The von Hippel–Lindau tumor suppressor protein is a component of an E3 ubiquitin–protein ligase activity (original) (raw)
Abstract
pVHL, the product of the VHL tumor suppressor gene, plays an important role in the regulation of cell growth and differentiation of human kidney cells, and inactivation of the VHL gene is the most frequent genetic event in human kidney cancer. The biochemical function of pVHL is unknown. Here we report that pVHL exists in vivo in a complex that displays ubiquitination-promoting activity in conjunction with the universally required components E1, E2, and ubiquitin. pVHL-associated ubiquitination activity requires, at a minimum, pVHL to bind elongin C and Cul-2, relatives of core components of SCF (Skp1–Cdc53/Cul-1–F-box protein) E3 ligase complexes. Notably, certain tumor-derived mutants of pVHL demonstrate loss of associated ubiquitination promoting activity. These results identify pVHL as a component of a potential SCF-like E3 ubiquitin–protein ligase complex and suggest a direct link between pVHL tumor suppressor and the process of ubiquitination.
Keywords: VHL, ubiquitination, tumor suppression, cullin, elongin, SCF E3 ligases
von Hippel–Lindau (VHL) disease is a hereditary cancer syndrome leading to the development of a variety of tumors, including clear cell carcinomas of the kidney and pheochromocytomas and vascular tumors of the central nervous system and retina (Maher and Kaelin 1997; Kaelin and Maher 1998). The VHL susceptibility gene is a tumor-suppressor gene, and germ-line mutations affecting this gene have been documented in ∼80% of VHL patients (Latif et al. 1993). Functional inactivation of both VHL alleles also occurs in the majority of sporadic clear cell renal carcinomas (Gnarra et al. 1994). Therefore, inactivation of the VHL tumor-suppressor gene appears to be a requisite step in the development of clear cell renal carcinoma.
The VHL gene, which contains three exons, encodes a 213-amino-acid polypeptide (Latif et al. 1993; Iliopoulos et al. 1995). The biochemical function of pVHL is as yet unknown. pVHL resides mostly in the cytoplasm and, to a lesser extent, in the nucleus and in association with cell membranes (Duan et al. 1995a; Iliopoulos et al. 1995; Pause et al. 1997; Ohh et al. 1998; Lee et al. 1999). In addition, many cells also produce a shorter variant of pVHL protein, pVHL19, which appears to arise as a result of alternative translation initiation (Iliopoulos et al. 1998; Schoenfeld et al. 1998). Cell fractionation studies suggest that pVHL19, unlike pVHL, is equally distributed between the nucleus and cytoplasm and is not found in association with membranes (Iliopoulos et al. 1998). Recent evidence suggests that pVHL shuttles between the nuclear and the cytoplasmic compartments in a transcription-dependent manner (Lee et al. 1999).
pVHL displays no similarity to other known proteins, thus giving no clues about its function. Hence, most studies aimed at elucidating pVHL function have focused on the identification and functional analysis of interacting proteins. pVHL associates with a number of cellular proteins including Fibronectin (Ohh et al. 1998), Elongin B and Elongin C (Duan et al. 1995b; Kibel et al. 1995; Takagi et al. 1997), Cullin-2 (Cul-2) (Pause et al. 1997; Lonergan et al. 1998) and Rbx1 (Kamura et al. 1999). The interaction of pVHL with Fibronectin is thought to be required for the proper assembly of an extracellular fibronectin matrix and is abolished by all disease-causing mutations analyzed to date, suggesting that the binding of pVHL to fibronectin is likely to contribute to the ability of pVHL to suppress tumor growth (Ohh et al. 1998). Similarly, the ability of pVHL to form a stable complex with Elongin B, Elongin C, and Cul-2 has been linked to pVHL’s capacity to regulate the stability of hypoxia-inducible mRNAs (Lonergan et al. 1998), including mRNAs that encode angiogenic proteins [e.g., vascular endothelial growth factor (VEGF)] (Gnarra et al. 1996; Iliopoulos et al. 1996; Siemeister et al. 1996), mitogenic factors [e.g., platelet-derived growth factor-B (PDGF-B) and transforming growth factor-α (TGF-α)] (Iliopoulos et al. 1996; Knebelmann et al. 1998), and proteins involved in cell metabolism (e.g., Glut-1 glucose transporter) (Iliopoulos et al. 1996).
The pVHL sequence includes a carboxy-terminal BC-box motif (Aso et al. 1995; Kamura et al. 1998) and GEExE repeats in its amino terminus (Latif et al. 1993). The BC-box motif within pVHL, a short ∼12-amino-acid-long degenerate sequence, is a region frequently mutated in pVHL, suggesting there resides a critical domain for pVHL tumor-suppressor function (Stebbins et al. 1999). The BC-box motif is also present in a number of other cellular proteins including the F-box protein elongin A (Aso et al. 1996) and members of the SOCS (suppressors of cytokine signaling) family (Kamura et al. 1998). As in the case of Elongin A and SOCS-1, the BC-box motif in pVHL serves as an interaction site for the elongin BC complex. Interestingly, the BC box of SOCS-family members is embedded within the SOCS-box motif (Kamura et al. 1998).
The identification of Cul-2 and Rbx1 as pVHL-associated proteins prompted speculations about a possible role for pVHL in the process of ubiquitination (Pause et al. 1997; Lonergan et al. 1998; Kamura et al. 1999). Cul-2 is a member of a recently identified evolutionary-conserved gene family referred to as the Cullins (Kipreos et al. 1996). In higher eukaryotes, six members of the Cullin family have been identified, namely Cul-1, -2, -3, -4A, -4B, and -5. Little is known about their respective gene products and function(s). The best-characterized member of this family is Cul-1, a structural and functional homolog of budding yeast Cdc53, which has a key role in regulated ubiquitin-mediated proteolysis (Patton et al. 1998; Koepp et al. 1999). Cdc53/Cul-1 assembles with Skp1 and distinct F-box proteins into E3 ubiquitin-protein ligase complexes, referred to as SCF (for Skp1/Cdc53(Cul-1)/F-box protein) complexes (Bai et al. 1996; Feldman et al. 1997; Skowyra et al. 1997). In this type of E3 ligase, the F-box protein is believed to function as the substrate-specific receptor (Krek 1998; Patton et al. 1998; Koepp et al. 1999). A recently identified evolutionary conserved protein, Rbx1/Roc1, the fourth subunit of SCF complexes, associates with Cdc53/Cul-1 and is critical for efficient recruitment of an E2 enzyme to Cdc53/Cul-1 and, hence, essential for SCF function (Kamura et al. 1999; Ohta et al. 1999; Skowyra et al. 1999; Tan et al. 1999). Interestingly, the SCF subunit Rbx1/Roc1 has also been identified as a pVHL-associated protein (Kamura et al. 1999). In addition, the primary sequence of Elongin C, which bridges the interaction of pVHL and Cul-2, displays homology to Skp1 (Bai et al. 1996; Stebbins et al. 1999). Elongin B interacts with Elongin C in the pVHL complex and harbors a ubiquitin-like domain (Garrett et al. 1995; Stebbins et al. 1999). Taken together, these findings suggest similarities in the overall architecture of the pVHL complex to SCF E3 ligases.
Biochemical evidence supporting a role for pVHL in the process of ubiquitination is at present lacking. Here we show that recombinant pVHL recruits ubiquitination-promoting activity from human cell extracts. The appearance of this activity can be specifically blocked by peptides that block pVHL/Elongin BC/Cul-2 association. We also show that antibodies specific for pVHL immunoprecipitate pVHL-associated ubiquitination-promoting activity from unperturbed VHL+/+ cells. Likewise, anti-hemagglutinin (HA) immunoprecipitates derived from VHL−/− renal cell carcinoma (RCC) cell lines that stably express a HA-tagged version of wild-type pVHL exhibit ubiquitination activity. In contrast, selected, naturally occurring mutants of pVHL lack this activity. Finally, sequences surrounding and including the BC box in pVHL share sequence homology with an F-box motif. Our data imply that the pVHL may function, at least in part, as a component of an E3 ubiquitin–protein ligase complex.
