Location, Location, Location: Geographic Clustering of Lower-Extremity Amputation Among Medicare Beneficiaries With Diabetes (original) (raw)
Abstract
OBJECTIVE
Lower-extremity amputation (LEA) is common among persons with diabetes. The goal of this study was to identify geographic variation and the influence of location on the incidence of LEA among U.S. Medicare beneficiaries with diabetes.
RESEARCH DESIGN AND METHODS
We conducted a cohort study of beneficiaries of Medicare. The geographic unit of analysis was hospital referral regions (HRRs). Tests of spatial autocorrelation and geographically weighted regression were used to evaluate the incidence of LEA by HRRs as a function of geographic location in the U.S. Evaluated covariates covered sociodemographic factors, risk factors for LEA, diabetes severity, provider access, and cost of care.
RESULTS
Among persons with diabetes, the annual incidence per 1,000 of LEA was 5.0 in 2006, 4.6 in 2007, and 4.5 in 2008 and varied by the HRR. The incidence of LEA was highly concentrated in neighboring HRRs. High rates of LEA clustered in contiguous portions of Texas, Oklahoma, Louisiana, Arkansas, and Mississippi. Accounting for geographic location greatly improved our ability to understand the variability in LEA. Additionally, covariates associated with LEA per HRR included socioeconomic status, prevalence of African Americans, age, diabetes, and mortality rate associated with having a foot ulcer.
CONCLUSIONS
There is profound “region-correlated” variation in the rate of LEA among Medicare beneficiaries with diabetes. In other words, location matters and whereas the likelihood of an amputation varies dramatically across the U.S. overall, neighboring locations have unexpectedly similar amputation rates, some being uniformly high and others uniformly low.
In 1969, W.R. Tobler, perhaps the most influential geographer of the last century, stated what became known as the first law of geography, “Everything is related to everything else, but near things are more related than distant things” (1,2). However, one could expect that diabetic foot abnormalities, such as lower-extremity amputation (LEA), should arise as a function of an individual’s health and not geography (3,4). It is estimated that between 10 and 25% of patients with diabetes will develop a foot ulcer in their lifetime (3,5,6). While the average annual cost of care for a Medicare beneficiary with diabetes is close to 10,000,about10,000, about 10,000,about33,000 is reimbursed annually for Medicare beneficiaries with diabetes and a foot ulcer (7). Further, having a foot ulcer is the major risk factor for an LEA (6,8). About 5% per year of those patients with a foot ulcer require an amputation (3).
While many LEAs are thought to be preventable, an estimated 80,000 LEAs are performed on patients with diabetes in the U.S. each year (3,6,9). In 2008, more than 15,000 LEAs were performed on Medicare beneficiaries with diabetes (3). The annual incidence of LEA in Medicare beneficiaries with diabetes was 5 per 1,000 in 2006 and 2007 and 4 per 1,000 in 2008 (3). About $52,000 is reimbursed annually for a Medicare beneficiary with diabetes and an LEA (7). Previous studies have shown that the incidence of LEA among beneficiaries with diabetes in Medicare exhibits stark geographic variation (3,7,10). The goal of this study was to explore the nature of and potential reasons for the geographic variation of incident LEA among diabetic beneficiaries of Medicare. In particular, finding evidence that LEA rates are disproportionately similar within clusters of neighboring locations could help identify causes and thus opportunities for prevention.
RESEARCH DESIGN AND METHODS
Population and study design
We performed a study of the full population of U.S. Medicare beneficiaries with diabetes to explore geographic variation in incident LEA. U.S. Medicare in essence is the largest health care insurance provider in the U.S. and the largest government-funded medical entitlement program. Our cohort was restricted to those individuals enrolled in Medicare Parts A and B fee-for-service with diabetes in 2006, 2007, or 2008. A beneficiary was included if, at any time, s/he had at least a 12-month period of continuous enrollment. Beneficiaries were considered alive up to and including the month of their death. Enrollment was determined using the Medicare Enrollment Database. Additional information concerning the population studied is available online (http://www.effectivehealthcare.ahrq.gov/) as summarized in the Data Points publications series briefs produced as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program funded by the U.S. Department of Health & Human Services Agency for Healthcare Research and Quality (3,7,11).
Identification of patients with diabetes
Beneficiaries were defined as having diabetes if they had two or more claims with ICD-9 codes consistent with diabetes or at least one inpatient claim with ICD-9 codes consistent with diabetes in the 12-month period of continuous enrollment, a method similar to that used by the Centers for Disease Control and Prevention for the study of large administrative datasets (Supplementary Appendix) (3,7,11).
