tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c (original) (raw)
. 2000 Aug 15;14(16):2060–2071.
Abstract
TNFR1/Fas engagement results in the cleavage of cytosolic BID to truncated tBID, which translocates to mitochondria. Immunodepletion and gene disruption indicate BID is required for cytochrome c release. Surprisingly, the three-dimensional structure of this BH3 domain-only molecule revealed two hydrophobic α-helices suggesting tBID itself might be a pore-forming protein. Instead, we demonstrate that tBID functions as a membrane-targeted death ligand in which an intact BH3 domain is required for cytochrome c release, but not for targeting. _Bak_-deficient mitochondria and blocking antibodies reveal tBID binds to its mitochondrial partner BAK to release cytochrome c, a process independent of permeability transition. Activated tBID results in an allosteric activation of BAK, inducing its intramembranous oligomerization into a proposed pore for cytochrome c efflux, integrating the pathway from death receptors to cell demise.
Keywords: BID, cytochrome c, BAK, mitochondrial targeting, apoptosis
BID is a member of the BH3-domain-only subgroup of BCL-2 family members proposed to connect proximal death and survival signals to the core apoptotic pathway at the level of the classic family members that bear multiple Bcl-2 homology (BH) domains (Adams and Cory 1998; Gross et al. 1999a). This set of pro-apoptotic proteins shares their only sequence homology within the BH3 amphipathic α-helical domain that is essential for killing activity and heterodimerization with other BCL-2 family members. Evidence that these proteins reside within a conserved cell death pathway was strongly supported by the demonstration that egl-1, the upstream negative regulator of the anti-apoptotic ced-9 gene in Caenorhabditis elegans, encodes a BH3-domain-only protein (Conradt and Horvitz 1998). Several of these proteins appear to exist in an inactive conformation in viable cells, but undergo a post-translational modification in response to select death signals to assume an active conformation. These modifications dictate the subcellular location and the binding partners of such proteins. For example, BAD in response to survival factor signaling is robustly phosphorylated on serine residues, which inactivates the molecule. Phosphorylated BAD does not bind BCL-2 or BCL-XL, and is sequestered in the cytosol bound to 14-3-3 (Zha et al. 1996). BAD connects the core death pathway to upstream signaling in that survival pathways that activate the PI3-K pathway phosphorylate BAD on Ser136 (Datta et al. 1997; del Peso et al. 1997), whereas survival factors that activate a mitochondrial-anchored PKA holoenzyme complex result in phosphorylation of the Ser112 site (Harada et al. 1999). BIM, in response to several death stimuli, moves from microtubules to the mitochondria where it appears to bind BCL-XL to promote cell death (Puthalakath et al. 1999).
BID is a BH3-domain-only pro-apoptotic member first noted for its capacity to bind either BCL-2 or BAX and promote cell death. Mutational analysis indicated that an intact BH3 domain was required for binding BCL-2 and BAX, and this activity correlated with the ability of BID to induce cell death. This suggested a model in which BID served as a death ligand that moved from the cytosol to the mitochondrial membrane to inactivate BCL-2 or activate BAX (Wang et al. 1996). More recently, this model has been refined by the recognition that cytosolic p22 BID is activated by caspase-8 cleavage following engagement of Fas or TNFR1 receptors on cells (Li et al. 1998; Luo et al. 1998; Gross et al. 1999b). The truncated p15 BID (tBID) translocates to mitochondria, where it inserts into the mitochondrial outer membrane. Immunodepletion of BID from cytosolic preparations argued that tBID is required for the release of cytochrome c from mitochondria (Luo et al. 1998; Gross et al. 1999b). The release of cytochrome c from mitochondria has been shown to promote the oligomerization of a cytochrome c/Apaf-1/Caspase-9 complex, which activates caspase−9 to result in the cleavage of downstream effector caspase−3 and caspase−7 (Liu et al. 1996; Li et al. 1997; Zou et al. 1997). An absence of similar cell deaths in Apaf-1 and caspase-9-deficient mice lends support to their position in a linear pathway of developmental apoptosis (Cecconi et al. 1998; Hakem et al. 1998; Kuida et al. 1998; Yoshida et al. 1998).
_Bid_-deficient mice revealed that BID was a critical caspase substrate in vivo (Yin et al. 1999). BID proved important in hepatocytes for the release of cytochrome c, dysfunction of mitochondria, and even the death of cells following Fas activation in vivo. Other cell types that do not absolutely require BID for FasL- or TNFα-induced death still demonstrate lack of cytochrome c release, diminished effector caspase activity, and an altered pattern of substrate cleavage in _Bid_−/− mice. Thus, certain cell types such as hepatocytes appear to require a BID-dependent mitochondrial amplification loop that releases cytochrome c, oligomerizing Apaf-1 and caspase-9 to activate sufficient effector caspases to execute apoptosis.
However, the precise mechanism whereby cytochrome c is released from mitochondria remains uncertain, and observations have varied with different cell types and death signals. Following growth factor withdrawal, mitochondrial swelling has been noted. In this model, defective exchange of ADP results in hyperpolarization of the inner membrane, an increase in matrix volume, and nonspecific rupture of the outer membrane, releasing intermembrane space proteins including cytochrome c (Vander Heiden et al. 1999). Following death signals, the pro-apoptotic protein BAX has been shown to translocate to mitochondria, where it inserts as an apparent homo-oligomerized integral membrane protein (Wolter et al. 1997; Gross et al. 1998). Recent studies using recombinant BAX in pure liposomes indicate its capacity to form a pore utilizing four BAX molecules that will transport cytochrome c (Saito et al. 2000). Other studies of BAX or BID suggest that they could result in a more global permeabilization of the outer mitochondrial membrane, releasing multiple intermembrane space proteins (Basanez et al. 1999; Kluck et al. 1999).
Two broad categories of mechanisms might account for how BID results in cytochrome c release. As noted, BID might serve as a death ligand to activate other resident mitochondrial receptor proteins to release cytochrome c. Alternatively, it is also conceivable that BID would itself function as a downstream effector participating in an intramembranous pore that released cytochrome c. To date, BID is the one molecule absolutely required for the release of cytochrome c in loss-of-function approaches including immunodepletion and gene knockout. Moreover, tBID becomes an alkali-resistant, integral membrane protein following translocation to mitochondria. Despite sequence homology limited to only the BH3 domain, BID's overall α-helical content and three-dimensional structure proved remarkably similar to the anti-apoptotic BCL-XL protein (Muchmore et al. 1996; Chou et al. 1999; McDonnell et al. 1999). This includes the presence of two central hydrophobic core helices that constitute potential pore-forming domains, as they are similar to those in BCL-XL and the bacterial pore-forming toxins of diptheria toxin fragment B and colicin. Finally, p15 BID (Schendel et al. 1999) like BCL-XL, BCL-2, and BAX (Antonsson et al. 1997; Minn et al. 1997; Schlesinger et al. 1997) has been noted to form ion-conductive pores in vitro in artificial lipid bilayers. This constellation of findings lends credence to the hypothesis that tBID itself could be a downstream death effector.
