Activation of Extracellular Transglutaminase 2 by Thioredoxin (original) (raw)
Background: Extracellular transglutaminase 2 is oxidized and inactive.
Results: Thioredoxin can recognize and reduce disulfide bond of transglutaminase 2 with high specificity.
Conclusion: Extracellular transglutaminase 2 can be activated by both recombinant and cellular secreted thioredoxin.
Significance: This study suggests the physiological mechanism of redox-dependent regulation of transglutaminase 2.
Keywords: Enzyme Inactivation, Extracellular Matrix Proteins, Inflammatory Bowel Disease, Thioredoxin, Transglutaminases
Abstract
The mechanism of activation of transglutaminase 2 (TG2) in the extracellular matrix remains a fundamental mystery in our understanding of the biology of this multifunctional mammalian enzyme. Earlier investigations have highlighted the role of a disulfide bond formed by vicinal Cys residues in maintaining calcium-bound TG2 in an inactive state. Here, we have shown that the redox potential of this disulfide bond is approximately −190 mV, a high value for a disulfide bond in proteins. Consistent with this observation, TG2 activity in a freshly wounded fibroblast culture depends upon the redox potential of the environment. We sought to identify a physiological mechanism for the activation of oxidized TG2. With a _k_cat/Km of 1.6 μm−1 min−1, human thioredoxin (Trx) was a highly specific activator of oxidized human TG2. Trx-mediated activation of TG2 was blocked by PX-12, a small molecule Trx inhibitor that is undergoing clinical trials as a cancer chemotherapeutic agent. In a mixed culture containing fibroblasts and monocytic cells, interferon-γ stimulated Trx release from monocytes, which in turn activated TG2 around the fibroblasts. Recombinant human Trx could also activate extracellular TG2 in cryosections of human and mouse small intestinal biopsies. In addition to explaining how TG2 can be activated by dietary gluten in the small intestinal mucosa of celiac sprue patients, our findings reveal a new strategy for inhibiting the undesirable consequences of TG2 activity in this widespread, lifelong disease.
Introduction
Transglutaminase 2 (TG2), a ubiquitous member of the mammalian transglutaminase family, can be found in the intracellular and extracellular environment of many organs. Its catalytic activity is tightly regulated in response to a wide spectrum of biological cues (1, 2). Therefore, understanding how these diverse signals are integrated and transduced by this multifunctional protein represents an important unsolved problem in chemical biology.
Calcium ions and guanine nucleotides are two well known allosteric regulators of mammalian TG2 activity. In the presence of calcium and the absence of guanine nucleotides, TG2 adopts an “open” and active conformation that cross-links selected glutamine residues on the surfaces of target proteins to a variety of small molecules or macromolecular amines (3). Conversely, in the absence of calcium and the presence of guanine nucleotides, TG2 assumes a “closed” and catalytically inactive conformation (4, 5). Recent studies have revealed a third allosteric regulatory mechanism. Specifically, the formation of a disulfide bond between vicinal Cys residues in the open conformation of the protein reversibly inhibits its enzyme activity (3, 6). Thus, our present knowledge of regulation of TG2 activity can be summarized as shown in Scheme 1. The first of these two reversible processes is perhaps most important when cytosolic TG2 is activated or when it is exported from the cytosol into the extracellular environment. In contrast, the latter process is presumably responsible for allosterically regulating the activity of the vast reservoir of inactive TG2 in the extracellular matrix (7). Notwithstanding these biochemical insights, the physiological mechanism by which redox-dependent regulation of TG2 is achieved remains a mystery. This study sought to address this problem.
SCHEME 1.
EXPERIMENTAL PROCEDURES
Cell Culture
THP-1 cells were cultured as suspension cells in RPMI 1640 medium (Invitrogen A10491) without β-mercaptoethanol; the culture medium was changed every 2–3 days. THP-1 cell density was maintained between 0.4 and 1.0 × 106/ml. WI-38 cells were cultured in T-75 flasks, as described previously (7).
Chemicals and Other Reagents
Cbz-Gln-Gly (ZQG) was from Bachem (Torrance, CA) and glutamate dehydrogenase was from BBI Enzymes (Madison, WI). All other reagents (buffer salts, α-ketoglutaric acid, calcium chloride, and NADH) for assaying TG2 activity were from Sigma. 5-(Biotinamido)pentylamine (5BP)2 was from Pierce. Bovine pancreas insulin (I6634) and rat liver thioredoxin reductase (T9698) were from Sigma. SDS-polyacrylamide gradient gels (5–20%) were from Bio-Rad; nickel-nitrilotriacetic acid-agarose was from Qiagen, and the HiTrap-Q anion exchange column was from GE Healthcare.
Preparation of Recombinant Human TG2
Recombinant human TG2 was expressed and purified as described previously (3). To prepare oxidized TG2 (oxTG2), 10 mg of TG2 was incubated in 5 ml of 20 mm Tris-HCl buffer (pH 7.2) with 10 mm oxidized glutathione (GSSG) for 6 h at room temperature, followed by anion exchange chromatographic purification of the oxidized protein.
