Autophagy and Cartilage Homeostasis Mechanisms in Joint Health, Aging and Osteoarthritis (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 1.
Published in final edited form as: Nat Rev Rheumatol. 2011 Aug 2;7(10):579–587. doi: 10.1038/nrrheum.2011.109
Abstract
Osteoarthritis (OA) represents the most prevalent joint disease but neither preventive measures nor disease-modifying OA drugs (DMOADs) are available and a continuing need exists for safe and more effective symptom-modifying therapies. Failures of previous clinical trials on DMOADs in patients with established or advanced disease motivate investigation of mechanisms that maintain joint health. Enhancing such mechanisms may be a novel approach to OA risk reduction. Aging is one of its most important OA risk factors. However, aging of joint cartilage is a process that is distinct from the subsequent cartilage changes that develop as OA is initiated. This review is focused on mechanisms that maintain cell and tissue homeostasis, and how these mechanisms fail during the aging process. Augmentation of homeostasis mechanisms will be discussed as a novel avenue to delay joint aging and reduce OA risk.
Keywords: Cartilage, superficial zone, autophagy, aging, osteoarthritis
Introduction
Osteoarthritis (OA) is the most prevalent musculoskeletal disorder and currently affects more than 30 million Americans. With population aging, the number of arthritis patients will double by 2030 1. In 2002, approximately 500,000 hip and knee replacements were performed in the US, the majority for OA, and this is expected to double by 2015 2,3. OA causes more lower extremity dysfunction and related complications than any other disease. It compromises overall health, quality of life and life expectancy of the affected patient 4,5.
Disease-modifying OA drugs (DMOADs) are not available 6 as a large number of drug candidates in clinical trails either failed to show efficacy or were associated with adverse events 7. Current pharmacological management of OA is limited to pain medications and many OA patients use complementary and alternative medicines, with similar expenses as for pharmaceuticals 8,9.
Substantial progress has been made in identifying central pathogenesis pathways that mediate the progressive joint destruction in established OA 10-12 (Figure 1). OA clearly is a heterogeneous disease with patient subpopulations that differ risk factor profiles and in the specific initiating mechanisms. Degradation of the articular cartilage is at the center of OA pathogenesis but other joint tissues are invariably affected. The temporal and mechanistic relationship of changes in the different tissue compartments, in particular between bone and cartilage, is a subject of current discussion 13. Joint inflammation of varying severity is present in OA patients 14. Lesions in ligaments and menisci can develop simultaneously with cartilage lesions while primary traumatic injuries to menisci or ligaments can lead to secondary cartilage degradation and OA in at least 50% of cases 15.
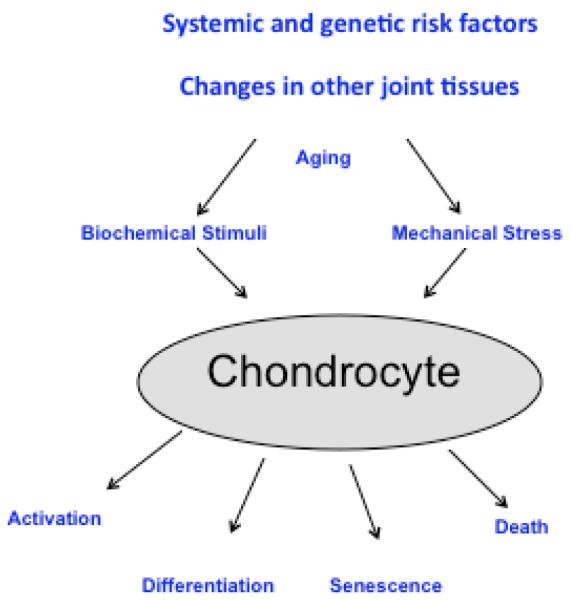
Figure 1. Pathogenesis pathways in established osteoarthritis.
Cartilage degradation is influenced by systemic risk factors (genetic risk, obesity, joint disuse) changes in other joint tissues. Once OA is established OA, cartilage lesion progression is mediated through biochemical stimuli and mechanical stress acting on chondrocytes that are altered in their gene expression patterns due to aging-related intracellular changes. Effector mechanisms that are activated include production of inflammatory mediators, matrix degradation, abnormal differentiation, and cell death.
