CD4+CD25+Foxp3+ regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion (original) (raw)
Abstract
CD4+CD25+Foxp3+ regulatory T (Treg) cells are generated during thymocyte development and play a crucial role in preventing the immune system from attacking the body's cells and tissues. However, how the formation of these cells is directed by T-cell receptor (TCR) recognition of self-peptide:major histocompatibility complex (MHC) ligands remains poorly understood. We show that an agonist self-peptide with which a TCR is strongly reactive can induce a combination of thymocyte deletion and CD4+CD25+Foxp3+ Treg cell formation in vivo. A weakly cross-reactive partial agonist self-peptide could similarly induce thymocyte deletion, but failed to induce Treg cell formation. These studies indicate that CD4+CD25+Foxp3+ Treg cell formation can require highly stringent recognition of an agonist self-peptide by developing thymocytes. They also refine the “avidity” model of thymocyte selection by demonstrating that the quality of the signal mediated by agonist self-peptides, rather than the overall intensity of TCR signaling, can be a critical factor in directing autoreactive thymocytes to undergo CD4+CD25+Foxp3+ Treg cell formation and/or deletion during their development.
Keywords: affinity, immune regulation, specificity, tolerance, transgenic mice
CD4+ and CD8+ T cells use the α/β T-cell receptor (TCR) to recognize peptides (which can be derived from foreign or self-peptides) as complexes with molecules of the major histocompatibility complex (MHC) (1). A key feature of this recognition is the ability of the TCR to distinguish between subtle structural variations in peptide:MHC ligands; thus, rather than acting as a binary “on–off” signal, the TCR can interpret differences between optimal and suboptimal ligands that substantially affect the intracellular signaling pathways that are activated (2). For effector CD4+ and CD8+ T cells, foreign peptides that stimulate robust proliferation and additional effector functions (e.g., cytokine production) when present at low concentrations are typically termed agonist peptides. Partial agonists (which usually differ from the agonist by one or a few amino acid substitutions) require higher concentrations to induce proliferation and/or induce only a subset of effector functions, and antagonist peptides specifically inhibit the response(s) that can be induced by an agonist (2). This capacity of the TCR to transmit distinct signals in response to peptide:MHC complexes also plays a pivotal role in directing thymocyte development. Seminal studies using TCR transgenic mice and in vitro thymocyte culture systems showed that positive selection of CD4−CD8+ (CD8SP) thymocytes could be induced by low doses of agonist and/or antagonist peptides, whereas higher doses of agonist peptides led to thymocyte deletion (3–5). These studies led to the “avidity” model of thymocyte selection, which has been extended in recent years to characterize an affinity threshold that can direct positive selection versus deletion of CD8SP thymocytes through activation of distinct signaling pathways (6). Similar approaches have been used to identify endogenous self-peptides that can promote positive selection of TCR transgenic CD8SP and CD4+CD8− (CD4SP) thymocytes in vitro (7). However, it has also become clear that thymocytes can undergo selection to become CD4+CD25+Foxp3+ regulatory T (Treg) cells (8, 9), and how signaling through the TCR induces the formation of Treg cells is not yet understood.
Firm evidence that TCR interactions with self-peptides can induce Treg cell formation came from studies using transgenic mice expressing a TCR that recognizes a MHC class II-restricted peptide from the influenza hemagglutinin (HA) and coexpressing the HA as a self-antigen (10). A large proportion of the HA-specific CD4SP thymocytes and CD4+ T cells in such mice were initially found to express CD25 and to exert regulatory function in vitro and were subsequently shown to also express the transcription factor Foxp3, which is closely associated with Treg cell formation and function (9, 11). The formation of self-antigen–specific Treg cells has since been described in additional mice expressing HA as a self-antigen (12–14) and in mice coexpressing an ovalbumin-specific TCR and ovalbumin as a self-antigen (15, 16). A notable feature of these experimental systems has been that the self-peptide that induces Treg cell formation is recognized by the TCR as an agonist; agonist peptides are typically associated with strong signals through the TCR, and in the avidity model of thymocyte development, such signals might be expected to induce thymocyte deletion. Indeed, it has been difficult to place Treg cell formation within this avidity model; one idea has been that Treg cell formation might be induced by TCR signals that lie in an affinity/avidity range below that necessary to induce thymocyte deletion, but above that necessary for positive selection (17). However, this model has received little direct experimental analysis, and how distinct or overlapping signals transmitted by the TCR in response to self-peptides might direct thymocyte deletion and/or Treg cell formation remains to be determined.
