Polyubiquitin Binding to Optineurin Is Required for Optimal Activation of TANK-binding Kinase 1 and Production of Interferon β (original) (raw)
Background: Optineurin is a polyubiquitin-binding protein of unknown function.
Result: Macrophages from mice expressing a polyubiquitin-binding defective mutant of optineurin show reduced activation of TANK-binding kinase 1 (TBK1) and reduced production of interferon β.
Conclusion: The binding of polyubiquitin to optineurin is required for optimal activation and function of TBK1.
Significance: This study identifies a new physiological role for optineurin.
Keywords: Interferon, Lipopolysaccharide (LPS), Macrophages, Toll-like Receptors (TLR), Ubiquitylation, TBK1, Optineurin, Poly(I:C)
Abstract
TANK-binding kinase (TBK1) is essential for transcription of the interferon (IFN) β gene in response to lipopolysaccharide (LPS) and double-stranded RNA, but the molecular mechanisms that underlie the activation of TBK1 are incompletely understood. Previously, we identified the NF-κB essential modulator (NEMO)-related polyubiquitin-binding protein, optineurin (OPTN), as a novel binding partner of TBK1. To determine whether the ubiquitin-binding function of OPTN is involved in regulating TBK1 and IFNβ production, we generated a mouse in which wild-type optineurin was replaced by the polyubiquitin binding-defective mutant, OPTND477N/D477N. In this study, we found that LPS or poly(I:C)-induced TBK1 activity was significantly reduced in bone marrow-derived macrophage (BMDM) from OPTND477N/D477N mice. Consistent with this, the phosphorylation of IFN regulatory factor 3 (IRF3) and the production of IFNβ mRNA and secretion were reduced. Stimulation of BMDMs with LPS triggered the phosphorylation of OPTN, which was reversed by phosphatase treatment and prevented by pharmacological inhibition of both the canonical IκB kinases (IKKα/β) and the IKK-related kinases (TBK1/IKKϵ). In contrast, LPS-stimulated phosphorylation of OPTN(D477N) was markedly reduced in BMDMs from OPTND477N/D477N mice, and inhibition of the canonical IKKs alone prevented phosphorylation, providing further evidence that ubiquitin binding to OPTN contributes to LPS-induced TBK1 activation. TBK1 and IKKβ phosphorylated OPTN preferentially at Ser-177 and Ser-513, respectively, in vitro. In conclusion, our results suggest that OPTN binds to polyubiquitylated species formed in response to LPS and poly(I:C), enhancing the activation of TBK1 that is required for optimal phosphorylation of IRF3 and production of IFNβ.
Introduction
The significance of nondegradative protein ubiquitylation events in regulating innate immunity has become increasingly evident in recent years. Ligands that bind to Toll-like receptors (TLRs)2 or the interleukin-1 receptor induce the activation of TRAF6, an E3 ubiquitin ligase that catalyzes the formation of Lys-63-linked polyubiquitin (K63-pUb) chains (1, 2), whereas LUBAC, an E3-ligase that catalyzes the formation of linear polyubiquitin (linear pUb) chains is required for signaling by TNFα (3–5). These polyubiquitin chains then regulate “downstream” signaling events by interacting with polyubiquitin-binding proteins. For example, the binding of K63-pUb chains to the TAB2 and TAB3 components of the TAK1 complex (6–8) and the binding of K63-pUb chains and/or linear pUb chains to the NF-κB essential modulator (NEMO) regulatory component of the canonical IκB kinase (IKK) complex (9–11) are thought to induce conformational changes that activate these protein kinases (12). These polyubiquitin chains may also act as scaffolds to co-localize the canonical IKK complex with its activator TAK1 and other regulatory components of this signaling pathway.
The importance of polyubiquitin binding to NEMO in regulating innate immunity has been established by the discovery of human mutations (e.g. the NEMO(D311N) mutation) (13) that abrogate binding to polyubiquitin (9, 11) and prevent activation of the canonical IKK complex or NF-κB-dependent gene transcription (14) by inflammatory stimuli. These mutations in NEMO also cause a severe immunodeficiency disease and increased susceptibility to infection by mycobacteria (13).
A polyubiquitin-binding domain present in NEMO, originally termed ABIN homology domain 2 (AHD2) (15), but later renamed the ubiquitin-binding domain in ABINs (A20-binding inhibitors of NF-κB) and NEMO (UBAN) (16), is found in four other human proteins, termed ABIN1, ABIN2, ABIN3, and optineurin (OPTN). We recently generated a knock-in mouse in which wild-type ABIN1 was replaced by ABIN1(D485N), a mutation equivalent to the conversion of Asp-311 in NEMO to Asn, and which also abrogates binding to K63-pUb or linear-pUb chains (17). Interestingly, we found that ABIN1D485N/D485N knock-in mice developed all the hallmarks of lupus. Moreover, in response to a variety of TLR ligands, TAK1-dependent signaling events, such as the activation of the canonical IKK complex and MAPKs, were enhanced in both bone marrow-derived dendritic cells and macrophages from ABIN1D485N/D485N mice, and pro-inflammatory cytokine production was similarly elevated. From this study, we were able to demonstrate that autoimmunity in the ABIN1D485N/D485N mice was driven by the hyper-activation of the TLR-MyD88 signaling pathway and, importantly, established that the interaction of ABIN1 with polyubiquitin chains limits the strength of TLR signaling and the production of cytokines (17).
The protein most closely related to NEMO is OPTN, and for this reason, it has also been termed NEMO-related protein. OPTN, like NEMO, contains a zinc finger at its C terminus, which is reported to facilitate binding of K63-pUb chains to NEMO (18). Although important roles for NEMO and ABINs have been elucidated, the physiological roles of OPTN have yet to be defined.
Previously, we identified the noncanonical IKK, TBK1 (TANK-binding kinase 1), a central kinase involved in production of type I IFNs (19–22), as a novel binding partner for OPTN and showed that it binds to the N-terminal region of OPTN (23). Type I IFNs, such as IFNβ, are induced during bacterial and viral infection and stimulate the transcription of a large set of genes important for the host defense via signaling through the type 1 IFNα/β receptor (24). The recognition of viral and bacterial components, such as dsRNA and LPS, is mediated by host pattern recognition receptors, including TLR3, TLR4, and the cytosolic RNA and DNA receptors (25–27). When engaged, these receptors stimulate the phosphorylation of IRF3, which is catalyzed by TBK1, and the related IκB kinase family member IKKϵ. This induces the dimerization of IRF3 and its translocation to the nucleus where it stimulates IFNβ gene transcription (19, 21). Studies utilizing TBK1-deficient BMDMs determined that TBK1 is specifically required for IFNβ production in response to activation of TLR3 and TLR4 (22), whereas TBK1 is not required for type I IFN production in response to RNA virus infection in BMDMs and dendritic cells (22, 28). Several forms of TBK1 are thought to be present in cells in which it is complexed to different proteins such as TANK, NAP1, and SINTBAD (29). Based largely on overexpression and knockdown studies in nonimmune cells, these complexes were thought for many years to be the major forms of TBK1 controlling the production of IFNβ (30–33). However, IFNβ production induced by viral infection was subsequently found to be unimpaired in BMDCs from TANK−/− mice (34). Thus, the critical TBK1-adaptor complex(es) responsible for activation of TBK1 and production of type I IFNs in innate immune cells remains to be defined.
Here, we investigate the physiological role of OPTN using a knock-in mouse in which the wild-type protein has been replaced by OPTN(D477N), a polyubiquitin-binding defective mutant. We find that nearly all the OPTN in BMDMs is associated with TBK1. We further show that the activation of TBK1 and the phosphorylation of its substrate, interferon regulatory factor 3 (IRF3), are reduced in BMDMs from OPTND477N/D477N mice, leading to decreased production of IFNβ mRNA and IFNβ secretion. Thus, by utilizing a genetic approach, our findings describe an in vivo role for OPTN and demonstrate the importance of polyubiquitin-binding to OPTN for optimal activation of TBK1 and IFNβ production.
