The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells (original) (raw)
. Author manuscript; available in PMC: 2011 Oct 28.
Published in final edited form as: Cancer Cell. 2005 Oct;8(4):287–297. doi: 10.1016/j.ccr.2005.09.006
Summary
Tumor cells with mutated PTEN proliferate in an EGFR-independent manner. Induction of PTEN sensitizes cells to EGFR inhibition, and the combination causes synergistic apoptosis. Synergy is due to inhibition of two parallel pathways that phosphorylate the proapoptotic protein BAD at distinct sites. Serine 112 phosphorylation is EGFR/MEK/MAPK dependent, whereas serine 136 phosphorylation is PI3K/Akt dependent. Either phosphorylation is sufficient to sequester BAD to 14-3-3. BAD is released and apoptosis is induced only if both serines are dephosphorylated in response to inhibition of both pathways. Reduction of BAD expression by RNA interference prevents apoptosis in response to pathway inhibition. Thus, BAD integrates the antiapoptotic effects of both pathways. Combined inhibition of EGFR and PI3K signaling may be a useful therapeutic strategy.
Introduction
In normal cells, proliferation is regulated by a complex network of pathways that transduces signals from activated growth factor receptors. Stimulation of these receptor-activated pathways results in progression through the cell cycle but also acts to suppress the apoptotic machinery and has many other consequences that result in regulated proliferation. The EGFR tyrosine kinase is an important regulator of the proliferation of normal epithelial cells (Olayioye et al., 2000). EGFR (HER1) is one of four members of the HER kinase family of receptors. These receptors are activated by ligand-dependent homo and hetero-dimerization, which leads to kinase activation, auto- and _trans_-phosphorylation of their intracellular domains, and initiation of signaling. HER kinase activation leads to stimulation of diverse pathways, including those comprised of Ras/Raf/MEK/MAPK, phospholipase C, and STAT (Yarden and Sliwkowski, 2001), _trans_-phosphorylation of HER3 causes potent activation of phosphatidylinositol-3 kinase (PI3K)/Akt signaling, but phosphorylation of EGFR itself is only a weak activator of this pathway (Soltoff et al., 1994).
EGFR-activated signaling stimulates cell cycle progression, regulates cell shape and motility, and inhibits apoptosis (Woodburn, 1999; Yarden and Sliwkowski, 2001). Mutational or autocrine activation of the receptor leads to unregulated proliferation and malignant transformation in model systems and in specific cellular contexts (De Luca et al., 1999; Huang et al., 1997; Moscatello et al., 1998; Salomon et al., 1995). Naturally occurring mutations of EGFR have been detected in a significant proportion of patients with glioblastomas (Frederick et al., 2000) and in non-small cell lung cancer (Paez et al., 2004; Pao et al., 2004). The level of expression of EGFR has also been correlated with poor prognosis in a variety of tumors.
These studies on the physiologic role of EGFR in normal cells, the functional consequences of its activation, and its association with various malignancies led to the hypothesis that it may be a therapeutic target. A variety of inhibitors of HER family tyrosine kinase activity, some selective for EGFR, and several antibodies that bind to the extracellular domains of EGFR and HER2 have been developed (Harari, 2004; Mendelsohn and Baselga, 2000). However, the clinical activity of these strategies has been restricted to a small number of patients. Small molecule inhibitors of EGFR have antitumor activity in the subset (10%–30%) of patients with lung cancer whose tumors harbor EGFR mutations (Paez et al., 2004). Overt antitumor activity is rare in patients with wild-type EGFR and in most tumor types. Anti-EGFR antibodies have antitumor activity in very few patients and marginally sensitize some tumors to chemotherapy and radiation, but the effects are subtle (Baselga et al., 2000; Shin et al., 2001).
The data suggest that EGFR may not play an important role in most carcinomas. Indeed, in several model systems, oncogenic transformation of normal epithelial cells abrogates their dependence on EGFR for proliferation or survival (Markowitz et al., 1994). This is perhaps understandable; Ras, B-Raf, PI3K, PTEN, and Akt are all downstream targets of EGFR that are mutated at high frequency in various malignancies (Li et al., 1997; Samuels et al., 2004; Tsao et al., 2004). The proliferation of tumor cells with PTEN mutation is not dependent on EGFR, but restoration of PTEN expression sensitizes them to EGFR inhibition (Bianco et al., 2003; She et al., 2003b). We have now uncovered a mechanism for this phenomenon. Induction of PTEN expression inhibits Akt activity in these cells, slows their growth, and markedly sensitizes them to induction of apoptosis by gefitinib (Iressa, ZD1839), a selective inhibitor of EGFR tyrosine kinase. Synergy is due to inhibition of two parallel pathways, EGFR/MEK/MAPK and PI3K/Akt, that regulate the function of the proapoptotic protein BAD by phosphorylating it on distinct sites, serines 112 and 136, respectively. Phosphorylation at either site is sufficient to cause BAD to bind to 14-3-3, sequestering it from its effector molecules. BAD is released from 14-3-3 and apoptosis is induced maximally only if both serines are dephosphorylated in response to inhibition of both pathways. Our data demonstrate that the BAD protein is a switch integrating the antiapoptotic effects of multiple pathways that suppress apoptosis in PTEN-deficient tumor cells.
Results
Proliferation of tumor cells with PTEN mutation is not dependent on EGFR
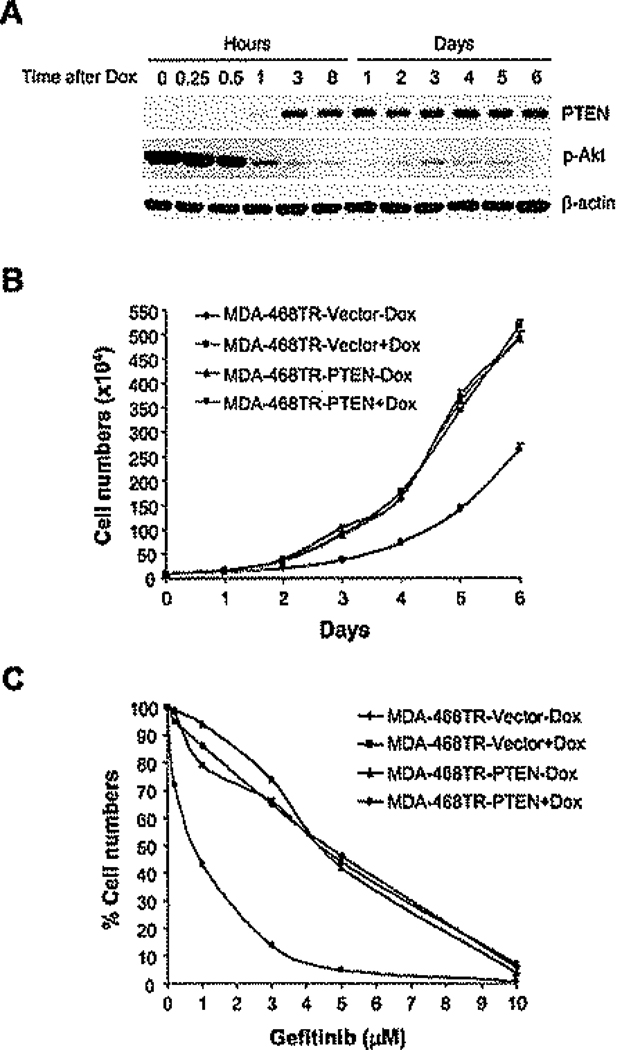
MDA-468 is a breast cancer cell line in which EGFR expression is elevated and the PTEN gene is mutated (Li et al., 1997). In order to study whether cellular dependence on EGFR is dependent on PTEN, we established MDA-468TR-PTEN cells in which PTEN expression is inducible by tetracycline (She et al., 2003b). PTEN expression was maximally induced 3 hr after exposure to 100 ng/ml doxycycline (Figure 1A). PTEN was induced to levels similar to those in SkBr3 breast cancer cells and slightly lower than those in the BT474 cell line (data not shown). Induction of PTEN expression effectively inhibited (>90%) phosphorylation of Akt and caused a slowing of growth in tissue culture, but proliferation did continue (Figures 1A and 1B). We and others have previously shown that expression of PTEN sensitizes these cells to EGFR inhibition (Bianco et al., 2003; She et al., 2003b). The proliferation of normal epithelia is EGFR dependent, and sensitive tumor cells are inhibited by the EGFR inhibitor gefitinib over a concentration range of 50–700 nM (Baselga et al., 2002; Moasser et al., 2001), whereas MDA-468 cells were inhibited by concentrations (5–6 µM) far exceeding those required to inhibit EGFR (Figure 1C). Induction of PTEN sensitized MDA-468 cells to much lower doses of gefitinib consistent with those required for EGFR inhibition (IC50s in 600–700 nM range).
