Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy (original) (raw)
Abstract
Radiotherapy is one of the most successful cancer therapies. Here the effect of irradiation on antigen presentation by MHC class I molecules was studied. Cell surface expression of MHC class I molecules was increased for many days in a radiation dose-dependent manner as a consequence of three responses. Initially, enhanced degradation of existing proteins occurred which resulted in an increased intracellular peptide pool. Subsequently, enhanced translation due to activation of the mammalian target of rapamycin pathway resulted in increased peptide production, antigen presentation, as well as cytotoxic T lymphocyte recognition of irradiated cells. In addition, novel proteins were made in response to γ-irradiation, resulting in new peptides presented by MHC class I molecules, which were recognized by cytotoxic T cells. We show that immunotherapy is successful in eradicating a murine colon adenocarcinoma only when preceded by radiotherapy of the tumor tissue. Our findings indicate that directed radiotherapy can improve the efficacy of tumor immunotherapy.
MHC class I molecules present endogenous peptides to CTLs. Many of these peptides are generated by the proteasome from newly synthesized but rapidly degraded proteins called rapidly degraded proteins (RDPs) or defective ribosomal products (1, 2). The proteasome products are trimmed by tripeptidyl peptidase II (3) and downstream peptidases, and only a small fraction will escape complete degradation by binding the transporter associated with antigen processing (TAP) (4–6). TAP translocates peptides into the ER lumen for further trimming and binding to MHC class I molecules (7). Upon peptide binding, the MHC class I molecules traffic to the cell membrane, allowing T cells to examine the intracellular peptide content and eliminate those cells presenting foreign peptides. TAP is not fully active under normal circumstances, as the peptide pool forms the limiting factor in the antigen presentation pathway (7). This may change under stressful conditions. For example, during an acute influenza infection the generation of viral proteins leads to a rapid increase in peptide generation enabling swift CTL responses to the infection (1). Here we have examined the effect on the intracellular peptide products and MHC class I expression of another clinically relevant condition—irradiation.
The best-known effect of ionizing radiation is the induction of double-stranded DNA breaks, which can result in mutations leading to transformation and tumor formation if DNA repair fails (8, 9). Cells respond to DNA damage by activating complex pathways to arrest the cell cycle, allowing DNA repair, or inducing programmed cell death (10). Microarray analysis of cells after ionizing radiation has revealed up-regulation of nucleotide excision repair genes, cell cycle genes, and genes involved in apoptosis (11). In addition, irradiation-induced radical formation modifies proteins by radical-induced cross-linking, breakage of disulphide bonds, and amino acid side-chain oxidation (12), which may result in protein unfolding and degradation. Absorption of radiation can occur directly within proteins but mostly causes radiolysis of water, which constitutes up to 90% of the volume of cells. The resulting short-living radicals can modify intracellular proteins and DNA. It is unknown if γ-irradiation modifies the intracellular protein pool in vivo and whether the resulting MHC class I peptide repertoire is altered by this treatment. We show that γ-irradiation enhanced peptide production and surface expression of MHC class I for many days at higher doses. In addition, the MHC class I peptide repertoire now includes radiation-specific peptides. The radiation-induced effects on MHC class I antigen presentation may have important consequences because combining radiotherapy with immunotherapy results in superior antitumor responses.
RESULTS
Ionizing radiation enhances surface expression of MHC class I molecules
The antigen presentation pathway is far from saturated under normal conditions, and changes in the intracellular protein pool will induce rapid changes in peptide–MHC class I complexes, enabling the immune system to respond swiftly to changes in the intracellular protein content (1, 13). Ionizing radiation is known to induce radicals, which oxidize proteins and DNA, resulting in a plethora of biological effects. We exposed the human melanoma cell line MelJuSo to different doses of γ-radiation, and 18 h later quantified cell surface–MHC class I complexes by flow cytometry. Consistent with previous reports (14–16), we observed a radiation-induced increase in MHC class I expression in vitro. This increase was dose dependent (Fig. 1 A), whereas the expression of another protein, the transferrin receptor, remained unaffected. Identical increases were observed after γ-irradiation of transfected MelJuSo cells expressing HLA-A2 under the control of the CMV promoter, indicating that the increase in HLA expression was not caused by transcriptional activation of the MHC locus (unpublished data).
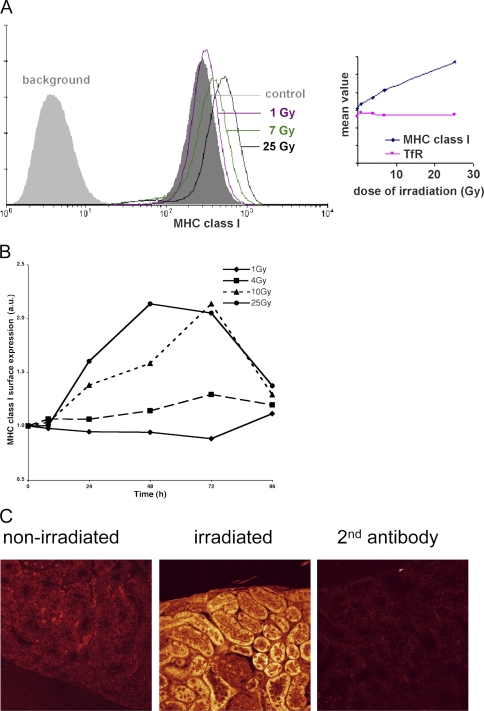
Figure 1.
Ionizing radiation increases the expression of peptide– MHC class I complexes at the cell surface. (A) Cells were exposed to different doses of irradiation as indicated, and levels of peptide–MHC class I complexes at the cell surface were measured 18 h after γ-irradiation by flow cytometry. Fluorescence in the absence of the first antibody (W6/32) is indicated (background). (Inset) The mean fluorescence intensity (MFI) plotted for MHC class I as well as the transferrin receptor (TfR) after an 18 h culture after various doses of γ-irradiation (representative experiment). (B) Time course of radiation-induced MHC class I up-regulation. MelJuSo cells were exposed to various doses of γ-radiation and cultured for the indicated times before analysis of surface MHC class I expression by FACS. The MFI was determined and related to MHC class I expression in control MelJuSo cells, and the ratio was plotted. Marked long-term increases in MHC class I expression are observed at higher doses of radiation. (C) An HLA-A2 transgenic mouse was locally exposed to γ-irradiation at a dose of 25 Gy. 24 h later, the mouse was killed and sections of a kidney exposed to γ-irradiation and a kidney outside the radiation field were stained with a rabbit anti–MHC class I H chain serum followed by a secondary antibody coupled to Cy5. Background fluorescence was detected with the second antibody only. Images were made under identical settings.
To test the temporal effect of radiation on MHC class I expression, MelJuSo cells were irradiated with different doses and cultured for various times before analysis of MHC class I expression by FACS (Fig. 1 B). A marked increase in MHC class I expression was observed at higher doses (10–25 Gy) over a period of 3 d. To test whether similar increases in MHC class I expression could be observed in vivo, we irradiated HLA-A2 transgenic mice (17), such that one kidney was outside and the other kidney in the field of radiation with a dose of 25 Gy. 24 h after irradiation, kidneys were isolated and sections of the respective kidneys were stained with antibodies against human MHC class I H chains (Fig. 1 C). Quantification of fluorescence revealed a two- to threefold increase in HLA expression in the irradiated kidney compared with the nonirradiated kidney, similar to the effects in tissue culture (Fig. 1, A and B). MHC class I levels also increased in subcutaneous tissues of transgenic mice upon irradiation (unpublished data). Thus, γ-radiation results in a dose-dependent cellular response by increasing MHC class I expression in vitro as well as in vivo.
