Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential (original) (raw)
Abstract
Antiviral compounds that increase the resistance of host tissues represent an attractive class of therapeutic. Here, we show that squalamine, a compound previously isolated from the tissues of the dogfish shark (Squalus acanthias) and the sea lamprey (Petromyzon marinus), exhibits broad-spectrum antiviral activity against human pathogens, which were studied in vitro as well as in vivo. Both RNA- and DNA-enveloped viruses are shown to be susceptible. The proposed mechanism involves the capacity of squalamine, a cationic amphipathic sterol, to neutralize the negative electrostatic surface charge of intracellular membranes in a way that renders the cell less effective in supporting viral replication. Because squalamine can be readily synthesized and has a known safety profile in man, we believe its potential as a broad-spectrum human antiviral agent should be explored.
Keywords: innate immunity, hepatitis B virus, eastern equine encephalitis virus, dengue virus, yellow fever virus
Squalamine (Fig. 1_A_) was first discovered in the tissues of the dogfish shark (Squalus acanthias) as a broad-spectrum antimicrobial agent in 1993 (1, 2) and later identified within the circulating white blood cells of the sea lamprey (Petromyzon marinus) (3). It was found to have pharmacological activity in endothelial cells, inhibiting several growth factor-dependent processes (such as angiogenesis, migration, and proliferation) both in vitro and in vivo (4–11). Recently, squalamine was discovered to enter cells and cause displacement of proteins that are associated through electrostatic interactions with the inner face of the cytoplasmic membrane (12–14). Squalamine (3β-_N_-1-{_N_-[3-(4-aminobutyl)]-1,3-diaminopropane)-7α, 24R-dihydroxy-5α-cholestane 24 sulfate; molecular weight = 628) (2) carries a net positive charge by virtue of its spermidine moiety and exhibits a high affinity for anionic phospholipids (15, 16); on entry into a eukaryotic cell, it neutralizes the negative charge of the surface to which it binds (12, 13). Surprisingly, this disruption of electrostatic potential can occur without obvious structural damage to the cell membrane as measured by changes in permeability (13). After it has gained entry into a cell, squalamine subsequently exits over the course of hours, which was shown by its pharmacokinetic properties in numerous mammalian species, including humans (17–19).
Fig. 1.
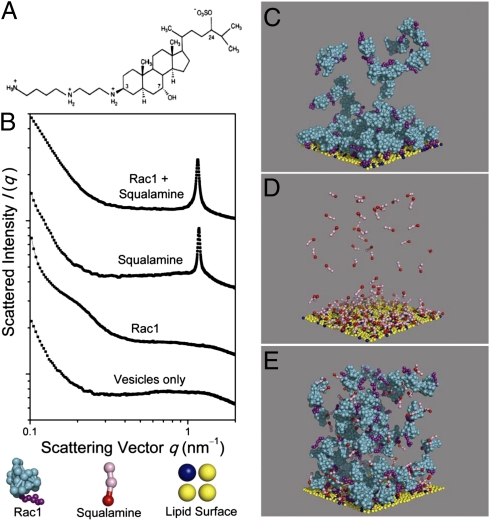
Squalamine strongly displaces Rac1 from model membranes because of charge density matching. (A) Squalamine chemical structure. (B) Diffraction patterns from lipid membrane vesicles, Rac1–membrane complexes, squalamine–membrane complexes, and membranes incubated with both Rac1 and squalamine (bottom to top). The diffraction pattern of the final case is nearly identical to the diffraction signature of squalamine–membrane complexes, indicating strong suppression of Rac1–membrane binding [lipid compositions 1,2-Dioleoyl-sn-Glycero-3-[Phospho-l-Serine] (sodium salt)/1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (DOPC)/1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine = 20/20/60, Rac1 to lipid ratio = 1:50, squalamine to lipid ratio = 1:15; all are molar ratios]. (C–E) Representative configurations from molecular dynamics simulations of solutions of Rac1 (C), squalamine (D), and both Rac1 and squalamine (E), respectively, in the presence of a coarse-grained membrane in which 20% of the lipids are charged. Rac1 and squalamine are present at concentrations that yield the same net charge on all molecules, each higher than needed to neutralize the membrane. Squalamine was found to exhibit nearly two times stronger electrostatic membrane binding than Rac1, and it displaced 56% of Rac1 from the membrane.