Figure 1.
Induction of PTEN in tumor cells with PTEN mutation sensitizes them to EGFR inhibition
A: MDA-468TR-PTEN cells were treated with 100 ng/ml doxycycline (Dox) and harvested at the indicated times. Cell lysates were immunoblotted with the indicated antibodies.
B and C: MDA-468TR-PTEN and vector control cells were treated with or without Dox (100 ng/ml) (B), or with Dox in combination with gefitinib for 6 days (C). Results represent mean ± SE from three independent experiments (B) or are shown as a percentage of cell number relative to getitinib-untreated control cells (C).
PTEN restoration sensitizes cells to induction of apoptosis by EGFR inhibitors
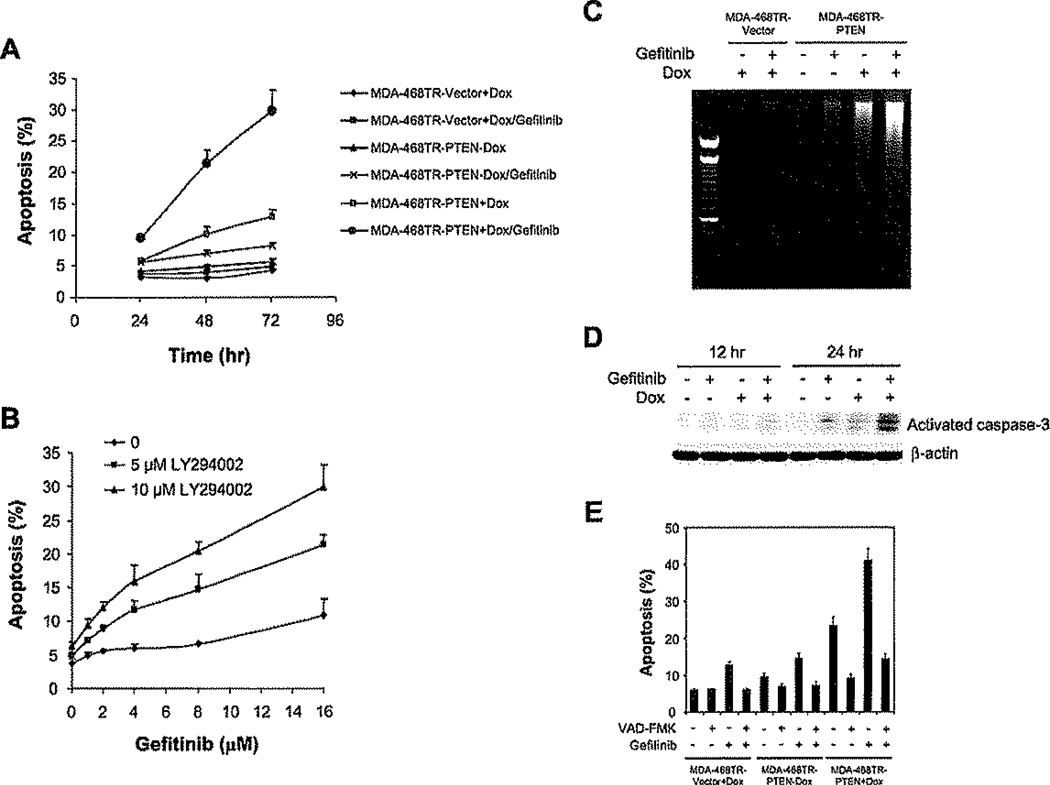
The sensitization of MDA-468 cells to gefitinib by PTEN was not due to an effect on their proliferation. Neither PTEN induction nor the PI3K inhibitor LY294002, alone or in combination with gefitinib, affects the cell cycle distribution of MDA-468 (data not shown). Instead, a synergistic induction of apoptosis was observed when gefitinib was administrated in combination with PTEN induction (Figure 2A) or LY294002 (Figure 2B). Similar results were obtained in SKMG-3 glioblastoma cells, which also overexpress EGFR and are PTEN null (Thomas et al., 2001) (Figure S1 in the Supplemental Data available with this article online). Gefitinib alone had little effect on apoptosis, and PTEN induction had a modest effect (increase from 5% to 13% at 72 hr), but the combination induced a marked and synergistic induction (31%) of apoptosis as assessed by increased sub-G1 fraction or by DNA fragmentation (Figures 2A–2C). Induction of PTEN in combination with gefitinib treatment caused synergistic activation of caspase-3, a key effector of apoptosis, by 24 hr (Figure 2D) and substantially increased levels of cleaved PARP, a caspase-3 substrate (data not shown) (Nicholson et al., 1995). Furthermore, the induction of apoptosis by the combination was markedly inhibited by the broad spectrum caspase inhibitor Z-VAD-FMK (Figure 2E). Thus, inhibition of PI3K renders these cells dependent on EGFR for suppression of caspase-3-dependent apoptosis.
Figure 2.
Inhibition of both PI3K and EGFR synergistically induces apoptosis via activation of caspase-3
A and B: MDA-468TR-PTEN and control cells were treated with 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml (A). MDA-468 cells were treated with gefitinib and LY294002 for 72 hr (B). The fraction of apoptotic cells (sub-G1) was determined by flow cytometry, and the results represent mean ± SE from three independent experiments.
C: MDA-468TR-PTEN and control cells were treated with 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml) for 8 hr and then assessed for DNA fragmentation.
D: MDA-468TR-PTEN cells were treated with 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml). Cell lysates were immunoblotted for the active fragment of caspase-3 and β-actin.
E: MDA-468TR-PTEN and control cells were pretreated with 100 µM caspase inhibitor Z-VAD-FMK, for 1 hr followed by 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml) for 72 hr. Apoptotic cells (sub-G1) were determined as in A, and the results represent mean ± SE from two independent experiments.
Inhibition of Akt signaling is required to sensitize PTEN-deficient tumor cells to EGFR inhibitors
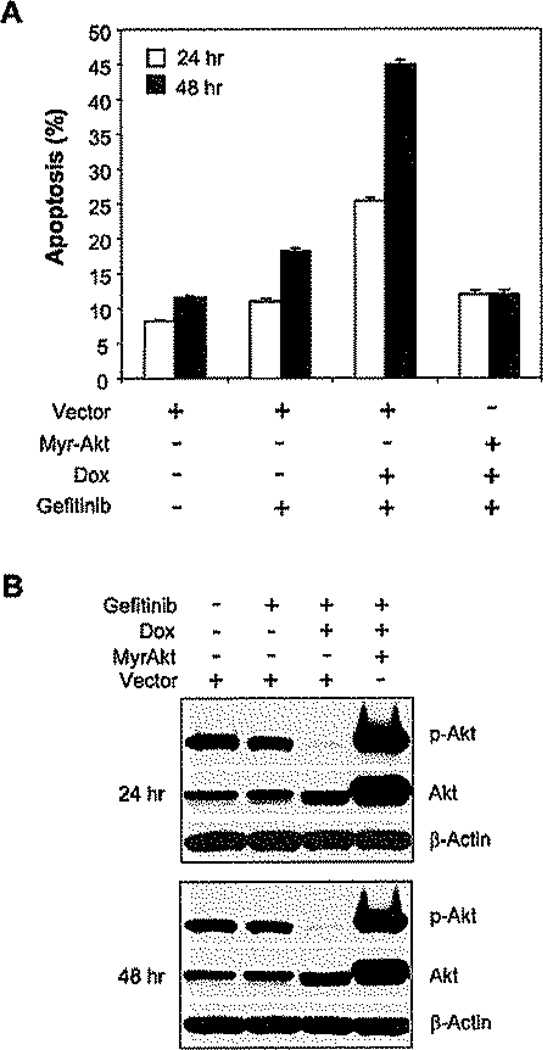
Induction of PTEN expression results in a marked decline in intracellular Akt activity. Myristoylated Akt (Myr-Akt) is constitutively localized to the membrane (Alessi and Cohen, 1998), and when it was transiently expressed in MDA-468 cells, induction of PTEN no longer inhibited the phosphorylation of Akt (Figure 3B). Furthermore, induction of PTEN did not sensitize Myr-Akt-expressing cells to gefitinib (Figure 3A). Thus, induction of apoptosis requires PTEN-dependent downregulation of Akt kinase activity.
Figure 3.