Preparation of Recombinant Trx Proteins
The cDNA encoding the full-length human thioredoxin gene (GenBankTM Accession NM_003329) was obtained from Open Biosystems (Huntsville, AL). The gene was PCR-amplified using primers 5′-AAAAAACATATGAAAATCCATCACCATCACCATCACATGGTGAAGCAGATCGAGAG-3′ (forward primer) and 5′-TTTTTTCTCGAGTTAGACTAATTCATTAATGG-3′ (reverse primer). The amplimer was restriction-digested and inserted between the NdeI and XhoI sites of the vector pQE-T7 (Qiagen, Valencia, CA), yielding expression plasmid pCK11, which encoded for full-length human thioredoxin with an N-terminal His6 tag (MKIHHHHHH-Trx). Plasmid pCK11 was introduced into the Escherichia coli Rosetta 2 strain by electroporation. The resulting cells were grown in LB medium containing 50 μg/ml kanamycin and 34 μg/ml chloramphenicol at 37 °C, until the _A_600 of the culture reached 0.6. The culture was then cooled to 18 °C, induced with 0.2 mm isopropyl 1-thio-β-d-galactopyranoside, and grown for another 12 h. Cells were harvested by centrifugation (4420 × g, 15 min). The cell pellet was resuspended in lysis/wash buffer (50 mm phosphate, 10 mm imidazole, 500 mm NaCl, 1 mm DTT (pH 7.6)) and lysed by sonication (six times for 30 s, on ice). After the cell debris was removed by centrifugation (42,700 × g, 45 min), the supernatant was incubated with nickel-nitrilotriacetic acid-agarose for 0.5 h. The resin was washed with 10 column volumes of lysis/wash buffer, and the bound protein was eluted with 4 column volumes of elution buffer (50 mm phosphate, 250 mm imidazole, 50 mm NaCl, 1 mm DTT (pH 7.6)). The eluate was diluted to 50 ml with water and applied to a HiTrap-Q anion exchange column. Recombinant human Trx was eluted in the flow-through. The protein was exchanged into a buffer containing 20 mm Tris-HCl (pH 7.2) and 1 mm DTT and stored at −80 °C.
As a control protein, E. coli thioredoxin was purified from E. coli BL21(DE3)/pET32c, which harbors the full-length thioredoxin gene fused to an N- and C-terminal His6 tag. The protein was expressed and purified similarly as human Trx.
TG2 Activity Assay
TG2 activity was assayed using the glutamate dehydrogenase-coupled assay described previously (8). In brief, enzymatic deamidation of 20 mm ZQG was spectrophotometrically monitored at 340 nm in the presence of 1 μm TG2 and a buffer containing 200 mm MOPS buffer (pH 7.2), 4 mm CaCl2, 0.35 mm NADH, glutamate dehydrogenase (36 units/ml), and 10 mm α-ketoglutarate. The reaction rate was calculated from the slope of the absorbance curve, using the extinction coefficient of NADH (ϵ = 6220 liters·mol−1·cm−1).
Thioredoxin Activity Assay
Steady-state kinetic analysis of Trx-mediated reduction of insulin and oxTG2 was performed via a coupled assay containing 0.5 μm thioredoxin reductase (TrxR), an appropriate concentration of Trx, and 0.3 mm NADPH, in a buffer containing 50 mm Tris-HCl and 2 mm EDTA (pH 7.5) (9). In all such assays, care was taken to ensure that the rate of NADPH oxidation was limited by the concentration of Trx. The reaction rate was calculated from the slope of the absorbance curve, using the extinction coefficient of NADPH (ϵ = 6220 liter·mol−1·cm−1). Michaelis-Menten kinetic parameters were determined by fitting the kinetic data, using GraphPad Prism 5.
WI-38 Fibroblast Scratch Assay
Wounding assays were performed as described previously (7) with minor modifications. Briefly, WI-38 cells were seeded on fibronectin-coated 8-well chambered cover slides and grown until near confluency. GSSG was added to the medium of some wells, and wounds were inflicted using a pipette tip. After 60 min of incubation at 37 °C, the medium was exchanged with or without 2 mm DTT, and samples were further incubated for 30 min at 37 °C. Activity was assessed via a 60-min incubation with medium containing 0.3 mm 5BP. Cells were washed in PBS, fixed for 10 min in ice-cold methanol, and blocked for 1 h with 5% BSA in PBS. Incorporated 5BP was visualized using Cy2-streptavidin (1:1000, GE Healthcare). TG2 protein was stained with mAb CUB7402 (1:500, Thermo Scientific), followed by goat anti-mouse Cy3-IgG1 (1:1500 Southern Biotech) exposure. Nuclei were stained with DAPI. Images were acquired on a Nikon inverted fluorescence microscope.