EXPERIMENTAL PROCEDURES
Materials
MRT67307 (35), BI605906 (35), and 5_Z_-7-oxozeanol (36) were dissolved in dimethyl sulfoxide and stored at −20 °C as 10 mm solutions. LPS (Escherichia coli strain O5:B55) was from Alexis Biochemicals (San Diego), and poly(I:C) was from InvivoGen (San Diego). Mouse colony-stimulating factor (M-CSF) was purchased from R&D Systems (Minneapolis, MN). Fusion proteins in which glutathione transferase (GST) was linked covalently to the N termini of human interferon regulatory factor 3, OPTN, OPTN(D474N), NEMO, and NEMO(D311N), were expressed in E. coli and purified on glutathione-Sepharose. Human TBK1 and IKKβ were expressed from baculovirus vectors in Sf21 cells and purified by the Division of Signal Transduction Therapy, Medical Research Council Protein Phosphorylation Unit, University of Dundee, Dundee, Scotland, UK.
Generation of OPTND477N/D477N Knock-in Mice
The optn gene consists of 14 exons. OPTN knock-in mice were generated by TaconicArtemis (Hudson, NY) using standard protocols. Briefly, a targeting vector was constructed to introduce a point mutation corresponding to an Asp-477 to Asn mutation in exon 12 of the optn gene. This vector was used to target an ES cell line derived from C57/B16N mice, and correct targeting was confirmed by Southern analysis. Positive clones were used to generate chimeric mice. These mice were crossed to FLPe transgenic mice (Taconic Artemis), which were also on a C57/B16 background. Crossing to the FLPe strain resulted in deletion of the FLP recognition target site-flanked selectable marker (neo). Germ line transmission was confirmed by PCR of ear biopsies using the primer sets listed in supplemental Table 1, and the mice crossed away from the FLPe transgene in subsequent matings. The OPTN+/+ and OPTND477N/D477N mice were maintained on a C57BL/6 background. All mice were maintained in accordance with United Kingdom and European Union regulations. All animal work was carried out under a project license granted by the United Kingdom Home Office under the Animals (Scientific Procedures) Act 1986. Before being submitted to the Home Office, the application for the project license (60/3923), which described and justified the protocols to be used, was first reviewed and approved by the University of Dundee Ethical Review Committee. The University of Dundee is a designated establishment for breeding and for scientific procedures under the Animals (Scientific Procedures) Act 1986.
Antibodies
Antibodies against mouse OPTN (sheep number S308C, second bleed) or human OPTN (sheep number S009C, third bleed), human TBK1 (sheep S041C, second bleed), and the C-terminal peptide of mouse IKKϵ (NRLIERLHRVPSAPDV) (sheep S277C, second bleed), which were used to immunoprecipitate mouse OPTN, TBK1, and IKKϵ, respectively, were generated by the Division of Signal Transduction Therapy, the Medical Research Council Protein Phosphorylation Unit, University of Dundee, Dundee, UK. Anti-OPTN antibody (for immunoblotting) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Abcam (Cambridge, UK). Antibodies that recognize TBK1 phosphorylated at Ser-172, IRF3 phosphorylated at Ser-396, IKKα/β phosphorylated at Ser-180/Ser-181, p105/NF-κB1 phosphorylated at Ser-933, STAT1 at Tyr701 and IκBα were from Cell Signaling Technology (Beverly, MA). Antibodies that recognize JNK phosphorylated at Thr-183 and Tyr-185 and all forms of IRF3 were from Invitrogen, and an antibody that recognizes K63-pUb chains was from Millipore (clone number HWA4C4), and an antibody that recognizes ubiquitin was from Dako (Dako Glostrup, Denmark). Mouse monoclonal antibodies against α-tubulin was from Sigma. Antibodies recognizing all forms of IKKβ were from BD Biosciences, and rabbit-, mouse-, and sheep-specific secondary antibodies conjugated to horseradish peroxidase were from Pierce.
Binding of OPTN and NEMO to Polyubiquitin
To study the binding of polyubiquitin, GST-OPTN and GST-NEMO (8 μg), as well as the GST-OPTN(D474N) and GST-NEMO(D311N) mutants, were immobilized individually on glutathione-Sepharose (10 μl packed volume) and incubated for 1 h at 21 °C with 2 μg of Lys-48- and Lys-63-linked (Boston Biochem, Cambridge, MA) or linear ubiquitin oligomers (Enzo Life Sciences, Plymouth Meeting, PA) in 0.3 ml of 25 mm HEPES, pH 7.5, 1 mm EGTA, 0.5% (v/v) Triton X-100, 2 mm MgCl2 (Buffer A) plus 150 mm NaCl. The beads were washed five times with Buffer A plus 250 mm NaCl and once with Buffer A without Triton X-100, and the polyubiquitin chains associated with GST-OPTN and GST-NEMO were examined by SDS-PAGE followed by immunoblotting with anti-ubiquitin.
Cell Culture
BMDMs were obtained by differentiation of marrow from the femur and tibia of mice as described previously (37). One hour prior to stimulation with TLR agonists, aliquots of pharmacological inhibitors dissolved as 10 mm solutions in DMSO, or an equivalent volume of DMSO for control incubations, were added to the culture media. The BMDMs were then stimulated with the TLR ligands indicated in the figure legends. Primary mouse embryonic fibroblasts (MEFs) were generated by trypsinization of embryonic day 12.5 fetus from either OPTN+/+ or OPTND477N/D477N pregnant mice. MEFs were used between passages 3 and 6 for all experiments.
Immunoblotting and Immunoprecipitation
Cells were lysed and centrifuged, and the supernatant (cell extract) was removed. Cell extract (20–35 μg of protein) was subjected to SDS-PAGE and immunoblotting as described previously (35). To immunoprecipitate OPTN, 50 μg of cell extract protein was incubated overnight at 4 °C with 8 μg of the sheep anti-mouse OPTN antibody. After end-over-end rotation for an additional 60 min at 4 °C with protein-G-Sepharose (15 μl), the Sepharose beads were collected by centrifugation, washed three times with cell lysis buffer plus 0.15 m NaCl, denatured in SDS, subjected to SDS-PAGE, and immunoblotted.
Immune Complex Kinase Assays
Endogenous TBK1 was immunoprecipitated from 50 μg of cell extract protein using 1 μg of antibody (TBK1-S041C; IKKϵ-S277C). The TBK1 immune complexes were washed twice with lysis buffer and once with 50 mm Tris/HCl, pH 7.5, 0.1 mm EDTA, 10 mm magnesium acetate, and 1 mm DTT. The washed beads were resuspended in 20 μl of the same buffer containing 2 μm GST-IRF3 and 0.1 mm [γ-32P]ATP (6 μCi/μl). The protein kinase reactions were carried out for 20 min at 30 °C and terminated by the addition of 5 μl of 5% SDS. The samples were heated at 100 °C, separated by SDS-PAGE, transferred to a PVDF nylon membrane, and the membrane autoradiographed to detect phosphorylated GST-IRF3. The levels of TBK1 present in the immunoprecipitation and the GST-IRF3 present in the reactions were detected by immunoblotting.
Quantitative RT-PCR and ELISA
Total RNA was extracted from BMDMs using the RNeasy micro kit (Ambion, Austin, TX). RNA was reverse-transcribed using random and oligo(dT) primers, qScript reverse transcriptase, and the accompanying reagents (Quanta Biosciences, Gaithersburg, MD) following the manufacturer's directions. PCR mixes were assembled using the PerfeCT SYBR Green Fast mix (Quanta Biosciences). Reactions were performed with the SYBR Green (plus melting curve analysis) program on either the Bio-Rad iCycler or C1000 thermal cycler quantitative PCR system (Bio-Rad). All reactions were performed in duplicate. The primer sequences were as follows: IFNβ-FOR, 5′-GGAAAAGCAAGAGGAAAGATTGAC-3′, and IFNβ-REV, 5′-CCACCATCCAGGCGTAGC-3′; IL-12p40-FOR, 5′-TCATCAGGGACATCATCAAACC-3′, and IL-12p40-REV, 5′-TGAGGGAGAAGTAGGAATGGG-3′. The concentrations of IFNβ and IL-12p40 released into the cell culture media were determined by ELISA using the Verikine mouse IFNβ kit (PBL Interferon Source, Piscataway, NJ) or Quantikine mouse IL-12/IL-23p40 non-allele-specific kit (R&D Systems, Minneapolis, MN), respectively.