Radiation expands the intracellular peptide pool in a dose-dependent manner
To understand the molecular basis for radiation-induced MHC class I expression, we analyzed quantitative changes in the intracellular peptide pool by a TAP mobility assay using MelJuSo cells stably transfected with TAP1-GFP. The lateral mobility of the TAP transporter tagged with the GFP can be followed using a time-lapse protocol visualizing fluorescence recovery after photobleaching (FRAP). TAP transports peptides into the ER in an ATP-dependent manner, and its activity is inversely related to its mobility. Fully active TAP transporters diffuse at a slow rate (possibly caused by the opened pore), whereas inactive TAP molecules (in a closed conformation) diffuse more rapidly (1). The diffusion rate of TAP transporters in nonirradiated cells is increased in cells depleted for peptides (after proteasome inhibition) and decreased in peptide-saturated cells (Fig. 2 A). When cells were irradiated with a dose of 4 Gy and assayed 1 h later, TAP mobility decreased to levels comparable to peptide-saturated cells. This was dependent both on proteasomal activity and ATP. ATP depletion increased TAP mobility in irradiated cells to a level similar to ATP-depleted control cells (Fig. 2 A). The human CMV protein US6 inhibits and arrests conformational changes in TAP (18, 19). Ionizing radiation did not alter the mobility of TAP in cells transfected with the ER luminal part of US6 (Fig. 2 B). This excludes possible effects of irradiation on TAP mobility caused by alterations in the ER membrane, indicating that irradiated cells have an increased TAP activity because of higher levels of intracellular peptides.
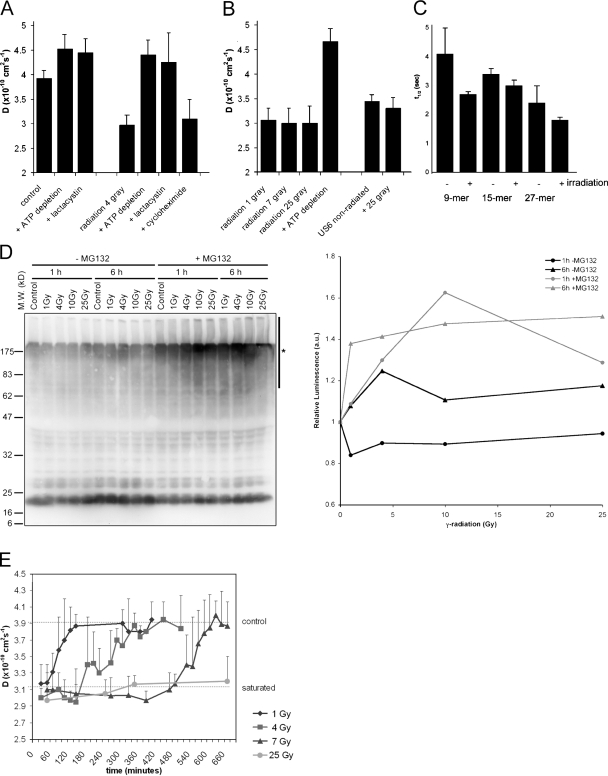
Figure 2.
Ionizing radiation increases intracellular peptide levels in a dose-dependent manner. (A) TAP activity (which correlates with the amount of intracellular peptides) was measured through its lateral mobility in the ER membrane using FRAP. Lactacystin was used to deplete cells for peptides by inhibiting the proteasome. ATP depletion and proteasome inhibition inactivate TAP. Cycloheximide was used to inhibit protein synthesis. TAP activity was measured 1 h after a dose of 4 Gy. (n = 9; mean ± SD). (B) TAP activity measured 1–2 h after different exposures to γ-irradiation as indicated (n = 9; mean ± SD). (C) Control cells and cells exposed to 25 Gy radiation were microinjected with peptide substrates of different length (as indicated) and the half-life was determined (n = 8; mean ± SD). (D) (Left) MelJuSo cells are exposed to various doses of γ-radiation followed by a culture for 1 or 6 h, as indicated. In the last 30 min, cells were cultured in the presence or absence of the proteasome inhibitor MG132 to visualize the polyubiquitinated species produced but not converted by the proteasome. _N_-ethylmaleimide was added before lysis to inhibit deubiquitinating enzymes, and cell lysates were separated by 10% SDS-PAGE and transferred to polyvinylidene flouride membranes for detection of ubiquitin with antibody FK2. The position of the marker proteins is indicated and the polyubiquitinated protein fraction quantified indicated by a bar (marked with an asterisk) on the right site of the gel. (Right) Quantification of the effects of radiation on polyubiquitination. The luminescence for the polyubiquitin pool (area indicated by an asterisk) of the Western blot was quantified, corrected for protein input, and related to the signal in the control cells. A marked increase in the polyubiquitin pool is observed after radiation when proteasomes are inhibited by MG132. (E) TAP activity measured over time after γ-irradiation at time point t = 0. The top line indicates TAP activity in nonirradiated cells; the bottom line indicates TAP activity in peptide saturated cells (each data point n > 3; mean ± SD).
An increase in peptide levels could be the result of reduced peptidase activity; therefore, we tested whether peptidase activity was inhibited by γ-irradiation by introducing internally quenched fluorescent peptides in control cells or cells 2–3 h after radiation by 25 Gy. These peptides will become fluorescent after degradation by intracellular peptidases (3, 4). We determined the stability of peptides of different length, including a 9- and 14-mer peptide, and a 27-mer tripeptidyl peptidase II substrate (Fig. 2 C). No substantial difference in the half-life of these peptides was observed after irradiation. If any, the peptide half-life was shorter in cells exposed to γ-irradiation. This suggests that the increase in TAP activity and MHC class I expression does not result from inactivation or saturation of intracellular peptidase activity, but from an increase in protein degradation in response to ionizing radiation.
To independently validate increased protein turn-over in response to γ-radiation, MelJuSo cells were exposed to different doses of γ-radiation and the extent of polyubiquitination determined 1 or 6 h later by Western blotting with antiubiquitin antibodies (Fig. 2 D). Because any effect on polyubiquitination may be quenched by enhanced proteasomal degradation, the proteasome was inhibited by MG132 30 min before cell lysis. Increased polyubiquitination is observed in response to radiation. The Western blot was quantified by luminescence detection and the polyubiquitin signal (Fig. 2 D, vertical line) related to input protein. The relative luminescence visualizes an increase in polyubiquitinated species only after proteasome inhibition (Fig. 2 D, right), indicating radiation-enhanced protein ubiquitination followed by proteasomal degradation. This should result in more degradation fragments as detected in the experiments directly measuring TAP activity (Fig. 2, A–C).
Because MHC class I expression increased in a radiation dose-dependent manner (Fig. 1 A), we investigated whether the peptide pool increased similarly. Cells were irradiated with an increasing dose of γ-irradiation, and TAP mobility was determined. All cells show peptide-saturated levels at 1 h after irradiation (Fig. 2 B), which does not explain the dose-dependent increase in MHC class I expression. Therefore we tested the temporal effects of different doses of γ-radiation on the intracellular peptide pool, again using the TAP mobility assay (Fig. 2 E). The increase in TAP activity lasted only 1 h when cells were irradiated with 1 Gy. A dose of 4 Gy resulted in peptide-saturated levels for around 3 h before the cells returned to nonsaturated levels, whereas peptide saturation occurred for up to 7 h in cells irradiated with 7 Gy. Cells irradiated with 25 Gy remained peptide saturated for more than 24 h (Fig. 2 E, lower line). These kinetics may explain the dose-dependent effect of irradiation on MHC class I expression.