Trx Secretion and TG2 Activity in Interferon-γ-stimulated THP-1 Cell Cultures
THP-1 cells were grown as described above and incubated with 1000 units/ml IFN-γ for 48 h. Trx levels in the extracellular medium and the cell lysate were assayed by Western blotting, as follows. All samples were diluted 1:1 with 2× Laemmli sample buffer containing β-mercaptoethanol and boiled for 10 min. Samples were applied to 4–20% SDS-polyacrylamide gels and transferred onto a PVDF membrane at 80 V for 2 h in transfer buffer (2.42 g of Tris base, 11.2 g of glycine to 800 ml of water plus 200 ml of methanol). Membranes were blocked with 5% nonfat dry milk in 20 mm Tris-Cl buffer containing 150 mm NaCl and 0.1% Tween 20 for 1 h at room temperature and incubated with mouse anti-Trx mAb (A-5 clone from Santa Cruz Biotechnology, 1:2000 dilution) overnight at 4 °C. After three washes with TBS-T, the membrane was exposed to HRP-conjugated goat anti-mouse IgG (Invitrogen, 1:5000 dilution), and Trx bands were visualized using the ECL-Plus substrate (GE Healthcare). Blots were quantified using a Typhoon fluorescence imager (GE Healthcare).
The ability of the secreted Trx to activate extracellular TG2 was assayed in a co-culture system with WI-38 fibroblast cells, which are known to harbor high levels of inactive TG2 in their environment (7). THP-1 cells (500,000 cells/ml) were pretreated with or without 1000 units/ml IFN-γ for 36 h, concentrated to 1/16th of the original volume, and co-cultured with confluent WI-38 monolayers in an 8-well chamber for another 6 h. THP-1 cells were then carefully washed off by PBS, and Tris-HCl buffer containing 5 mm CaCl2 and 0.5 mm 5-BP was added to each well. Cultures were further incubated for 60 min at 37 °C, washed three times with PBS, and fixed with 4% paraformaldehyde at room temperature for 30 min. After two washes with PBS (5 min each), the contents of each well were blocked with 1% BSA in PBS for 5 min and washed twice with PBS. Alexa Fluor 555-conjugated streptavidin (1:500 dilution in blocking buffer) was added to each well for 30 min at room temperature. The fixed and stained cells were washed again four times with PBS and visualized by fluorescence microscopy.
In Situ TG2 Activity and Tissue Staining
4- and 6-μm cryosections were cut from OCT-embedded small intestinal biopsies. Unfixed sections were preincubated for 20 min at room temperature in 100 mm Tris-HCl (pH 7.4) with 5 mm CaCl2 in the presence or absence of 1 mm DTT or varying amounts of recombinant human Trx. Sections were then incubated in the same buffers with 0.5 mm 5BP at 37 °C for 40 min, followed by a 2-min wash in PBS. The sections were fixed for 10 min with 4% paraformaldehyde and stained for TG2 protein (mAb CUB7402, 1:200) in 1.25% BSA in PBS at room temperature for 1 h. TG2 protein was detected with goat anti-mouse Cy3-IgG1 (1:1500), and TG2 activity was visualized with Cy2-streptavidin (1:500). Nuclei were stained with Hoechst dye. Images were acquired with a Nikon inverted fluorescence microscope. Sections from the biopsies of two healthy controls, two treated celiac disease patients, two untreated celiac disease patients, and from C57/B6 wild-type and TG2 knock-out mice (10) were analyzed. Mice were handled according to a locally approved protocol, and the use of human material was approved by the Norwegian regional ethics committee (REK Sør, Project S-97201).
RESULTS
Redox Potential of the Disulfide Bond in Human TG2
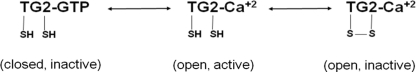
The catalytic activity of TG2 is tightly regulated by the formation of a disulfide bond between Cys370 and Cys371 (6). No residual enzymatic activity was detectable in the disulfide-bonded state in the presence of Ca2+ ions (hereafter referred to as oxTG2). Based on the resolution limit of our assay, we estimated that the specific activity of oxTG2 was no more than 1% of the reduced form of TG2, and therefore we used this activity assay to determine the redox potential of the disulfide bond in human TG2. Purified, fully reduced TG2 was pre-equilibrated with a 10 mm GSH/GSSG redox buffer over a wide potential range (−70 to −230 mV) for 1 h. The steady-state activity of the resulting enzyme was measured in the same redox buffer. The GSH/GSSG ratio was determined to be unchanged over the course of activity assay by analytical HPLC (data not shown). As expected, TG2 was inactivated when the redox potential increased, although the kinetics of inactivation appeared to be relatively slow (Fig. 1A). Because enzymatic activity attained steady state only after ∼4 h, the slopes of individual reaction progress curves were calculated from 4- to 5-h data and compared with the activity of DTT-treated TG2 (Fig. 1B). The redox potential _E_0 of TG2 was calculated by fitting the plot to Nernst Equation 1 for a two-electron process and was determined to be −184 ± 4 mV.

FIGURE 1.
Redox potential of the disulfide bond formed by vicinal Cys residues in oxTG2. A, time dependence of TG2 activity upon exposure to buffers with varying redox potentials. Fully reduced TG2 was preincubated for 1 h in buffers containing varying GSH/GSSG ratios, subjected to a total [GSH] + [GSSG] concentration of 10 mm. Thereafter, TG2 activity was spectrophotometrically monitored over 6 h in the presence of 20 mm ZQG substrate at room temperature. In each case, steady state was achieved after ∼4 h. B, steady-state enzyme activity as a function of redox potential. Specific activity was normalized to the activity of TG2 following 5 mm DTT treatment. C, steady-state fraction of reduced TG2 as a function of redox potential. The fraction of reduced TG2 was determined by mass spectrometry based on the alkylation status of Cys370 and Cys371.
