Role of β4 integrin phosphorylation in human invasive squamous cell carcinoma: regulation of hemidesmosome stability modulates cell migration (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 1.
Published in final edited form as: Lab Invest. 2011 Jul 18;91(10):1414–1426. doi: 10.1038/labinvest.2011.104
Abstract
Hemidesmosomes (HDs) are multiprotein structures that anchor epithelia to the basement membrane. During squamous cell carcinoma (SCC) invasion there is a reduction in the number of HDs, which may facilitate dissemination. Mechanisms of HD disassembly are incompletely understood. Previous work has shown that EGF-induced phosphorylation of the β4 integrin on three of its serines, S1356S1360S1364, can induce HD disassembly in normal cells. Here we examine the role of β4 integrin serine phosphorylation in SCC. We have found that around 60% of invasive cutaneous SCC show increased β4 phosphorylation on S1356 when compared to carcinoma in situ or normal tissue. To assess mechanisms by which SCC increases β4 phosphorylation we performed in vitro analyses. Compared to keratinocytes, SCC cells showed increased levels of S1356 phosphorylation in the absence of EGF, correlating with reduced HD-like structures. In addition, phospho-S1356 signal was largely segregated from other HD components. EGFR and PKC inhibitors inhibited basal levels of S1356 phosphorylation in SCC, suggesting that cells use intrinsic mechanisms to activate the EGF signaling pathway to induce β4 phosphorylation. Moreover, these inhibitors stabilized HD-like structures in SCC cells and reduced their migratory ability. Mutation of S1356S1360S1364 in SCC cells to non-phosphorylatable alanines stabilized HD-like structures and substantially reduced migration, while mutation into phosphorylation mimicking aspartate reduced HD-like structures but had no effect on migration, suggesting that serine phosphorylation function is releasing anchorage rather than promoting migration. Altogether these results suggest that β4 serine phosphorylation may have an important role during SCC invasion by destabilizing HDs and facilitating migration.
Keywords: Integrins, hemidesmosome, cell migration, invasion, phosphorylation
INTRODUCTION
Squamous-cell carcinomas (SCC) are highly invasive tumors capable of metastatis (1–3). Stratified squamous epithelia, where most of the SCCs originate from, are strongly attached to the basal lamina through hemidesmosomes (HDs), multiprotein structures that provide stability (4, 5). During wound healing or SCC invasion there is an increase in HD disassembly (6, 7). In some SCC types, HD disassembly has been shown to correlate with metastatic potential (7). For this reason, there is a considerable interest to understand the mechanisms of HD disassembly.
The mechanisms of HD disassembly are not completely understood. Studies suggest that phosphorylation of the α6β4 integrin, plays an important role in HD disassembly (8–12). The α6β4 integrin is the main organizer of HDs (13). α6β4 connects to laminin on the basal lamina and facilitates the assembly of other HD components, including plectin and BPAG1, which link α6β4 to cytokeratins (4, 5, 13).
The chain of events that leads to HD disassembly in SCC is not well understood. Growth factors might trigger the initial events. Supporting this idea, EGF and MSP have been shown to induce HD disassembly in vitro (14–16). These factors activate signaling pathways that result in β4 phosphorylation. EGF induces phosphorylation of β4 on serine and tyrosine residues several of which have been identified and shown to be involved in HD disassembly (8, 9, 10, 11, 14). Around 95% of β4 phosphorylation induced by EGF occurs on serine (8, 15), mostly on four sites, S1356, S1360, S1364 and S1424 (8, 9, 11). In normal cells, substitution of these serines with alanine impedes phosphorylation and in a cooperative manner, can inhibit EGF-induced HD disassembly (8, 9, 11). The mechanism by which β4 serine phosphorylation induces disruption of HDs is unclear, although evidence suggests that S1356S1360S1364 phosphorylation controls α6β4 /plectin interaction (11).
One possible scenario to explain reduced HDs in SCC, is that β4 phosphorylation may be altered, changing the balance towards disassembly. There is little information about β4 phosphorylation in SCC or its impact on HDs in cell migration. In this study, we analyzed β4 phosphorylation in primary SCC as well as in SCC cells in vitro. SCC frequently shows alterations in EGFR signaling (18, 19), so we analyzed phospho-S1356, a β4 residue whose phosphorylation is EGF-dependent. We found that S1356 phosphorylation in primary SCC correlates with invasiveness. In vitro analysis showed that SCC cells have intrinsic mechanisms to increase the basal level of β4 phosphorylation in the absence of EGF, reducing HD-like structures stability. Interestingly, SCC cells still use EGFR and PKC in the absence of exogenous EGF. Gefitinib, an EGFR kinase inhibitor, increased HD-like structures stability by reducing β4 phosphorylation, affecting cell migration as well. Mutation of β4 S1356S1360S1364 into alanines stabilized HD-like structures and hindered SCC migration. Our results suggest that β4 phosphorylation plays an important role in SCC progression by altering HD stability and the ability of cells to migrate. Targeting HD stability may be a method to reduce the ability of SCC to disseminate.
MATERIALS AND METHODS
Cells and reagents
Squamous cell carcinoma cell lines: A431 cells were obtained from ATCC; Colo-16 were obtained from Dr. N. Hail (University of Colorado, Denver, CO); SCC-25 were provided by Dr. A. M. Mercurio (UMass Med, Worcester, MA). HaCaT keratinocytes were obtained from Dr. S. La Flamme (Albany Medical College, Albany, NY). All cells were maintained in DMEM with 10% fetal calf serum, except SCC25 that was maintained in a 1:1 mixture of DMEM and Ham’s F12 medium supplemented with 400 ng/ml hydrocortisone and 10% fetal bovine serum. Antibodies: 3E1 (β4, Chemicon); GoH3 (α6, Chemicon); rabbit anti-β4 (15); anti-BPAG1 (20); anti-plectin (Santa Cruz Biotechnology); affinity-purified phospho-specific rabbit polyclonal Ab (anti-phosphoS1356Ab) raised against β4 peptide DDVLR(pS)PSGSQ (custom-made, QBC, Hopkinton, MA).