Pathways of cartilage destruction in OA include cell death, activation and abnormal differentiation of remaining cartilage cells, degradation of the extracellular matrix, and production of inflammatory mediators (Figure 1). Within each of these pathways, critical signaling and effectors molecules have been identified 10-12. Pharmacologically targeting these pathways has been successful in animal models. Examples of classes of drug candidates that showed DMOAD activities in animal models include inhibitors of matrix degrading enzymes (MMPs, aggrecanases), inhibitors of inflammatory mediators (IL-1, TNF, COX-2, iNOS), growth factors (BMP-7, FGF-18), drugs targeting bone (bisphosphonates, calcitonin, osteoprotegerin), kinase inhibitors, lubricants (hyaluronic acid, lubricin), and vitamins. Nutraceuticals such as glucosamine, chondroitin sulfate, and avocado extracts also showed efficacy in these models. Explanations for the failure to translate this large number of treatment candidates into clinical applications are being discussed and focus on the pathogenesis pathways and molecular targets, the predictive value of animal models, the selection of patients for clinical trials, and endpoints 16.
New perspectives discussed here are mechanisms that maintain joint health, how such mechanisms may decompensate with aging and in individuals at risk for early onset of OA. A hypothesis is proposed for augmentation of homeostasis mechanisms to maintain joint health and decrease the risk of developing OA.
Aging and OA-related changes in cartilage begin at the superficial zone
Joint health is dependent on normal structure and function of all joint tissues, including cartilage, subchondral bone, ligaments, menisci, synovium, and synovial fluid. OA is a whole joint disorder and manifests with loss of joint cartilage, subchondral bone remodeling, osteophyte formation, synovial hyperplasia, changes in synovial fluid, fibrosis of joint capsule and changes in menisci and ligaments. Among the joint tissues, articular cartilage is the most susceptible to damage and shows the most profound aging-related changes. With advancing age, articular cartilage undergoes changes in extracellular matrix structure, mechanical properties, as well as changes in chondrocyte survival, mitotic, and synthetic activity. Importantly, these changes differ in many respects from those seen in OA, which is characterized by cell activation with increased proliferation and gene expression 17-22.
It is now recognized that disruption of the articular surface or superficial zone (SZ) is a key triggering event for the chronic and progressive extracellular matrix remodeling process that ultimately manifests as OA 19,23-30. Based on this critical role of the SZ, this review is focused on mechanisms that are required for SZ homeostasis and the earliest events that lead to its disruption.
The principal functions of articular cartilage are to provide the surface for movement of the two articulating bones and to dissipate mechanical load 31. These biomechanical functions are enabled by the unique structure of the cartilage SZ, which spans the first 10-20% of full thickness articular cartilage and forms a fluid-tissue interface in the synovial cavity. During normal joint use, the cartilage SZ is exposed to compressive and shear forces and it is able to absorb normal levels of mechanical stress without the occurrence of wear because of its unique composition and properties. The outermost layer of molecules on the cartilage surface contains the joint lubricants lubricin, also named superficial zone protein (SZP), hyaluronic acid, and phospholipids. In cartilage, the production of lubricin is a specific function of cells in the SZ but not cells in the mid and deep zones. The repertoire of SZ chondrocyte proteins includes several other secreted and cellular proteins that are distinct from those produced by cells in the mid and deep zone 32,33.
An important more recently discovered feature of the SZ is that it contains the majority of mesenchymal stem cells in adult cartilage 34-36. Conceptually, the presence of stem cells would endow the SZ with the capacity for self-renewal, which may be required to sustain its integrity under exposure to mechanical stress.
The SZ can be compromised by excessive acute or chronic mechanical stress and by aging-related declines in cell function or cell viability. Results from in vitro and animal models demonstrate that the SZ is more susceptible to damage induced by mechanical stress than the other cartilage zones 37. Once the SZ is disrupted, cartilage cells are activated and, through the production of matrix degrading enzymes, lesions expand in depth and diameter, subsequently causing joint inflammation, pain, and joint dysfunction, the major manifestations of OA. Thus, understanding mechanisms that maintain SZ integrity, and the causes of their failure, will identify approaches to maintain joint health.
Loss of cells, via apoptosis and other types of cell death are among the major changes that occur in the SZ due to aging and exposure to mechanical stress. The number of chondrocytes near the articular surface of macroscopically normal cartilage from human femoral condyles decreases by ~50% between age 20 and 90 30 and this is associated with biomechanical softening of cartilage 38.