In an alternative and more direct experiment designed to estimate the _E_0 of the disulfide bond, recombinant human TG2 was pre-equilibrated in appropriate redox buffers for 8 h, after which all of its free cysteine residues were covalently labeled by iodoacetamide. The denatured protein was digested with trypsin, separated by C18 liquid chromatography, and analyzed by electrospray ionization-mass spectroscopy. From a quantitative analysis of the relative alkylation of Cys370, Cys371, and Cys230 (6), the fractional oxidation state of TG2 was deduced. In turn, these data were fitted to the same two-electron Nernst Equation 1 to calculate _E_0. The results (_E_0 = −198 ± 5 mV, see Fig. 1C) were in good agreement with the redox potential measured by the activity assay.
TG2 Activity in Freshly Wounded Fibroblast Monolayers Depends upon Redox Potential
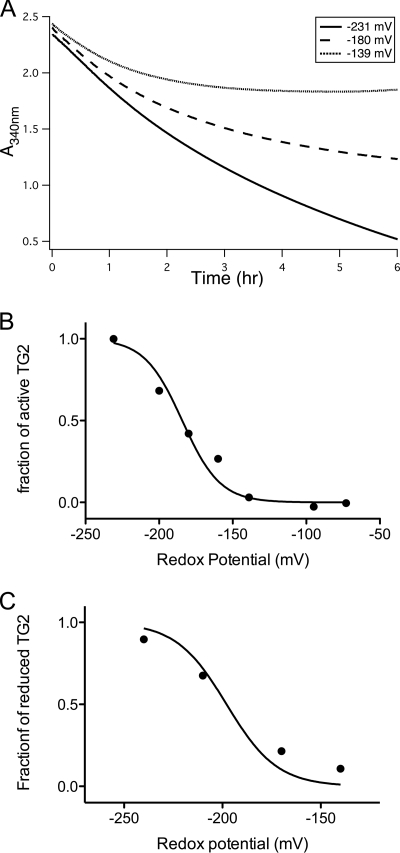
Wounding of WI-38 fibroblast monolayers results in strong and rapid activation of TG2 in the vicinity of the wound (7, 11). This activity is lost over time (∼4 h, data not shown) (7). In the presence of the oxidizing agent GSSG, TG2 activity along the wound boundary was markedly reduced (Fig. 2). Subsequent addition of the reducing agent dithiothreitol (DTT) restored TG2 activity (Fig. 2), suggesting that enzyme inactivation was a reversible process. The dependence of this TG2 activity on the redox potential of the environment was confirmed by titration of the GSH/GSSG ratio (supplemental Fig. S1). Because a majority of TG2 in the extracellular matrix was tightly bound to fibronectin, we also evaluated the activity of recombinant TG2 immobilized on the extracellular fibronectin fibrils of a WI-38 monolayer. As shown in supplemental Fig. S2, the presence of DTT increased the activity of the deposited TG2, suggesting that fibronectin-bound TG2 is also subject to redox regulation.
FIGURE 2.
Redox-dependent TG2 activity in a wounded WI-38 fibroblast monolayer. As shown in the top row (center, left panel), TG2 is rapidly activated along the wound edge of a scratched WI-38 monolayer. In the presence of GSSG (0.5 mm, 2nd row, or 1.5 mm, 3rd row), TG2 activity at the scratch boundary is blocked. Activity is regained upon changing to a medium containing 2 mm DTT (4th and 5th rows). Panels in the left column show TG2 activity as imaged by streptavidin staining, and those in the right column show TG2 protein as imaged by staining with the CUB7402 monoclonal antibody. Each image is a representative of 3–4 wounds; the entire experiment was repeated three times. Scale bars, 100 μm.
In Vitro Activation of oxTG2 by Mammalian Thioredoxin
The high redox potential of the disulfide bond in oxTG2 suggested that this enzyme could be a physiological sensor of subtle changes in the redox potential of the extracellular matrix, which ordinarily is an oxidizing environment. However, even under thermodynamically favorable conditions, the rate of this intramolecular redox reaction is relatively slow (Fig. 1A). We therefore hypothesized that oxTG2 in the extracellular matrix was activated through a specific molecular recognition event involving another redox-active protein.
In theory, any disulfide bond reducing agent with an _E_0 value lower than −190 mV could activate oxTG2. For several reasons, we targeted thioredoxin as such a candidate. First, Trx has a much lower _E_0 value (−230 mV) than TG2 (12), and it can therefore be expected to provide an adequate driving force for the reaction. Second, although extracellular Trx/Trx80 has been thought to have cytokine and chemokine properties (13, 14), recent data have revealed its role in several redox regulatory processes (15, 16). Third, although Trx is predominantly a cytosolic protein in mammals, it can be found at appreciable concentrations (1–10 nm) in extracellular fluids such as plasma (17). Last, but not least, the plasma levels of Trx are known to undergo significant increases in response to various disease states (18, 19), a phenomenon that has also motivated clinical targeting of this extracellular protein for the treatment of cancer (20).
