-diameter DRG neurons
Before determining the mechanosensory role of TRPA1 we first had to determine in which DRG neurons it is expressed. This in itself is a controversial issue and depends on which sensory neurons are investigated (Story et al. 2003; Nagata et al. 2005; Kwan et al. 2009). Firstly, utilising in situ hybridisation for TRPA1 on DRG sections revealed that in TL DRG, TRPA1 mRNA is predominantly localised in smaller-diameter neurons (<25 μm). Indeed we found that in DRGs from the TL region ∼90% of the TRPA1 expressing neurons had small diameters (Fig. 1_A_). To determine if this was also the case in the setting in which patch clamp recordings would be performed, we also performed laser capture microdissection of dissociated neurons. Subsequent QRT-PCR analysis revealed a significantly greater expression of TRPA1 transcript in neurons with soma diameters <20 μm compared with larger soma diameters >30 μm (Fig. 1_B_). Taken together these results confirmed TRPA1 is enriched within small-diameter DRG neurons. To confirm that mRNA expression translated to protein expression we also performed immunohistochemistry on dissociated neurons. This also revealed that neurons expressing TRPA1 protein were predominantly of smaller diameter (Fig. 1_C_). As _Trpa1_−/− mice have the placental alkaline phosphatase gene (PLAP) substituted for Trpa1, we also used a PLAP antibody, which also confirmed expression in small-diameter DRG neurons (Fig. 1_C_). On the basis of these results patch clamp recordings were performed on two different populations of neurons, (i) smaller neurons with soma diameters <20 μm, a TRPA1-rich population, or (ii) larger neurons with soma diameters >30 μm, a TRPA1-poor population.
Figure 1. TRPA1 mRNA and protein is predominantly localised in smaller-diameter neurons.
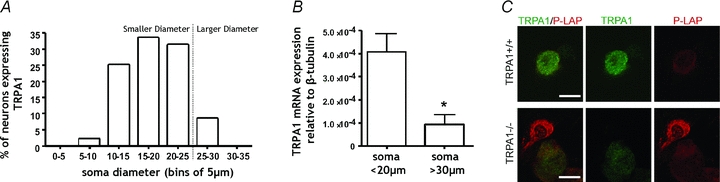
A, in situ hybridisation revealed that in thoracolumbar (TL) dorsal root ganglion (DRG) TRPA1 mRNA is predominantly localised in smaller-diameter neurons (<25 μm). _B_, laser capture microdissection of dissociated neurons and subsequent QRT-PCR analysis revealed a significantly greater expression of TRPA1 transcript in neurons with soma diameters <20 μm compared with larger soma diameters >30 μm (*P < 0.05, unpaired _t_ test, _n_ = 3 mice for each population). _C_, immunohistochemistry on dissociated neurons revealed TRPA1 protein expression in small-diameter _Trpa1_+/+ DRG neurons. As _Trpa1_−/− mice have placental alkaline phosphatase (_PLAP_) substituted for _Trpa1_, we also used a PLAP antibody, which confirmed TRPA1 expression in small-diameter DRG neurons. Note the lack of TRPA1 expression in the larger-diameter neuron. On the basis of these results patch clamp recordings were performed on 2 different populations of neuron, (i) smaller neurons with soma diameters <20 μm and (ii) larger neurons with soma diameters >30 μm. Scale bar 15 μm.
Mechanically activated currents elicited by neurite displacement in small-diameter DRG neurons
We recorded mechanosensory currents in response to probing the neurites of isolated TL DRG neurons, which provides an improved environment for testing of direct mechanosensory function (Hu & Lewin, 2006; Wetzel et al. 2007). Neurite stimulation induced three different types of current (Fig. 2_A_) as previously described (Hu & Lewin, 2006). The mechanically activated currents were termed slowly adapting (SAMCs), rapidly adapting (RAMCs) and intermediate-adapting (IAMCs) based on their significantly differing half-inactivation times (Fig. 2_B_). SAMCs, RAMCs and IAMCs were all evoked at sub-millisecond latency as observed previously (Hu & Lewin, 2006; Wetzel et al. 2007). However, the time to half-activation of IAMCs was significantly shorter than RAMCs (Fig. 2_C_). By contrast some neurons did not respond to mechanical neurite probing and were termed no response (NR) neurons. In small-diameter neurons IAMC (67.6%) was the predominant current followed by SAMC (11.7%), RAMC (10.3%) and NR (10.2%).
Figure 2. Mechanically activated currents elicited by neurite displacement.
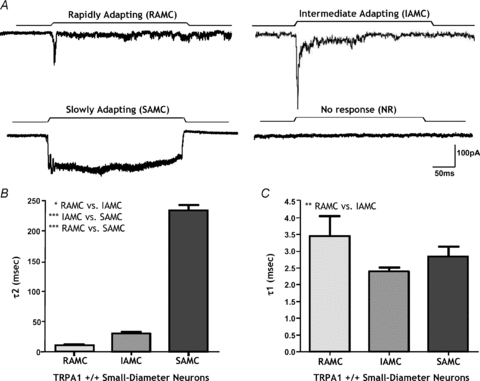
A, 4 distinct types of mechanically activated current termed rapidly adapting (RAMC), intermediately adapting (IAMC), slowly adapting (SAMC) and no response (NR) were elicited in response to neurite probing. B, the inactivation currents for each type of response were distinct and significantly different from one another based on their time of inactivation (*P < 0.05, RAMC: 6.5 ± 0.6 ms vs. IAMC: 29.9 ± 3.6 ms; ***P < 0.001, IAMC: 29.9 ± 3.6 ms vs. SAMC: 233.1 ± 9.1 ms n = 7–46; ***P < 0.001, RAMC 6.5 ± 0.6 ms vs. SAMC: 233.1 ± 9.1 ms, n = 7–8). C, the time taken for IAMC to reach half-peak amplitude is significantly quicker than RAMC (**P < 0.01, 2.39 ± 0.13 ms vs. 3.45 ± 0.59 ms after probe onset, n = 7–46).