Protein degradation and synthesis after irradiation
To test whether stable proteins or RDPs contributed to the increased intracellular peptide pool, cells were pretreated with the translation inhibitor cycloheximide before irradiation to inhibit protein synthesis during and after irradiation. RDPs are defined as translation products that failed to become functional and are rapidly degraded after translation (5, 7). This pool is immediately affected by translation inhibition unlike the stable protein pool (1, 2). In contrast to nonirradiated cells, treatment with cycloheximide had no effect on TAP activity, indicating that irradiation induces the degradation of stable proteins (Fig. 2 A). To visualize the breakdown of proteins after irradiation, we stably expressed enhanced GFP (EGFP) under the CMV promotor in MelJuSo cells. Cells were irradiated at 25 Gy, and GFP fluorescence was compared with control cells by flow cytometry. Fluorescence did not decrease in cells 4 h after irradiation (when GFP would have been degraded), probably because the affected protein pool is too small to detect directly by FACS. Surprising, GFP fluorescence increased in cells 24 h after irradiation (Fig. 3 A). The apparent increase in protein expression after irradiation may be caused by increased rates of protein translation after radiation-induced activation of the kinase mammalian target of rapamycin (mTOR), a critical regulator of protein translation (20). mTOR activates S6K which releases the initiation factor 4E-binding protein 1, resulting in enhanced ribosomal translation. As the name indicates, mTOR can be specifically inhibited by the drug rapamycin (21). When GFP-expressing MelJuSo cells were irradiated in the presence of rapamycin, no increase in GFP expression in response to ionizing radiation was observed, suggesting that the mTOR pathway plays a critical role in the responses to irradiation (Fig. 3 A). Rapamycin also decreased GFP expression in nonirradiated cells, suggesting that mTOR activity is also required for optimal protein expression under control conditions.
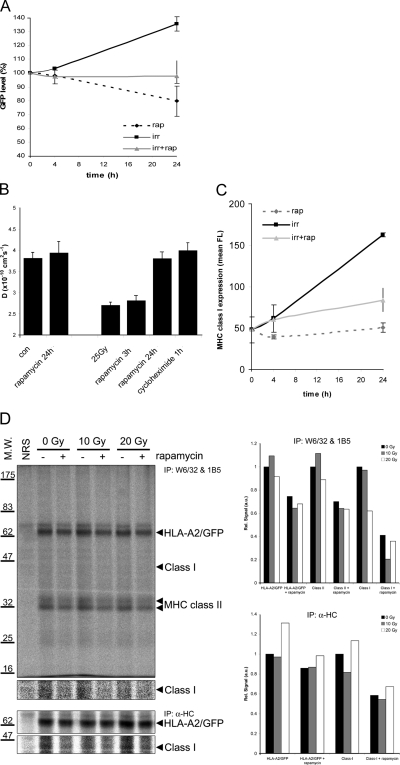
Figure 3.
The mTOR pathway, ionizing radiation, and antigen presentation by MHC class I. (A) MelJuSo cells stably expressing GFP under the control of the CMV promoter were exposed to 25 Gy γ-irradiation in the presence or absence of the mTOR inhibitor rapamycin. GFP expression was determined by flow cytometry at 0, 4, or 24 h after γ-irradiation (n = 6; mean ± SD). (B) MelJuSo cells expressing TAP1-GFP were exposed to 25 Gy radiation and cultured for 24 h before analysis. Rapamycin was either present during the 24 h culture period or only during the last 3 h. Alternatively, translation was inhibited by cycloheximide added during the last hour before analysis. The intracellular peptide pool was detected by FRAP. (C) MelJuSo cells were exposed to 25 Gy radiation in the presence or absence of the mTOR inhibitor rapamycin before labeling the cells with the MHC class I antibody W6/32 for flow cytometric analysis. (n = 4; mean ± SD). (D) MelJuSo cells stably expressing HLA-A2/GFP under control of the CMV promoter were radiated at the doses indicated and cultured for another 6 h in the presence or absence of rapamycin, as indicated. Cells were labeled with 35S-methionine/cystein 60 min before lysis, and endogenous and GFP-tagged MHC class I molecules, MHC class II molecules, and the free MHC class I H-chains were isolated, as indicated. The position of the molecules as well as the marker proteins on the 10% gel is indicated. NRS, normal rabbit serum control immunoprecipitation. (Right) Quantitation of the SDS-PAGE signals related to that from nonradiated control cells after subtraction of the same Mr area in the NRS lane.
To measure the effect of radiation-induced activation of the mTOR pathway on the intracellular peptide pool, MelJuSo cells were irradiated with a dose of 25 Gy and cultured for 24 h. The increased peptide pool was sensitive to rapamycin treatment as deduced from TAP activity measurements by FRAP, and also to cycloheximide when added during the last hour of the culturing period (Fig. 3 B). This is in contrast with the initial increase in intracellular peptides that was not inhibited by treatment with cycloheximide or rapamycin, when measured 1 h after irradiation (Fig. 2 A; unpublished data). This suggests that after the initial phase of breakdown of existing proteins, protein synthesis increased because of mTOR activation resulting in higher levels of (RDP-derived) peptides. To determine the contribution of mTOR activation by irradiation on enhanced MHC class I expression, cells were exposed to 25 Gy followed by a further culture for 4 or 24 h in the absence or presence of rapamycin. No effect of rapamycin on MHC class I expression was observed after 4 h, but the radiation-induced increase in MHC class I expression 24 h after irradiation was markedly reduced when cells were treated with rapamycin (Fig. 3 C). This indicates that mTOR activation is important in the later phase of the radiation-induced enhanced MHC class I presentation.
To test whether the radiation-induced mTOR pathway also enhanced translation of MHC class I molecules, HLA-A2-GFP–expressing MelJuSo cells were biosynthetically labeled 6 h after exposure to 10 or 20 Gy radiation. To assess the role of the mTOR pathway, cells were cultured in the presence or absence of the inhibitor rapamycin. Because radioactive labeling after amino acid starvation will activate the mTOR pathway, we added 35S-methionine/cystein to the normal culture medium for 1 h before lysis and isolation of MHC class I complexes, free H chains, and MHC class II molecules (Fig. 3 D). Translation of GFP-tagged and endogenous MHC class I molecules, H chains as well as MHC class II molecules was not altered in response to radiation but was still inhibited by rapamycin and thus affected by constitutive mTOR activity.
Radiation generates unique MHC class I binding antigenic peptides
The increased pool of MHC class I molecules after irradiation may be loaded with similar peptides as nonirradiated cells, or with a unique set of peptides generated in response to radiation-induced mTOR-enhanced translation and de novo protein production. To analyze the peptide profile after ionizing radiation, MHC class I molecules were purified from control cells and cells 18 h after irradiation with 25 Gy. Peptides were eluted and separated into 50 fractions by reverse phase HPLC (rpHPLC). Each fraction isolated from the radiated cells was analyzed by mass spectometry (MS) and compared with the peptide content in the corresponding fraction of control cells (Fig. 4 A). Comparison of respective fractions revealed ∼1% unique peptides in irradiated cells, implying that the majority of the peptides presented after irradiation were derived from proteins also expressed and degraded in control cells. Several peptides that were unique to the irradiated peptide pool were sequenced by tandem MS/MS analysis (Fig. 4 B). All peptides have the correct anchor residues for binding to HLA-A1 molecules of MelJuSo cells (Fig. 4 B, bold) (22). Two peptides are derived from proteins involved in DNA repair (MSH6 and PCNA [23]), one in protein breakdown (nuclear F-box protein Fbl7 involved in the recognition and ubiquitin tagging of [unknown] substrates) and one protein (CGI 51 or SAM50) that is located at the outer mitochondrial membrane (unpublished data) and probably part of the mitochondrial protein import system (24). These peptides are derived from proteins most likely up-regulated in response to γ-irradiation.
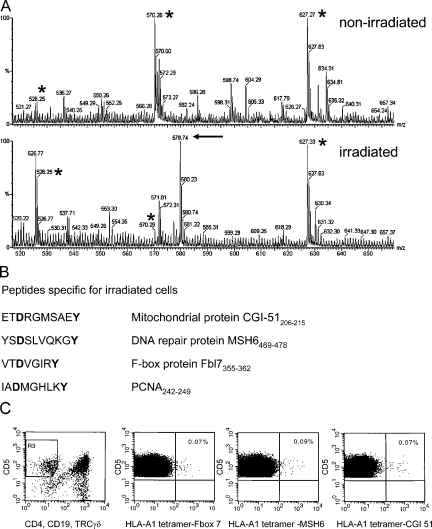
Figure 4.