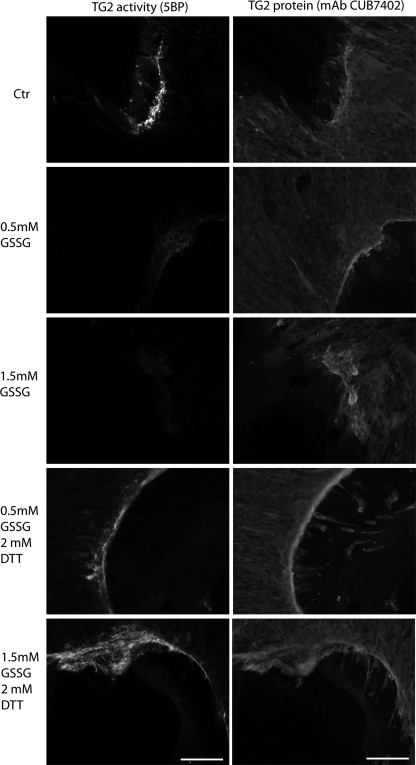
As an initial test of our hypothesis, we conducted a preliminary investigation into the kinetics of Trx-mediated activation of oxTG2. Reduced forms of Trx from E. coli and humans were prepared, as described under “Experimental Procedures.” DTT was used as a reference small molecule reducing agent. Under comparable conditions, recombinant human Trx reduced oxTG2 with a second order rate constant that was at least 100 times higher than DTT and at least 150 times higher than E. coli Trx (data not shown). No transglutaminase activity was detectable in our preparations of recombinant Trx, TrxR, or Trx + TrxR (data not shown). We therefore quantified the specificity of human Trx for human oxTG2 by measuring its Michaelis-Menten parameters. Insulin, a well characterized extracellular substrate of Trx, was used as a reference (21), and human thioredoxin reductase was used as a catalyst to achieve turnover under steady-state conditions. The _k_cat/Km values of Trx for insulin (Fig. 3A) and oxTG2 (Fig. 3B) were 3.6 and 1.6 μm−1 min−1, respectively, and the Km values were 30 and 21 μm, respectively. The observed parameters for insulin were in good agreement with data reported previously (22). Thus, as little as 2.5 nm Trx in the extracellular matrix should be able to activate 10% of the local oxTG2 within 30 min.
FIGURE 3.
Steady-state kinetic analysis of Trx-mediated reduction of insulin (A) and oxTG2 (B). The assay mixture contained 10 nm Trx, 0.5 μm TrxR, 0.3 mm NADPH, and varying substrate concentrations in a buffer containing 50 mm Tris-HCl and 2 mm EDTA. The change in absorbance from 10 to 20 min was used to calculate the rates. Solid lines are best fit curves to the Michaelis-Menten equation. A control experiment verified the inability of TrxR to reduce oxTG2 in the absence of Trx (data not shown).
The small molecule PX-12 (1-methylpropyl 2-imidazolyl disulfide) inhibits human Trx by irreversible thioalkylation of Cys73 (23, 24). In our assay for Trx-mediated activation of oxTG2, PX-12 blocked TG2 activation in a dose-dependent manner (Table 1).
TABLE 1.
PX-12 inhibits Trx-mediated activation of oxTG2
1 μm oxTG2 was incubated with 5 μm Trx (prereduced and free of contaminating DTT) and varying concentrations of PX-12. TG2 activity was measured by a spectrophotometric assay in which NADH consumption was monitored. (For details, see under “Experimental Procedures.”) The extent to which Trx was inhibited by PX-12 was calculated by comparing the (linear) rates of NADH consumption between 90 and 110 min.
| PX-12 | Inhibition of Trx activity |
|---|---|
| μ_m_ | % |
| 0 | 0 |
| 3 | 66 |
| 10 | 92 |
| 30 | >99.9 |
Biological Assay for Interferon-γ-mediated Activation of Extracellular TG2 by Trx
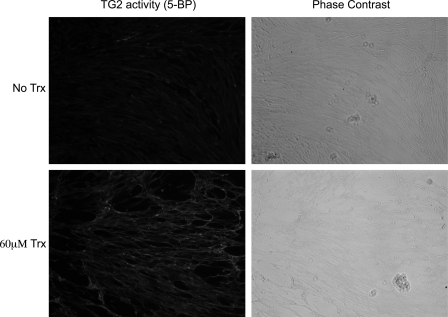
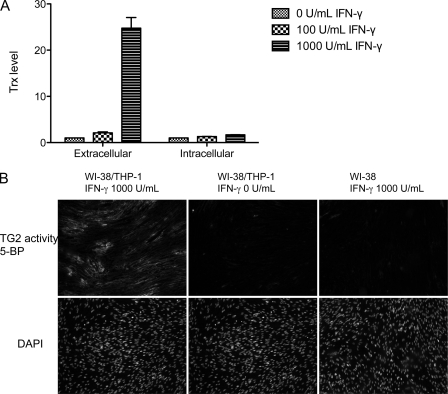
To assess the biological relevance of the above observations, we first added recombinant human Trx to cultured WI-38 fibroblast cells. WI-38 fibroblasts are known to harbor large quantities of inactive TG2 in their extracellular matrix (7). As seen in Fig. 4, cells treated with 60 μm Trx showed significant extracellular TG2 activity relative to controls. We therefore co-cultured the THP-1 monocytic cell line with WI-38 fibroblasts. It is known that exposure to IFN-γ increases the levels of extracellular Trx in cultured THP-1 cells (Fig. 5A) (25). When IFN-γ-treated THP-1 cells were co-incubated with WI-38 monolayers for 6 h, TG2 activity could be detected around many fibroblasts (Fig. 5B). Control experiments verified that monocytes and IFN-γ were required for eliciting TG2 activity in this assay. Thus, it appears that IFN-γ mediates the extracellular release of Trx, which in turn activates extracellular TG2.