Outcome of interest: LEA
A beneficiary was defined as having an incident LEA if s/he had any of a group of specific Current Procedural Terminology (CPT) codes and the immediate previous 6-month period without any of these CPT codes or ICD-9 codes consistent with LEA (Supplementary Appendix). Codes were also created to separately study minor and major amputations (3,7,11).
Unit of analysis: geographic units
The unit of analysis was the Dartmouth Atlas of Health Care geographic units (n = 306) called hospital referral regions (HRRs). Subjects were assigned to an HRR based on zip code of residence (www.dartmouthatlas.org).
Explanatory or confounding variables
Group-level explanatory variables, all of which were measured at the level of the HRR, included (measured per diabetic population): prevalence of diabetic foot ulcer (DFU), size of the Medicare fee-for-service population, prevalence of diabetes, mortality rate among those with diabetes and a foot ulcer, history of peripheral arterial disease (PAD), obesity, prevalence of microvascular complications of diabetes (3), prevalence rate of macrovascular complications of diabetes (3), and Medicare-reimbursed cost for the care of an individual with diabetes and cost for care of a foot ulcer. Further, 2000 data from the Health Resources and Service Administration were evaluated that estimated the number of physicians per HRR (i.e., all physicians, then separately endocrinologists, general physicians, vascular surgeons, general surgeons, cardiovascular surgeons, and podiatrists) (12). The following HRR level variables for the geographic regions were based on 2000 census data (the most currently available data at the time of our analysis): mean age; percent African American; and a composite measure of socioeconomic status that was validated for a similar population and based on income, housing value, monthly rent, education level, and employment (13). All variables were centered and standardized using z scores.
Analysis
Descriptive analysis.
The incidence rate of LEA by HRR was calculated per year. These incidences were expressed as events per 1,000 person-years and summarized as means or medians across HRR per year. Comparisons among HRRs were made using linear regression. A goal of this study was to evaluate the importance of Tobler’s first law of geography as an explanation for geographic variation in LEA rates. To this end, we evaluated spatial autocorrelation, the condition in which outcomes at nearer locations are more correlated than outcomes at locations that are farther apart. To test for spatial autocorrelation, we used Moran’s I test and local tests of spatial autocorrelation using the local index of spatial autocorrelation statistic (14,15). These tests are more fully explained in the Supplementary Appendix online.
Regression analysis.
Ordinary least squares (OLS) regression, both simple (unadjusted) and multiple (adjusted), and spatially weighted OLS regression with a spatial weights matrix were used. It is important to realize that if any form of autocorrelation exists, it is important to account for it. For this study, our data were potentially spatially autocorrelated. _R_2 (the proportion of variation explained by the model results) values are presented to reflect the proportion of LEA variability in HRRs that was explained by the model. Multiple regression models were fit based on purposeful variable selection, the inclusion of variables with a P value of <0.10, maximization of _R_2. Standard diagnostics were used to make the decision to use spatially weighted regression rather than spatial-lagged models and to assess the model fit (15). As part of sensitivity analyses, similar analyses were conducted based on codes for minor and major LEA. All analyses, including the production of maps, were conducted using STATA 11 (StataCorp LP, College Station, TX), GeoDa (Arizona State University, Tempe, AZ), and/or ArcGIS 9 (Esri, Redlands, CA). Values were mainly reported for 2008 but were similar for 2006 and 2007.
RESULTS
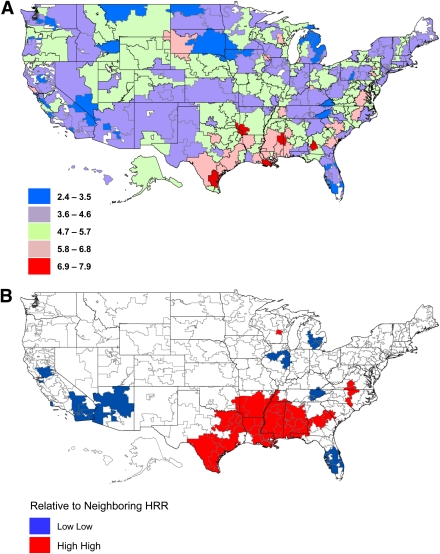
There were about 5 million beneficiaries with diabetes enrolled in Medicare each year between 2006 and 2008 who met our inclusion criteria. The annual incidence of LEA varied by calendar year. The overall mean (median) annual incidence per 1,000 persons trended downward over time and was 5.0 (4.9) in 2006, 4.6 (4.4) in 2007, and 4.5 (4.5) in 2008 (P < 0.0001). Among HRRs, the annual incidence varied approximately three- and fivefold within a year. As an example, the rate of LEA in HRRs in 2008 ranged from 2.4 to 7.9 per 1,000 (P < 0.0001) (Fig. 1_A_).