Ionizing radiation alters the MHC class I–associated peptide profile and immunological responses. (A) Mass spectrometry profiles of double-charged peptides eluted from corresponding rpHPLC fractions from nonirradiated and irradiated cells as indicated. The peptides marked by an asterisk are observed in both profiles, whereas the arrow indicates peptide CGI-51 that is observed only in the peptide fraction after irradiation. (B) Peptide sequences from proteins induced by γ-radiation as determined by MS and the corresponding proteins. Note that all peptides contain the anchor residues for HLA-A1 (in bold). (C) Identification of CTLs recognizing HLA-A1 tetramers containing irradiation-induced peptides. Flow cytometric analysis of human blood mononuclear cells gated on CD5+ and CD4−/CD19− TCRγδ− staining (leftmost panel) to determine the CTL population.
The identification of radiation-induced peptides on irradiated tumor cells may allow a combination of radiotherapy and immunotherapy to induce specific responses, providing that T cells recognize some of these uniquely expressed peptides. To identify CTLs recognizing these peptides, HLA-A1 tetramers were generated and loaded with radiation-induced peptides CGI 51(206–215), MSH6(469–478), and F-box protein Fbl7(335–362) (Fig. 4 B). Two out of three healthy HLA-A1–positive donors had a small population (∼0.08%) of tetramer-reactive CD8+ T lymphocytes when PBMCs were incubated with tetramers containing one of the three peptides (Fig. 4 C). Influenza-specific T cells could be detected in all three donors (∼0.14%) using HLA-A1 tetramers containing influenza virus peptide, whereas no reactivity (<0.02%) was found with HLA-A1 tetramers loaded with peptide derived from the melanoma-associated antigen MAGE-A1 (25), which is not expressed in normal HLA class I–expressing tissues (unpublished data). When CD8+ T cells were isolated from donor lymphoid cells and stimulated for 1 wk with CD8-negative lymphoid cells that were preloaded with radiation-induced peptides, the percentage of tetramer-binding T cells did not increase in these cultures, despite the presence of radiation-induced antigen-specific T cells in two of these donors. It is possible that CTL specific for radiation-induced peptides are “anergic” as has been shown for Mart-1/HLA-A2–specific CTL in both healthy donors and patients (26, 27). These T cells have to be stimulated more extensively to leave their “anergic state” before becoming responsive to radiation-induced cells, a phenomenon which has frequently been observed in tumor vaccination studies (28).
Irradiation of tumor cells enhances T cell recognition in vitro and boosts the efficacy of adoptive CTL immunotherapy in vivo
To test if the radiation-induced up-regulation of MHC class I results in better recognition of target cells, we irradiated mouse colon adenocarcinoma MC38 cells (20 Gy) that express the tumor antigen gp70. The effect of radiation and the mTOR-dependent MHC class I expression was determined. Similar to MelJuSo cells, MC38 cells up-regulate MHC class I expression upon irradiation, which can be inhibited by rapamycin (Fig. 5, A–D). Subsequently the manipulation of recognition of the gp70 epitope on cells by specific CTL was tested. Irradiation of MC38 cells leads to enhanced lysis in a rapamycin-dependent fashion (Fig. 5 E). Rapamycin had no effect on CTL recognition of (nonirradiated) cells. This suggests that the radiation-induced increase in MHC class I expression leads to increased sensitivity to antigen-specific cytotoxic T cell killing.
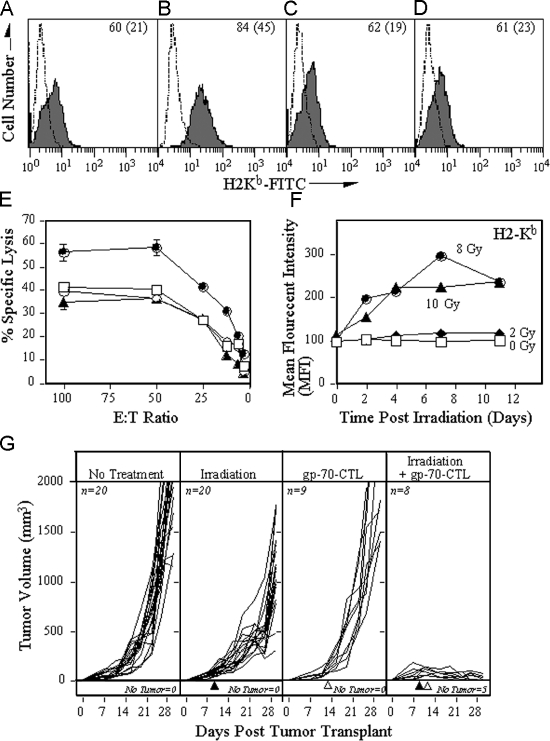
Figure 5.
Irradiation of tumor cells boosts the efficacy of CTL in vitro and in vivo. MC38 cells were treated with (A) buffer, (B) 20 Gy radiation, (C) rapamycin, or (D) radiation and rapamycin and recultured. After 24 h, the MHC levels were analyzed by flow cytometry. (A–D) The dashed line depicts isotype control antibody; the solid line depicts H2-Kb. Inset numbers depict the percentage of positive cells (mean fluorescent intensity). (E) 111In-labeled MC38 cells treated with buffer (□), 20 Gy radiation (•), rapamycin (◯), or the combination of radiation and rapamycin (▴). After 24 h, the cells were cultured with different numbers of gp70-specific CTL for 18 h. (F) Temporal effects of H-2Kb expression after radiation. MC38 cells were exposed to various doses of radiation followed by culture for the times indicated. The surface expression of H2-Kb was subsequently determined by FACS analyses and the MFI plotted after being related to the MFI of H2-Kb expression of control MC38 cells. (G) C57BL/6 mice were injected with 3 × 105 MC38 tumor cells s.c., and the volume of the tumor (in mm) was measured daily and plotted. (First panel) Mice receiving no additional treatment. (Second panel) Tumors in mice were subjected to external-beam irradiation (10 Gy) in situ on day 9 of tumor transplant (▴). (Third panel) Mice were adoptively transferred (IV) with 3 × 106 gp70-specific CTL at day 10 (▵). (Fourth panel) Tumors in mice were subjected to external-beam irradiation (10 Gy) in situ at day 9 (▴) followed by adoptive transfer of gp70-specific CTL at day 10 (▵). The number of mice in each arm of the experiment and the number of mice without any detectable tumor is indicated in the figure.
To examine if this phenomenon also occurs in vivo, we first tested the temporal effect of radiation on expression of H2-Kb molecules. MC38 cells were exposed to various doses of radiation, followed by culture and FACS analyses for H2-Kb molecules (Fig. 5 F). MHC class I expression is increased for over 11 d after 8 or 10 Gy radiation. To then test the effects of radiation on antitumor immune responses, we used an adoptive transfer paradigm. MC38 cells were injected subcutaneous at the right hind leg of C57/BL6 mice. After 9 d, mice were divided into four groups that received either no treatment, 10Gy radiation of the tumor tissue, only adoptive transfer of CTL, or the combination of irradiation of the tumor, followed 24 h later by transfer of CTL. Tumors of mice that did not receive any treatment (Fig. 5 G) grew progressively, ultimately causing the death of the animals (100% by day 30). Exposure of tumors to irradiation at day 9 reduced tumor outgrowth only, and treatment of tumors with adoptively transferred gp70-specific CTL at day 10 did not significantly inhibit tumor outgrowth as well. However, treatment of tumors with the combination of irradiation and CTL adoptive transfer, markedly inhibited tumor outgrowth (P < 0.001 vs. no treatment; P < 0.001 vs. irradiation alone; P < 0.001 vs. adoptive transfer alone). Moreover, 62.5% of the mice treated with this combination of tumor irradiation and CTL adoptive transfer resolved their tumor and became tumor-free during the experiment, suggesting that radiotherapy is an important supplement to current anticancer immunotherapy protocols.