The studies herein examined the ability of an agonist versus a partial agonist self-peptide to induce thymocytes expressing MHC class II-restricted TCRs to undergo Treg cell selection in vivo. We provide evidence that Treg cells can be selected on the basis of highly stringent recognition of an agonist self-peptide and suggest that the quality of the TCR signal that is delivered by agonist versus partial agonist self-peptide(s) can play a critical role in determining whether autoreactive thymocytes undergo deletion and/or Treg cell formation during their development.
Results
Partial Agonist Self-Peptide Induces Thymocyte Deletion Without Foxp3 Induction.
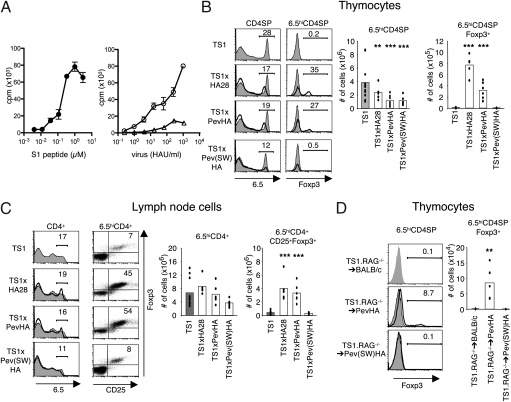
To examine how specificity for self-peptides directs thymocyte development, we used TS1 mice, which express a transgenic TCR (detected with the monoclonal antibody 6.5) that was isolated from an influenza virus PR8-infected BALB/c mouse (18). The TS1 TCR recognizes the major I-Ed–restricted CD4+ T-cell determinant of PR8 HA termed S1 (19) and reacts with a synthetic S1 peptide with a sensitivity (1/2max = ∼0.1 μM) that is typical of agonist peptides (20) (Fig. 1_A_). Synthetic peptides may not fully recapitulate the range of peptides that can be processed and presented from protein molecules (21), and CD4+ T cells from TS1 mice also react strongly with splenocytes presenting naturally processed S1-related peptide(s) derived from the intact PR8 HA molecule (Fig. 1_A_). By contrast, CD4+ T cells from TS1 mice are more weakly cross-reactive with splenocytes presenting peptides derived from the SW strain of influenza virus, which contains an analog of the S1 peptide, termed S1(SW), which differs from the S1 peptide by two amino acid substitutions (19) (Fig. 1_A_). Collectively, these data indicate that the TS1 TCR recognizes naturally processed S1 and S1(SW) determinants generated from intact PR8 and SW HA molecules with relative reactivities that are typical of agonist and partial agonist peptides, respectively.
Fig. 1.
The partial agonist S1(SW) peptide induces 6.5hi thymocyte deletion but not Treg cell formation. (A) Proliferation of 6.5hiCD4+ T cells from TS1 mice incubated with graded doses of S1 peptide (Left) or with graded numbers of HAU of PR8 virus (circles) or SW virus (triangles) (Right) shown as means ± SEM. n = 3. (B) Representative histograms show 6.5 and Foxp3 expression by CD4SP thymocytes from TS1 × HA28 (n = 5), TS1 × PevHA (n = 6), and TS1 × Pev(SW)HA (n = 6) mice (black lines) overlaying thymocytes from TS1 (n = 11) mice (filled), with mean percentages in gate indicated. Graphs show numbers of 6.5hiCD4SP and 6.5hiCD4SPFoxp3+ thymocytes, with bars denoting means and individual values shown. **P ≤ 0.05, ***P ≤ 0.01. (C) Same as in B except showing 6.5, CD25, and Foxp3 expression by CD4+ LN cells. (D) Representative histograms show Foxp3 expression on 6.5+CD4SP thymocytes from chimeras of Pev(SW)HA (n = 6) or PevHA (n = 6) mice (black lines) overlaying filled histograms from BALB/c (n = 6) mice (shaded) reconstituted with BM from TS1.RAG−/− mice. Mean percentages in indicated gates are shown. Graph shows numbers of thymocytes with bars denoting means and individual values shown. **P ≤ 0.05. All data are representative of at least three independent experiments.