Plasmids
β4 shRNA-A431cells: pLKO.1β4-shRNA TRCN0000057768 (Open Biosystems) against an untranslated region of β4 was used to inhibit β4 endogenous expression in A431. pLKO.1GFP-shRNA was used as control. Cells were puromycin selected. β4-PCLXSN and triple mutants: β4 integrin cDNA fused to a C-terminus myc tag was inserted in PCLXSN retroviral vector (Imgenex). A triple mutation ser→ala or ser→asp on S1356S1360S1364 was introduced into β4-myc using standard techniques (9). Retroviral particles were used to infect β4 shRNA-A431cells. As control, we used the empty vector. Cells were selected using G418 and β4-negative cells were eliminated by FACS-sorting.
Indirect immunofluorescence
Cells were stained as described previously (15, 21). Briefly, cells grown on coverslips were extracted or not with detergent buffer containing 0.5% Triton-X-100, 100mM KCl, 200mM sucrose, 10mM EGTA, 2mM MgCl2, and 10mM PIPES at pH6.8 for 1min,, then fixed using paraformaldehyde or methanol. Cells were rinsed, blocked and stained with indicated Abs and Cy2/Cy3-conjugated secondaries. Slides were analyzed using fluorescence microscopy. Analysis of HD-like structures: collected images were background-subtracted, thresholded and fluorescence integrated density per cell was calculated using ImageJ software (NIH).
In vitro wound healing assay
Cells grown to confluency were scratched with a yellow tip and new medium containing inhibitors or not was added. Image records were collected at time 0. Wounded plates were incubated for 8–24h. Images were collected and percentage of wound closure was determined by digital analysis.
Human tissue samples
Tissue sections were obtained from the Cancer Center Tissue and Tumor Bank of UMASSMed with IRB approval. FFPE sections were stained with indicated antibodies using standard immunoperoxidase technique. Frozen sections were fixed in methanol, rinsed, blocked and stained with indicated antibodies followed by cy2/cy3-conjugated secondaries. Slides were analyzed by fluorescence microscopy. Staining intensities were scored from 0 (absent) to 4 (very strong) by two observers (SL and IR). Colocalization analysis in frozen sections was performed using ImageJ (NIH) and Colocalization Threshold plugin (ref.22 and www.uhnresearch.ca/facilities/wcif/software/Plugins/colocalisation_threshold.html). Statistical analysis was performed using T-test.
RESULTS
Phosphorylation of the β4 integrin in primary human SCC correlates with invasion
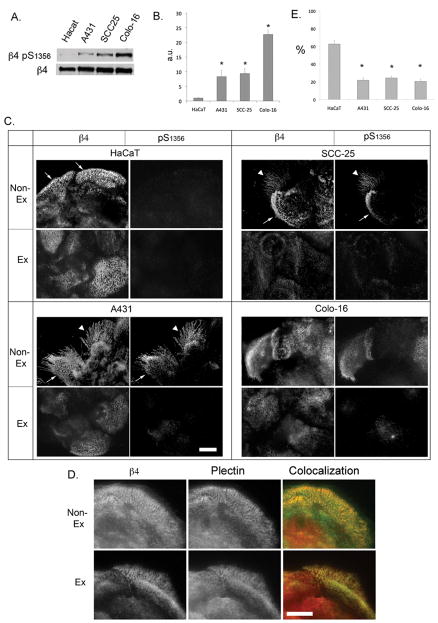
EGF signaling is frequently altered in SCC (18, 19). We have previously identified S1356 as one of the main phosphorylation sites on the β4 integrin that is phosphorylated upon EGF stimulation in a PKC-dependent manner using peptide mapping analysis, results that have been confirmed by other groups (8, 11). We and others have also shown that S1356 phosphorylation contributes importantly in HD disassembly (8, 11). Therefore, we generated a phosphospecific Ab against phospho-S1356. To assess Ab specificity, we mutated β4 S1356→A1356 to prevent Ab recognition of phospho-S1356. As shown in Fig 1A, mutation of the residue eliminated the phospho-S1356 Ab signal, confirming specificity. To confirm that the Ab acts in accordance to previous studies, we evaluated the kinetics of S1356 phosphorylation in HaCaT keratinocytes during EGF stimulation using phospho-S1356 Ab and Western analysis. As shown in Fig. 1B, there is little phosphorylation in non-stimulated cells, rapidly increasing to high levels after 5min and maintaining levels for 2h. Previous work suggests that phosphorylation of some of the serines in S1356S1360S1364 serine cluster are PKC-dependent (8, 11). Therefore we analyzed the effects of PKC stimulators and inhibitors on S1356 phosphorylation. As shown in Fig. 1C, EGF-dependent phosphorylation can be inhibited with conventional PKC inhibitor Go6976. Furthermore, PKC stimulator PMA induces S1356 phosphorylation, suggesting PKC-dependence. Altogether these results indicate that the Ab is specific and performs as expected in accordance to previous work.
Figure 1. Regulation of β4 phosphorylation (phospho-S1356) by EGF in HaCaT keratinocytes.
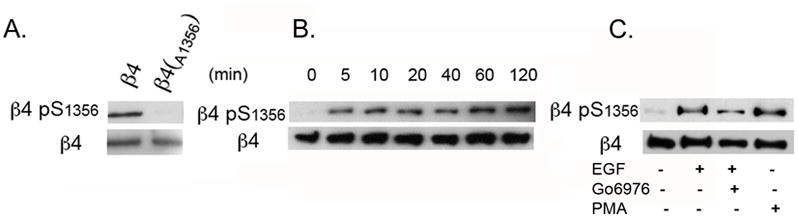
A. Anti-phospho-S1356 Ab specificity. Lysates obtained from EGF-stimulated Cos 7 cells transfected with wt β4 or mutant β4 A1356 were probed with Abs against β4 and phospho-S1356. B. Time course of S1356 phosphorylation in HaCaT keratinocytes. Cells were serum-starved and EGF-stimulated (50ng/ml) for different times, and analyzed by Western blotting using phospho-S1356 and β4 Abs. C. S1356 phosphorylation is PKC-dependent. HaCaT keratinocytes were EGF-stimulated in the presence or absence of conventional PKC inhibitor Go6976, or with PMA, then analyzed by Western blotting using phospho-S1356 and β4 Abs.
To assess the prevalence of β4 phosphorylation in human SCC, we analyzed S1356 phosphorylation in normal skin and primary SCC. Archival formalin fixed paraffin embedded (FFPE) or frozen sections were analyzed using immunohistochemistry (IHC) or immunofluorescence (IF) analysis, respectively.