TRPA1 deletion alters specific mechanically activated currents in small-diameter neurons
We then determined whether the absence of TRPA1 alters the response properties of mechanically activated currents. We found that the rapid peak of the IAMC was significantly reduced in amplitude by ∼43% in small-diameter DRG neurons from _Trpa1_−/− mice (Fig. 3_A_ and B, Table 1). Data shown in Fig. 3_A_ and B are for responses to a submaximal (2 μm) mechanical stimulus, although responses were proportional in amplitude to the size of graded stimuli, all of which were reduced in _Trpa1_−/− small-diameter neurons (Fig. 3_C_). Responses of DRG neurons to a different type of stimulus – probing of the cell soma – were evoked at higher displacement thresholds than those to neurite deformation, but were likewise significantly reduced in the _Trpa1_−/− neurons (Fig. 3_D_). By contrast, we found that TRPA1 deletion had no effect on RAMC (Fig. 4_A_) or SAMC (Fig. 4_B_) amplitude in small-diameter neurons. Overall, the electrical properties of _Trpa1_−/− DRG neurons, including resting membrane potential, voltage gated Na+ and K+ currents and I–V relationships, were unaffected (Table 1, and data not shown). We also encountered similar proportions of the different types of mechanosensory currents in Trpa1+/+ and _Trpa1_−/− neurons (Fig. 4_C_, Table 1), indicating no difference in basic mechanosensory phenotype.
Figure 3. Deficits in mechanotransduction occur in intermediately adapting mechanically activated currents (IAMCs) of small-diameter TRPA1−/− neurons.
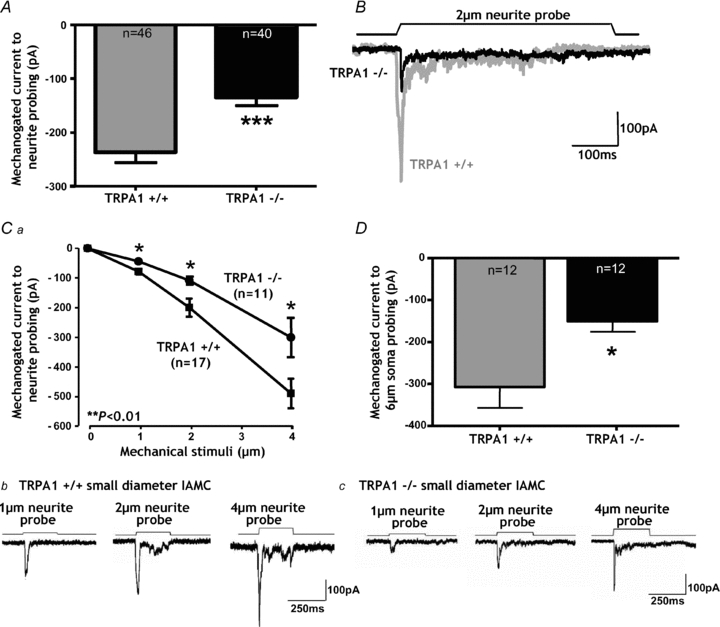
A, in _Trpa1_−/− small diameter neurons the amplitude of the IAMCs generated by 2 μm neurite probing was significantly reduced compared with Trpa1+/+ (***P < 0.001, unpaired _t_ test, _n_ = 40–46). _B_, examples of original traces demonstrating the extent of IAMC deficit in TRPA1−/− small-diameter neurons. _Ca_, the deficits observed in IAMCs from _Trpa1_−/− small-diameter neurons occur across a range of probing intensities (**_P_ < 0.01, 2-way ANOVA; *_P_ < 0.05, for 1 μm, 2 μm and 4 μm _post hoc_ tests). _Cb_ and _c_, original recordings of _Trpa1_+/+ (_b_) and _Trpa1_−/− small-diameter neurons (_c_) in response to 1 μm, 2 μm and 4 μm neurite displacements. _D_, IAMCs were evoked in small-diameter neurons by probing of the cell soma >4 μm as found previously (Hu & Lewin, 2006). With a 6 μm displacement the peak IAMC was significantly reduced in _Trpa1_−/− small-diameter neurons (*P < 0.05, unpaired t test, n = 12).
Table 1.
Properties of mechanically activated currents evoked by neurite probing
| Smaller-diameter neurons | Larger-diameter neurons | |||
|---|---|---|---|---|
| Trpa1+/+ | _Trpa1_−/− | Trpa1+/+ | _Trpa1_−/− | |
| Intermediately adapting mechanically activated currents | n = 46 | n = 40 | n = 13 | n = 8 |
| Mean current amplitude (pA) | −237.3 ± 19.14 | −134.1 ± 15.93*** | −249.8 ± 34.20 | −281.1 ± 53.47 |
| Mean RMP (mV) | −54.39 ± 0.96 | −52.86 ± 1.249 | −58.69 ± 2.13 | −60.00 ± 2.20 |
| Mean soma size (μm) | 17.22 ± 0.48 | 17.14 ± 0.49 | 33.46 ± 1.28 | 32.83 ± 1.72 |
| Mean soma area (μm2) | 263.777 ± 17.02 | 277.06 ± 15.13 | 972.72 ± 58.92 | 1013.14 ± 116.69 |
| Rapidly adapting mechanically activated currents | n = 7 | n = 8 | n = 3 | n = 3 |
| Mean current amplitude (pA) | −167.3 ± 33.62 | −147.6 ± 27.36 | −212.4 ± 82.04 | −262.4 ± 9.287 |
| Mean RMP (mV) | −57.29 ± 2.327 | −51.63 ± 3.746 | −51.00 ± 5.360 | −55.00 ± 1.155 |
| Mean soma size (μm) | 17.33 ± 0.66 | 17.33 ± 1.11 | 29 ± 1.5 | 31.33 ± 0.66 |
| Mean soma area (μm2) | 301.33 ± 22.66 | 270.66 ± 34.60 | 920 ± 80 | 986.66 ± 13.33 |
| Slowly adapting mechanically activated currents | n = 8 | n = 13 | n = 5 | n = 3 |
| Mean current amplitude (pA) | −236.9 ± 71.35 | −182.8 ± 22.46 | −358.4 ± 70.63 | −323.0 ± 85.03 |
| Mean RMP (mV) | −51.63 ± 1.812 | −49.54 ± 1.457 | −58.20 ± 1.463 | −63.57 ± 4.028 |
| Mean soma size (μm) | 16.50 ± 3.44 | 16.30 ± 0.63 | 32.4 ± 1.16 | 35 ± 2.88 |
| Mean soma area (μm2) | 258.4 ± 27.26 | 263.33 ± 22.97 | 923.2 ± 61.85 | 1166.66 ± 218.58 |
| No mechano response currents | n = 7 | n = 9 | n = 4 | n = 3 |
| Mean RMP (mV) | −51.71 ± 1.629 | −51.00 ± 2.858 | −59.50 ± 1.936 | −61.00 ± 3.055 |
| Mean soma size (μm) | 12.61 ± 2.62 | 16.10 ± 1.8 | 32.50 ± 2.21 | 34.12 ± 3.05 |
| Mean soma area (μm2) | 226 ± 30 | 268.44 ± 26.86 | 927 ± 67.96 | 1206.66 ± 277.20 |
Figure 4. TRPA1 does not contribute to other mechanically activated currents in small-diameter neurons.
