The magnitude of the T cell response to a clinically-significant dose of influenza virus is regulated by TRAIL (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 1.
Published in final edited form as: J Immunol. 2011 Sep 21;187(9):4581–4588. doi: 10.4049/jimmunol.1002241
Abstract
An immune response of appropriate magnitude should be robust enough to control pathogen spread, but not simultaneously lead to immunopathology. Primary infection with influenza A virus (IAV) results in a localized pulmonary infection and inflammation, and elicits an IAV-specific CD8 T cell immune response necessary for viral clearance. Clearance of IAV-infected cells, and recovery from infection, is mediated by perforin/Granzyme B- and Fas/FasL-mediated mechanisms. We recently reported that TNF-related apoptosis-inducing ligand (TRAIL) is another means by which IAV-specific CD8 T cells can kill IAV-infected cells. The current study examined the role of TRAIL in the pulmonary CD8 T cell response to a clinically-significant IAV [A/PR/8/34 (PR8; H1N1)] infection (i.e., leads to observable, but limited, morbidity and mortality in WT mice). Compared to WT mice, IAV-infected _Trail_−/− mice experienced increased morbidity and mortality despite similar rates of viral clearance from the lungs. The increased morbidity and mortality in _Trail_−/− mice correlated with increased pulmonary pathology and inflammatory chemokine production. Analysis of lung-infiltrating lymphocytes revealed increased numbers of IAV-specific CD8 T cells in infected _Trail_−/− mice, which correlated with increased pulmonary cytotoxic activity and increased pulmonary expression of MIG and MIP-1α. In addition, there was decreased apoptosis and increased proliferation of IAV-specific CD8 T cells in the lungs of _Trail_−/− mice compared to WT mice. Together, these data suggest that TRAIL regulates the magnitude of the IAV-specific CD8 T cell response during a clinically-significant IAV infection to decrease the chance for infection-induced immunopathology.
Keywords: TRAIL, influenza virus, apoptosis, chemokine
INTRODUCTION
Primary infection with influenza A virus (IAV) results in a localized pulmonary infection that induces an IAV-specific CD8 T cell immune response essential for efficient viral clearance (1–8). Recruitment of IAV-specific T cells into the lung after their initial priming in lung-draining LN is dependent on chemokine expression in the lung. A plethora of chemokines have been implicated in T cell homing to the airway after IAV infection, including MIP-1α, MIP-1β, MIP-3α, RANTES, and IP-10 (9–13). Similarly, expression of the corresponding chemokine receptors – including CCR2, CCR5 and CXCR3 – have been detected on activated, IAV-specific T cells after IAV infection. The interactions of these chemokines with their respective receptors enhance integrin expression, which is essential for IAV-specific T cell migration into the lung environment (9, 13–16).
The initiation of the T cell response to primary IAV infection also leads to the expression of effector molecules (such as FasL and perforin/Granzyme B) used by the T cells to kill IAV-infected cells (7). In addition to these well-characterized cytotoxic pathways, previous work from our laboratory identified a role for TNF-related apoptosis-inducing ligand (TRAIL)-expressing CD8 T cells in the primary immune response to IAV infection (17). TRAIL has classically been studied in tumor immunology settings because it selectively induces apoptosis in transformed cells while leaving normal cells and tissues unaffected (18–20), but TRAIL-based immunity is becoming more appreciated as a key component in the immune response to viral infections – including responses to cytomegalovirus, human immunodeficiency virus, and respiratory syncytial virus (21–28). While TRAIL-expressing IAV-specific CD8 T cells participate in the killing of virally-infected cells (17), TRAIL expression on alveolar Mϕ has also been associated with increased lung damage and susceptibility to IAV pneumonia (29, 30). Thus, depending on the pathogenicity of the virus or the size of the initial inoculum, TRAIL may have beneficial or detrimental roles in the immune response to IAV.
Seasonal IAV infections affect 10–20% of the U.S. population each winter, resulting in substantial morbidity (~114,000 hospitalizations) and mortality (~36,000 deaths) (31). Utilizing an experimental model that mimics the typical infection characteristics of humans is imperative to understanding the properties of the immune response that result in IAV clearance while limiting immunopathology. The infectious dose of IAV [A/PR/8/34 (PR8; H1N1)] utilized in our previous investigation did not induce mortality in either WT or _Trail_−/− mice, but it did result in significant morbidity in the _Trail_−/− mice (17). In the current study, we altered our infection protocol to examine the role of TRAIL in the CD8 T cell-mediated immunity induced during a clinically-significant IAV infection that results in limited morbidity and mortality in WT mice. Our results suggest that TRAIL plays a significant role in limiting the magnitude of the IAV-specific CD8 T cell response in the lungs during a clinically-significant IAV infection through alterations in IAV-specific CD8 T cell recruitment to the site of infection as well as the proliferation and survival of these T cells in the lung.
MATERIALS and METHODS
Mice, virus, infections, and peptides
Wild-type (WT) C57BL/6 (B6) and BALB/c mice were purchased from the National Cancer Institute (Frederick, MD) and the Jackson Laboratory (Bar Harbor, ME), respectively. _Trail_−/− C57BL/6 and BALB/c mice were obtained from Amgen (Seattle, WA) (32) and Dr. Thomas Sayers (NCI, Frederick, MD), respectively. Both strains of _Trail_−/− mice have been backcrossed onto the C57BL/6 or BALB/c background >10 generations. _CXCR3_−/− mice were obtained from Dr. Steven Varga (University of Iowa). All mice were used at 16–24 weeks of age, and all experiments followed approved University of Iowa IACUC protocols. The mouse-adapted A/PuertoRico/8/34 (PR8; H1N1) and X-31 (H3N2) IAV strains were grown in the allantoic fluid of 10 d old embryonated chicken eggs for 2 d at 37°C, as previously described (5, 33, 34). Allantoic fluid was harvested and stored at −80°C. Groups of 20.5–22.5 g WT and _Trail_−/− mice were given 1500 egg-infectious units (EIU) of mouse-adapted PR8 or X-31 in Iscove's media intranasally (i.n.) following anesthesia with isofluorane. The IAV peptides NP366 (ASNENMETM) and PA224 (SSLENFRAYV) for C57BL/6, and HA529 (IYSTVAGSL) and NP147 (TYQRTRALV) for BALB/c were purchased from Biosynthesis Inc. (Lewisville, TX). IAV tetramers NP366 (H-2Db/ASNENMETM) and PA224 (H-2Db/SSLENFRAYV) for C57BL/6, and HA529 (H-2Kd/IYSTVAGSL) and NP147 (H-2Kd/TYQRTRALV) for BALB/c were obtained from the National Institutes of Health Tetramer Core Facility.
Determination of lung virus titer
Pulmonary IAV titers were determined via endpoint dilution assay and expressed as Tissue Culture Infections Dose50 (TCID50) as previously described (35). Briefly, 10-fold dilutions of homogenized and clarified lung from IAV-infected mice were mixed with 105 MDCK cells in DMEM. After 24 h incubation at 37°C, the inoculum was removed and DMEM media containing 0.0002% L-1-(tosylamido-2-phenyl)ethyl chloromethyl ketone (TPCK)-treated trypsin (Worthington Diagnostics, Freehold, NJ) and penicillin (100U/ml)/streptomycin (100 µg/ml) was added to each well. After 3 d incubation at 37°C, supernatants were mixed with an equal volume of 0.5% chicken RBC, the agglutination pattern read, and the TCID50 values calculated.