The ability of squalamine to alter the electrostatic charge in so drastic of a fashion as to cause displacement of membrane-anchored proteins, suggested to one of us (M.Z.) that squalamine might have, as a consequence, antiviral properties. Many viruses enter cells through engagement of the ρ-GTPases, such as Rac1, to influence the actin cytoskeleton (20–23). Displacement of key proteins anchored through electrostatic forces (of host or viral origin) from the cytoplasmic face of the plasma membrane might interfere with entry (20, 21, 24), protein synthesis (25), virion assembly (26, 27), virion budding (28), or other steps in the viral replication cycle (29). Certain viruses seem to require the presence of the anionic phospholipid phosphatidylserine in the target cell plasma membrane as part of the fusion process, and charge neutralization of the anionic phospholipid could, in principle, interrupt these events (24, 25, 30–33). Because squalamine, after parenteral administration, is cleared from the circulation over the course of hours by the liver, from which it is excreted through the biliary system, we focused our initial investigations on viruses that infect the liver.
Results
The charge density on squalamine enables it to have unusual interactions with cell membranes. Because its surface charge density is nearly equal and opposite to the charge density of lipid components of the anionic cytoplasmic surface of typical eukaryotic membranes, squalamine should exhibit anomalously strong membrane binding because of the maximal recovery of counterion entropy (34). We show that, owing to this effect, squalamine can strongly displace membrane-bound cationic proteins such as Rac1, a ρ-GTPase recruited to the inner leaflet of the eukaryotic cytoplasmic membrane for the actin remodeling necessary for endocytosis (35). By incubating liposomes [1,2-Dioleoyl-sn-Glycero-3-[Phospho-l-Serine] (sodium salt)/1,2-Dioleoyl-sn-Glycero-3-Phosphocholine/1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine = 20/20/60; similar to the inner leaflet lipid composition of the plasma membrane of mammalian cells] (36) with Rac1 and squalamine, we obtain the diffraction signatures of Rac1–membrane complexes and squalamine–membrane complexes, respectively, using synchrotron small-angle X-ray scattering (Fig. 1_B_). Rac1–membrane complexes exhibit a broad diffraction feature at scattering vector q ∼0.22 nm−1, which is consistent with weakly correlated bilayer membranes with electrostatically associated Rac1 on both sides. In contrast, squalamine–membrane complexes exhibit a strong, sharp diffraction feature at q ∼1.19 nm−1, reflecting sheets of bilayers with nearly flat squalamine molecules sandwiched between the polar faces of the packed bilayers at a spatial periodicity of 5.3 nm. Incubation of the membrane with both Rac1 and squalamine at the isoelectric point shows a diffraction pattern nearly identical to the pattern of squalamine–membrane complexes (spacing = 5.44 nm), with no evidence of Rac1–membrane complexes, indicating that squalamine–membrane binding has occurred at the expense of such complexes (Fig. 1_B_). This striking displacement of Rac1 from anionic membranes by squalamine is confirmed in molecular dynamics simulations based on a coarse-grained model of this system (Fig. 1 C–E, SI Materials and Methods, and Movies S1, S2, S3, and S4). At concentrations high enough for either Rac1 or squalamine to saturate the membrane, squalamine displaces 56% of all Rac1 from the membrane. Squalamine is found to exhibit much stronger electrostatic binding than Rac1 to a flat charged bilayer, thus nearly doubling the counterion release that dominates the free-energy change on binding. Together, these data illustrate a general property of squalamine. Because of its charge and shape, squalamine can electrostatically displace membrane-bound proteins and potentially influence attending intracellular processes, with the specific interactions perturbed dependent on the cellular context. We suggest that, by transiently altering the electrostatic interactions of the intracellular membranes of a host cell, we place that cell into a state that is incompatible with efficient replication of certain viruses.
Activity in Vitro.
To determine whether squalamine exhibits antiviral activity in vitro, we studied the infection of a line of human microvascular endothelial cells (HMEC-1) with dengue virus. We chose this model for several reasons. Dengue virus, which infects the microvascular and hepatic endothelium in the human disease (37), has been recently shown to engage a Rac1-dependent pathway in the endothelial cell during its entry phase (22, 38); the early fusion process between the virus and the endosomal membrane is believed to involve electrostatic interactions between the viral E protein and anionic phospholipids (39). Our hypothesis predicts that squalamine should interrupt this infection. At concentrations between 20 and 60 μg/mL, squalamine has been shown to inhibit a broad array of growth factor-induced, actin-dependent responses in endothelial cells, including cell migration, cell division, and vascular tube formation in a 3D matrix (4–11).