Constitutively active Akt prevents apoptosis induced by PTEN induction in combination with gefitinib
A and B: MDA-468TR-PTEN cells were transfected with myristoylated Akt (Myr-Akt) or vector control. Cells were then treated with 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml). Apoptotic cells (sub-G1) were determined as in Figure 2A, and the results represent mean ± SE from three independent experiments (A). Levels of phosphorylated Akt, total Akt, and β-actin were assessed by immunoblotting (B).
EGFR inhibition affects MAP kinase but not Akt kinase activation
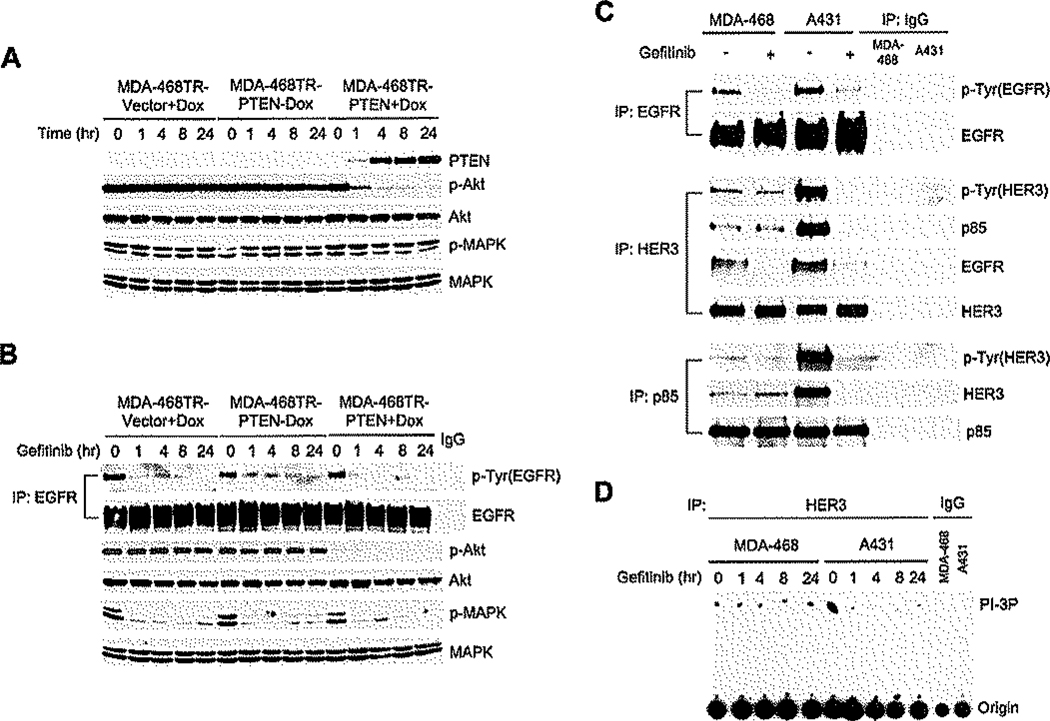
Loss of Akt activity is necessary, but not sufficient, for the synergistic induction of apoptosis by PTEN and gefitinib. Induction of PTEN in MDA-468 cells markedly reduced Akt phosphorylation but not MAPK phosphorylation (Figure 4A). Three micro-molar gefitinib, a concentration in excess of that required for maximal reduction of EGFR and HER2 phosphorylation in these cells (Bianco et al., 2003; Moasser et al., 2001), inhibited MAPK phosphorylation in either PTEN-expressing or control cells (Figure 4B). However, phosphorylation of Akt was unaffected by gefitinib in PTEN-deficient cells, and it did not further reduce Akt activity after PTEN induction. Similarly, in SKMG-3 cells, phosphorylation of Akt was affected by the PI3K inhibitor LY294002, but not by gefitinib (Figures S1 and S2).
Figure 4.
Activation of PI3K and Akt is independent of EGFR in PTEN-deficient tumor cells
A and B: MDA-468TR-PTEN and control cells were treated with or without Dox (100 ng/ml) (A). MDA-468TR-PTEN and control cells were pretreated with Dox (100 ng/ml) overnight to induce PTEN and then treated with 3 µM gefitinib (B). Cell lysates were immunoblotted with the indicated antibodies. IP was done with EGFR antibody or control IgG followed by immunoblotting of tyrosine-phosphorylated EGFR and total EGFR (B).
C: MDA-468 and A431 cells were treated with 3 µM getitinib or DMSO (<0.1%) for 4 hr. IP was done with the indicated antibodies or control IgG followed by immunoblotting with the indicated antibodies.
D: MDA-468 and A431 cells were treated with 3 µM gefitinib or DMSO (<0.1%). Cell lysates were immunoprecipitated with HER3 or control IgG antibody, and PI3K assays were performed as described.
The data suggest that PI3K/Akt is EGFR independent in these cells. To confirm this conclusion, the effect of gefitinib on the association of PI3K with HER kinases in MDA-468 cells was assessed. HER3 has no tyrosine kinase activity, but contains multiple tyrosines that, when phosphorylated, are high affinity docking sites for PI3K (Soltoff et al., 1994). Treatment of MDA-468 cells with gefitinib inhibited EGFR phosphorylation and markedly reduced the association of EGFR with HER3 (Figure 4C). However, the tyrosine phosphorylation of HER3 was not inhibited by gefitinib, and the interaction of HER3 with the PI3K p85 regulatory subunit was insensitive to gefitinib. Furthermore, HER3-associated PI3K activity was also unaffected (Figure 4D). In contrast, in A431 tumor cells with wild-type PTEN that are sensitive to EGFR inhibitors, gefitinib inhibited the interaction of p85 with HER3, HER3-associated PI3K activity (Figures 4C and 4D), and Akt phosphorylation (Moasser et al., 2001). Thus, in MDA-468 cells, the inhibition of Akt that is required for the induction of apoptosis by the combination of gefitinib and PTEN induction is entirely due to the latter. In these cells, the PI3K/Akt pathway is not driven by EGFR.
How, then, does EGFR inhibition affect tumor cells in which PI3K is inhibited by PTEN? Gefitinib effectively inhibits MAPK phosphorylation in MDA-468 cells, whether or not PTEN expression has been induced (Figure 4B). The selective MEK kinase inhibitor CI-1040 (Sebolt-Leopold, 2004) was used to determine whether the consequences of MEK/MAPK inhibition were similar to those of EGFR inhibition. Induction of PTEN or inhibition of PI3K with LY294002 caused 2- to 3-fold sensitization of MDA-468 cells to MEK inhibition (Figures 5A and 5B). CI-1040 also synergized with PTEN or LY294002 to induce apoptosis (Figures 5C and 5D). We tested the effects of combined inhibition of MEK and PI3K in two other tumor cell lines with PTEN deletion. SKMG-3 is a glioblastoma with high levels of expression of EGFR (Thomas et al., 2001); SKMel-11 is a melanoma with PTEN deletion in which MAPK is driven by mutated B-Raf (Tsao et al., 2004). CI-1040 and LY294002 induced synergistic apoptosis in both of the cell lines as well (Figures 5E and 5F). These data show that in tumor cells with PTEN deletion and significant activation of MAPK, inhibition of PI3K/Akt signaling alone is insufficient to induce marked apoptosis, but inhibition of both pathways together has synergistic effects. Further, these results suggest that the effects of EGFR inhibition are mediated, at least in part, by downstream inhibition of MAPK. However, other pathways are likely to be also involved, as inhibition of EGFR is more effective than inhibition of MEK (comparison of IC50 in Figure 1C versus Figure 5A).
Figure 5.
The MEK/MAPK pathway in part mediates sensitization to EGFR inhibition
A and B: MDA-468TR-PTEN and control cells were treated with CI-1040 (MEK inhibitor) or DMSO (<0.1%) with or without Dox (100 ng/ml) for 5 days (A). MDA-468 cells were treated with CI-1040 and LY294002 for 5 days (B). Results are shown as a percentage of cell number relative to CI-1040-untreated control cells.
C–F: MDA-468TR-PTEN and control cells were treated with CI-1040 or DMSO (<01%) with or without Dox (100 ng/ml) for 72 hr (C). MDA-468 (D). SKMG-3 (E), and SKMel-11 (F) cells were treated with CI-1040 and LY294002 for 72 hr (D and E) or the indicated times (F). Apoptotic cells (sub-G1) were determined as in Figure 2A, and the results represent mean ± SE from two or three independent experiments.