Recent findings advanced concepts on the maintenance of SZ cell survival and regulation of the unique differentiation status of SZ cells (Figure 2). The chromatin protein HMGB2 is uniquely expressed in SZ cells in human and animal cartilage 39. With advancing age, there is a reduction of cells expressing HMGB2 and a reduction in SZP. Mice with deletion of the Hmgb2 gene show increased SZ cell death and reduced SZP production. The onset of degenerative changes in the SZ occurs at an earlier age in these modified mice and is followed by a more severe OA-like joint pathology 39. The major functions of HMGB2 are to support SZ cell survival through interactions with beta-catenin signaling 40 and to control the unique gene expression profile and differentiation status of the SZ cells, including adult stem cells (Figure 2).
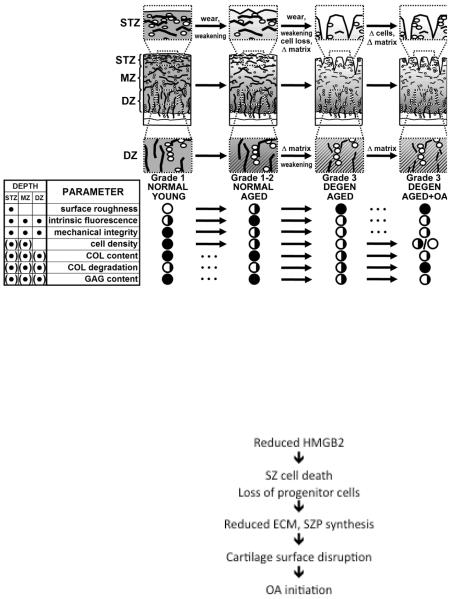
Figure 2. Mechanisms of cartilage superficial zone disruption.
A. The normal organization of extracellular matrix and cells is first disrupted in the cartilage superficial zone, leading to biomechanical weakening and cell loss. Once the superficial zone is disrupted tissue destruction extends into the deeper zones of articular cartilage.
B. Molecular changes that precede cell and extracellular matrix destruction involve the chromatin protein HMGB2 is uniquely expressed in the cartilage superficial zone. It supports chondrocyte survival and regulates the specific differentiation status of SZ cells, including progenitor cells. Aging-related loss of HMGB2 expression or deletion of the Hmgb2 gene leads to death of SZ cells and reduced synthesis of the important lubricant superficial zone protein (SZP). This causes the initial disruption of the cartilage SZ and initiates OA pathogenesis pathways shown in Figure 1.
Cartilage homeostasis: autophagy and the unfolded protein response (UPR)
Cartilage biomechanical function is dependent on normal tissue structure, which is maintained by the presence of an appropriate number of cells with normal biosynthetic function (Figure 3). Cartilage homeostasis is also supported by mechanical loading within physiological range. Cellular homeostasis is dependent on intracellular mechanisms that maintain functional organelles and macromolecules that are required for cell survival and normal biosynthetic function. In tissues with a high rate of cell turnover, cellular constituents are continuously renewed. In contrast, cartilage is a postmitotic tissue with very low and barely detectable rates of cell replication. Cells in such tissues depend on autophagy as a principal mechanism that removes damaged and dysfunctional organelles and macromolecules 41. The unfolded protein response (UPR), also called endoplasmic reticulum (ER) stress response, is a related mechanism that assures normal protein folding during synthesis in the ER 42-45. In cells exposed to ER stress the activation of the UPR can initiate autophagy to remove misfolded proteins and this promotes cell survival 46. Consequences of abnormal autophagy and UPR are deficiencies or dysfunctions in secreted molecules, inflammation, and cell death (Table 1). The significance of these control mechanisms is clearly demonstrated by observations that their dysfunction accelerates development of aging-related diseases 47,48 and this has led to the identification of new therapeutic targets 49,50.
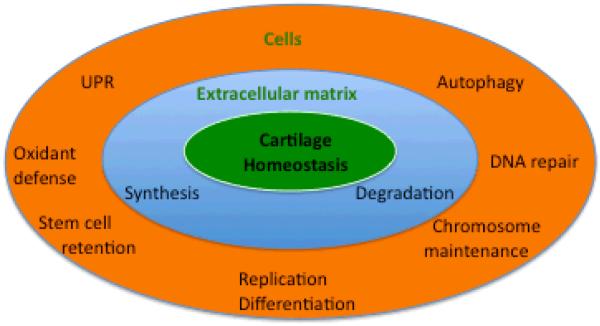
Figure 3. Cartilage homeostasis.
Cartilage homeostasis depends on normal extracellular matrix structure, which is maintained by a balanced rate of synthesis and degradation. Cellular homeostasis requires defense mechanisms that protect cells against oxidants and prevents DNA mutations and telomeric DNA damage. The UPR ascertains production of normally folded proteins and prevents protein dysfunction and aggregation. Autophagy serves roles in nutrient and energy homeostasis and is essential for the removal of dysfunctional cellular organelles and macromolecules. Normal function of cellular homeostasis mechanisms ascertains a physiological rate of cell replication, retention of adult stem cell populations and normal differentiation.