Histology
Whole lungs with the heart attached were harvested from WT or _Trail_−/− mice on various days after IAV infection. Lungs were inflated and placed in 10% formalin. After 10 d, fixed lungs were processed and embedded in paraffin. Five (5) µm thick sections were H&E stained, or stained with an anti-influenza NP mAb (clone H16-L10-4R5, obtained from W. Gerhard; Wistar Institute, Philadelphia, PA) and counter-stained with hematoxylin. The identity of the slides was blinded, and the slides were then scored by a board-certified veterinary pathologist. The intensity of the cellular infiltration in the H&E stained slides was scored as follows: 0 – none detected; 1 – rare to uncommon; 2 - detectable extravasated neutrophils in small aggregates in airway and or alveoli; 3 - multiple moderate foci/aggregates in airway and/or alveoli; and 4 – Severe coalescing foci/aggregates that efface alveoli.
Cytotoxicity Assays
In vivo. Splenocytes were resuspended in NycoPrep 1.077A (Axis-Shield; Norton, MA) and then purified according to the manufacturer's instructions. NycoPrep-purified splenic mononuclear cells (107/ml) were labeled with either 2 µM CFSE (Invitrogen; Eugene, OR) at 37°C for 10 min or 2 µM PKH-26 (Sigma; St. Louis, MO) at room temperature for 5 min. After labeling, residual non-cell-associated CFSE and PKH-26 were neutralized by adding an equal volume of fetal calf serum to the cell suspension. CFSE-labeled splenic mononuclear cells (107/ml) were pulsed with 10 µM NP366 and PA224 peptide for 1 h at 37°C. PKH-26+ splenic mononuclear cells (107/ml) were similarly incubated but without peptide. The cells were then washed and mixed at a 1 pulsed:1 unpulsed ratio, and 107 cells were adoptively transferred i.v. into WT or _Trail_−/− mice 8 d after IAV infection. After 8 h, the lungs were removed, digested, and analyzed by flow cytometry, as previously described (5), to enumerate the number of remaining target cells. Uninfected mice were used as controls. The reduction in the number of recovered peptide-pulsed target cells in the IAV-infected versus uninfected mice was considered the percent specific lysis. In vitro. On d 8 post-IAV infection, lungs were harvested from WT or _Trail_−/− mice, homogenized, and CD8 T cells were MACS purified (>95% purity). A portion of the purified T cells was then stained with anti-CD8α, NP336 tetramer, and PA224 tetramer [NP366 and PA224 tetramers were obtained from the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility (Germantown, MD)]. The percentage of tetramer+ CD8 T cells was used to calculate the number of IAV-specific effectors. For target cells, C57BL/6 splenocytes were prepared and pulsed with 10 µM NP366 and PA224 peptide for 1 h at 37°C as described above. The cells were then labeled with 100 µCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. The effector CD8 T cells were mixed with the peptide-pulsed target cells at a 50:1 effector:target ratio, and cultured for 18 h in a 96-well round-bottom plate. The percent specific lysis was calculated as: 100 × (experimental c.p.m. − spontaneous c.p.m.)/(total c.p.m. − spontaneous c.p.m.). Spontaneous and total 51Cr release was determined in the presence of either medium alone or 1% NP-40, respectively.
Flow Cytometry
Quantitation of IAV-specific CD8 T cells was performed as follows. Lungs were harvested from IAV-infected WT or _Trail_−/− mice. The isolated cells were then stained with FITC-conjugated anti-CD3ε (145-C11; eBioscience, San Diego, CA), PerCP-Cy5-conjugated anti-CD8α (53-6.7; eBioscience), and either APC-conjugated NP366 or PA224 tetramer. The number of CD3+CD8+tetramer+ T cells from the infected WT or _Trail_−/− mice was enumerated using total pulmonary cell counts and flow cytometry. Surface Labeling. In addition to the above mAb and tetramers, isolated lung cells were stained with PE-conjugated anti-mouse CXCR3 (CXCR3-173; eBioscience), or CCR5 (HM-CCR5; eBioscience) mAb. To examine FasL expression, cells were first blocked with 1:100 rat and hamster serum as well as unconjugated streptavidin (Molecular Probes, Eugene, Oregon). Cells were washed and then stained with biotinylated FasL (MFL3; eBioscience), followed by streptavidin PE. Stained cells were fixed and erythrocytes lysed with FACS lysing solution (BD Biosciences), and subsequently analyzed on a FACSCalibur flow cytometer. Intracellular Staining. Granzyme B: Isolated lung cells (106) were surfaced stained with anti-CD3ε, -CD8α, and NP366 or PA224 tetramer as described above. Subsequently, the cells were fixed, permeablized, and stained with the PE-conjugated anti-human Granzyme B mAb (GB11; Invitrogen), or isotype control. IFN-γ/TNF: Isolated lung cells were cultured at 2 × 106 cells/well in a 24-well plate in the presence of 1 µM of either NP336 or PA224 peptide or media control, 400 U/ml recombinant human IL-2, and 1 µg/ml brefeldin A. After 6 h, the cells were surface stained with PE-conjugated anti-mouse CD8α, fixed, permeablized, and stained with APC-conjugated anti-mouse IFN-γ (XMG1.2; eBioscience) and either FITC-conjugated anti-mouse TNF mAb (MP6-XT22; eBioscience) or isotype control. CD107a: Isolated lung cells were cultured with NP336 or PA224 peptide, IL-2, and brefeldin A as described above, as well as FITC-conjugated anti-CD107a (1D45; eBioscience) or isotype control. After 6 h, cells were surface stained with PE-conjugated anti-mouse CD8α, fixed, permeablized, and stained with APC-conjugated anti-mouse IFN-γ or isotype control as above. Caspase 3/7: The FAM caspase 3/7 kit (Vybrant) was purchased from Invitrogen and used according to the manufacturer’s instruction. BrdU: As previously described (36), WT and _Trail_−/− mice were infected with 1500 EIU PR8 as above. On d 7 p.i., the lung resident cells were initially labeled by i.n. CFSE administration (33, 36). Two (2) h after CFSE administration, the mice were given 0.8 mg/mouse BrdU i.n. After another 4 h, the number of CFSE+ BrdU+ NP366- and PA244-tetramer+ cells were determined by flow cytometry (36). All flow cytometry data were acquired on a LSR II (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc.)
Measurement of pulmonary chemokines
Lungs were harvested from IAV-infected WT or _Trail_−/− mice on day 6 p.i., and homogenized in 3 ml of DMEM. Subsequently, the pulmonary chemokine expression was determined using a mouse chemokine array (Invitrogen, Carlsbad, CA).
Statistical analysis
For each analysis, normal distribution of data was first verified. To assess the difference between two sets of data with normal distribution, statistical significance was assessed using an unpaired, one-tailed t-test or a paired t-test for control and experimental data groups that could be paired. If normality test failed, Mann-Whitney Rank Sum tests were completed to compare data sets. To assess the differences among multiple sets of data with normal distribution, statistical significance was assessed using an ANOVA analysis of the data sets. If normality test failed, Kruskal-Wallis One Way Analysis of Variance on Ranks test was used to determine overall significance with subsequent pair wise comparisons completed using Dunn’s Method. To determine differences in interstitial inflammation after infection, a Wilcoxon two-sided two-sample exact test was run. When appropriate, subsequent pair-wise multiple comparisons were completed using the Holm-Sidak method. Differences were considered to be statistically significant at p ≤ 0.05.