Inhibition of BAD phosphorylation integrates the effects of EGFR/MEK/MAPK and PI3K/Akt signaling on apoptosis
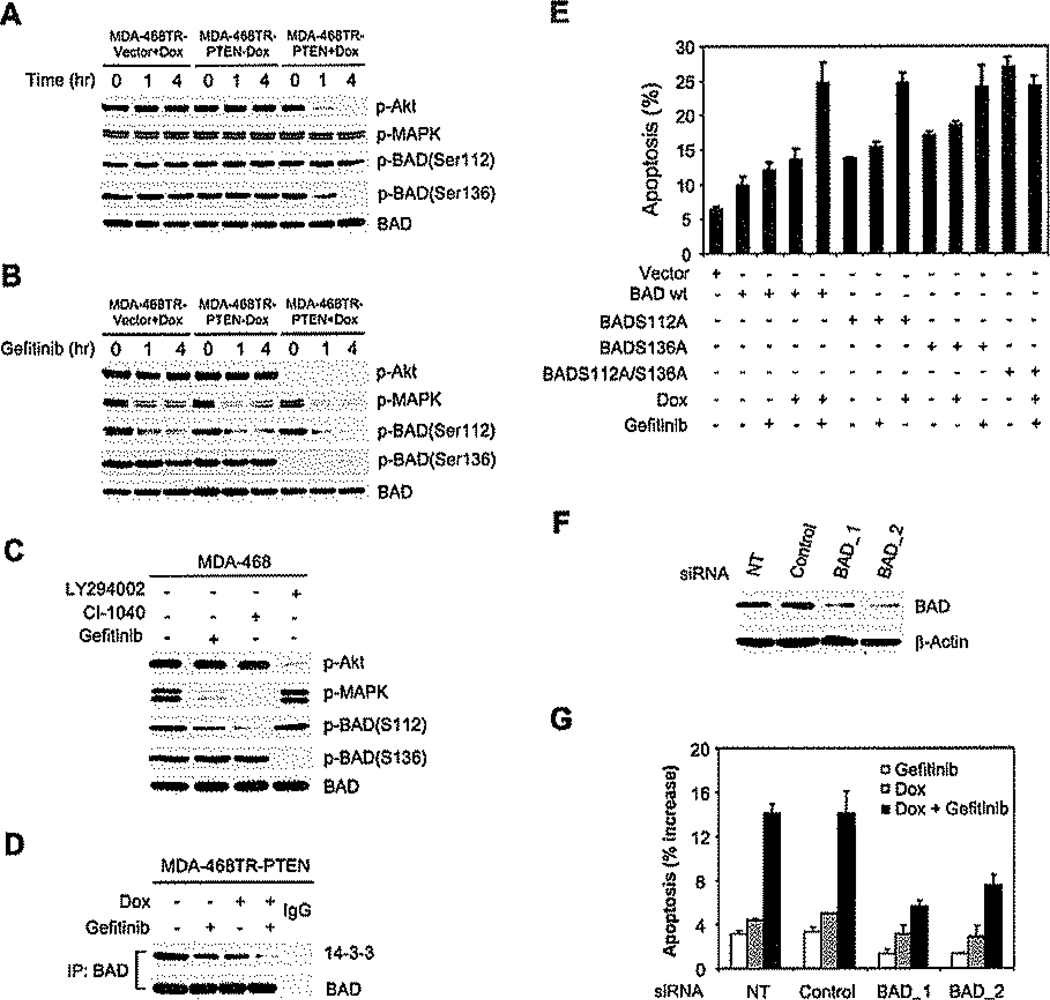
The BAD protein plays an important role in mediating the apoptotic signal in cells deprived of growth factors. BAD is a BH3 domain protein that induces apoptosis by dimerizing with and inactivating the antiapoptotic proteins Bcl-2 and Bcl-XL (Yang et al., 1995). Growth factors prevent apoptosis, in part, by activating signaling pathways that result in the serine phosphorylation of BAD (Bonni et al., 1999; Zha et al., 1996). Phosphorylated BAD is bound to 14-3-3 protein and is thus unavailable to interact with members of the Bcl-2 family (Zha et al., 1996). In many cells, BAD is predominantly phosphorylated on serine 112, a site that is MAPK dependent (Bonni et al., 1999; Fang et al., 1999; Scheid et al., 1999). BAD is phosphorylated on other sites as well, including serine 136, an Akt-dependent site (Datta et al., 1997). It was difficult to determine the phosphorylation state of endogenous BAD in MDA-468 cells with available reagents. In MDA-468 cells in which it was transiently overexpressed, BAD was phosphorylated on serine 112 and serine 136 (Figure 6A) and was found to be associated with the 14-3-3 protein (Figure 6D). Serine 112 phosphorylation was sensitive to EGFR and MEK inhibitors (Figures 6B and 6C), but not to PI3K inhibitors or to PTEN induction (Figures 6A–6C). In contrast, serine 136 was dephosphorylated in response to manipulations that result in Akt inhibition (Figures 6A–6C), but not to those that cause a decline in MAPK activation (Figures 6B and 6C), Similar results were also obtained in SKMG-3 cells (Figure S2). Neither inhibition of serine 112 nor inhibition of serine 136 was sufficient to cause a significant decrease in BAD-14-3-3 association (Figure 6D). However, the binding of BAD to 14-3-3 was efficiently abrogated by the combination (Figure 6D).
Figure 6.
Inhibition of MAPK-dependent serine 112 phosphorylation and Akt-dependent serine 136 phosphorylation of BAD causes synergistic induction of apoptosis
A–C: MDA-468TR-PTEN, MDA-468TR-Vector, or MDA-468 cells were transfected with wild-type BAD. The transfected cells were treated with or without Dox (100 ng/ml)(A); or pretreated with Dox (100 ng/ml) overnight to induce PTEN and then treated with 3 µM gefitinib (B); or treated with 3 µM gefitinib, 1 µM CI-1040, or 5 µM LY294002 for 4 hr (C). Cell lysates were immunoblotted for the indicated antibodies. Samples were also immunoprecipitated with BAD antibody and immunoblotted for phosphorylated BAD(Ser136).
D: MDA-468TR-PTEN cells transfected with BAD were pretreated with Dox (100 ng/ml) overnight to induce PTEN and then treated with 3 µM gefitinib for 4 hr. Cell lysates were immunoprecipitated with BAD antibody or control IgG and immunoblotted for 14-3-3 protein.
E: MDA-468TR-PTEN cells were transfected with empty vector or wild-type BAD, the BAD mutants S112A and S136A, or the double mutant S112A/S136A. The transfected cells were treated with 3 µM gefitinib or DMSO (<0.1 %) with or without Dox (100 ng/ml) for 72 hr. Apoptotic cells (sub-G1) were determined as in Figure 2A, and the results represent mean ± SE from three independent experiments.
F and G: MDA-468TR-PTEN cells were either not transfected (NT) or stably transfected with negative control vectors or a mixture of four different siRNA vectors targeted against BAD. Immunoblotting was used to analyze the indicated proteins in two different clones with stable decreases in BAD expression (F). The transfected cells were treated with 3 µM gefitinib or DMSO (<0.1%) with or without Dox (100 ng/ml) For 72 hr. Apoptotic cells (sub-G1) were determined as in Figure 2A. The results are expressed as the increased levels of apoptosis by subtracting each of the untreated controls and represent mean ± SE from three independent experiments (G).
To establish that activation of BAD requires inhibition of both pathways and confirm that dephosphorylation of BAD is responsible for the synergistic induction of apoptosis, we transfected wild-type BAD, the BAD mutants S112A and S136A, or the double mutant S112A/S136A into MDA-468TR-PTEN cells (Figure 6E). Maximal induction of apoptosis in wild-type transfectants required inhibition of both pathways. BADS112A transfectants had higher baseline apoptosis than wild-type, and apoptosis was enhanced by induction of PTEN but not by gefitinib. The BADS136A mutant had the opposite phenotype: insensitivity to PTEN induction and enhancement by gefitinib. The double mutant induced maximal apoptosis, which was insensitive to inhibition of both pathways.
To determine whether BAD is directly involved in the apoptotic response to gefitinib and PTEN expression, we used siRNA to knock down expression of endogenous BAD protein in MDA-468TR-PTEN cells. In two different clones with stable expression of BAD siRNA and 70%–80% decreased BAD expression, induction of apoptosis by gefitinib and PTEN expression was significantly reduced (Figures 6F and 6G). These data support the idea that synergistic apoptosis induced by combined inhibition of EGFR/MAPK and PI3K/Akt signaling is due to activation of the BAD protein.