Results
Recombinant pVHL recruits ubiquitination activity from human cell extracts
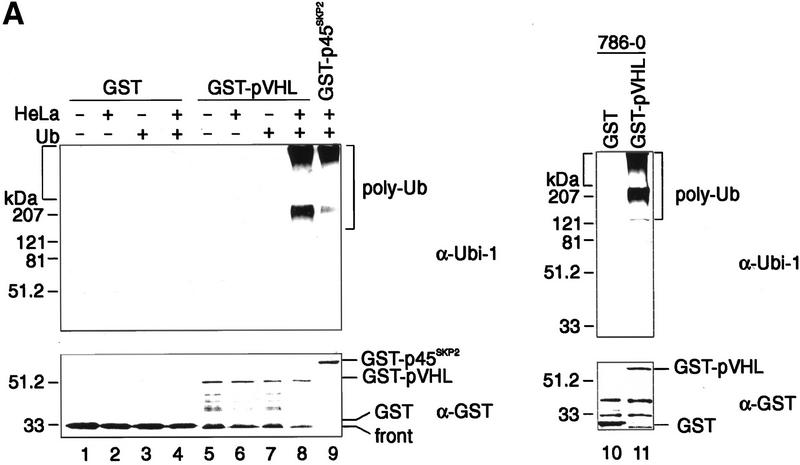
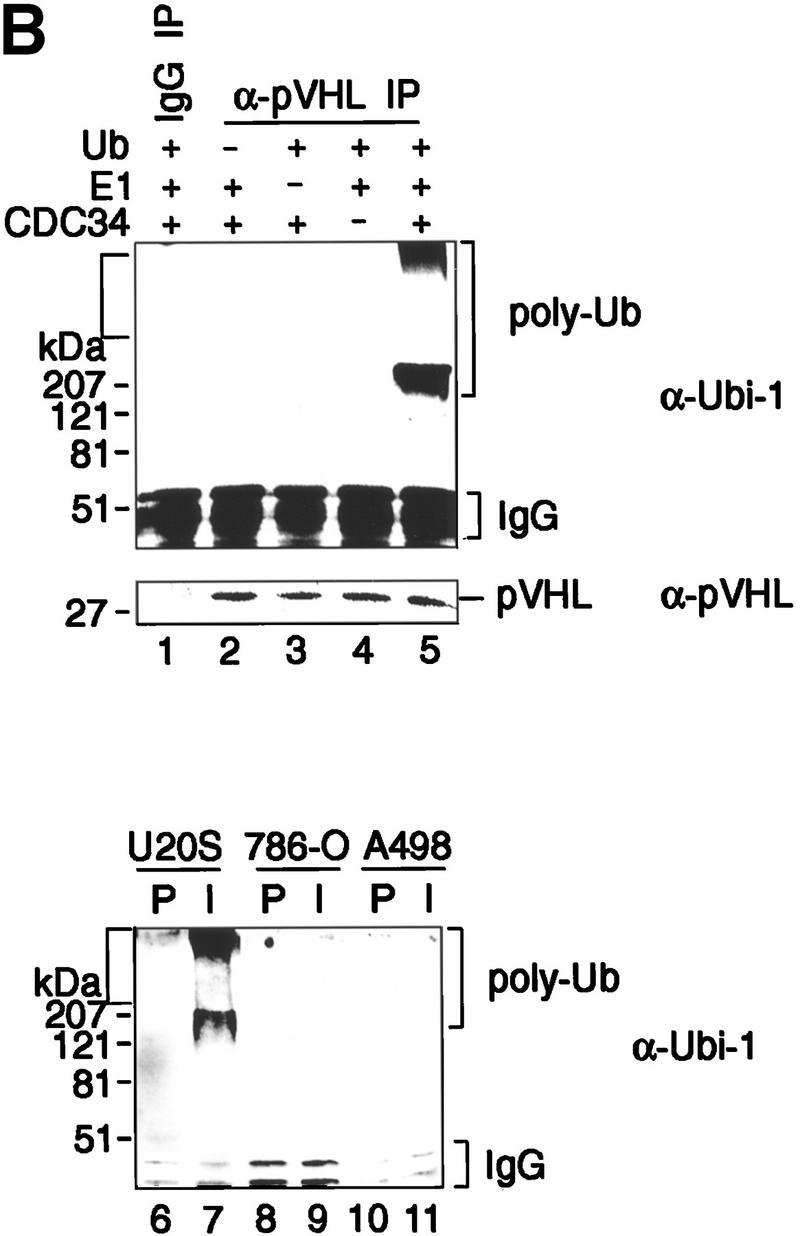
To test whether pVHL is a component of a potential E3 ligase activity, we used an assay that has been described previously on the occasion of the detection of p45Skp2 F-box protein (Marti et al. 1999) or Cul-1-associated ubiquitination-promoting activity (Lyapina et al. 1998). Specifically, glutathione–Sepharose beads were loaded with similar quantities of bacterially produced GST–pVHL or GST as control, and aliquots of each were either left untreated or were preincubated in lysates of HeLa cells as described in Materials and Methods. The latter step was included to allow the binding of cellular components, regulators, and substrates that might be required to detect pVHL-associated ubiquitination activity. After washing away unbound proteins, beads were supplemented with purified E1, purified human E2 enzyme Cdc34, ubiquitin, and an ATP-regenerating system. After an incubation period of 90 min at 30°C, the reaction mixture was separated by SDS-PAGE and analyzed by anti-ubiquitin immunoblotting to detect ubiquitin conjugates (Fig. 1A). GST–pVHL purified from bacterial lysates exhibited no ubiquitination activity (Fig. 1A, lane 7). In contrast, anti-ubiquitin antibodies detected a high molecular weight smear characteristic of ubiquitin conjugates when GST–pVHL was incubated in HeLa lysates prior to performing the assay (Fig. 1A, lane 8). The appearance of high-molecular weight migrating proteins was dependent on the addition of ubiquitin (Fig. 1A, cf. lanes 6 and 8), indicating that the high molecular weight smear was the result of multiubiquitination occurring during the in vitro reaction. As shown previously (Marti et al. 1999), GST–p45Skp2 isolated from insect cells displays associated ubiquitination activity under these experimental conditions (Fig. 1A, lane 9). No ubiquitination activity was detected when GST protein alone was preincubated in HeLa lysates (Fig. 1A, lane 4). Anti-GST immunoblotting confirmed that similar amounts of the indicated GST species were present in each assay (Fig. 1A, bottom). Similarly, GST–pVHL, but not GST protein alone, recruited ubiquitination-promoting activity from 786-O RCC cell lysates lacking wild-type VHL protein (Fig. 1A, lanes 11 and 10, respectively).
Figure 1.
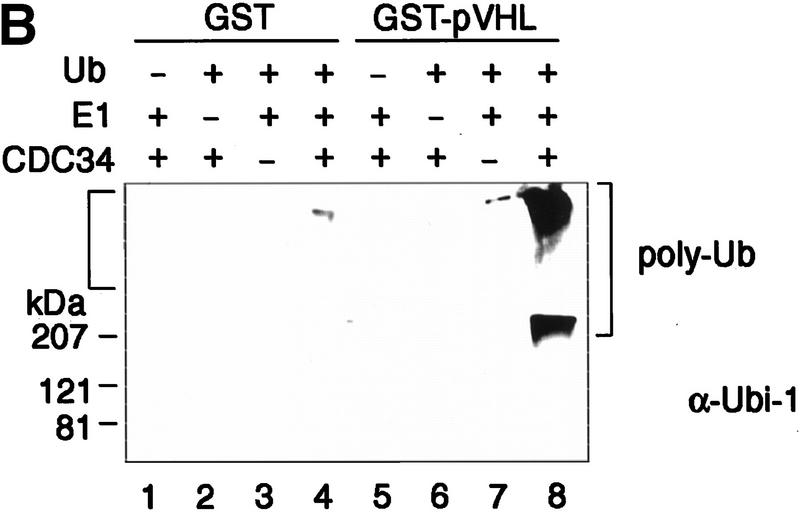
Recombinant pVHL purifies ubiquitination-promoting activity from human cell extracts. (A) GST (lanes 1_–_4,10), GST–pVHL (lanes 5_–_8,11), or GST–p45Skp2 (lane 9) was bound to glutathione–Sepharose beads and either directly exposed to the ubiquitination reaction as described in Materials and Methods in the absence (lanes 1,2,5,6) or presence (lanes 3,4,7,8,9) of ubiquitin (Ub) or were first incubated overnight in HeLa cell lysates as indicated by the plus and minus signs at top or in 786-O cell lysates (lanes 10,11). Reaction mixtures were analyzed on 10% SDS-gels and processed for Western blotting with anti-ubiquitin antibody (Ubi-1) (top) or reprobing with anti-GST antibody (bottom). (B) GST (lanes 1_–_4) or GST–pVHL (lanes 5_–_8) was bound to glutathione–Sepharose beads, incubated in HeLa lysates and exposed to the ubiquitination assay conditions as described in A in the absence or presence of either Ub, E1, or Cdc34 as indicated at top. Samples were analyzed on 10% SDS-gels and processed for Western blotting with anti-ubiquitin antibody (Ubi-1). Square bracket at left indicates the gel stack. Square bracket at right indicates the ladder of multi-ubiquitinated proteins.
To test further whether multiubiquitination of proteins that occurs during the in vitro reaction was dependent on the presence of an E1 enzyme and the human E2 enzyme Cdc34 in the reaction mixture, both enzymes were individually excluded from the reaction mixture containing GST–pVHL preincubated in HeLa lysate. As shown in Figure 1B, GST–pVHL-associated ubiquitination activity is strictly dependent on the presence of ubiquitin (lane 5), E1 (lane 6), and E2 Cdc34 (lane 7). These results suggest that human cell lysate-activated GST–pVHL displays ubiquitination-promoting activity in conjunction with the universally required components E1, E2, and ubiquitin. Finally, immunoblotting of these ubiquitination reaction mixtures with affinity-purified anti-Cdc34 antibodies that were raised against recombinant full-length human GST–Cdc34 fusion protein did not reveal any high molecular weight species that would be consistent with ubiquitinated Cdc34. Thus, it also appears that Cdc34 is not the major protein species ubiquitinated under these experimental conditions (data not shown).