Figure 1.
Maps of incidence of LEA among diabetic Medicare beneficiaries by HRR, 2008. A: Map of LEA incidence per 1,000 persons on Medicare with diabetes by HRR in 2008. B: Local index of spatial autocorrelation map of LEA incidence showing spatially correlated HRRs of highest incidence of LEA and lowest incidence of LEA in 2008.
In all years, using simple regression to estimate crude associations, the incidence of LEA increased within the HRR as a function of increasing prevalence of diabetes, prevalence of microvascular or macrovascular complication associated with diabetes, percentage of females, mortality rate associated with having a DFU, and the percentage of African Americans. The incidence of LEA was further increased within the HRR with decreasing average age, composite measure of socioeconomic status, and concentration of physicians. Surprisingly, LEA was not associated with the reimbursement from Medicare for the care of individuals with diabetes and a foot ulcer or with reimbursement for medications used to treat diabetes (Table 1). Many of these associations changed using regression models that accounted for spatial autocorrelation (Table 1 and below).
Table 1.
Selected standardized coefficients (all initially measured as rate per 1,000 persons with diabetes and based on z score and with 95% CIs) using simple, multiple, or spatially weighted regression to predict incidence of LEA among diabetic Medicare beneficiaries by HRR for 2008*
| Simple regression | Multiple regression | Multiple regression with spatial weights | |
|---|---|---|---|
| Medicare population | −0.06 (−0.16 to 0.03) | −0.16 (−0.41 to 0.09) | −0.11 (−0.34 to 0.12) |
| Prevalence of diabetes | 0.12 (0.03–0.22)# | −0.09 (−0.17 to 0.01) | −0.18 (−0.31 to −0.05)+ |
| Prevalence of PAD | −0.05 (−0.15 to 0.04) | 0.01 (−0.18 to 0.19) | 0.06 (−0.18 to 0.30) |
| Prevalence of DFU | 0.04 (−0.05 to 0.14) | 0.14 (0.07–0.21)# | 0.11 (0.02–0.20)# |
| Total medical costs paid by Medicare | 0.58 (−0.04 to 0.16) | −0.03 (−0.12 to 0.07) | −0.01 (−0.09 to 0.07) |
| Mortality rate among those with a DFU | 0.26 (0.17–0.36)^ | 0.13 (0.04–0.23)+ | 0.11 (0.01–0.21)# |
| Total medical costs paid by Medicare associated with DFU | −0.03 (−0.12 to 0.06) | −0.04 (−0.17 to 0.08) | −0.01 (−0.10 to 0.08) |
| Prevalence of macrovascular complications | 0.19 (0.09–0.28)^ | 0.04 (−0.09 to 0.16) | −0.01 (−0.13 to 0.11) |
| Prevalence of microvascular complications | 0.14 (0.04–0.24)+ | 0.09 (−0.02 to 0.18) | 0.04 (−0.05 to 0.13) |
| Age (mean) | −0.27 (−0.36 to −0.17)^ | −0.29 (−0.39 to −0.19)^ | −0.17 (−0.27 to −0.07)+ |
| Percent female | 0.10 (0.01–0.20)# | 0.02 (−0.08 to 0.13) | 0.01 (−0.09 to 0.11) |
| Percent African American | 0.23 (0.14–0.33)+ | 0.27 (0.16 to 0.39)^ | 0.18 (0.07–0.29)+ |
| Prevalence of physicians | −0.17 (−0.27 to −0.08)+ | 0.07 (−0.04 to 0.18) | 0.04 (−0.06 to 0.14) |
| Prevalence of podiatrists | 0.07 (−0.02 to 0.17) | 0.02 (−0.07 to 0.11) | 0.03 (−0.01 to 0.07) |
| Socioeconomic status (13) | −0.29 (−0.38 to −0.20)^ | −0.48 (−0.64 to −0.33)^ | −0.45 (−0.53 to −0.38)^ |
Spatial autocorrelation was found across HRRs for LEA rate and for many of the explanatory variables. That is, the LEA rate in HRRs was more similar among HRRs that were close together versus those farther apart, with high HRRs where LEA rates were high being generally nearby HRRs with similar high rates and HRRs with low rates being generally nearby HRRs with rates that were similarly low. Spatial autocorrelation was noted for LEA in HRRs in all years studied (P < 0.0001). Figure 1_B_ provides a better visual understanding of the specific pockets across the country where HRRs and their neighbors had rates of LEA that were similarly high or low. Disproportionately high rates (P < 0.0001) of LEA existed in contiguous portions of southeast Texas, southern Oklahoma, Louisiana, Arkansas, and Mississippi. Additionally, disproportionately low rates (P < 0.001) of LEA were localized through contiguous portions of southern Florida as well as parts of New Mexico, Arizona, and eastern Michigan (Fig. 1_B_).