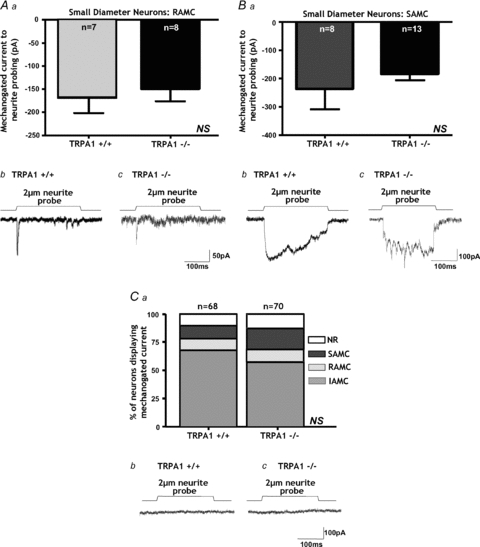
Aa, rapidly adapting (RAMCs, NS, P > 0.05, unpaired t test, n = 7–8) mechanically activated currents are unaltered in small-diameter _Trpa1_−/− neurons. B, original traces from small-diameter neurons showing no change in RAMCs between Trpa1+/+ and _Trpa1_−/− neurons. B, slowly adapting (SAMCs, NS, P > 0.05, unpaired t test, n = 8–13) mechanically activated currents were not significantly altered in small diameter _Trpa1_−/− neurons. B, original traces from small-diameter neurons showing no change in SAMCs between Trpa1+/+ and _Trpa1_−/− neurons. Ca, the proportions of each type of mechanically activated current were also unaltered by TRPA1 deletion as indicated by no significant difference in the proportion of each mechanically activated current type generated by neurite probing in _Trpa1_−/− neurons (NS, χ2 > 0.05). b, example of small-diameter neurons from Trpa1+/+ and _Trpa1_−/− mice which were unresponsive to neurite probing.
In contrast to data from small-diameter DRG neurons, large-diameter neurons, which we found to generally lack TRPA1, displayed no alterations in IAMC (Fig. 5_A_, Table 1). Furthermore, large-diameter _Trpa1_−/− neurons displayed no change in RAMC (Fig. 5_B_, Table 1) or SAMC (Fig. 5_C_, Table 1) amplitudes. Furthermore, similar proportions of the different types of mechanosensory currents were present in large-diameter Trpa1+/+ and _Trpa1_−/− DRG neurons (Fig. 5_D_). Taken together these data indicate TRPA1 plays a fundamental role in the initial process and amplitude of mechanical stimulus detection within a select population of small-diameter DRG neurons.
Figure 5. TRPA1 deletion does not alter mechanically activated currents evoked in large-diameter neurons.
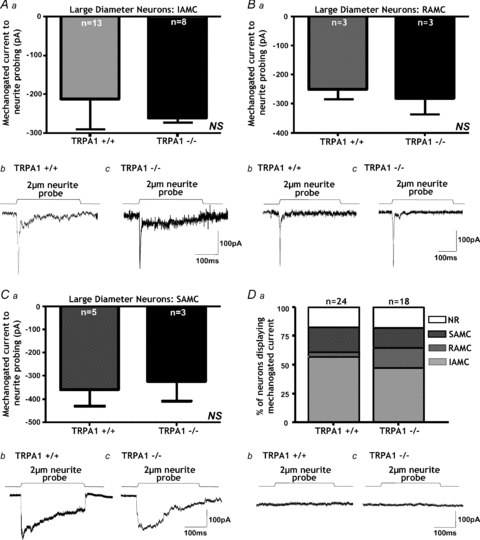
We showed that large diameter Trpa1+/+ DRG neurons generally lack TRPA1. Correspondingly, IAMCs (A, NS, P > 0.05, unpaired t test, n = 8–13), RAMCs (B, NS, P > 0.05, unpaired t test, n = 3) and SAMCs (C, NS, P > 0.05, unpaired t test, n = 3–5) were all unaltered in _Trpa1_−/− large-diameter neurons. Original traces from large-diameter neurons showing no change in IAMC (Ab), RAMC (Bb) or SAMC (Cb) between Trpa1+/+ and _Trpa1_−/− neurons. Da, similarly the proportions of the various types of mechanically activated current were not significantly changed by TRPA1 deletion (NS, χ2 > 0.05). b, example of large-diameter neurons from Trpa1+/+ and _Trpa1_−/− mice which were unresponsive to neurite probing.
A TRPA1 agonist activates small-diameter neurons and evokes mechanical hypersensitivity
Having shown that deletion of Trpa1 attenuates IAMC in small-diameter neurons we then investigated if +/+ neurons functionally expressed TRPA1. To do this we applied the TRPA1 agonist AITC (mustard oil, 40–400 μm) to both small- and large-diameter neurons. A recent study has suggested that AITC may also activate TRPV1 in addition to TRPA1 (Everaerts et al. 2011). These authors demonstrated that 100 μm AITC did not evoke responses in _Trpa1_−/− DRG neurons at a temperature of 25°C; however, this changed in experiments performed at 37°C whereby a percentage of _Trpa1_−/− DRG neurons started responding to 100 μm AITC. They also noted that in Trpa1+/+ DRG neurons AITC evokes TRPA1-mediated responses that are rapid and quickly desensitising, whereas TRPV1-mediated responses are slower but sustained (Everaerts et al. 2011). In the current study, performed at room temperature, AITC evoked a large rapid inward current (Fig. 6_A_) in 52% of the Trpa1+/+ small diameter neurons tested, compared with only 10% of Trpa1+/+ large diameter neurons. We also found that none of the _Trpa1_−/− DRG neurons tested responded to AITC at concentrations between 40 and 400 μm. Taken together these results strongly suggest that AITC only produces a TRPA1-dependent effect in the current study. Of the Trpa1+/+ responsive small-diameter neurons 73% displayed IAMCs to neurite displacement, whilst 27% displayed RAMCs. None of the AITC responsive neurons displayed SAMCs or NR to neurite probing. We also found that when Trpa1+/+ AITC responsive small-diameter neurons were subsequently re-tested with neurite probing they displayed significantly greater IAMC amplitudes (Fig. 6_A_), indicating pronounced mechanical hypersensitivity. This AITC-induced mechanical hypersensitivity required TRPA1 activation, since it occurred only in IAMC neurons that were responsive directly to AITC (Fig. 6_B_). IAMC maximal amplitudes in Trpa1+/+ small-diameter neurons were not significantly altered using low external Ca2+ solutions (Fig. 6_C_). However, we found that the mechanical hypersensitivity induced by AITC was lost in low Ca2+ conditions (Fig. 6_D_), indicating external calcium may play a key role in agonist-induced potentiation of mechanosensitivity. Furthermore, AITC-induced sensitisation did not occur in neurons with RAMC (data not shown).