RESULTS
Trail−/− mice exhibit increased morbidity and mortality during a clinically-significant IAV infection compared to WT mice
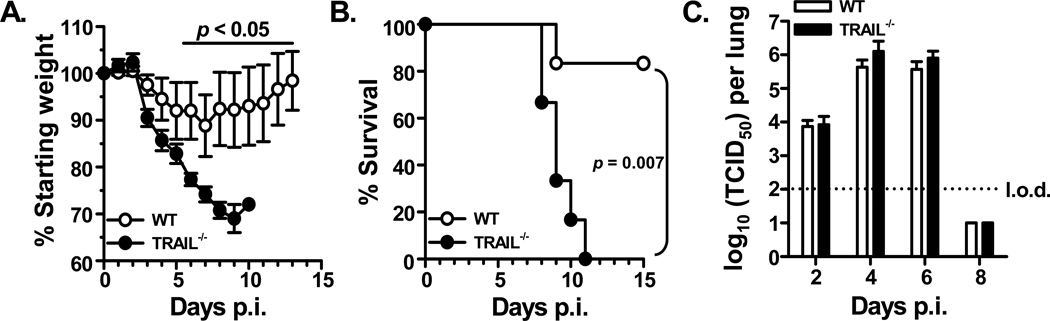
Our previous report examining the role of TRAIL in the immune response to IAV infections utilized a subclinical dose of IAV that induced minimal morbidity and no mortality in WT mice (17). While it is possible for humans to experience asymptomatic IAV infections, there is significant public health interest in better understanding the primary immune response to IAV infections that result in the development of clinical symptoms. Thus, we altered the infectious inoculum of the high virulent IAV stain PR8 and starting animal weight to induce observable morbidity in WT B6 mice (Figure 1A). In this setting, _Trail_−/− B6 mice showed significantly increased weight loss on d 6–10 p.i. compared to WT B6 mice that correlated with significantly increased mortality (Figure 1B). Reinforcing the importance for TRAIL in the immune response to IAV infection, increased mortality was also observed in _Trail_−/− BALB/c mice given a clinically-significant IAV compared to WT BALB/c mice (Supplemental Figure 1).
Figure 1. TRAIL deficiency correlates with increased disease severity during a clinically-significant IAV infection.
WT or _Trail_−/− C57BL/6 mice (n = 6 mice/group) were infected i.n. with 1500 EIU of A/PR/8/34 and weighed daily to assess morbidity (A) and mortality (B). In A, the values displayed represent the daily weight relative to the weight on day of infection. In B, data represent the percentage of mice surviving on the given day after infection; significantly increased mortality was observed in the _Trail_−/− mice. Data are representative of 2 separate experiments. C. Given a clinically-significant IAV infection (1500 EIU of A/PR/8/34), WT and _Trail_−/− mice have similar viral titers and clearance. At indicated days after infection, lungs were harvested, and pulmonary viral titers were assessed by determining the TCID50 in Madin-Darby canine kidney cell cultures. No significant difference was observed in the viral titers or the rate of viral clearance at the clinical dose of infection. Data are representative of 2 separate experiments with 3–5 mice per group.
Another aspect from our earlier studies with the subclinical IAV infection was a significant increase in lung viral titers and a significant delay in viral clearance in _Trail_−/− B6 mice compared to WT B6 mice (17). Since the IAV infection itself can cause some pathology, we examined the extent to which the above differences in morbidity and mortality were related to any differences in viral clearance. Despite the observed increased morbidity and mortality in _Trail_−/− B6 mice, the IAV titers and rate of viral clearance from the lungs of WT and _Trail_−/− B6 mice were surprisingly similar (Figure 1C). These data suggest the increased morbidity and mortality in _Trail_−/− B6 mice after a clinically-significant IAV infection was not due to an increased pulmonary viral load.
Trail−/− mice have increased pulmonary inflammation compared to WT mice during a clinically-significant high virulent IAV infection
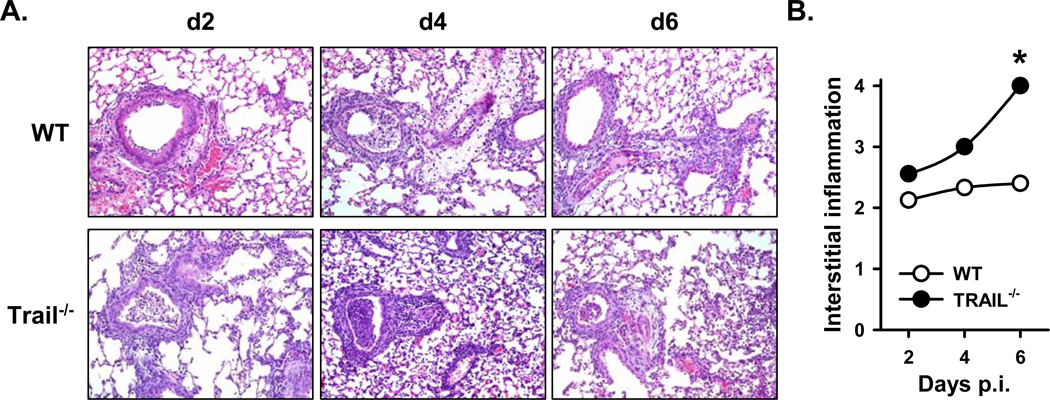
The extent of lung injury in the context of IAV clearance is influenced by viral load and the magnitude of the IAV-specific CD8 T cell response. For example, when the viral load is low CD8 T cells can potently limit IAV replication and protect IAV-induced lung injury, but significant immunopathology can occur when the IAV load is high (37). The efficiency with which _Trail_−/− B6 mice cleared the clinically-significant IAV infection, combined with the increased morbidity and mortality of these mice, prompted us to examine the lungs histologically. Consistent with the more extensive morbidity and increased mortality in _Trail_−/− B6 mice, histological evaluation of the lung revealed significantly increased interstitial inflammation in the _Trail_−/− B6 mice after IAV infection (Figure 2A and B). This increase in inflammation in the _Trail_−/− B6 mice correlated with increased pulmonary tissue damage and loss of organized tissue architecture. These data suggest the enhanced morbidity and mortality in _Trail_−/− B6 mice after clinical dose IAV infection may result from increased inflammation and immune-mediated tissue damage.
Figure 2. _Trail_−/− mice have increased pulmonary cellular infiltration and increased inflammation during a clinically-significant IAV infection.
WT or _Trail_−/− C57BL/6 mice (n = 6 mice/group) were infected with 1500 EIU of A/PR/8/34. On various days after infection, lungs were harvested and insufflated with 10% buffered formalin. Subsequently, the lung tissue was sectioned, mounted, and stained with Hematoxylin and Eosin (H&E). Images are 10X magnification. The identity of the slides were blinded and slides were evaluated; scores for each time point are indicated in the insert. Averaged results are presented, and statistical comparisons between WT and _Trail_−/− mice were done using the Wilcoxon two-sided two-sample exact test (* = p < 0.05).