Here we study the physiologically active p15 BID. The BH3 domain of tBID is not required for targeting but remains on the mitochondrial surface where it is required to trigger BAK to release cytochrome c. tBID functions not as a pore-forming protein but as a membrane-targeted and concentrated death ligand. tBID induces an oligomerization of BAK, and knockout mice indicate the importance of this event in the release of cytochrome c. An activation cascade of pro-apoptotic proteins from BID to BAK integrates the pathway from surface death receptors to the irreversible efflux of cytochrome c.
Results
BH3 domain of tBID is required for cytochrome c release but not mitochondrial targeting
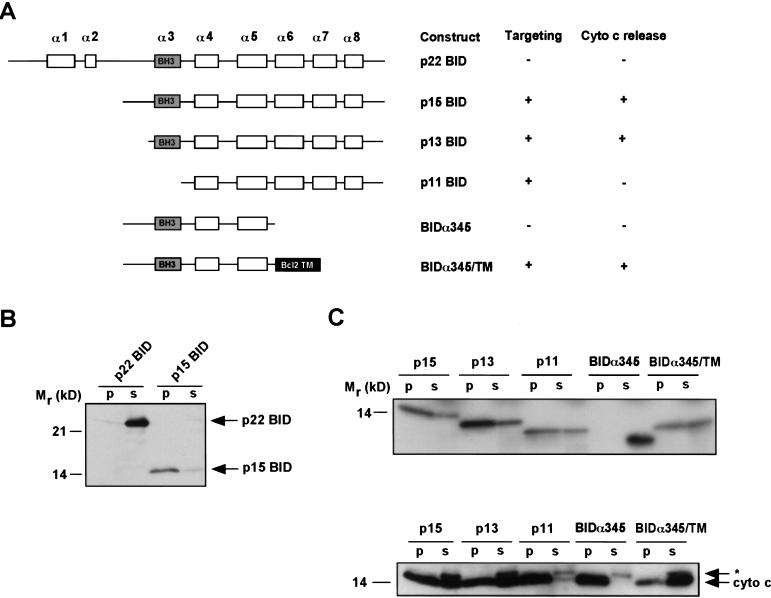
To dissect the mechanisms whereby BID targets mitochondria and results in the release of cytochrome c, we established a targeting system utilizing in vitro transcribed and translated (IVT) BID constructs and purified mouse liver mitochondria. These IVT proteins behaved similarly to purified, bacterially expressed recombinant BID and S100 fractions from activated cells used in previous experiments (Gross et al. 1999b). p22 BID did not target mitochondria or cause cytochrome c release, whereas p15 BID targeted mitochondria efficiently and resulted in cytochrome c release (Fig. 1A,B).
Figure 1.
In vitro targeting and cytochrome c release by various BID proteins. IVT proteins were incubated with purified mitochondria. The mitochondria were centrifuged, and pellet and supernatant were assayed for targeting and cytochrome c release. (A) Schematic of BID constructs showing α helices and capacity to target mitochondria and release cytochrome c. (B) Anti-BID Western blot of an in vitro targeting assay of p22 and p15 BID. Mitochondrial pellet (p) and supernatant (s). (C) Autoradiogram of 35S-labeled IVT BID proteins assayed by in vitro targeting (top). Anti-cytochrome c Western blot assessing cytochrome c release from pellet (p) into supernatant (s) (bottom). (*) A cross-reactive protein present in IVT mix.
In TNFα-activated cells, BID cleavage products of p13 and p11 are also found in mitochondria (Gross et al. 1999b). Similar to p15 BID, the p13 cleavage is also within the unstructured loop of BID and retains the BH3 domain, whereas p11 is cleaved at Asp98 and thus lacks the BH3 domain (Fig. 1A). p13 and p11 BID both targeted mitochondria and became integral membrane (as measured by resistance to alkali extraction), indicating that BH3 is not required for mitochondrial targeting (Fig. 1C). However, although p13 BID caused cytochrome c release comparable with p15 BID, p11 BID did not release cytochrome c (Fig. 1C). The G94E substitution mutant of the BH3 domain (mIII.4), originally noted to be incapable of binding BAX or BCL-2 or causing cell death (Wang et al. 1996) was able to target mitochondria but was defective in cytochrome c release (Fig. 2A,C). Thus, deletion and substitution mutants clarify that an intact BH3 domain is not required for targeting, but is essential for cytochrome c release.
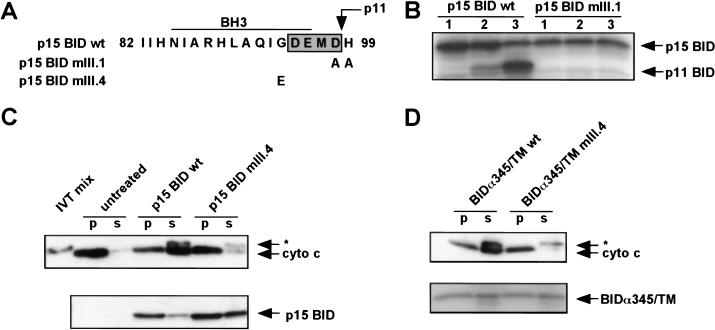
Figure 2.
Requirement for an intact BH3 domain on the cytoplasmic face of the mitochondrial outer membrane. (A) Sequence of p15 BID BH3 domain mutants mIII.1 and mIII.4. Shaded box indicates the caspase-3 cleavage site DEMD that generates the p11 fragment. (B) Treatment of mitochondria with recombinant caspase-3 after targeting wild-type (wt) and caspase-cleavage site mutant p15 BID mIII.1. (Lane 1) Amount of p15 BID targeted. (Lane 2) Treatment with caspase-3 at 4°C. (Lane 3) Treatment with caspase-3 at 30°C. p15 BID and p11 BID are visualized by 35S fluorography. (C) In vitro targeting and cytochrome c release by p15 BID wild type vs mIII.4. (*) A cross-reactive protein present in IVT mix. (D) In vitro targeting and cytochrome c release by BIDα345/TM wild-type and mutant mIII.4.