RESULTS
Generation of OPTND477N/D477N Mice
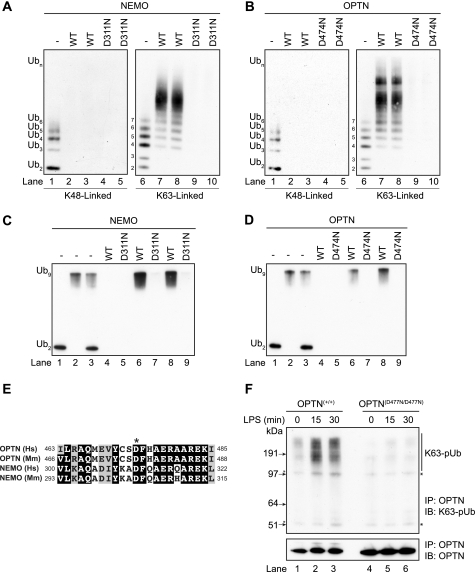
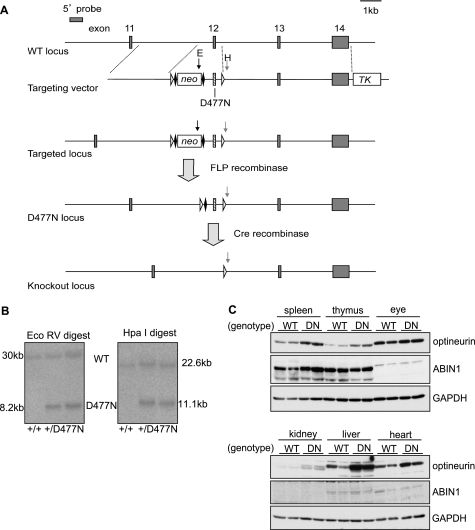
The mutation of Asp-311 of NEMO to Asn not only prevents it from binding to K63-pUb chains (9, 11) but also from binding to linear polyubiquitin chains (16, 17). Before making a knock-in mouse expressing a polyubiquitin-binding defective mutant of OPTN, we therefore investigated whether the equivalent mutation in human OPTN, Asp-474 to Asn, also prevented binding of human OPTN to polyubiquitin. When presented with a mixture of small ubiquitin oligomers linked via Lys-63, OPTN (Fig. 1B), like NEMO (Fig. 1A), preferentially captured the largest polyubiquitin chains present, even though they were trace components in the mixture of ubiquitin oligomers used in these experiments. OPTN, like NEMO, did not bind detectably to Lys-48-linked ubiquitin oligomers under the conditions tested (Fig. 1, A and B). OPTN, like NEMO, also captured a linear ubiquitin oligomer consisting of nine ubiquitins but did not capture linear di-ubiquitin in our assay (Fig. 1, C and D). An alignment of the amino acid sequences in human and mouse optineurin surrounding the critical Asp residue is compared with the corresponding sequences in NEMO in Fig. 1E. We therefore generated a knock-in mouse in which wild-type OPTN was replaced by the OPTN(D477N) mutant, equivalent to the OPTN(D474N) mutant of human OPTN (Fig. 2, A and B). The OPTND477N/D477N mice developed normally, were born at normal Mendelian frequencies, and were of normal size and weight. Interestingly, we observed that the OPTN(D477N) mutant in OPTND477N/D477N mice was expressed at a higher level than wild-type OPTN in OPTN+/+ mice in a number of tissues (Fig. 2C). A smaller increase in the expression of OPTN was also observed in BMDMs (e.g. Fig. 3A, bottom panel).
FIGURE 1.
Optineurin binds to Lys-63-linked and linear polyubiquitin chains. A and B, method used to study the binding of polyubiquitin chains has been described previously for GST-NEMO (17). Polyubiquitin chains captured by the immobilized human GST-NEMO (A) and human GST-OPTN (B) were released by denaturation in 1% (w/v) SDS, subjected to SDS-PAGE, and immunoblotted with an anti-ubiquitin antibody. Lanes 1 and 6 show, respectively, the Lys-48- and Lys-63-linked polyubiquitin preparations used in the experiment. The Lys-48-linked (lanes 2 and 3) and Lys-63-linked (lanes 7 and 8) polyubiquitin chains captured by NEMO (left-hand panel) or OPTN (right-hand panel) are shown. The OPTN(D474N) and NEMO(D311N) mutants did not bind to either Lys-48- or Lys-63-linked polyubiquitin chains under the conditions used (lanes 4, 5, 9, and 10 in the left- and right-hand panels). C and D, as in A and B except that binding to linear polyubiquitin oligomers was studied. Lanes 1–3 show the di-ubiquitin and nona-ubiquitin preparations used in the experiment. Lanes 4, 6, and 8 show that NEMO (C) and OPTN (D) bind to nona-ubiquitin but not to di-ubiquitin under the conditions used. Lanes 5, 7, and 9 show that NEMO(D311N) and OPTN(D474N) do not bind to linear polyubiquitin oligomers. Similar results were obtained in two different experiments. E, amino acid sequences in NEMO and OPTN surrounding the aspartic acid residue (*) in the UBAN that is critical for binding to polyubiquitin chains. Identities are shown by the white lettering on a black background and similarities by the black letters on a gray background. Abbreviations used are as follows: Hs, Homo sapiens; Mm, Mus musculus. F, BMDMs from OPTN+/+ or OPTND477N/D477N mice were stimulated with LPS (100 ng/ml) for the times indicated and extracted in lysis buffer containing 100 mm iodoacetamide to inhibit deubiquitylases. OPTN was then immunoprecipitated (IP) from 10 mg of cell extract protein using 40 μg of anti-human OPTN coupled covalently to protein-G-Sepharose. After 3 h at 4 °C, the immunoprecipitates were washed five times with lysis buffer plus 500 mm NaCl and once with 10 mm Tris/HCl, pH 8.0, before denaturation in SDS followed by SDS-PAGE and immunoblotting (IB) with antibodies that recognize K63-pUb chains or OPTN. The asterisks denote nonspecific bands.
FIGURE 2.
Generation of OPTND477N/D477N knock-in mice. A, strategy for generating the D477N knock-in mutation in the mouse optn gene. A vector was used to introduce a point mutation producing the D477N mutation into exon 12 of the OPTN locus and loxP sites (open triangles) either side of exon 12. The vector also contained a neomycin resistance cassette (neo) flanked by FLP recognition target sites (closed diamonds) for positive selection and a thymidine kinase gene (TK) for negative selection. The vector was used to target ES cells, which were screened by Southern blots using a probe 5′ to the targeting vector. The vector introduced an additional EcoRV site (black arrow, E) in the neo gene and an HpaI site (gray arrow, H) 3′ to the second loxP site. B, using the 5′ probe, EcoRV and HpaI digests gave bands of 30 or 22.6 kb, respectively, for a wild-type allele or 8.2 and 11.1 kb for a targeted locus. ES cells were used to generate knock-in mice from which the neo gene was removed by crossing to FLPe transgenic mice. C, comparison of the expression of the optineurin and ABIN1 proteins in various mouse tissues from OPTN+/+ (WT) or OPTND477N/D477N mice (DN) as judged by immunoblotting. Each lane represents tissue lysate (50 μg of protein) from an individual mouse (n = 2 per genotype). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control.
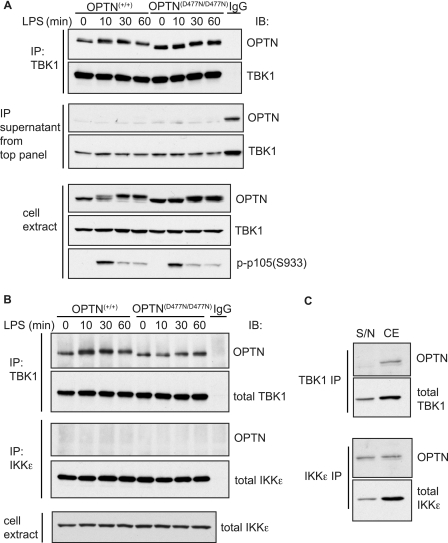
FIGURE 3.