When TS1 mice were mated with additional lineages of transgenic mice that express the PR8 HA as a self-antigen, 6.5hiCD4SP thymocytes underwent both deletion and selection to become 6.5hiCD4SPFoxp3+ thymocytes in response to the S1 self-peptide (14). Thus, TS1 × HA28 and TS1 × PevHA mice each coexpress the TS1 TCR and the PR8 HA [driven by SV40 promoter/enhancer (10) and β-globin locus control region sequences (22), respectively], and each contained both significantly lower numbers of 6.5hiCD4SP thymocytes and significantly higher numbers of 6.5hiCD4SPFoxp3+ thymocytes than were present in TS1 mice (Fig. 1_B_). The lymph nodes (LNs) of TS1 × HA28 and TS1 × PevHA mice also contained significantly higher numbers of 6.5hiCD4+Foxp3+CD25+ T cells than were present in TS1 mice (Fig. 1_C_), and 6.5hiCD4+CD25+ LN cells from these mice suppressed the proliferation of a bystander CD4+ T-cell population in vitro (Fig. S1).
To examine how interactions with the cross-reactive S1(SW) partial agonist self-peptide can shape 6.5hiCD4SP thymocyte development, we generated Pev(SW)HA mice, which express the SW HA as a self-antigen driven by the same β-globin locus control region sequences used to direct PR8 HA expression in PevHA mice. 6.5hiCD4SP thymocytes were significantly less abundant in TS1 × Pev(SW)HA mice than in TS1 mice; indeed, the average frequency of 6.5hiCD4SP thymocytes in TS1 × Pev(SW)HA mice was similar to that in TS1 × PevHA mice, indicating that thymocytes expressing the 6.5 TCR undergo a comparable degree of deletion in response to both agonist S1 and partial agonist S1(SW) self-peptides (Fig. 1_B_). Notably, however, there was no increase in the frequency of 6.5hiCD4SPFoxp3+ thymocytes or of 6.5hiCD4+Foxp3+CD25+ T cells in TS1 × Pev(SW)HA mice relative to TS1 mice, sharply contrasting the substantial increases that were observed in both TS1 × PevHA and TS1 × HA28 mice (Fig. 1, B and C). We had previously produced TS1.RAG−/−→HA28 radiation bone marrow (BM) chimeras to confirm that 6.5hiCD4SPFoxp3+ thymocyte formation is based on the specificity of the 6.5 TCR for the S1 self-peptide (10). As predicted from these earlier results, we found significantly increased 6.5hiCD4SPFoxp3+ thymocyte formation in TS1.RAG−/−→PevHA relative to TS1.RAG−/−→BALB/c chimeras, but notably, not in TS1.RAG−/−→Pev(SW)HA chimeras (Fig. 1_D_). Thus, both the agonist S1 and partial agonist S1(SW) self-peptides could induce deletion of 6.5hiCD4SP thymocytes, but only the agonist S1 self-peptide caused increased 6.5hiCD4+Foxp3+ T-cell formation.
As an Agonist, the S1(SW) Self-Peptide Induces Treg Cells.
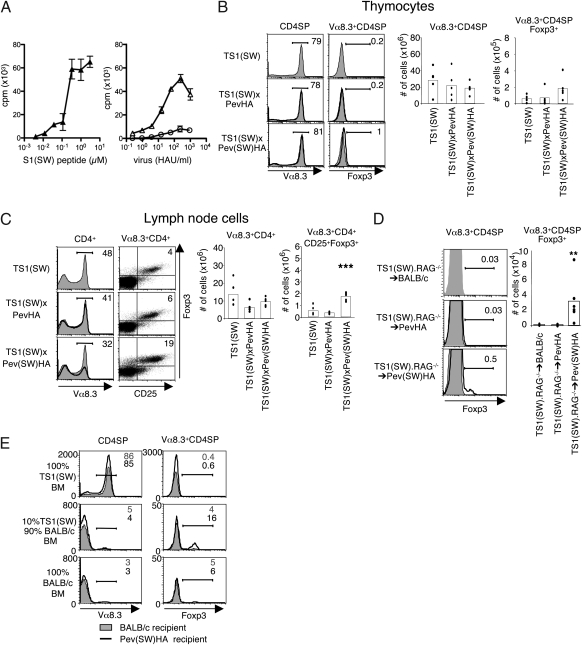
The preceding studies suggested that the specificity of the TCR for self-peptides can be an important factor in directing Treg cell formation, but it was also possible that some unknown characteristic of the Pev(SW)HA lineage renders the S1(SW) self-peptide incapable of inducing Foxp3+ T-cell formation. To examine whether the S1(SW) self-peptide can induce Treg cell formation when it is recognized as an agonist, we used TS1(SW) mice, which express a transgenic Vα8.3+/Vβ10+ TCR obtained from a SW virus-immunized