The HMEC-1 was inoculated with dengue virus Den V2 using a published procedure (22). At a squalamine concentration of 40 μg/mL, dengue infection was inhibited by about 60%, with complete inhibition observed at 100 μg/mL (Table 1). At these concentrations, squalamine was not cytotoxic, which was shown by phase contrast refractility, actin organization, and subsequent growth of uninfected cells after removal of squalamine. At concentrations of squalamine above 60 μg/mL, the cells detached more easily during the staining procedures, a consequence of the compound's known effect on cell adhesion (5).
Table 1.
Effect of squalamine on dengue virus infection of human endothelial cells
| Squalamine concentration (μg/mL) | Percent cells infected (percent inhibition) |
|---|---|
| 0 | 38 ± 1 (0) |
| 10 | 36 ± 4 (5) |
| 20 | 26 ± 1 (32) |
| 40 | 15 ± 1 (61) |
| 60 | 9 ± 1 (76) |
| 100 | 0 (100) |
Next, we examined the effect of squalamine on the infectivity of two viruses that cause human hepatitis, human hepatitis B virus (HBV) and human hepatitis δ-virus (HDV), on primary human hepatocytes. Monolayer cultures of primary human hepatocytes were infected with HBV using a modification of procedures described in the work by Taylor and Han (40). Squalamine effectively inhibited HBV replication in human primary hepatocytes when added either during the initial exposure of virus to the cells or at 24 h after infection (Table 2). There was no evidence of cytotoxicity associated with squalamine treatment based on the morphology of individual cells and the absence of visible damage to the physical integrity of the cellular monolayer. A similar study was performed to evaluate the effect of squalamine on the replication of HDV. Squalamine was introduced at 20 μg/mL during HDV exposure, and the effects were measured at day 7 when total RNA was extracted and assayed for HDV RNA sequences. Inhibition to 89 ± 4% was observed. With a higher concentration of 60 μg/mL squalamine, significant cell toxicity was observed. HDV was not inactivated by exposure to squalamine at the concentrations that exhibited antiviral effects (Materials and Methods).
Table 2.
Effect of squalamine on HBV infection of primary human hepatocytes
| Squalamine concentration (μg/mL) | Exposure to squalamine (h) | Percent HBV replication (percent inhibition) | Recovered RNA concentration (μg/mL) |
|---|---|---|---|
| 0 | 100 (0) | 187 | |
| 2 | −1 to +16 | 86 ± 41 (14) | 187 |
| 6 | −1 to +16 | 46 ± 10 (54) | 189 |
| 6 | +24 to +40 | 71 ± 18 (29) | 188 |
| 20 | −1 to +16 | 16 ± 6 (84) | 177 |
| 20 | +24 to +40 | 36 ± 8 (64) | 171 |
Activity in Vivo.
We selected three well-characterized animal models of viral infection: yellow fever (YF) in the Golden Syrian hamster (41), eastern equine encephalitis virus (EEEV) in the Golden Syrian hamster (42), and murine cytomegalovirus (MCMV) in the BALB/c mouse (43, 44). In each animal, viral infection of the liver and endothelium occurs. Both the YF (45) and MCMV (46) infection models have been used to evaluate human therapeutic candidates. No drug has been reported to impact survival or viremia during EEEV infection in the hamster.
Squalamine dosing regimens in mice (4–6, 9, 10), monkeys (8), and humans (17–19, 47) have been established for the control of pathological angiogenesis, and the dose-dependent toxicology for the compound is known in each of these species. The compound distributes widely throughout the body (excluding the brain) and exits the bloodstream with a half-life between 1 and 5 h. It is cleared by the liver and excreted through the biliary tract into the feces; in the mouse, squalamine's half-life in the liver is about 12–24 h. For the antiviral studies reported here, we chose a single daily dose of 10–15 mg/kg administered over 6–8 consecutive d as our starting regimen, a regimen that has been proven to be well-tolerated in previous rodent studies (e.g., cancer and ophthalmic angiopathy). In most of the studies described in this report, the compound was administered s.c.
YF.
The YF virus (YFV) is an ssRNA virus of the Flaviviridae family responsible for over 30,000 deaths worldwide (45). Although an effective vaccine exists, periodic outbreaks continue to occur, and no antiviral agent has as yet been developed to treat human YF. We conducted experiments to evaluate the efficacy of squalamine for both the prevention and treatment of YF. In the hamster, YFV directly infects the hepatocyte, causing a severe necrotizing hepatitis that reaches maximum severity around days 4–5 postinoculation (47). Serum concentrations of the hepatic enzyme alanine amino transferase (ALT) are used to monitor hepatic disease and reflect in magnitude both the hepatic pathology and hepatic viral titers (48). Viral titers in various organs peak between days 4 and 6 and progressively decrease as the adaptive immune response accelerates (48).