BH3 domain of p15 BID is on the cytoplasmic surface of the mitochondrial outer membrane
Next, we examined the topology of BH3 to assess whether this critical domain might itself be buried within the mitochondrial membrane. Mitochondria with targeted p15 BID were treated with recombinant caspase-3, which cleaved at the canonical DEMD site, releasing the NH2 terminus including BH3 and leaving p11 BID in the mitochondria (Fig. 2B). This conclusion was supported by a D98A mutant (mIII.1) of p15 BID, which targeted mitochondria but failed to be cleaved by caspase-3 (Fig. 2A,B). This demonstrates that this cleavage site in the BH3 domain is exposed on the mitochondrial surface.
p15 BID functions as a membrane-targeted ligand
Location of the critical BH3 domain on the cytoplasmic face of the mitochondria suggests that it could exert its effects by binding to other proteins on the mitochondrial surface. To test this model directly, we constructed truncated BIDα345 that deleted the COOH terminus including the two hydrophobic α6, α7 helices, which appear to be the transmembrane domains responsible for tBID's integral membrane position (Fig. 1A,C). At comparable concentrations, this mutant failed to target mitochondria and did not cause cytochrome c release (Fig. 1C).
Consequently, we created a chimeric molecule fusing BIDα345 with the mitochondrial signal-anchor sequence from BCL-2 (Nguyen et al. 1993) to restore mitochondrial targeting (Fig. 1A). BIDα345/TM targeted mitochondria and exposed the BH3 domain of BID on the mitochondrial surface. The chimeric BIDα345/TM restored cytochrome c release in the absence of the potential pore-forming α6, α7 helices (Fig. 1C). Consistently, the mutant BIDα345/TMmIII.4 containing the G94E BH3 mutation did not release cytochrome c (Fig. 2D). These results indicate that the hydrophobic α6, α7 helices are required for membrane targeting but not cytochrome c release. Moreover, directing the BID BH3 domain to the mitochondrial surface appears sufficient to cause cytochrome c release.
BAK is required for tBID-induced cytochrome c release
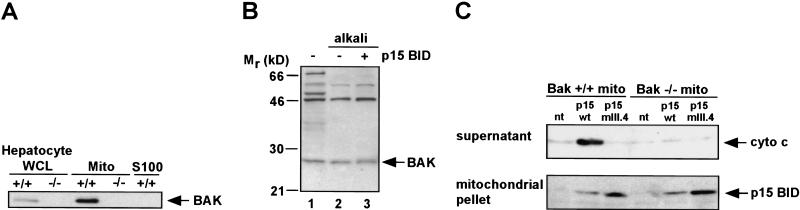
The requirement that an intact BH3 domain be present on the surface of mitochondria for pro-apoptotic activity lends support to the original model that BID is a death ligand. Candidates for BID's potential receptor included its documented binding partners the anti-apoptotic BCL-2 and the pro-apoptotic BAX. However, mouse liver mitochondria used here have no substantial BCL-2 or BAX. Instead, a survey of BCL-2 members revealed that BAK, a pro-apoptotic member structurally similar to BAX, was present at substantial levels as also noted in other cell types (Griffiths et al. 1999). Even in viable liver cells, BAK was present on the mitochondrial outer membrane as an alkali-resistant, integral membrane protein (Fig. 3A,B).
Figure 3.
Requirement for BAK in p15 BID-induced cytochrome c release. (A) Subcellular fractionation of _Bak_-deficient (−/−) and wild-type ( +/+) hepatocytes, immunoblotted with an anti-BAK Ab. (WCL) Whole cell lysate, (Mito) heavy membrane, (S100) cytosol fraction. Equal amounts of protein were loaded in the paired WCL and Mito fractions. (B) Alkali-resistance of mitochondrial BAK in the presence or absence of p15 BID. Wild-type mitochondria +/− 0.5 ng/μl recombinant p15 BID were resuspended in 0.1 m Na2CO3 (pH 11.5) at 4°C for 30 min, and the mitochondrial membranes were then pelleted and analyzed by an anti-BAK Western blot. (C) In vitro targeting and cytochrome c release by recombinant p15 BID wild-type and mutant p15 mIII.4 in either _Bak_-deficient (−/−) or wild-type (+/+) mitochondria.
To assess whether BAK was involved in tBID-induced cytochrome c release, we turned to _Bak_-deficient mice generated by gene targeting (T. Lindsten and C.B. Thompson, in prep.). Bak deficiency was confirmed by the lack of BAK protein in hepatocyte cells and mitochondria (Fig. 3A). tBID does not require BAK for mitochondrial targeting, as recombinant p15 BID and mutant p15 BIDmIII.4 targeted wild-type and _Bak_-deficient mitochondria (Fig. 3C). However, BAK proved necessary for cytochrome c release as p15 BID could not cause cytochrome c release from _Bak_-deficient mitochondria (Fig. 3C).
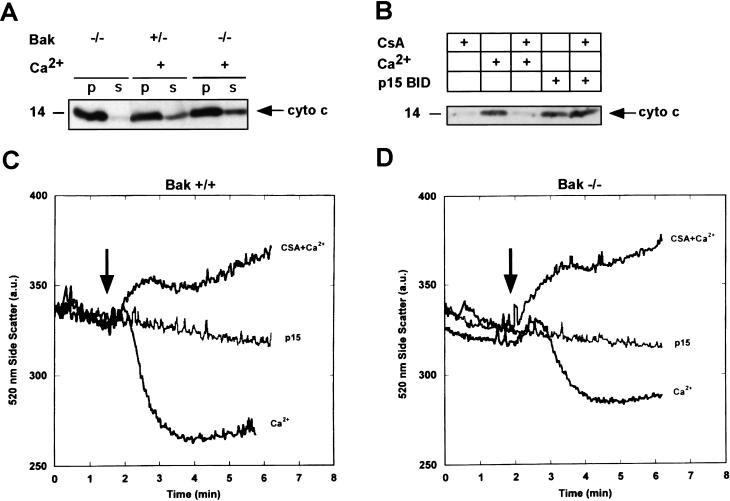
_Bak_-deficient mitochondria proved competent to release cytochrome c by the independent stimulus Ca2+ that induces permeability transition (PT), resulting in mitochondrial swelling as measured by a decrease in real-time side scatter and cytochrome c release comparable with wild-type mitochondria (Fig. 4A,C,D). The swelling and cytochrome c release by Ca2+ was blocked by the PT inhibitor cyclosporin A in both Bak+/− and _Bak_−/− mitochondria (Fig. 4B–D). In contrast, p15 BID does not cause permeability transition of Bak+/+ or _Bak_−/− mitochondria, and p15 BID-induced cytochrome c release is not inhibited by cyclosporin A (Fig. 4B–D). These studies indicate that (1) BAK is not required for PT and (2) p15 BID requires BAK to release cytochrome c in a PT-independent manner.