DISCUSSION
Radiotherapy is—after surgery—the most successful cancer treatment. Besides its cytotoxic properties, radiation can induce multiple effects on cells and tissues as a function of dosage. In animal models and clinical studies, lower doses of radiation may induce immunomodulatory activities by up-regulating tumor-associated antigens (29, 30), adhesion molecules (31, 32) and IFN-γ secretion (33, 34), and CTL may be attracted to irradiated tissue to induce local responses (34) and improve tumor responses (16). Ionizing radiation can also inhibit distant tumors after local radiation therapy, a phenomenon known as the abscopal effect. This phenomenon has been reported in various malignancies but remains a poorly understood event. After irradiation of a tumor, T cells are probably required to mediate distant tumor growth inhibition (35, 36). Our data on the effect of γ-irradiation on MHC class I molecules provides an explanation for the immune-mediated abscopal effect.
We applied doses between 1–25 Gy, which is lower or comparable with doses received by patients, and observed that ionizing radiation induces a dose-dependent increase in MHC class I presentation in two phases (Fig. 6). The first phase represents peptides derived from existing proteins, because inhibition of translation does not affect the generation of these peptides. More polyubiquitinated proteins for proteasomal degradation are observed swiftly after radiation even at doses of 1–4 Gy, resulting in more peptides for MHC class I antigen presentation and enhanced MHC class I expression as peptides are the limiting step in complex formation (37). Irradiation will result in the formation of free radicals even at low doses, and proteins will be modified directly by radiation or indirectly by radicals formed after water radiolysis, resulting in oxidation of various amino acids. These modifications may target the affected proteins for rapid degradation by the proteasome. Although this will reflect a small fraction of the cellular protein pool, it still raises the amount of peptides and thus MHC class I expression. In the second phase, the mTOR kinase is activated resulting in enhanced protein synthesis. As a result, more proteins are generated of which a fraction is immediately degraded as RDPs, resulting in increased intracellular peptide levels. MHC class I molecules are better translated after mTOR activation, but no additional increase in MHC class I translation is observed. In addition, a substantial pool of free MHC class I H chains is still observed even when cells are exposed to 20 Gy radiation, suggesting that more MHC class I molecules could have been loaded with peptide, if around. Because peptides are the limiting factor in MHC class I assembly (37), an increased peptide pool would be most important for increased MHC class I expression. The data suggest that mTOR-increased “general” translation rather than specific increases in MHC class I translation are important for the radiation-increased surface MHC class I expression, as observed by FACS. In addition to these two responses, irradiated cells also respond by expressing unique proteins, and peptides from these proteins are subsequently loaded by MHC class I molecules. Various proteins may be selectively up-regulated and/or degraded in response to radiation, including DNA repair proteins like PCNA, MSH2, and MSH6, but also proteins involved in cell cycle checkpoints, apoptosis, and protein degradation (11). Fragments of these proteins are found on MHC class I after irradiation. Our experimental protocol did not allow quantitation of the total peptide pool given the many experimental steps between MHC class I peptide isolation, peptide elution, concentration, and HPLC fractionation before MS analysis (which is also not a quantitative technique). Although the input peptides should have been two- to threefold more in the irradiation pool (based on FACS analysis for surface MHC class I peptide combination), this difference may be lost after the various procedures. Still, it is conceivable that mTOR activation results in altered protein synthesis and thus peptide profile. The MHC class I antigen presentation pathway is extraordinary inefficient, generating a threshold for self-antigens that may only be presented after strong up-regulation. The resulting self-peptides are presented on irradiated tissue and may be recognized by specific CTLs in the patient. We have identified such CTLs in blood after labeling with tetramers loaded with three identified radiation-specific self-peptides, but these CTLs could not be expanded and stimulated ex vivo. Possibly, these CTLs are present in an anergic state. Because IFN-γ is locally secreted in response to irradiation (33, 34), such CTL may undergo further activation in irradiated tissue. Specific vaccination protocols may be required to reawaken these anergic CTLs, as has recently been described for successful in vivo antitumor responses (28), before the local induction of the peptides by irradiation.
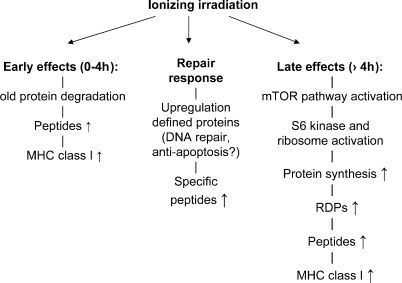
Figure 6.
A model summarizing the three effects of ionizing radiation on MHC class I antigen presentation. The early effects are caused by the degradation of proteins that may be triggered or damaged by irradiation. Later effects are caused by activation of the mTOR pathway, which results in increased protein translation of proteins, and an increased generation of peptides from (the RDPs of) these new proteins. The increased peptide pool will enhance MHC class I assembly because peptides are the limiting factor. In addition, unique proteins will be expressed/up-regulated in response to ionizing radiation, resulting in novel peptides presented by MHC class I molecules.
An alternative strategy to combine radiotherapy and immunotherapy is to apply local irradiation to increase expression of MHC class I molecules, as shown here in kidneys of locally irradiated HLA-A2 transgenic mice. Radiation-induced increase in MHC class I levels leads to enhanced susceptibility of tumor cells to lysis by CTL in vitro. Notably, we previously reported radiation-enhanced killing by CTL of five human colon carcinoma cell lines. Analysis of their surface antigens showed only class I up-regulation as the common denominator (16). In fact, we now show that activation of the mTOR pathway by radiation is critical for the radiation-enhanced CTL killing. We examined whether irradiation and immune cells can also work in concert in vivo. Neither radiation nor adoptive transfer of specific CTL was curative in the mouse model. The combination of irradiation of the tumor and adoptive CTL transfer strongly reduced the tumor volume, and most mice receiving this combination therapy completely eradicated the tumor mass. This is probably not due only to up-regulation of MHC class I expression because mTOR activation will have more general effects on protein expression including up-regulation of Fas, ICAM-1 (38), and interferon expression (33, 34). Higher doses of radiation enhances MHC class I expression for days or even weeks. This may depend on cell proliferation. Slowly dividing cells may have a longer half-life of MHC class I molecules explaining why the rapidly dividing MelJuSo cells have a shorter radiation-induced increase in MHC class I expression than MC-38 cells. Up-regulation of other proteins may be acting in concert with MHC class I molecules in the local induction of CTL responses.
This combination of therapies is based on the concept that targeted radiation strongly supports adoptive immunotherapy of cancer. The synergistic effect of the combination of radiotherapy and immunotherapy may explain the earlier mentioned abscopal effects, as the locally induced up-regulation of particular tumor-related antigens in MHC class I may lift the expression of these antigens above the threshold level needed for the activation of CTL. Once activated, these T cells might recognize and assault distant tumors.
The effect of radiation on MHC class I expression, by enhancing the intracellular peptide pool, may be used in a new combination of radiotherapy and immunotherapy that could be attractive in the treatment of radiation-resistant tumors but also to selectively boost antitumor responses of activated or adoptively introduced tumor-specific CTLs. CTLs may then recognize the unique radiation-induced antigenic peptides or enhanced tumor-related self-antigens resulting in better tumor responses. Because radiation can be locally applied to the tumor without touching surrounding tissue, the specificity of radiotherapy can be combined with the specificity of immunotherapy, as shown in this study. This novel combination of radio-immunotherapy may add a new perspective to existing cancer treatments.
MATERIALS AND METHODS
Mice, constructs, cell lines, and antibodies
TAP1-GFP was made by fusing full-length TAP1 to EGFP (Clontech Laboratories) and stably transfected in the melanoma cell line MelJuSo as described (1). MelJuSo cells expressing HLA-A2-GFP have been described (39). MelJuSo was DNA typed and expresses HLA-A1, -B8, -Cw7, -DR3, and -DQ2 (40). The murine colon adenocarcinoma cell line, MC38 (H-2b), has been described previously (41). The H-2Kb–restricted, gp70-specific CD8+ CTL line, designated gp70-CTL, was generated from C57BL/6 as described (42), and recognizes the peptide epitope p15 (KSPWFTTL). The CTL lines were maintained by weekly in vitro stimulation with irradiated, syngeneic naive splenocytes in RPMI 1640 with fetal calf serum, supplemented with 10 IU/ml murine IL-2 (Boehringer Mannheim). The irradiated syngeneic splenocytes were pulsed with 1 μg/ml gp70 peptide for 1 h and then washed before culture with the CTL (43). For cytotoxicity assays and adoptive transfer, the CTL were recovered at day 6 of the stimulation cycle by centrifugation through a density gradient (LSM; Organon Teknika).