FIGURE 4.
Effect of recombinant Trx on TG2 activity in WI-38 cells. WI-38 fibroblasts were incubated at 37 °C with or without recombinant human Trx. Thereafter, the medium was replaced with fresh medium containing 0.5 mm 5BP for 1 h at 37 °C. The incorporated 5BP was detected as described under the “Experimental Procedures.”
FIGURE 5.
A, secretion of Trx by cultured THP-1 cells. Relative abundance of Trx in the extracellular versus intracellular environments of cultured THP-1 monocytes treated with IFN-γ for 48 h. Representative data are average values from experiments performed in triplicate; the entire experiment was repeated with equivalent results. B, activation of TG2 in a co-culture of WI-38 fibroblasts and THP-1 cells. As detailed under “Experimental Procedures,” THP-1 cells were pretreated with (left panel) or without (middle panel) 1000 units/ml IFN-γ for 36 h and then co-cultured with confluent WI-38 fibroblasts for 6 h. In a control experiment, WI-38 cells were cultured alone with 1000 units/ml IFN-γ (right panel).
Trx Can Also Activate TG2 in Small Intestine
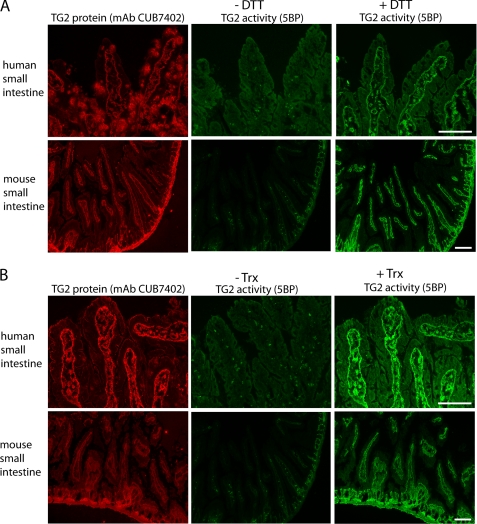
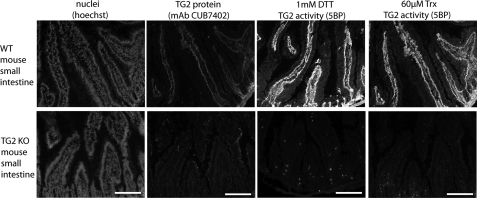
As is the case in WI-38 fibroblast cultures, extracellular TG2 is also largely inactive in the small intestine (7). DTT pretreatment of cryosections of human or mouse small intestine leads to activation of TG2 in these histological samples (Fig. 6A). Comparable TG2 activity could also be observed upon exposure to recombinant human Trx (Fig. 6B). No TG2 activity was observed in biopsies derived from TG2 knock-out mice (Fig. 7).
FIGURE 6.
Activation of TG2 in small intestinal cryosections. A, TG2 activity, measured as 5BP incorporation (green), was observed in mouse and human small intestinal cryosections following incubation with the reducing agent DTT. TG2 protein was visualized with mAb CUB7402 (red) and is only shown for the sections not treated with DTT. The human histological sample was derived from a small bowel biopsy of a treated celiac disease patient. B, incubation of similar cryosections with recombinant human Trx also led to TG2 activation. In both panels TG2 protein staining is only shown for sections treated with Trx. No significant differences were observed between biopsies of treated celiac patients, untreated celiac patients, or disease controls. Scale bars, 100 μm.
FIGURE 7.
Incorporation of 5BP in small intestinal cryosections of wild-type (WT) and TG2 knock-out (KO) mice. Following incubation with DTT or Trx, incorporation of 5BP was only seen in cryosections from WT mice but not TG2 KO mice. No staining of TG2 protein was seen in TG2 KO mice.
DISCUSSION
The mechanism by which TG2 is activated by injury or inflammation is a fundamental mystery in mammalian biology. We have determined that the redox potential of the disulfide bond formed by vicinal Cys residues in human TG2 is approximately −190 mV, higher than the corresponding value for most protein disulfide bonds (26). In many organs, large amounts of TG2 are transported across the mammalian plasma membrane via an unidentified mechanism (27) and deposited in the extracellular matrix in a form that is tightly bound to fibronectin (28). The absence of significant quantities of reducing agents in this environment (29) should lead to relatively rapid oxidation and concomitant inactivation of this pool of TG2. Here, we have shown that active TG2 released into the extracellular space of cultured cells is inactivated by oxidation and that inactive TG2 can be reactivated by reducing agents. We have also verified this reversible phenomenon in small intestinal mucosa.