Optineurin-TBK1 interaction does not depend on polyubiquitin binding and occurs independently of stimulation. BMDMs from OPTN+/+ and OPTND477N/D477N mice were stimulated with 100 ng/ml LPS for 10, 30, or 60 min or left unstimulated (0 min). A, top two panels, TBK1 was immunoprecipitated (IP) from the cell lysates using anti-TBK1 or control IgG, and the presence of OPTN and TBK1 in the immunoprecipitates was analyzed by immunoblotting (IB). A, middle two panels, supernatants obtained after immunoprecipitation of TBK1 were also analyzed by immunoblotting to assess the extent of depletion of OPTN and TBK1 from the cell extracts. A, bottom panels, cell lysates were immunoblotted with the indicated antibodies. B, TBK1 or IKKϵ was immunoprecipitated (IP) from 50 μg of cell extract protein using anti-TBK1, anti-IKKϵ, or control IgG, and the presence of OPTN, TBK1, or IKKϵ in the immunoprecipitates was analyzed by immunoblotting (IB). Cell extracts (30 μg) were immunoblotted to measure total levels of IKKϵ. C, amount of OPTN and TBK1 remaining in the supernatant (S/N) and cell extract (CE) obtained after immunoprecipitation of TBK1 (top two panels) or IKKϵ (bottom two panels) from the unstimulated OPTN+/+ samples. Results for A–C are representative of at least three separate experiments.
To investigate whether the endogenous OPTN(D477N) mutant in the knock-in mice could bind K63-pUb chains, we immunoprecipitated this protein from BMDM extracts. OPTN immunoprecipitated from the extracts of wild-type mice was able to immunoprecipitate the K63-pUb chains generated in response to LPS, but the OPTN(D477N) mutant could not (Fig. 1F). This establishes that the endogenous OPTN(D477N) mutant is defective in binding to K63-pUb chains.
TBK1 Interacts with OPTN
We have shown previously that TBK1 interacts with OPTN in a yeast two-hybrid analysis or co-transfection/immunoprecipitation experiments carried out in HEK293 cells (23). In this study, we showed that the endogenous OPTN was also associated with the endogenous TBK1, when the latter was immunoprecipitated from BMDM cell extracts. The interaction between TBK1 and optineurin was not affected by stimulation of the cells with the TLR4 ligand, LPS, and was similar in BMDMs from either OPTN+/+ mice or OPTND477N/D477N mice (Fig. 3A, top panel). Importantly, nearly all of the OPTN in BMDM extracts was immunodepleted by immunoprecipitation with the anti-TBK1 antibody (Fig. 3B, middle panel), demonstrating that TBK1 is a major binding partner of OPTN in these cells. In contrast, the immunoprecipitation of IKKϵ, the protein kinase to which TBK1 is most closely related, did not deplete any OPTN from the extracts (Fig. 3, B and C).
Interaction of Polyubiquitin Chains with OPTN Is Required for Optimal Activation of TBK1 and IFNβ Production by LPS or Double-stranded RNA in BMDMs
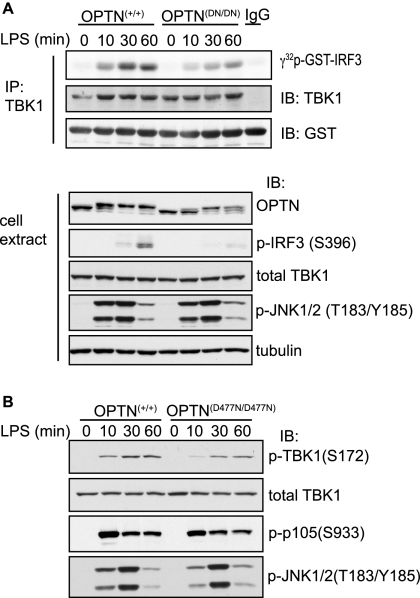
Studies in macrophages (22) and MEFs (19–21) from TBK1−/− mice have established that this protein kinase is required for the activation of interferon-regulatory factor 3 (IRF3) and the transcription of the IFNβ gene by ligands that activate TLR4 and TLR3 (19–22). We therefore studied whether these signaling pathways were affected in the OPTND477N/D477N mice. We found that the LPS-stimulated activation of TBK1 was decreased in BMDMs from the OPTND477N/D477N mice, as judged by both an immune complex kinase assay (Fig. 4A) and by immunoblotting with antibodies that recognize TBK1 phosphorylated at its activation loop residue Ser-172 (Fig. 4B). In addition, the phosphorylation of IRF3, a TBK1 substrate, was diminished (Fig. 4A). Consistent with the observed decrease in TBK1 activity and phosphorylation of IRF3, the production of IFNβ mRNA (Fig. 5A) and the secretion of IFNβ (Fig. 5B) were also reduced significantly in BMDMs from the OPTND477N/D477N mice compared with OPTN**+/+** mice.
FIGURE 4.
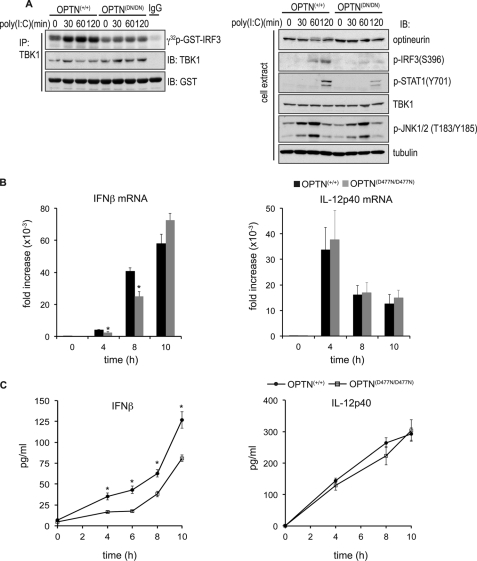
Polyubiquitin binding to optineurin is required for full TBK1 activation in response to LPS. OPTN+/+ and OPTND477N/D477N BMDMs were stimulated with 100 ng/ml LPS for the times indicated. A, in the upper panel, TBK1 was immunoprecipitated (IP) from 50 μg of cell extract protein and assayed with GST-IRF3 and Mg-[γ-32P]ATP as the substrates (see “Experimental Procedures”). The reactions were stopped by denaturation in SDS, subjected to SDS-PAGE, and after transfer to a PVDF membrane were autoradiographed to detected the 32P-labeled GST-IRF3 formed during the assay. The total levels of TBK1 and GST-IRF3 present in each assay were assessed by immunoblotting (IB). In the lower panel, cell extract from each experiment (30 μg of protein) was analyzed by immunoblotting for the total levels of OPTN, TBK1, and tubulin and for phosphorylated IRF3 and phosphorylated JNK1/2. B, experiment was carried out as in A, except that the cell extracts were immunoblotted for TBK1 phosphorylated at Ser-172 as well as for total TBK1 and the phosphorylation of p105 and JNK. A and B, results are representative of at least three separate experiments.
FIGURE 5.
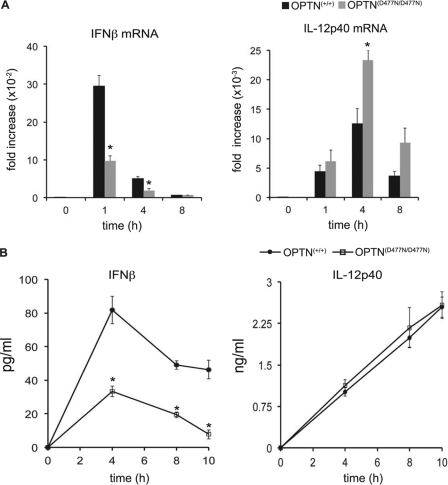
LPS-stimulated production of IFNβ is reduced in BMDMs from OPTND477N/D477N mice. BMDMs from OPTN+/+ and OPTND477N/D477N mice were stimulated with 100 ng/ml LPS for the times indicated. A, total RNA was extracted and analyzed by quantitative PCR for expression of IFNβ (left-hand panel) and IL-12p40 mRNA levels (right-hand panel). mRNA levels were normalized for 18 S rRNA expression, and the results are presented as fold-increase relative to the mRNA levels present in wild-type unstimulated cells. B, concentration of IFNβ (left-hand panel) or IL-12p40 (right-hand panel) present in the cell culture medium as measured by ELISA. Results are representative of at least two separate experiments with n = 4 mice per genotype. The error bars represent the mean ± S.E. Statistical analysis was performed by one-way analysis of variance with p < 0.05 considered statistically significant (*).