Table 1.
Consequences of normal and defective autophagy and UPR.
| Normal Autophagy and UPR | Defective Autophagy and UPR |
|---|---|
| Maintenance of functional organelles | Dysfunctional proteins and organelles |
| Functional proteome | Mitochondrial dysfunction, ROS production |
| Normal cell survival and function | DNA damage |
| Abnormal gene expression | |
| Cell death |
Autophagy is a lysosomal degradation pathway that is essential for survival, differentiation, development, and homeostasis 51. However, in certain physiological and pathological conditions, it can also lead to a form of cell death that is characterized by cytoplasmic vacuolation and is termed type II programmed cell death or cell death by autophagy 52-56. Stressors such as nutrient and energy deprivation, reactive oxygen species, or hypoxia, induce autophagy. The morphological hallmark of autophagy is the formation of the sequestering vesicle, the autophagosome. The molecular components of the autophagy machinery, the Atg genes, were first identified in yeast, and corresponding homologues have been identified in higher eukaryotes. Among the Atg genes, Atg1, Atg6, Atg8 (ULK1, Beclin1, and LC3 in mammals, respectively), and Atg5 are four major regulators of the autophagy pathway. A key regulator of autophagy initiation is the target of Rapamycin complex 1 (TORC1) (Figure 4). High nutrient availability leads to activation of TORC1 to stimulate protein synthesis through activation of p70s6K and the eukaryotic initiation factor 4E binding proteins (4E-BP1). At the same time, TORC1 inhibits autophagy by phosphorylating ULK1 57. ULK1 transduces pro-autophagic signals to autophagosome formation 45. Activation of ULK1 through inhibition of TORC1 stabilizes complex formation with FIP200 and Atg13 and leads to subcellular redistribution to initiate the formation of autophagosomes. First, an isolated double membrane structure (phagophore) is formed (nucleation) and this is driven by the Beclin 1 associated class III PI3 kinase with phosphatidylinositol3-phosphate-containing vesicles 58,59. Substrate recognition occurs during this process. The phagophore undergoes elongation and completion driven by two ubiquitin-related conjugation systems, the LC3-phosphatidylethanolamine conjugation of LC3, resulting in the formation of the autophagy marker LC3-II, and the Atg12 and Atg5 conjugation. The autophagosome that is enclosed by double or multilayered membranes then fuses with the lysosome. The enclosed cargo is degraded and the constituents are released and re-utilized.
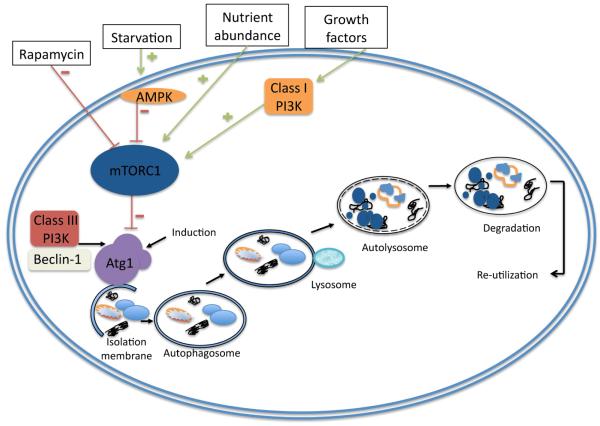
Figure 4. Regulation and execution of autophagy.
The mTORC1 complex is the central regulator of autophagy as it inhibits Atg1, the initiator of autophagy. mTOR integrates input from growth factor pathways, and from mechanisms that sense hypoxia, energy and nutrient status. Nutrient deficiency or low energy status (high AMP concentrations) or drugs such as rapamycin cause mTOR inhibition and Atg1 activation. Autophagy then proceeds through a series of steps including membrane nucleation, elongation, formation of the autophagosome, fusion with the lysosome and degradation of the cargo and recycling of the resulting molecular constituents. When mTOR is active it forms a complex with Atg1 (mammalian ULK1), FIP200 and Atg13 and phosphorylates ULK1 and Atg13 to repress autophagy. mTOR inhibition leads to Ulk1 dissociation and initiation of the autophagy cascade. First an isolated double membrane structure (phagophore) is formed (nucleation) and this is driven by the Beclin 1 associated class III PI3 Kinase with phosphatidylinositol3-phosphate-containing vesicles. Substrate recognition occurs during this process. The phagophore undergoes elongation and completion driven by two ubiquitin-related conjugation systems, the LC3-phosphatidylethanolamine conjugation of LC3 (resulting in the formation of the autophagy marker LC3-II) and the Atg12 and Atg5 conjugation. The autophagosome that is enclosed by double or multilayered membranes then fuses with the lysosome. The enclosed cargo is degraded, the constituents are released and re-utilized.