Ectopically produced pVHL associates with ubiquitination activity in renal cell carcinoma cells
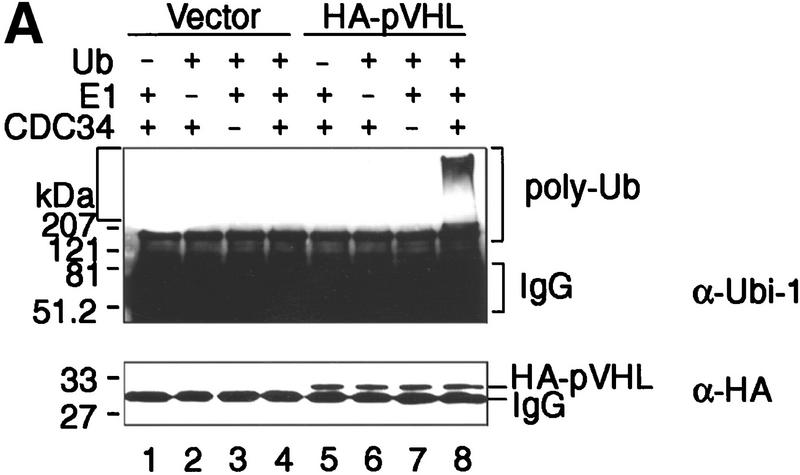
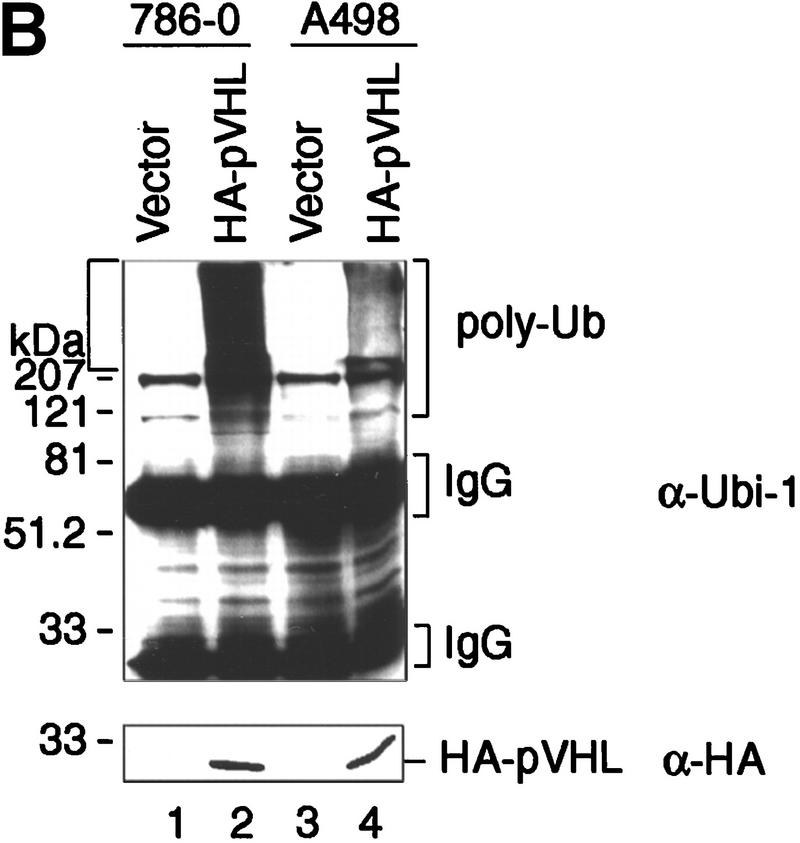
786-O and A498 RCC cells lack wild-type VHL protein. To assess whether immunopurified pVHL displays ubiquitination activity, stable cell lines ectopically producing HA–pVHL(wt) were generated. As a control, 786-O and A498 cells were stably transfected with a backbone expression plasmid (Vector). These cells were lysed and pVHL immunoprecipitated with a monoclonal anti-HA antibody. When anti-HA immunoprecipitates were tested for ubiquitination activity, only the HA–pVHL-producing 786-O cells displayed strong ubiquitination activity (Fig. 2A, lane 8). Ubiquitination-promoting activity associated with anti-HA immunoprecipitates was not detected when HA antibody was first preincubated with HA peptide (data not shown). Likewise, no such activity was seen when ubiquitin, E1, or E2 Cdc34 was omitted from the reaction mixture (Fig. 2A, lanes 5, 6, and 7, respectively). Identical results were obtained with different 786-O cell clones (data not shown). Western blotting of anti-HA immunoprecipitates showed that comparable quantities of HA–pVHL were present in this experiment (Fig. 2A, bottom, lanes 5–8). As shown in Figure 2B, anti-HA immunoprecipitates derived from HA–pVHL-producing A498 cells also possessed ubiquitination activity (cf. lanes 2 and 4). These results show that a potent ubiquitination activity also immunopurifies with ectopically produced pVHL from different RCC cells.
Figure 2.
Ectopically produced pVHL displays associated ubiquitination-promoting activity. (A) VHL−/− 786-O cell lines stably transfected with a plasmid encoding HA-tagged pVHL (lanes 5_–_8) or with the backbone expression plasmid (lanes 1_–_4) were lysed and immunoprecipitated with anti-HA antibody. Immunoprecipitates were processed for ubiquitination assay reactions as described in Material and Methods, omitting Ub (lanes 1,5), E1 (lanes 2,6), or Cdc34 (lanes 3,7) from reaction mixtures. Samples were analyzed on 10% SDS gels and processed for Western blotting with anti-ubiquitin antibody (Ubi-1) (top) or anti-HA antibody (bottom). (B) VHL−/− 786-O and A498 cell lines stably transfected with a plasmid encoding HA-tagged pVHL (lanes 2,4) or with the backbone expression plasmid (lanes 1,3) were lysed, immunoprecipitated with anti-HA antibody and immunoprecipitates were processed for ubiquitination assay reactions and Western blot analysis as described above with anti-ubiquitin antibody (Ubi-1) (top) or anti-HA antibody (bottom).
Immunoprecipitates of endogenous pVHL display ubiquitination activity
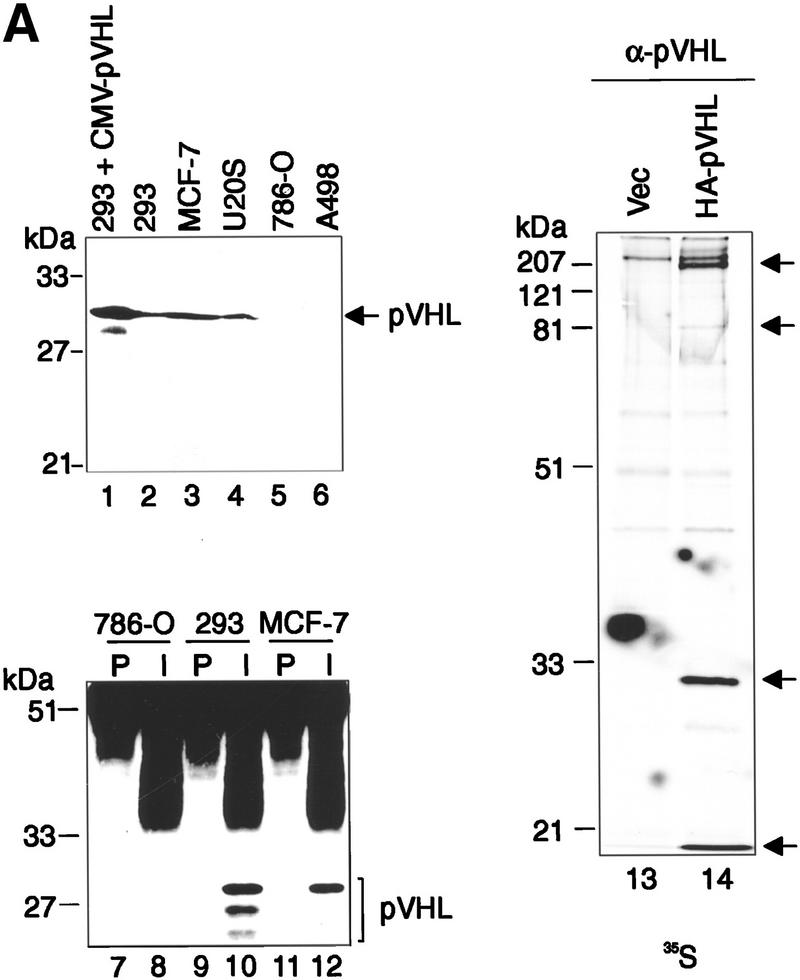
In an effort to extend the above finding, polyclonal rabbit antisera were raised against GST–pVHL, affinity purified, and tested for their ability to immunoprecipitate endogenous pVHL-associated ubiquitination activity. The specificity of the anti-pVHL antibody is illustrated in Figure 3A. Affinity-purified anti-pVHL antibodies recognized a protein of the expected molecular weight on immunoblotting of whole cell lysates from 293 (lane 2), MCF-7 (lane 3), and U2-OS (lane 4) cells, but failed to detect a gene product of that size in either 786-O or A498 RCC cells lacking wild-type pVHL (lanes 5 and 6, respectively). Importantly, the abundance of the endogenous detected protein was significantly increased in whole cell lysates prepared from 293 cells transfected previously with an expression plasmid encoding untagged pVHL (lane 1). Likewise, endogenous pVHL was detected in anti-pVHL immunoprecipitates from 293 (lane 10) and MCF-7 (lane 12) cells but not in 786-O cells (lane 8). Moreover, affinity-purified anti-pVHL antibodies are capable of immunoprecipitating HA–pVHL and associated proteins from [35S]methionine cell lysates derived from HA–pVHL-producing 786-O cells but not from corresponding control cell lysates (Fig. 3A, lanes 14 and 13, respectively). Hence, this antibody specifically detects bona fide pVHL.
Figure 3.