Trail−/− mice have enhanced pulmonary recruitment of IAV-specific CD8 T cells during a clinically-significant IAV infections compared to WT mice
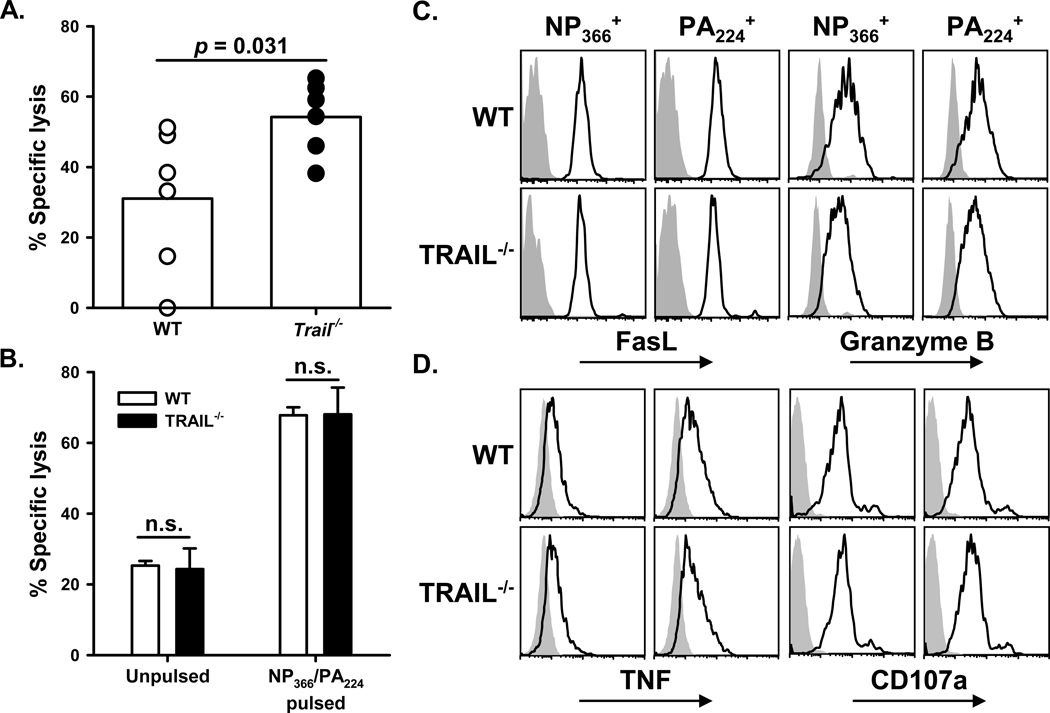
The clearance of IAV from the lung, as well as the increased mortality of _Trail_−/− B6 mice, is consistent with the kinetics of CD8 T cell infiltration into the lungs after IAV infection (4, 5). During primary IAV infections, CD8 T cells contribute to viral clearance (6, 38) by triggering apoptosis in the infected cells through FasL:Fas interactions, perforin/Granzyme B secretion (7), and TRAIL:DR5 interactions (17). Our previous analysis of subclinical IAV infections revealed that inefficient viral clearance in _Trail_−/− B6 mice was attributed to decreased cytotoxic function by IAV-specific CD8 T cells (17). The efficiency with which _Trail_−/− mice cleared a clinically-significant IAV infection prompted us to measure IAV-specific CD8 T cell cytolytic function. To our surprise, the in vivo cytotoxic activity of IAV-specific CD8 T cells was significantly higher in _Trail_−/− mice versus WT B6 mice (Figure 3A). In contrast, the in vitro killing activity of IAV-specific CD8 T cells from the lungs of infected WT or _Trail_−/− B6 mice was nearly identical, when measured on a per-cell basis (Figure 3B). To determine the extent to which a change in the effector phenotype of the _Trail_−/− IAV-specific CD8 T cells responding to the infection contributed to the observed cytotoxic difference, we examined the expression of several cytotoxic proteins known to be critical for the cytotoxic function of IAV-specific T cells. Phenotypic analysis of the IAV-specific CD8 T cells showed no significant difference in the expression of FasL, granzyme B, and TNF, and had a similar ability to degranulate, as measured by CD107a staining (Figure 3C and D).
Figure 3. _Trail_−/− mice display enhanced IAV-specific CD8+ T cell-mediated in vivo cytotoxicity compared to WT mice, despite similar cytotoxic molecule expression.
A. The pulmonary IAV-specific CD8 T cell response in WT or _Trail_−/− C57BL/6 mice infected with 1500 EIU of A/PR/8/34 was measured by in vivo cytotoxicity assay on d 8 p.i. Symbols represent killing in individual mice, and bars represent mean killing. Percentage IAV-specific killing was calculated by comparing unpulsed target lysis to IAV peptide-pulsed target lysis. Target cells were verified to be DR5+ by flow cytometry (data not shown), and target cell frequencies were normalized to ratios harvested from transfers into naïve mice. B. In contrast to the in vivo cytotoxicity, the in vitro cytotoxic activity of WT and _Trail_−/− IAV-specific CD8 T cells was similar. IAV-specific CD8 T cells were MACS-purified from the lungs of WT or _Trail_−/− C57BL/6 mice infected with 1500 EIU of A/PR/8/34 on 8 d p.i. The T cells were then cultured with unpulsed or IAV peptide-pulsed 51Cr-labeled splenocytes at a 50:1 E:T ratio for 18 h. Bars represent the mean (± S.D.) specific lysis measured from triplicate wells. No significant (n.s.) difference was observed between groups containing WT and _Trail_−/− effector cells. C–D. Pulmonary T cells from WT and _Trail_−/− mice have similar expression of effector molecules. WT or _Trail_−/− C57BL/6 mice were infected with 1500 EIU of A/PR/8/34 and then lungs were harvested on d 8 p.i. Isolated cells were stained with anti-CD8α, NP366 tetramer or PA224 tetramer, anti-CD3ε, anti-granzyme B or isotype control antibody, and anti-FasL or isoptye control antibody. Solid line histograms represent FasL or Granzyme B staining on CD8+tetramer+ T cells. Gray histograms represent isotype control staining. For TNF and CD107a analysis (D), isolated cells were incubated with NP366 or PA224 peptides or control media, brefeldin A, and anti-CD107a for 5 h. After incubation, the cells were stained with anti-CD8α, anti-IFNγ or isotype control antibody, and anti-TNF or isotype control antibody. Solid line histograms represent TNF or CD107a expression on CD8+IFNγ+ cells. Gray histograms represent isotype control staining.