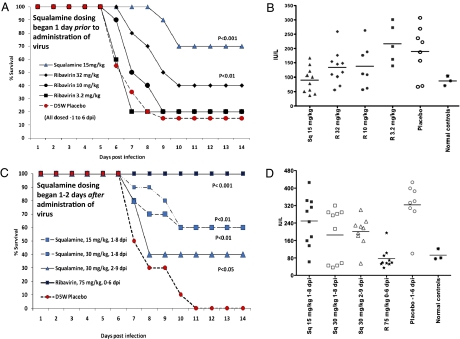
In the prevention study, squalamine administration began 1 d before virus inoculation, and daily dosing (15 mg/kg s.c.) continued through day 6 postinoculation. By day 9, 85% of the untreated cohort had died. Serum ALT measured on the untreated group was elevated on day 6, indicating hepatitis (Fig. 2_B_). Of the group administered squalamine, 100% survived through day 8, 2 d after the last day of drug administration; 70% were protected from mortality and survived through the remainder of the experiment (day 21) (Fig. 2). Serum ALT in this cohort was within the normal range, suggesting minimal virus infection of the liver. We compared the effect of squalamine with ribavirin, an antiviral agent that has been well-studied in this model (45, 48, 49). Ribavirin was administered in a two times daily dosing regimen at 3.2, 10, and 32 mg/kg through the normal i.p. route. The highest ribavirin dose protected about 40% of the cohort, whereas the lower doses were ineffective (Fig. 2). By raising the total daily dose of ribavirin (50–75 mg/kg), this compound can control infection in the hamster quite effectively (45).
Fig. 2.
Treatment of YF in Syrian hamsters. Survival of Syrian hamsters treated before (A) or after (C) viral inoculation. Dosing regimens are indicated and described in Materials and Methods. Serum ALT was sampled at day 6 postinfection for the group receiving squalamine before (B) or after (D) viral inoculation [treatment groups (n = 10) and D5W (5% dextrose) placebo (n = 20)]. All animals that had survived 14 d remained alive until day 21 and were designated as cured. [We should note that, in the treatment experiment (C), a cohort that received 15 mg/kg 1 d before virus inoculation (a control prevention study) achieved a 40% survival rate and has been omitted from the graph for the sake of clarity.]
To determine whether squalamine can treat an existing YFV infection in the hamster model, animals were infected with a lethal inoculum of virus. Then, one time daily treatment with squalamine (15 or 30 mg/kg per d s.c.) was started beginning on day 1 or 2 after viral administration and continuing until day 8 or 9, respectively. Survival was monitored, and animals that remained alive by day 21 were considered cured (Fig. 2_C_). By day 11 after infection, 100% of untreated animals had died. In contrast, 60% of the animals that had received 15 mg/kg per d (from day 1 to 8) or 30 mg/kg per d (from day 1 to 8) were cured. Serum ALT activity, measured on day 6, was lower in the treated animals compared with the untreated cohort (Fig. 2_D_). Delay of treatment (30 mg/kg per d) until day 2 still resulted in a cure rate of 40% (Fig. 2_C_). Treatment with ribavirin at its optimal dosing regimen (beginning on the day of viral infection) achieved a cure rate of 100% (Fig. 2_C_).
EEEV.
EEEV is an enveloped RNA virus of the Alphavirus family genus in the family Togaviridae for which neither an effective antiviral drug nor a licensed human vaccine is available (50). The case fatality rate is between 30% and 80% for humans and up to 95% for horses, and EEEV is regarded as a potential biodefense threat (51).
EEEV causes widespread vascular disease in the hamster, involving all of the major organs (42). Infection of the vasculature of the brain followed by direct infection of neuronal cells results in fatal encephalitis. From day 3 after peripheral inoculation, viral titers within the brain continue to increase, despite rising levels of neutralizing antibodies. Because squalamine cannot enter the brain after systemic administration, we would not expect it to directly influence EEEV infection of the hamster brain or circumvent the onset of encephalitis. However, in the hamster (but not the mouse) (52), EEEV infects the liver early after viral inoculation (42). We hypothesized that, although squalamine would not be expected to directly influence the viral infection within the brain, the substance might influence the clinical course of the peripheral disease by increasing the viral resistance of organs such as the liver and perhaps, the systemic vascular endothelium.
