The LIN-2/LIN-7/LIN-10 Complex Mediates Basolateral Membrane Localization of the C. elegans EGF Receptor LET-23 in Vulval Epithelial Cells (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 27.
Abstract
In C. elegans, the LET-23 receptor tyrosine kinase is localized to the basolateral membranes of polarized vulval epithelial cells. lin-2, lin-7, and lin-10 are required for basolateral localization of LET-23, since LET-23 is mislocalized to the apical membrane in lin-2, lin-7, and lin-10 mutants. Yeast two-hybrid, in vitro binding, and in vivo coimmunoprecipitation experiments show that LIN-2, LIN-7, and LIN-10 form a protein complex. Furthermore, compensatory mutations in lin-7 and let-23 exhibit allele-specific suppression of apical mislocalization and signaling-defective phenotypes. These results present a mechanism for basolateral localization of LET-23 receptor tyrosine kinase by direct binding to the LIN-2/LIN-7/LIN-10 complex. Each of the binding interactions within this complex is conserved, suggesting that this complex may also mediate basolateral localization in mammals.
Introduction
The establishment and maintenance of epithelial cell polarity is crucial for polarized cell functions such as specific signaling to the basolateral sides of epithelial cells (reviewed in Rodriguez and Nelson, 1989; Rodriguez and Powell, 1992). Polarized epithelial cells are separated into apical and basolateral membrane domains that contain different subsets of transmembrane proteins, causing these domains to be biochemically and functionally distinct. The tight junctions maintain cell polarity by preventing lateral diffusion of lipids and transmembrane proteins from one membrane compartment to the other. The tight junctions also separate the apical and basolateral extracellular environments by creating a tight seal between epithelial cells (reviewed in Rodriguez and Nelson, 1989).
Transmembrane proteins are targeted to the basolateral membrane domain via _cis_-acting signals present in their cytoplasmic tails (Hunziker et al., 1991). Several different basolateral signals have been identified for proteins such as LDL receptor, polyimmunoglobulin receptor, and epidermal growth factor receptor (EGFR) (Hobert and Carlin, 1995, and reviewed in Mellman, 1995). However, little is known about the _trans_-acting proteins that bind to the transmembrane protein and mediate basolateral targeting.
LET-23 is the homolog of the EGFR in C. elegans, and it is preferentially localized to the basolateral membranes of the six vulval precursor cells (P3.p to P8.p), which are polarized epithelial cells (reviewed in Kim, 1997 and Kornfeld, 1997). During the second larval stage, the anchor cell secretes an EGF-like signal (LIN-3) into the basal extracellular space of the vulval precursor cells that activates LET-23 receptor tyrosine kinase (RTK) on the basolateral surfaces of the vulval precursor cells. Activation of LET-23 RTK causes the cells to proliferate and differentiate into vulval cells. Loss-of-function let-23 mutations prevent vulval induction, resulting in a vulvaless phenotype in which all of the vulval precursor cells express a nonvulval fate instead of vulval fates.
In C. elegans, genes involved in targeting LET-23 RTK to the basolateral membrane domain can be identified in genetic screens for vulvaless mutants. Vulval signaling requires basolateral expression of LET-23 RTK, as cells that lack LET-23 RTK in the basolateral membrane domain presumably cannot respond to LIN-3 EGF in the basal extracellular space. Mutations in lin-2, lin-7, and lin-10 were initially isolated because they decrease vulval signaling and cause a vulvaless phenotype (Ferguson and Horvitz, 1985). Subsequently, these mutations were shown to result in apical mislocalization of LET-23 RTK (Simske et al., 1996, and C. W. W., unpublished data).
LIN-7, LIN-2, and LIN-10 each contain protein domains that may mediate interactions with other proteins. LIN-7 contains a single PDZ domain (Simske et al., 1996), and PDZ domains are known to mediate protein–protein interactions with C-terminal tails of trans-membrane proteins as well as with other PDZ domains (reviewed in Kim, 1997). LIN-2 contains a CaM kinase domain, a calmodulin-binding domain, a PDZ domain, an SH3 domain, and a guanylate kinase domain (Hoskins et al., 1996). LIN-2 is highly similar to mammalian Lin2/CASK (52% identical overall) (Hata et al., 1996; Cohen et al., 1998). Lin2/CASK is expressed in epithelia and neurons and has been shown to bind to neurexin, syndecan, and protein 4.1 (Hata et al., 1996; Cohen et al., 1998; Hsueh et al., 1998). LIN-2 is related to a family of proteins called membrane-associated guanylate kinases (MAGUKs) that include discs-large (DlgA) and PSD-95 (reviewed in Kim, 1997). In mammals, DlgA binds the cytoplasmic tail of the Shaker-type K+ channel (Marfatia et al., 1996); in Drosophila, dlg mutations prevent synaptic localization of the Shaker channel (Tejedor et al., 1997; Zito et al., 1997). PSD-95 binds the NMDA glutamate receptor and the Shaker-type K+ channel, and it has been proposed that PSD-95 functions to either localize these receptors to the synapse or to cluster them within the synapse (reviewed in Sheng, 1996). Thus, three MAGUKs (LIN-2, DlgA, and PSD-95) may each function in receptor localization; specifically, LIN-2 localizes an RTK to the basolateral membrane domain of epithelia, and Dlg and possibly PSD-95 localize neurotransmitter receptors at neuronal synapses.
LIN-10 contains two PDZ domains and a phosphotyrosine-binding domain (PTB) (C. W. W., unpublished data). LIN-10 is similar to three mammalian proteins called Mint1/X11α, Mint2/X11β, and Mint3/X11γ (for _M_unc18-_int_eracting proteins) (Duclos et al., 1993; Okamoto and Sudhof, 1997). Mint/X11 proteins were identified as proteins that bind to the Munc18/syntaxin complex, which is involved in synaptic vesicle fusion (Okamoto and Sudhof, 1997).
In this report, we begin to elucidate the biochemical mechanism for basolateral localization of LET-23 RTK by LIN-2, LIN-7, and LIN-10. We demonstrate that LIN-7 binds to LET-23 RTK in vivo and show that this binding is required for LET-23 basolateral localization and for vulval induction. We also demonstrate that LIN-2 and LIN-10 form a protein complex with LIN-7, indicating that all three proteins play a direct role in basolateral localization of LET-23 RTK. In addition, we have identified mammalian LIN-7 homologs (mLin7A, -B, and -C) and show that the binding interactions between mammalian Lin2/CASK and mLin7A are conserved. Results from others reveal that mammalian Lin2/CASK binds to mammalian Lin10/Mint/X11, demonstrating that the Lin2/Lin7/Lin10 ternary complex is conserved in mammals (J.-P. Borg, personal communication; Butz et al., 1998 [this issue of _Cell_]). These results provide a mechanism for basolateral localization of the LET-23 RTK in C. elegans vulval epithelia and indicate that a similar mechanism may be involved in basolateral localization of other transmembrane proteins in polarized epithelial cells of all animals.