Similar to the results obtained with LPS, we also observed reduced activation of TBK1 and phosphorylation of IRF3 when BMDMs from OPTND477N/D477N mice were stimulated with the TLR3 ligand, poly(I:C) (Fig. 6A), which was accompanied by a decrease in the production of IFNβ mRNA (Fig. 6B) and IFNβ secretion (Fig. 6C). The poly(I:C)-stimulated phosphorylation of STAT1 at Tyr-701 was reduced in BMDMs from the OPTND477N/D477N mice after 2 h (Fig. 6A), presumably as a consequence of the reduced production of IFNβ and reduced IFNβ-stimulated activation of the JAK-STAT1 pathway. In contrast the production of IL-12p40 mRNA and IL-12 secretion were unaffected.
FIGURE 6.
Poly(I:C)-induced TBK1 activity and IFNβ production are reduced in OPTND477N/D477N BMDMs. OPTN+/+and OPTND477N/D477N BMDMs were stimulated with 10 μg/ml poly(I:C) for the times indicated. A, TBK1 activities (left-hand panel) measured as described in Fig. 4. Further aliquots of the extracts (30 μg of protein) were immunoblotted with the same antibodies used in Fig. 4A, as well as for STAT1 phosphorylated at Tyr-701. B, total RNA was extracted and analyzed by quantitative RT-PCR for expression of IFNβ and IL-12p40 mRNA levels. The mRNA levels were normalized for 18 S rRNA expression, and the results are presented as the fold-increase from wild-type unstimulated cells. C, concentrations of IFNβ or IL-12p40 in the culture media were determined by ELISA. Results are representative of at least three separate experiments with n = 4 mice per genotype. The error bars represent the mean ± S.E. Statistical analysis was performed by one-way analysis of variance with p < 0.05 considered statistically significant (*).
Interaction of Polyubiquitin Chains with OPTN Is Required for Optimal Activation of TBK1 and Type I IFN Production by Transfected Poly(I:C) in Embryonic Fibroblasts
The binding of double-stranded RNA to cytoplasmic RNA sensors (25–27) also induces the activation of TBK1, the phosphorylation of IRF3, and the production of type interferons (38). Therefore, to study the role of optineurin in this non-TLR pathway, we transfected primary MEFs with poly(I:C), which is thought to stimulate interferon production by binding to cytosolic RNA receptors (38). Transfected poly(I:C) induced a weaker activation of TBK1 in MEFs from the OPTND477N/D477N mice than in wild-type mice (supplemental Fig. S1_A_), and the production of interferon β and α was also decreased (supplemental Fig. S1_B_). In contrast, the production of IL-12p40 mRNA was increased. These experiments show that OPTN plays a role in the activation of TBK1 and type I IFN production by TLR-independent as well as TLR-dependent pathways. It has been reported that, in contrast to MEFs, the production of IFNβ mRNA is not reduced in BMDMs from TBK1−/− mice following infection by Sendai virus (22). Consistent with this finding, we found that IFNβ production induced by Mumps virus was similar in BMDMs from OPTND477N/D477N and wild-type mice (results not shown).
LPS-stimulated Activation of NF-κB and NF-κB-dependent Gene Transcription Is Not Impaired in OPTND477N/D477N Mice
Based on overexpression and miRNA “knockdown” studies, it was reported that OPTN suppresses TNF-stimulated, NF-κB-dependent gene transcription and IL-6 production in HEK293 cells (39), and we therefore investigated whether this was also the case in LPS-stimulated macrophages. We found that the LPS-stimulated phosphorylation of the canonical IKKs (IKKα and IKKβ), the phosphorylation of p105 (an established substrate of the IKKβ (40, 41)), the degradation of IκBα, and the production of IL-6 mRNA were similar in OPTND477N/D477N and OPTN+/+ mice (supplemental Fig. S2 and Figs. 4 and 7). In addition, the LPS-stimulated production of IL-12p40 mRNA and secretion, which is also thought to be dependent on NF-κB, was similar in OPTND477N/D477N and OPTN+/+ mice (Fig. 5). The phosphorylation (activation) of c-Jun N-terminal kinase (JNK) induced by LPS (Fig. 4B) or poly(I:C) (Fig. 6A) was also similar in BMDMs from OPTND477N/D477N and OPTN+/+ mice.
FIGURE 7.
Optineurin is phosphorylated in response to LPS in a TBK1- and TAK1-IKKα/β-dependent manner. BMDMs from OPTN+/+ and OPTND477N/D477N mice were stimulated for 30 min with 100 ng/ml LPS. A, wild-type optineurin was immunoprecipitated from 50 μg of cell extract protein of OPTN+/+ BMDMs using a sheep α-mouse optineurin antibody. The resuspended immunoprecipitates (30 μl) were then treated with (+) or without (−) the protein phosphatase from bacteriophage λgt10 (λ_-ppase_) (200 units). The change in mobility of OPTN following treatment with λppase was analyzed by immunoblotting with an anti-OPTN antibody. B, prior to stimulation with LPS, BMDMs from OPTN+/+ and OPTND477N/D477N mice were incubated for 1 h with or without (−) the TBK1/IKKϵ inhibitor MRT67307 (mrt), the IKKβ inhibitor BI605906 (bi), or both inhibitors. The cell extracts were then subjected to SDS-PAGE followed by immunoblotting with the antibodies indicated. The inhibition of IKKβ was confirmed by immunoblotting for phosphorylated p105 (S933). C, same as B, except that the TAK1 inhibitor 5_Z_-7-oxozeanol (ox) was used instead of BI605906 to inhibit the activation of both IKKα and IKKβ. The inhibition of IKKβ or TAK1 was confirmed by immunoblotting for phosphorylated p105 (S933). A–C, results are representative of at least two separate experiments.
OPTN Is Phosphorylated by IKK Family Members in Response to LPS
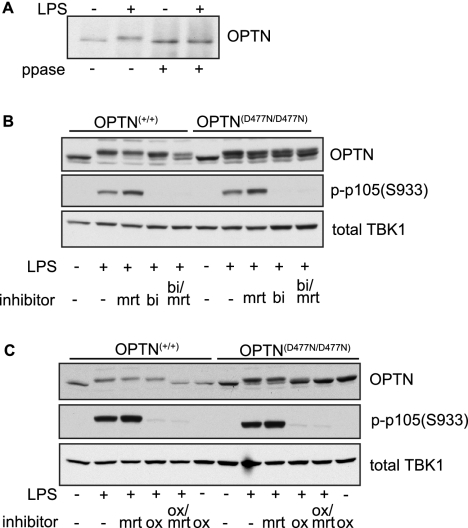
LPS induced a striking decrease in the electrophoretic mobility of OPTN (Fig. 3A, bottom panel) suggesting that OPTN might become phosphorylated in response to this ligand. To determine whether the LPS-induced mobility shift of OPTN was due to phosphorylation, we immunoprecipitated the endogenous OPTN from BMDM extracts followed by incubation with the protein phosphatase from phage λgt10. This treatment reversed the LPS-stimulated decrease in the mobility of OPTN but had no effect on the mobility of OPTN from unstimulated cells (Fig. 7A), establishing that LPS had indeed induced the phosphorylation of OPTN.
Because OPTN interacts with TBK1, this protein kinase was a candidate to mediate OPTN phosphorylation. We therefore studied the effect of MRT67307, a relatively specific inhibitor of TBK1 and IKKϵ that does not inhibit the canonical IKKs, IKKα and IKKβ (35). We also studied the effect of BI605906, a specific inhibitor of IKKβ (35). Interestingly, incubation with either MRT67307 or BI605906 alone had little effect on the LPS-stimulated decrease in the mobility of OPTN, but when added together, the decrease in mobility was partially suppressed (Fig. 7B).