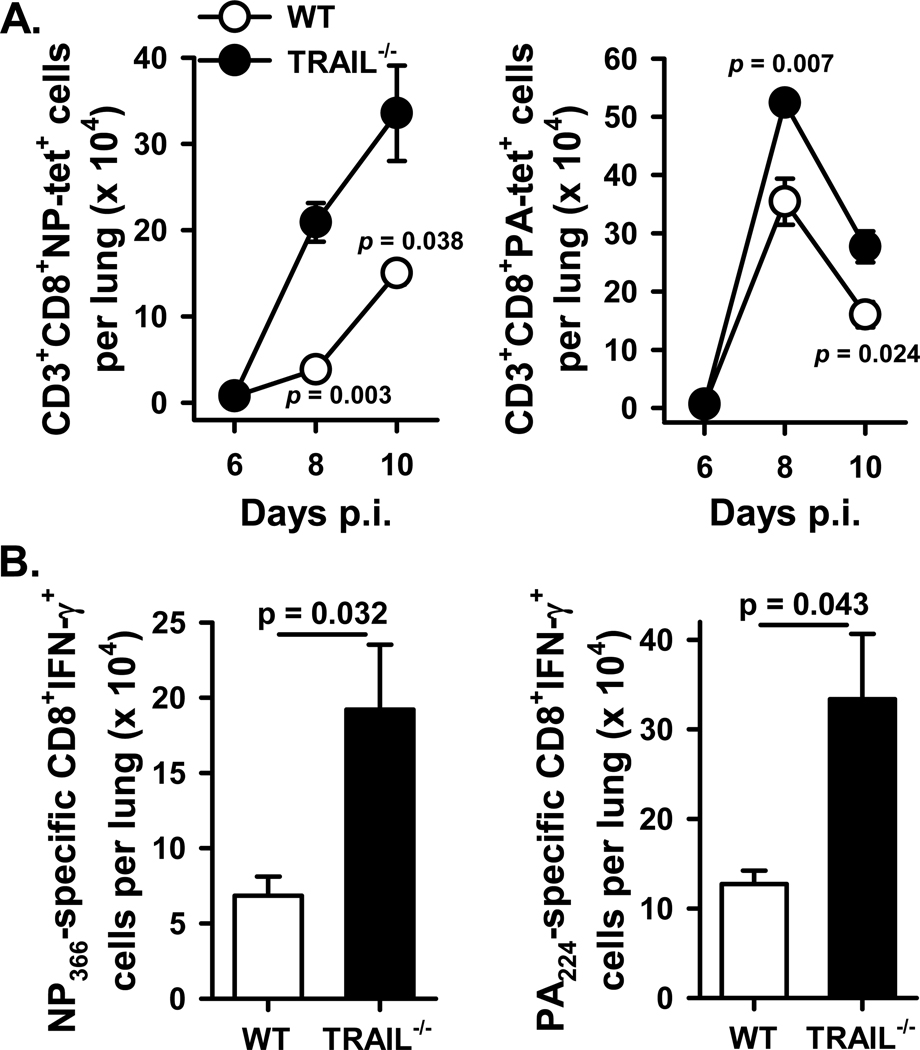
Having observed minimal phenotypic differences between the IAV-specific WT and _Trail_−/− CD8 T cells on a per cell basis, we quantitated the magnitude of the IAV-specific T cell response to determine the extent to which T cell numbers might have contributed to the enhanced in vivo pulmonary T cell cytotoxicity in _Trail_−/− B6 mice. This analysis revealed a significant increase in the number of NP366- and PA224-specific CD8 T cells in the lungs of _Trail_−/− versus WT B6 mice (Figure 4A). Consistent with these findings, analysis of pulmonary HA529- and NP147-specific CD8 T cells in WT and _Trail_−/− BALB/c mice showed similar increases in the IAV-specific CD8 T cell response (Supplemental Figure 2). The increase in the IAV-specific CD8 T cell response in _Trail_−/− B6 mice was also seen when determining the number of IFNγ-producing CD8 T cells after Ag-specific in vitro restimulation (Figure 4B). Interestingly, increased numbers of IAV-specific CD8 T cells were also detected in the lungs of _Trail_−/− B6 mice after infection with the low virulent IAV strain, X-31 (Supplemental Figure 3), suggesting that the exaggerated CD8 T cell response in _Trail_−/− mice may be independent on the IAV strain used for infection. Together, these data suggest that the increased in vivo cytotoxicity of pulmonary IAV-specific CD8 T cells in _Trail_−/− mice given a clinically-significant IAV infection likely results from a difference in the pulmonary environment that leads to greater numbers of IAV-specific CD8 T cells in the lungs of _Trail_−/− mice compared to WT mice.
Figure 4. _Trail_−/− mice given a clinically-significant IAV infection show enhanced pulmonary T cell recruitment compared to WT mice.
WT or _Trail_−/− C57BL/6 mice were infected with 1500 EIU of A/PR/8/34. A. On d 6, 8, and 10 p.i., lungs were harvested and homogenized, and the isolated cells were stained with anti-CD3ε, anti-CD8α, and NP366 tetramer or PA224 tetramer. The number of CD8+tetramer+ T cells from the infected WT or _Trail_−/− mice was enumerated using total pulmonary cell counts and flow cytometry. Data are averaged from 5 mice per group. B. On d 8 p.i., lungs were harvested and homogenized, and the isolated cells were incubated in vitro with NP336 or PA224 peptide for 6 h. The number of Ag-specific CD8+ T cells, based on IFNγ production after restimulation, from the infected WT or _Trail_−/− mice was enumerated using total pulmonary cell counts and flow cytometry. Data are averaged from 5 mice per group.
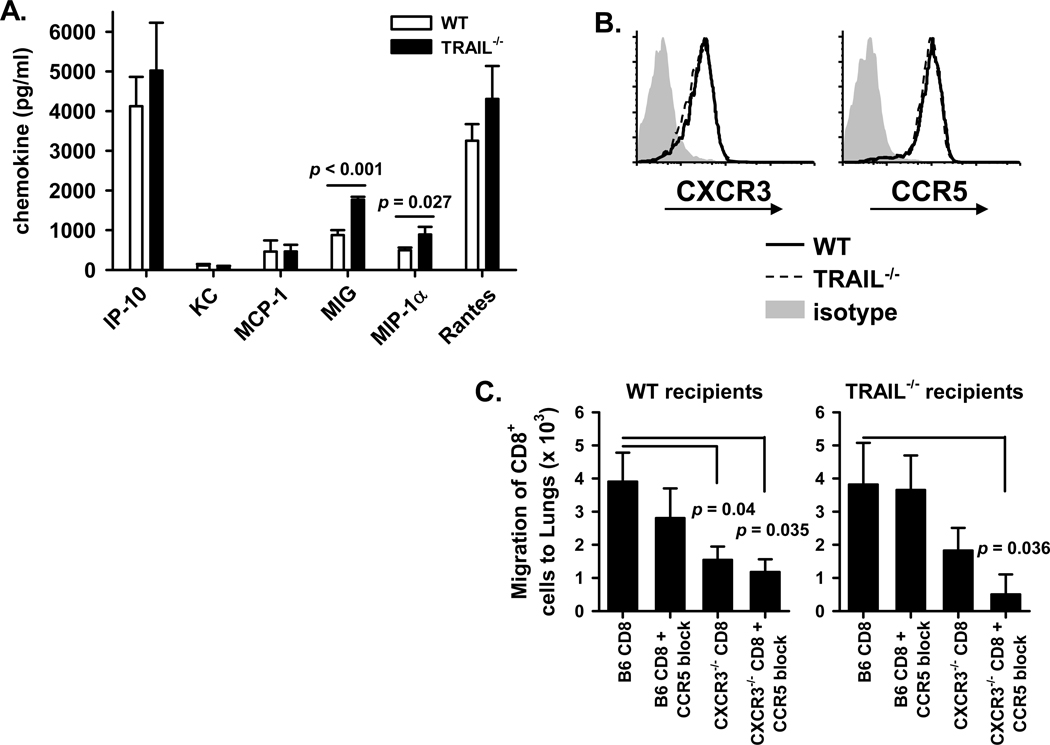
Trail−/− mice have increased pulmonary chemokine expression during a clinically-significant IAV infection compared to WT mice
The increase in IAV-specific CD8 T cell numbers in the lungs of _Trail_−/− mice given a clinically-significant IAV infection could be explained by 3 potential mechanisms: 1) altered pulmonary chemokine expression leading to increased recruitment; 2) differential apoptosis of the T cells in the lungs; and/or 3) increased proliferation of T cells within the lung. To determine the possibility of altered chemokine expression causing enhanced recruitment of IAV-specific CD8 T cells to the lungs, we first measured the pulmonary expression of chemokines associated with T cell recruitment. We observed significantly increased expression of MIG and MIP-1α in the lungs of _Trail_−/− mice compared to WT B6 mice (Figure 5A). Both of these chemokines are critical for CD8 T cell migration into the lungs during IAV infection (9–12). For chemokines to effectively act on T cells and enhance their migration into the lungs, the T cells need to express the corresponding receptor for the chemokine. Examination of the Ag-specific CD8 T cells responding to IAV infection revealed that CXCR3 (receptor for MIG) and CCR5 (receptor for MIP-1α) were similarly expressed on IAV-specific pulmonary T cells from WT and _Trail_−/− B6 mice (Figure 5B).