Finally, we created multiple regression models to see if we could identify characteristics of the populations in and features of HRRs that could explain our findings. The HRR-based variables included in these models were age, sex, percentage African American, prevalence of PAD, prevalence of diabetes, prevalence of DFU, prevalence of microvascular complication, prevalence of macrovascular complication, a composite of socioeconomic status, reimbursement for medications used for diabetes care, reimbursement for foot ulcer therapy, prevalence of physicians, prevalence of podiatrists, and yearly mortality among those with diabetes and a foot ulcer (Table 1). In all years, the ability of our regression models to properly predict LEA incidence were markedly enhanced when the geographic dependence in the data were managed using statistical models that accounted for spatial autocorrelation. As an example, in 2008, the conventional regression model composed of our final covariate set had an _R_2, which is a measure of the accuracy of the regression model, of 0.35. The model that accounted for the geographic nature of the data had an _R_2 of 0.46. Similar results were noted when we conducted sensitivity analyses based on minor and major LEA as our outcome.
Finally, in our “spatial” model, incidence of LEA was increased with decreasing size of the Medicare population, prevalence of diabetes, mean age, and socioeconomic status all measured within the HRR. Further, the incidence of LEA increased with increasing prevalence of DFU, mortality rate associated with DFU, and percentage of the HRR that is African American, all measured within the HRR (Table 1).
CONCLUSIONS
LEA often results from a catastrophic failure to heal a lower-extremity diabetic foot ulcer. Within the Medicare population, the incidence of LEA among patients with diabetes declined between 2006 and 2008 from 5.0 to 4.5 per 1000 person-years. This gradual decline in the rate of LEA has been observed by the Centers for Disease Control and Prevention. However, regardless of the year that we studied, the rate of LEA varied greatly, with similar geographic patterns that were statistically clustered within pockets of neighboring HRRs in every year that we studied. That is, individuals with diabetes living in neighboring HRRs were more similar in terms of their likelihood to have an amputation than individuals living elsewhere. HRRs with high amputation rates were surrounded by HRRs with similarly high rates, and HRRs with low amputation rates were surrounded by HRRs with similarly low rates. This characteristic of LEA is indeed consistent with Tobler’s first law of geography. As a result, our findings suggest that the risk of LEA among those Medicare beneficiaries with diabetes is associated with where people live. We tried to explain this observation using spatially weighted multiple regression techniques to identify characteristics of individuals and locations that could explain the clustering of LEA. LEA appears to be less common in HRRs with lower diabetes prevalence but appears more common in HRRs with lower socioeconomic status, a higher percentage of African Americans, a higher prevalence of DFU, and a higher rate of mortality among those with DFU. Somewhat unexpectedly, the cost of care either to treat DFU (a precursor to LEA) or medically treat those with diabetes was not associated with the prevention of LEA. However, the effect of location could not be fully explained, and allowing the models to compensate for the autocorrelation associated with location always resulted in a better statistical model. In other words, Tobler’s law does extend to amputation risk but the reasons for this neighborly clustered effect are not fully apparent. Potentially this effect might be explained by physician or patient preferences common to neighboring regions, but confirmation will require further study.