Starting from the hypothesis that biological activation of extracellular TG2 involves an elaborately regulated molecular recognition event, we have demonstrated that thioredoxin, a ubiquitous redox-active chaperone protein, has strong specificity for oxTG2 as a substrate. With an intracellular concentration in the 5–10 μm range (30), Trx is an abundant cytosolic protein. Therefore, upon injury-mediated tissue damage, a high local concentration of Trx can be expected to transiently appear in the extracellular matrix, leading to rapid activation of TG2.
It is less obvious how TG2 activity is triggered in response to inflammatory signals. IFN-γ is a potent pro-inflammatory cytokine with a central role in celiac disease pathogenesis. This cytokine is secreted by gluten-reactive, disease-specific T cells that reside in the small intestinal mucosa of celiac patients. Although the consequences of IFN-γ release remain to be established, studies in model systems have shown that IFN-γ can increase the trans-epithelial flux of antigen-sized peptides, thereby establishing a potentially autocrine process for gluten-induced toxicity (8). However, this self-potentiating mechanism depends upon the activity of TG2 in the extracellular matrix, because gluten peptides must be deamidated by the enzyme to transform into strong T cell antigens (31). Here, we have shown that IFN-γ triggers Trx release from monocytes, which in turn activates TG2. Trx secretion does not follow the classical Golgi-endoplasmic reticulum route, but it occurs by an unknown leaderless mechanism (32).
A number of reports indicate that local redox changes play an important role in inflammation, both in lymphoid and nonlymphoid tissues (33–36). It remains to be definitively established whether the IFN-γ produced in the celiac intestinal mucosa causes thermodynamic activation of oxTG2 via the onset of a reducing environment or Trx-promoted kinetic activation of oxTG2. Our biochemical and cellular data presented here support the latter model. If so, then a simple yet powerful picture emerges for IFN-γ-mediated potentiation of intestinal inflammation in celiac disease through deamidation of immunogenic gluten peptides.
Last but not least, to the extent our findings are applicable to celiac disease pathogenesis, it is reasonable to expect that extracellular Trx activity in the small intestinal mucosa will be an attractive target for nondietary therapy of celiac disease. In this regard, we note that PX-12, an irreversible Trx inhibitor that blocks TG2 activation in a dose-dependent fashion, has been safely administered to patients at high doses (24). Improved models for characterizing the anti-inflammatory activity of Trx inhibitors are under development in our laboratories.
CONCLUSION
We have determined that the redox potential of the disulfide bond formed by vicinal Cys residues in human transglutaminase 2 (TG2) is approximately −190 mV and that oxidation indeed is a relevant mechanism for regulation of biological TG2. We also show that human Trx recognizes and reduces the disulfide bond of TG2 with high specificity. Finally, we demonstrate that the pro-inflammatory cytokine interferon-γ can induce the secretion of Trx at levels capable of activating oxidized TG2. Our findings have implications for celiac disease pathogenesis and therapy. They also suggest a potential biological role for extracellular Trx, an emerging disease biomarker.
Acknowledgments
We thank Rebekah Silva for help with mass spectrometry analysis of TG2 redox status, Bita Sahaf for helpful discussions regarding thioredoxin and thioredoxin reductase, Gerry Melino for the gift of transglutaminase 2 knock-out mice, and Fleur Du Prè and Shuo-Wang Qiao for harvesting of mouse small intestines.
*
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK63158 (to C. K.). This work was also supported by the Research Council of Norway (to L. M. S.).
2
The abbreviations used are:
5BP
5-biotinamidopentylamine
Trx
thioredoxin
TrxR
thioredoxin reductase
ox
oxidized.