Figure 4.
p15 BID-induced cytochrome c release is independent of permeability transition (PT), and BAK is not required for PT. (A) Cytochrome c release in response to 200 μm Ca2+, which induces permeability transition in _Bak_-deficient (−/−) and wild-type or heterozygous (+/−) isolated mitochondria. (B) Comparison of Ca2+ vs p15 BID-induced cytochrome c release and response to 1 μm cyclosporin A (CsA) in isolated mitochondria. (C,D) Mitochondrial swelling as determined by a real time measure of side scatter in response to 75 μm Ca2+, or 75 μm Ca2+ plus 1.5 μm CsA, or p15 BID (0.5 ng/μl) in Bak+/+ (C) or _Bak_−/− isolated mitochondria (18 μg mitochondrial protein in 2 ml of targeting buffer). (D) Arrows indicate time of p15 BID or Ca2+ addition.
p15 BID physically interacts with BAK
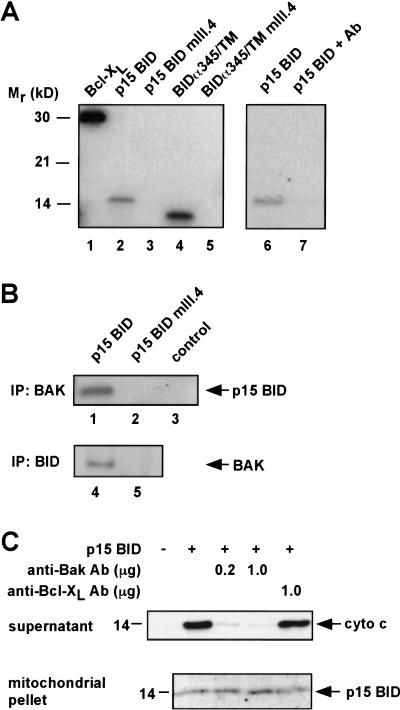
Because p15 BID appears to function at the membrane surface and require BAK for cytochrome c release, we investigated whether BAK might be the mitochondrial partner whose function is regulated by tBID. In vitro binding assays using recombinant GST–BAK demonstrated that wild-type but not mutant p15 BIDmIII.4 bound BAK directly. GST–BAK also bound the wild-type but not mutant chimera BIDα345/TM (Fig. 5A).
Figure 5.
BID and BAK physically interact, and a BAK-blocking Ab prevents cytochrome c release. (A) In vitro binding between GST–BAK and IVT BCL-XL, p15 BID, and BIDα345/TM, but not p15 BID mIII.4 or BIDα345/TM mIII.4 (lanes 1–5). None of the IVT proteins bound GST itself (data not shown). Preincubation with an anti-BAK Ab inhibits GST–-BAK in vitro binding to p15 BID (lanes 6,7). (B) Coimmunoprecipitation of BAK and wild-type p15 BID. 35S-labeled p15 BID wild-type (lane 1) and mutant p15 BID mIII.4 (lane 2) were targeted to mitochondria in vitro. The mitochondria were then solubilized and immunoprecipitated with an anti-BAK Ab. Coimmunoprecipitated p15 was detected by autoradiography. The anti-BAK Ab did not directly immunoprecipitate p15 BID wild type from solution (lane 3). BAK (24 kD) comigrates with light chain (25 kD), precluding detection of BAK by IP Western. Consequently, 35S-labeled IVT BAK was mixed with IVT p15 wild type or p15 mIII.4 in buffer B. BID was immunoprecipitated with an anti-BID Ab, and coimmunoprecipitated BAK was detected by autoradiography (lanes 4,5). (C) Inhibition of the p15 BID/BAK interaction prevents cytochrome c release. Wild-type mitochondria were incubated with the indicated amounts of anti-BAK Ab (G-23) or anti-BCL-XL Ab (SC-18) for 20 min at room temperature. A total of 25 ng of recombinant p15 BID was targeted to the mitochondria. The cytochrome c released into the supernatant and the p15 BID targeted to the mitochondrial pellet are shown.
Next we examined whether BAK and p15 BID interact at the level of the mitochondrion. Wild-type or mutant p15 BIDmIII.4 was targeted to mitochondria, which were then solubilized and immunoprecipitated with an anti-BAK antibody (Ab). Wild-type but not mutant p15 BID coimmunoprecipitated with BAK (Fig. 5B). In the reciprocal experiment, BAK coimmunoprecipitated with wild-type but not mutant p15 BID (Fig. 5B). Thus, independent binding assays demonstrate a physical interaction between p15 BID and BAK that correlates with the functional activity of cytochrome c release.
Inhibiting p15 BID/BAK interaction prevents cytochrome c release
To test whether interfering with the physical interaction between p15 BID and BAK would inhibit the release of cytochrome c, we incubated mitochondria with a polyclonal Ab to residues of BAK located near the proposed BH3-binding pocket. This antibody did not prevent p15 BID targeting to mitochondria but inhibited p15 BID-induced cytochrome c release in a dose-dependent manner (Fig. 5C). A control Ab against BCL-XL did not inhibit p15 BID-induced cytochrome c release. Inclusion of the anti-BAK Ab also prevented GST–BAK from binding p15 BID in vitro (Fig. 5A, lanes 6,7). These data are consistent with the BAK Ab preventing p15 BID from interacting with BAK by obstructing its binding and strongly support the importance of a tBID/BAK interaction in the release of cytochrome c.