Autophagy occurs at low basal levels in mammalian cells to perform homeostatic functions such as facilitating protein and organelle turnover 60. Loss of this basal function leads to deposition of aggregation prone proteins that stimulate production of reactive oxygen species (ROS). Both ROS accumulation 61 and TORC1 activation 62 are associated with accelerated aging and development of age-associated pathologies. Conversely, reduction of ROS levels with antioxidants or as a result of TORC1 inhibition causes an extension of life span 63.
Autophagy also prevents accumulation of defective mitochondria that produce high levels of ROS 41. By controlling these detrimental processes, autophagy plays an important anti-aging function and reduces aging-associated cell death, dysfunction, and disease. Genetic studies in model organisms support the importance of autophagy in physiological and pathological events. Loss of autophagy genes leads to neurodegeneration, cardiomyopathies, abnormalities in skeletal development, and death and is associated with the accumulation of cytoplasmic protein aggregates 64.
In articular cartilage, a post-mitotic tissue characterized by a very low rate of cell turnover 65, autophagy would appear to be essential to maintain normal cell function and survival as it removes aggregate-prone or misfolded proteins, dysfunctional organelles, including mitochondria, peroxisomes, and ribosomes 66. Mitochondrial dysfunction has been demonstrated in OA and is associated with excessive oxygen radical production 67. Furthermore, while autophagy changes in various models and tissues with aging 68, this has not been sufficiently investigated in articular cartilage.
Our recent study was the first to demonstrate that autophagy is a constitutively active and apparently protective process for the maintenance of articular cartilage homeostasis. The cartilage SZ shows the strongest expression of all autophagy regulators examined thus far, including ULK1, Beclin1, LC3. Human OA and aging-related and surgically-induced OA in mice are associated with a reduction and loss of ULK1, Beclin1, and LC3 expression in articular cartilage 69. Furthermore, the reduction of these key regulators of autophagy is accompanied by increased apoptosis. These observations in cartilage are consistent with the notion that the basal autophagic activity decreases with age, thus contributing to the accumulation of damaged macromolecules and susceptibility to aging-related OA 70.
Enhancing homeostasis mechanisms to decelerate joint aging
Changes in cartilage cells and extracellular matrix are an inevitable consequence of aging, but not all older individuals are diagnosed with OA or have typical OA pathology at autopsy. These aging-related changes have been described as ‘normal aging’, but this does not imply normal tissue function and, importantly, they have adverse consequences as they increase the risk for OA. The onset of aging-related changes in human cartilage begins in the fifth decade 29 and with progression can ultimately lead to overt OA. In combination with other risk factors, such as acute or chronic mechanical injury, obesity, or malalignment, the aging-related changes may lead to an earlier onset or development of a more severe form of OA. Decelerating aging-related changes, thus, has potential to reduce risk of early OA development.
The principal aging-related changes in cartilage are similar to those in other tissues and include compromised cellular biosynthetic capacity and cell death. The progressive accumulation of damaged macromolecules and organelles in somatic cells, leading to the decreased ability of cells to function normally and survive, has been identified as a common mechanism in various tissues and abnormal protein aggregation and formation of characteristic pathological structures are central features of aging-related diseases 71.
Autophagy and UPR, which maintain normal cell function and survival, are regulated by signaling pathways that control longevity in model organisms. Recent evidence indicates that autophagy is required for life span extension in various model organisms, and that numerous autophagy-related genes or proteins are directly regulated by longevity pathways 72. The mechanisms by which autophagy is lost with aging, reduced expression of autophagy proteins or failure of lysosomal hydrolases 73,74, result in an increase of toxic protein products and slow clearance of autophagosomes 75. The effects of oxidative stress on the insulin receptor-signaling pathway seem to play a critical role in decreased autophagy in aged organisms 76. The signaling network involving longevity factors SIRT1, mTOR, FoxO3, NF-κB, and p53 regulate autophagy. mTOR and NF-κB are repressors of the autophagy pathway under input signals of stress and inflammation, while SIRT1, a stress resistance and longevity factor, and FoxO3, a major regulator of cellular metabolism, proliferation, and oxidant stress resistance, enhance autophagy 77.