FFPE sections from 20 cutaneous SCC were stained for phospho-S1356. Tissues were divided into 5 categories: normal skin (n=4); carcinoma in situ (n=7); well (n=4), moderately (n=5), or poorly differentiated (n=4) invasive carcinoma. Staining intensities were scored 0=absent to 4=very strong. In normal skin, β4 was expressed in basal cells (Fig 2A) as previously described (28). Phospho-S1356 stain was mostly negative (Fig. 2B). In carcinoma in situ, β4 was highly expressed (Fig 2C), sometimes observed in suprabasal levels, and there was little β4 phosphorylation (Fig 2D). In invasive carcinomas, β4 expression remained high, sometimes extending beyond the basal layer (Fig. 2E and G). In contrast to normal skin and carcinoma in situ, invasive SCC showed increased phospho-S1356 (Fig. 2F and H). The signal was specific because it was eliminated by incubating the primary antibody in the presence of competing phospho-S1356 peptide, or by treating the tissue with alkaline phosphatase before adding the primary antibody (results not shown). Around 60% of the invasive tumors showed high levels of phospho-S1356 with IHC scores higher than normal skin (Fig. 2I). However, we found no difference between invasive carcinomas according to their degree of differentiation. β4 phosphorylation could be found along the tumor/stroma interface (Fig. 2H), or extending deeply into the tumor (Fig. 2F) usually co-distributing with β4. The increased phosphorylation of the β4 integrin in invasive SCC suggests that phosphorylation may play a role during this tumor progression phase.
Figure 2. Phosphorylation of the β4 integrin is increased in human SCC.
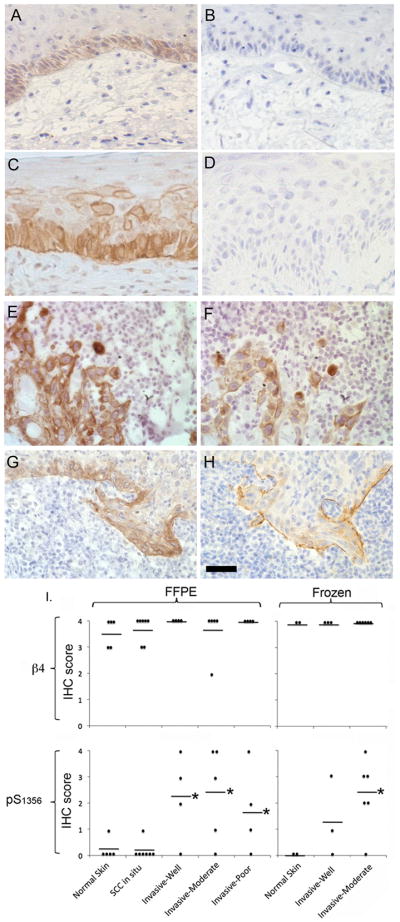
A–H. FFPE sections stained with β4 integrin (A,C,E,G) and phospho-S1356 (B,D,F,H). Abs were revealed by IHC in normal skin (A,B), carcinoma in situ (C,D), and well differentiated (E,F) or poorly differentiated (G,H) invasive cutaneous SCC. Bar=100um. I. Frequency plots of predominant IHC score for β4 and phospho-S1356 in tissue sections. FFPE (left panels) or frozen sections (right panels) were scored based on the observed staining intensity of indicated Abs, using a scale of 0 to 4. Each symbol represents a separate section and the horizontal line represents the mean score for each category. *p<0.05.
Distribution pattern of β4 phosphorylation in relation to total β4, basement membrane and other HD components in human SCC
To assess more closely the relationship between the β4 phosphorylation signals and total β4, other HD components or the basement membrane in human SCC, we analyzed 9 cutaneous SCC (all invasive, 3 well and 6 moderately differentiated) and 2 normal skin frozen sections using double IF. Consistent with IHC results, normal skin showed high levels of β4 expression and little β4 phosphorylation (Fig. 3A–C). About 60% of the invasive SCC sections showed moderate to high levels of β4 phosphorylation (Figs 3E,H,K and Fig. 2I). β4 phosphorylation varied within regions, frequently showing gaps in relation to the band-like signal of β4 (arrows Fig. 3E,F,H and I) suggesting that phosphorylation is regionally modulated. Phosphorylation was stronger at the tumor/stroma interface sometimes extending more deeply (Fig. 3E and H), similar to FFPE sections.
Figure 3. Distribution pattern of β4 phosphorylation in human SCC frozen sections: discontinuous patches that can exclude other HD components.
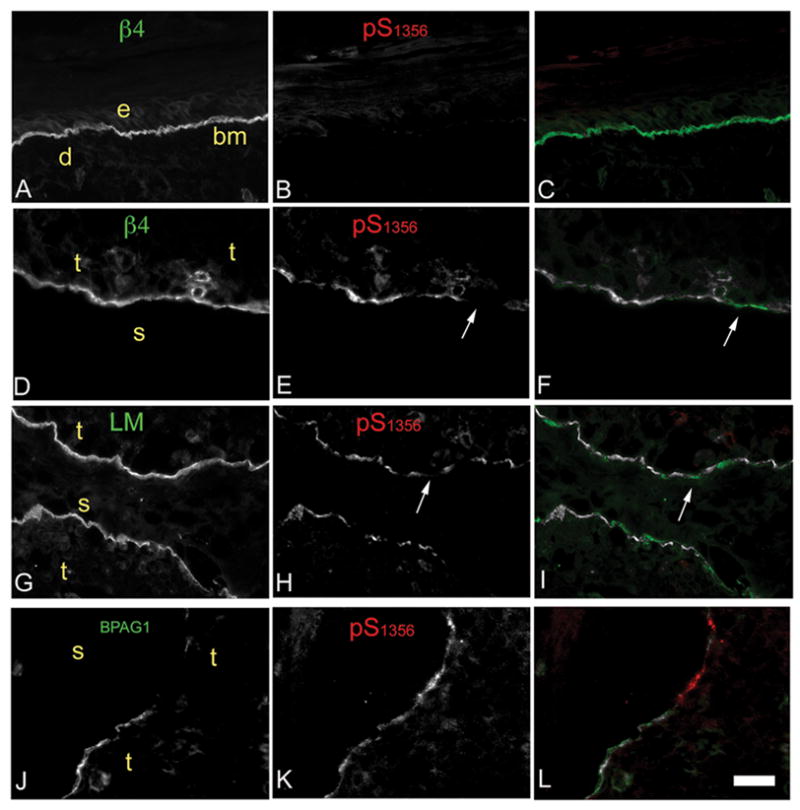
Frozen sections from skin (A–C) or invasive SCC (D–L) were dual-immunostained using Abs against (indicated within image): phospho-S1356 (red); and β4, Laminin-332 or BPAG1 (green). Colocalization in the third column was determined by threshold correlation analysis (22), which shows correlating areas above threshold in white (colocalizing) or below threshold in red or green (non-colocalizing). e, epidermis; d, dermis; bm, basement membrane; t, tumor; s, stroma. Bar=100um. Notice that β4 phosphorylation may range from mildly discontinuous (E) to highly discontinuous (H). Laminin-332 is mostly continuous and does not follow phospho-S1356 gaps (G–I). While both BPAG1 and phospho-S1356 phosphorylation are discontinuous they show segregation more frequently (J–L).