mouse (10). Vα8.3+CD4+ T cells from TS1(SW) mice recognize both a synthetic S1(SW) peptide and naturally processed S1(SW) peptide(s) derived from SW virus as agonists and are weakly cross-reactive with PR8-derived S1 peptide(s) (Fig. 2_A_). When TS1(SW) mice were mated with Pev(SW)HA mice to produce TS1(SW) × Pev(SW)HA mice, there was a modest increase in the frequency of Vα8.3+CD4SPFoxp3+ thymocytes relative to either TS1(SW) or TS1(SW) × PevHA mice (1 versus 0.2%, respectively) (Fig. 2_B_). Moreover, the number of Vα8.3+CD4+Foxp3+CD25+ LN cells was significantly higher in TS1(SW) × Pev(SW)HA mice than in either TS1(SW) or TS1(SW) × PevHA mice (Fig. 2_C_), and Vα8.3+CD4+CD25+ LN cells from these mice suppressed the proliferation of a bystander CD4+ T-cell population in vitro (Fig. S2). We also generated radiation chimeras in which BM from TS1(SW).RAG−/− mice was transplanted into lethally irradiated BALB/c, PevHA, and Pev(SW)HA recipients. There was an ∼30-fold increase in the number of Vα8.3+CD4SPFoxp3+ thymocytes in TS1(SW).RAG−/−→Pev(SW)HA chimeras relative to TS1(SW).RAG−/−→BALB/c chimeras, but no such increases were found in TS1(SW).RAG−/−→PevHA chimeras (Fig. 2_D_). Thus, the S1(SW) self-peptide in Pev(SW)HA mice can induce Foxp3 up-regulation among CD4SP thymocytes and CD4+ T cells, but only when it is recognized as an agonist peptide by the TS1(SW) TCR.
Fig. 2.
The TS1(SW) TCR undergoes Treg cell formation in response to agonist S1(SW) peptide. (A) Vα8.3+CD4+ T cells from TS1(SW) mice incubated with graded doses of S1(SW) peptide (Left) or with graded numbers of HAU of PR8 virus (circles) or SW virus (triangles) (Right), shown as means ± SEM. n = 3. (B) Representative histograms showing Vα8.3 and Foxp3 expression by CD4SP thymocytes from TS1(SW) × PevHA (n = 5) and TS1(SW) × Pev(SW)HA (n = 5) mice (black lines) overlaying thymocytes from TS1(SW) (n = 12) mice (filled), with mean percentages in gate indicated. Graphs show numbers of Vα8.3+CD4SP and Vα8.3+CD4SPFoxp3+ thymocytes, with bars denoting means and individual values shown. (C) Same as in B except showing Vα8.3, CD25 ,and Foxp3 expression by CD4+ LN cells. ***P ≤ 0.01. (D) Representative histograms showing Vα8.3 and Foxp3 expression by CD4SP thymocytes in chimeric Pev(SW)HA (n = 6) or PevHA (n = 6) mice (black lines) overlaying filled histograms from BALB/c (n = 6) mice (shaded) reconstituted with BM from TS1(SW).RAG−/− mice. Mean percentages in indicated gates are shown. Graph shows numbers of thymocytes with bars denoting means and individual values shown. **P ≤ 0.05. (E) Representative histograms showing Vα8.3 and Foxp3 expression by CD4SP thymocytes in chimeric BALB/c (filled) or Pev(SW)HA (black) mice reconstituted with 100% BM from TS1(SW) mice (n = 4) (Top), 10% BM from TS1(SW) mice plus 90% BM from BALB/c mice (n = 4) (Middle), or 100% BM from BALB/c mice (n = 4) (Bottom). Mean percentages in indicated gates in BALB/c (gray) and Pev(SW)HA (black) recipients are shown. All data are representative of at least three independent experiments.
Precursor Frequency Affects Agonist-Induced Foxp3+ Thymocyte Formation in TS1(SW) × Pev(SW)HA Mice.