Figure 6. Activation of TRPA1 increases IAMC amplitude.
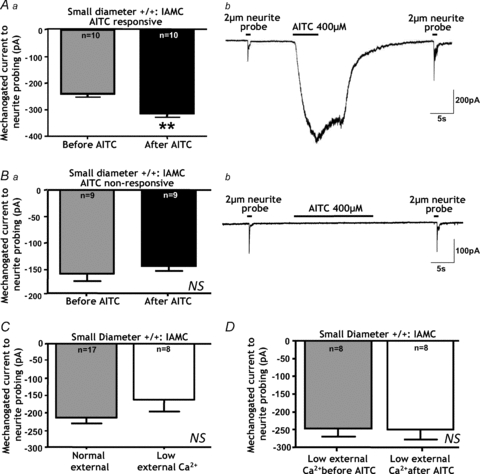
The TRPA1 agonist AITC (mustard oil) predominantly actives +/+ small-diameter neurons. Fifty-two per cent of Trpa1+/+ small diameter neurons responded to AITC (40–400 μm) with the vast majority displaying IAMCs in response to neurite displacement. Aa, Trpa1+/+ small-diameter neurons that were responsive to AITC subsequently displayed significantly greater IAMC maximal amplitudes (**P < 0.01, paired t_ test, n = 10) indicating pronounced mechanical hypersensitivity. b, representative example of a whole cell recording from a Trpa1+/+ small-diameter neuron showing IAMC evoked in response to 2 μm neurite probing, then an inward current to AITC and a subsequent increase in IAMC amplitude. Ba, Trpa1+/+ small-diameter neurons that were non-responsive to AITC did not display enhanced IAMC amplitudes (NS, P > 0.05, paired t test, n = 9). b, representative example of whole cell recording from a Trpa1+/+ small-diameter neuron showing IAMC evoked in response to neurite 2 μm probing, then an lack of response to AITC and no subsequent change in IAMC amplitude. C, IAMC in Trpa1+/+ small-diameter neurons were not significantly altered using low external Ca2+ solutions (0.1 mm CaCl2; 4 mm MgCl2_vs. 2 mm CaCl2; 2 mm MgCl2; NS, P > 0.05, unpaired t test, n = 8–17). D, however, using low external Ca2+ solutions prevented the TRPA1 agonist AITC from causing enhanced IAMCs in Trpa1+/+ small-diameter neurons (NS, P > 0.05, paired t test, n = 8).
TRPA1 antagonism selectively reduces intermediately adapting mechanically activated currents, but only in neurons expressing functional TRPA1
A recent study found inconsistencies in their mechanosensory findings when comparing data obtained using _Trpa1_−/− mice and the selective TRPA1 antagonist HC-030031 (Vilceanu & Stucky, 2010). Therefore, we decided to study the effect of TRPA1 pharmacological blockade on our mechanically activated currents. Somewhat surprisingly HC-030031 (10 μm) had no significant effect on IAMC amplitude in small-diameter neurons, although there was a trend to decrease IAMC amplitude (Fig. 7_C_). Following a 15 min washout of the TRPA1 antagonist we applied AITC to confirm the functional expression of TRPA1 in these neurons. When we then separated neurons based on AITC responsiveness, this revealed HC-030031 does in fact reduce IAMC amplitude, but only in Trpa1+/+ small-diameter neurons which respond to AITC (Fig. 7_A_). By contrast, HC-030031 had no effect on small-diameter IAMC neurons which were unresponsive to AITC (Fig. 7_B_). None of the small-diameter neurons with SAMC were responsive to AITC and correspondingly HC-030031 had no effect on SAMC amplitude (Fig. 7_D_). Although some small-diameter neurons with RAMC responded weakly to AITC, the TRPA1 antagonist had no effect on RAMC amplitude (Fig. 7_E_), which corresponded with our findings in _Trpa1_−/− neurons. Overall these results confirmed that TRPA1 contributes to IAMC in small-diameter neurons.
Figure 7. Pharmacological blockade of TRPA1 reduces IAMCs.