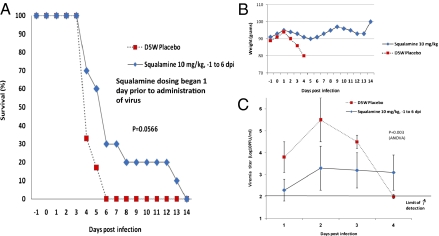
Golden Syrian hamsters were infected with a lethal s.c. inoculum of EEEV; 1 d before infection, animals received 10 mg/kg squalamine s.c., which continued daily for 6 d postinfection. Blood was drawn from the retroorbitus during the first 4 d to monitor viral concentration within the vascular space. Treatment with squalamine extended the survival of the infected animals compared with those animals receiving vehicle alone (Fig. 3_A_). Treated animals maintained body weight compared with the untreated animals (Fig. 3_B_). Initial viral titers in the blood stream of squalamine-treated animals were about 100-fold lower than the titers in animals receiving vehicle, showing antiviral activity of the compound when administered systemically (Fig. 3_C_).
Fig. 3.
Treatment of EEEV in Syrian hamsters. Survival (A) and average body weight (B) of Syrian hamsters infected with EEEV and treated s.c. with either squalamine (10 mg/kg) or placebo (D5W) on days −1 to 6 after infection. (C) Viremia of Syrian hamsters on days 1–4 after infection with EEEV that were treated with either squalamine or placebo. Error bars indicate the SDs of the means. The limit of detection for the assay was 100 pfu/mL [treatment group (n = 10) and D5W placebo (n = 6)]. P = 0.003 by one-way ANOVA.
MCMV.
MCMV is an enveloped DNA virus of the Herpeseviridae family. The dynamics of infection of MCMV have been extensively studied in the mouse (44). When introduced into the mouse i.v., MCMV initially propagates in two tissue compartments, the liver parenchyma and the vascular endothelium (44). Surprisingly, the virus that replicates in the liver does not spread throughout the body but likely is excreted into the feces. The virus that propagates within the vascular endothelium seems responsible for spread through the body. Based on the known tissue distribution of squalamine in the mouse, we hypothesized that squalamine should influence the natural history of an MCMV infection.
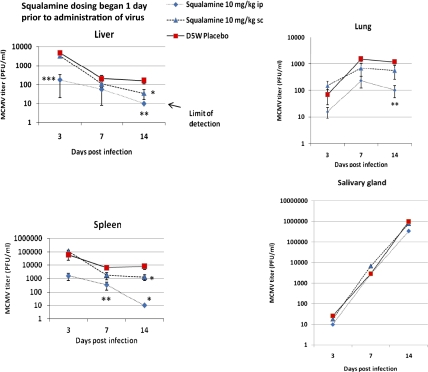
We inoculated BALB/c mice through the i.p. route with a sublethal inoculum of MCMV. Squalamine was administered at a dose of 10 mg/kg daily, beginning 1 d before infection and continuing daily through day 6, by either the i.p. or s.c. routes. An infected cohort received only vehicle. We chose to compare i.p. and s.c. routes to determine how the pharmacokinetic profile of the dosed squalamine might influence outcome. s.c. dosing in the mouse results in a slow release of compound from the injection site, reaching a peak blood and tissue concentration within 5–8 h; i.p. dosing results in a rapid rise in blood and tissue levels, peaking within 1 h of administration and generally achieving levels 10-fold higher than the s.c. route (53). For this study, on days 3, 7, and 14 postinfection, animals were euthanized, and the concentration of infectious virus present in various tissues was measured. Because dosing ended by day 6, reduction in tissue viral titers at later times was likely to be a result of the action of host defenses. Administration of squalamine through the i.p. route was the most effective, resulting in undetectable viral titers in both the liver and spleen at day 14 (Fig. 4). Dosing through the s.c. route was also effective in reducing viral titers in the liver and spleen but less effective than the i.p. route. Viral titers continued to increase in the lung between days 3 and 7 after infection and plateaued between days 7 and 14, which is likely a consequence of the animals’ immune response. Viral infection within the submaxillary salivary gland continued to increase without abatement.
Fig. 4.
Treatment of MCMV in BALB/c mice. MCMV titers from organs harvested at 3, 7, or 14 d after viral inoculation (n = 6 animals for each data point). The limit of detection was 10 pfu/mL. Error bars indicate SDs of the means. *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA compared with D5W control.