Figure 5. _Trail_−/− mice have increased pulmonary chemokine expression during a clinically-significant IAV infection compared to WT mice.
WT or _Trail_−/− C57BL/6 mice were infected with 1500 EIU of A/PR/8/34. A. Lungs were harvested on d 6 p.i. and homogenized in 3 ml of DMEM. Subsequently, the pulmonary chemokine expression was determined using multiplex analysis. Data presented are the average chemokine concentration measured from 4 WT or _Trail_−/− mice, and are representative of two independent experiments. B. Lungs were harvested on d 8 p.i., and the isolated cells were stained with anti-CD8α, NP366 tetramer, PA224 tetramer, anti-CD3ε, anti-CXCR3, anti-CCR5, or isotype control. Histograms show CXCR3 and CCR5 expression on CD3+CD8+ tetramer+ T cells from WT (solid line) and _Trail_−/− (dashed line) mice, or the isotype control (shaded histogram). Data are representative of 5 mice from 2 independent experiments. C. Abrogation of chemokine signals to T cells blocks their migration to the lung after IAV infection. Details of the experimental design are presented in Supplemental Figure 4.
Because of the established importance for these chemokine receptors in recruiting T cells to the lungs during IAV infection (9–12), we examined the extent to which CXCR3 and CCR5 were also required for the migration of IAV-specific CD8 T cells into the lungs of clinical dose-infected _Trail_−/− B6 mice (experimental design depicted in Supplemental Figure 4). CD8 T cells were isolated from the lung-draining LN of WT and _CXCR3_−/− B6 mice. After pretreatment with a CCR5 blocking mAb or isotype control, these T cells were then transferred into IAV-infected WT or _Trail_−/− B6 mice. The T cells lacking CXCR3 migrated to the lungs of WT or _Trail_−/− B6 mice ~50% less efficiently than WT T cells, but there was only a minimal alteration in the migration of T cells that had CCR5 blocked (Figure 5C). Loss of signal through both CXCR3 and CCR5 did not further inhibit the migration of the transferred cells to the lung of infected WT B6 recipients, but the loss of both CXCR3 and CCR5 significantly reduced the recruitment of these cells into the lungs of infected _Trail_−/− B6 recipients. These data reinforce the role these receptors play in T cell migration to the lung after IAV infection and suggest that increased chemokine production in the _Trail_−/− pulmonary environment contributes to the enhanced number of Ag-specific CD8 T cells that are recruited into the lung to respond to a clinically-significant IAV infection.
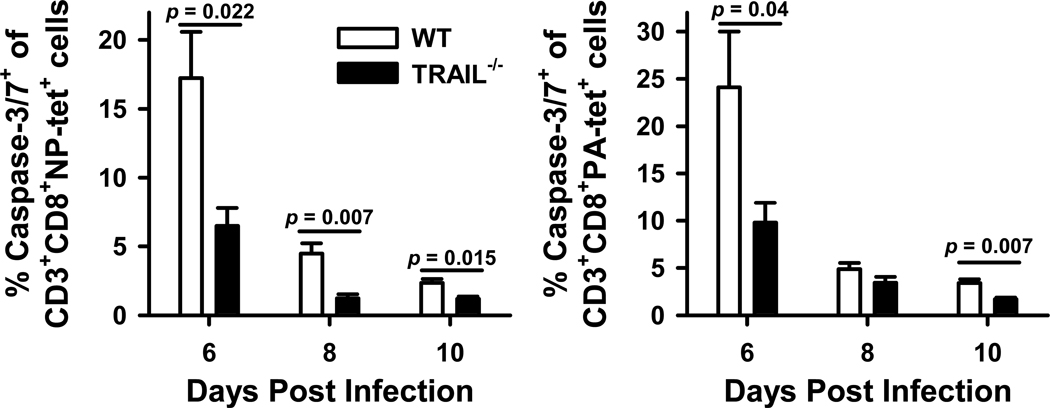
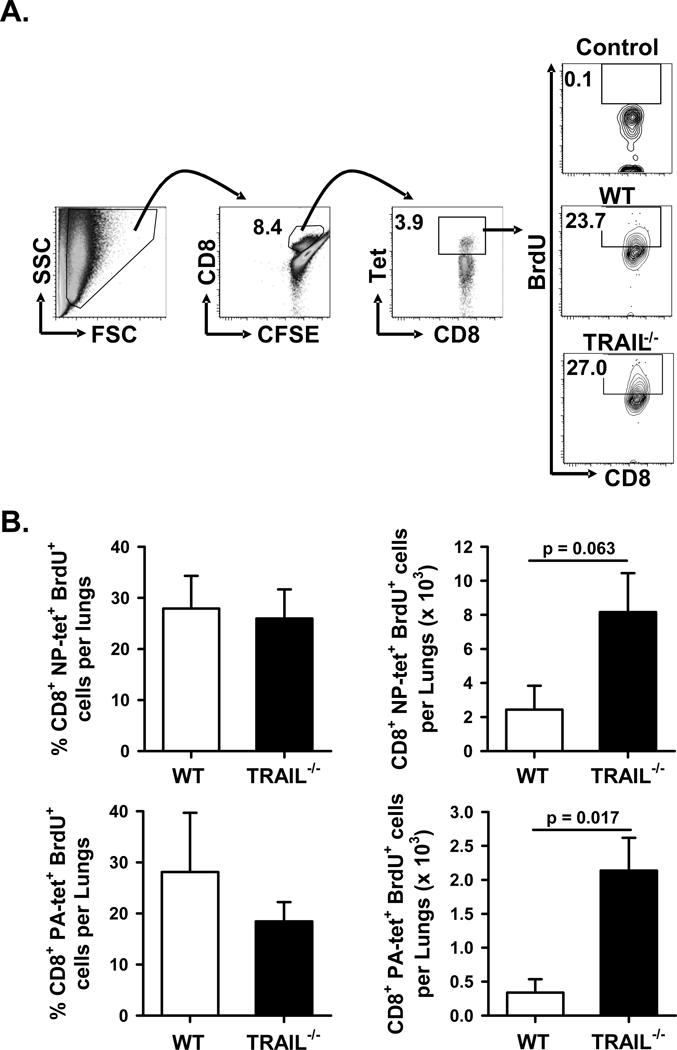
Trail−/− mice have decreased apoptotic death and increased proliferation of the infiltrating IAV-specific CD8 T cells in the lung after infection compared to WT mice
To investigate the potential impact of alterations in T cell apoptosis or proliferation affecting the magnitude of the IAV-specific T cell response in this system, the following experiments were performed. First, we determined the frequency of NP366- and PA224-specific CD8 T cells within the lungs of WT and _Trail_−/− B6 mice undergoing apoptosis (based on active caspase 3/7) during the course of infection. Interestingly, there was a significant decrease in the frequency of apoptotic NP366- and PA224-specific CD8 T cells in _Trail_−/− B6 mice given a clinically-significant IAV infection compared to WT B6 mice (Figure 6). These data correlate with the difference in numbers of NP366- and PA224-specific CD8 T cells in WT and _Trail_−/− B6 mice shown in Figure 4A. Next, we measured the proliferative status of the NP366- and PA224-specific CD8 T cells within the lung on d 7 p.i. by sequential i.n. administration of CFSE and BrdU (36). Using this procedure, the data shows that while there was no significant difference in the frequency of NP366- and PA224-specific CD8 T cells proliferating (based on BrdU incorporation) within the lung of the infected WT and _Trail_−/− mice, there were increased numbers of these populations of CD8 T cells actively proliferating in the lungs of _Trail_−/− B6 mice given a clinically-significant IAV infection compared to WT B6 mice (Figure 7). These results suggest that in addition to increased pulmonary chemokine expression and IAV-specific CD8 T cell recruitment, enhanced pulmonary T cell responses in _Trail_−/− mice given a clinically-significant IAV infection also result from increased proliferation and decreased apoptosis of the IAV-specific CD8 T cells in the lungs.