Previous studies have shown wide variation between and within countries in the rates of LEA (3,5,6,16,17). For example, in the U.S. in 2001, a study by Wrobel et al. (10) of Medicare beneficiaries using data from 1996 to 1997 described an 8.6-fold regional variation in the rate of LEA. Their method for calculating rates were adjusted for age, race, and sex, but proceeded without respect to the geographic nature of the data and thus may have led to results that were biased due to spatial autocorrelation (10). They opined that one reason for the observed variation in rates of LEA could have stemmed from regional variation in health care provider management decisions (10). Similarly, Moxey et al. (17) analyzed variation in LEA of patients treated by the U.K. National Health Service. They also descriptively evaluated LEA variation among distinct regions in the U.K. (17). Variation was noted in the rate of LEA, but this variation was not as profound (less than twofold variation) as that reported in the U.S. by Wrobel et al. (10) or in our study.
There are many potential explanations for our findings and limitations. First, it is important to realize that our study is of the Medicare population, one of the largest entitlement programs offered by the U.S. government, and may not generalize to those who are not beneficiaries of this program. In addition, our study focused on any LEA and not separately on minor and major amputations. Our results may not generalize nor inform about practice variation with respect to the level of LEA. Second, it seems unlikely that the physical geographic area, as described by an HRR, by itself is a risk factor for LEA. More likely, the location of the HRR is a marker for a factor that was not measured. While some known factors were not accounted for or could have been inaccurately measured, it is unlikely that many known factors would have had a large impact on our findings. For example, chronic kidney disease is associated with LEA and may be a reason for Medicare eligibility for those <65 years of age, and this was not directly measured (18). HRRs that are correlated with increased risk of LEA span parts of several states making it hard to imagine the presence of a disease-based risk factor that follows this precise geographic distribution. Further, some have described an increased risk of LEA in Hispanic populations (6,19). Indeed, two areas with large Hispanic populations (southern Texas and southern Florida) were located in spatially correlated high-risk and lower-risk of LEA zones. Access to care and how care is used are often cited as concerns with respect to severe complications (13,20–23). Our study population included only Medicare beneficiaries who, by definition, all have insurance and therefore have access to some form of care. The cost to treat DFU (a precursor of LEA) was not associated with a decrease in LEA incidence. In other words, treatment for DFU requires access to care, and treatment is often thought to prevent LEA; but in this study, treatment as represented by reimbursement was not associated with reduced rates of LEA, and adjustment did not alter the final results.
A potential and likely explanation stems from the fact that health care providers in different regions have differing management styles and thresholds for performing an LEA as well as for treating diabetes. Currently, there are no agreed upon guidelines for the treatment of DFUs or for determining when an LEA is necessary (22–24). It is possible that health care providers in adjacent HRRs are more likely to manage patients in a similar fashion. Management decisions may correlate with training whereby health care providers are likely to practice geographically close to where they were trained. Geographically associated clinical practices may even take precedence over an individual physician’s experience. With respect to LEA, the prevalence of diabetes and the rate of DFU (i.e., markers of increased disease experience) explained less of the variance in LEA rate per HRR than spatial correlation. Interestingly, a recent study on the incidence of the use of dialysis for end-stage renal disease, which is often associated with diabetes, revealed that those who chose dialysis were less likely to be under the care of a nephrologist or be prepared for dialysis (12). It is also possible that patient-based cultural or educational differences are more similar in geographically adjacent areas, and these attributes create preferences for certain medical therapies such as LEA. In addition, it is possible that treatment variation, such as a tendency to treat patients by diet alone or inadequately with medications and thereby increasing the risk of LEA, varies by the HRR. We were not able to measure treatment variation or the effectiveness of treatment.
In conclusion, in this study we confirm that there is regional variation in the rate of LEA among those with diabetes who are beneficiaries of Medicare. Further, and not previously described, the variation in risk for those with diabetes who are beneficiaries of Medicare is not geographically random but is systematically high or low in many distinct locations in the U.S. We evaluated variations in demographic factors, socioeconomic factors, and disease-related factors within HRRs. While some of the regional variation in the incidence of LEA was explained by these factors, a key predictor of the LEA rate in a given HRR was simply the rate of LEA observed among its neighbors. Areas of the U.S. that are high risk or low risk for LEA appear to border each other, and there is a strong neighborly influence on the rate of LEA in adjacent areas. The ultimate reason for the geographic-based variation and the existence of these clusters is not clear but certainly could be related to several modifiable factors such as health care provider management decisions and training, access and use of preventive care, and patient health–based education and treatment preferences. However, with a more than fivefold variation in the incidence of LEA, future studies to further reveal the factors driving this high and low geographic clustering of amputation rates are indicated. To the degree that these differences are not inherent to a patient’s disease process, development and implementation of practice guidelines for the management of LEA could have profound effects on the incidence of LEA among Medicare beneficiaries.