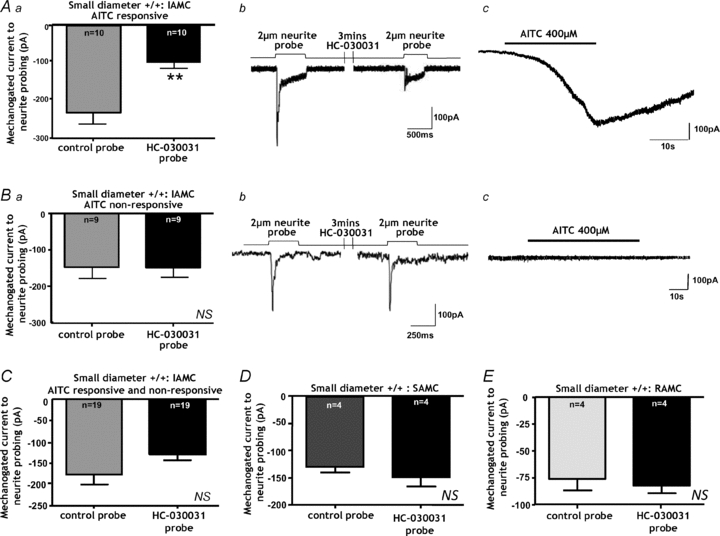
Aa, the TRPA1 antagonist HC-030031 (10 μm) reduces IAMCs but only in small-diameter neurons responding to AITC (**P < 0.01, paired _t_ test, _n_ = 10). _b_, representative example of a whole cell recording showing IAMC in response to 2 μm neurite probing. Application of the TRPA1 antagonist HC-030031 (10 μm) for 3 min subsequently reduced IAMC amplitude. _c_, after washout of HC-030031 this neuron was confirmed to be AITC responsive. _Ba_ and _b_, by contrast HC-030031 had no effect on IAMC amplitude (NS, _P_ > 0.05, paired t test, n = 9) in small-diameter neurons which were unresponsive to AITC (c). C, when data were not differentiated based on responsiveness to AITC, HC-030301(10 μm) tended to decrease IAMCs amplitude in Trpa1+/+ small-diameter, but did not reach statistical significance (NS, P = 0.07, paired t test, n = 19). D, the TRPA1 antagonist had no effect on SAMC amplitude in small-diameter neurons (NS, P > 0.05, n = 4), and correspondingly none of these neurons with SAMC were responsive to AITC. E, although some small-diameter neurons with RAMCs responded weakly to AITC, the HC-030301(10 μm) had no effect on RAMC amplitude (NS, P > 0.05, paired t test, n = 4).
Transfection of TRPA1 into small-diameter neurons alters intermediate adapting mechanically activated currents
In order to determine the mechanosensory role of TRPA1 in isolation, we expressed TRPA1 recombinantly in HEK cells. However, preliminary experiments revealed that this resulted in a constitutively active TRPA1 current, as has been described previously (Karashima et al. 2008), which could be reversed by the TRPA1 antagonist HC-030031 (data not shown). As such we decided instead to transfect DRG neurons to achieve our aim with a different approach. Using a nucleoporation technique (Harty & Waxman, 2007; Wetzel et al. 2007) we first transfected small-diameter +/+ neurons with a control GFP plasmid cDNA and fluorescent neurons were selected for recordings. Their IAMC amplitudes did not differ from those of untransfected +/+ neurons (Fig. 8_A_ and B), indicating that the transfection process alone did not alter mechanically activated current properties. We then transfected DRG neurons with a TRPA1-GFP plasmid, thus over-expressing TRPA1 above native levels. This significantly increased the IAMC amplitude compared with normal Trpa1+/+ small-diameter neurons (Fig. 8_A_ and C) and those expressing control-GFP alone (Fig. 8_A_), demonstrating that the extent of TRPA1 expression determines the size of the IAMC. We also found that this increase in IAMC could be reversed to normal levels by the addition of HC-030031 (10 μm) for 5 min (Fig. 8_A_), indicating TRPA1 had retained its pharmacological profile in recombinant expression.
Figure 8. Altering the expression of TRPA1 in small-diameter neurons determines IAMC amplitude.
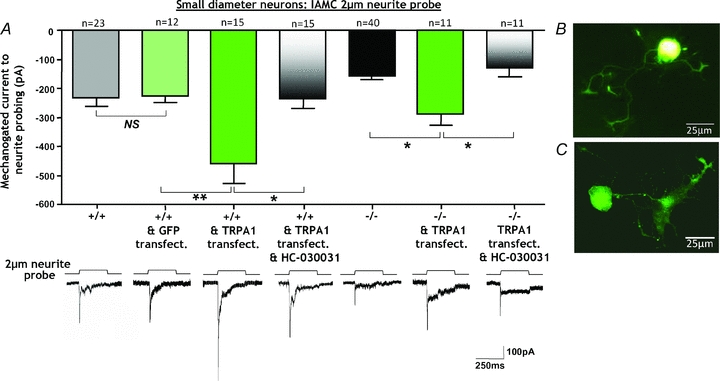
Trpa1+/+ small-diameter neurons transfected with control-GFP had IAMC amplitudes that did not differ from normal IAMC amplitudes of untransfected Trpa1+/+ small diameter neurons (NS, P > 0.05, n = 12–23). By contrast, over-expression of TRPA1 into Trpa1+/+ small-diameter neurons significantly increased IAMC amplitude compared with control-GFP alone (**P < 0.01, n = 12–15). In Trpa1+/+ small-diameter neurons over-expressing TRPA1, the subsequent application of the TRPA1 antagonist HC-030031 (10 μm) reversed the increase in IAMC amplitude (*P < 0.05, n = 15). The introduction of TRPA1 into _Trpa1_−/− small-diameter neurons significantly increased IAMC amplitude above untransfected _Trpa1_−/− small-diameter neuron levels (*P < 0.05, n = 11–40) and restored IAMC amplitude to Trpa1+/+ small-diameter neuron levels. In _Trpa1_−/− small-diameter neurons transfected with TRPA1, the subsequent application of HC-030031 reversed IAMC amplitude (*P < 0.05, n = 11). Lower panels show original representative traces demonstrating the changes in IAMC amplitude in the respective conditions. Scale bars apply throughout. B and C, examples of a Trpa1+/+ small-diameter neuron transfected with control-GFP (normal fluorescent image; B) and TRPA1-GFP (confocal image; C). Scale bars 25 μm.
We also determined if the phenotype of _Trpa1_−/− neurons could be restored by introducing TRPA1 into these neurons via transfection. Correspondingly, we found that the IAMC amplitude of _Trpa1_−/− small-diameter neurons expressing TRPA1-GFP was significantly increased compared with untransfected _Trpa1_−/− neurons (Fig. 8_A_). Furthermore, these currents were restored to the same amplitude as in untransfected +/+ neurons, whilst HC-030031 reduced IAMC amplitude back to _Trpa1_−/− levels (Fig. 8_A_). Overall, these results suggest that TRPA1 not only contributes to normal IAMC amplitude in small-diameter neurons but over-expression of TRPA1 results in mechanical hypersensitivity.