To assess whether the discontinuous pattern of β4 phosphorylation corresponds to basement membrane disruptions along the tumor/stroma interface, we analyzed colocalization of phospho-S1356 and Laminin-332. As shown in Fig. 3G–I, Laminin-332 was continuous and did not correlate with the patchy pattern of β4 phosphorylation, suggesting that β4 phosphorylation is unrelated to the basement membrane. To assess if β4 phosphorylation affects the distribution of other HD components, we evaluated colocalization of BPAG1 and phospho-S1356. The patchy pattern of both BPAG1 and β4 phosphorylation frequently excluded each other (Fig. 3J–L), suggesting that β4 phosphorylation may affect the distribution of this HD component.
Increased basal level of S1356 phosphorylation in SCC cells is associated with reduced HD-like structure stability
The higher levels of phosphorylation observed in invasive primary SCC and previous observations that HDs are reduced in SCC (7), prompted us to assess in vitro for possible mechanisms explaining these observations. Reduced HDs in SCC may be related to alterations in β4 phosphorylation. We therefore analyzed the levels and distribution of S1356 phosphorylation in three SCC cell lines: A431(23), SCC-25 (24) and Colo-16 (25) in relation to HaCaT keratinocytes, which are immortalized keratinocytes capable of differentiation in organotypic cultures and formation of HD-like structures in vitro (29). Western analysis showed that, in comparison to HaCat keratinocytes, all SCC cells had elevated basal levels of phospho-S1356 in the absence of EGF (Fig. 4A and B), suggesting intrinsic mechanisms to increase phosphorylation. We then compared the phospho-S1356 distribution between HaCaT keratinocytes and SCC cells using IF (Fig 4C and E). The cells were dual-immunostained with β4 and phospho-S1356 Abs. HaCat keratinocytes showed characteristic HD-like structures stained with the β4 Ab that largely survived extraction with detergent buffer before fixation (Fig. 4C; Non-Ex: nonextracted; Ex: extracted). The resistance to detergent buffer is mostly conferred by the connection of the β4 integrin with the cytokeratins through plectin and BPAG1 (30, 31). As expected, most of the β4 that remains after detergent extraction in HD-like structures colocalizes with plectin (Fig. 4D). Using quantitative IF, we determined that about 60% of HaCaT β4 is resistant to detergent (Fig. 4E). The phospho-S1356 signal was low in HaCaT keratinocytes (Fig. 4C). In contrast, SCC cells showed a higher phospho-S1356 signal in all three SCC cell lines (Fig 4C), which colocalized with β4 in HD-like structures and retraction fibers (Fig. 4E and 5A–C). However, β4 signal was less resistant than HaCaT keratinocytes to detergent extraction (~20%, Fig 4E). Most of the phosphorylation signal in the SCC cells was not resistant to detergent (Fig 4E). These findings are consistent with the notion that β4 phosphorylation weakens HD stability.
Figure 4. Increased basal levels of β4 phosphorylation on S1356 in SCC cells correlates with a reduction of HD-like structures.
A. Cell lysates from serum-starved HaCaT keratinocytes or SCC cells (A431, SCC-25, Colo-16) were analyzed by Western blotting using phospho-S1356 and β4 Abs. B. Bands were quantified by densitometry. C. IF analysis of total β4 and phospho-S1356 spatial distribution in HaCaT keratinocytes and SCC cells. Cells grown on coverslips were serum-starved and detergent-extracted to identify HD-associated β4 (“extracted”), or not to assess total β4 (“non-extracted”), then fixed and stained using phospho-S1356 and β4 Abs. HaCat keratinocytes show characteristic HD-like structures identified by β4 staining on the basal aspect of the cell (thin arrows). Phospho-S1356 signal can be observed in SCC cells (A431, SCC-25 and Colo-16) in HD-like structures (thin arrows) and retraction fibers (arrowheads). Bar=10um. D. Dual immunostaining analysis of detergent-extracted or non-extracted Hacat cells using anti-β4 (green) and anti-plectin antibodies (red). After detergent extraction most of the detergent-resistant β4 colocalizes with plectin in HD-like structures. Bar=10um. E. Percentage of detergent-resistant β4 in HD-like structures: Using IF analysis, the integrated fluorescence density for β4 was calculated for cells extracted or not with detergent buffer before fixation as described in Methods, and expressed as % of detergent-resistant β4 / total β4. Data shown are means±SE of >200 cells. *p<0.05.
Figure 5. Phospho-S1356 is partly segregated from other HD components in SCC cells.