Results
The C Terminus of LET-23 RTK Is Required for Basolateral Membrane Localization
The following genetic observations involving a unique let-23 allele, (sy1), suggest that the C terminus of LET-23 is necessary for basolateral localization. This mutation generates a premature stop codon that removes the last six amino acids of LET-23 (Aroian et al., 1994) and results in a phenotype that closely resembles that of lin-2, lin-7, and lin-10 mutants. First, let-23(sy1) and loss-of-function mutations in lin-2, lin-7, and lin-10 appear to affect the activity of LET-23 RTK only in the vulval precursor cells, since they display a vulvaless phenotype but do not display phenotypes associated with defective LET-23 signaling in other cells (Aroian and Sternberg, 1991). In contrast, strong let-23 reduction-of-function mutations cause lethality and disrupt several other develop- mental processes (such as formation of the vulva, the posterior ectoderm, the male tail, and oocyte fertilization) (Aroian and Sternberg, 1991). Second, let-23(sy1), lin-2, lin-7, and lin-10 mutants display an incompletely penetrant vulvaless phenotype (86%–95% of homozygous animals are vulvaless), whereas strong let-23 reduction-of-function mutations result in a completely penetrant vulvaless phenotype (Ferguson and Horvitz, 1985; Aroian and Sternberg, 1991). One possibility is that the phenotype of let-23(sy1) animals is similar to that of lin-2, lin-7, and lin-10 mutants because let-23(sy1) prevents localization of LET-23 to the basolateral membrane domain.
To test this possibility, we analyzed the membrane localization of LET-23 in let-23(sy1) animals using anti-LET-23 antibodies in immunocytochemical experiments to stain wild-type, let-23(sy1), and lin-7(null) animals. We found that LET-23 staining is in the apical plasma membrane in let-23(sy1) mutants, similar to the staining pattern observed in lin-7(null) mutants (Figure 1A). In contrast, LET-23 staining is observed in the basolateral plasma membrane in wild-type animals. This result indicates that the last six amino acids of LET-23 are required for basolateral membrane localization. Thus, the C terminus of LET-23 may be involved in a common mechanism for basolateral membrane localization along with LIN-2, LIN-7, and LIN-10.
Figure 1. The C Terminus of LET-23 RTK Is Required for Basolateral Membrane Localization and for Binding to LIN-7.
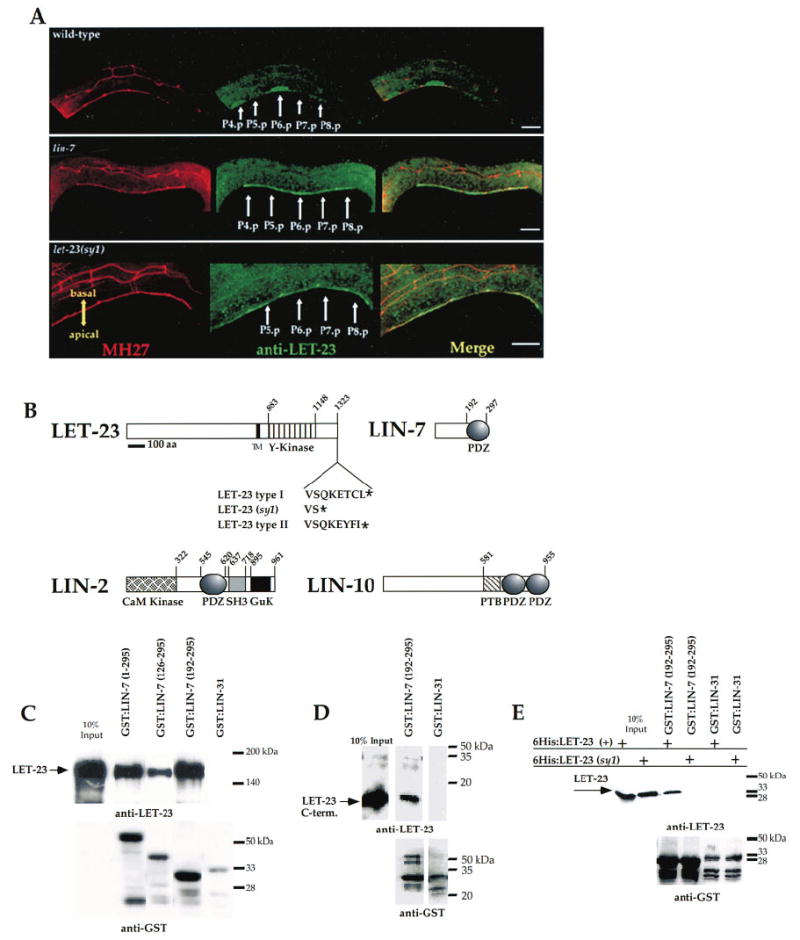
(A) LET-23 is mislocalized to the apical membrane in the vulval precursor cells in let-23(sy1) mutants. Immunocytochemical staining of LET-23 in L2 hermaphrodites in wild-type animals (top), lin-7(e1449) mutants (middle), and let-23(sy1) mutants (bottom). LET-23 staining is shown in green, and staining for the cell junctions using the monoclonal antibody MH27 is shown in red. Anterior is to the left, and ventral is down in all figures. Scale bar = 10 μm.
(B) Protein structures of LET-23, LIN-7, LIN-2, and LIN-10. Transmembrane domain (TM), tyrosine kinase (Y-kinase), CaM kinase, PDZ, SH3, GuK, and PTB domains are shown. The positions of the first and last amino acid of protein domains are indicated. LET-23(type I), LET-23(sy1), and LET-23(type II) C-terminal sequences are shown.
(C–E) LET-23 was detected by Western blotting using anti-LET-23 antibodies (top), and GST fusion proteins were detected using anti-GST antibodies (bottom). The amino acids present in the fusion proteins are shown in parentheses. Ten percent of total LET-23 used in binding experiments are shown in the first lanes. (C) The LIN-7 PDZ domain binds LET-23 in vitro. GST:LIN-7 or GST:LIN-31 fusion proteins were incubated with LET-23 in cell lysates from Sf9 cells. (D) The C terminus of LET-23 binds to the LIN-7 PDZ domain in vitro. GST:LIN-7 PDZ domain or GST:LIN-31 fusion proteins were incubated with a purified 6His fusion protein that contains the last 50 amino acids of LET-23. (E) The LIN-7 PDZ domain does not bind to LET-23(sy1) in vitro. GST:LIN-7 PDZ domain fusion proteins were incubated with 6His fusion proteins containing the C-terminal 196 amino acids from type I LET-23 or with a similar region of LET-23(sy1).
The PDZ Domain of LIN-7 Binds to the C Terminus of LET-23 RTK
Previous results showed that LIN-7 binds to LET-23 RTK in vitro, suggesting that LIN-7 plays a direct role in basolateral localization of LET-23 RTK (Simske et al., 1996). In order to characterize the regions of these proteins that interact, we first defined the region of LIN-7 that binds to LET-23 by fusing various regions of LIN-7 to GST and testing them for binding to LET-23 in vitro. Each GST:LIN-7 fusion protein, bound to glutathioneagarose beads, was combined with an Sf9 cell lysate containing full-length LET-23 expressed from a baculovirus vector. After washing and eluting the proteins from the beads, LET-23 was detected by Western blotting using anti-LET-23 antibodies. We observed that a fusion protein containing only the PDZ domain of LIN-7 (amino acids 192–295) bound to LET-23. LET-23 bound to LIN-7 specifically, as it did not bind to the negative control GST:LIN-31 (Figure 1C).
Type I PDZ domains (such as the LIN-7 PDZ domain) bind to type I consensus motifs (S/T-X-I/L/V) at the C terminus of proteins (Songyang et al., 1997). The C terminus of LET-23 contains a type I PDZ-binding motif (T-C-L), and we tested whether this C terminus binds to LIN-7 in vitro. We purified bacterially expressed hexa-histidine (6His) fusion proteins that contained either the C-terminal 196 or 50 amino acids of LET-23. Each LET-23 fusion protein was mixed with the LIN-7 PDZ domain (as a GST fusion protein bound to glutathioneagarose beads), and levels of bound LET-23 were determined by Western blotting with anti-LET-23 antibodies. These experiments showed that both LET-23 C termini fusion proteins bound LIN-7 (Figures 1D and 1E). This protein interaction is specific, as neither LET-23 fusion protein bound the negative control GST:LIN-31.