Induction of PTEN expression synergizes with EGFR inhibitors to suppress MDA-468 xenograft growth in vivo
The synergistic proapoptotic effect of EGFR inhibition and PTEN induction suggests that targeting both PI3K and EGFR signaling may be a rational strategy for the treatment of tumors with defective PTEN and active EGFR. We sought to determine whether inhibition of both pathways in vivo would effectively suppress tumor growth. Nude mice were injected subcutaneously with MDA-468TR-PTEN and vector control cells. Mice with established tumors were treated with doxycycline in the drinking water. Doxycycline effectively induced PTEN and caused profound reduction in Akt phosphorylation but had no effect on EGFR or MAPK phosphorylation (Figures 7B and 7C). In contrast, gefitinib had no effect on Akt phosphorylation but markedly inhibited phosphorylation of EGFR and MAPK, which fell within 5 hr of drug administration and remained depressed for at least 24 hr (Figure 7C).
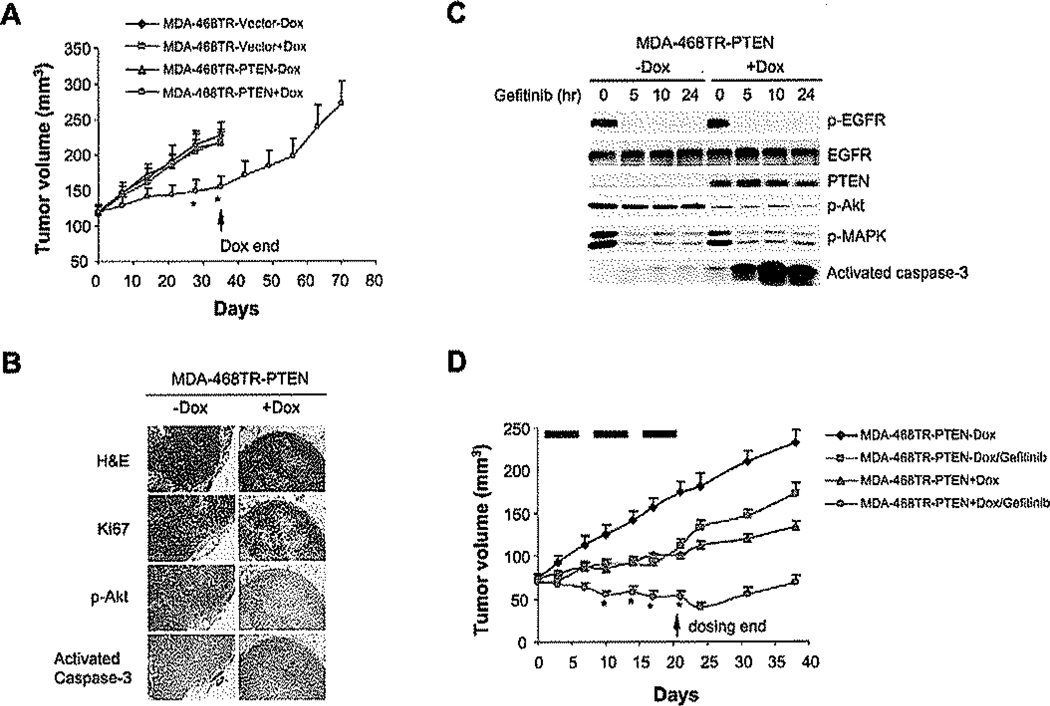
Figure 7.
Induction of PTEN in combination with the EGFR inhibitor gefitinib synergistically suppresses PTEN-deficient and EGFR-overexpressing tumor growth in vivo
A: Mice with established MDA-468TR-PTEN and vector control xenografts were treated with Dox (0.5 mg/ml) in the drinking water. The arrow indicates withdrawal of Dox beginning on day 35. The results represent the mean tumor volume ± SE (n = 5 mice/group) from two independent experiments. *p < 0.01, MDA-468TR-PTEN + Dox versus MDA-468TR-Vector-Dox, MDA-468TR-Vector + Dox, or MDA-468TR-PTEN-Dox.
B: Mice with established MDA-468TR-PTEN xenografts were maintained with or without Dox (0.5 mg/ml) in the drinking water for 24 hr. Tumors were excised, and H&E, Ki67, phosphorylated Akt, and activated caspase-3 were assessed by immunohistochemical staining.
C: Mice with established MDA-468TR-PTEN xenografts were maintained with or without Dox (0.5 mg/ml) in the drinking water and treated with gefitinib 150 mg/kg/day × 3 days or vehicle only (<0.3% lactic acid) as control. Tumors were excised pretreatment and at various times after the last dose of gefitinib administration. The levels of phosphorylated EGFR(Tyr1068), Akt and MAPK, and total EGFE, PTEN, and activated caspase-3 were assessed by immunoblotting.
D: Mice with established MDA-468TR-PTEN xenografts were maintained with or without Dox (0.5 mg/ml) in the drinking water and treated with gefitinib 150 mg/kg/day p.o. × 5 days/week × 3 weeks or vehicle only (<0.3% lactic acid) as control. Dox was given to mice until the gefitinib treatment was finished. Black rectangles represent gefitinib treatment weeks. The results represent the mean tumor volume ± SE (n = 5 mice/group) from two independent experiments, *p < 0.01, MDA-468TR-PTEN + Dox/gefitinib versus MDA-468TR-PTEN-Dox, MDA-468TR-PTEN-Dox/gefitinib, or MDA-468TR-PTEN + Dox.
Induction of PTEN slowed tumor growth (p < 0.01), but the growth suppression was reversible upon withdrawal of doxycycline and loss of PTEN expression (Figure 7A). Doxycycline treatment had no significant effects on tumor growth in vector control mice (p > 0.05). In mice, the maximally tolerated dose of gefitinib given daily for 5 days ranged from 150 to 200 mg/kg. Treatment with gefitinib also slowed tumor growth, but neither PTEN induction nor gefitinib caused tumor regression or completely prevented tumor growth (Figure 7D). However, gefitinib in combination with PTEN induction synergistically suppressed the growth of the tumor xenografts and caused tumor regression (p < 0.01). Furthermore, caspase-3 was activated synergistically by PTEN induction in combination with gefitinib (Figure 7C). These results recapitulate the tissue culture data and show that PTEN reexpression sensitizes tumor cells to induction of apoptosis by EGFR inhibition in vivo as well as in tissue culture.
Discussion
The EGFR receptor plays an important role in regulating the proliferation and survival of normal epithelial cells. In various model systems, overexpression, autocrine stimulation, and mutation of EGFR have been shown to be transforming (De Luca et al., 1999; Huang et al., 1997; Salomon et al., 1995). Mutations in the EGFR gene have recently been identified in a minority of non-small cell lung cancers, and their presence is strongly correlated with clinical response to EGFR inhibitors (Paez et al., 2004). A truncated, alternative form of EGFR is often expressed in glioblastoma and is felt to play a role in their pathogenesis (Ekstrand et al., 1994). However, it is difficult to show that EGFR plays a role in the overwhelming majority of tumors in which it is wild-type. Potent, selective inhibitors of the EGFR tyrosine kinase have been developed, and they have limited activity in tumor types that do not harbor EGFR mutations (Paez et al., 2004).
EGF induces proliferation by activating a network of signaling elements, including members of the STAT family, PLCγ, Ras, and PI3K (Yarden and Sliwkowski, 2001). Genes encoding components of these pathways are often mutated in malignancy, especially _K_- and N-Ras, B-Raf, PI3K, and PTEN (Li et al., 1997; Samuels et al., 2004; Tsao et al., 2004). Mutational activation of pathways downstream of EGFR in cancer cells may relieve their dependence on at least some aspects of EGFR signaling. Indeed, it has been noted that, whereas normal colon and breast epithelial cell models are quite dependent on EGFR for proliferation (Markowitz et al., 1990; Ram et al., 2000), oncogenic transformation or malignant progression are associated with increasing EGFR independence (Markowitz et al., 1994). Furthermore, it has been recently shown that EGFR and Ras mutations are mutually exclusive in lung cancer (Paez et al., 2004; Pao et al., 2005).