Conceptually, intervening at a critical level in the regulation of autophagy or modulating general longevity pathways has potential to decelerate specific aging effects that increase the risk for development of disease. Model organisms are being used at the first stage of in vivo testing of interventions that have potential to delay aging-related dysfunction and extend life span 78. Candidate interventions in diverse chemical and functional categories have been considered and these include pharmaceuticals, nutriceuticals, hormones, vitamins, anti-oxidants, chelators, and extracts from various natural materials. Caloric restriction also has been shown to extend life span in various species, including mice, rats, and dogs 79. Life span extension in dogs was associated with reduced severity of hip OA 80. Discussion continues on mechanisms that mediate effects of caloric restriction but attenuation of oxidative damage and changes in insulin-like signaling are likely to be important. Autophagy is required for the beneficial effects of caloric restriction in some models 81.
An interventions testing program, coordinated by the National Institute on Aging is currently evaluating more than 10 different agents for life span extension in populations of genetically heterogeneous mice 82. Results published thus far indicate that the lipoxygenase inhibitor nordihydroguaiaretic acid, aspirin 83, and rapamycin 84, but not resveratrol or simvastatin 85 prolong life span. The study on rapamycin is remarkable as it demonstrated life span extension by 28% in males and 38% in females even when treatment was initiated in mice as late as 20 months, corresponding to approximately age 60 in humans. While these observations are encouraging, it is currently unknown whether therapies that prolong life span also prevent aging related disease in these model organisms. Translation into human applications poses additional challenges, in particular in regards to safety. Rapamycin may not be feasible for long-term administration as it may cause adverse events due to its potent immunosuppressive and anti-proliferative activities 86. Alternative small molecules that activate autophagy in mTOR-dependent and mTOR-independent pathways have been identified and this includes compounds that are already in clinical use 87. Glucosamine, which has a long history of use as a dietary supplement, and can activate both, the UPR and autophagy 88-91. In liver cells the UPR activation was related to a change in hexosamine pathway flux 88, and, interestingly, the induction of autophagy appeared to occur by an mTOR-independent mechanism 91.
Conclusions
Recent findings on aging-related changes in joint cartilage suggest that cellular homeostasis mechanisms decompensate, setting the stage for structural and mechanical tissue failure. Subsequent cartilage cell death and activation of abnormal gene expression patterns initiates the cartilage remodeling process that ultimately leads to OA pathology and symptoms. Mechanisms such as autophagy, UPR, or pathways that directly regulate SZ cell viability are critical for maintaining cartilage and joint health. Declines in the function of these mechanisms represent the earliest currently known aging-related changes.
Autophagy and the UPR are linked to signaling pathways that mediate aging and longevity. It can be hypothesized that general mechanisms that govern organismal or global aging also contribute to tissue-specific changes. This has been confirmed in aging-related disease of the brain, muscle, and heart. The general paradigm that emerges is that the triggers of homeostasis failure include organ specific factors but that the same homeostasis mechanisms are affected. In articular cartilage, mechanical stress had been established as a risk factor for OA and it is now apparent that it impairs autophagy.
Candidate compounds that modulate homeostasis and longevity pathways have been identified in vitro and a substantial number have been shown to prolong life span in invertebrate model organisms, mice, and other vertebrates. However, such approaches have yet to be tested in animal models for their potential to prevent failure of cartilage homeostasis and decrease risk of OA development. Animal models are available to test candidate compounds for general aging phenotypes and tissue specific pathologies, including several spontaneous OA models, such as the Hartley guinea pig or Str/ort mice 92.
Translation of measures that enhance joint health and decelerate joint aging into clinical application will require approaches that differ from those used in prior clinical trials on pharmaceuticals with potential disease-modifying activity in patients with established or advanced OA. Subsets of individuals can be identified that are at risk for OA development, for example based on the presence of risk factors such as history of joint injury, malalignment, or age. Conceptually, such patient populations may benefit from interventions that augment homeostasis mechanisms. The failure of promising pharmaceuticals in clinical trials that enrolled patients with advanced disease supports the notion that supporting homeostasis may decrease the severity or the risk of developing OA.
Clinical trials to demonstrate that such interventions reduce risk for OA development would require long-term observations in large patient populations. Biomarkers for indicative of early stages of cartilage degradation are available and could be tested in clinical studies with patients at risk for developing early age-related or mechanically induced changes in cartilage homeostasis.