This type I PDZ domain–binding site in LET-23 is important for basolateral receptor localization, since the PDZ-binding motif is deleted and LET-23 is mislocalized in let-23(sy1) animals. To directly test whether these amino acids of LET-23 are required for binding to LIN-7, we mixed either a wild-type LET-23 C terminus (amino acids 1127–1323) or a C terminus lacking the last six amino acids (amino acids 1127–1317) with the PDZ domain of LIN-7 (expressed as a GST fusion protein and bound to glutathione-agarose beads). Levels of LET-23 bound to GST:LIN-7 PDZ domain were determined by Western blotting using anti-LET-23 antibodies. These results demonstrate that the LIN-7 PDZ domain binds to wild-type LET-23 but does not bind to the truncated form, indicating that the last six amino acids of LET-23 are required for binding (Figure 1E). In summary, the above results show that binding between LIN-7 and the C terminus of LET-23 in vitro correlates with LET-23 basolateral membrane localization in vivo.
Interactions between Compensatory Mutations in let-23 RTK and lin-7
We took a genetic approach to demonstrate conclusively that LIN-7 binds to LET-23 RTK in vulval precursor cells and that this binding is functionally required for basolateral receptor localization and vulval induction. First, we altered the C terminus of LET-23 so that it could not bind to LIN-7 and could not localize to the basolateral membrane domain of vulval precursor cells. We then made a compensatory change in LIN-7 that restores its ability to bind LET-23 in the vulval precursor cells. If these compensatory mutations restore LIN-7 and LET-23 binding in vivo and if this binding is functionally important for receptor localization, then these mutations should restore basolateral receptor localization and vulval induction.
We deduced an amino acid substitution in LIN-7 that could compensate for a change in LET-23 based on the cocrystal structure of a type I PDZ domain bound to a peptide ligand (Cabral et al., 1996; Doyle et al., 1996). This study revealed that a particular contact between the first residue of the αB helix in the PDZ domain (corresponding to position 259 in LIN-7) and an amino acid in the −2 position of the peptide may regulate PDZ binding specificity (Figure 2A). PDZ domains have been categorized into at least two different types based on their specificity for binding different peptide ligands (Songyang et al., 1997). In type I PDZ domains, the interaction is between a histidine in the PDZ domain (corresponding to position 259 in LIN-7) and a threonine or a serine in the bound peptide. In type II PDZ domains, a valine in the PDZ may interact with a tyrosine or phenyl-alanine in the bound peptide (Daniels et al., 1998). For example, LIN-7 has a type I PDZ domain (His-259) that binds to the type I consensus sequence (T-C-L) at the C terminus of LET-23, and p55 has a type II PDZ domain (p55 contains a valine at the first residue of the αβ helix) that binds to a type II consensus sequence (Y-F-I) at the C terminus of glycophorin C (Marfatia et al., 1997).
Figure 2. Compensatory Mutations in LET-23 RTK and LIN-7 Show Allele-Specific Suppression of Basolateral Receptor Mislocalization and Vulval Induction Phenotypes.
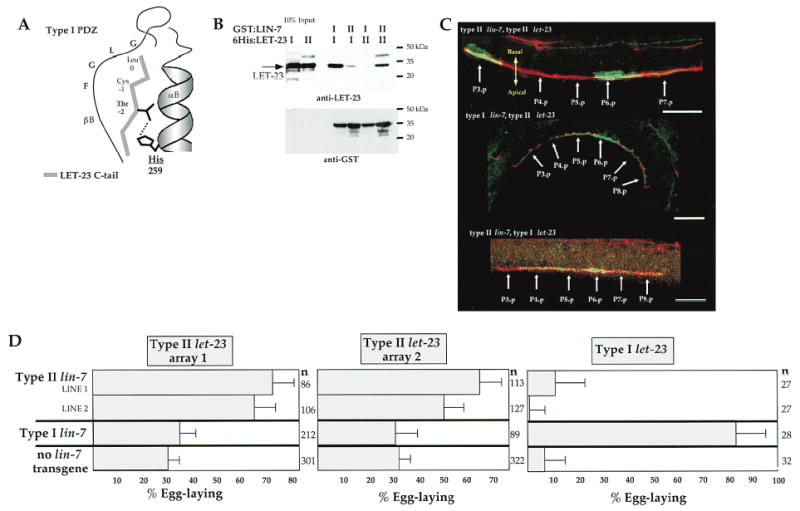
(A) Diagram of the predicted interactions between the type I PDZ domain of LIN-7 and a bound peptide, showing interaction between histidine (corresponding to amino acid 259 of LIN-7) of the αlB helix of the PDZ domain and threonine in the −2 position of the bound peptide (diagrammed after Doyle et al., 1996).
(B) Allele-specific binding between LIN-7 and LET-23 in vitro. Purified type I and type II 6His:LET-23 fusion proteins were incubated with type I and type II GST:LIN-7 PDZ fusion proteins. LET-23 was detected by immunoblotting with anti-LET-23 antibodies (top), and GST fusion proteins were detected with anti-GST antibodies (bottom). Ten percent of total LET-23 used in binding experiments is shown in the first two lanes.
(C) Allele-specific suppression of apical mislocalization phenotype. Lateral view of L2 hermaphrodites that coexpresses either type II lin-7 with type II let-23 (top), type I lin-7 with type II let-23 (middle), or type II lin-7 with type I let-23 (bottom). LET-23 staining is shown in green, and MH27 staining of the cell junctions is shown in red. Scale bar = 10 μm.
(D) Allele-specific suppression of the vulvaless phenotype. Shown are the percent of egg-laying animals (+/− 95% confidence interval) expressing the pairwise combinations of type I or type II lin-7 with type I or type II let-23. Data are from two independent type II lin-7 extrachromosomal arrays (lines 1 and 2), a single type I lin-7 extrachromosomal array, two independent type II let-23 arrays (array 1 and array 2), and endogenous type I let-23. The type II lin-7 with type II let-23 and type I lin-7 with type II let-23 transgene combinations were expressed in lin-7(e1449) let-23(sy1); unc-29(e1072) mutants. The type I and type II lin-7 transgenes were expressed with type I let-23 in lin-7(e1449), let-23(+)/let-23(sy1) heterozygotes. lin-7 activity is not required in animals expressing high levels of let-23 expression (Simske et al., 1996), so we used low-copy type II let-23 extrachromosomal arrays that presumably express let-23 at low levels. n, number of animals scored.
To generate compensatory mutations in LIN-7 and LET-23, we replaced His with Val at position 259 of LIN-7 to change the binding specificity from a type I to a type II PDZ domain (we refer to this as type II LIN-7). Likewise, we replaced the last three amino acids of the type I C terminus in LET-23 (T-C-L) with those of the type II C terminus from glycophorin C (Y-F-I) (we refer to this as type II LET-23).