HLA-A2.1 transgenic mice (17) were a gift from F. Lemonier (Institute Pasteur, Paris, France) and maintained germ-free in the animal facility of the Netherlands Cancer Institute. For the radio-immunotherapy experiments, female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility. Mice were housed and maintained under pathogen-free conditions in micro-isolator cages until used for experiments at 6–8 wk of age.
The anti–human transferring mAb 66Ig10 (44), the anti–human MHC class I mAb W6/32 (45), the anti–human class I H-chain serum (13), the anti–human MHC class II mAb 1B5 (40), and rabbit anti-GFP serum (40) were used. Ubiquitin was detected with monoclonal FK2 (Stressgen). Mouse cell surface staining was performed with primary FITC-labeled mAb H-2Kb, purchased from PharMingen. Rabbit anti–human class I H-chain serum (46) was used for the immunostaining of the HLA-A2 transgenic mice. The mAb CD5-FITC, CD4-PE, CD19-PE, and TCRγδ-PE were obtained from Becton Dickinson.
Biochemistry
Western blot analysis.
MelJuSo cells were irradiated at the indicated doses 1 and 6 h before lysis. Where indicated, 10 μM MG132 was added to cells 30 min before lyses. All cells were treated with 50 μM _N_-ethylmaleimide 5 min before lysis to prevent postlyses deubiquitylation of substrate proteins. Cells were lysed in 1× reducing SDS sample buffer, boiled, and analyzed by 10% SDS-PAGE, followed by Western blotting onto polyvinylidene flouride membranes. Membranes were blocked and stained for multiubiquitin with the FK2 antibody. Western blots were quantified by luminescence detection (MultiImage; Alpha Innotech Corp.).
Biosynthetic labeling.
HLA-A2/GFP-expressing MelJuSo cells (39) were irradiated with 0, 10, or 20 Gy and cultured in the presence or absence of 10 nM rapamycin for 6 h in IMDM supplemented with 8% FCS. Cells were subsequently labeled with 100 μCi 35S-methionine/cystein (added to the normal culture medium) for 1 h before lysis in NP-40–containing lysis mixture. 5% of the lysate was used to quantify total protein input using Bradford assay (BioRad Laboratories), and immunoprecipitates were isolated from equal amounts of total protein. MHC class I as well as class II complexes were isolated first with mAb W6/32 and 1B5 followed by isolation of the free MHC class I H chains, and samples were analyzed by 10% SDS-PAGE. A normal serum control was included. Gels were quantified by phosphoimage analysis (Fuji film FLA-3000).
Ionizing radiation
Cells were exposed to γ-irradiation from a 137Cs source. The indicated doses were given at a rate of 0.75 Gy per min. HLA-A2 transgenic mice were locally exposed to 25 Gy γ-radiation. Therefore, the mice were maintained in a leaden tube with holes during radiation, and the areas of radiation were indicated on the skin with black ink (47). The mice were killed 24 h after irradiation, and tissues from the radiated and nonradiated areas were isolated and fixed in formalin for immunocytochemistry. Sections were cut and incubated with rabbit anti–human HLA class I H chain serum that recognizes HLA class I molecules under these conditions (46). In addition, no cross-contamination occurred using rabbit antibodies for staining mouse tissue. A second goat anti–rabbit Ig coupled to the far-red dye Cy5 (Molecular Probes) was used to minimize detection of tissue autofluorescence. Images were made by the Leica SP2 confocal system using the glow-over/glow-under mode, and quantification of fluorescence was performed using standard Leica software. Background (secondary antibody only) fluorescence was subtracted to determine the increase in HLA-A2 expression after ionizing radiation.
FACS and confocal fluorescence microscopy analysis for FRAP experiments
MelJuSo cells were stably transfected with a construct expressing EGFP in pcDNA3. Cells were exposed to 0 or 25 Gy radiation in the presence or absence of 20 nM rapamycin and cultured for the times indicated. Alternatively, MelJuSo cells were γ-irradiated with 0–25 Gy and cultured for the indicated time spans in the presence or absence of 20 nM rapamycin, followed by staining the MHC class I complexes with W6/32 and flow cytometry. For long-term experiments, MelJuSo or MC38 cells were exposed to 1–25 Gy radiation and cultured for up to 7 d (MelJuSo) before quantitation of MHC class I expression by W6/32 and FACS analyses (gated on the living cell population only). Cells were seeded such that they did not require dilution (replating) during the culture period. MC38 cells were cultured for 11 d after radiation. MC38 cells were treated with 20 Gy and cultured for 24 h in the presence or absence of 20 nM rapamycin, followed by staining with FITC-labeled mAb H-2Kb and flow cytometry.
Confocal analysis of living cells and FRAP experiments were performed as described previously (1). To inhibit proteasomes, cells were incubated at 37°C in the presence of 10 μM lactacystin for 30 min. For ATP depletion, cells were incubated for 30 min with a mixture of NaAz (0.05%) and 2-deoxyglucose (50 μM). Diffusion of TAP-GFP was determined as described (1). In brief, a circular spot in the ER was bleached at full laser intensity, and an attenuated laser beam was used to monitor recovery of fluorescence in the bleached region (using Leica TCS time-lapse). The half-time for recovery was calculated from each recovery curve after correction for loss of fluorescence caused by imaging (which was usually less than 4%). The diffusion coefficient D was determined from at least three independent FRAP experiments (generally at least 10 measurements per experiment) and depicted as mean value ± SD. As a standard, FRAP experiments of nonmanipulated cells were included as internal control. Temperature in the culture chamber at the CLSM was 37°C and continuously controlled.
Peptide degradation
Internally quenched peptides were introduced by microinjection in control MelJuSo cells or irradiated MelJuSo cells (1–2 h after receiving a dose of 25 Gy), whereas fluorescence emission was detected as described (4). The peptide sequences were T[K-dabcyl]NKTER[C-fluorescein]Y (9-mer), T[K-dabcyl]NKTER[C-fluorescein]YFKDLGP (15-mer), and T[K-dabcyl]NKTER[C-fluorescein]YFKDLGPFKDLGPFKDLGP (27-mer). Peptides were HPLC purified and sequence verified by MS.
MHC class I peptide isolation, rpHPLC, MS, and peptide sequencing
MHC class I–peptide complexes were purified from >1010 MelJuSo cells that were either control cells or cells harvested 18 h after γ-irradiation with a dose of 25 Gy. Peptides were purified by affinity chromatography using anti–class I W6/32 antibodies. Peptides were eluted with TFA from the isolated MHC class I molecules and passed through a 10-kD filter as described (22). The resulting peptide pool was separated by RP-HPLC on a SMART system equipped with a reversed phase C18 column (Pharmacia Biotech) using an acetonitrile gradient in 0.1% TFA as previously described (48). Partial RP-HPLC profiles were obtained from the respective class I eluates of control or irradiated cells. Aliquots were sampled from each HPLC fraction and analyzed by electrospray MS on a Q-TOF mass spectrometer (Micromass) as previously described (49). After comparison of the corresponding fractions, peptides unique to the irradiated cell fraction were subjected to tandem MS (approximate collision energy 25–30V). Amino acid sequences were interactively interpreted with the program PeptideSearch (50).