Supplementary Material
Supplementary Data
Acknowledgments
This study was funded in part by K24AR02212 from the National Institutes of Health to D.J.M.
No potential conflicts of interests relevant to this article were reported.
D.J.M., O.H., C.E.L., C.P.F., S.H., and D.J.W. had full access to all of the data and researched the study data. D.J.M., J.N., and D.J.W. wrote the manuscript. D.J.M., O.H., C.E.L., J.N., S.H., and D.J.W. edited the manuscript and approved the final version of the manuscript.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
References
- 1.Sui DZ. Tobler's first law of geography: a big idea for a small world? Ann Assoc Am Geogr 2004;94:269–277 [Google Scholar]
- 2.Tobler WR. A computer movie simulating urban growth in the Detroit region. Econ Geogr 1970;46:234–240 [Google Scholar]
- 3.Margolis D, Malay DS, Hoffstad OJ, et al. Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. Data Points #2. Rockville, MD, Agency for Healthcare Research and Quality, U.S. Dept. of Health and Human Services, January 2011 (AHRQ Publ. No. 10[11]-EHC009-1-EF) [PubMed] [Google Scholar]
- 4.Iversen MM, Tell GS, Riise T, et al. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care 2009;32:2193–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev 2008;24(Suppl. 1):S3–S6 [DOI] [PubMed] [Google Scholar]
- 6.Reiber GE, Lemaster JW. Epidemiology and Economic Impact of Foot Ulcers and Amputations in People with Diabetes. In Levin and O'Neal's The Diabetic Foot. 7th ed. Bowker JH, Pfeifer MA, Eds. Philadelphia, Mosby Elsevier, 2008, p. 3-22 [Google Scholar]
- 7.Margolis D, Malay DS, Hoffstad OJ, et al. Economic burden of diabetic foot ulcers and amputation: diabetic foot ulcers. Data Points #3. Rockville, MD, Agency for Healthcare Research and Quality, U.S. Dept. of Health and Human Services, January 2011 (AHRQ Publ. No. 10[11]-EHC009-2-EF) [Google Scholar]
- 8.Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157–162 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007 [Internet], 2008. Atlanta, GA, U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed 4 January 2011
- 10.Wrobel JS, Mayfield JA, Reiber GE. Geographic variation of lower-extremity major amputation in individuals with and without diabetes in the Medicare population. Diabetes Care 2001;24:860–864 [DOI] [PubMed] [Google Scholar]
- 11.Margolis D, Malay DS, Hoffstad OJ, et al. Prevalence of diabetes, diabetic foot ulcer, and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. Data Points #1. Rockville, MD, Agency for Healthcare Research and Quality, U.S. Dept. of Health and Human Services, February 2011 (AHRQ Publ. No. 10[11]-EHC009-EF) [PubMed] [Google Scholar]
- 12.O’Hare AM, Rodriguez RA, Hailpern SM, Larson EB, Kurella Tamura M. Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA 2010;304:180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis 2008;51:563–572 [DOI] [PubMed] [Google Scholar]
- 14.Anselin L. Local indicators of spatial association—LISA. Geogr Anal 1995;27:93–115 [Google Scholar]
- 15.Anselin L. Exploring spatial data with GeoDa: a workbook. Urbana, IL, Center for Spatially Integrated Social Science, 2005 [Google Scholar]
- 16.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 17.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg 2010;97:1348–1353 [DOI] [PubMed] [Google Scholar]
- 18.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 2008;31:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaturvedi N, Abbott CA, Whalley A, Widdows P, Leggetter SY, Boulton AJ. Risk of diabetes-related amputation in South Asians vs. Europeans in the UK. Diabet Med 2002;19:99–104 [DOI] [PubMed] [Google Scholar]
- 20.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA 1992;268:2388–2394 [PubMed] [Google Scholar]
- 21.Ward MM. Access to care and the incidence of end-stage renal disease due to diabetes. Diabetes Care 2009;32:1032–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004;351:48–55 [DOI] [PubMed] [Google Scholar]
- 23.Brem H, Sheehan P, Boulton AJ. Protocol for treatment of diabetic foot ulcers. Am J Surg 2004;187:1S–10S [DOI] [PubMed] [Google Scholar]
- 24.Steed DL, Attinger C, Brem H, et al. Guidelines for the prevention of diabetic ulcers. Wound Repair Regen 2008;16:169–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data