Immunoprecipitates of endogenous pVHL possess ubiquitination-promoting activity. (A) Specificity of anti-pVHL antibody. Whole cell extracts were prepared by the addition of SDS-sample buffer to 293 cells transfected with expression plasmids encoding untagged pVHL (lane 1) as well as to untransfected 293 (lane 2), MCF-7 (lane 3), U2-OS (lane 4), 786-O (lane 5), and A498 (lane 6) cells. Protein mixtures were separated by SDS-PAGE and processed for Western blotting with affinity-purified anti-pVHL antibody. The exposure time of lane 1 is ten times less than that of lanes 2_–_6. Note that anti-pVHL antibodies do not recognize pVHL, as expected, in VHL−/− 786-O or A498 cells. Whole cell extracts prepared from 786-O (lanes 7,8), 293 (lanes 9,10), or MCF-7 (lanes 11,12) cells were subjected to immunoprecipitation with either 15 μg of affinity-purified anti-pVHL antibody (lanes 8,10,12) or affinitypurified rabbit IgG (lanes 7,9,11) and processed for Western blotting with affinity-purified anti-pVHL antibody. VHL−/− 786-O cells ectopically producing HA-tagged pVHL wild-type (lane 14) or containing backbone expression plasmid (lane 13) were metabolically labeled with [35S]methionine, lysed, and immunoprecipitated with 10 μg of affinity-purified anti-pVHL antibodies. Bound proteins were resolved by SDS-PAGE and detected by fluorography. Arrows at right mark the positions of coimmunoprecipitating fibronection, Cul-2, HA–pVHL, and Elongin B (from top to bottom). Note that the pattern of coimmunoprecipitating proteins are similar to work published previously (for references see Results). (B) Lysates were prepared from 293 (lanes 1_–_5), U2-OS (lanes 6,7), 786-O (lanes 8,9), and A498 (lanes 10,11) cells and subjected to immunoprecipitations with 5 μg of either affinity-purified rabbit IgG (lanes 1,6,8,10) or affinity-purified anti-pVHL antibody (lanes 2_–_5,7,9,11) and processed as described in the legend to Fig. 2A. Samples were analyzed on 10% SDS gels and processed for Western blotting with anti-ubiquitin antibody (Ubi-1) or anti-pVHL antibody.
Ubiquitination assays performed on anti-pVHL immunoprecipitates recovered from 293 cell lysates revealed potent ubiquitination activity (Fig. 3B, lane 5, top) that was dependent on the presence of ubiquitin (lane 2), E1 (lane 3), or E2 Cdc34 (lane 4) in the reaction mixture. No ubiquitination activity was detected in control immunoprecipitates (lane 1). Likewise, anti-pVHL immunoprecipitates recovered ubiquitination activity from cell lysates of wild-type pVHL-expressing U2-OS cells (Fig. 3B, lane 7) but not from cell lysates of two pVHL-negative cell lines (lanes 9,11). In addition, immunoblotting of ubiquitination reactions with anti-pVHL antibodies did not reveal the existence of ubiquitinated pVHL. Similar results were obtained when anti-HA or anti-GST antibodies were used to detect possible HA- or GST–pVHL-associated ubiquitin conjugates (data not shown). Collectively, these results suggest that endogenous pVHL is also part of a complex that possesses ubiquitination activity in conjunction with Cdc34. This implies that pVHL is a component of, or is associated with, a complex that possesses E3 ubiquitin–protein ligase activity toward other as-yet-unidentified substrates.
BC-box motif peptides block the ability of pVHL to recruit ubiquitination activity
A synthetic peptide comprising the BC-box motif of pVHL can efficiently block the interaction of GST–pVHL with Elongin BC and Cul-2, whereas an identical peptide carrying a single point mutation is inactive in this regard (Kibel et al. 1995). Hence, glutathione–Sepharose beads preloaded with GST–pVHL were incubated with HeLa lysates containing either wild-type or mutant BC-box peptide and tested for associated ubiquitination activity. As shown in Figure 4A (top), GST–pVHL recruited ubiquitination activity (lane 2). The ability of pVHL to recruit ubiquitination activity from cell lysates was efficiently blocked by wild-type BC-box peptide (lane 3) but not mutant peptide (lane 4). In keeping with the known ability of a BC-box peptide to inhibit pVHL–Elongin BC association, and consequently block the recruitment of Cul-2 into the complex, anti-Cul-2 and anti-Elongin C immunoblotting revealed that endogenous Cul-2 as well as Elongin C copurified efficiently with GST–pVHL in the presence of mutant BC peptide (middle, lane 4) but not wild-type BC peptide (middle, lane 3). The specificity of this approach is further demonstrated by the inability of a synthetic peptide encompassing the 16 most carboxy-terminal amino acid residues of pVHL to block recruitment of ubiquitination activity (top, lane 5) as well as Cul-2 and elongin C copurification (middle, lane 5). These results establish a correlation between pVHL’s ability to display ubiquitination activity and to bind Elongin C–Cul-2.
Figure 4.
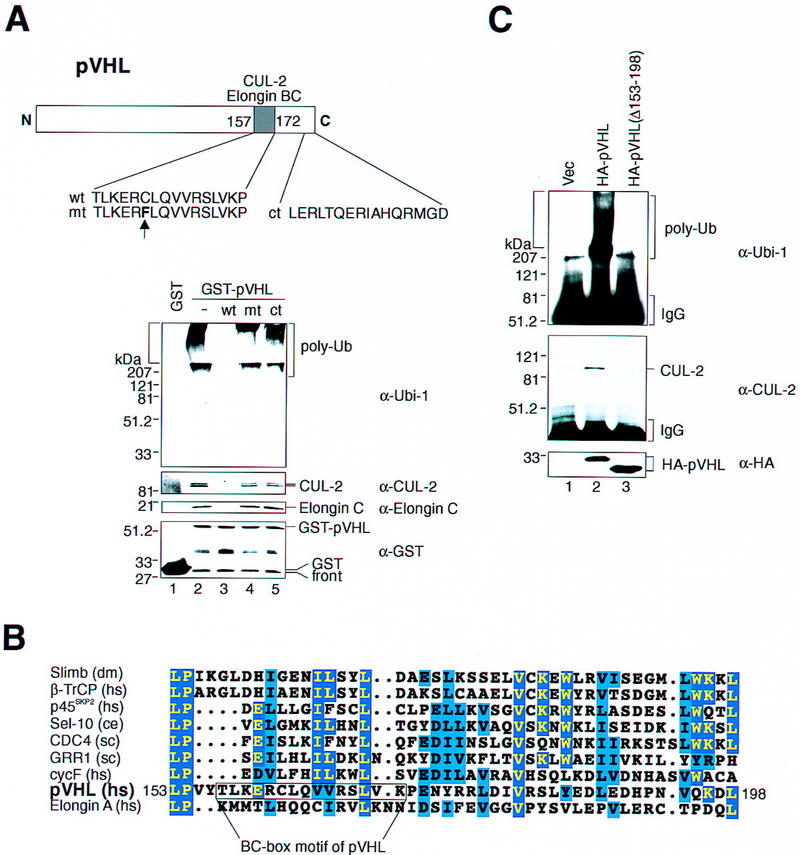
Effects of BC-box peptides on pVHL-associated ubiquitination activity. (A) Structure of pVHL(wt). The schematic depicts the carboxy-terminal BC-box motif that serves as an interaction site for Elongin BC/Cul-2 complexes. The amino acid sequence of peptides encoding the wild-type (wt) and mutant (mt) BC-box domain and the most carboxy-terminal 16 amino acids (ct) are displayed below. The vertical arrow highlights the single point mutation present in the mt peptide. GST (lane 1) or GST–pVHL (lanes 2_–_5) was bound to glutathione–Sepharose beads, incubated in HeLa lysates in the absence (lanes 1,2) or presence of either wt (lane 3), mt (lane 4), or ct (lane 5) peptides and exposed to ubiquitination assay conditions as described in the legend to Fig. 1A. Reaction mixtures were analyzed on 10% SDS gels and processed for Western blotting with either anti-ubiquitin (Ubi-1) (top panel), anti-Cul-2 (second panel), anti-Elongin C (third panel), or anti-GST (bottom panel) antibodies. Note, that HeLa Cul-2 and Elongin C failed to associate with GST–pVHL in the presence of wt but not mt peptide. (B) Sequence alignment of the main region of homology between pVHL and the F-box motif of selected F-box proteins of different origins. Identical residues are indicated by yellow letters on blue boxes and functionally similar residues are in black letters on blue boxes. The BC-box motif of pVHL comprising 15 amino acid residues is shown. (C) VHL−/− 786-O cells producing the indicated HA-tagged pVHL species (lanes 2,3) or containing backbone expression plasmid (lane 1) were lysed and immunoprecipitated with anti-HA antibody and immunoprecipitates were processed for ubiquitination assay reactions and Western blot analysis with anti-ubiquitin antibody (Ubi-1) (top) or anti-Cul-2 antibody (middle). Aliquots of whole cell extracts of the aforementioned cell lines were processed for Western blotting with anti-HA antibody (bottom).
The F-box motif is a critical structural element of a class of cellular proteins that function as substrate-specific receptors of SCF E3 ligase complexes (Bai et al. 1996). Given the above-noted results and the observation that Elongin BC complexes bridge Cul-2 to pVHL in a manner analogous to the SCF E3 ubiquitin-protein ligase configuration, we asked whether sequences surrounding the BC-box motif bear any structural similarity to an F-box motif. Previous computer-assisted similarity searches of databases for the F-box motif with generalized profiles revealed a large number of proteins containing the F-box motif, including Elongin A that is known to associate with Elongin BC (Bai et al. 1996). These database searches on the other hand, did not uncover an F-box motif within pVHL. However, a careful inspection of the primary sequence of pVHL by eye revealed a segment within pVHL comprising residues 153–198 (which includes the BC-box identified previously) that displays similarity to the F-box motif present in a number of known F-box proteins from different organisms (Fig. 4B). Hence, pVHL may be a distant representative of the F-box protein family.
Consistent with the findings described above, anti-HA immunoprecipitates from 786-O cells producing a mutant of HA–pVHL, in which amino acid residues 153–198 (see Fig. 4B) were deleted, possessed no detectable ubiquitination activity (Fig. 4C, top, lane 3) and failed to coimmunoprecipitate Cul-2 (Fig. 4C, middle, lane 3). Anti-HA immunoprecipitates derived from 786-O cells producing wild-type HA–pVHL were active in this regard (Fig. 4C, top and middle, lane 2). Anti-HA immunoblotting confirmed that the cell lines under investigation express similar amounts of the relevant HA–pVHL species (Fig. 4C, bottom, lanes 2,3). These results suggest that the specific segment of pVHL that comprises the BC box and that shares similarity to the F-box motif is critically required for the manifestation of pVHL-associated ubiquitination activity.