The PTEN gene is frequently mutationally inactivated in human cancer, often in glioblastoma and prostate cancer (Li et al,, 1997), tumors in which truncated or wild-type EGFR is over-expressed (Ekstrand et al., 1994; Thomas et al., 2001). We and others have shown that these tumors are not dependent on EGFR but are sensitized to EGFR inhibitors when PTEN is reexpressed (Bianco et al., 2003; She et al., 2003b). In this paper, we confirm these data in a system in which PTEN expression is under inducible control in tissue culture and in vivo. We have gone on to demonstrate that reexpression of PTEN or pharmacological inhibition of PI3K causes PTEN-deficient tumor cells to depend on EGFR for survival. Others have shown that PTEN expression affects cell cycle progression as well (Ramaswamy et al., 1999). The RB protein is mutated in MDA-468 (Srethapakdi et al., 2000), which may explain the absence of an anti-proliferative effect of PTEN. Inhibition of EGFR with gefitinib synergizes with induction of PTEN or inhibition of PI3K to induce caspase 3-dependent apoptosis in tissue culture and in a xenograft model.
Apoptosis is marked and dependent upon inhibition of Akt activity. Induction of PTEN expression downregulates Akt kinase activity. Introduction of constitutively active, membrane bound myr-Akt prevents PTEN-induced Akt inhibition and sensitization of cells to gefitinib. Prior studies suggested the possibility that reexpression of PTEN sensitizes cells to EGFR inhibitors because it restores the EGFR dependence of PI3K/Akt kinase signaling (Bianco et al., 2003; She et al., 2003b). Our data here do not support this view. We show that induction of PTEN causes a profound inhibition of the phosphorylation of Akt and its substrates that is not further enhanced by EGFR inhibitors. Furthermore, activated EGFR does not bind with high affinity to the p85 regulatory subunit of PI3K and, in most systems, PI3K activity is not dependent on EGFR signaling (Olayioye et al., 2000; Soltoff et al., 1994). In MDA-468 cells, we have demonstrated that both PI3K activity and p85 binding to phosphotyrosine-containing proteins are not dependent on EGFR. These data strongly suggest that the induction of apoptosis by gefitinib is not due to further inhibition of PI3K or Akt kinase. It is interesting to note that in MDA-468 we have demonstrated (Figure 4) that p85 binding to HER3 is resistant to doses of gefitinib that effectively inhibit both EGFR and HER2. This suggests that HER3 is capable of being phosphorylated by an unknown tyrosine kinase, which, in these cells, is responsible for PI3K activation.
The ability of gefitinib to cooperate with inhibition of PI3K/Akt signaling is due, in part, to its inhibition of MAP kinase activity. EGFR activation leads to efficient activation of Ras signaling, and levels of MAP kinase activity are often elevated in tumors in which EGFR is mutated or overexpressed (Chakravarti et al., 2002; Rojas et al., 1996). We show that reexpression of PTEN or inhibition of PI3K also sensitizes cells to induction of apoptosis by a potent and selective inhibitor of MEK kinase activity. The data suggest that the survival of PTEN-deficient tumors does not require signaling through Ras/MAP kinase but that they rely on this pathway when PTEN expression is restored and Akt is inhibited. In melanoma, activating mutations of B-Raf constitutively activate MEK/MAPK signaling (Wan et al., 2004) and commonly occur in association with inactivating mutations of PTEN (Tsao et al., 2004). In Figure 5F, we show in one such tumor cell, SKMel-11, that inhibition of PI3K alone does not induce apoptosis but that it synergizes with MEK inhibition.
Others have noted that the antitumor effects of inhibition of two pathways, particularly MAP kinase- and PI3 kinase-dependent pathways, are much greater than those elicited by inhibiting either alone (Bedogni et al., 2004; Lee et al., 2003; Uzgare and Isaacs, 2004). However, the molecular mechanism underlying this phenomenon is poorly understood. BAD is a BH3 domain-containing protein that activates apoptosis, in part by binding to and inactivating the antiapoptotic proteins Bcl-2 and Bcl-XL (Yang et al., 1995). BAD is negatively regulated by pathways under the control of extracellular growth factors, which thereby inhibit apoptosis. BAD may be phosphorylated at multiple sites, prominently serine 112, a MAP kinase-dependent site (Bonni et al., 1999; Fang et al., 1999; Scheid et al., 1999), and serine 136, a site phosphorylated by Akt kinase (Datta et al., 1997). Phosphorylation of these sites causes BAD to bind to 14-3-3, sequestering it in the cytoplasm and preventing its proapoptotic effects (Datta et al., 2000; Zha et al., 1996).
In primary breast epithelial cells, EGFR has been shown to phosphorylate BAD on serine 112 (Gilmore et al., 2002). Gefitinib selectively inhibits serine 112 phosphorylation and induces apoptosis in these cells. We show here that, in MDA-468, transfected BAD is highly phosphorylated on both serine 112 and serine 136 and forms a complex with 14-3-3. In agreement with other reports (Fernando and Wimalasena, 2004; Gilmore et al., 2002; Hayakawa et al., 2000), inhibition of EGFR or MEK abrogates serine 112, but not serine 136 phosphorylation, whereas, in contrast, induction of PTEN expression or inhibition of PI3K activity inhibits serine 136 but not serine 112. Inhibiting either pathway alone slightly affects BAD association with 14-3-3 and has little effect on apoptosis. Inhibiting both pathways abolishes BAD binding to 14-3-3 and is associated with dramatic apoptosis. Reduction of the expression of endogenous BAD with siRNA markedly attenuates induction of apoptosis in response to inhibition of both pathways.
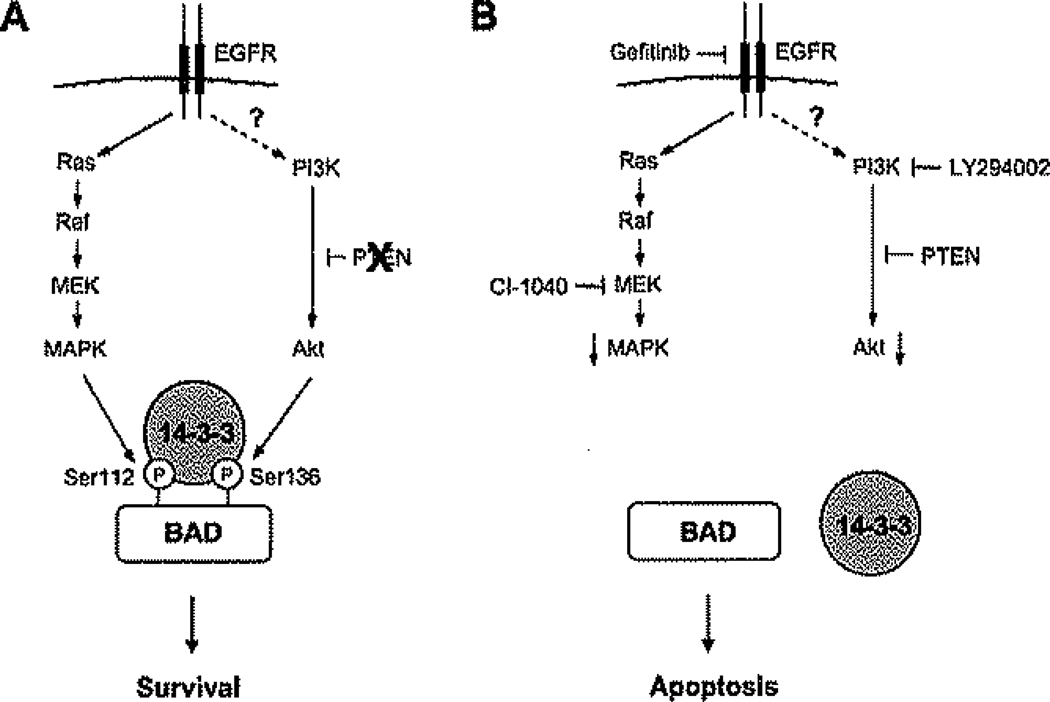
The results suggest a model (Figure 8) in which, in normal cells, BAD phosphorylation at several sites integrates the anti-apoptotic effects of multiple growth factor-mediated signaling pathways. EGFR-dependent MAPK signaling plays a prominent role in this regulation, and in experimental systems, inhibition of serine 112 phosphorylation by gefitinib induces apoptosis in normal mammary epithelia. However, in tumors in which transformation is mediated by constitutive activation of Akt, such as those in which PTEN is mutationally inactivated, serine 136 phosphorylation is sufficient to sequester BAD and inhibit apoptosis. Normal MAPK-dependent serine 112 phosphorylation is rendered superfluous. Indeed, we have shown that inhibiting MAPK has little or no effect in tumor cells in which the pathway is not activated by mutation (data not shown). In systems terminology, the BAD protein acts as an “Or” logic gate or switch; phosphorylation at either site in response to either pathway is sufficient for its inactivation.
Figure 8.