The finding that agonist-induced Foxp3+CD4+ T-cell formation occurred less efficiently in mice expressing the TS1(SW) TCR than the TS1 TCR was notable because the TS1(SW) TCR is expressed by a higher proportion of thymocytes (∼80% versus ∼25% of CD4SP thymocytes, respectively). CD4SPFoxp3+ thymocyte formation can be impaired under conditions of high precursor frequency (23, 24), and we examined whether reducing the frequency of thymocytes expressing the TS1(SW) TCR might enhance the representation of Vα8.3+CD4SPFoxp3+ thymocytes in mice expressing the S1(SW) self-peptide. In Pev(SW)HA radiation chimeras that had received TS1(SW) BM cells diluted 1:10 with BALB/c BM cells (and that therefore contained a reduced frequency of Vα8.3+CD4SP thymocytes), 16% of the Vα8.3+CD4SP thymocytes were Foxp3+ (Fig. 2_E_). We also mated TS1(SW) mice with HACII mice, which express high levels of PR8 HA driven by a MHC class II promoter (25); in TS1(SW) × HACII mice, Vα8.3+CD4SP thymocytes were subjected to substantial deletion by the partial agonist S1 peptide, but little or no Vα8.3+CD4SPFoxp3+ thymocyte formation occurred (Fig. S3). Thus, decreasing the frequency of thymocytes expressing the TS1(SW) TCR could accentuate the formation of Vα8.3+CD4SPFoxp3+ thymocytes in response to the agonist S1(SW) self-peptide, whereas enhanced deletion mediated by high levels of the partial agonist S1 could not.
Differential Thymocyte Signaling by Agonist/Partial Agonist Self-Peptides.
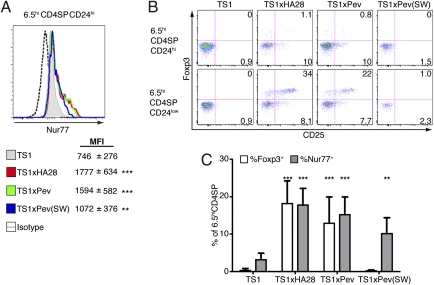
To further analyze how TCR signaling in response to self-peptides can direct thymocyte deletion and/or Treg cell formation, we examined expression of Nur77, CD25, and Foxp3 at different stages of thymocyte development. Previous studies have demonstrated that Nur77 participates in thymocyte deletion (26, 27). Consistent with this finding, immature 6.5hiCD4SPCD24hi thymocytes from TS1 × HA28, TS1 × PevHA, and TS1 × Pev(SW)HA mice (in which 6.5hi thymocytes undergo self-peptide–induced deletion) all expressed significantly higher levels of Nur77 than were found in TS1 mice (Fig. 3_A_). Notably, however, only the 6.5hiCD4SPCD24hi thymocytes from TS1 × HA28 and TS1 × PevHA mice exhibited up-regulation of CD25 (Fig. 3_B_), which has been shown to identify CD4SP thymocytes that have received an appropriate TCR signal for commitment to Treg cell development and can differentiate into CD4SPFoxp3+ thymocytes in response to common γ-chain cytokine signaling (28). Indeed, the more mature 6.5hiCD4SPCD24low thymocytes from TS1 × HA28 and TS1 × PevHA mice, but not those from TS1 × Pev(SW)HA mice, also exhibited up-regulation of Foxp3 relative to TS1 mice (Fig. 3_B_). As a result of these processes, there were significant increases in the frequencies of 6.5hiCD4SPNur77+ thymocytes in TS1 × HA28, TS1 × PevHA, and TS1 × Pev(SW)HA mice relative to TS1 mice, but significantly increased frequencies of 6.5hiCD4SPFoxp3+ thymocytes were only found in TS1 × HA28 and TS1 × PevHA mice (Fig. 3_C_). It is notable that Nur77 levels were highest on thymocytes from TS1 × HA28 and TS1 × PevHA mice, because studies with a Nur77 reporter mouse containing otherwise unmanipulated TCR repertoires revealed higher Nur77 expression on CD4SPFoxp3+ thymocytes (29). Thus, agonist S1 and partial agonist S1(SW) self-peptides induced distinct phenotypic changes in thymocytes expressing the TS1 TCR; both ligands activated a pathway(s) associated with thymocyte deletion, but only the agonist S1 self-peptide induced signals that lead to Foxp3 up-regulation.
Fig. 3.