tBID induces a conformational change and oligomerization of BAK
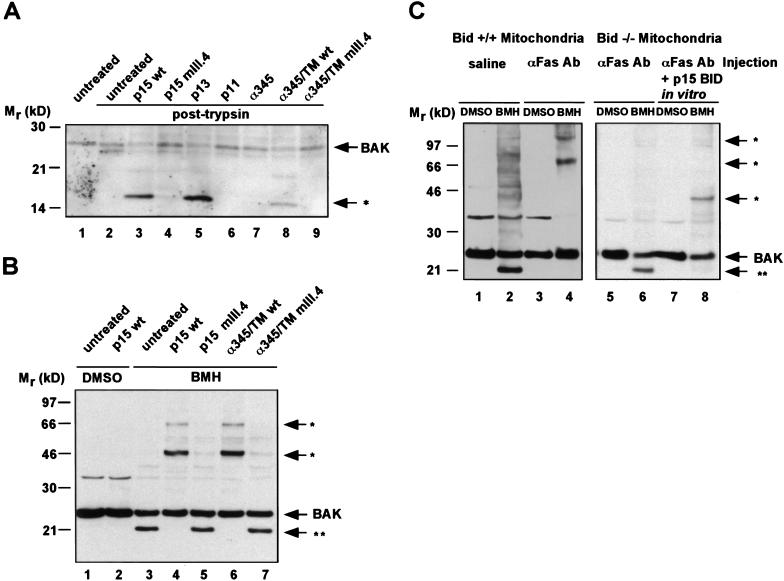
Ligand binding often causes a conformational change of a receptor protein, thereby regulating the activity of this partner and initiating downstream signaling events (Schlessinger 1988). Consequently, we tested whether BAK would undergo a conformational change in response to tBID. First, we assessed the protease sensitivity of BAK in the presence of p15 BID. Trypsin treatment of isolated mitochondria from viable hepatocytes cleaves BAK from 24 kD to a ∼22-kD fragment (Fig. 6A, lanes 1,2). Mitochondria bearing targeted p15 BID displayed an altered pattern of trypsin-digested BAK, revealing a ∼16-kD fragment indicating the exposure of a new trypsin site in this BAK conformation (Fig. 6A, lane 3). The capacity of BID mutant and chimeric molecules to cause this conformational change in BAK correlated precisely with their ability to trigger cytochrome c release (Fig. 6A, lanes 4–9).
Figure 6.
p15 BID induces a conformational change and oligomerization of BAK in vitro and in vivo. (A) The IVT BID proteins denoted were targeted to wild-type mitochondria. The mitochondria were then treated with 30 μg/ml trypsin (lanes 2–9). The trypsin sensitivity pattern of BAK was assessed by an anti-BAK Ab Western blot. (B) The IVT BID proteins denoted were targeted to mitochondria. Mitochondria were then treated with a DMSO control buffer or with 10 mm BMH cross-linker. Pattern of cross-linked BAK was determined by an anti-BAK Ab Western blot. (*) Higher molecular mass BMH-cross-linked BAK complexes. (**) An intramolecularly cross-linked BAK monomer displaying faster mobility. (C) Mitochondria were compared from Bid+/+ versus _Bid_−/− mice following intravenous injection of saline or anti-Fas Ab (Jo2, Pharmingen, 0.5 μg/gm body mass) for 2 hr. Fas-activated Bid+/+ mitochondria lost the faster mobility BAK species and demonstrated BMH cross-linked higher molecular mass BAK complexes (lanes 3,4). _Bid_−/− mitochondria do not oligomerize BAK in response to in vivo Fas activation (lanes 5,6). However, BAK oligomerization can be restored by in vitro targeting of p15 BID (lanes 7,8).
We utilized a series of chemical cross-linkers to assess whether the tBID-induced conformational change in BAK was accompanied by the formation of higher order BAK complexes within the mitochondrial membrane. Bismaleimidohexane (BMH), a 16 Å, membrane permeable, homobifunctional maleimide that covalently cross-links sulfhydryl groups, proved instructive. Mouse BAK has two cysteine residues at positions 14 and 153. Of note, the majority of BAK in untreated mitochondria displayed a faster, ∼21-kD mobility following BMH cross-linking presumably reflecting an intramolecular cross-linked BAK monomer (Fig. 6B, lane 3). Upon addition of p15 BID to isolated mitochondria, the BMH irreversible cross-linker shifted BAK into three distinct complexes at ∼48 kD (major species) and ∼72 and ∼96 kD (minor species) that were detected by an anti-BAK Ab on Western blot (Fig. 6B, lane 4). These bands were not detected in the absence of cross-linker (Fig. 6B, lanes 1,2). Moreover, addition of tBID eliminated the BAK conformation represented by the faster mobility ∼21-kD species at the same time BAK shifted into higher molecular mass complexes (Fig. 6B, lane 4). Mitochondria treated with the nonfunctional BH3 mutants, p15 BIDmIII.4, and BIDα345/TMmIII.4 retained the faster mobility BAK conformation and did not form higher molecular mass complexes (Fig. 6B, lanes 5,7). Consistently, the wild-type BIDα345/TM chimera did eliminate the faster mobility BAK species and initiated the formation of the same BAK complexes as p15 BID (Fig. 6B, lane 6). Thus, the ability of these BID proteins to induce the apparent oligomerization of BAK mirrored their ability to bind BAK, cause a conformational change in BAK, and release cytochrome c. On the basis of the results of the chimeric molecules, we conclude that targeting an intact BH3 domain to the mitochondrial surface causes the formation of BAK complexes.
The ∼48-kD complex was most predominant and its size consistent with that of a BAK dimer. However, we did test whether any of these BAK cross-linked complexes possessed other known mitochondrial protein candidates. For example, Western blot analysis did not detect the presence of any BID, VDAC, ANT, or BCL-XL in the BAK cross-linked complexes. The estimated sizes of these BAK cross-linked complexes are consistent with an evolving homo-oligomerization of BAK similar to what we have observed for recombinant BAX in pure liposomes (Saito et al. 2000), although we cannot formally exclude the presence of other non-identified proteins.
tBID induces the activation of BAK in vivo
Injection of anti-Fas Jo2 Ab into mice results in the cleavage and translocation of tBID to mitochondria and subsequent massive hepatocellular apoptosis, in a process that requires BID as evidenced by _Bid_-deficient mice (Ogasawara et al. 1993; Yin et al. 1999). We injected wild-type mice with anti-Fas Ab to determine whether their mitochondria had altered BAK. BAK on such mitochondria displayed an altered trypsin sensitivity, as compared with saline-injected controls (data not shown). Treatment of mitochondria from such Fas-activated hepatocytes with the irreversible cross-linker BMH revealed the elimination of the faster mobility BAK and the movement of BAK into higher molecular mass complexes again consistent with trimers (72 kD) and tetramers (≥96 kD) (Fig. 6C). Importantly, no alteration in BAK conformation was noted in mitochondria from the anti-Fas Ab-treated livers of _Bid-_deficient mice. Treatment of mitochondria from _Bid_−/− hepatocytes with recombinant p15 BID restored the BAK conformational change.