The failure of MRT67307 and BI605906 to completely prevent the LPS-stimulated phosphorylation of OPTN in BMDMs could be explained by the inability of these compounds to inhibit IKKα, as shown previously for other substrates of IKKα and IKKβ (35). To investigate whether this was the case, we studied the effect of the TAK1 inhibitor, 5_Z_-7-oxozeanol, which prevents the activation of IKKα as well as IKKβ (36), in the presence or absence of MRT67307. These experiments showed that the combined addition of the TAK1 inhibitor and the TBK1/IKKϵ inhibitor completely suppressed the LPS-stimulated decrease in mobility of OPTN, whereas either inhibitor alone had little effect (Fig. 7C). Control experiments confirmed that, as expected, MRT67307 prevented the phosphorylation of IRF3 (results not shown), whereas BI605906 or 5_Z_-7-oxozeanol prevented the phosphorylation of p105/NF-κB1. Taken together, these experiments indicate that the LPS-stimulated phosphorylation of wild-type OPTN is catalyzed by both the canonical IKKs (IKKα/β) and the IKK-related kinases (TBK1/IKKϵ).
Importantly, the smaller LPS-stimulated decrease in the electrophoretic mobility of the OPTN(D477N) mutant in BMDMs from OPTND477N/D477N mice was largely suppressed by the TAK1 inhibitor alone (Fig. 7C). This is consistent with the reduced activation of TBK1 by LPS in BMDMs from the knock-in mice, leading to decreased TBK1-dependent phosphorylation of OPTN. Thus, the LPS-stimulated decrease in the mobility of the OPTN(D477N) mutant in BMDMs from OPTND477N/D477N mice is largely mediated by the canonical IKKs.
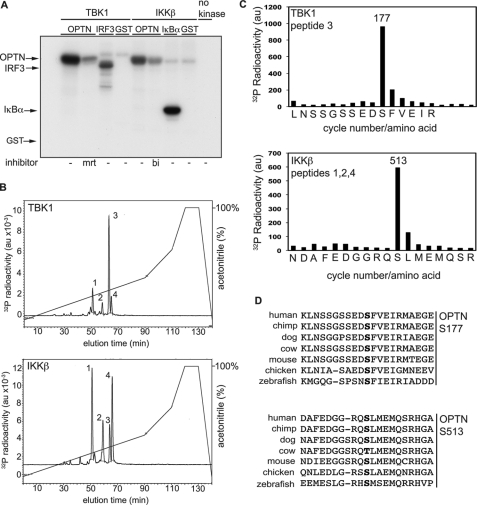
To identify the sites of phosphorylation, we phosphorylated OPTN in vitro. TBK1 phosphorylated GST-OPTN with similar efficiency to GST-IRF3 (Fig. 8A), the level of phosphorylation approaching 1 mol/mol protein under the conditions used. In contrast, GST was not phosphorylated. IKKβ also phosphorylated OPTN (Fig. 8A), the level of phosphorylation approaching 0.5 mol/mol. OPTN phosphorylated by TBK1 or IKKβ was digested with trypsin, and the tryptic peptides generated were separated by chromatography on a C18 column, which resolved four phosphopeptides termed 1, 2, 3, and 4. The same four peptides were observed whether OPTN had been phosphorylated by TBK1 or IKKβ, although their proportions differed markedly after phosphorylation by each kinase (Fig. 8B). Peptide 3, the major tryptic phosphopeptide obtained by phosphorylation with TBK1, was subjected to mass spectrometry, which showed that it was a monophosphorylated derivative of the peptide comprising amino acid residues 168–182. Fragmentation data were consistent with phosphorylation at Ser-177, an assignment confirmed by subjecting the peptide to solid phase sequencing, which resulted in the release of 32P radioactivity after the 10th cycle of Edman degradation corresponding to Ser-177 (Fig. 8C).
FIGURE 8.
Identification of sites on optineurin phosphorylated by TBK1 or IKKβ in vitro. A, wild-type GST-OPTN (2 μm) was incubated for 30 min with TBK1 (2 units/ml) or IKKβ (2 units/ml) in the absence (−) or presence of MRT67307 (mrt) or BI605906 (bi) prior to initiating the protein kinase reactions with Mg-[γ-32p]ATP. The phosphorylation of OPTN was compared with the phosphorylation of GST-IRF3 or GST-IκBα(2–54) (2 μm), which are established physiological substrates of TBK1 and IKKβ, respectively. GST (2 μm) was used as control. The reactions were terminated in SDS and subjected to SDS-PAGE, and the gel was autoradiographed. B, 32P-labeled OPTN from A obtained by phosphorylation with TBK1 (upper panel) or IKKβ (lower panel) were digested with trypsin and subjected to chromatography on a Vydac C18 column as described under “Experimental Procedures.” 32P radioactivity in arbitrary units (au) is shown by the full line, and the acetonitrile gradient is indicated by the diagonal lines. C, solid phase sequencing of peptide 3 generated after phosphorylation by TBK1 (upper panel of B) and the pooled peptides 1, 2, and 4 generated after phosphorylation by IKKβ (lower panel of B). Peptides were subjected to solid phase sequencing (49) to identify the cycles of Edman degradation at which 32P radioactivity (filled bars) was released from the phosphopeptides present in these fractions. D, alignment of amino acid sequences of OPTN from various species demonstrating conservation of the sites phosphorylated by TBK1 or IKKβ in vertebrate species. The phosphorylated serine residues are shown in boldface type.
The minor phosphopeptide 3 obtained after tryptic digestion of OPTN phosphorylated by IKKβ (Fig. 8B) was also the peptide comprising residues 168–182 phosphorylated at Ser-177 (results not shown). The other three phosphopeptides 1, 2, and 4 phosphorylated by IKKβ (Fig. 8B) were all found by mass spectrometry to be monophosphorylated derivatives of the same peptide comprising amino acid residues 502–520, but in different states of oxidation with both (peptide 1), one (peptide 2) or neither (peptide 4) of the two methionine residues oxidized to methionine sulfoxide. Analysis of the fragmentation patterns indicated that Ser-513 was the site of phosphorylation, an assignment confirmed by solid phase sequencing that was carried out after first removing the N-terminal glutamic acid residue by performing one cycle of Edman degradation prior to coupling the peptide for solid phase sequencing (Fig. 8C). The removal of N-terminal glutamic acid residues is needed to prevent their cyclization during the procedure for coupling the peptide to the solid phase sequencing support (42). The serine residues phosphorylated by TBK1 and IKKβ are conserved in vertebrates (Fig. 8D).
DISCUSSION
The production of type I IFNs in response to bacterial and viral infection is an important component of the host immune defense system. Although recent studies have established an essential role for ubiquitin-binding adaptor proteins such as NEMO and ABIN1 in modulating pro-inflammatory cytokine production via regulation of key kinases, such as IKKα/β and TAK1, much less is known regarding the contribution of K63-pUb chains and ubiquitin-binding adaptor proteins in the regulation of kinases, such as TBK1, that are critical for induction of type 1 IFNs. OPTN is a TBK1-binding partner and a member of the UBAN-containing ubiquitin-binding protein family. The function of OPTN in innate immune cells, including how OPTN might influence TBK1 activity, and a role for its ubiquitin binding activity is not known. In this study, we have generated knock-in mice expressing a polyubiquitin-binding defective mutant of optineurin, OPTND477N/D477N, to examine in a physiological context the role of OPTN in innate immune cells and to assess the contribution of K63-pUb-binding to the production of type 1 IFN. We have found that TBK1 activity is reduced significantly in response to activation of TLR4 (LPS) and TLR3 ((poly)I:C) in BMDMs that express the polyubiquitin-binding defective mutant of OPTN. Consistent with reduced TBK1 activity in OPTND477N/D477N BMDMs, the LPS or poly(I:C)-stimulated phosphorylation of IRF3 was also decreased significantly, and production of IFNβ mRNA and IFNβ secretion was impaired. We also found that the activation of TBK1 and the production of type 1 IFNs induced by the binding of poly(I:C) to cytosolic RNA sensors was reduced in MEFs from OPTND477N/D477N mice compared with wild-type mice. Our results suggest that the binding of OPTN to K63-pUb chains and/or linear pUb chains is important for optimal activation of TBK1 by these signaling pathways and demonstrate that OPTN acts in a positive manner to promote an effective IFN response from BMDMs and MEFs. The identification of the polyubiquitylated protein(s) that is(are) formed in response to LPS and poly(I:C) and whose interaction with OPTN enhances the activation of TBK1 will therefore be an important aspect of future research.