Agonist and partial agonist self-peptides activate distinct signaling pathways during 6.5hiCD4SP thymocyte development. (A) Representative histograms showing intracellular Nur77 expression by 6.5hiCD4SPCD24hi thymocytes from TS1 × HA28 (red) (n = 4), TS1 × PevHA (green) (n = 4), and TS1 × Pev(SW)HA (blue) (n = 4) mice are shown relative to TS1 (filled) (n = 4) mice. Mean fluorescent intensities (MFIs) of Nur77 staining are shown ± SEM. **P ≤ 0.05, ***P ≤ 0.01. (B) Representative (n = 4) dot plots showing Foxp3 versus CD25 expression by 6.5hiCD4SPCD24hi (Upper) and 6.5hiCD4SPCD24low (Lower) thymocytes from TS1, TS1 × HA28, TS1 × PevHA, and TS1 × Pev(SW)HA mice. Mean percentages of cells in the indicated quadrants are shown. (C) Graph shows mean percentages ± SEM of 6.5hiCD4SP thymocytes that are Foxp3+ (open bars) or Nur77+ (filled bars) in TS1 (n = 4), TS1 × HA28 (n = 4), TS1 × PevHA (n = 4), and TS1 × Pev(SW)HA (n = 4) mice. **P ≤ 0.05, ***P ≤ 0.01. All data are representative of at least three independent experiments.
Discussion
We have examined how specificity for self-peptides can instruct autoreactive thymocytes to undergo deletion and/or differentiation to become Treg cells. Previous studies in this and other systems have shown that TCR recognition of agonist self-peptides can promote Treg cell formation (10, 12–16); however, interactions with self-peptides can also induce thymocyte deletion (7), and how signaling through the TCR might specify these different fates has been unclear. The findings here support a model of thymocyte development in which both agonist and partial agonist peptides can induce thymocyte deletion, but development into a Treg cell requires a TCR-mediated signal that an agonist self-peptide is uniquely able to provide.
In its simplest form, the avidity model of thymocyte development posits that individual thymocytes interpret quantitative differences in the TCR signals that accumulate during their interaction either with specific self-peptides or with combinations of self-peptides, and certain threshold levels of signaling trigger discrete outcomes (3–5). Thus, low-intensity signaling through the TCR has been found to promote thymocyte development (positive selection), whereas more intense TCR signaling can induce thymocyte death (deletion), and it has been proposed that Treg cell formation may be induced by TCR signals above the threshold necessary for positive selection, but below that which induces deletion (17). However, the data here are difficult to fit into a simple model in which TCR signaling thresholds direct thymocyte deletion versus Treg cell development. It is clear that the TS1 TCR reacts more weakly with the S1(SW) peptide than the S1 peptide; conventional CD4+ T cells proliferate much more weakly when activated with S1(SW) versus S1 determinants (Fig. 1_A_), and lower levels of Nur77 were expressed by 6.5+CD4SP thymocytes that had encountered the partial agonist S1(SW) versus the S1 agonist (Fig. 3_A_), consistent with weaker TCR stimulation. Significantly, these low levels of TCR stimulation induced by S1(SW) are sufficient to cause 6.5+CD4SP thymocyte deletion in TS1 × Pev(SW)HA mice, but they fail to induce Foxp3 up-regulation. In light of this finding, it is incongruous to propose that a higher signaling threshold alone (such as can be provided by the agonist S1 peptide) is necessary for Foxp3 induction in TS1 × HA28 and TS1 × PevHA mice, because potential Treg cells would have been eliminated at the signaling threshold that causes deletion. Instead, the data here are more consistent with a model in which an agonist self-peptide delivers a qualitatively distinct signal that can induce thymocytes to develop along a Treg cell differentiation pathway.