Initially, we wished to show that the amino acid substitution altered the specificity of binding in vitro. We purified type I and type II LIN-7 PDZ domains as GST fusion proteins coupled to glutathione-agarose beads. Then, we incubated the LIN-7 PDZ domains with purified 6His fusion proteins containing either the type I or type II C termini of LET-23. After Western blotting with anti-LET-23 antibodies, we observed that the type I LIN-7 PDZ domain could bind strongly to the type I LET-23 C terminus but weakly to the type II C terminus. Furthermore, the type II LIN-7 PDZ domain bound to the type II but not to the type I LET-23 C terminus (Figure 2B). These results show that the type II amino acid substitutions in LIN-7 and LET-23 compensate for each other and permit allele-specific binding in vitro.
Next, if binding of LET-23 to LIN-7 is functionally important for basolateral localization, then LIN-7 of one type should direct LET-23 of the same type to the baso-lateral membrane (type I with type I or type II with type II), but not LET-23 of a different type (type I with type II or type II with type I). We generated transgenic animals that express all pairwise combinations of type I and type II LET-23 RTK and type I and type II LIN-7 and stained them with anti-LET-23 antibodies to determine whether LET-23 was present on the basolateral membrane domain. Animals that coexpress the same types of LIN-7 and LET-23 (type I LIN-7 with type I LET-23 or type II LIN-7 with type II LET-23) show basolateral expression of LET-23 RTK (Figure 2C and data not shown). However, animals that coexpress different types of LIN-7 and LET-23 (type I LIN-7 with type II LET-23 or type II LIN-7 with type I LET-23) showed apical distribution of LET-23 (Figure 2C).
Finally, if LIN-7 binding to LET-23 RTK is functionally important for vulval induction, then a vulva should be induced in animals expressing similar LIN-7 and LET-23 types, but not different types. We determined levels of vulval induction in strains expressing each of the pairwise combinations of type I and type II LIN-7 with type I and type II LET-23. In these experiments, both types of LIN-7 and type II LET-23 were expressed from an extrachromosomal array, whereas type I LET-23 was expressed from the chromosome. We observed that transgenic animals expressing type I LIN-7 with type I LET-23 showed high levels of vulval induction, that transgenic animals expressing different types showed low levels of vulval induction, and that transgenic animals expressing both type II LIN-7 and type II LET-23 showed relatively high levels of vulval induction (Figure 2D). These results provide compelling evidence that LIN-7 binds to LET-23 in vulval precursor cells and that this binding is functionally important for basolateral receptor localization and vulval induction.
LIN-2, LIN-7, and LIN-10 Interact in the Yeast Two-Hybrid System
In addition to lin-7, two other genes (lin-2 and lin-10) are genetically required for basolateral localization of LET-23 RTK (Simske et al., 1996; C. W. W., unpublished data). One possibility is that LIN-2 and LIN-10 function with LIN-7 as a protein complex that binds LET-23 RTK. We tested this possibility using the yeast two-hybrid system and discovered interactions between LIN-7 and LIN-2 and between LIN-2 and LIN-10 (Figure 3). We did not observe any other yeast two-hybrid interactions; specifically, LET-23 did not interact with LIN-2 or LIN-10 and LIN-7 did not interact with LIN-10.
Figure 3. LIN-2, LIN-7, and LIN-10 Interactions in the Yeast Two-Hybrid System.
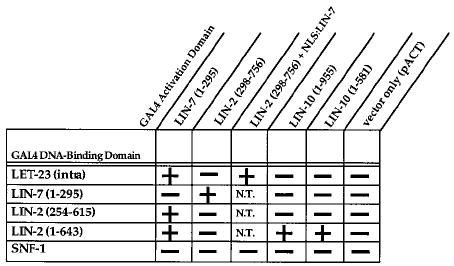
LIN-7 (nearly full-length), LET-23 (the intracellular domain), LIN-2 (three different fragments), and LIN-10 (full-length or an N-terminal fragment) were expressed as fusion proteins with either the GAL4 DNA-binding domain or the GAL4 transcriptional activation domain in the yeast two-hybrid system as indicated. Amino acids present in the fusion protein are shown in parentheses. Pluses or minuses refer to expression of the two reporter genes lacZ and HIS3, indicating protein–protein interaction. NLS:LIN-7 refers to LIN-7 fused with the SV40 nuclear localization signal. SNF-1 is an S. cerevisiae protein kinase and is used as a negative control. Only lacZ activity was scored in the yeast three-hybrid experiments. The HIS3 reporter gene could not be scored, since the third plasmid expressing NLS:LIN-7 carried wild-type HIS3 as a selectable marker. N.T., not tested.
Coimmunoprecipitation of LIN-2, LIN-7, and LIN-10 from S2 Cells
In order to investigate LIN-2, LIN-7, and LIN-10 binding interactions in vivo, we expressed these proteins in heterologous Drosophila Schneider (S2) cells and performed coimmunoprecipitation studies. Cell lysates were made from a stable S2 cell line that expresses LIN-2, LIN-7, and LIN-10, and LIN-7 was immunoprecipitated with anti-LIN-7 antibodies or with preimmune sera as a negative control. We found that LIN-2 specifically coimmunoprecipitated with LIN-7 by immunoblotting with anti-LIN-2 antibodies (Figure 4, lanes 3 and 7). In a similar manner, we immunoprecipitated LIN-10 from the S2 cell extract with anti-LIN-10 antibodies and observed that LIN-2 was able to coimmunoprecipitate specifically with LIN-10 (Figure 4, lane 1). These results indicate that LIN-7 binds to LIN-2 and that LIN-2 binds to LIN-10 in vivo.
Figure 4. Coimmunoprecipitation of LIN-2, LIN-7, and LIN-10 from S2 Cells.
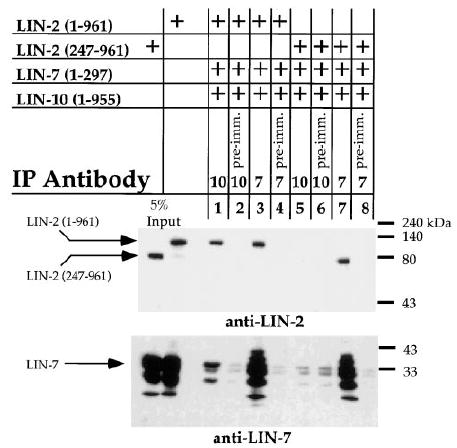
LIN-7 or LIN-10 was immunoprecipitated from Schneider S2 cell lysates (IP antibody), and levels of coimmunoprecipitated LIN-2 or LIN-7 were determined by Western blotting using anti-LIN-2 (top) or anti-LIN-7 antibodies (bottom). Amino acids present in the fusion proteins are indicated in parentheses. LIN-10 or LIN-7 preimmune sera were used as negative controls (preimm.). Total amounts of LIN-2 or LIN-7 present in the cell extracts are shown (5% input).
To determine whether LIN-7 and LIN-10 can simultaneously bind to LIN-2 to form a ternary complex, we immunoprecipitated the complex with anti-LIN-10 antibodies. We observed that LIN-7 was present in the complex by Western blotting with anti-LIN-7 antibodies (Figure 4, lane 1). Furthermore, this coimmunoprecipitation was dependent on LIN-2 acting as an adaptor, as LIN-7 did not coimmunoprecipitate with LIN-10 in cells that express a truncated form of LIN-2 that does not bind LIN-10 (Figure 4, lane 5). These results indicate that LIN-2, LIN-7, and LIN-10 can form a ternary complex in vivo.