Discussion
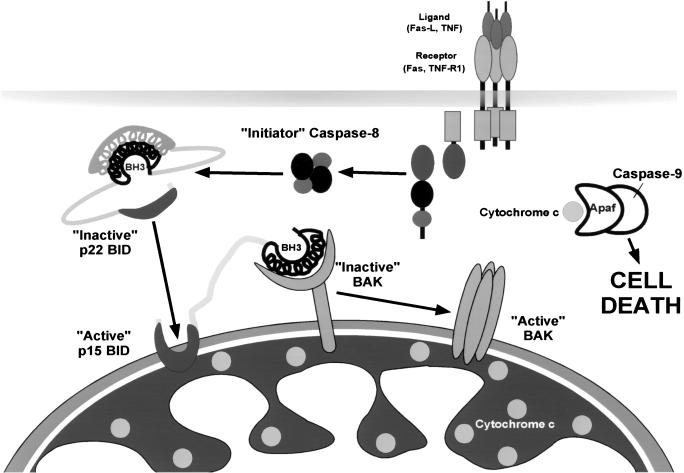
These studies integrate an apoptotic pathway from a surface death receptor, through the sequential activation of pro-apoptotic BCL-2 family members, to the release of cytochrome c and caspase activation. Two loss-of-function mouse models, Bid deficiency and Bak deficiency, were used to establish a cascade in which Fas engagement on hepatocytes activates BID, which activates BAK to release cytochrome c (Fig. 7). The BH3-domain-only tBID serves as an upstream death ligand that functions to allosterically regulate the full pro-apoptotic molecule BAK constitutively present on mitochondria. The BH3 domain of tBID must be intact and able to interact with resident mitochondrial BAK to release cytochrome c. The α6, α7 hydrophobic helices confer an integral mitochondrial membrane position to tBID, which does not itself form a pore capable of releasing cytochrome c. Instead, the membrane targeting of tBID appears to represent a localized, concentrating mechanism to present the BH3 domain to resident BAK.
Figure 7.
An activation cascade of pro-apoptotic BID to BAK integrates the apoptotic pathway from death receptors to mitochondrial release of cytochrome c.
Peptide ligand-induced oligomerization of transmembrane receptors is a common mechanism for initiating signal transduction (Wells 1994). The homomultimeric cyclic-nucleotide-gated ion channel displays allosteric coupling between binding sites, which governs the multiple subconductance states of the channel (Tierney and Stowell 1998). By analogy, BAK can be envisioned as a resident mitochondrial membrane-based partner that undergoes allosteric activation as a result of a conformational change induced upon tBID engagement. A unique aspect of this tBID/BAK model is the ligand-induced homo-oligomerization that results in an allosteric, global conformational change of the activated BAK complex. BAK activation was noted both in vitro and in vivo and manifests as altered protease sensitivity and oligomerization into higher-order multimers as detected by cross-linking. Although we cannot completely exclude the presence of other proteins in such complexes, the sizes are compatible with homo-oligomerization, and we have not detected other candidates tested to date. We cannot formally exclude an indirect mechanism involving anti-apoptotic BCL-2 members; however, the addition of BCL-2 to mitochondria prevents rather than promotes tBID-induced activation, and a p15 BID mutant that does not bind BCL-2 or BCL-XL still oligomerizes BAK and releases cytochrome c (data not shown). Of note, tBID does not appear to stay in association with BAK after oligomerization, suggesting a hit-and-run model in which tBID no longer binds after inducing the BAK conformational change. Following Fas activation of hepatocytes in vivo, the most prominent BAK oligomers (Fig. 6) generated appear to be ≥ tetramers by size. We have noted that the very closely related full pro-apoptotic member BAX is capable of oligomerizing in pure lipid membranes to form a pore of ≥22 Å comprised of four BAX molecules that transports 17 Å cytochrome c (Saito et al. 2000) (Fig. 7). Moreover, the tBID/BAK interaction in vitro released cytochrome c before any obvious permeability transition. The inability of cyclosporin A to prevent tBID-induced cytochrome c release here and in other studies (Shimizu and Tsujimoto 2000) argues tBID can function independent of the permeability transition pore. Together these results suggest that oligomerized BAK itself provides a pore for cytochrome c release. Conversely, the capacity of _Bak_-deficient mitochondria to undergo a permeability transition indicates BAK is not a critical component of a Ca2+-induced PTP.
The physiologic relevance of BAK oligomerization is supported by _Bid_- and _Bak_-null cells. _Bid_-deficient hepatocytes indicate BID is singularly required to oligomerize BAK and release cytochrome c following Fas activation. Consistent with the importance of a BID/BAK axis, _Bak_-deficient mitochondria show no response to integrated tBID. The formation of BAK oligomers is highly reminiscent of the BAX oligomerization noted following growth factor deprivation (Gross et al. 1998). One major difference is that these pro-apoptotic molecules are initially in separate subcellular compartments in viable hepatocytes. The BAX present in viable hepatocytes is rather exclusively in the cytosol, whereas BAK is an integral membrane protein even prior to a death signal. Other studies have indicated an association between p22 BID and BAX. The only BH3 mutants of BID that retained activity-bound BAX, not BCL-2, suggesting BID could also function as a BAX activator (Wang et al. 1996). Studies of staurosporine-treated HeLa cells indicate that BID in this system will induce the oligomerization and membrane insertion of BAX and a conformational change in BAK (Desagher et al. 1999; Eskes et al. 2000). Our preliminary assessment of intact hepatocytes from _Bak_-deficient mice indicates tBID may also activate the BAX present in cytosol, indicating the existence of multiple pro-apoptotic targets. However, in this purified mitochondrial organelle system, it is BAK that we can establish as being essential for cytochrome c release. We expect that tBID's activation of full pro-apoptotic members BAX and/or BAK will prove to be cell type and death signal selective.