Discussion
It is clear that TRPA1 contributes to chemically induced pain as it is activated by numerous pungent chemicals and environmental irritants such as mustard oil (Bandell et al. 2004; Macpherson et al. 2007_a_) acrolein and formalin (Bautista et al. 2006; Macpherson et al. 2007_a_; McNamara et al. 2007). However, the contribution of TRPA1 to mechanosensory function and mechanically evoked pain remains unresolved due to a series of contrasting results. Although it is clear TRPA1 is not involved in auditory mechanosensation (Bautista et al. 2006; Kwan et al. 2006; Prober et al. 2008), its mechanosensory role in other structures, particularly DRG neurons, is equivocal, with studies both for (Kwan _et al._2006, 2009; Brierley et al. 2009; Kerstein et al. 2009; Vilceanu & Stucky, 2010) and against (Bautista et al. 2006; Bhattacharya et al. 2008; Kwan et al. 2009; Rugiero & Wood, 2009). Due to contrasting findings the advent of two different lines of _Trpa1_−/− mice and a selective TRPA1 antagonist have added to the debate rather than resolved it. In particular a mechanosensory role for TRPA1 in cutaneous C-fibres has been clouded by the observation that firing rates of low-threshold Aβ and D-hair mechanoreceptive fibres are increased in _Trpa1_−/− fibres (Kwan et al. 2009). Such discrepancies exist because of a lack of direct evidence for a relationship between the level of expression of the channel in native sensory neurons and the size of the mechanosensory response elicited from them. In the current study we have addressed this issue, using a specific mechanosensory system, in conjunction with _Trpa1_−/− mice, a TRPA1 antagonist, transfection techniques and grouping data based on neurons' responsiveness to the TRPA1 agonist AITC. Our results provide new evidence that TRPA1 plays a key role in the mechanosensory function of a specific set of small-diameter nociceptive neurons. We also demonstrate that other DRG neurons do not require TRPA1 for normal mechanosensory function. Therefore, our results help to bridge the current discrepancies in the literature by detailing how TRPA1 tunes the mechanosensory function of certain DRG neurons.
Our results specifically demonstrate that TRPA1 contributes to intermediately adapting currents (IAMCs), the most abundant type of mechanically activated current we observed in murine small-diameter DRG neurons in response to neurite probing. We observed in TRPA1−/− small-diameter neurons that maximum IAMC amplitudes were significantly reduced by 43%. We found this deficit occured across a range of stimulation intensities and in response to differing mechanical stimuli. Furthermore, we also found that a TRPA1 antagonist reduced IAMCs, but only in small-diameter neurons and only in neurons that were responsive to the TRPA1 agonist AITC. Notably, this deficit in _Trpa1_−/− IAMCs could be reversed by TRPA1 transfection into _Trpa1_−/− small-diameter neurons, whilst IAMCs were enhanced by over-expressing TRPA1 into Trpa1+/+ small-diameter neurons. Finally, the effect of Trpa1 gene deletion, or the mechanical hypersensitivity induced by over-expression of TRPA1, could be mimicked or reversed by the TRPA1 antagonist HC-030031. Taken together these data suggests TRPA1 makes a positive contribution to mechanosensory function. A positive contribution to mechanosensation is consistent with gut- and skin-based afferent recordings (Brierley et al. 2009; Kwan et al. 2009) and concurs with findings in lower species, where the Drosophila gene painless, the homologue of mammalian Trpa1, is expressed in polymodal nociceptor endings and contributes to the detection of intense mechanical stimuli (Tracey et al. 2003). Similarly, mutations to TRPA-1 in C. elegans cause deficits in sensory neurons required for mechanosensory behaviours (Kindt et al. 2007). Whilst this manuscript was in preparation another study also found mechanosensory deficits in DRG neurons from _Trpa1_−/− mice (Vilceanu & Stucky, 2010). However, the exact deficits reported differ, potentially due to the spinal level of DRGs studied or to the differing experimental protocols utilised. In particular this previous study utilised soma probing, whilst we mainly utilised neurite probing, a technique which has previously been shown to be more sensitive and correlates well with results obtained with nerve-fibre recordings (Wetzel et al. 2007). In the current study we found similar proportions of small-diameter Trpa1+/+ and _Trpa1_−/− neurons displaying IAMCs; however there was a 43% reduction in IAMC amplitude in _Trpa1_−/− small diameter neurons. This reduction correlates well with the deficits in mechanosensory function observed in gut- and skin-based nerve-fibre afferent recordings, which demonstrated no overt loss of afferent subtype in _Trpa1_−/− mice, but a 40–50% reduction in firing in high-threshold colonic afferent nociceptors and cutaneous C-fibres across a wide range of force intensities (Brierley et al. 2009; Kwan et al. 2009). Similarly, we found that the TRPA1 antagonist HC-030031 significantly reduced IAMC amplitude in AITC responsive Trpa1+/+ small diameter neurons, a reduction comparable to the reduced firing rate induced by HC-030031 in cutaneous C-fibres (Kerstein et al. 2009). By contrast, Vilceanu & Stucky 2010, showed a complete loss of mechanically activated current phenotype in _Trpa1_−/− neurons (Vilceanu & Stucky, 2010). In the current study we included NGF in our culture medium as it has been previously demonstrated to be critical in maintaining TRPA1 function (Akopian et al. 2007). As such we were able to determine which neurons in our mechanosensory system specifically displayed TRPA1 transcript and protein expression and critically which neurons displayed functional responses to the TRPA1 agonist AITC. The potential down side of NGF incubation is that it has been shown to be effective in inducing IAMCs (Lechner et al. 2009), perhaps explaining the relatively higher proportion of IAMCs observed in the current study. NGF has also been shown to enhance the response of nociceptors to mechanical stimuli (Di Castro et al. 2006), although it should be noted that in the current study all neuronal cultures were incubated identically.
A contribution of TRPA1 to mechanosensitivty contrasts with other DRG based studies, which have either stretched or probed the cell soma (Bhattacharya et al. 2008; Rugiero & Wood, 2009). We found that the mechanosensory role of TRPA1 was revealed after differentiating data on the basis of (i) mechanically activated current type, (ii) size of neuron and (iii) responsiveness to a TRPA1 agonist. We also found that our data on the effects of a TRPA1 antagonist HC-030031 on mechanosensory currents had to be differentiated based on whether or not neurons could be shown to functionally express TRPA1, via the use of an agonist. Finally, it was also necessary to implement a transfection method that did not adversely affect mechanically activated currents due to the transfection process alone. Our data agree with some other aspects of these studies (Bhattacharya et al. 2008; Rugiero & Wood, 2009) in that not all neurons require TRPA1 for normal mechanosensation. We observed that other types of mechanically activated current (SAMC and RAMC) in small-diameter neurons do not require TRPA1 for normal mechanosensory function, whilst large-diameter neurons appear to not require TRPA1 at all. These findings would therefore suggest the possibility of a specific pharmacotherapy targeting TRPA1 expressing nociceptors to reduce their mechanosensory function.