A recent study reported that the poly(I:C)- and virus-stimulated activation of an IFNβ reporter gene and IFNβ secretion was inhibited by the overexpression of OPTN and enhanced by siRNA knockdown of OPTN in HEK293 cells that stably express TLR3 (43). It was concluded from these experiments that OPTN is a negative regulator of the signaling pathways by which double-stranded RNA and viruses stimulate transcription of the IFNβ gene. Additionally, these investigators reported that the human wild-type OPTN bound to TBK1 in co-transfection/immunoprecipitation experiments but that human OPTN(D474N) (equivalent to mouse OPTN(D477N)) did not and was unable to inhibit IFNβ production when overexpressed (43). These results contrast with those presented here in BMDMs in which the endogenous OPTN(D477N) mutant was shown to bind to TBK1 as efficiently as the endogenous wild-type OPTN and that IFNβ production was reduced in BMDMs from OPTND477N/D477N mice. The reasons for the discrepancy with our results are unclear and could be due in part to the different cells used. However, it is also well known that the specificity of signaling can break down when components are overexpressed and that siRNA knockdown can cause off-target effects, leading to erroneous conclusions being drawn. For example, based on siRNA knockdown studies, it was concluded that the TANK-TBK1 complex mediated the production of IFNβ after viral infection (30). However, the IFNβ produced in response to viral infection was subsequently found to be unimpaired in BMDCs from TANK−/− mice, and a quite different role for TANK was revealed by these studies (34). The nonphysiological overexpression of OPTN in HEK293 cells may cause competition for binding to K63-pUb and/or linear pUb chains, preventing them from binding to other polyubiquitin-binding proteins involved in the activation of TBK1.
It is intriguing that several key proteins that regulate innate immunity have recently been found to be phosphorylated by both the IKK-related kinases and the canonical IKKs, including NEMO and TANK, as well as the catalytic subunits of the four IKK family members themselves (35). We found that the endogenous wild-type OPTN was also phosphorylated by both the canonical IKKs and the IKK-related kinases because the LPS-stimulated decrease in the electrophoretic mobility of OPTN in BMDMs could only be prevented in the presence of both a TAK1 inhibitor (to suppress the activation of both the canonical IKKs, IKKα and IKKβ) and a TBK1/IKKϵ inhibitor. In contrast, the LPS-stimulated phosphorylation of the OPTN(D477N) mutant in BMDMs from OPTND477N/D477N mice was suppressed by the TAK1 inhibitor alone. Because the LPS-stimulated activation of TBK1 is reduced in BMDMs from OPTND477N/D477N mice, but the activation of the related IKKϵ is not (results not shown), these experiments indicate that it is TBK1, rather than IKKϵ, which phosphorylates the endogenous OPTN in response to LPS. Furthermore, the dramatic reduction in the mobility shift for OPTN(D477N) in the presence of the TAK1 inhibitor alone provides an additional read-out of the impaired activation of TBK1 in LPS-stimulated OPTND477N/D477N BMDMs.
We identified Ser-177 and Ser-513, as the major residues in OPTN phosphorylated by TBK1 and IKKβ, respectively, in vitro, and in a separate study we have found that the TLR1/2 ligand Pam3Csk4 stimulates phosphorylation of the endogenous OPTN at Ser-177 in the RAW macrophage cell line, which is prevented by pharmacological inhibition of TBK1.3 The serine residues in OPTN phosphorylated by the canonical IKKs and the IKK-related kinases are conserved in vertebrates, consistent with important roles for these modifications. Understanding the function of OPTN phosphorylation will be an important problem to address in the future. While this paper was under review, OPTN was shown to bind to the autophagy receptor LC3, and the TBK1-dependent phosphorylation of OPTN at Ser-177 was shown to enhance its interaction with LC3 and promote the clearance of ubiquitylated cytosolic Salmonella enterica (44). Thus the role of OPTN and its phosphorylation are not confined to the regulation of interferon production.
Recently, mutations in OPTN have been shown to cause amyotrophic lateral sclerosis (ALS) (45), a form of motor neuron disease. Interestingly, one of the mutations in human OPTN that causes ALS is OPTN(E478G) (45), which lies only four amino acid residues C-terminal to Asp-474 of human OPTN/Asp-477 of mouse OPTN. We have found that, like the OPTN(D474N) mutant, OPTN(E478G) does not bind to K63-pUb or linear-pUb chains (supplemental Fig. S3). The impaired binding of the OPTN(E478G) mutant to polyubiquitin may therefore impair the activation of TBK1 and underlie ALS in man. However, the OPTND477N/D477N mice that we have generated do not show any overt signs of ALS even after 12 months.
Mutations in OPTN have also been shown to cause Paget disease of bone (46), a relatively common familial disorder characterized by enlarged and deformed bones. Paget disease of bone is thought to be caused by abnormalities in signaling by the TNF family member RANKL. Interestingly, the RANKL-stimulated production of IFNβ has been reported to have a key role in limiting the differentiation of osteoclasts by enhancing the synthesis of the transcription factor c-Fos (47) or inducible nitric-oxide synthetase (48). Moreover, mice that do not express the type 1 interferon receptor have bone abnormalities (47). Although the RANKL-stimulated production of IFNβ does not require IRF3, IRF7, or IRF9 (47), it would be of great interest to know whether TBK1 is required for RANKL-stimulated production of IFNβ, whether RANKL-stimulated activation of TBK1 is reduced in cells from the OPTN(D477N) mice, and whether these mice develop Paget disease of bone as they age.
Supplementary Material
Supplemental Data
Acknowledgments
We thank colleagues for the DNA vectors encoding GST-NEMO and GST-OPTN (Mark Peggie) and for performing phosphopeptide separations by HPLC and advice about protein sequencing (David Campbell), and the protein and antibody production teams of the Division of Signal Transduction Therapy, Medical Research Council Protein Phosphorylation Unit (coordinated by Hilary McLauchlan and James Hastie) for purified TBK1 and IKKβ and antibodies that immunoprecipitate TBK1, IKKϵ, and OPTN.
*
This work was supported by the United Kingdom Medical Research Council, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck-Serono, and Pfizer.
3
J. Zhang, K. Clark, and P. Cohen, unpublished results.
2
The abbreviations used are:
TLR
Toll-like receptor
OPTN
optineurin
BMDM
bone marrow-derived macrophage
IKK
IκB kinase
NEMO
NF-κB essential modulator
MEF
mouse embryonic fibroblast
ALS
amyotrophic lateral sclerosis
RANKL
receptor activator of NF-κB ligand
FLPe
Flippase recombination enzyme.