Structural data support the observation that BID can bind either BAK or BAX. The BCL-XL residues involved in a BCL-XL/BH3 peptide interaction (Sattler et al. 1997) are generally conserved in both full pro-apoptotic BAX and BAK, but are not present in the BH3 domain-only BID. The predicted orientation of helices in BAX or BAK are very similar to BCL-XL, indicating that they would form a BH3-binding pocket. However, no substantial pocket was found in BID reflecting in part insertions in loop 4–5 and deletions in loop 5–6 that alter the orientation of the helices. This is consistent with BID serving as a donor but not a recipient of BH3 domains. Moreover, the removal of the NH2-terminal 7 kD of BID following caspase-8 cleavage would eliminate a strong hydrophobic interaction between the α1 helix and the α3 BH3 helix and be expected to further expose the BH3 domain (Chou et al. 1999; McDonnell et al. 1999).
The function of other BH3-domain-only molecules has been equated with inactivating anti-apoptotic members such as BCL-2 or BCL-XL. Whereas high levels of BCL-2 or BCL-XL can bind and possibly sequester tBID, it was BAK and not the BCL-XL present in hepatocytes that was required for cytochrome c release. In contrast, BAD only binds anti-apoptotic members; its pro-death activity correlated with the necessity of an intact BH3 domain to bind and presumably inactivate BCL-2 or BCL-XL (Kelekar et al. 1997; Ottilie et al. 1997; Zha et al. 1997). Similarly, BIM, when released from microtubules, is felt to bind and inactivate BCL-XL (Puthalakath et al. 1999). However, these interactions could also displace available BAX or BAK (Yang et al. 1995). In C. elegans the BH3-domain-only egl-1 is an upstream negative regulator of the Bcl-2 homolog ced-9 (Conradt and Horvitz 1998). Moreover, EGL-1 will bind CED-9, which can also bind CED-4, the APAF-1 homolog, which activates the downstream caspase, CED-3. This might likewise create a displacement reaction that, at least in part, regulates the pathway. tBID is unique in that it activates an essential pro-apoptotic member BAK emphasizing the evolutionary diversification and complexity of the BH3-domain-only molecules that interconnect death and survival signals with the core apoptotic pathway.
Materials and methods
DNA constructs
All expression constructs for IVT were cloned into pcDNA3 (Invitrogen) and sequenced. p15 BID wild type, mIII.1, and mIII.4 were amplified by PCR from p22 BID versions of these mutants (Wang et al. 1996). p13 BID, p11 BID, and BIDα345 were amplified from wild-type p22 BID.
For the chimeric proteins BIDα345/TM and BIDα345/TMmIII.4, a sequence encoding BID amino acids 60–142 was amplified with a 3′ extension that overlapped the 5′ end of the mouse BCL-2 transmembrane sequence. The BCL-2 transmembrane sequence encoding amino acids 215–239 was amplified with a 5′ extension that overlapped the 3′ end of the BID fragment. The two fragments were mixed and amplified together to create the final chimeric proteins, BIDα345/TM and BIDα345/TMmIII.4.
Recombinant proteins
IVT proteins were in vitro transcribed and translated by use of a T7-coupled wheat germ extract system (Promega) and 35S protein labeling mix (New England Nuclear) according to the manufacturers' protocols.
For recombinant p15 BID wild type and mIII.4, a full-length cDNA coding for mouse p22 BID was cloned into pET-15b (Novagen). The fusion protein was expressed in the Escherichia coli strain BL21 (DE3) (Novagen) and contained an NH2-terminal polyhistidine tag followed by a thrombin cleavage site. This protein was soluble and was purified to homogeneity by nickel affinity chromatography (Qiagen) using the manufacturer's protocol.
Active recombinant caspase-8 containing a non-cleavable polyhistidine tag at the NH2 terminus was expressed and purified by a similar method. Most of the caspase-8 fusion protein was expressed as an insoluble precursor protein. However, a small fraction of the protein had undergone autocatalysis to its active, two-subunit form, which was soluble and readily purified.
p22 BID was incubated with the active caspase-8 preparation at a mass ratio of 100:1 (p22:caspase 8). The reaction was carried out in 20 mm PIPES, 100 mm NaCl, 10 mm DTT, 1 mm EDTA, 0.1% CHAPS, and 10% sucrose at room temperature for 18 hr at a final p22 Bid concentration of 1 mg/ml. The reaction mixture was diluted 1:10 in 20 mm Tris, 500 mm NaCl, 5 mm imidazole, and then applied to a nickel affinity column. p15 remained associated to the polyhistidine-tagged p7. Elution of p15 from the column was carried out with 6 m guanidine HCl, 20 mm Tris, 500 mm NaCl, 5 mm imidazole (pH 7.4). Undigested p22, p7, and caspase-8, all polyhistidine tagged, remained bound to the column. The eluted p15 was diluted to 50 μg/ml and dialyzed against 10 mm HEPES (pH 7.6), 1 mm DTT, and 50 mm KCl.
For recombinant GST–BAK, a recombinant fusion protein containing GST fused to the NH2 terminus of human BAK lacking the transmembrane domain was expressed in E. coli and purified with glutathione–agarose beads (Sigma). The beads with bound GST–BAK were then resuspended in buffer B (142 mm KCl, 5 mm MgCl2, 10 mm HEPES at pH 7.6, 1 mm EDTA, and 0.1% NP-40).
Generation of Bak-deficient mice
A targeting construct was made that deleted 4.8 kb, encompassing exons 3–6 of the Bak gene (T. Lindsten, E. Ulrich, G. Evan, A. Ma, and C. Thompson, in prep.). The _Bak_-targeting construct was linearized with _Xho_I and electroporated into R1 ES cells (Nagy et al. 1993) by use of standard techniques (Joyner 1993). Four independent clones were identified and injected into C57BL/6 blastocysts. All four clones showed germ-line transmission. _Bak_-null mice were born at the expected Mendelian ratio.
In vitro targeting and cytochrome c release
Mitochondria were isolated from mouse liver according to published protocols (McBride et al. 1995). Liver from one mouse was homogenized with two up and down strokes in a Wheaton glass homogenizer with type B pestle in HIM buffer (200 mm mannitol, 70 mm sucrose, 10 mm HEPES, 1 mm EGTA at pH 7.5) plus 0.2% defatted BSA (Sigma). The homogenate was centrifuged at 600_g_ for 10 min. The supernatant was centrifuged at 7000_g_ for 15 min. The pellet containing mitochondria was resuspended in HIM and recentrifuged at 600_g_ for 10 min. The supernatant was centrifuged at 7000_g_ for 10 min, and the mitochondrial pellet was resuspended in MRM-S (250 mm sucrose, 10 mm HEPES, 1 mm ATP, 5 mm succinate, 0.08 mm ADP, 2 mm K2HPO4 at pH 7.4) to a final concentration of 2 mg/ml protein. The supernatant from the first 7000_g_ spin was centrifuged at 100,000_g_ for 30 min to collect the soluble S-100 fraction.