REFERENCES
- 1.Fesus L., Piacentini M. (2002) Trends Biochem. Sci. 27, 534–539 [DOI] [PubMed] [Google Scholar]
- 2.Lorand L., Graham R. M. (2003) Nat. Rev. Mol. Cell Biol. 4, 140–156 [DOI] [PubMed] [Google Scholar]
- 3.Pinkas D. M., Strop P., Brunger A. T., Khosla C. (2007) PLoS Biol. 5, e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S., Cerione R. A., Clardy J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2743–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begg G. E., Carrington L., Stokes P. H., Matthews J. M., Wouters M. A., Husain A., Lorand L., Iismaa S. E., Graham R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19683–19688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamnaes J., Pinkas D. M., Fleckenstein B., Khosla C., Sollid L. M. (2010) J. Biol. Chem. 285, 25402–25409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel M., Strnad P., Watts R. E., Choi K., Jabri B., Omary M. B., Khosla C. (2008) PLOS One 3, e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klöck C., Jin X., Choi K., Khosla C., Madrid P. B., Spencer A., Raimundo B. C., Boardman P., Lanza G., Griffin J. H. (2011) Bioorg. Med. Chem. Lett. 21, 2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arner E. S., Holmgren A. (2001) Curr. Protoc. Toxicol. Chapter 7, Unit 7.4 [DOI] [PubMed] [Google Scholar]
- 10.De Laurenzi V., Melino G. (2001) Mol. Cell. Biol. 21, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upchurch H. F., Conway E., Patterson M. K., Jr., Maxwell M. D. (1991) J. Cell. Physiol. 149, 375–382 [DOI] [PubMed] [Google Scholar]
- 12.Watson W. H., Pohl J., Montfort W. R., Stuchlik O., Reed M. S., Powis G., Jones D. P. (2003) J. Biol. Chem. 278, 33408–33415 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H., Masutani H., Yodoi J. (2006) Semin. Cancer Biol. 16, 444–451 [DOI] [PubMed] [Google Scholar]
- 14.Pekkari K., Holmgren A. (2004) Antioxid. Redox. Signal. 6, 53–61 [DOI] [PubMed] [Google Scholar]
- 15.Xu S. Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C. J., Bahnasi Y. M., Al-Shawaf E., Porter K. E., Jiang L. H., Emery P., Sivaprasadarao A., Beech D. J. (2008) Nature 451, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azimi I., Matthias L. J., Center R. J., Wong J. W., Hogg P. J. (2010) J. Biol. Chem. 285, 40072–40080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura H., Vaage J., Valen G., Padilla C. A., Björnstedt M., Holmgren A. (1998) Free Radic. Biol. Med. 24, 1176–1186 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H., De Rosa S., Roederer M., Anderson M. T., Dubs J. G., Yodoi J., Holmgren A., Herzenberg L. A., Herzenberg L. A. (1996) Int. Immunol. 8, 603–611 [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki K., Noda N., Okada S., Hagiwara Y., Miyata M., Sakurabayashi I., Yamaguchi N., Sugimura T., Terada M., Wakasugi H. (1998) Biotherapy 11, 277–288 [DOI] [PubMed] [Google Scholar]
- 20.Tonissen K. F., Di Trapani G. (2009) Mol. Nutr. Food Res. 53, 87–103 [DOI] [PubMed] [Google Scholar]
- 21.Holmgren A. (1979) J. Biol. Chem. 254, 9627–9632 [PubMed] [Google Scholar]
- 22.Holmgren A. (1979) J. Biol. Chem. 254, 9113–9119 [PubMed] [Google Scholar]
- 23.Kirkpatrick D. L., Kuperus M., Dowdeswell M., Potier N., Donald L. J., Kunkel M., Berggren M., Angulo M., Powis G. (1998) Biochem. Pharmacol. 55, 987–994 [DOI] [PubMed] [Google Scholar]
- 24.Baker A. F., Dragovich T., Tate W. R., Ramanathan R. K., Roe D., Hsu C. H., Kirkpatrick D. L., Powis G. (2006) J. Lab. Clin. Med. 147, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S. H., Oh J., Choi J. Y., Jang J. Y., Kang M. W., Lee C. E. (2008) BMC Immunol. 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azimi I., Wong J. W., Hogg P. J. (2011) Antioxid. Redox. Signal. 14, 113–126 [DOI] [PubMed] [Google Scholar]
- 27.Zemskov E. A., Mikhailenko I., Hsia R. C., Zaritskaya L., Belkin A. M. (2011) PLOS One 6, e19414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radek J. T., Jeong J. M., Murthy S. N., Ingham K. C., Lorand L. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 3152–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiswing L., Oberley T. D. (2010) Antioxid. Redox. Signal. 13, 449–465 [DOI] [PubMed] [Google Scholar]
- 30.Holmgren A., Luthman M. (1978) Biochemistry 17, 4071–4077 [DOI] [PubMed] [Google Scholar]
- 31.Shan L., Molberg Ø., Parrot I., Hausch F., Filiz F., Gray G. M., Sollid L. M., Khosla C. (2002) Science 297, 2275–2279 [DOI] [PubMed] [Google Scholar]
- 32.Rubartelli A., Bajetto A., Allavena G., Wollman E., Sitia R. (1992) J. Biol. Chem. 267, 24161–24164 [PubMed] [Google Scholar]
- 33.Bertini R., Howard O. M., Dong H. F., Oppenheim J. J., Bizzarri C., Sergi R., Caselli G., Pagliei S., Romines B., Wilshire J. A., Mengozzi M., Nakamura H., Yodoi J., Pekkari K., Gurunath R., Holmgren A., Herzenberg L. A., Herzenberg L. A., Ghezzi P. (1999) J. Exp. Med. 189, 1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelini G., Gardella S., Ardy M., Ciriolo M. R., Filomeni G., Di Trapani G., Clarke F., Sitia R., Rubartelli A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sido B., Giese T., Autschbach F., Lasitschka F., Braunstein J., Meuer S. C. (2005) Eur. J. Immunol. 35, 408–417 [DOI] [PubMed] [Google Scholar]
- 36.Gelderman K. A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12831–12836 [DOI] [PMC free article] [PubMed] [Google Scholar]