Discussion
The results presented here unambiguously show that squalamine is active in vitro and in vivo against a broad spectrum of human viral pathogens, including both RNA- and DNA-enveloped viruses. The shark seems to be surprisingly immune to viral infection (54), despite lacking a rapidly responsive adaptive immune system (55). Neither an IFN gene nor its receptor has been identified within the recently sequenced elephant shark genome (56), leaving open the possibility that the shark lacks an IFN-based antiviral defense. Curiously, the elephant shark genome also lacks identifiable retroviral sequences, which are abundantly represented in boney fish species (57), providing additional evidence that the antiviral defenses of the shark are likely to be remarkably effective. We propose that squalamine might serve an antiviral function in the shark.
We believe that squalamine exerts its antiviral effects by altering the infectivity of the tissues into which it is transported through its capacity to perturb the electrostatic potential of the cellular membranes onto which it binds. On entry into a cell, squalamine would be expected to disturb the intracellular arrangement of membrane-associated proteins positioned by electrostatic forces, possibly including some of host or viral origin that are required for the viral replication cycle. Our recently published studies that relate to the mechanism proposed in this manuscript were conducted in various cells transiently expressing specifically designed probes and proteins, and they illustrate the diverse pleiotropic effects that can be caused by the entry of this molecule into a cell; we have shown that squalamine causes displacement of a fluorescent probe bearing the cationic tail of a Ras protein from the plasma membrane (12), squalamine activates an ion channel by displacing an inhibitory cationic segment from the anionic inner face of the plasma membrane (13), and squalamine inhibits a sodium proton exchanger by displacing a cationic control sequence from the cytoplasmic face of the plasma membrane (14). Hence, by modifying the electrostatic interactions of a membrane, squalamine could influence many diverse intracellular processes, making that cell less capable of supporting the replication of certain viruses. Squalamine does not seem to be related in chemical structure or mechanism of action to any chemotherapeutic substance currently in use. By targeting host membranes, squalamine differs in mechanism from the recently described broad-spectrum antiviral compound LJ100, which seems to act by irreversibly damaging the membranes of enveloped viruses and interrupting effective entry (58). If the proposed mechanism of action of squalamine is correct, development of viral resistance would be expected to be unlikely. We have not yet optimized squalamine dosing in any of the animal models presented in this report, and we do not know the maximum prophylactic or therapeutic benefit that can be achieved in these systems. Because squalamine has been studied in humans in several phase II clinical trials for cancer and retinal vasculopathies (59, 60), can be readily synthesized (61), and has a known safety profile in man (17–19, 60), its potential as a broad-spectrum human antiviral agent should be explored.
Materials and Methods
Squalamine (as the dilactate salt) was synthesized by the route shown in the work by Zhang et al. (61) and was greater than 97% pure. A 1-mg/mL solution of squalamine in 5% dextrose and 40 mM sodium phosphate (pH 7.4) was used for dosing. For in vitro experiments, squalamine was dissolved in 99% ethanol and diluted in water as required. Details of the scattering and simulation studies are presented in SI Materials and Methods. Each of the viral experiments reported was conducted within the specific laboratory of the designated investigator using published protocols, with modifications as noted.
Full methods and associated references are available in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
M.Z. wishes to acknowledge the support and encouragement of Drs. Christopher Tseng and Heather Greenstone of the National Institute of Allergy and Infectious Extramural Antiviral Screening Program. M.Z. thanks Aaron Nelson for his careful reading of the manuscript. We thank the expert assistance of M. C. Dominguez with HMEC-1/DenV2 assays. E.L. acknowledges support through National Science Foundation Grant DMR-1006430 and allocation of computing time on Northwestern University's Quest cluster. The dengue studies were partially supported by a Conacyt (Mexico) grant (to I.M.). The studies on EEEV were supported by a grant from the National Institute of Allergy and Infectious Disease through Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research National Institutes of Health Grant U54 AIO57156 (to A.M. and S.C.W). This work was supported by National Science Foundation Grant DMR-0409769 (to G.C.L.W.) and National Institutes of Health Grant 1UO1-AI082192-01 (to G.C.L.W.).
Footnotes
Conflict of interest statement: M.Z. has filed a patent application that involves the use of squalamine for the treatment and prevention of viral infections.
*This Direct Submission article had a prearranged editor.