Figure 6. Decreased apoptosis of IAV-specific CD8 T cells in the lungs of _Trail_−/− mice given a clinically-significant IAV infection compared to WT mice.
WT or _Trail_−/− C57BL/6 mice were infected with 1500 EIU of A/PR/8/34. On d 6, 8, and 10 p.i., lungs were harvested and the isolated cells were analyzed by flow cytometry for apoptosis of IAV-specific CD8 T cells as measured by the frequency of active caspase3/7+ cells of the CD3+CD8+NP366 or PA224 tetramer+ cells. Data are averaged from 4 mice/group/timepoint.
Figure 7. Increased number of actively proliferating IAV-specific CD8 T cells in _Trail_−/− mice given a clinically-significant IAV infection compared to WT mice.
WT or _Trail_−/− B6 mice were infected with 1500 EIU of PR8. On d 7 p.i., mice were given CFSE i.n., followed by BrdU i.n. 2 h later. Lungs were harvested 4 h later, homogenized, and the isolated cells were analyzed by flow cytometry for proliferation of IAV-specific CD8 T cells as measured by the frequency of BrdU+ cells of the CFSE+CD8+NP366 or PA224 tetramer+ cells. The gating strategy, representative plots gated on CFSE+CD8+ cells (A), and averaged data (B) based on 4 mice/group are shown.
DISCUSSION
TRAIL is best known as being a potent inducer of apoptosis in a number of tumor systems, where it selectively induces the death of transformed cells (20, 39). More recently, TRAIL-expressing CD8 T cells, NK cells, Mϕ, and plasmacytoid DC have all been implicated in the cytotoxicity and control of virus infections (17, 28, 30, 40, 41). We previously demonstrated that CD8 T cells utilize TRAIL as a means of killing IAV-infected cells, and IAV-infected epithelial cells are sensitized to TRAIL-induced apoptosis during a subclinical (i.e., one that does not lead to weight loss or death in WT mice) high virulent IAV (PR8) infection (17). The results presented herein extend these previous findings by investigating the role of TRAIL in the immune response during a clinically-significant IAV infection. These data demonstrate some striking differences to our subclinical infection model, and suggest an additional role for TRAIL in shaping the magnitude of the CD8 T cell response to IAV infections. Consistent with our previous study, _Trail_−/− mice showed increased morbidity after infection; not surprisingly, this increased morbidity correlated with increased mortality at the clinically-significant dose. However, the increase in morbidity and mortality in the _Trail_−/− mice did not result from delayed viral clearance or increased viral load, as observed in our subclinical dose infection model. Instead, the data suggest that the increased morbidity and mortality resulted from immunopathology caused (at least in part) by the recruitment of significantly increased numbers of IAV-specific CD8 T cells.
The immune response to a primary IAV infection is composed early by innate immune cells (neutrophils, NK cells, Mϕ, γδ T cells), which are followed by IAV-specific CD4 and CD8 T cells and finally antibodies. CD8 T cells eliminate IAV-infected cells via FasL, cytolytic granule secretion (perforin/granzyme), and TRAIL-mediated mechanisms (7, 17, 28). Despite the loss of one of these three pathways, _Trail_−/− mice were still able to control the infection and clear virus similarly to WT mice after a clinically-significant IAV infection. In fact, IAV-specific CD8 T cells from WT and _Trail_−/− mice exhibited similar in vitro killing capacity, suggesting equivalent cytotoxic ability on a per cell basis. Examination of the in vivo cytotoxicity mediated by IAV-specific CD8 T cells, in contrast, revealed enhanced target cell killing in clinical dose-infected _Trail_−/− mice. Interestingly, these results are in opposition to those observed during the response to a sub-clinical IAV infection (17). Further, the increased T cell cytotoxicity observed in the _Trail_−/− mice did not result from a compensatory increase in FasL expression or granzyme B production, but instead correlated with an increased number of IAV-specific T cells in the lungs because of increased pulmonary recruitment, decreased apoptosis, and increased proliferation. These data suggest quite different functions for TRAIL during the immune response to IAV depending on the initial infection conditions. Thus, understanding why TRAIL functions differently based on the IAV dose is a challenge for future studies.
One possibility may be that there are TRAIL-dependent differences in DC function in the lung-draining LN, as well as in the lung environment itself, that contribute to the differential T cell responses observed in the WT and _Trail_−/− mice after IAV infection – including differences in MHC and costimulatory molecule expression, cytokine/chemokine production, ability to prime naïve IAV-specific T cells in the lung-draining LN, and the ability to stimulate T cell survival in the lung (36, 42, 43). Some of these differences in DC function may be related to the way IAV-derived pathogen-associated molecular patterns (PAMPs) and proinflammatory damage-associated molecular patterns (DAMPs (44)) released from dying IAV-infected respiratory epithelial cells are perceived by TLR and NLR expressed by different phagocytes in the lung at the time of infection. Diehl et.al. (45) reported that TRAIL:DR5 interactions contributed the negative regulation of proinflammatory cytokine production by DC and Mϕ stimulated with various TLR agonists. DC and Mϕ can also upregulate TRAIL expression after cytokine or TLR agonist stimulation (45–48). Given the strong evidence for TRAIL as an inducer of apoptosis and the emerging evidence for non-apoptotic TRAIL signaling (49), it is tempting to speculate that TRAIL may serve as a negative regulator of chemokine/cytokine production in these APC/phagocytes during a clinically-significant IAV infection through direct apoptotic and non-apoptotic signals. For example, TRAIL expression could result in the killing of the cells via the canonical apoptotic signaling pathway. Alternatively, TRAIL expression could induce signaling pathways that shut down chemokine production without inducing death. Considering the data suggesting that cellular inhibitor of apoptosis protein (cIAP) is required for inflammasome activation (50), that TRAIL signaling can downregulate cIAP (50), and that IAV activates the inflammasome through NLRP3 (51, 52), the potential intersection of the TRAIL receptor and inflammasome signaling pathways provides an intriguing possibility for how TRAIL might affect this aspect of the immune response during a clinically-significant IAV infection.