Coimmunoprecipitation of ubiquitination activity is abrogated by certain naturally occurring loss-of-function mutations of pVHL
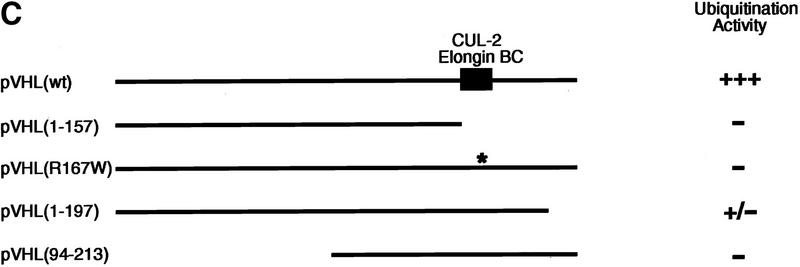
Given that pVHL is a component of a complex that exhibits ubiquitination activity, we asked whether selected naturally occurring mutants have lost this capacity. To this end, mammalian expression plasmids encoding HA-tagged versions of the following pVHL mutants were generated: a naturally occurring truncation mutant of pVHL lacking the carboxy-terminal 56 amino acids, which fails to bind Elongin BC–Cul-2 and Fibronectin [HA–pVHL (1–157)]; the most frequently occurring point mutation, which dramatically reduces pVHL–Elongin BC–Cul-2 complex formation and pVHL/fibronection binding [HA–pVHL(R167W)]; a carboxy-terminal deletion mutant, which retains Elongin BC–Cul-2 binding but lacks the ability to interact with Fibronectin [HA–pVHL(1–197)]; and an amino-terminal deletion mutant, which also interacts with Elongin BC–Cul-2 but fails to bind Fibronectin [HA–pVHL(94–213)] (see Fig. 5C). 786-O subclones were generated that ectopically produce these various mutants. The production of the various pVHL species and their association with cellular proteins was assessed by metabolically labeling these cell lines with [35S]methionine, followed by immunoprecipitation with an anti-HA antibody. As shown in Figure 5A, anti-HA immunoprecipitates contained HA–pVHL(wt) and additional proteins with molecular masses of ∼220, 90, and 16 kD (lane 2), all of which were specifically absent in anti-HA immunoprecipitates derived from cells containing the backbone plasmid alone (lane 1). It has been shown previously that the 220-, 90-, and 16-kD proteins correspond to Fibronectin (Ohh et al. 1998), Cul-2 (Pause et al. 1997; Lonergan et al. 1998), and Elongin B (Duan et al. 1995b; Kibel et al. 1995), respectively. On this particular SDS gel, Elongin C migrated with the front. The pattern of Glut-1 inhibition by these pVHL mutants was similar to work published previously (data not shown), as was the pattern of the proteins that coimmunoprecipitated with either pVHL(wt) or the various mutants analyzed here (lanes 3–6) (Duan et al. 1995b; Kibel et al. 1995; Pause et al. 1997; Lonergan et al. 1998; Ohh et al. 1998), suggesting that the pVHL species under investigation behave as expected with respect to their ability to interact with known cellular proteins.
Figure 5.
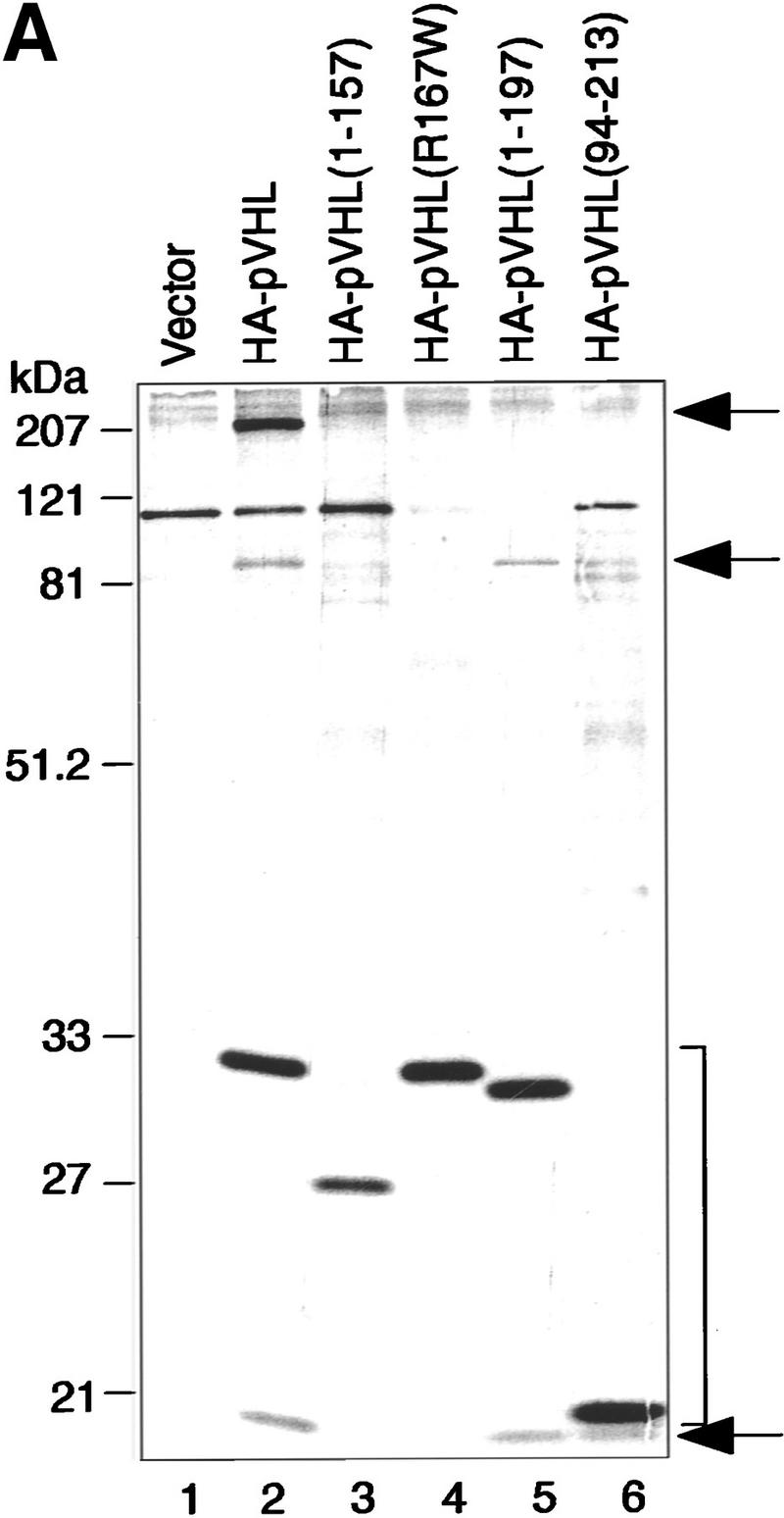
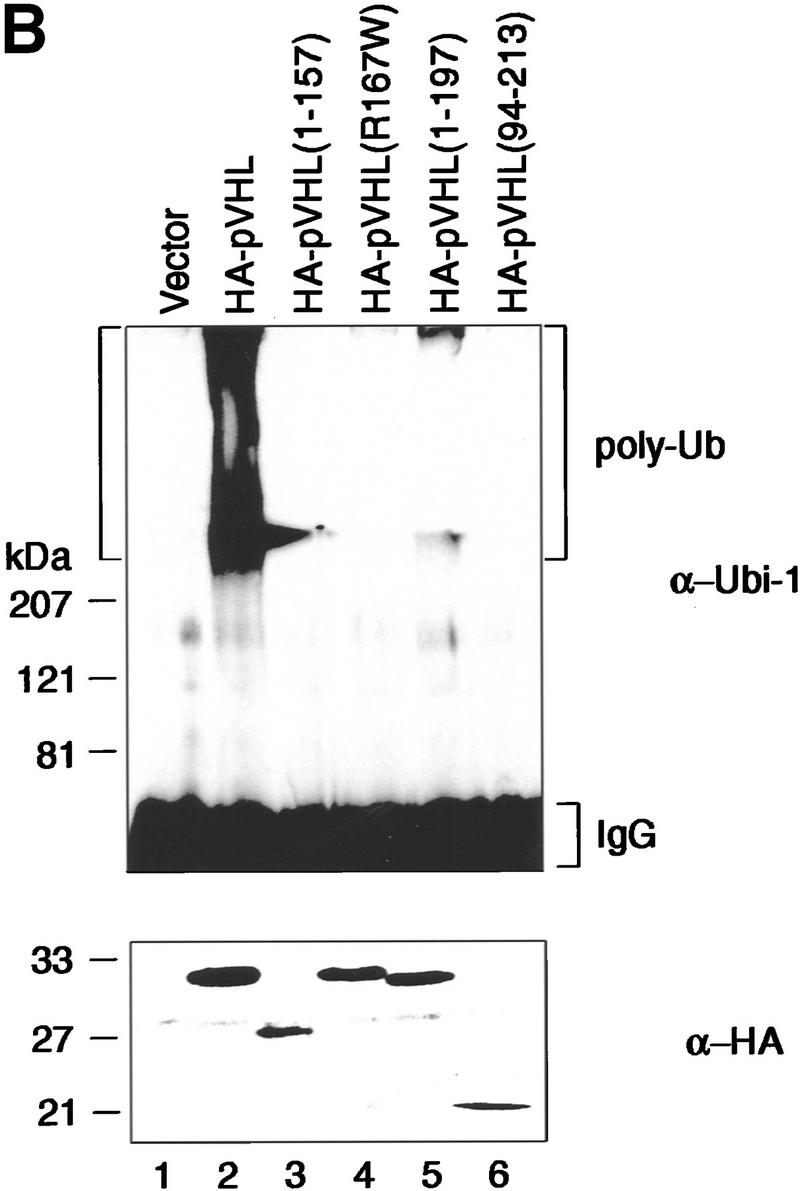
Tumor-derived mutations of pVHL fail to display ubiquitination-promoting activity. (A) VHL−/− 786-O cells ectopically producing the indicated HA-tagged pVHL species (lanes 2_–_6) or containing backbone expression plasmid (lane 1) were metabolically labeled with [35S]methionine, lysed, and immunoprecipitated with anti-HA antibody. Bound proteins were resolved by SDS-PAGE and detected by fluorography. Arrows at right mark the positions of coimmunoprecipitating Fibronection, Cul-2, and Elongin B (from top to bottom). Note that the pattern of coimmunoprecipitating proteins are similar to previously published work (for references see Results). The bracket indicates the positions of the various pVHL species. Elongin C is not visible on this SDS gel because it migrated with the front. (B) VHL−/− 786-O cell lines analyzed in A were lysed, immunoprecipitated with anti-HA antibody, and processed for ubiquitination assay reactions as described in the legend to Fig. 3A. Samples were analyzed on 10% SDS gels and processed for Western blotting with anti-ubiquitin antibody (Ubi-1) (top) or anti-HA antibody (bottom). (C) Schematic representation of the various pVHL mutants tested and their corresponding ubiquitination activity (+ and −). (█) The BC box in pVHL; (*) the location of the point mutation.