References
- 1.Moore KS, et al. Squalamine: An aminosterol antibiotic from the shark. Proc Natl Acad Sci USA. 1993;90:1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wehrli SL, Moore KS, Roder H, Durell S, Zasloff M. Structure of the novel steroidal antibiotic squalamine determined by two-dimensional NMR spectroscopy. Steroids. 1993;58:370–378. doi: 10.1016/0039-128x(93)90040-t. [DOI] [PubMed] [Google Scholar]
- 3.Yun SS, Li W. Identification of squalamine in the plasma membrane of white blood cells in the sea lamprey, Petromyzon marinus. J Lipid Res. 2007;48:2579–2586. doi: 10.1194/jlr.M700294-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Williams JI, Pietras RJ. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene. 2002;21:2805–2814. doi: 10.1038/sj.onc.1205410. [DOI] [PubMed] [Google Scholar]
- 5.Williams JI, et al. Squalamine treatment of human tumors in nu/nu mice enhances platinum-based chemotherapies. Clin Cancer Res. 2001;7:724–733. [PubMed] [Google Scholar]
- 6.Schiller JH, Bittner G. Potentiation of platinum antitumor effects in human lung tumor xenografts by the angiogenesis inhibitor squalamine: Effects on tumor neovascularization. Clin Cancer Res. 1999;5:4287–4294. [PubMed] [Google Scholar]
- 7.Yin M, Gentili C, Koyama E, Zasloff M, Pacifici M. Antiangiogenic treatment delays chondrocyte maturation and bone formation during limb skeletogenesis. J Bone Miner Res. 2002;17:56–65. doi: 10.1359/jbmr.2002.17.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Genaidy M, et al. Effect of squalamine on iris neovascularization in monkeys. Retina. 2002;22:772–778. doi: 10.1097/00006982-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Higgins RD, Sanders RJ, Yan Y, Zasloff M, Williams JI. Squalamine improves retinal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:1507–1512. [PubMed] [Google Scholar]
- 10.Higgins RD, Yan Y, Geng Y, Zasloff M, Williams JI. Regression of retinopathy by squalamine in a mouse model. Pediatr Res. 2004;56:144–149. doi: 10.1203/01.PDR.0000128977.55799.34. [DOI] [PubMed] [Google Scholar]
- 11.Sills AK, Jr, et al. Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature. Cancer Res. 1998;58:2784–2792. [PubMed] [Google Scholar]
- 12.Yeung T, et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 13.Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander RT, et al. Membrane surface charge dictates the structure and function of the epithelial Na+/H+ exchanger. EMBO J. 2011;30:679–691. doi: 10.1038/emboj.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selinsky BS, Smith R, Frangiosi A, Vonbaur B, Pedersen L. Squalamine is not a proton ionophore. Biochim Biophys Acta. 2000;1464:135–141. doi: 10.1016/s0005-2736(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 16.Selinsky BS, et al. The aminosterol antibiotic squalamine permeabilizes large unilamellar phospholipid vesicles. Biochim Biophys Acta. 1998;1370:218–234. doi: 10.1016/s0005-2736(97)00265-4. [DOI] [PubMed] [Google Scholar]
- 17.Hao D, et al. A Phase I and pharmacokinetic study of squalamine, an aminosterol angiogenesis inhibitor. Clin Cancer Res. 2003;9:2465–2471. [PubMed] [Google Scholar]
- 18.Bhargava P, et al. A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin Cancer Res. 2001;7:3912–3919. [PubMed] [Google Scholar]
- 19.Herbst RS, et al. A phase I/IIA trial of continuous five-day infusion of squalamine lactate (MSI-1256F) plus carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2003;9:4108–4115. [PubMed] [Google Scholar]
- 20.Pelkmans L, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 21.Pelkmans L, Helenius A. Insider information: What viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 22.Zamudio-Meza H, Castillo-Alvarez A, González-Bonilla C, Meza I. Cross-talk between Rac1 and Cdc42 GTPases regulates formation of filopodia required for dengue virus type-2 entry into HMEC-1 cells. J Gen Virol. 2009;90:2902–2911. doi: 10.1099/vir.0.014159-0. [DOI] [PubMed] [Google Scholar]
- 23.Harmon B, Campbell N, Ratner L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010;6:e1000956. doi: 10.1371/journal.ppat.1000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 25.Ahola T, Lampio A, Auvinen P, Kääriäinen L. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J. 1999;18:3164–3172. doi: 10.1093/emboj/18.11.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J Virol. 2008;82:2405–2417. doi: 10.1128/JVI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chukkapalli V, Oh SJ, Ono A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc Natl Acad Sci USA. 2010;107:1600–1605. doi: 10.1073/pnas.0908661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stansell E, et al. Basic residues in the Mason-Pfizer monkey virus gag matrix domain regulate intracellular trafficking and capsid-membrane interactions. J Virol. 2007;81:8977–8988. doi: 10.1128/JVI.00657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agirre A, Barco A, Carrasco L, Nieva JL. Viroporin-mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J Biol Chem. 2002;277:40434–40441. doi: 10.1074/jbc.M205393200. [DOI] [PubMed] [Google Scholar]