Agonist-induced formation of CD4SPFoxp3+ thymocytes was also observed in TS1(SW) × Pev(SW)HA mice, but only when the frequency of thymocytes expressing the TS1(SW) TCR was reduced in mixed BM chimeras. Efficient suppression of endogenous TCR gene rearrangement causes the TS1(SW) TCR to be highly represented in mice expressing the Vα8.3/Vβ10 transgenes, and the inability to readily detect Vα8.3+CD4SPFoxp3+ thymocyte formation in intact TS1(SW) × Pev(SW)HA mice resembles recent studies showing that “niches” for Treg cell development can be saturated under conditions of high precursor frequency (23, 24). In this regard, one possible explanation for the efficient formation of 6.5+CD4SPFoxp3+ thymocytes in TS1 × HA28 and TS1 × PevHA mice could be that S1-induced deletion lowers 6.5+ thymocyte precursor numbers and creates a niche for the formation of 6.5+CD4SPFoxp3+ thymocytes. However, 6.5+CD4SP thymocytes are subjected to a comparable degree of deletion by the S1(SW) partial agonist in TS1 × Pev(SW)HA mice, but this deletion does not in itself lead to increased 6.5+CD4SPFoxp3+ thymocyte formation. Similarly, elevated expression of the partial agonist S1 self-peptide induced deletion of Vα8.3+CD4SP thymocytes without inducing Treg cell formation in TS1(SW) × HACII mice, because partial agonist self-peptides fail to provide an appropriate signal for Treg cell induction even though deletion led to lower thymocyte frequencies. Another possible explanation for the efficient formation of 6.5+CD4SPFoxp3+ thymocytes in TS1 × HA28 and TS1 × PevHA mice could be that the presence of a strong agonist peptide induces IL-2 expression from the 6.5+CD4SPFoxp3− thymocytes and/or 6.5+CD4+Foxp3− effector T cells that also develop in these mice, and this IL-2 in turn promotes the formation of Treg cells. This is unlikely, however, because elevated expression of IL-2 would be expected to act on all Treg cells irrespective of TCR specificity, and no increases were found in the numbers or percentages of 6.5−CD4SPFoxp3+ thymocytes, or of 6.5−CD4+Foxp3+ splenocytes, in TS1 × HA28 and TS1 × PevHA mice relative to TS1 × Pev(SW)HA mice (although both 6.5+CD4+Foxp3+ and 6.5−CD4+Foxp3+ splenocyte numbers did increase in response to IL-2:anti-IL2 complex injection) (Fig. S4).
The conclusion that qualitative differences in signaling events induced by self- peptides can shape thymocyte development is consistent with studies showing that CD8SP thymocytes that have been positively selected by agonist versus antagonist self-peptides can exhibit distinct phenotypes and have differing abilities to respond to antigenic stimulation (30). It is likely that these phenotypes are a reflection of variations in the TCR-proximal signaling events that are induced by different peptide ligands, which in turn activate distinct signaling pathways. Significantly, whereas the agonist S1 and partial agonist S1(SW) peptides could both induce Nur77 up-regulation and deletion, only the agonist S1 peptide caused a significant increase in the formation of 6.5hiCD4SPFoxp3−CD25+ thymocytes. The ability to induce CD25 up-regulation is noteworthy because CD4SPFoxp3−CD25+ thymocytes have been identified as a precursor population that has received a TCR-mediated signal, and that will differentiate into CD4SPFoxp3+ thymocytes in response to IL-2 or IL-15 (28). The nature of the TCR signal that is required to initiate CD25 up-regulation was not known, however, and we have shown here that an agonist self-peptide is uniquely able to activate signaling pathways that initiate the formation of CD4SPFoxp3−CD25+ thymocytes. This requirement for specific recognition of a self-peptide in Treg cell formation also resonates with recent evidence that positive selection of conventional CD4+ thymocytes can be mediated by TCR recognition of specific self-peptides that can also act as coagonists for activation and/or homeostasis of mature CD4+ T cells in the periphery (31, 32). For Treg cells, a requirement for specific recognition of agonist peptides during thymocyte selection may play an important role in establishing a link between the formation of Treg cells in response to tissue-specific antigens in the thymus and their activity at sites in the periphery where the self-antigen is expressed.
Materials and Methods
Viruses and Peptides.
Influenza viruses A/Puerto Rico/8/34 (PR8) and A/Swine/31 (SW) were propagated by growth in the allantoic cavity of hens’ eggs and purified by sucrose gradient centrifugation. Virus titers were determined by agglutination of chicken erythrocytes and expressed as hemagglutinating units (HAU). Peptides S1 (SFERFEIFPKE), S1(SW) (SFEKFEIFPKT), and S2 (HNTNGVTAACSHE) were synthesized and purified by HPLC (Genscript).
Mice.