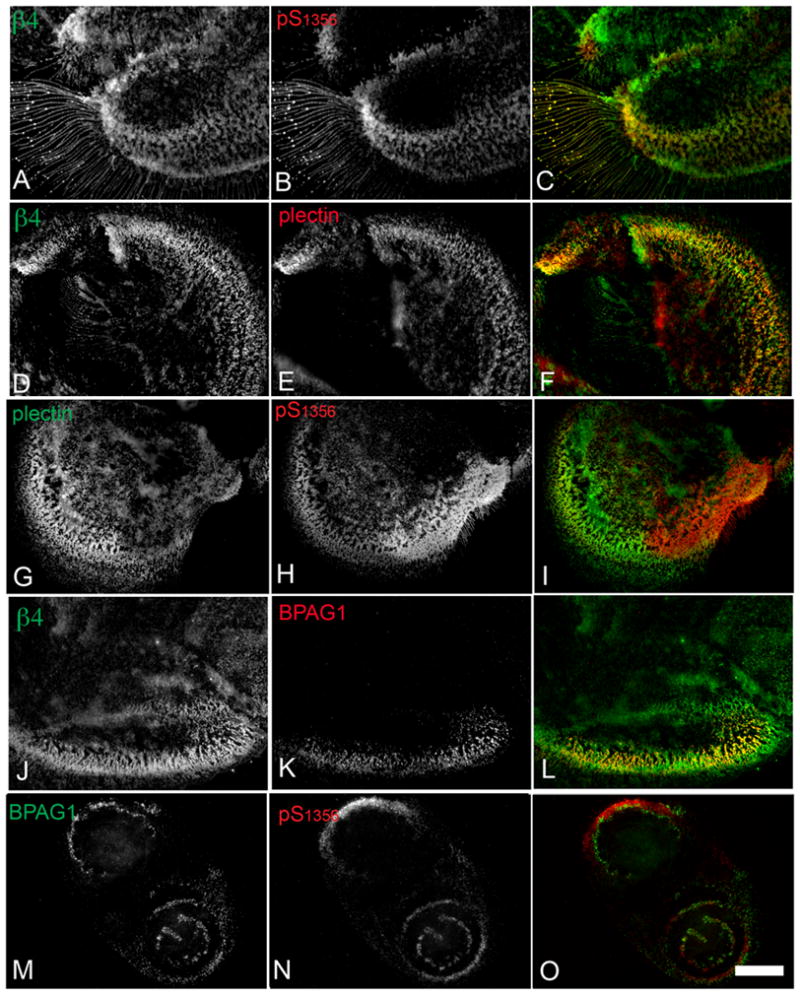
SCC-25 cells were grown in coverslips, fixed and stained for IF analysis using the indicated Ab. The third column shows colocalization in yellow. β4 and phospho-S1356 colocalize in retraction fibers and a portion of the HD-like structures (A–C). While HD component plectin colocalizes well with total β4 in HD-like structures (D–F), it is partially segregated from the phospho-S1356 signal (G–I). Notice inverse gradients between phospho-S1356 and plectin signals in colocalized areas. HD component BPAG1 colocalizes well with β4 (J–L) whereas it is highly segregated from phospho-S1356 signal (M–O). Bar= 10um.
Previous studies suggest that phosphorylated S1356S1360S1364 promotes disruption of β4-plectin interactions (11). We therefore assessed phospho-S1356 /plectin and plectin/total β4 colocalization in SCC cells. A similar pattern was found for all SCC types (exemplified by SCC25 in Fig. 5), showing that while plectin usually colocalized with total β4 in stabilized HD-like structures as previously described (Fig 5D–F; (32)), phospho-S1356 only partially overlapped with plectin and interestingly, within colocalized areas, an inverse gradient between the 2 signals was frequently observed (Fig. 5G–I), suggesting that plectin may be gradually removed as more β4 becomes phosphorylated. We also assessed colocalization of phospho-S1356 or total β4 with BPAG1, another linker of β4 with cytokeratins. While BPAG1 always colocalized with parts of the total β4 Fig 5J–L), a sharper segregation was seen between phospho-S1356 and BPAG1 (Fig. 5M–O) suggesting that other HD components are affected by phospho-S1356 as well. Altogether these results suggest that SCC cells have intrinsic mechanisms to induce β4 phosphorylation that might disrupt interactions among HD components, decreasing HD-like structure stability.
Intrinsic activation of EGFR signaling pathway in SCC cells raises phospho-S1356 basal levels affecting HD-like structure stability and cell migration
To address the mechanisms that could generate an increase in the basal levels of β4 phosphorylation in SCC, we determined whether this increase still depends on the EGF/PKC pathway. We evaluated phospho-S1356 in the presence or absence of EGFR inhibitor Gefitinib or PKC inhibitor Go6976. As shown in Fig. 6A, both Gefitinib and Go6976 inhibit phospho-S1356 basal levels in SCC cells, suggesting that activation of EGFR and PKC is still necessary in the absence of an external source of EGF and that SCC cells have intrinsic mechanisms to activate these kinases.
Figure 6. EGFR and PKC inhibitors reduce S1356 basal phosphorylation in SCC cells, stabilizing HD-like structures and inhibiting cell migration.
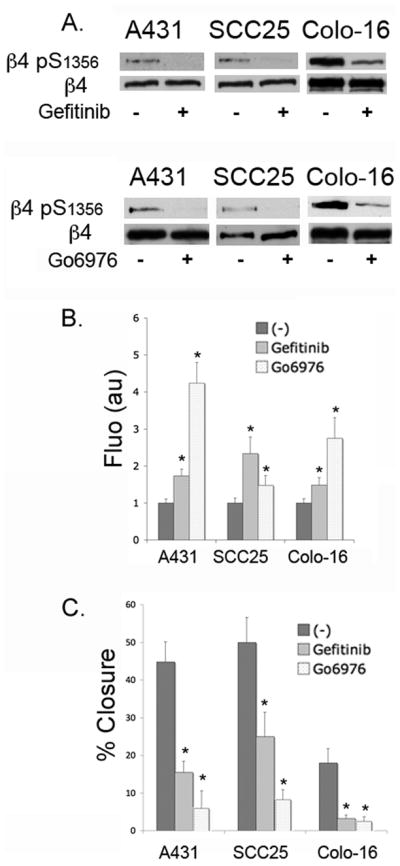
A. Serum-starved SCC cells were treated or not with EGFR inhibitor Gefitinib (10uM, upper panel) or conventional PKC inhibitor Go6976 (2uM, lower panel) for one hour, then lysed and analyzed by Western blotting using phospho-S1356 and β4 Abs. B. Effects of Gefitinib and Go6976 on HD-like structures stability. SCC cells were grown on coverslips and treated or not with inhibitor for 1h, then detergent-extracted, fixed and processed for IF using anti-β4 Ab. The integrated fluorescence density for the HD-like structures was quantified as described in Methods, and expressed in arbitrary units per cell. Data shown are means±SE of >200 cells. *p<0.05. C. Effects of Gefitinib and Go6976 on SCC cell migration using in vitro wound healing assay. Confluent SCC cells were scratched with a yellow tip. Inhibitors were added or not and the wound was allowed to close. Images captured at the start and end of the experiment were used to quantify wound closure (%). Data shown are means±SE of three independent experiments *p<0.05.