When anti-HA immunoprecipitates from these various cell lines were tested for ubiquitination activity, only the HA–pVHL-producing 786-O cells displayed strong ubiquitination activity (Fig. 5B, lane 2). The two tumor-derived species of pVHL, namely HA–pVHL(1–157) (lane 3) and HA–pVHL (R167W) (lane 4), failed to display ubiquitination-promoting activity. HA–pVHL(1–197), although able to interact with Elongin BC/Cul-2, displayed significantly reduced ubiquitination activity (lane 5), suggesting that HA–pVHL(1–197) is largely (albeit not wholly) defective in ubiquitination activity. Likewise, HA–pVHL(94–213) failed to exhibit ubiquitination activity (lane 6), although it contains an intact BC box and interacts with Elongin BC and Cul-2. Independent clones of each pVHL species were tested and behaved identically (data not shown). These results suggest that certain naturally occurring mutations affect pVHL’s ability to associate with ubiquitination activity. Moreover, the manifestation of pVHL-associated ubiquitination activity not only requires pVHL–Elongin BC–Cul-2 complex formation, but is also influenced by the integrity of distinct elements in pVHL’s primary structure, particularly those in the carboxyl and amino termini.
Discussion
The data presented here provide biochemical evidence that pVHL tumor suppressor participates in the process of ubiquitination. Specifically, we found that recombinant pVHL associates with ubiquitination-promoting activity in human cell extracts. The ability of pVHL to recruit ubiquitination activity was specifically blocked with a BC-box peptide that inhibited binding of pVHL to Elongin C and Cul-2, whereas a point-mutant derivative, corresponding to a naturally occurring VHL missense mutation, had no effect. Moreover, antibodies specific for pVHL immunopurified ubiquitination activity from VHL+/+ but not from VHL−/− human cells. Finally, examination of various mutants of pVHL stably expressed in RCC cells revealed that certain naturally occurring mutants of pVHL demonstrate loss of ubiquitination activity in conjunction with the universally required components E1, E2, and ubiquitin. This argues that the capacity of pVHL to recruit ubiquitination-promoting activity may be important for its role as a tumor suppressor. Taken together, these biochemical data imply that the pVHL tumor suppressor may function, at least in part, as a component of an E3 ubiquitin–protein ligase complex. In further support of this, we showed Elongin C–Cul-2 binding to pVHL to be necessary, although not sufficient, to recruit ubiquitination activity. Examination of pVHL sequences surrounding and including the BC-box motif (i.e., the domain involved in Elongin BC–Cul-2 binding) revealed certain hallmarks of an F-box motif. We therefore hypothesize that pVHL may be a distant member of the F-box protein family and that pVHL–Elongin C–Elongin B–Cul-2 complexes may exemplify a subclass of the SCF superfamily of E3 ligases, in which pVHL acts as the substrate-specific receptor.
The F-box motif was identified originally as a ∼40-amino-acid-long degenerate sequence common to a collection of otherwise structurally unrelated proteins that all shared the property to interact with Skp1 (Bai et al. 1996). As demonstrated previously, pVHL interacts with the Skp1-related protein Elongin C (Duan et al. 1995b; Kibel et al. 1995; Takagi et al. 1997), and so far several of our attempts to demonstrate the existence of a pVHL/Skp1 complex in vivo have failed (data not shown). One possibility is that the presence of the BC-box motif embedded within an F-box homology region favors the binding of Elongin C over Skp1 to the F-box motif. In this regard, it is interesting to note that Elongin A, which contains an F-box motif (Bai et al. 1996), also carries a BC-box motif (Kamura et al. 1998) and binds Elongin BC (Aso et al. 1995). The BC-box motif is present in a collection of other proteins that do not contain an obvious F-box motif, among them SOCS-1, in which the BC-box is embedded in the SOCS-box (Kamura et al. 1998). While this paper was under review, Pavletich and colleagues reported on the resolution of the crystal structure of the pVHL–Elongin C–Elongin B complex (Stebbins et al. 1999) and proposed that the pVHL domain comprising the BC box, referred to as the pVHL α domain, the SOCS box, and the F box are structurally related. Because pVHL displays associated ubiquitination activity that is dependent on its ability to interact with Elongin C and Cul-2, one hypothesis that remains to be tested is whether the presence of a BC-box motif imparts certain functional properties on proteins that relate to the process of ubiquitination.
We found that pVHL associates with a potent ubiquitination activity in human cells. Bacterially produced pVHL lacked this activity. pVHL-associated ubiquitination activity became only apparent following incubation of pVHL in human cell extracts. There are at least two possibilities that can explain this observation: Either pVHL associates with certain E3 ubiquitin–protein ligases as part of its tumor-suppressing function or pVHL itself is a core component of an E3 activity and the HeLa cell lysate provides the necessary additional cofactors/substrates for pVHL-associated ubiquitination activity to manifest. In favor of the latter possibility is the observation that one set of proteins that appear to play an important contributory role in the above-noted ubiquitination assay are the BC-box-binding proteins Elongin C and Cul-2, relatives of the SCF family of E3 ligases. This is evidenced by the fact that peptides blocking the association of these proteins with pVHL also blocked the recruitment of pVHL-associated ubiquitination activity. In addition, a mutant of pVHL that lacked the Elongin BC-binding domain and thus, failed to associate with Cul-2, likewise, was defective in recruiting ubiquitination-promoting activity. Hence, one might argue that the pVHL–Elongin C–Elongin B–Cul-2 assemblage is a close relative of, but is distinct from, the archetype SCF E3 ligase complexes. In keeping with this notion, the pVHL complex and SCF ligases share yet another common component, the Rbx1/Roc1 ring-finger protein (Kamura et al. 1999; Ohta et al. 1999; Skowyra et al. 1999; Tan et al. 1999). At least in the case of the SCF family, Rbx1/Roc1 bound Cdc53/Cul-1 and promoted binding of the E2 enzyme Cdc34 to the SCF–Rbx1/Roc1 holoenzyme (Kamura et al. 1999; Ohta et al. 1999; Skowyra et al. 1999; Tan et al. 1999). In this setting, Rbx1/Roc1 stimulated substrate-dependent ubiquitination by the Cdc34–SCF complex. In the absence of a relevant substrate, however, recombinant Rbx1/Roc1 stimulated autoubiquitination of Cdc34 in vitro when SCF components were present. Recent experiments suggest, however, that neither Cdc34 nor pVHL are the major ubiquitinated proteins in our assay (data not shown). Consistent with the view that other proteins are being ubiquitinated under our conditions is the fact that pVHL(94–213), a mutant that lacks the amino-terminal 94-amino acid residues, measurably interacts with Elongin B, Elongin C, and Cul-2 (Lonergan et al. 1998) but lacks associated ubiquitination activity. In this regard, analysis of the crystal structure of pVHL revealed that the ∼100-residue amino-terminal domain is rich in β-sheets and represents a macromolecular-binding site within pVHL frequently mutated in tumors (Stebbins et al. 1999). Hence, it is possible that one or more proteins/substrates ubiquitinated in our assay are recruited, at least in part, via pVHL’s amino-terminal residues.
Previous results linked the formation of pVHL–Elongin BC–Cul-2 complexes to a biological activity of pVHL, namely, the ability of pVHL to regulate hypoxia-inducible mRNAs (Maher and Kaelin 1997; Kaelin and Maher 1998). Naturally occurring mutants of pVHL, such as pVHL(1–157) or pVHL(R167W), lack this biological activity. Studies here show that these mutants, likewise, fail to display ubiquitination-promoting activity. It should be noted, however, that there is a large cohort of pVHL mutants that map outside of the Elongin BC-binding domain of pVHL and give rise to proteins capable of interacting with the Elongin BC–Cul-2 complex. In this regard, the amino-terminal truncation mutant of pVHL, pVHL(94–213), measurably interacts with Elongin BC and Cul-2 complexes (Lonergan et al. 1998) but lacks associated ubiquitination activity. Similarly, pVHL(1–197) lacks the most carboxy-terminal 16-amino-acid residues, is capable of suppressing the production of hypoxia-inducible mRNA production under normoxic conditions and interacting with Elongin BC–Cul-2 complexes (Lonergan et al. 1998), but displays significantly reduced ubiquitination-promoting activity (this report). Interestingly, this pVHL mutant fails to bind to fibronectin. Taken at face value, it appears that pVHL’s capacity to inhibit the production of hypoxia-inducible mRNAs under normoxic conditions cannot be directly correlated with its ability to display associated ubiquitination-promoting activity. However, the sensitivities of the biochemical assay described here and the functional assays used in the past may differ siginficantly. On the other hand, one should take into account that the approach taken for the analysis of pVHL(1–197) with respect to inhibition of Glut-1 expression used overexpression strategies. Finally, the amino-terminal truncation mutant of pVHL, pVHL(94–213), measurably interacts with Elongin BC–Cul-2 complexes but is defective in both suppression of Glut-1 expression and fibronectin binding and as shown here, lacks associated ubiquitination activity. Given these results, one might argue that not only sequences including the BC-box motif, but also those extending to the amino and carboxyl termini of pVHL, are required for pVHL to display associated ubiquitination-promoting activity. Whether these sequences are required for structural integrity of the VHL protein, or for binding of substrates, remains to be shown. More significantly, this biochemical activity may be of critical importance for its tumor-suppressing function and any clinical phenotype associated with a given pVHL mutant may then be correlated, at least in part, to the degree by which this biochemical activity is quantitatively or qualitatively altered.