A model for role of BAD as an apoptotic switch in PTEN-deficient fumor cells
A: In PTEN-deficient tumor cells, activation of PI3K and Akt is EGFR independent. The BAD protein acts as a switch that integrates the antiapoptotic effects of the EGFR/MEK/MAPK and PI3K/Akt pathways.
B: Dephosphorylation of BAD abolishes BAD binding to 14-3-3 and is required for the synergistic induction of apoptosis in response to inhibition of both pathways.
It follows that inhibition of the activated Akt signaling pathway is not sufficient to activate apoptosis. When Akt is inhibited in response to PTEN induction, the cell relies on EGFR/MAPK signaling, the default physiologic pathway, to inactivate BAD and prevent apoptosis. Under these conditions, inhibition with gefitinib or an inhibitor of MEK signaling causes cell death. It seems unlikely that BAD is the sole target that integrates the effects of these pathways on survival. These data are important as a heuristic model to explain interactions between normal and oncogenically activated pathways (Figure 8). However, data obtained with S112A and S136A mutants and BAD siRNAs suggest that, in the MDA-468 system, BAD is the dominant target responsible for the effects of both pathways on apoptosis.
Whether or not BAD plays this dominant role in other systems is unknown. We have demonstrated synergistic effects of pharmacologic inhibition of PI3K in combination with inhibition of EGFR or MEK in multiple other cellular systems (Figures 5E and 5F, Figure S1, and data not shown). Inhibition of these pathways has the predicted effect on phosphorylation of BAD on serine 112 and serine 136 (Figure S2). However, the role played by BAD in mediating the phenotypic consequences of combined pathway inhibition in other models will require more detailed mechanistic studies in tissue culture and in vivo models.
These data do have several important clinical implications. They suggest that monotherapy with EGFR inhibitors would not be effective in tumors with PTEN inactivation, even if they express an activated form of EGFR. PTEN inactivation together with EGFR overexpression occurs commonly in glioblastoma, prostate cancer, and perhaps other tumors. Furthermore, in tumors in which both pathways are mutationally activated, such as some melanomas, inhibition of neither alone would be expected to affect tumor survival. The data provide a rationale for therapy with combinations of signaling inhibitors. Although transformation may be exquisitely dependent on a pathway activated by a mutated oncogene, tumor cells may survive inhibition of this pathway by relying on the normal physiologic pathway. The data suggest that, in tumors with PTEN loss that are derived from EGFR-dependent lineages, it may be necessary to block both EGFR/MAPK and PI3K/Akt kinase signaling. No drugs that efficiently inhibit the PI3K/Akt kinase signaling in vivo are currently available for clinical use, although many are in early stages of development. Currently, the only potential ways to inhibit this pathway clinically are with inhibitors of tyrosine kinases that are known to drive PI3K in particular tumors (HER2 IGF1R) (Garcia-Echeverria et al., 2004; Moasser et al., 2001) or with inhibitors of Hsp90 that cause Akt degradation (Basso et al., 2002).
Recent clinical results with EGFR inhibitors have questioned whether wild-type EGFR is a significant therapeutic target (Paez et al., 2004). The data presented here suggest three scenarios in which inhibition of wild-type EGFR could be useful: first, as described here, in combination with drugs that inhibit the oncogenic target in situations in which the EGFR drives physiologic signaling; second, in tumors in which Akt activity is low and EGFR-driven MAPK is responsible for cell survival; third, in the occasional tumor in which Akt kinase is dependent upon wild-type EGFR. Rational therapy will depend on developing the means to classify tumors in these terms.
Experimental procedures
Cell culture and reagents
The human cancer cell lines MDA-468 and A431 (ATCC, Manassas, VA) were grown as described (Moasser et al., 2001). The glioblastoma cell line SKMG-3 was provided by Christopher Thomas (Thomas et al., 2001) and maintained in DMEM with 10% FBS. The melanoma cell line SKMel-11 was obtained from Alan Houghton (MSKCC, New York, NY) and maintained in RPMI 1640 medium with 10% FBS. Gefitinib was obtained from AstraZeneca Pharmaceuticals (Cheshire, United Kingdom); CI-1040 was obtained from Pfizer (New York, NY); LY294002 and the caspase inhibitor Z-VAD-FMK were from Calbiochem (La Jolla, CA); and doxycycline was from Sigma (St. Louis, MO).
Cell proliferation and apoptosis assays
Cells were seeded in six-well plates at a density of 50,000 cells per well in duplicate. The following day, cells were placed in fresh medium containing indicated concentrations of drugs and allowed to grow for 4–6 days, and subsequently harvested by trypsinization and counted using a Zeiss Coulter Counter (Beckman Coulter, Miami, FL). To measure apoptosis, cells were seeded in 100 mm dishes at a density of 8 × 105 cells per dish, and the following day, cells were treated with drug or vehicle (DMSO) for the indicated times. Both adherent and floating cells were harvested and stained with ethidium bromide (Nusse et al., 1990). Detection and quantitation of apoptotic cells (sub-G1 fraction) were performed by flow cytometric analysis. To assay DNA fragmentation, both adherent and floating cells were harvested. The fragmented DNA was extracted and determined as described (She et al., 2003a).
Immunoblotting and immunoprecipitation
Cells were washed with PBS once, disrupted on ice for 30 min in NP-40 lysis buffer as described (Basso et al., 2002), and cleared by centrifugation. Protein concentration was determined with BCA reagent (Pierce Chemical Co., Rockford, IL). Equal amounts of protein (50 µg) in cell lysates were separated by SDS-PAGE, transferred to membranes, immunoblotted with specific primary and secondary antibodies, and detected by chemilumines-cence with the ECL detection reagents (Amersham Biosciences, Piscataway, NJ). Antibodies for p-EGFR(Tyr1068), p-Akt(Ser473), p-MAPK, p-BAD(Ser112) and BAD(Ser136), activated (cleaved) caspase-3, cleaved PARP, total Akt, and MAPK were from Cell Signaling Technology (Beverly, MA). Phosphotyrosine (PY99), PTEN, and 14-3-3β (FL-246) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The monoclonal BAD and EGFR antibodies were from BD Biosciences (San Diego, CA). Other antibodies include anti-PI3K p85 (Upstate Biotechnology, Waltham, MA), HER3 (Neomarkers; Ab-2, Fremont, CA), and β-actin (Sigma). For immunoprecipitation, 500–1000 µg of cell lysate protein were incubated overnight at 4°C with the designated antibody, and then protein G-Sepharose (Amersham Biosciences) was added for 3 hr while being rocked. Precipitates were washed three times with lysis buffer and once with PBS, resuspended in 2× Laemmli buffer, and resolved by SDS-PAGE followed by immunoblotting analysis.
Stable and transient transfections
MDA-468 cells were stably transfected with a tet-inducible PTEN vector and named MDA-468TR-PTEN as described (She et al., 2003b). In this vector system, doxycycline binds to and interferes with the tet repressor, leading to unhindered activity of the CMV promoter and expression of the PTEN cDNA insert. MDA-468 cells were also stably transfected with a tet-inducible parent vector and used as vector-only controls. Transient transfections were performed with Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The active myristoylated Akt construct, pUSEamp/myr-Akt, was from Upstate Biotechnology. Wild-type BAD and its mutants (BADS112A, BADS136A, and BADS112A/S136A) were provided by Michael Greenberg (Datta et al., 1997). MDA-468, MDA-468TR-Vector, and MDA-468TR-PTEN cells were seeded in 100 mm dishes at a density of 3 × 106 cells/dish and transfected with 10 µg of pUSEamp/myr-Akt, wild-type BAD, or its mutants and Lipofectamine Plus reagent. After 24 hr, cells were placed in medium containing indicated concentration of drugs for the indicated times, and then cells were harvested and analyzed by immunoblotting or apoptosis assays as described above.
Knockdown of BAD protein by stable expression of siRNAs
MDA-468TR-PTEN cells were transfected with a pGB BAD siRNA vector mixture of four different siRNA vectors targeted against BAD or pGB negative control vector (BioVison, Mountain View, CA) by using Lipofectamine Plus reagent (Invitrogen). The stable transfectants were obtained by selection for G418 resistance (400 µg/ml) and further analyzed by immunoblotting BAD protein.
PI3K activity assay
PI3K activity was determined as described previously (She et al., 2003a). In brief, cells were treated with 3 µM gefitinib, harvested, and lysed. HER3-associated PI3K was coimmunoprecipitated with monoclonal HER3 antibody (Neomarkers; Ab-4). PI3K activity was assayed in 250 µM ATP containing 10 µCi [γ-32P]ATP with phosphatidylinositol as substrate. The product, PI3 phosphate, was resolved by thin layer chromatography and detected by autoradiography.