Identification of the Region of LIN-7 that Binds LIN-2
We delineated the region of LIN-7 that binds to LIN-2 by generating a series of LIN-7 fusion proteins that contained successively larger N-terminal truncations. A Cos7 cell lysate containing LIN-2 (amino acids 247–961) was incubated with the GST:LIN-7 fusion proteins bound to glutathione-agarose beads. Levels of bound LIN-2 were detected by Western blotting using anti-LIN-2 antibodies. Amino acids 109–295 of LIN-7 could bind LIN-2, but amino acids 126–295 or the PDZ domain of LIN-7 could not bind LIN-2 (Figure 5A). Since the LIN-7 PDZ domain fusion protein is functional and binds LET-23, we infer from this data that LIN-2 binds to a region of LIN-7 N-terminal to the PDZ domain.
Figure 5. Delineation of the Region of LIN-2 that Binds to LIN-7.
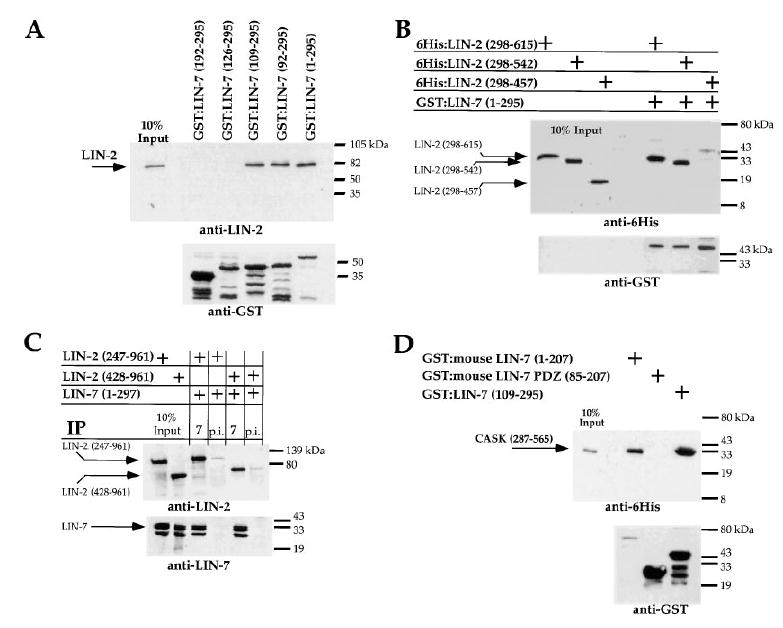
Top gels show levels of bound LIN-2 or human Lin2/CASK protein using immunoblotting with either anti-LIN-2 or anti-6His antibodies. Bottom gels are loading controls showing levels of GST fusion proteins, using anti-GST antibodies. Amino acids present in fusion proteins are shown. Ten percent of total protein present in the cell extract is shown.
(A) Cos7 cell lysate containing LIN-2 (amino acids 247–961) was incubated with purified GST:LIN-7 fusion proteins bound to glutathione-agarose beads.
(B) Purified 6His:LIN-2 fusion proteins were incubated with purified GST:LIN-7 fusion proteins coupled to glutathione-agarose beads.
(C) N-terminally truncated LIN-2 proteins were analyzed for their ability to coimmunoprecipitate with LIN-7 or preimmune sera (p.i.) in S2 cell lysates.
(D) Human Lin2/CASK binds to mouse Lin7 in vitro. Purified 6His:human Lin2/CASK was incubated with purified GST:mouse Lin7 fusion proteins coupled to glutathione-agarose beads.
LIN-2, LIN-7, and LET-23 Can Bind Simultaneously in a Yeast Three-Hybrid System
Our results suggest that LIN-7 has two distinct binding sites, since the PDZ domain of LIN-7 binds to LET-23 RTK and, presumably, a region N-terminal to the PDZ of LIN-7 binds to LIN-2. To show that LET-23 and LIN-2 bind simultaneously to two distinct sites in LIN-7, we employed a yeast three-hybrid system. In the yeast three-hybrid system, we expressed a third protein (LIN-7) in combination with the bait (LET-23) and prey (LIN-2) fusion proteins. LIN-2 and LET-23 do not interact in the yeast two-hybrid system, but an interaction was observed with concomitant expression of LIN-7 (containing an SV40 nuclear localization signal) in these yeast (Figure 3). This interaction requires coexpression of LIN-2, LIN-7, and LET-23, since interactions were not observed when LET-23 and LIN-2 were expressed without LIN-7 or when LIN-2 and LIN-7 were expressed without LET-23. These results suggest that LET-23 and LIN-2 can bind simultaneously to distinct sites of LIN-7.
Defining the Regions of LIN-2 that Bind to LIN-7 and to LIN-10
To map the region of LIN-2 that binds LIN-7, we first expressed different portions of LIN-2 as 6His fusion proteins and determined whether they could bind to LIN-7 in vitro. These experiments showed that amino acids 298–542 of LIN-2 could bind to LIN-7 (Figure 5B). Next, we found that a LIN-2 fragment containing amino acids 428–961 coimmunoprecipitates with LIN-7 from S2 cell lysates (Figure 5C). The combination of these in vitro and in vivo results indicate that a region between the CaM kinase/calmodulin–binding domain and the PDZ domain of LIN-2 (amino acids 428–542) binds to LIN-7.
Finally, we used the yeast two-hybrid system to map the regions of LIN-2 and LIN-10 that bind one another. We found that the N terminus of LIN-2 (amino acids 1–643) interacts with LIN-10 (Figure 3). LIN-2 fragments lacking the CaM kinase domain were unable to bind LIN-10 but were able to bind LIN-7; specifically, amino acids 254–615 of LIN-2 failed to interact with LIN-10 in the yeast two-hybrid system, and amino acids 247–961 of LIN-2 did not coimmunoprecipitate with LIN-10 from S2 cells (Figures 3 and 4). Thus, LIN-10 likely binds to a region in the N-terminal 247 amino acids of LIN-2 that contains the CaM kinase domain. We also found that the N terminus of LIN-10 (amino acids 1–581) interacts with LIN-2 in the yeast two-hybrid system (Figure 3).
Mammalian LIN-2, LIN-7, and LIN-10 Form a Protein Complex
Previous work showed that mammalian CASK and Mint/X11 are highly similar to worm LIN-2 and LIN-10, respectively (Hata et al., 1996; Okamoto and Sudhof, 1997; Cohen et al., 1998). Here, we report three mammalian genes that are highly similar to worm lin-7. Public databases of cDNA clones currently contain three mouse clones predicted to encode proteins similar to worm LIN-7 (I.M.A.G.E. clones 391220, 870825, and 779032), and we named these proteins mLin7A (amino acid sequence is 73% identical to worm LIN-7), mLin7B (73% identical), and mLin7C (63% identical), respectively.
We tested whether the protein interaction between LIN-2 and LIN-7 is conserved in mammals by analyzing whether human Lin2/CASK binds to mouse Lin7A. We purified human Lin2/CASK as a hexahistidine fusion protein and incubated it with mouse GST:Lin7A fusion proteins bound to glutathione-agarose beads. We determined the levels of bound human Lin2/CASK using Western blots with anti-6His antibodies. This experiment showed that human Lin2/CASK binds to full-length mouse Lin7A, but not to the PDZ domain (Figure 5D). Furthermore, we found that human Lin2/CASK could bind to worm LIN-7 in vitro. These results demonstrate that the binding interaction between LIN-2 and LIN-7 is conserved, not only between mammalian homologs, but also between proteins derived from animals as distantly related as mammals and worms.