Since Gefitinib is used in some types of SCC chemotherapy (27), we addressed whether this drug could exert some of its anti-tumor effects through HD stabilization and modifying cell migration. As shown in Fig. 6Bβ4 in HD-like structures is substantially increased using Gefitinib or Go6976. We then assessed their effect on SCC migration using in vitro wound healing assays. Both inhibitors efficiently reduced migration in all cells (Fig. 6C). Go6976 effect was more pronounced, suggesting that alternative signaling pathways may converge with EGF-signaling to activate PKC. These results suggest that anti-tumor activity of Gefitinib might include inhibition of SCC cell migration through HD stabilization and inhibition of β4 phosphorylation.
Prevention of β4 phosphorylation on S1356S1360S1364 restores HD-like structures in SCC cells and slows migration
Cell migration is an important component of carcinoma invasion and metastasis (33). Considering that SCC invasion correlates with β4 phosphorylation and reduction of HD-like structures, we hypothesized that by preventing S1356S1360S1364 phosphorylation in SCC, we would replenish HD-like structures and hinder migration. Previous work in normal cells has shown that triple mutation is needed to increase HD stability (8, 11). We therefore expressed in A431 a non-phosphorylatable triple mutant β4 A1356A1360A1364-myc or a phosphorylation-mimicking mutant β4 D1356D1360D1364-myc, using wild type (wt)β4-myc as control. We first silenced β4 endogenous expression in A431 using shRNA technology (targeting β4 non-translated region). We then expressed wt and mutant β4 at equivalent levels (Fig. 7A). Using IF analysis and detergent extraction to assess HD-associated β4, we found that β4-myc and β4-A1356A1360A1364-myc incorporated well into HD-like structures (Fig 7B non-extracted vs. extracted). A quantitative analysis showed that β4-A1356A1360A1364-myc produced twice the amount of HD-like structures as β4-myc (Fig 7C). In contrast, incorporation of β4 D1356D1360D1364-myc into HD-like structures was reduced (Fig 7B, C). We addressed whether HD-like structure stabilization induced by β4 A1356A1360A1364-myc can counter EGF-induced HD disruption. As shown in Fig 7C, β4 A1356A1360A1364-myc mutant efficiently resisted EGF-induced HD-like structure disruption while β4–myc was largely mobilized. These data suggest that low number of HD-like structures in SCC can be reverted by preventing β4 phosphorylation.
Figure 7. Abrogation of β4 phosphorylation sites S1356S1360S1364 in SCC cells increases HD-like structure stability and hinders cell migration.
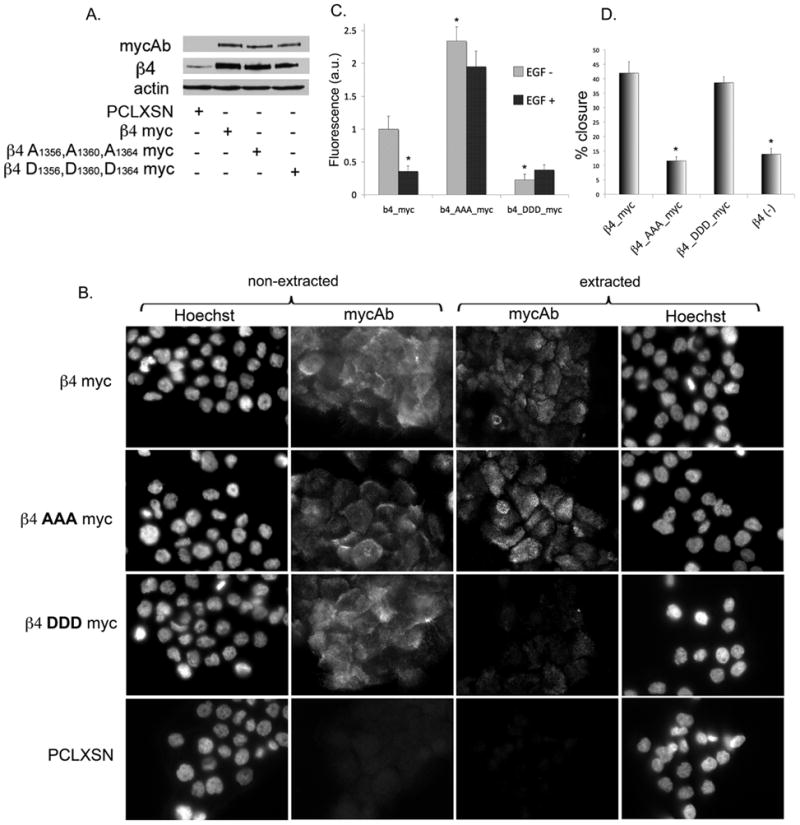
Wild type β4 myc or triple mutants containing either a Ser→Ala (β4-AAA-myc) or Ser→Asp (β4-DDD-myc) substitutions on S1356S1360S1364, were stably expressed in β4 shRNA-silenced A431 cells. A. Cells expressing the β4 constructs were analyzed by Western blotting using anti-myc Ab, showing similar level of expression. B. Analysis of HD-like structures stability in β4 phosphorylation mutants. Cells grown on coverslips were detergent-extracted or not before fixation, and processed for IF using anti-myc Ab. Notice that only wtβ4-myc and β4-AAA-myc mutant were substantially retained in HD-like structures after extraction. C. Resistance of β4 phosphorylation mutants to EGF-induced HD-like structures disassembly. Cells on coverslips were treated or not with EGF for 30 minutes before being detergent-extracted, fixed and processed for IF using anti-myc Ab to analyze incorporation into HD–like structures. Quantitation of HD-like structures by digital image analysis is expressed in arbitrary units of fluorescence integrated density per cell. *p<0.05. D. Effect of β4 mutants on cell migration using in vitro wound healing assay. Cells were assayed as in previous figure.
We then assessed the ability of β4 phosphorylation mutants to influence cell migration using wound healing assay. As shown in Fig. 7D, β4 A1356A1360A1364-myc substantially reduced A431 migration. However, the phosphorylation mimicking mutant did not affect migration. These results suggest that regulation of β4 phosphorylation can modulate cell movement through HD destabilization, although once β4 leaves HDs, serine phosphorylation provides no further advantage.