TRPA1 contributes to enhanced mechanosensory function
As we showed TRPA1 contributed to IAMCs we also wanted to investigate the potential role of TRPA1 in mechanical hypersensitivity. We found that acute TRPA1 sensitisation, via the addition of the TRPA1 agonist AITC, subsequently evoked enhanced IAMC amplitude in AITC responsive neurons. This is consistent with our previous findings using in vitro afferent fibre recordings whereby AITC causes pronounced acute mechanical hypersensitivity in TRPA1+/+ mice, which is lost in _Trpa1_−/− fibres (Brierley et al. 2009). The current study now demonstrates that this process can occur via direct activation of neuronal TRPA1 as opposed to, or in addition to, an intermediate released by non-neuronal structures in response to AITC. Although the exact mechanism for this is not fully known, we have shown in the present study that it depends on extracellular Ca2+. Recent studies have shown that TRPA1 can be directly activated by Ca2+ (Zurborg et al. 2007), whilst agonist activation of TRPA1 results in pore dilatation (Chen et al. 2009; Banke et al. 2010), which could potentially contribute to this process. However, this is a complicated issue as although Ca2+ directly activates TRPA1, changing external Ca2+ alone can have a modulatory effect on mechanogated current amplitude (Drew et al. 2002; McCarter & Levine, 2006; Lechner et al. 2009). In particular increasing external Ca2+ inhibits mechanically activated currents (Drew et al. 2002; McCarter & Levine, 2006; Lechner et al. 2009), although this effect is significantly greater in capsaicin unresponsive neurons relative to capsaicin responsive neurons (Drew et al. 2002) and many TRPA1 neurons also co-express TRPV1. A recent study has shown RAMC responses are potentiated via a G-protein-dependent mechanism (Lechner & Lewin, 2009). Irrespective of the exact mechanism involved in IAMC sensitisation these novel results demonstrate transient chemical stimuli lead to acute mechanical hypersensitivity via TRPA1 in sensory neurons. As such TRPA1 may be crucial in tuning the mechanosensory function of nociceptors in response to environmental challenges. Interestingly, a recently identified TRPA1 gain-of-function mutation causes familial episodic pain syndrome (Kremeyer et al. 2010). These patients demonstrate normal baseline sensory thresholds but an enhanced hyperalgesia to punctate stimuli on treatment with AITC, providing evidence that enhanced TRPA1 function can induce human pain perception (Kremeyer et al. 2010). Our data suggest such hyperalgesia could occur via sensitisation of neuronal TRPA1 and an enhancement of mechanically activated currents in select nociceptors. Additionally, TRPA1 translocation to the cellular membrane occurs in response to acute activation or inflammatory signals (Schmidt et al. 2009). Along these lines we have also identified a mechanism which could underlie chronic mechanical hypersensitivity. Not only have we shown that TRPA1 transfection into _Trpa1_−/− small-diameter neurons rescues IAMCs to Trpa1+/+ levels, but we have also shown over-expression of TRPA1 increases IAMC amplitudes. Notably both of these effects can be reversed by HC-030031, providing strong evidence for a role of TRPA1 in mechanical hyperalgesia. This would be consistent with previous studies showing AITC induced mechanical hypersensitivity in Trpa1+/+ is potentiated in gut-innervating nociceptors during inflammation (Brierley et al. 2009), whilst a TRPA1 antagonist has been beneficial in attenuating somatic inflammatory and neuropathy induced mechanical hypersensitivity (Petrus et al. 2007; Eid et al. 2008). Critically, data from the current study also suggests neuronal TRPA1 contributes directly to this process, a mechanism independent of TRPA1 functioning in non-neuronal structures (Purhonen et al. 2008; Doihara et al. 2009; Kwan et al. 2009). These findings, combined with the observation that mice lacking Trpa1 (Bautista et al. 2006; Kwan et al. 2006; Macpherson et al. 2007_b_; McNamara et al. 2007; Bessac et al. 2008) display reduced pain behaviours indicate a key role for TRPA1 in both chemical and mechanical pain perception.
A critical question remains as to the exact involvement of TRPA1 in this mechanosensory process. Indeed whether or not TRPA1 expression alone confers mechanosensitivity remains unclear. Although C. elegans TRPA-1 is activated by mechanical stimuli when expressed in a cell line (Kindt et al. 2007), more recent studies are ambiguous (Rugiero & Wood, 2009; Vilceanu & Stucky, 2010), with the most recent study concluding TRPA1 expression in HEK cells is not sufficient to confer mechanical sensitivity (Vilceanu & Stucky, 2010). Although we cannot rule out that TRPA1 is a direct mechanosensor, given that our data indicate a contribution of TRPA1 to IAMC amplitude but only in small diameter neurons, without a lack of overall IAMC phenotype in _Trpa1_−/− neurons; it is possible that TRPA1 has an indirect rather than a direct role in mechanosensitivity. If this is the case TRPA1 could be activated concurrently with the mechanosensor or alternatively TRPA1 could be rapidly activated by calcium entering via a mechanosensitive channel, leading to TRPA1 contributing to the overall IAMC amplitude in small diameter neurons. These scenarios could explain why deletion or pharmacological blockade of TRPA1 reduces IAMCs, whilst introduction of TRPA1 into –/– neurons or over-expression of TRPA1 in +/+ neurons restores or increases IAMC amplitudes, respectively. We also showed that Trpa1+/+ RAMC small-diameter neurons responded to AITC, although these responses were much weaker than those observed in IAMC neurons, but deletion or pharmacological blockade of TRPA1 failed to alter RAMC amplitude, suggesting although TRPA1 is functionally expressed in these RAMC neurons it doesn't contribute to their overall mechanosensory function. Indeed a recent study has identified a critical role for Piezo 1 and Piezo 2 in these RAMCs in DRG neurons (Coste et al. 2010). We also show that other neurons do not require TRPA1 for normal mechanosensitivity providing further evidence that a heterogeneity of channels are involved in determining mechanosensory function, which is likely to underlie the specific mechanosensory properties of the plethora of sensory afferent subtypes innervating cutaneous and visceral structures (Lewin & Moshourab, 2004; Lumpkin & Caterina, 2007; Blackshaw et al. 2010; Brierley, 2010).