What are the potential substrates for pVHL-linked ubiquitination in human cells? The ability of pVHL to bind to Elongin BC–Cul-2 has been linked to its ability to suppress the production of hypoxia-inducible mRNA under normoxic conditions. Hence, among the substrates there might be ones that contribute to the regulation of mRNA stability. The RNA-binding protein HuR has been reported to stabilize VEGF mRNA markedly at least in vitro (Levy et al. 1998). In addition, three proteins with molecular masses of 32, 28, and 17 kD have been identified as part of an hypoxia-inducible RNA–protein complex that is constitutively elevated in tumor cell lines lacking wild-type pVHL (Levy et al. 1996). Finally, Kaelin and collaborators linked pVHL to the control of fibronection matrix assembly (Ohh et al. 1998). Their experiments showed that pVHL interacts with Fibronectin, possibly in the endoplasmatic reticulum (ER), and that every pVHL mutant tested in their study (also including those studied here) were shown to fail to interact with fibronection. Whether pVHL interacts directly with fibronectin or whether other proteins mediate this interaction remains to be determined. A hypothesis put forward at the time was that pVHL may be involved in the processing of misfolded ER-resident proteins. Of note, several lines of evidence would suggest ER-mediated degradation involves the ubiquitin/proteasome pathway (Brodsky and McCracken 1997) and recent evidence points to a role of at least one SCF E3 ligase in this process (Margottin et al. 1998). A role for pVHL in the elimination of misprocessed proteins, such as those arising in the cell due to the unavailability of glucose or in response to certain stresses, has also been proposed recently by Gorospe et al. (1999). In light of the results reported here, it is tempting to envision a model in which one function of pVHL is to direct the ubiquitination of ER-resident substrates. The ubiquitination assay presented here may therefore prove useful in the future as a means to identify specific target proteins of this potential SCF-like E3 ubiquitin–protein ligase complex and to determine the degree to which pVHL tumor suppression relies on this activity.
Materials and methods
Plasmid constructions
The plasmids pRcCMV–HA–pVHL(wt), pRcCMV–HA–pVHL (R167W), pGEX2TK–pVHL(wt) (Iliopoulos et al. 1995; Kibel et al. 1995), and pGST4T1–p45Skp2 (Lisztwan et al. 1998) have been described. To generate pRcCMV–HA–pVHL(1–157), pRcCMV–HA–pVHL(wt) was digested with _Bst_I107–_Xba_I, and the resulting vector fragment combined with the oligonucleotide dimer: 5′-TACTTGAATTCTGCAGATATCCATCACACTGG-CGGCCGCTCGAGCATGCAT-3′ and 5′-CTAGATGCATGC-TCGAGCGGCCGCCAGTGTGATGGATATCTGCAGAAT-TCAAGTA-3′. To generate pcDNA3–HA–pVHL(wt), pRcCMV–HA–pVHL(wt) was digested with _Hin_dIII–_Eco_RI and the resulting fragment subcloned into pcDNA3–HA–E2F1 vector fragment, derived from a _Hin_dIII–_Eco_RI digest. To generate pcDNA3–pVHL(wt) (untagged pVHL), pcDNA3–HA–pVHL(wt) was digested with _Bam_HI–_Eco_RI and the resulting fragment subcloned into pcDNA3 vector fragment, derived from a _Bam_HI–_Eco_RI digest. To generate pcDNA3–HA–pVHL(1–197) and pcDNA3–HA–pVHL(94–213), a one-step PCR method was used. The PCR products were digested with _Bam_HI–_Xba_I, and subcloned into a vector fragment isolated from a _Bam_HI–_Xba_I digest of pcDNA3–HA–pVHL. The following primers were used: pcDNA3–HA–pVHL(1–197), 5′-TCCCTCGGATCCGCCACCATGCCCCGGAGGGCGGAG-3′ and 5′-CGCCAGTCTAGAGGATATCTGCAGAATTCAGTCTTTCTGCACATTTGG-3′; pcDNA3–HA–pVHL(94–213), 5′-TCCCTCGGATCCGCCAC-CATGGAGCCGCAGCCCTACCCAACGCTGCCGCCTGGC-3′ and 5′-CGCCAGTCTAGAGGATATCTGCAGAATTCAATCTCC-3′. A two-step PCR method was used to generate pcDNA3–HA–pVHL(Δ153–198). The following primers were used: 5′-CAATATCACAGAGCGGCTGACACA-3′ and 5′-TGTCAGCCGCTCTGTGATATTGGC-3′ to generate a deletion of amino acid residues 153–198 of pVHL. pcDNA3–HA–pVHL(wt) served as a template. All PCR-derived regions in the newly generated mutants were confirmed by sequence analysis.
Antibodies
Polyclonal rabbit serum for full-length human pVHL was raised against a GST–pVHL fusion protein purified from bacteria essentially as described previously (Lisztwan et al. 1998). Affinity purification of polyclonal rabbit serum to pVHL was achieved by incubation with first a GST affinity column, followed by a GST–pVHL column, prepared previously by covalently cross-linking the respective proteins to glutathione–Sepharose with demethylpimelimidate (Harlow and Lane 1988). Anti-Cul-2 peptide antibodies were raised against a synthetic peptide VLIDKQYIERSQASADEYSY, corresponding to the carboxy-terminal region of human Cul-2. The peptide was coupled to keyhole limpet hemocyanin by glutaraldehyde coupling and injected into rabbits. Anti-peptide antibodies were affinity purified as described (Lisztwan et al. 1998). Polyclonal rabbit serum for full-length Elongin C was raised against Elongin C protein purified from bacteria essentially as described previously (Krek et al. 1992). The mouse mAb 12CA5 and HA11 recognizing the HA epitope were purchased from Boehringer Mannheim and BAbCo, respectively. Anti-Ubi-1 antibody was purchased either from Zymed or from BAbCo. Anti-GST antibodies were obtained from Sigma.
Cell culture and transfection
Human 293 embryonic kidney cells, U2-OS, MCF-7, HeLa, 786-O, and A498 cells were maintained in DMEM supplemented with 10% fetal calf serum (FCS) (GIBCO-BRL). 786-O cells stably transfected with pRcCMV vector, pRcCMV–HA–pVHL(wt), pRcCMV–HA–pVHL(1–157), pRcCMV–HA–pVHL(R167W), pcDNA3–HA–pVHL(1–197), pcDNA3–HA–pVHL(94–213), and pcDNA3–HA–pVHL(Δ153–198) were generated exactly as described (Iliopoulos et al. 1995) and maintained in DMEM supplemented with 10% FCS and 0.5 mg/ml G418. A498 cells stably expressing pRcCMV vector or pRcCMV–HA–pVHL(wt) were generated in an identical fashion.
Metabolic labeling, Western blotting, and immunoprecipitation
Radioisotopic labeling was performed by methionine starvation for 30 min followed by growth in 3 ml of methionine-free DMEM supplemented with [35S]methionine [250 μCi] and 10% dialyzed FCS for 4 hr at 37°C in a humidified 10% CO2 atmosphere. Immunoblotting was performed as described (Lisztwan et al. 1998). Blots were processed by enhanced chemiluminescence (Amersham) according to the manufacturer’s instructions. Immunoprecipitations were performed essentially as described previously (Lisztwan et al. 1998).
Ubiquitination assay
The E1-activating enzyme used in ubiquitination assays was either from Affinity, Inc. (Exeter, UK) or purified from Sf9 insect cells infected with a baculovirus-expressing His6-tagged E1 from Arapidopsis thaliana. For purification of His6-E1 from infected Sf9 cells, cells were lysed in ubiquitination assay lysis (UAL) buffer [20 mm HEPES (pH 7.4), 100 mm NaCl, 0.1% Triton X-100, 5 mm MgCl2, 5 mm EDTA, 0.2 mm DTT, 1 mm PMSF, 10 μg/ml aprotinin], incubated with Ni–NTA-beads (Qiagen), washed twice with UAL buffer, once with wash buffer (50 mm NaH2PO4, 100 mm NaCl, 10 mm imidazole, 0.2 mm DTT), and eluted with 600 μl of elution buffer [100 mm imidazole, 20 mm Tris (pH 8.0), 100 mm NaCl, 0.2 mm DTT]. The purified protein was dialyzed overnight at 4°C against dialysis buffer [20 mm HEPES (pH 7.4), 100 mm KAOc, 0.2 mm DTT] and concentrated by centricon columns (Amicon). Glycerol was added to a final concentration of 10%. Aliquots were stored at −70°C. Human E2 enzyme Cdc34 was expressed in Escherichia coli and purified as described (Banerjee et al. 1993). Both enzymes were tested for thioester formation capacity prior to use.