- 30.Coil DA, Miller AD. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J Virol. 2004;78:10920–10926. doi: 10.1128/JVI.78.20.10920-10926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coil DA, Miller AD. Enhancement of enveloped virus entry by phosphatidylserine. J Virol. 2005;79:11496–11500. doi: 10.1128/JVI.79.17.11496-11500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coil DA, Miller AD. Phosphatidylserine treatment relieves the block to retrovirus infection of cells expressing glycosylated virus receptors. Retrovirology. 2005;2:49. doi: 10.1186/1742-4690-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14:1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang H, Whited G, Nguyen C, Stucky GD. The directed cooperative assembly of proteorhodopsin into 2D and 3D polarized arrays. Proc Natl Acad Sci USA. 2007;104:8212–8217. doi: 10.1073/pnas.0702336104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 36.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 38.Wang JL, et al. Roles of small GTPase Rac1 in the regulation of actin cytoskeleton during dengue virus infection. PLoS Negl Trop Dis. 2010;4:e809. doi: 10.1371/journal.pntd.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stauffer F, et al. Interaction between dengue virus fusion peptide and lipid bilayers depends on peptide clustering. Mol Membr Biol. 2008;25:128–138. doi: 10.1080/09687680701633091. [DOI] [PubMed] [Google Scholar]
- 40.Taylor JM, Han Z. Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS One. 2010;5:e15784. doi: 10.1371/journal.pone.0015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesh RB, et al. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 42.Paessler S, et al. The hamster as an animal model for eastern equine encephalitis—and its use in studies of virus entrance into the brain. J Infect Dis. 2004;189:2072–2076. doi: 10.1086/383246. [DOI] [PubMed] [Google Scholar]
- 43.Cavanaugh VJ, Deng Y, Birkenbach MP, Slater JS, Campbell AE. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J Virol. 2003;77:1703–1717. doi: 10.1128/JVI.77.3.1703-1717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacher T, et al. The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe. 2008;3:263–272. doi: 10.1016/j.chom.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardin RD, et al. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virol J. 2009;6:214. doi: 10.1186/1743-422X-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- 48.Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 2007;73:140–146. doi: 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sbrana E, et al. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am J Trop Med Hyg. 2004;71:306–312. [PubMed] [Google Scholar]
- 50.Wang E, et al. Chimeric Sindbis/eastern equine encephalitis vaccine candidates are highly attenuated and immunogenic in mice. Vaccine. 2007;25:7573–7581. doi: 10.1016/j.vaccine.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrigo NC, Watts DM, Frolov I, Weaver SC. Experimental infection of Aedes sollicitans and Aedes taeniorhynchus with two chimeric Sindbis/Eastern equine encephalitis virus vaccine candidates. Am J Trop Med Hyg. 2008;78:93–97. [PMC free article] [PubMed] [Google Scholar]
- 52.Vogel P, Kell WM, Fritz DL, Parker MD, Schoepp RJ. Early events in the pathogenesis of eastern equine encephalitis virus in mice. Am J Pathol. 2005;166:159–171. doi: 10.1016/S0002-9440(10)62241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zasloff M. US Patent. 1997 6,143,738. [Google Scholar]
- 54.Villarreal LP. Viruses and the Evolution of Life. Washington, DC: ASM Press; 2005. [Google Scholar]
- 55.Adelman MK, Schluter SF, Marchalonis JJ. The natural antibody repertoire of sharks and humans recognizes the potential universe of antigens. Protein J. 2004;23:103–118. doi: 10.1023/b:jopc.0000020077.73751.76. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesh B, et al. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol. 2007;5:e101. doi: 10.1371/journal.pbio.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Basta HA, Cleveland SB, Clinton RA, Dimitrov AG, McClure MA. Evolution of teleost fish retroviruses: Characterization of new retroviruses with cellular genes. J Virol. 2009;83:10152–10162. doi: 10.1128/JVI.02546-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf MC, et al. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci USA. 2010;107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietras RJ, Weinberg OK. Antiangiogenic steroids in human cancer therapy. Evid Based Complement Alternat Med. 2005;2:49–57. doi: 10.1093/ecam/neh066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connolly B, Desai A, Garcia CA, Thomas E, Gast MJ. Squalamine lactate for exudative age-related macular degeneration. Ophthalmol Clin North Am. 2006;19:381–391. doi: 10.1016/j.ohc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Zhang XH, et al. Synthesis of squalamine utilizing a readily accessible spermidine equivalent. J Org Chem. 1998;63:8599–8603. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information