In conclusion, experience from previous clinical trials indicates that in established OA it is not only impossible to reverse existing damage but even preventing disease progression is a challenge. OA prevention should be pursued as an alternative approach to contain the predicted large increase in OA prevalence as the population ages. Aging is recognized as the major OA risk factors and mechanisms and molecular targets to delay aging-related decompensation of cellular homeostasis have been identified. A concerted effort of investigators from various disciplines is required to utilize recent insight to develop effective approaches towards decelerating joint aging and OA risk reduction.
Key points.
- Previous clinical trials on disease modifying drugs enrolled patients with established or advanced OA and failed to show efficacy.
- Aging represents a main risk factor for OA and pharmacological approaches to delay cartilage aging may reduce OA risk.
- Failure of cellular homeostasis mechanisms is among the earliest events that precede cartilage cell death and extracellular matrix damage.
- Autophagy is a central cellular homeostasis mechanism that is compromised in aging cartilage.
- Approaches to enhance autophagy and other homeostasis mechanisms may protect against aging-related cell dysfunction and be effective in reducing risk for aging-related degenerative diseases such as OA.
Acknowledgments
This manuscript was supported by NIH grants AG007996, AR056026 and a grant from Cargill, Incorporated.
Footnotes
Author contributions B. Carames and M. Lotz compiled data and wrote the article. All authors made substantial contributions to the manuscript. All authors performed review/editing of the manuscript prior to submission.
LITERATURE CITED
- 1.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997-2004. Arthritis Rheum. 2008;59:481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 3.Mahomed NN, et al. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85-A:27–32. doi: 10.2106/00004623-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Guccione AA, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989;44:M112–117. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Le Graverand-Gastineau M.P. Hellio. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–1401. doi: 10.1016/j.joca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Herman CJ, Allen P, Hunt WC, Prasad A, Brady TJ. Use of complementary therapies among primary care clinic patients with arthritis. Prev Chronic Dis. 2004;1:A12. [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey SD, Spencer AC, Topolski TD, Belza B, Patrick DL. Use of alternative therapies by older adults with osteoarthritis. Arthritis Rheum. 2001;45:222–227. doi: 10.1002/1529-0131(200106)45:3<222::AID-ART252>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Loeser RF. Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact. 2008;8:303–306. [PubMed] [Google Scholar]
- 11.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valdes AM, Spector TD. The genetic epidemiology of osteoarthritis. Curr Opin Rheumatol. 2010;22:139–143. doi: 10.1097/BOR.0b013e3283367a6e. [DOI] [PubMed] [Google Scholar]
- 13.Goldring SR, Goldring MB. Bone and cartilage in osteoarthritis: is what’s best for one good or bad for the other? Arthritis Res Ther. 2010;12:143. doi: 10.1186/ar3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanzello CR, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: Molecular characterization and relationship with symptoms. Arthritis Rheum. 2010 doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 16.Le Graverand-Gastineau MP. Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr Drug Targets. 2010;11:528–535. doi: 10.2174/138945010791011893. [DOI] [PubMed] [Google Scholar]
- 17.Aigner T, Rose J, Martin J, Buckwalter J. Aging theories of primary osteoarthritis: from epidemiology to molecular biology. Rejuvenation Res. 2004;7:134–145. doi: 10.1089/1549168041552964. [DOI] [PubMed] [Google Scholar]
- 18.Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton WE, Jr., Bennion P, Yang L. Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:379–381. [PubMed] [Google Scholar]
- 20.Davidson E.N. Blaney, Scharstuhl A, Vitters EL, van der Krann PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbero A, et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.van der Krann PM, van den Berg WB. Osteoarthritis in the context of ageing and evolution: Loss of chondrocyte differentiation block during ageing. Ageing Res Rev. 2008;7:106–113. doi: 10.1016/j.arr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Poole AR, Guilak F, Abramson S. Etiopathogenesis of osteoarthritis. In: Moskowitz RW, Altman RW, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis. Lippincott, Williams & Williams; Philadelphia: 2007. [Google Scholar]
- 24.Hollander AP, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994;12:474–484. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- 26.Panula HE, et al. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis. 1998;57:237–245. doi: 10.1136/ard.57.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- 28.Saarakkala S, et al. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage. 2009 doi: 10.1016/j.joca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Temple MM, et al. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15:1042–1052. doi: 10.1016/j.joca.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Temple-Wong MM, et al. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2009;17:1469–1476. doi: 10.1016/j.joca.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter DR, et al. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004:S69–77. doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 32.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14:889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 35.Dowthwaite GP, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 36.Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otsuki S, et al. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58:1076–1085. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 38.Bae WC, et al. Indentation testing of human cartilage: sensitivity to articular surface degeneration. Arthritis Rheum. 2003;48:3382–3394. doi: 10.1002/art.11347. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi N, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–1186. doi: 10.1073/pnas.0806062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi N, et al. Chromatin protein HMGB2 regulates articular cartilage surface maintenance via beta-catenin pathway. Proc Natl Acad Sci U S A. 2009;106:16817–16822. doi: 10.1073/pnas.0904414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010;12:503–535. doi: 10.1089/ars.2009.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fribley A, Zhang K, Kaufman RJ. Regulation of apoptosis by the unfolded protein response. Methods Mol Biol. 2009;559:191–204. doi: 10.1007/978-1-60327-017-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 45.Uchiyama Y, Shibata M, Koike M, Yoshimura K, Sasaki M. Autophagy-physiology and pathophysiology. Histochem Cell Biol. 2008;129:407–420. doi: 10.1007/s00418-008-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogata M, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Austin RC. The unfolded protein response in health and disease. Antioxid Redox Signal. 2009;11:2279–2287. doi: 10.1089/ars.2009.2686. [DOI] [PubMed] [Google Scholar]
- 49.Kim DH, Davis RC, Furukawa R, Fechheimer M. Autophagy contributes to degradation of Hirano bodies. Autophagy. 2009;5:44–51. doi: 10.4161/auto.5.1.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong DS, Kelly JW. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizushima N. Physiological functions of autophagy. Curr Top Microbiol Immunol. 2009;335:71–84. doi: 10.1007/978-3-642-00302-8_3. [DOI] [PubMed] [Google Scholar]
- 52.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 53.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu L, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci U S A. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 56.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 57.Chan EY, Tooze SA. Evolution of Atg1 function and regulation. Autophagy. 2009;5:758–765. doi: 10.4161/auto.8709. [DOI] [PubMed] [Google Scholar]
- 58.Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- 59.Ohsumi Y, Mizushima N. Two ubiquitin-like conjugation systems essential for autophagy. Semin Cell Dev Biol. 2004;15:231–236. doi: 10.1016/j.semcdb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 61.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 62.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 65.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7 doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 68.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carames B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 71.Martinez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 73.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 74.Raben N, et al. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet. 2008;17:3897–3908. doi: 10.1093/hmg/ddn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vellai T, Takacs-Vellai K, Sass M, Klionsky DJ. The regulation of aging: does autophagy underlie longevity? Trends Cell Biol. 2009;19:487–494. doi: 10.1016/j.tcb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuervo AM, et al. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 77.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Hadley EC, Lakatta EG, Morrison-Bogorad M, Warner HR, Hodes RJ. The future of aging therapies. Cell. 2005;120:557–567. doi: 10.1016/j.cell.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 79.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 80.Smith GK, et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. J Am Vet Med Assoc. 2006;229:690–693. doi: 10.2460/javma.229.5.690. [DOI] [PubMed] [Google Scholar]
- 81.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 82.Nadon NL, et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) 2008;30:187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strong R, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller RA, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sofroniadou S, Goldsmith D. Mammalian target of rapamycin (mTOR) inhibitors: potential uses and a review of haematological adverse effects. Drug Saf. 2011;34:97–115. doi: 10.2165/11585040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J Biol Chem. 2010;285:11061–11067. doi: 10.1074/jbc.R109.072181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sage AT, et al. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am J Physiol Endocrinol Metab. 2010;298:E499–511. doi: 10.1152/ajpendo.00507.2009. [DOI] [PubMed] [Google Scholar]
- 89.Matthews JA, Belof JL, Acevedo-Duncan M, Potter RL. Glucosamine-induced increase in Akt phosphorylation corresponds to increased endoplasmic reticulum stress in astroglial cells. Mol Cell Biochem. 2007;298:109–123. doi: 10.1007/s11010-006-9358-5. [DOI] [PubMed] [Google Scholar]
- 90.Qiu W, Su Q, Rutledge AC, Zhang J, Adeli K. Glucosamine-induced endoplasmic reticulum stress attenuates apolipoprotein B100 synthesis via PERK signaling. J Lipid Res. 2009;50:1814–1823. doi: 10.1194/jlr.M800343-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shintani T, et al. Glucosamine induces autophagy via an mTOR-independent pathway. Biochem Biophys Res Commun. 2010;391:1775–1779. doi: 10.1016/j.bbrc.2009.12.154. [DOI] [PubMed] [Google Scholar]
- 92.Poole R, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2010;18(Suppl 3):S10–16. doi: 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]