TS1, TS1(SW), HA28, PevHA, HACII, and TS2 mice have been described previously (10, 11, 18, 22, 25). To generate Pev(SW)HA mice, a 770-bp fragment encoding amino acids 38–294 of the HA1 domain of the PR8 HA gene was removed from the construct used to generate PevHA mice and replaced with the corresponding sequence from the SW HA gene. Transgenic mice were backcrossed at least six generations to BALB/c mice before use. Mice were killed at 8–12 wk of age. BM chimeras were generated by injecting 5 × 106 T-cell–depleted BM cells i.v. into 6- to 8-wk-old irradiated (900 rad) mice and analyzed 8 wk later. All mice were housed under specific pathogen-free conditions in the Wistar Institute Animal Facility. IL-2/anti–IL-2 complexes were prepared and injected 5 d before sacrifice as described (33).
Flow Cytometry.
Single cell suspension of thymus, spleen, or LN were prepared and stained with labeled antibodies by incubation on ice. Anti-clonotypic antibody 6.5 (18) was biotinylated and detected with streptavidin-allophycocyanin, and remaining antibodies were obtained from BD Pharmingen or eBioscience. Intracellular staining for Foxp3 and Nur77 were performed according to the eBioscience protocol for Foxp3 staining. For intracellular IFN-γ staining, cells were first stimulated with phorbol myristate acetate and ionomycin and incubated with brefeldin A, according to the eBioscience protocol.
In Vitro T Cell Assays.
Proliferation assays used FACS-purified 6.5+CD4+ or Vα8.3+CD4+ T cells (2 × 105 cells/mL) incubated with BALB/c splenocytes (106 cells/mL) and varying amounts of S1 or S1(SW) peptide or differing numbers of HAU of either PR8 or SW virus. Assays were carried out in supplemented Iscove's modified Dulbecco's medium at 37 °C in 5% CO2 for 3 d, with the addition of [3H]thymidine for the last 16 h. For suppression assays CD4+ T cells from TS2 mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes) and incubated for 4 d with FACS-purified 6.5+CD4+CD25+ LN cells from TS1 × HA28 mice or with FACS-purified Vα8.3+Vβ10+CD4+CD25+ LN cells from TS1(SW) × Pev(SW)HA mice (1 Treg:2 effector cells) and with either BALB/c splenocytes, 1 μM S2 peptide, and the indicated amount of S1 or S1(SW) peptide or with 1 μM S2 peptide and splenocytes from HACII mice.
Statistical Analysis.
Unpaired, two-tailed t tests were used for data analysis and the generation of P values.
Supplementary Material
Supporting Information
Acknowledgments
We thank Laura Panarey, Alissa Basehoar, and Abigail Liebow for superb assistance and Avinash Bhandoola for helpful comments on the manuscript. This work was supported by the National Institutes of Health (AI-59166 to A.J.C.) and by the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.R. is a guest editor invited by the Editorial Board.
References
- 1.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 2.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 3.Ashton-Rickardt PG, et al. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 4.Sebzda E, et al. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 5.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 6.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 7.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 10.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, 3rd, et al. CD4+CD25+ regulatory T cell repertoire formation shaped by differential presentation of peptides from a self-antigen. J Immunol. 2008;180:2149–2157. doi: 10.4049/jimmunol.180.4.2149. [DOI] [PubMed] [Google Scholar]
- 12.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 13.Aschenbrenner K, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 14.Picca CC, et al. Thymocyte deletion can bias Treg formation toward low-abundance self-peptide. Eur J Immunol. 2009;39:3301–3306. doi: 10.1002/eji.200939709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahata K, et al. Generation of CD4(+)CD25(+) regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 16.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 18.Kirberg J, et al. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerasoli DM, Riley MP, Shih FF, Caton AJ. Genetic basis for T cell recognition of a major histocompatibility complex class II-restricted neo-self peptide. J Exp Med. 1995;182:1327–1336. doi: 10.1084/jem.182.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kersh GJ, Allen PM. Structural basis for T cell recognition of altered peptide ligands: A single T cell receptor can productively recognize a large continuum of related ligands. J Exp Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudensky AYu, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–627. [PubMed] [Google Scholar]
- 22.Lerman MA, Larkin J, 3rd, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J Immunol. 2004;173:236–244. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 23.Bautista JL, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J Exp Med. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed AJ, et al. Alloreactive CD4 T cell activation in vivo: An autonomous function of the indirect pathway of alloantigen presentation. J Immunol. 2003;171:6502–6509. doi: 10.4049/jimmunol.171.12.6502. [DOI] [PubMed] [Google Scholar]
- 26.Zhou T, et al. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183:1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lio C-WJ, Hsieh C-S. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature. 1994;369:750–752. doi: 10.1038/369750a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo WL, et al. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information