Work from others demonstrates that mammalian Lin2/CASK binds to mammalian Lin10/Mint/X11 (J.-P. Borg, personal communication; Butz et al., 1998). Together with our results, these data indicate that Lin2/CASK, mLIN-7, and Lin10/Mint/X11 form a ternary protein complex in mammals. The high degree of amino acid sequence conservation, the conserved protein interactions, and the clear role in basolateral localization in worms indicates that the mammalian Lin2/Lin7/Lin10 ternary complex may play a role in basolateral localization of transmembrane proteins in polarized epithelial cells.
Discussion
lin-2, lin-7, and lin-10 are required for basolateral localization of LET-23 RTK, since mutations in lin-2, lin-7, or lin-10 result in mislocalization of LET-23 RTK to the apical membrane domain and cause a vulvaless phenotype. Our work suggests that the mechanism of basolateral localization of LET-23 RTK involves formation of a LIN-2/LIN-7/LIN-10 ternary complex that directly binds to the C terminus of LET-23 RTK (Figure 6). In this protein complex, LIN-10 binds to LIN-2, LIN-2 binds to LIN-7, and LIN-7 binds to LET-23 RTK.
Figure 6. Model for Basolateral Localization of LET-23 RTK by the LIN-2/LIN-7/LIN-10 Protein Complex.
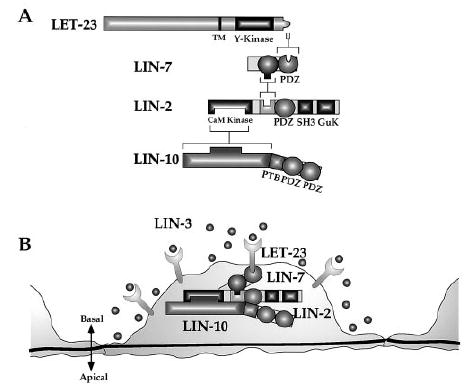
(A) The C terminus of LET-23 binds to the PDZ domain of LIN-7, an internal region of LIN-7 binds to an internal region of LIN-2, and the CaM kinase domain of LIN-2 binds to an N-terminal region of LIN-10.
(B) Localization of LET-23 RTK to the basolateral membrane of the vulval precursor cells by the LIN-2/LIN-7/LIN-10 protein complex.
A combination of genetic and biochemical data strongly support the model presented in Figure 6. First, expression of lin-2, lin-7, lin-10, and let-23 are required in the vulval precursor cells for vulval development (Simske and Kim, 1995; Hoskins et al., 1996; Simske et al., 1996; C. W. W., unpublished data), indicating that LIN-2, LIN-7, LIN-10, and LET-23 RTK are each present to form a protein complex in the vulval precursor cells during vulval induction. Second, the sy1 mutation in let-23 (that deletes the LIN-7-binding site on LET-23) or mutations that eliminate lin-2, lin-7, or lin-10 activity each cause mislocalization of LET-23 RTK and result in a highly penetrant vulvaless phenotype (Aroian et al., 1994). These results indicate that these genes and this binding site have a similar or identical cellular function. Third, LIN-2, LIN-7, and LET-23 can directly bind to each other in vitro, and LIN-2, LIN-7, and LIN-10 coimmunoprecipitate when expressed in S2 cells in vivo.
Fourth, we altered the C terminus of LET-23 so that it could not bind to wild-type LIN-7 and then altered LIN-7 with a compensatory mutation that restored LET-23 binding in vitro. We showed that these compensatory mutations in let-23 and lin-7 exhibit allele-specific suppression of both the apical receptor mislocalization and the vulvaless phenotypes. These results provide compelling evidence that LIN-7 directly binds to LET-23 RTK in vulval precursor cells and that this binding is functionally required for basolateral distribution of LET-23 and vulval signaling.
Finally, worm LIN-2, LIN-7, and LIN-10 are highly similar to mammalian Lin2/CASK, mLin7 proteins, and Mint/X11 proteins, respectively. The mammalian proteins also form a ternary complex in which Lin10/Mint1/X11α binds to Lin2/CASK and Lin2/CASK binds to mLin7 (Figure 5; J.-P. Borg, personal communication; Butz et al., 1998). Thus, the protein–protein interactions in this complex are conserved from worms to mammals, strongly supporting the idea that these protein interactions are functionally important. Our work in C. elegans clearly shows that this complex functions in basolateral localization of a transmembrane protein, indicating that this complex may have a similar function in mammalian polarized epithelia. Interestingly, the human EGF receptor (HER-4) contains a type I PDZ-binding site, yet we were unable to detect binding between HER-4 and mLin7A (data not shown).
There are likely to be additional proteins not yet identified that bind to the LIN-2/LIN-7/LIN-10 protein complex in C. elegans, since LIN-2 and LIN-10 can potentially bind other proteins via their SH3, PDZ, and PTB domains. These domains may bind to proteins that localize the LIN-2/LIN-7/LIN-10 complex within the cell or that regulate the function of this complex in receptor localization.
Specificity of Function of the LIN-2/LIN-7/LIN-10 Complex
lin-2, lin-7, and lin-10 do not have a general role in the organization of the polarized cytoarchitecture of vulval precursor cells, but rather seem to be involved in the basolateral localization of LET-23 RTK specifically. In lin-2, lin-7, or lin-10 mutants, the cell junctions are intact (as determined by staining with the MH27 cell junctional marker) and there are no obvious defects in the basal basement membrane or the apical cuticle (as determined by observation using Nomarski optics). These observations suggest that the apical and basolateral membrane compartments of the vulval precursor cells in lin-2, lin-7, and lin-10 mutants remain functionally distinct, since components of the extracellular matrix are basolaterally secreted and components of the cuticle are apically secreted. Thus, basolateral targeting and secretion of proteins other than LET-23 occurs correctly in lin-2, lin-7, and lin-10 mutants, indicating that they are targeted by a different biochemical mechanism than the one studied here. Sequence comparisons of basolateral targeting signals from different mammalian proteins reveal that there are several types of sequence motifs, implying that there are multiple basolateral targeting mechanisms employing different _trans_-acting proteins that can bind to these sequences (reviewed in Mellman, 1995).
When the LIN-2/LIN-7/LIN-10 protein complex is defective, it is interesting that LET-23 RTK becomes apical rather than diffusely expressed over the entire plasma membrane. Individual transmembrane proteins may have apical targeting signals, such as _N_-linked glycosylation, as well as basolateral sorting signals (Scheiffele et al., 1995). In several cases, the basolateral sorting signal appears to be dominant so that the transmembrane protein is predominantly basolateral, but becomes predominantly apical when the basolateral sorting sequence is mutated or deleted (Matter and Mellman, 1994). In a similar manner, LET-23 RTK may also have an apical targeting motif that functions in the absence of basolateral targeting by LIN-2/LIN-7/LIN-10.
Common Receptor Localization Mechanisms in Epithelia and Neurons
Both neurons and epithelia are polarized cells and must localize transmembrane proteins to distinct regions on their cell surfaces (reviewed in Rodriguez and Powell, 1992). Polarized epithelial cells localize distinct trans-membrane proteins to either the apical or basolateral membrane domains, and neurons localize proteins required for synaptic transmission to pre- and postsynaptic sites. Transmembrane proteins that are localized to the basolateral membranes in epithelia are localized to the dendrites when expressed in neurons, suggesting that similar machinery may be used to localize these proteins to distinct membrane compartments in these two cell types (Dotti and Simons, 1990; Jareb and Banker, 1998).