DISCUSSION
HDs disassemble during wound healing and carcinoma invasion. In some types of SCC, the reduction of HDs is also associated with high metastastic potential (7). In this paper we have explored the possibility that reduction of HDs in SCC is due to β4 integrin serine phosphorylation. Consistent with this idea we have found a correlation between an increase in β4 integrin phosphorylation and carcinoma invasion in a group of 29 primary cutaneous SCC.
This is the first report showing that β4 phosphorylation occurs in human tissue. The analysis of the β4 phosphorylation in FFPE and frozen tissues showed a clear increase in phospho-S1356 signal in ~60% of the invasive SCC. However we did not detect significant differences between the various degrees of differentiation among the invasive tumors. The phosphorylation signal was distributed unevenly, showing frequent gaps in the interface between the SCC and stroma, suggesting that the phosphorylation may be dependent on the context of the tumor location. Considering that EGFR activation is frequently seen in SCC and that β4 can be phosphorylated through EGF signaling, one possible scenario to explain regional variations of β4 phosphorylation would be a concomitant activation of EGFR within the same areas. We made efforts to detect phospho-EGFR and, although we found that SCC expressed EGFR, we were not able to detect phospho-EGFR (results not shown), a problem that has also been encountered by others studying cutaneous SCC (19). Interestingly, in other types of SCC, the existence of regional variations in phospho-EGFR has been reported (34), reminiscent of β4 phosphorylation pattern. Another possibility is that regional phosphorylation of the β4 integrin might reflect gaps in the basement membrane, a phenomenon that frequently occurs in invasive carcinoma. However, we found no relationship between the β4 phosphorylation pattern and the basement membrane, which mostly showed a continuous distribution of Laminin-332, suggesting that β4 phosphorylation is not determined by the basement membrane organization.
To gain more insight into what causes an increase in β4 phosphorylation in SCC, we used an in vitro model of SCC. Our in vitro results suggest that SCC cells acquire intrinsic mechanisms to increase β4 phosphorylation in the absence of any added growth factor. This increase of β4 basal phosphorylation in SCC cells is accompanied by a reduction of HD-like structures and segregation of the phosphorylation signal in relation to the HD components plectin and BPAG1, results that are consistent with the notion that β4 phosphorylation promotes disassembly of HDs through the disruption of interactions with other HD components (8, 11). Interestingly, we also observed segregation of BPAG1 and β4 phosphorylation signal in SCC tissues, although the complete disappearance of BPAG1 within groups of cells in SCC suggests a different mechanism of segregation whereby continuous β4 phosphorylation might eventually affect BPAG1 turnover or expression. Clearly more studies are necessary to understand long-term effects of β4 phosphorylation on other HD components. One shared mechanism that the three SCC cell lines use to increase the basal phosphorylation of the β4 integrin is the maintenance of an active EGFR in the absence of added growth factor, indicated by the inhibition of β4 phosphorylation with Gefitinib, an EGFR kinase inhibitor. An active EGFR in such conditions would suggest the possibility of autocrine secretion of EGFR ligands or sensitizing mutation/amplification of EGFR. Constitutive activation of EGFR through expression of autocrine EGFR ligands, gain-of-function mutations, or protein overexpression is found in nearly 90% of all oral cavity and head and neck SCCs (2, 27, 35). In the specific case of A431, overexpression of EGFR and autocrine stimulation with TGFα is well documented and may explain the increase in β4 phosphorylation (36, 37). Importantly, the inhibition of β4 phosphorylation by Gefitinib correlated with stabilized HD-like structures and reduced cell migration in all SCC cells, suggesting that EGF effect on migration may be in part due to the regulation of β4 phosphorylation promoting HD disassembly and allowing cells to move. Downstream EGFR, PKC still seems to mediate increased S1356 phosphorylation in SCC as shown by the inhibitory action of Go6976, a conventional PKC inhibitor (38). Therefore SCC cells may only differ from keratinocytes in some type of disregulation of the EGF signaling pathway rather than the activation of an alternative route.
Our data suggest that β4 phosphorylation might be involved in regulating the invasive ability of SCC through modulation of HD stability and cell migration. First, our results show that by inhibiting β4 phosphorylation with Gefitinib in SCC cells, there was clear stabilization of HD-like structures, which correlated with cell migration inhibition. The effect on HD-like structure stability and cell migration was somewhat stronger using Go6976, suggesting that alternative signaling pathways may converge in the activation of PKC. Second, by preventing the phosphorylation of β4 through mutation of the serine cluster S1356S1360S1364 to alanines, we were able to increase HD-like structure stability and inhibit migration even in the presence of EGF. However, a phosphorylation mimicking mutation of the serine cluster to aspartate failed to increase migration. This suggests that the role of β4 integrin serine phosphorylation in SCC migration is to release the cell from migration-hindering attachment rather than actively promote migration. It is worth mentioning that β4 per se was still necessary for cell migration, since silencing β4 reduced migration, which is in agreement with previous studies suggesting that β4 itself promotes migration and invasion through a variety of signaling pathways (39, 40). In this regard, a role of β4 tyrosine phosphorylation in the migration-promoting function of β4 has been previously shown (41–43, 44).
An interesting implication of our findings is that some of the antitumorigenic effects of EGFR inhibitors, such as Gefitinib, might be exerted through the stabilization of HDs by inhibiting β4 serine phosphorylation and consequently affecting the migration capability of the tumor which ultimately impacts metastatic potential. Future studies that evaluate the restoration of HDs and reduction of β4 phosphorylation during Gefitinib treatments could be potentially useful in assessing treatment efficiency and providing additional prognosis value.
Acknowledgments
This work was sponsored by National Institutes of Health grant numbers CA120202 (I.R.) and CA118916 (S.L.). We thank Dr. Arthur Mercurio and Dr. Don Senger for their useful discussions.