REFERENCES
- 1.Walsh M. C., Kim G. K., Maurizio P. L., Molnar E. E., Choi Y. (2008) PLoS One 3, e4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Deng L., Hong M., Akkaraju G. R., Inoue J., Chen Z. J. (2001) Nature 412, 346–351 [DOI] [PubMed] [Google Scholar]
- 3.Haas T. L., Emmerich C. H., Gerlach B., Schmukle A. C., Cordier S. M., Rieser E., Feltham R., Vince J., Warnken U., Wenger T., Koschny R., Komander D., Silke J., Walczak H. (2009) Mol. Cell 36, 831–844 [DOI] [PubMed] [Google Scholar]
- 4.Kirisako T., Kamei K., Murata S., Kato M., Fukumoto H., Kanie M., Sano S., Tokunaga F., Tanaka K., Iwai K. (2006) EMBO J. 25, 4877–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tokunaga F., Sakata S., Saeki Y., Satomi Y., Kirisako T., Kamei K., Nakagawa T., Kato M., Murata S., Yamaoka S., Yamamoto M., Akira S., Takao T., Tanaka K., Iwai K. (2009) Nat. Cell Biol. 11, 123–132 [DOI] [PubMed] [Google Scholar]
- 6.Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004) Mol. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 7.Kulathu Y., Akutsu M., Bremm A., Hofmann K., Komander D. (2009) Nat. Struct. Mol. Biol. 16, 1328–1330 [DOI] [PubMed] [Google Scholar]
- 8.Sato Y., Yoshikawa A., Yamashita M., Yamagata A., Fukai S. (2009) EMBO J. 28, 3903–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ea C. K., Deng L., Xia Z. P., Pineda G., Chen Z. J. (2006) Mol. Cell 22, 245–257 [DOI] [PubMed] [Google Scholar]
- 10.Rahighi S., Ikeda F., Kawasaki M., Akutsu M., Suzuki N., Kato R., Kensche T., Uejima T., Bloor S., Komander D., Randow F., Wakatsuki S., Dikic I. (2009) Cell 136, 1098–1109 [DOI] [PubMed] [Google Scholar]
- 11.Wu C. J., Conze D. B., Li T., Srinivasula S. M., Ashwell J. D. (2006) Nat. Cell Biol. 8, 398–406 [DOI] [PubMed] [Google Scholar]
- 12.Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009) Nature 461, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Döffinger R., Smahi A., Bessia C., Geissmann F., Feinberg J., Durandy A., Bodemer C., Kenwrick S., Dupuis-Girod S., Blanche S., Wood P., Rabia S. H., Headon D. J., Overbeek P. A., Le Deist F., Holland S. M., Belani K., Kumararatne D. S., Fischer A., Shapiro R., Conley M. E., Reimund E., Kalhoff H., Abinun M., Munnich A., Israël A., Courtois G., Casanova J. L. (2001) Nat. Genet. 27, 277–285 [DOI] [PubMed] [Google Scholar]
- 14.Windheim M., Stafford M., Peggie M., Cohen P. (2008) Mol. Cell. Biol. 28, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyninck K., Kreike M. M., Beyaert R. (2003) FEBS Lett. 536, 135–140 [DOI] [PubMed] [Google Scholar]
- 16.Wagner S., Carpentier I., Rogov V., Kreike M., Ikeda F., Löhr F., Wu C. J., Ashwell J. D., Dötsch V., Dikic I., Beyaert R. (2008) Oncogene 27, 3739–3745 [DOI] [PubMed] [Google Scholar]
- 17.Nanda S. K., Venigalla R. K., Ordureau A., Patterson-Kane J. C., Powell D. W., Toth R., Arthur J. S., Cohen P. (2011) J. Exp. Med. 208, 1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laplantine E., Fontan E., Chiaravalli J., Lopez T., Lakisic G., Véron M., Agou F., Israël A. (2009) EMBO J. 28, 2885–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 20.Hemmi H., Takeuchi O., Sato S., Yamamoto M., Kaisho T., Sanjo H., Kawai T., Hoshino K., Takeda K., Akira S. (2004) J. Exp. Med. 199, 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWhirter S. M., Fitzgerald K. A., Rosains J., Rowe D. C., Golenbock D. T., Maniatis T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 233–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry A. K., Chow E. K., Goodnough J. B., Yeh W. C., Cheng G. (2004) J. Exp. Med. 199, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton S., Hesson L., Peggie M., Cohen P. (2008) FEBS Lett. 582, 997–1002 [DOI] [PubMed] [Google Scholar]
- 24.Pietras E. M., Saha S. K., Cheng G. (2006) J. Endotoxin Res. 12, 246–250 [DOI] [PubMed] [Google Scholar]
- 25.Creagh E. M., O'Neill L. A. (2006) Trends Immunol. 27, 352–357 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z., Kim T., Bao M., Facchinetti V., Jung S. Y., Ghaffari A. A., Qin J., Cheng G., Liu Y. J. (2011) Immunity 34, 866–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaoka A., Wang Z., Choi M. K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K., Ohba Y., Taniguchi T. (2007) Nature 448, 501–505 [DOI] [PubMed] [Google Scholar]
- 28.Matsui K., Kumagai Y., Kato H., Sato S., Kawagoe T., Uematsu S., Takeuchi O., Akira S. (2006) J. Immunol. 177, 5785–5789 [DOI] [PubMed] [Google Scholar]
- 29.Chau T. L., Gioia R., Gatot J. S., Patrascu F., Carpentier I., Chapelle J. P., O'Neill L., Beyaert R., Piette J., Chariot A. (2008) Trends Biochem. Sci. 33, 171–180 [DOI] [PubMed] [Google Scholar]
- 30.Guo B., Cheng G. (2007) J. Biol. Chem. 282, 11817–11826 [DOI] [PubMed] [Google Scholar]
- 31.Ryzhakov G., Randow F. (2007) EMBO J. 26, 3180–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasai M., Oshiumi H., Matsumoto M., Inoue N., Fujita F., Nakanishi M., Seya T. (2005) J. Immunol. 174, 27–30 [DOI] [PubMed] [Google Scholar]
- 33.Sasai M., Shingai M., Funami K., Yoneyama M., Fujita T., Matsumoto M., Seya T. (2006) J. Immunol. 177, 8676–8683 [DOI] [PubMed] [Google Scholar]
- 34.Kawagoe T., Takeuchi O., Takabatake Y., Kato H., Isaka Y., Tsujimura T., Akira S. (2009) Nat. Immunol. 10, 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark K., Peggie M., Plater L., Sorcek R. J., Young E. R., Madwed J. B., Hough J., McIver E. G., Cohen P. (2011) Biochem. J. 434, 93–104 [DOI] [PubMed] [Google Scholar]
- 36.Ninomiya-Tsuji J., Kajino T., Ono K., Ohtomo T., Matsumoto M., Shiina M., Mihara M., Tsuchiya M., Matsumoto K. (2003) J. Biol. Chem. 278, 18485–18490 [DOI] [PubMed] [Google Scholar]
- 37.Ananieva O., Darragh J., Johansen C., Carr J. M., McIlrath J., Park J. M., Wingate A., Monk C. E., Toth R., Santos S. G., Iversen L., Arthur J. S. (2008) Nat. Immunol. 9, 1028–1036 [DOI] [PubMed] [Google Scholar]
- 38.Miyahira A. K., Shahangian A., Hwang S., Sun R., Cheng G. (2009) J. Immunol. 182, 2248–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu G., Wu C. J., Zhao Y., Ashwell J. D. (2007) Curr. Biol. 17, 1438–1443 [DOI] [PubMed] [Google Scholar]
- 40.Waterfield M., Jin W., Reiley W., Zhang M., Sun S. C. (2004) Mol. Cell. Biol. 24, 6040–6048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waterfield M. R., Zhang M., Norman L. P., Sun S. C. (2003) Mol. Cell 11, 685–694 [DOI] [PubMed] [Google Scholar]
- 42.Campbell D. G., Morrice N. A. (2002) J. Biomol. Tech. 13, 119–130 [PMC free article] [PubMed] [Google Scholar]
- 43.Mankouri J., Fragkoudis R., Richards K. H., Wetherill L. F., Harris M., Kohl A., Elliott R. M., Macdonald A. (2010) PLoS Pathog. 6, e1000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C., Dötsch V., Bumann D., Dikic I. (2011) Science 333, 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H., Komure O., Matsuura S., Kobatake K., Morimoto N., Abe K., Suzuki N., Aoki M., Kawata A., Hirai T., Kato T., Ogasawara K., Hirano A., Takumi T., Kusaka H., Hagiwara K., Kaji R., Kawakami H. (2010) Nature 465, 223–226 [DOI] [PubMed] [Google Scholar]
- 46.Albagha O. M., Visconti M. R., Alonso N., Langston A. L., Cundy T., Dargie R., Dunlop M. G., Fraser W. D., Hooper M. J., Isaia G., Nicholson G. C., del Pino Montes J., Gonzalez-Sarmiento R., di Stefano M., Tenesa A., Walsh J. P., Ralston S. H. (2010) Nat. Genet. 42, 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takayanagi H., Kim S., Matsuo K., Suzuki H., Suzuki T., Sato K., Yokochi T., Oda H., Nakamura K., Ida N., Wagner E. F., Taniguchi T. (2002) Nature 416, 744–749 [DOI] [PubMed] [Google Scholar]
- 48.Zheng H., Yu X., Collin-Osdoby P., Osdoby P. (2006) J. Biol. Chem. 281, 15809–15820 [DOI] [PubMed] [Google Scholar]
- 49.Morton S., Yang H. T., Moleleki N., Campbell D. G., Cohen P., Rousseau S. (2006) Biochem. J. 399, 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data