For one ubiquitination reaction, GST, GST–pVHL, or GST–p45Skp2 fusion protein was affinity purified from bacterial cultures (or insect cell cultures in the case of GST–p45Skp2) with 30 μl of a 50% slurry of glutathione–Sepharose beads per reaction and washed four times in UAL buffer. Alternatively, anti–HA or anti-pVHL immunoprecipitates from stably transfected RCC cell lysates, 293 or U2–OS cell lysates, respectively, were performed as described previously (Lisztwan et al. 1998) and washed four times in UAL buffer. Where indicated, glutathione–Sepharose beads loaded with the relevant fusion proteins were incubated overnight at 4°C in 1 ml of HeLa cell lysate (1 mg/ml total protein in UAL buffer) prior to performing an ubiquitination assay. For BC-box peptide competition experiments, HeLa cell extracts were preincubated for 30 min on ice with either 1 μm wild-type BC-box peptide (TLKERCLQVVRSLVKP), mutant BC-box peptide (TLKERFLQVVRSLVKP), or carboxy-terminal pVHL peptide (LERLTQERIAHQRMGD) prior to addition of glutathione–Sepharose beads preloaded with the indicated GST proteins.
Ubiquitination reactions were carried out in a total volume of 25 μl containing 15 μl of either glutathione–Sepharose beads loaded with indicated GST fusion proteins or protein A–Sepharose-beads-collected immune complexes and 10 μl of assay buffer containing 1 μl of 10× reaction buffer [40 mm MgAOc, 5 mm DTT, and 1 mm PMSF], 1 μl of 10× ATP-regenerating system [20 mm HEPES (pH 7.4), 10 mm ATP, 10 mm MgAOc, 300 mm creatine-phosphate, 0.5 mg/ml creatine-phosphokinase], 1 μl of 1× HEPES/KAOc buffer [20 mm HEPES (pH 7.4), 100 mm KAOc, 0.5 mm DTT], 5 pmoles of ubiquitinaldehyde (Affiniti, Inc.) [in 50 mm HEPES (pH 6.9)], 6 μg of ubiquitin (Sigma) [in 1× HEPES/KAOc buffer without DTT], 1 μg of purified human Cdc34 [in 20 mm HEPES (pH 7.4), 100 mm K acetate, 1 mm DTT, and 10% glycerol], and 0.2 μg of purified E1 protein. The reaction mixture was incubated at 30°C for 1.5 hr. Fifteen microliters of sample buffer was added to stop the reaction. Samples were boiled for 10 min, analyzed by SDS-PAGE, and processed for Western blotting with the indicated antibody.
Acknowledgments
We thank members of our laboratory for helpful discussions and advice. We are thankful to Dr. W.G. Kaelin for providing pVHL cDNA and Drs. J.W. and R.C. Conaway for Elongin B and Elongin C cDNAs. We thank P. Müller for synthesis of oligonucleotides and F. Fischer for the synthesis of peptides. We also thank Dr. Martin Scheffner for critical reading of the manuscript and for helpful comments. This work was supported by a predoctoral fellowship to J.L. from the Roche Foundation and the Basler Cancer League. G.I. is supported by a grant from the Human Frontier Science Program Organization (HFSPO) and M.G. is supported by a grant from the Swiss Cancer League. C.W. is supported by the Novartis Research Foundation. W.K. is a START-fellow and is supported by the Swiss National Science Foundation and by the Novartis Research Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL wilhelm.krek@fmi.ch; FAX 41 61 6973976.
References
- Aso T, Lane WS, Conaway JW, Conaway RC. Elongin (SIII): A multisubunit regulator of elongation by RNA polymerase II. Science. 1995;269:1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- Aso T, Haque D, Barstead RJ, Conaway RC, Conaway JW. The inducible elongin A elongation activation domain: Structure, function and interaction with the elongin BC complex. EMBO J. 1996;15:5557–5566. [PMC free article] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gregori L, Xu Y, Chau V. The bacterially expressed yeast CDC34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. J Biol Chem. 1993;268:5668–5675. [PubMed] [Google Scholar]
- Brodsky JL, McCracken AA. ER-associated proteasome-mediated protein degradation: How two topologically restricted events come together. Trends Cell Biol. 1997;7:151–156. doi: 10.1016/S0962-8924(97)01020-9. [DOI] [PubMed] [Google Scholar]
- Duan DR, Humphrey JS, Chen DY, Weng Y, Sukegawa J, Lee S, Gnarra JR, Linehan WM, Klausner RD. Characterization of the VHL tumor suppressor gene product: Localization, complex formation, and the effect of natural inactivating mutations. Proc Natl Acad Sci. 1995a;92:6459–6463. doi: 10.1073/pnas.92.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan DR, Pause A, Burgess WH, Aso T, Chen DY, Garrett KP, Conaway RC, Conaway JW, Linehan WM, Klausner RD. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995b;269:1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- Feldman RM, Correll CC, Kaplan KB, Deshaies RJ. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell. 1997;91:221–230. doi: 10.1016/s0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Garrett K, Aso T, Bradsher J, Foundling S, Lane W, Conaway R, Conaway J. Positive regulation of general transcription factor SIII by a tailed ubiquitin homolog. Proc Natl Acad Sci. 1995;92:7172–7176. doi: 10.1073/pnas.92.16.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumor suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Zhou S, Merrill MJ, Wagner JR, Krumm A, Papavassiliou E, Oldfield EH, Klausner RD, Linehan WM. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci. 1996;93:10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M, Egan JM, Zbar B, Lerman M, Geil L, Kuzmin I, Holbrook NJ. Protective function of von Hippel-Lindau protein against impaired protein processing in renal carcinoma cells. Mol Cell Biol. 1999;19:1289–1300. doi: 10.1128/mcb.19.2.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Iliopoulos O, Kibel A, Gray S, Kaelin WG., Jr Tumor suppression by the human von Hippel-Lindau gene product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos O, Ohh M, Kaelin WG., Jr pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc Natl Acad Sci. 1998;95:11661–11666. doi: 10.1073/pnas.95.20.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Maher ER. The VHL tumor-suppressor gene paradigm. Trends Genet. 1998;14:423–426. doi: 10.1016/s0168-9525(98)01558-3. [DOI] [PubMed] [Google Scholar]
- Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr, Conaway RC, Conaway JW. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes & Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, Lane WS, Kaelin WG, Jr, Elledge SJ, Conaway RC, Harper JW, Conaway JW. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- Knebelmann B, Ananth S, Cohen HT, Sukhatme VP. Transforming growth factor alpha is a target for the von Hippel-Lindau tumor suppressor. Cancer Res. 1998;58:226–231. [PubMed] [Google Scholar]
- Koepp DM, Harper JW, Elledge SJ. How the cyclin became a cyclin: Regulated proteolysis in the cell cycle. Cell. 1999;97:431–434. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- Krek W. Proteolysis and the G1-S transition: The SCF connection. Curr Opin Genet Dev. 1998;8:36–42. doi: 10.1016/s0959-437x(98)80059-2. [DOI] [PubMed] [Google Scholar]
- Krek W, Maridor G, Nigg EA. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992;116:43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lee S, Neumann M, Stearman R, Stauber R, Pause A, Pavlakis GN, Klausner RD. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Mol Cell Biol. 1999;19:1486–1497. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Goldberg MA. Hypoxia-inducible protein binding to vascular endothelial growth factor mRNA and its modulation by the von Hippel-Lindau protein. J Biol Chem. 1996;271:25492–25497. doi: 10.1074/jbc.271.41.25492. [DOI] [PubMed] [Google Scholar]
- Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273:6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Lisztwan J, Marti A, Sutterluety H, Gstaiger M, Wirbelauer C, Krek W. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45SKP2: Evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW, Kaelin WG., Jr Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyapina SA, Correll CC, Kipreos ET, Deshaies RJ. Human CUL1 forms an evolutionarily conserved ubiquitin ligase complex (SCF) with SKP1 and an F-box protein. Proc Natl Acad Sci. 1998;95:7451–7456. doi: 10.1073/pnas.95.13.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Kaelin WG., Jr von Hippel-Lindau disease. Medicine. 1997;76:381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between the ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, Louis DN, Gavin BJ, Kley N, Kaelin WG, Jr, Iliopoulos O. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell. 1998;1:959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- Ohta T, Michel JJ, Schottelius AJ, Xiong Y. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol Cell. 1999;3:535–541. doi: 10.1016/s1097-2765(00)80482-7. [DOI] [PubMed] [Google Scholar]
- Patton EE, Willems AR, Tyers M. Combinatorial control in ubiquitin-dependent proteolysis: Don’t Skp the F-box hypothesis. Trends Genet. 1998;14:236–243. doi: 10.1016/s0168-9525(98)01473-5. [DOI] [PubMed] [Google Scholar]
- Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM, Klausner RD. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld A, Davidowitz EJ, Burk RD. A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci. 1998;95:8817–8822. doi: 10.1073/pnas.95.15.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996;56:2299–2301. [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–219. doi: 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- Skowyra D, Koepp DM, Kamura T, Conrad MN, Conaway RC, Conaway JW, Elledge SJ, Harper JW. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGrr1 and rbx1. Science. 1999;284:662–665. doi: 10.1126/science.284.5414.662. [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Kaelin WG, Jr, Pavletich NP. Structure of the VHL-ElonginC-ElonginB complex: Implications for VHL tumor suppressor function. Science. 1999;284:455–461. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Pause A, Conaway RC, Conaway JW. Identification of elongin C sequences required for interaction with the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 1997;272:27444–27449. doi: 10.1074/jbc.272.43.27444. [DOI] [PubMed] [Google Scholar]
- Tan P, Fuchs SY, Chen A, Wu K, Gomez C, Ronai Z, Pan ZQ. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]