Animal studies
Six-week-old athymic BALB/c female mice (NCI-Frederick Cancer Center) were maintained in pressurized ventilated cages. MDA-468TR-Vector or MDA-468TR-PTEN cells (1 × 107) were mixed 1:1 with Matrigel (Collaborative Research, Bedford, MA) and injected subcutaneously into the right flank (200 µl/mouse). After 7–10 days, mice bearing tumors 6–7 mm in diameter were randomized among control and treated groups. For induction of the tetracycline-responsive promoter, mice received doxycycline (0.5 mg/ml) in the drinking water. Gefitinib was administrated orally as a lactate salt (pH 5.2) at a dose of 150 mg/kg/day × 5 consecutive days each week for 3 weeks. Mice were weighed and tumors were measured with vernier calipers. Tumor volumes were calculated with the following formula: π/6 × larger diameter × (smaller diameter)2. Unpaired Student’s t test was used to assess statistical significance.
To analyze cellular markers, mice with established tumors were given doxycycline (0.5 mg/ml) in the drinking water or treated with gefitinib at a dose of 150 mg/kg/day for 3 consecutive days. Mice were sacrificed pretreatment and at the indicated times posttreatment. For immunoblotting, tumor tissue was homogenized in RIPA buffer as described (She et al., 2003b). For immunohistochemical staining, tumors to be examined were excised promptly after euthanasia and immediately placed in 4% paraform-aldehyde. The tumors were fixed overnight in paraformaldehyde and then dehydrated and embedded in paraffin. Sections of 8 µm were cut for hematoxylin and eosin staining and Ki-67, p-Akt(Ser473), and activated caspase-3 immunochemical staining.
SIGNIFICANCE.
We demonstrate that restoration of PTEN expression causes PTEN-deficient tumor cells to depend on EGFR for survival. The BAD protein acts as a switch that integrates the antiapoptotic effects of the EGFR/MAPK and PI3K/Akt pathways. Inhibition of both pathways is required to release BAD from 14-3-3 and activate its proapoptotic functions. The data provide a heuristic model for understanding pathway interactions that allows the development of rational strategies for combination therapy and reveals a potential clinical role for inhibition of wild-type EGFR. Thus, EGFR inhibitors may be useful in combination with inhibitors of PI3K/Akt kinase signaling for the treatment of glioblastomas, prostate cancer, and other tumors in which EGFR activation and PTEN mutation coexist.
Acknowledgments
We wish to thank Mark Moasser for helping establish the MDA-468TR-PTEN cells; Michael Greenberg for the wild-type BAD and its mutants, BADS112A, BADS136A, and BADS112A/S136A; and Christopher Thomas for SKMG-3 cell line. This work was supported by NIH grant PO1-CA94060, the Taub Foundation, the William H. Goodwin and Alice Goodwin Foundation for Cancer Research, and the MSKCC Experimental Therapeutics Program.
Footnotes
References
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D’Andrea G, Seidman A, Norton L, Gunnett K, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J. Clin. Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, Kaye SB, Gianni L, Harris A, Bjork T, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J. Clin. Oncol. 2002;20:4292–4302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, O’Neill MS, Welford SM, Bouley DM, Giaccia AJ, Denko NC, Powell MB. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–2560. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, Tsurutani J, Dennis PA, Mills GB, Arteaga CL. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the anti-tumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307–4315. [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- De Luca A, Casamassimi A, Selvam MP, Losito S, Ciardiello F, Agrawal S, Salomon DS, Normanno N. EGF-related peptides are involved in the proliferation and survival of MDA-MB-468 human breast carcinoma cells. Int. J. Cancer. 1999;80:589–594. doi: 10.1002/(sici)1097-0215(19990209)80:4<589::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, Longo N, Hamid ML, Olson JJ, Liu L, Collins VP, James CD. Functional characterization of an EGF receptor with a truncated extracellular domain expressed in glioblastomas with EGFR gene amplification. Oncogene. 1994;9:2313–2320. [PubMed] [Google Scholar]
- Fang X, Yu S, Eder A, Mao M, Bast RC, Jr, Boyd D, Mills GB. Regulation of BAD phosphorylation at serine 112 by the Rasmitogen-activated protein kinase pathway. Oncogene. 1999;18:6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- Fernando RI, Wimalasena J. Estradiol abrogates apoptosis in breast cancer cells through inactivation of BAD: Ras-dependent nonge-nomic pathways requiring signaling through ERK and Akt. Mol. Biol. Cell. 2004;15:3266–3284. doi: 10.1091/mbc.E03-11-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O'Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J. Biol. Chem. 2002;277:27643–27650. doi: 10.1074/jbc.M108863200. [DOI] [PubMed] [Google Scholar]
- Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr. Relat. Cancer. 2004;11:689–708. doi: 10.1677/erc.1.00600. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000;60:5988–5994. [PubMed] [Google Scholar]
- Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J. Bioi. Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- Lee HY, Srinivas H, Xia D, Lu Y, Superty R, LaPushin R, Gomez-Manzano C, Gal AM, Walsh GL, Force T, et al. Evidence that phosphatidylinositol 3-kinase- and mitogen-activated protein kinsase kinase-4/c-Jun NH2-terminal kinase-dependent pathways cooperate to maintain lung cancer cell survival. J. Biol. Chem. 2003;278:23630–23638. doi: 10.1074/jbc.M300997200. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Molkentin K, Gerbic C, Jackson J, Stellato T, Willson JK. Growth stimulation by coexpression of transforming growth factor-α and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J. Clin. Invest. 1990;86:356–362. doi: 10.1172/JCI114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz SD, Myeroff L, Cooper MJ, Traicoff J, Kochera M, Lutterbaugh J, Swiriduk M, Willson JK. A benign cultured colon adenoma bears three genetically altered colon cancer oncogenes, but progresses to tumorigenicity and transforming growth factor-β independence without inactivating the p53 tumor suppressor gene. J. Clin. Invest. 1994;93:1005–1013. doi: 10.1172/JCI117048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J. Biol. Chem. 1998;273:200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Nusse M, Beisker W, Hoffmann C, Tarnok A. Flow cytometric analysis of G1- and G2/M-phase subpopulations in mammalian cell nuclei using side scatter and DNA content measurements. Cytometry. 1990;11:813–821. doi: 10.1002/cyto.990110707. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram TG, Hosick HL, Ethier SP. Heregulin-β is especially potent in activating phosphatidylinositol 3-kinase in nontransformed human mammary epithelial cells. J. Cell. Physiol. 2000;183:301–313. doi: 10.1002/(SICI)1097-4652(200006)183:3<301::AID-JCP2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J. Biol. Chem. 1996;271:27456–27461. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J. Biol. Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS. MEK inhibitors: a therapeutic approach to targeting the Ras-MAP kinase pathway in tumors. Curr. Pharm. Des. 2004;10:1907–1914. doi: 10.2174/1381612043384439. [DOI] [PubMed] [Google Scholar]
- She QB, Ma WY, Wang M, Kaji A, Ho CT, Dong Z. Inhibition of cell transformation by resveratrol and its derivatives: differential effects and mechanisms involved. Oncogene. 2003a;22:2143–2150. doi: 10.1038/sj.onc.1206370. [DOI] [PubMed] [Google Scholar]
- She QB, Solit D, Basso A, Moasser MM. Resistance to gefitinib in PTEN-null HER-overexpresssing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3′-kinase/Akt pathway signaling. Clin. Cancer Res. 2003b;9:4340–4346. [PubMed] [Google Scholar]
- Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, Lawhorn K, Khuri FR, Glisson BS, Myers J, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin. Cancer Res. 2001;7:1204–1213. [PubMed] [Google Scholar]
- Soltoff SP, Carraway KL, III, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylnositol 3-kinase by epidermal growth factor. Mol. Cell. Biol. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srethapakdi M, Liu F, Tavorath R, Rosen N. Inhibition of Hsp90 function by ansamycins causes retinoblastoma gene product-dependent G1 arrest. Cancer Res. 2000;60:3940–3946. [PubMed] [Google Scholar]
- Thomas C, Ely G, James CD, Jenkins R, Kastan M, Jedlicka A, Burger P, Wharen R. Glioblastoma-related gene mutations and over-expression of functional epidermal growth factor receptors in SKMG-3 glioma cells. Acta Neuropathol. (Berl.) 2001;101:605–615. doi: 10.1007/s004010000332. [DOI] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64:6190–6199. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol. Ther. 1999;82:241–250. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]