Irrespective of TRPA1's direct or indirect contribution to mechanosensitivity this study clearly shows TRPA1 contributes to determining IAMC amplitude, whilst sensitisation of TRPA1 in these neurons leads to acute mechanical hypersensitivity. Furthermore, over-expression of TRPA1 leads to long-term enhancement of IAMCs. These results demonstrate that TRPA1 contributes selectively to normal mechanosensation in addition to acute and chronic mechanical hyperalgesia. Therefore, blocking mechanically activated TRPA1 could be beneficial in the treatment of numerous pathophysiological conditions.
Acknowledgments
This work was supported by a National Health and Medical Research Council of Australia (NHMRC) Australian Biomedical Research Fellowship to S.M.B. and P.A.H., NHMRC Principal Research Fellowship to L.A.B., NHMRC Senior Research Fellowship to G.Y.R. and by NHMRC project grant no. 508103 (L.A.B., S.M.B., G.Y.R.) and NHMRC Project Grant no. 1008100 (S.M.B., L.A.B., G.Y.R.). We thank Prof. David Corey and Dr Kelvin Kwan (Harvard Medical School, USA) for supplying lines of Trpa1+/+ and _Trpa1_−/− mice and the mouse TRPA1-IRES-GFP plasmid DNA.
Glossary
Abbreviations
AITC
allyl-isothiocyanate
DRG
dorsal root ganglion
GFP
green fluorescent protein
IAMC
intermediately adapting mechanically activated current
NR
no response
RAMC
rapidly adapting mechanically activated current
SAMC
slowly adapting mechanically activated current
TL
thoracolumbar
TRP
transient receptor potential
Author contributions
S.B. performed the patch clamp experiments and analysis and wrote the manuscript. S.B., G.R. and A.B. contributed to conception, design of the experiments and interpretation of data. J.C. performed the neuronal transfections and cell cultures. A.H. designed and performed the immunohistochemistry, laser capture microdissection and quantitative RT-PCR experiments. P.H. designed and performed the in situ hybridisation experiments. A.P. contributed to cell cultures. All authors contributed to revising the article and evaluating it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol. 2010;298:C1457–C1468. doi: 10.1152/ajpcell.00489.2009. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt S-E, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brierley SM, Hughes PA. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135. doi: 10.1136/gut.2009.179523. [DOI] [PubMed] [Google Scholar]
- Brierley SM. Molecular basis of mechanosensitivity. Auton Neurosci. 2010;153:58–68. doi: 10.1016/j.autneu.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. A selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, Kym PR, Reilly RM. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin S-Y, Vollrath MA, Amalfitano A, Cheung EL-M, Derfler BH, Duggan A, Geleoc GSG, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang D-S. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley T, Ranade S, Petrus M, Dubin A, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doihara H, Nozawa K, Kojima R, Kawabata-Shoda E, Yokoyama T, Ito H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem. 2009;331:239–245. doi: 10.1007/s11010-009-0165-7. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Wood JN, Cesare P. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J Neurosci. 2002;22:RC228. doi: 10.1523/JNEUROSCI.22-12-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield MD, Rychkov GY, Bretag AH, Roberts ML. Zinc inhibits human ClC-1 muscle chloride channel by interacting with its common gating mechanism. J Physiol. 2005;568:5–12. doi: 10.1113/jphysiol.2005.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Harty TP, Waxman SG. Inactivation properties of sodium channel Nav1.8 maintain action potential amplitude in small DRG neurons in the context of depolarization. Mol Pain. 2007;3:12. doi: 10.1186/1744-8069-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Chiang LY, Koch M, Lewin GR. Evidence for a protein tether involved in somatic touch. EMBO J. 2010;29:855–867. doi: 10.1038/emboj.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw L. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol. 2007;500:863–875. doi: 10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Meseguer V, Owsianik G, Voets T, Nilius B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflugers Arch. 2008;457:77–89. doi: 10.1007/s00424-008-0493-6. [DOI] [PubMed] [Google Scholar]
- Keating DJ, Rychkov GY, Giacomin P, Roberts ML. Oxygen-sensing pathway for SK channels in the ovine adrenal medulla. Clin Exp Pharmacol Physiol. 2005;32:882–887. doi: 10.1111/j.1440-1681.2010.04279.x. [DOI] [PubMed] [Google Scholar]
- Kerstein P, del Camino D, Moran M, Stucky C. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenaorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, Villegas A, Acosta N, Pineda-Trujillo NG, Ramirez JD, Zea J, Burley MW, Bedoya G, Bennett DL, Wood JN, Ruiz-Linares A. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Frenzel H, Wang R, Lewin GR. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO J. 2009;28:1479–1491. doi: 10.1038/emboj.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Lewin GR. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol. 2009;587:3493–3503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol. 2004;61:30–44. doi: 10.1002/neu.20078. [DOI] [PubMed] [Google Scholar]
- Lin S-Y, Corey DP. TRP channels in mechanosensation. Curr Opin Neurobiol. 2005;15:350–357. doi: 10.1016/j.conb.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Litjens T, Nguyen T, Castro J, Aromataris EC, Jones L, Barritt GJ, Rychkov GY. Phospholipase C-γ1 is required for the activation of store-operated Ca2+ channels in liver cells. Biochem J. 2007;405:269–276. doi: 10.1042/BJ20061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Macpherson L, Dubin A, Evans M, Marr F, Schultz P, Cravatt B, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007a;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt BF, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007b;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Levine JD. Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol Pain. 2006;2:28. doi: 10.1186/1744-8069-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2 and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Zimmerman S, Myers BR, McDermott BM, Jr, Kim S-H, Caron S, Rihel J, Solnica-Krezel L, Julius D, Hudspeth AJ, Schier AF. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen AK, Louhivuori LM, Kiehne K, Kerman KE, Herzig KH. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008;582:229–232. doi: 10.1016/j.febslet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. J Physiol. 2010;588:301–314. doi: 10.1113/jphysiol.2009.182360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugiero F, Wood JN. The mechanosensitive cell line ND-C does not express functional thermoTRP channels. Neuropharmacology. 2009;56:1138–1146. doi: 10.1016/j.neuropharm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Dubin AE, Petrus MJ, Earley TJ, Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. 2009;64:498–509. doi: 10.1016/j.neuron.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring: elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang S, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Tracey J, WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13 519–13 524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]