Identification of specific CTL with tetramers
PBMCs isolated from a buffycoat preparation of healthy donor blood were thawed and incubated with 10 μg/ml DNase I in Iscoves medium containing 8% FCS for 1 h at room temperature. Tetramers were generated by refolding the HLA-A1 heavy chain with peptides CGI-51(206–215), MSH6(469–478), and F-box protein Fbl7(335–362) and human β2m as described (51). 106 PBMCs were incubated with APC-conjugated HLA-A1 tetramers for 10 min at 37°C, washed, and incubated on ice with anti-CD5–FITC antibody, anti-CD4–PE, anti-CD19–PE, and anti-TCRγδ PE antibodies for 20 min. Cells were washed and analyzed by flow cytometry (FACScalibur). Dead cells were excluded from analysis after detection with propidium iodide. The population of cells gated on CD5+ and CD4−, CD19−, TCRγδ− staining consisted of 93% CD8+ T lymphocytes (Fig. 4 C, R3). The percentage of cells that bind the respective tetramers was determined in this population.
CTL recognition
MC38 cells were exposed to 20 Gy radiation, 20 nM rapamycin (maintained throughout the 24 h culture), or a combination of radiation and rapamycin, and recultured for 24 h. These cells were used as targets in a standard cytotoxicity assay using 111In. These radiolabeled cells (1 × 103 cells/well) were cultured with gp70-specific CTL at effector to target ratios ranging from 100:1 to 3.125:1 in 96-well U-bottomed plates (Costar) and cocultured for 18 h at 37°C with 5% CO2. After incubation, supernatants were collected using a Supernatant Collection System (Skantron), and radioactivity was quantitated using a γ-counter (Cobra Autogamma; Packard). The percentage of specific release of 111In was determined by the standard equation: % specific lysis = ([experimental–spontaneous]/[maximum–spontaneous]) × 100. The nonspecific lysis ranged between 6–11% for all groups.
Tumor therapy studies
C57BL/6 mice were injected with 3 × 105 MC38 tumor cells s.c. in the quadriceps area of the right hind-limb. 9 d after tumor transplant, tumors were irradiated with 10 Gy. The mice were restrained in a customized jig and placed in a leaded holder such that only the tumor was exposed to the irradiation beam. The irradiation was performed using a Pantax machine at 300 kV, 10 mA, and a dose rate of 157 cGy/min. The dose used (10 Gy) was predetermined to have a minimal effect on the tumors s.c. growth rate.
24 h after tumor irradiation, a subset of mice received 3 × 106 gp70-specific CTLs i.v. by adoptive transfer. Tumor size was measured daily by digital caliper in two dimensions, and the volumes were calculated. Animals were killed when any tumor measurement (length or width) exceeded 20 mm. The animals were housed and maintained in accordance with the guidelines of the National Cancer Institute Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services Publication National Institutes of Health 85–23).
Acknowledgments
We thank M. Verhey for advice and critical reading of the manuscript, E. Kueter and P. Weder for generating HLA-A1 tetramers, F. Couwenberg for help with FACS, F. Lemonnier, J. Haanen, and A. Bins for HLA-A2 transgenic mice, J.W. Drijfhout for peptide chemistry, M. van der Valk for help with the sectioning, and F. Steward for help with the radiation of the HLA-A2 transgenic mice.
This work was supported by grants from the Netherlands Cancer Society KWF and the Centre for Medical Systems Biology. This research was in part supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute Center for Cancer Research.
The authors have no conflicting financial interests.
Abbreviations used in this paper: EGFP, enhanced GFP; FRAP, fluorescence recovery after photobleaching; MS, mass spectrometry; mTOR, mammalian target of rapamycin; RDP, rapidly degraded proteins; rpHPLC, reverse phase HPLC; TAP, transporter associated with antigen processing.
J.W. Hodge and C.A. Herberts contributed equally to this work.
H. Spit's and E.A. Reits' present address is Division of Cell Biology and Histology, Academic Medical Center, 1105 AZ Amsterdam, Netherlands.
References
- 1.Reits, E.A., J.C. Vos, M. Gromme, and J. Neefjes. 2000. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 404:774–778. [DOI] [PubMed] [Google Scholar]
- 2.Schubert, U., L.C. Anton, J. Gibbs, C.C. Norbury, J.W. Yewdell, and J.R. Bennink. 2000. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 404:770–774. [DOI] [PubMed] [Google Scholar]
- 3.Reits, E., J. Neijssen, C. Herberts, W. Benckhuijsen, L. Janssen, J.W. Drijfhout, and J. Neefjes. 2004. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 20:495–506. [DOI] [PubMed] [Google Scholar]
- 4.Reits, E., A. Griekspoor, J. Neijssen, T. Groothuis, K. Jalink, P. van Veelen, H. Janssen, J. Calafat, J.W. Drijfhout, and J. Neefjes. 2003. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity. 18:97–108. [DOI] [PubMed] [Google Scholar]
- 5.Yewdell, J.W. 2001. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 11:294–297. [DOI] [PubMed] [Google Scholar]
- 6.Princiotta, M.F., D. Finzi, S.B. Qian, J. Gibbs, S. Schuchmann, F. Buttgereit, J.R. Bennink, and J.W. Yewdell. 2003. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 18:343–354. [DOI] [PubMed] [Google Scholar]
- 7.Yewdell, J.W., E. Reits, and J. Neefjes. 2003. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat. Rev. Immunol. 3:952–961. [DOI] [PubMed] [Google Scholar]
- 8.Khanna, K.K., and S.P. Jackson. 2001. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27:247–254. [DOI] [PubMed] [Google Scholar]
- 9.Rosen, E.M., S. Fan, I.D. Goldberg, and S. Rockwell. 2000. Biological basis of radiation sensitivity. Part 2: cellular and molecular determinants of radiosensitivity. Oncology (Huntingt) 14:741–757. [PubMed] [Google Scholar]
- 10.Bartek, J., and J. Lukas. 2001. Cell cycle. Order from destruction. Science. 294:66–67. [DOI] [PubMed] [Google Scholar]
- 11.Tusher, V.G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA. 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaich, K.M. 1980. Free radical initiation in proteins and amino acids by ionizing and ultraviolet radiations and lipid oxidation–part I: ionizing radiation. Crit. Rev. Food Sci. Nutr. 13:89–129. [DOI] [PubMed] [Google Scholar]
- 13.Neefjes, J.J., and H.L. Ploegh. 1988. Allele and locus-specific differences in cell surface expression and the association of HLA class I heavy chain with beta 2-microglobulin: differential effects of inhibition of glycosylation on class I subunit association. Eur. J. Immunol. 18:801–810. [DOI] [PubMed] [Google Scholar]
- 14.Santin, A.D., P.L. Hermonat, J.C. Hiserodt, M. Chiriva-Internati, J. Woodliff, J.W. Theus, D. Barclay, S. Pecorelli, and G.P. Parham. 1997. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int J Radiat Oncol Biol Phys. 39:737–742. [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty, M., S.I. Abrams, K. Camphausen, K. Liu, T. Scott, C.N. Coleman, and J.W. Hodge. 2003. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J. Immunol. 170:6338–6347. [DOI] [PubMed] [Google Scholar]
- 16.Garnett, C.T., C. Palena, M. Chakraborty, K.Y. Tsang, J. Schlom, and J.W. Hodge. 2004. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64:7985–7994. [DOI] [PubMed] [Google Scholar]
- 17.Firat, H., F. Garcia-Pons, S. Tourdot, S. Pascolo, A. Scardino, Z. Garcia, M.L. Michel, R.W. Jack, G. Jung, K. Kosmatopoulos, et al. 1999. H-2 class I knockout, HLA-A 2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 29:3112–3121. [DOI] [PubMed] [Google Scholar]