Our work and recent work from others illustrate that the LIN-2/LIN-7/LIN-10 complex may be used for localizing transmembrane proteins to specific sites of the plasma membrane in both neurons and epithelia. First, LIN-10 localizes the glutamate receptor GLR-1 to neuronal synapses in C. elegans. In lin-10 mutants, GLR-1 is not localized to the synapses, and the animals are touch insensitive (indicative of a defect in glr-1 function) (Rongo et al., 1998 [this issue of _Cell_]). Second, Discs-large (Dlg) and PSD-95 are two MAGUKs related to LIN-2, and both have been implicated in localizing or clustering of neurotransmitter receptors at synapses (reviewed in Sheng, 1996; Zito et al., 1997). Third, mammalian Lin2/CASK, mLin7, and mammalian Lin10/Mint/X11 are expressed in neurons and form a ternary protein complex (Butz et al., 1998; J.-P. Borg, personal communication). This result suggests that this protein complex may function in receptor localization in neurons, as it does in epithelia. In summary, there appears to be a common mechanism of receptor localization in neurons and epithelia that involves LIN-10, MAGUKs (such as LIN-2, DlgA, and PSD-95), and possibly LIN-7 as well. Furthermore, this mechanism of protein targeting has been conserved from worms to mammals.
Possible Mechanisms of Protein Localization by the LIN-2/LIN-7/LIN-10 Complex
How might the LIN-2/LIN-7/LIN-10 complex localize transmembrane proteins in polarized cells? One possible model is that this complex acts to selectively retain proteins on the basolateral membrane domain of epithelial cells, perhaps by limiting endocytosis and recycling of these proteins from the membrane. Lin2/CASK binds to syndecans in mammalian epithelia and neurons and has been shown to bind to the actin-binding protein 4.1 in vitro (Cohen et al., 1998; Hsueh et al., 1998). Syndecans are basolaterally expressed transmembrane proteins that bind heparin in the extracellular matrix of epithelia (reviewed in Bernfield et al., 1992). The functional significance of the binding interactions between Lin2/CASK and either syndecan or protein 4.1 are not known. However, it is possible that binding to syndecan directs Lin2/CASK to the basolateral membrane of epithelia and that the interaction with protein 4.1 then tethers Lin2/CASK to the actin cytoskeleton. Once anchored to the basolateral membranes by syndecan and protein 4.1, the LIN-2/LIN-7/LIN-10 complex could then bind and retain transmembrane proteins, such as LET-23 RTK, to the basolateral membrane domain. Selective membrane retention may account for the basolateral localization of cadherins and the Na/K–ATPase in epithelia (Mays et al., 1995).
A similar model may account for synaptic localization of neurotransmitter receptors. Lin2/CASK binds to neurexin 1b and PSD-95 binds to neuroligins (Hata et al., 1996; Irie et al., 1997). Neurexins are neuronal trans-membrane proteins present at presynaptic sites that form intercellular junctions by binding to neuroligins at postsynaptic sites (Nguyen and Sudhof, 1997). Immunogold electron microscopy revealed that Lin2/CASK is present at both presynaptic and postsynaptic sites (Hsueh et al., 1998). These binding interactions may anchor Lin2/CASK (as well as mLin7 and Lin10/Mint/X11) to presynaptic sites and PSD-95 to postsynaptic sites. Once anchored, the LIN-2/LIN-7/LIN-10 protein complex or PSD-95 could cluster or retain neurotransmitter receptors at the synapse. It has been proposed that Dlg acts to cluster or selectively retain the Shaker K+ channel at neuromuscular synapses in Drosophila (Zito et al., 1997).
Another possible model is that LIN-2/LIN-7/LIN-10 could function in polarized secretion and could target transmembrane proteins for the basolateral membrane domain of epithelia or to the synapses of neurons. Mammalian Lin10/Mints bind to the Munc18/syntaxin 1 protein complex, which is involved in regulating secretory vesicle docking and fusion (Okamoto and Sudhof, 1997). Although the function of this protein–protein interaction is not known, Lin10/Mints could possibly specify which proteins are sorted to basolateral secretory vesicles or they could be involved in the docking and fusion of secretory vesicles with the basolateral plasma membrane.
Experimental Procedures
General Methods and Strains
Standard methods were used to culture C. elegans (variety Bristol strain N2) at 20°C (Wood, 1988). The following mutant alleles were used: linkage group I (LGI), unc-29(e1072) (Wood, 1988); LGII, let-23(sy1) (Aroian and Sternberg, 1991) and lin-7(e1449) (Ferguson and Horvitz, 1985); LGV, him-5(e1490) (Wood, 1988).
Molecular Biology
LIN-7 (amino acids 1–295, 92–295, 109–295, 126–295, and 192–295), mLin7A (1–207 and 85–207), and LIN-31 (1–190) were expressed in bacteria as GST fusion proteins from plasmids pGEX-1, pGEX-2T, or pGEX-3X (Smith and Johnson, 1988). LIN-2 (298–615, 298–542, and 298–457), hLin2/CASK (287–565), and LET-23 (1127–1323, 1127–1317, and 1274–1323) were expressed as bacterial hexahistidine fusion proteins from plasmids pTrcHis-A, -B, or -C (Invitrogen). LIN-2 (247–961) was expressed in Cos7 cells from plasmid pCMV (gift of A. Hajnal). LIN-2 (1–961, 247–961, or 428–961), LIN-7 (1–297), and LIN-10 (1–955) were expressed in S2 cells from plasmid pMK33 (Koelle et al., 1991). More details about plasmid construction are at http://cmgm.Stanford.edu/~kimlab.
Germline Transformation
Germline transformants were obtained by standard DNA microinjection as previously described (Simske et al., 1996). Concentrations of DNA used: type II let-23 (pK713.8-YFI, 4 μg/ml), unc-29(+) (F35D3, 60–80 μg/ml), col-10:GFP (pP09, 80 μg/ml, gift of G. Ruvkun), type I lin-7 (pJS149, 200 μg/ml), type II lin-7 (pJS149 H259V, 200 μg/ml). lin-7(e1449), let-23(sy1); unc-29(e1072); him-5(e1490) (strain SD729) animals were injected with either type II let-23 and unc-29(+), pP09 and type I lin-7, or pP09 and type II lin-7. Transgenic animals expressing type II let-23 are non-Unc, and those expressing lin-7 are green.
Yeast Two-Hybrid and Three-Hybrid Experiments
All yeast methods were performed as described (Simske et al., 1996). LET-23 (873–1323), LIN-2 (1–643 and 254–615), and LIN-7 (1–295) were expressed from pAS1 and LIN-2 (298–756); LIN-10 (1–955 and 1–581) and LIN-7 (1–295) were expressed from pACT (gift of S. Elledge). In the yeast three-hybrid, lin-7 cDNA was fused to the SV40 nuclear localization signal (PKKKRK) and was expressed from a modified version of pTS422 (gift of T. Doyle) where URA3 was replaced with HIS3.
Cell Transfection and Culture
Drosophila Schneider S2 cells were transfected using calcium phosphate (Krasnow et al., 1989). Stable lines were selected in 100 μg/ml Hygromycin B (Sigma). Cos7 cells were transiently transfected in 60 mm dishes with 3–6 μg DNA using lipofectamine (GIBCO-BRL). Cells were lysed 48 hr after transfection in 1 ml of binding buffer (150 mM NaCl, 25 mM Tris-HCl [pH 7.5], 1 mM MgCl2, 0.2% NP40, and 1× protease inhibitor cocktail [Pharmingen]). Cell debris was removed by centrifugation (3000 rpm in an Eppendorf microcentrifuge for 5 min).