For a targeting/cytochrome c release reaction, 25 μl of mitochondria was incubated with 20 μl of MRM-S/80 mm KCl/2 mm Mg(C2H3O2)2 and 0.5 ng/μl recombinant protein or 5 μl of IVT protein (0.1–0.5 ng/μl BID protein final concentration in reaction). The reaction was placed at 30 degrees for 30–60 min, and mitochondria were pelleted and solubilized in a volume of protein sample buffer equal to the volume of supernatant collected.
For post-trypsin treatment, trypsin was added to final concentration of 30 μg/ml, and the mitochondria were incubated on ice for 20 min. Soybean trypsin inhibitor (100 μg/ml) was then added, and the mitochondria were pelleted and solubilized in protein sample buffer. For post-caspase treatment, 0.1 μg of recombinant caspase-3 (Pharmingen) was added following the targeting reaction.
For cross-linking, dimethylsulfoxide (DMSO) (control) or BMH cross-linker (Pierce) dissolved in DMSO to a final concentration of 10 mm was added to mitochondria for 30 min at room temperature. The mitochondria were pelleted and resuspended in protein sample buffer. DTT in the sample buffer quenched the cross-linking reaction.
Western blotting and fluorography
Protein samples were separated on SDS–polyacrylamide gel or NuPAGE (Novex) according to manufacturer's protocols. Proteins were transferred to PVDF membranes (Immobilon-P, Millipore). Antibodies included anti-cytochrome c (75981A, Pharmingen), anti-BAK (G-23, Santa Cruz), anti-BAK (Upstate Biotechnology), anti-VDAC (Ab-4, Calbiochem), anti-BCL-XL (Pharmingen), anti-BID (Wang et al. 1996), and anti-ANT (generous gift of P. Schmid, University of Minnesota). Western blots were developed by use of a chemiluminescence reagent (New England Nuclear). To visualize 35S-labeled IVT proteins by fluorography, SDS–polyacrylamide gels were fixed, incubated in Amplify (Amersham), and dried before exposure to film.
GST-binding assay
For binding reaction, 35S-labeled IVT proteins were incubated with GST–BAK beads in buffer B for 2 hr at 4°C and washed extensively. For antibody blocking experiments, GST–BAK beads were incubated with anti-BAK antibody for 1 hr at 4°C and washed prior to addition of IVT proteins. The beads were then resuspended in protein sample buffer and analyzed by fluorography for bound proteins.
Coimmunoprecipitation
35S-labeled IVT proteins were incubated with mouse liver mitochondria for 30 min at 30°C. The mitochondria were then solubilized with buffer B for 30 min at 4°C. The samples were then incubated with anti-BAK antibody for 90 min at 4°C, followed by protein A-conjugated Sepharose beads (Sigma) for 60 min at 4°C. Following extensive washing, the beads were resuspended in protein sample buffer and analyzed by fluorography for bound proteins.
Mitochondrial swelling
Real-time mitochondrial swelling was followed by measuring 90° side scatter at 520 nm with a Perkin Elmer LS50B spectrophotometer. The reaction was continuously mixed with magnetic stir bar in a 30°C water jacket. Excitation and emission wavelengths were set to 520 nm with 2.5 nm slit widths.
Acknowledgments
We thank I. Goping and G. Shore for advice on mitochondrial targeting; J. McDonnell and D. Cowburn for advice on BID structure; A. Ross for advice on PCR; S. Kornfeld for advice on cross-linking; P. Schmid for ANT antibody; and E. Alnemri for caspase-8 expression plasmid. B. Avery and S. Wade provided excellent animal care, and E. Smith expertly coordinated figure and manuscript preparation. We thank K. Wang, J. Wang, and E. Cheng for assistance with DNA constructs. This work was supported by National Institutes of Health grant nos. 5T32CA09361–19 (M.C.W.) and CA50239 (S.J.K.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL stanley_korsmeyer@dfci.harvard.edu; FAX (617) 632-6401.
References
- Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell. 1998;93:519–529. doi: 10.1016/s0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;8:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes & Dev. 1999a;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999b;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Becknell B, Wilm M, Mann M, Huang LJ, Taylor SS, Scott JD, Korsmeyer SJ. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol Cell. 1999;3:413–422. doi: 10.1016/s1097-2765(00)80469-4. [DOI] [PubMed] [Google Scholar]
- Joyner AL. Gene targeting : A practical approach. Oxford, UK: Oxford University Press; 1993. [Google Scholar]
- Kelekar A, Chang BS, Harlan JE, Fesik SW, Thompson CB. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol Cell Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Esposti MD, Perkins G, Renken C, Kuwana T, Bossy-Wetzel E, Goldberg M, Allen T, Barber MJ, Green DR, et al. The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol. J Cell Biol. 1999;147:809–822. doi: 10.1083/jcb.147.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- McBride HM, Silvius JR, Shore GC. Insertion of an uncharged polypeptide into the mitochondrial inner membrane does not require a trans-bilayer electrochemical potential: Effects of positive charges. Biochim Biophys Acta. 1995;1237:162–168. doi: 10.1016/0005-2736(95)00088-k. [DOI] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: A structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Velez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Diaz JL, Horne W, Chang J, Wang Y, Wilson G, Chang S, Weeks S, Fritz LC, Oltersdorf T. Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins. J Biol Chem. 1997;272:30866–30872. doi: 10.1074/jbc.272.49.30866. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX dependent cytochrome-c transport reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, et al. Structure of Bcl-xL–Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Signal transduction by allosteric receptor oligomerization. Trends Biochem Sci. 1988;13:443–447. doi: 10.1016/0968-0004(88)90219-8. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Tsujimoto Y. Proapoptotic BH3-only bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci. 2000;97:577–582. doi: 10.1073/pnas.97.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney ML, Stowell MH. The functional significance of multimerization in ion channels. Curr Opin Struct Biol. 1998;8:186–188. doi: 10.1016/s0959-440x(98)80036-5. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999;3:159–167. doi: 10.1016/s1097-2765(00)80307-x. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: A novel BH3 domain-only death agonist. Genes & Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wells JA. Structural and functional basis for hormone binding and receptor oligomerization. Curr Opin Cell Biol. 1994;6:163–173. doi: 10.1016/0955-0674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]