- 18.Ahn, K., A. Gruhler, B. Galocha, T.R. Jones, E.J. Wiertz, H.L. Ploegh, P.A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 6:613–621. [DOI] [PubMed] [Google Scholar]
- 19.Hengel, H., J.O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G.J. Hammerling, U.H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 6:623–632. [DOI] [PubMed] [Google Scholar]
- 20.Sunavala-Dossabhoy, G., M. Fowler, and A. De Benedetti. 2004. Translation of the radioresistance kinase TLK1B is induced by gamma-irradiation through activation of mTOR and phosphorylation of 4E-BP 1. BMC Mol. Biol. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingar, D.C., and J. Blenis. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 23:3151–3171. [DOI] [PubMed] [Google Scholar]
- 22.Falk, K., O. Rotzschke, M. Takiguchi, B. Grahovac, V. Gnau, S. Stevanovic, G. Jung, and H.G. Rammensee. 1994. Peptide motifs of HLA-A1, -A11, -A31, and -A33 molecules. Immunogenetics. 40:238–241. [DOI] [PubMed] [Google Scholar]
- 23.Gu, L., Y. Hong, S. McCulloch, H. Watanabe, and G.M. Li. 1998. ATP-dependent interaction of human mismatch repair proteins and dual role of PCNA in mismatch repair. Nucleic Acids Res. 26:1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozjak, V., N. Wiedemann, D. Milenkovic, C. Lohaus, H.E. Meyer, B. Guiard, C. Meisinger, and N. Pfanner. 2003. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J. Biol. Chem. 278:48520–48523. [DOI] [PubMed] [Google Scholar]
- 25.Traversari, C., P. van der Bruggen, I.F. Luescher, C. Lurquin, P. Chomez, A. Van Pel, E. De Plaen, A. Amar-Costesec, and T. Boon. 1992. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 176:1453–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee, C., P.A. Savage, P.P. Lee, M.M. Davis, and P.D. Greenberg. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162:2227–2234. [PubMed] [Google Scholar]
- 27.Lee, P.P., C. Yee, P.A. Savage, L. Fong, D. Brockstedt, J.S. Weber, D. Johnson, S. Swetter, J. Thompson, P.D. Greenberg, et al. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5:677–685. [DOI] [PubMed] [Google Scholar]
- 28.Germeau, C., W. Ma, F. Schiavetti, C. Lurquin, E. Henry, N. Vigneron, F. Brasseur, B. Lethe, E. De Plaen, T. Velu, et al. 2005. High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J. Exp. Med. 201:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hareyama, M., K. Imai, T. Ban, H. Koshiba, K. Kubo, M. Shidou, A. Oouchi, and K. Morita. 1988. Effect of radiation on the expression of carcinoembryonic antigen on the membranes of human gastric adenocarcinoma cells–immunological study using monoclonal antibodies. Nippon Igaku Hoshasen Gakkai Zasshi. 48:1572–1574. [PubMed] [Google Scholar]
- 30.Ciernik, I.F., P. Romero, J.A. Berzofsky, and D.P. Carbone. 1999. Ionizing radiation enhances immunogenicity of cells expressing a tumor-specific T-cell epitope. Int J Radiat Oncol Biol Phys. 45:735–741. [DOI] [PubMed] [Google Scholar]
- 31.Gaugler, M.H., C. Squiban, A. van der Meeren, J.M. Bertho, M. Vandamme, and M.A. Mouthon. 1997. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int. J. Radiat. Biol. 72:201–209. [DOI] [PubMed] [Google Scholar]
- 32.Quarmby, S., R.D. Hunter, and S. Kumar. 2000. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res. 20:3375–3381. [PubMed] [Google Scholar]
- 33.Lugade, A.A., J.P. Moran, S.A. Gerber, R.C. Rose, J.G. Frelinger, and E.M. Lord. 2005. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174:7516–7523. [DOI] [PubMed] [Google Scholar]
- 34.Ganss, R., E. Ryschich, E. Klar, B. Arnold, and G.J. Hammerling. 2002. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62:1462–1470. [PubMed] [Google Scholar]
- 35.Nobler, M.P. 1969. The abscopal effect in malignant lymphoma and its relationship to lymphocyte circulation. Radiology. 93:410–412. [DOI] [PubMed] [Google Scholar]
- 36.Demaria, S., B. Ng, M.L. Devitt, J.S. Babb, N. Kawashima, L. Liebes, and S.C. Formenti. 2004. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 58:862–870. [DOI] [PubMed] [Google Scholar]
- 37.Neefjes, J.J., G.J. Hammerling, and F. Momburg. 1993. Folding and assembly of major histocompatibility complex class I heterodimers in the endoplasmic reticulum of intact cells precedes the binding of peptide. J. Exp. Med. 178:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty, M., S.I. Abrams, C.N. Coleman, K. Camphausen, J. Schlom, and J.W. Hodge. 2004. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 64:4328–4337. [DOI] [PubMed] [Google Scholar]
- 39.Gromme, M., F.G. Uytdehaag, H. Janssen, J. Calafat, R.S. van Binnendijk, M.J. Kenter, A. Tulp, D. Verwoerd, and J. Neefjes. 1999. Recycling MHC class I molecules and endosomal peptide loading. Proc. Natl. Acad. Sci. USA. 96:10326–10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ham, M., M. van Lith, B. Lillemeier, E. Tjin, U. Gruneberg, D. Rahman, L. Pastoors, K. van Meijgaarden, C. Roucard, J. Trowsdale, et al. 2000. Modulation of the major histocompatibility complex class II-associated peptide repertoire by human histocompatibility leukocyte antigen (HLA)-DO. J. Exp. Med. 191:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins, P.F., J.A. Kantor, M. Salgaller, P.H. Hand, P.D. Fernsten, and J. Schlom. 1991. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 51:3657–3662. [PubMed] [Google Scholar]
- 42.Kudo-Saito, C., J. Schlom, and J.W. Hodge. 2005. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin. Cancer Res. 11:2416–2426. [DOI] [PubMed] [Google Scholar]
- 43.Yang, J.C., and D. Perry-Lalley. 2000. The envelope protein of an endogenous murine retrovirus is a tumor-associated T-cell antigen for multiple murine tumors. J. Immunother. 23:177–183. [DOI] [PubMed] [Google Scholar]
- 44.van de Rijn, M., A.H. Geurts van Kessel, V. Kroezen, A.J. van Agthoven, K. Verstijnen, C. Terhorst, and J. Hilgers. 1983. Localization of a gene controlling the expression of the human transferrin receptor to the region q12 leads to qter of chromosome 3. Cytogenet. Cell Genet. 36:525–531. [DOI] [PubMed] [Google Scholar]
- 45.Parham, P., C.J. Barnstable, and W.F. Bodmer. 1979. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J. Immunol. 123:342–349. [PubMed] [Google Scholar]
- 46.Neefjes, J.J., V. Stollorz, P.J. Peters, H.J. Geuze, and H.L. Ploegh. 1990. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 61:171–183. [DOI] [PubMed] [Google Scholar]
- 47.Kruse, J.J., J.A. te Poele, A. Velds, R.M. Kerkhoven, L.J. Boersma, N.S. Russell, and F.A. Stewart. 2004. Identification of differentially expressed genes in mouse kidney after irradiation using microarray analysis. Radiat. Res. 161:28–38. [DOI] [PubMed] [Google Scholar]
- 48.de Bueger, M., F. Verreck, E. Blokland, J.W. Drijfhout, R. Amons, F. Koning, and E. Goulmy. 1993. Isolation of an HLA-A 2.1 extracted human minor histocompatibility peptide. Eur. J. Immunol. 23:614–618. [DOI] [PubMed] [Google Scholar]
- 49.Beekman, N.J., P.A. van Veelen, T. van Hall, A. Neisig, A. Sijts, M. Camps, P.M. Kloetzel, J.J. Neefjes, C.J. Melief, and F. Ossendorp. 2000. Abrogation of CTL epitope processing by single amino acid substitution flanking the C-terminal proteasome cleavage site. J. Immunol. 164:1898–1905. [DOI] [PubMed] [Google Scholar]
- 50.Mann, M., and M. Wilm. 1994. Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal. Chem. 66:4390–4399. [DOI] [PubMed] [Google Scholar]
- 51.Altman, J.D., P.A. Moss, P.J. Goulder, D.H. Barouch, M.G. McHeyzer-Williams, J.I. Bell, A.J. McMichael, and M.M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274:94–96. [DOI] [PubMed] [Google Scholar]