In Vitro Binding Assays
GST fusion proteins were purified on glutathione-agarose beads in 5 mM DTT as described (Simske et al., 1996). 6His:LET-23 and 6His:LIN-2 fusion proteins were purified native (for LET-23) or denatured (for LIN-2) on Ni2+-charged agarose beads as described (Janknecht et al., 1991). 6His:LIN-2 proteins were renatured in successive washes in 4 M, 2 M, and 1 M guanidine–HCl diluted in 1× PBS, 150 mM NaCl, and 0.5% Triton X-100. 6His fusion proteins were eluted in 1 M imidazole and dialyzed in PBS + 10% glycerol. Sf9 cell lysates were prepared as described (Simske et al., 1996), and 10–25 μl of the Cos7 cell lysates were used in binding assays. Soluble 6His:LIN-2 and 6His:LET-23 fusion proteins (1–2 μg) were mixed with 1 μg GST:LIN-7 fusion proteins in binding buffer in a final volume of 100 μl as previously described (Simske et al., 1996). Proteins were detected by Western blotting with anti-Xpress antibodies for hexahistidine fusions (1:5000 dilution, Invitrogen), anti-GST antibodies (1:500 dilution, Pharmacia), anti-LIN-2 (1:1000), anti-LIN-7 (1:1000), anti-LIN-10 (1:1000), and anti-LET-23 (1:1000).
Coimmunoprecipitation Experiments
Expression was induced in 3 × 107 of S2 cells (grown at 25°C) for 16 hr using 0.7 mM CuSO4. Cells were washed in PBS and once in buffer A (150 mM NaCl, 25 mM Tris-HCl [pH 7.5], 1 mM MgCl2), and then the cells were resuspended in 1 ml buffer A, 0.2% NP40, and protease inhibitor cocktail. Cells were briefly sonicated, and cell debris was removed by centrifugation (3000 rpm in an Eppendorf microcentrifuge for 5 min). Cell lysate (1 mg) was combined with binding buffer to a final volume of 100 μl. Six microliters of anti-LIN-7, anti-LIN-10, LIN-7 preimmune sera, or LIN-10 preimmune sera was added at 4°C for 90 min. Then, 50 μl of protein-A agarose beads (Pierce) was added for 1–2 hr at 4°C. The beads were washed rapidly four times in 1 ml binding buffer. Bound proteins were removed in Laemmli buffer, and the immunoprecipitates were resolved by SDS-PAGE and detected by Western blotting followed by chemiluminescence (SuperSignal, Pierce).
Immunofluorescent Staining
Mixed stage populations were fixed as in Simske et al. (1996) except Triton X-100 was used at 0.1%. MH27 antibody (gift of Bob Waterston) was used at 1:1000, and anti-LET-23 antibodies were used at 1:1000.
Acknowledgments
We thank A. Hajnal, R. Coffey, A. Candia, V. Reinke, P. Tan, and J. Wang for discussions and critical reading of the manuscript. We are grateful to A. Golden for providing LET-23; C. Ying for purifying LET-23 antibodies; P. Tan for GST:LIN-31 fusion protein; A. Cohen for hLin2 clones; A. Villeneuve for assistance with a dissecting fluorescence microscope; and K. Suyama for assistance with the confocal microscope. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was supported by grants from the NIH and NCI. S. M. K. was supported by an NSF predoctoral fellowship.
References
- Aroian RV, Sternberg PW. Multiple functions of let-23, a Caenorhabditis elegans receptor tyrosine kinase gene required for vulval induction. Genetics. 1991;128:251–267. doi: 10.1093/genetics/128.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroian RV, Lesa GM, Sternberg PW. Mutations in the Caenorhabditis elegans let-23 EGFR-like gene define elements important for cell-type specificity and function. EMBO J. 1994;13:360–366. doi: 10.1002/j.1460-2075.1994.tb06269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. this issue. [DOI] [PubMed] [Google Scholar]
- Cabral JH, Petosa C, Sutcliffe MJ, Raza S, Byron O, Poy F, Marfatia SM, Chishti AH, Liddington RC. Crystal structure of a PDZ domain. Nature. 1996;382:649–652. doi: 10.1038/382649a0. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 in epithelial cells potentially coordinating a functional link between the extracellular matrix and the actin cytoskeleton. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Cohen AR, Anderson JM, Brunger AT. Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition. Nat Struct Biol. 1998;5:317–325. doi: 10.1038/nsb0498-317. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Duclos F, Boschert U, Sirugo G, Mandel JL, Hen R, Koenig M. Gene in the region of the Friedreich ataxia locus encodes a putative transmembrane protein expressed in the nervous system. Proc Natl Acad Sci USA. 1993;90:109–113. doi: 10.1073/pnas.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson EL, Horvitz HR. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985;110:17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel Dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert M, Carlin C. Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J Cell Physiol. 1995;162:434–446. doi: 10.1002/jcp.1041620316. [DOI] [PubMed] [Google Scholar]
- Hoskins R, Hajnal AF, Harp SA, Kim SK. The C. elegans vulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development. 1996;122:97–111. doi: 10.1242/dev.122.1.97. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Janknecht R, de Martynoff G, Lou J, Hipskind RA, Nordheim A, Stunnenberg HG. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci USA. 1991;88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jareb M, Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- Kim SK. Polarized signaling: basolateral receptor localization in epithelial cells by PDZ-containing proteins. Curr Opin Cell Biol. 1997;9:853–859. doi: 10.1016/s0955-0674(97)80088-9. [DOI] [PubMed] [Google Scholar]
- Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Kornfeld K. Vulval development in Caenorhabditis elegans. Trends Genet. 1997;13:55–61. doi: 10.1016/s0168-9525(97)01005-6. [DOI] [PubMed] [Google Scholar]
- Krasnow MA, Saffman EE, Kornfeld K, Hogness DS. Transcriptional activation and repression by Ultrabithorax proteins in cultured Drosophila cells. Cell. 1989;57:1031–1043. doi: 10.1016/0092-8674(89)90341-3. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Cabral JH, Lin L, Hough C, Bryant PJ, Stolz L, Chishti AH. Modular organization of the PDZ domains in the human discs-large protein suggests a mechanism for coupling PDZ domain-binding proteins to ATP and the membrane cytoskeleton. J Cell Biol. 1996;135:753–766. doi: 10.1083/jcb.135.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfatia SM, Morais CJ, Kim AC, Byron O, Chishti AH. The PDZ domain of human erythrocyte p55 mediates its binding to the cytoplasmic carboxyl terminus of glycophorin C. J Biol Chem. 1997;272:24191–24197. doi: 10.1074/jbc.272.39.24191. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Molecular sorting of membrane proteins in polarized and nonpolarized cells. Cold Spring Harbor Symp Quant Biol. 1995;60:745–752. doi: 10.1101/sqb.1995.060.01.080. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Sudhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272:31459–31464. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- Rodriguez BE, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rodriguez BE, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. this issue. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Simske JS, Kim SK. Sequential signaling during Caenorhabditis elegans vulval induction. Nature. 1995;375:142–146. doi: 10.1038/375142a0. [DOI] [PubMed] [Google Scholar]
- Simske JS, Kaech SM, Harp SA, Kim SK. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell. 1996;85:195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Tejedor FJ, Bokhari A, Rogero O, Gorczyca M, Zhang J, Kim E, Sheng M, Budnik V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, W.B. (1988). The Nematode Caenorhabditis elegans (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Zito K, Fetter RD, Goodman CS, Isacoff EY. Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]