Abbreviations
BPAG
Bullous Pemphigoid Antigen
EGFR
epidermal growth factor receptor
FFPE
formalin fixed paraffin embedded
HD
hemidesmosome
IHC
immunohistochemistry
IF
immunofluorescence analysis
MSP
Macrophage-stimulating protein
SCC
squamous cell carcinoma
wt
wild type
References
- 1.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45(4–5):301–308. doi: 10.1016/j.oraloncology.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Green KJ, Jones JCR. Desmosomes and hemidesmosomes - structure and function of molecular components[Review] FASEB Journal. 1996;10(8):871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- 5.Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8(5):647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- 6.Gipson IK, Spurr-Michaud S, Tisdale A, Elwell J, Stepp MA. Redistribution of the hemidesmosome components alpha 6 beta 4 integrin and bullous pemphigoid antigens during epithelial wound healing. Experimental Cell Research. 1993;207(1):86–98. doi: 10.1006/excr.1993.1166. [DOI] [PubMed] [Google Scholar]
- 7.Herold-Mende C, Kartenbeck J, Tomakidi P, Bosch FX. Metastatic growth of squamous cell carcinomas is correlated with upregulation and redistribution of hemidesmosomal components. Cell Tissue Res. 2001;306(3):399–408. doi: 10.1007/s004410100462. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovitz I, Tsomo L, Mercurio AM. Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes. Mol Cell Biol. 2004;24(10):4351–4360. doi: 10.1128/MCB.24.10.4351-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain EC, Santos TM, Rabinovitz I. Phosphorylation of a novel site on the {beta}4 integrin at the trailing edge of migrating cells promotes hemidesmosome disassembly. Mol Biol Cell. 2009;20(1):56–67. doi: 10.1091/mbc.E08-06-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dans M, Gagnoux-Palacios L, Blaikie P, Klein S, Mariotti A, Giancotti FG. Tyrosine phosphorylation of the beta 4 integrin cytoplasmic domain mediates Shc signaling to extracellular signal-regulated kinase and antagonizes formation of hemidesmosomes. J Biol Chem. 2001;276(2):1494–1502. doi: 10.1074/jbc.M008663200. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelmsen K, Litjens SH, Kuikman I, Margadant C, van Rheenen J, Sonnenberg A. Serine phosphorylation of the integrin beta4 subunit is necessary for epidermal growth factor receptor induced hemidesmosome disruption. Mol Biol Cell. 2007;18(9):3512–3522. doi: 10.1091/mbc.E07-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol. 2008;20(5):589–596. doi: 10.1016/j.ceb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16(7):376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J Cell Biol. 1996;134(1):241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol. 1999;146(5):1147–1160. doi: 10.1083/jcb.146.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell. 2003;5(2):257–271. doi: 10.1016/s1534-5807(03)00201-6. [DOI] [PubMed] [Google Scholar]
- 17.Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J Cell Sci. 2003;116(Pt 2):387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- 18.Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–457. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 19.Rittie L, Kansra S, Stoll SW, Li Y, Gudjonsson JE, Shao Y, et al. Differential ErbB1 signaling in squamous cell versus basal cell carcinoma of the skin. Am J Pathol. 2007;170(6):2089–2099. doi: 10.2353/ajpath.2007.060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hieda Y, Nishizawa Y, Uematsu J, Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. Journal of Cell Biology. 1992;116(6):1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. Journal of Cell Biology. 1997;139(7):1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86(6):3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitrocultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 24.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures fromhuman squamous cell carcinomas. Cancer Res. 1981;41(5):1657–1663. [PubMed] [Google Scholar]
- 25.Moore GE, Merrick SB, Woods LK, Arabasz NM. A human squamous cell carcinoma cell line. Cancer Res. 1975;35(10):2684–2688. [PubMed] [Google Scholar]
- 26.Niessen CM, Hulsman EH, Oomen LC, Kuikman I, Sonnenberg A. A minimal region on the integrin beta4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. Journal of Cell Science. 1997;110(Pt 15):1705–1716. doi: 10.1242/jcs.110.15.1705. [DOI] [PubMed] [Google Scholar]
- 27.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21(2):177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 28.De Luca M, Tamura RN, Kajiji S, Bondanza S, Rossino P, Cancedda R, et al. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc Natl Acad Sci U S A. 1990;87(17):6888–6892. doi: 10.1073/pnas.87.17.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. Journal of Cell Biology. 1990;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nievers MG, Kuikman I, Geerts D, Leigh IM, Sonnenberg A. Formation of hemidesmosome-like structures in the absence of ligand binding by the (alpha)6(beta)4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the (beta)4 integrin subunit. Journal of Cell Science. 2000;113(Pt 6):963–973. doi: 10.1242/jcs.113.6.963. [DOI] [PubMed] [Google Scholar]
- 32.Niessen CM, Hulsman EH, Rots ES, Sanchez-Aparicio P, Sonnenberg A. Integrin alpha 6 beta 4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Molecular Biology of the Cell. 1997;8(4):555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annual Review of Cell Biology. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 34.Miyawaki M, Hijiya N, Tsukamoto Y, Nakada C, Kawahara K, Moriyama M. Enhanced phosphorylation of the epidermal growth factor receptor at the site of tyrosine 992 in esophageal carcinomas. Apmis. 2008;116(12):1097–1106. doi: 10.1111/j.1600-0463.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 35.Kupferman ME, Myers JN. Molecular biology of oral cavity squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39(2):229–247. doi: 10.1016/j.otc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Van de Vijver MJ, Kumar R, Mendelsohn J. Ligand-induced activation of A431 cell epidermal growth factor receptors occurs primarily by an autocrine pathway that acts upon receptors on the surface rather than intracellularly. J Biol Chem. 1991;266(12):7503–7508. [PubMed] [Google Scholar]
- 37.Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269(44):27595–27602. [PubMed] [Google Scholar]
- 38.Qatsha KA, Rudolph C, Marme D, Schachtele C, May WS. Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(10):4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91(7):949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the alpha6beta4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. Journal of Cell Biology. 1998;143(6):1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol. 2001;21(15):5082–5093. doi: 10.1128/MCB.21.15.5082-5093.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155(3):447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol. 2006;175(6):993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Dutta U, Shaw LM. SHP2 mediates the localized activation of Fyn downstream of the {alpha}6{beta}4 integrin to promote carcinoma invasion. Mol Cell Biol. 2010 doi: 10.1128/MCB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geuijen CA, Sonnenberg A. Dynamics of the alpha6beta4 integrin in keratinocytes. Molecular Biology of the Cell. 2002;13(11):3845–3858. doi: 10.1091/mbc.02-01-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuruta D, Hopkinson SB, Jones JC. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskeleton. 2003;54(2):122–134. doi: 10.1002/cm.10089. [DOI] [PubMed] [Google Scholar]
- 47.Koster J, van Wilpe S, Kuikman I, Litjens SH, Sonnenberg A. Role of binding of plectin to the integrin beta4 subunit in the assembly of hemidesmosomes. Mol Biol Cell. 2004;15(3):1211–1223. doi: 10.1091/mbc.E03-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]