The experimental system we chose for this investigation utilized a clinically-significant IAV infection to better model the clinical symptoms observed in IAV-infected humans. In particular, this clinically-significant IAV infection model increased morbidity and mortality in WT mice, symptoms that were not observed in WT mice given a subclinical infection with the same virus strain (17). One complicating factor in understanding the immune response to primary IAV infection is the highly variable nature of the virus itself. Even among the commonly used laboratory IAV strains, immune responses vary and their dependence/independence on/from regulation by other cell types. This challenge is broadened when one considers highly pathogenic strains like the 1918 strain or the recently-emerged H5N1 avian influenza strains. An important determinant of the extent of lung injury in the context of IAV clearance is the relationship between viral load and the magnitude of the IAV-specific CD8 T cell response. Significant lung injury, which can be mediated by the viral infection itself as well as the host immune response, commonly occurs during clinical and experimental IAV infection (53). However, protective immunity and clearance of pulmonary pathogens (including IAV) is tied to cellular immunity (5, 33, 54) – particularly the activity of pathogen-specific CD8 T cells. CD8 T cells potently block IAV replication and are protective against illness and IAV-induced lung injury when viral loads are low, but the CD8 T cell response to a high IAV load can result in significant immunopathology (37). Our data suggest that during a clinically-significant IAV infection TRAIL signals have the greatest impact on controlling the magnitude of the IAV-specific CD8 T cell response in the lungs by blunting IAV-specific CD8 T cell recruitment to the lungs, as well as reducing the viability/proliferative capacity of these cells. We have concentrated our experiments and discussion on the influence of TRAIL on the IAV-specific T cell response during a clinically-significant infection; however, it is important to note that other immune cell populations can contribute to the immunopathology seen during IAV infection. In particular, TRAIL-expressing Mϕ contribute to IAV-induced pneumonia by inducing apoptosis of airway epithelial cells after a lethal infection (30). Data of interest from this study showed that anti-TRAIL treatment attenuated lung epithelial apoptosis, lung leakage, and enhanced survival after infection – suggesting TRAIL directly contributed to the immunopathology (instead of the increased immunopathology seen in our system in the absence of TRAIL). Regardless of these differences, a better understanding of the positive (i.e. clearing virus efficiently and limiting virus-induced pathology) and negative roles (i.e. immunopathology) for TRAIL in the immune response to IAV might aid in improving intervention strategies for the treatment of IAV infections and the symptoms associated with them.
Supplementary Material
1
ACKNOWLEDGMENTS
We thank Dr. Steven Varga for critical reading of the manuscript. We also thank Dr. David Meyerholz for histological assessment of IAV-infected lungs.
This work was supported by the National Institutes of Health Grants AI 072032 (KLL) and AI 077565 (TSG), a University of Iowa Carver College of Medicine Collaborative Pilot Grant (KLL and TSG), and an American Heart Association Predoctoral Fellowship (ELB).
Footnotes
The authors declare no competing financial interests.
REFERENCES
- 1.Epstein SL, Lo CY, Misplon JA, Bennink JR. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 1998;160:322–327. [PubMed] [Google Scholar]
- 2.Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 2004;17:197–209. doi: 10.1089/0882824041310577. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 2004;173:1209–1218. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J. Immunol. 2005;174:5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 5.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr. Top. Microbiol. Immunol. 1996;206:1–14. doi: 10.1007/978-3-642-85208-4_1. [DOI] [PubMed] [Google Scholar]
- 7.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 8.Langlois RA, Legge KL. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J Immunol. 184:4440–4446. doi: 10.4049/jimmunol.0902984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creswell LL, Rosenbloom M, Cox JL, Ferguson TB, Sr, Kouchoukos NT, Spray TL, Pasque MK, Ferguson TB, Jr, Wareing TH, Huddleston CB. Intraaortic balloon counterpulsation: patterns of usage and outcome in cardiac surgery patients. Ann. Thorac. Surg. 1992;54:11–18. doi: 10.1016/0003-4975(92)91133-t. discussion 18–20. [DOI] [PubMed] [Google Scholar]
- 10.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, Woodland DL. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J. Immunol. 2009;183:4378–4384. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohlmeier JE, Woodland DL. Memory T cell recruitment to the lung airways. Curr. Opin. Immunol. 2006;18:357–362. doi: 10.1016/j.coi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadel SA, Bromley SK, Medoff BD, Luster AD. CXCR3-deficiency protects influenza-infected CCR5-deficient mice from mortality. Eur. J. Immunol. 2008;38:3376–3387. doi: 10.1002/eji.200838628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter MV, Topham DJ. The alpha1beta1 integrin and TNF receptor II protect airway CD8+ effector T cells from apoptosis during influenza infection. J Immunol. 2007;179:5054–5063. doi: 10.4049/jimmunol.179.8.5054. [DOI] [PubMed] [Google Scholar]
- 16.Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood. 2003;101:4916–4922. doi: 10.1182/blood-2002-10-3159. [DOI] [PubMed] [Google Scholar]
- 17.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 2008;181:4918–4925. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 19.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 20.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 21.Clarke P, Meintzer SM, Gibson S, Widmann C, Garrington TP, Johnson GL, Tyler KL. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotelkin A, Prikhod'ko EA, Cohen JI, Collins PL, Bukreyev A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 2003;77:9156–9172. doi: 10.1128/JVI.77.17.9156-9172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedger LM, Shows DM, Blanton RA, Peschon JJ, Goodwin RG, Cosman D, Wiley SR. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- 25.Vidalain PO, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washburn B, Weigand MA, Grosse-Wilde A, Janke M, Stahl H, Rieser E, Sprick MR, Schirrmacher V, Walczak H. TNF-Related Apoptosis-Inducing Ligand Mediates Tumoricidal Activity of Human Monocytes Stimulated by Newcastle Disease Virus. J. Immunol. 2003;170:1814–1821. doi: 10.4049/jimmunol.170.4.1814. [DOI] [PubMed] [Google Scholar]
- 27.Zeng J, Fournier P, Schirrmacher V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297:19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa E, Nakazawa M, Yoshinari M, Minami M. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J. Virol. 2005;79:7658–7663. doi: 10.1128/JVI.79.12.7658-7663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Law HK, Cheung CY, Ng IH, Peiris JS, Lau YL. Functional tumor necrosis factor-related apoptosis-inducing ligand production by avian influenza virus-infected macrophages. J. Infect. Dis. 2006;193:945–953. doi: 10.1086/500954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, Seeger W, Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. Fact Sheet: Kay facts about influenza and influenza vaccine. Department of Health and Human Services; 2005. [Google Scholar]
- 32.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 34.Kilbourne ED. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 35.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 36.McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–232. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Hida S, Takayanagi H, Yokochi T, Kayagaki N, Takeda K, Yagita H, Okumura K, Tanaka N, Taniguchi T, Ogasawara K. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur. J. Immunol. 2001;31:3138–3146. doi: 10.1002/1521-4141(200111)31:11<3138::aid-immu3138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Zilliox MJ, Parmigiani G, Griffin DE. Gene expression patterns in dendritic cells infected with measles virus compared with other pathogens. Proc Natl Acad Sci U S A. 2006;103:3363–3368. doi: 10.1073/pnas.0511345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207:521–534. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 45.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J. Exp. Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J. Exp. Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halaas O, Vik R, Ashkenazi A, Espevik T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol. 2000;51:244–250. doi: 10.1046/j.1365-3083.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 49.Lunemann JD, Waiczies S, Ehrlich S, Wendling U, Seeger B, Kamradt T, Zipp F. Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto)antigen-specific T cells. J Immunol. 2002;168:4881–4888. doi: 10.4049/jimmunol.168.10.4881. [DOI] [PubMed] [Google Scholar]
- 50.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 54.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1