Clinical Relevance of Multidrug Resistance Gene Expression in Ovarian Serous Carcinoma Effusions (original) (raw)
. Author manuscript; available in PMC: 2012 Dec 5.
Published in final edited form as: Mol Pharm. 2011 Jul 15;8(6):2080–2088. doi: 10.1021/mp200240a
Abstract
The presence of tumor cells in effusions within serosal cavities is a clinical manifestation of advanced-stage cancer and is generally associated with poor survival. Identifying molecular targets may help to design efficient treatments to eradicate these aggressive cancer cells and improve patient survival. Using a state-of-the-art Taqman-based qRT-PCR assay, we investigated the multidrug resistance (MDR) transcriptome of 32 unpaired ovarian serous carcinoma effusion samples obtained at diagnosis or at disease recurrence following chemotherapy. MDR genes were selected a priori based on an extensive curation of the literature published during the last three decades. We found three gene signatures with a statistically significant correlation with overall survival (OS), response to treatment (complete response - CR vs. other), and progression free survival (PFS). The median log-rank p-values for the signatures were 0.023, 0.034, and 0.008, respectively. No correlation was found with residual tumor status after cytoreductive surgery, treatment (with or without chemotherapy) and stage defined according to the International Federation of Gynecology and Obstetrics. Further analyses demonstrated that gene expression alone can effectively predict the survival outcome of women with ovarian serous carcinoma (OS: log-rank p=0.0000 and PFS: log-rank p=0.002). Interestingly, the signature for overall survival is the same in patients at first presentation and those who had chemotherapy and relapsed. This pilot study highlights two new gene signatures that may help in optimizing the treatment for ovarian carcinoma patients with effusions.
Keywords: ovarian serous carcinoma, effusion, multidrug resistance, gene signature
Introduction
Ovarian cancer is the fifth most deadly cancer in women and has the highest mortality rate of all gynecological cancers.1 In the U.S., 21,880 new patients were diagnosed with ovarian cancer in 2010 and approximately 13,850 succumbed to the disease.1 Despite aggressive surgical cytoreduction and first line combination chemotherapy of carboplatin and paclitaxel, there has been no significant improvement in survival of patients with ovarian cancer.2 The disease recurs in most cases such that the 5-year survival rate for patients with advanced ovarian cancer is approximately 20%.3 This high mortality rate is attributed to drug-resistant primary tumors and widespread metastasis to the serosal cavities through associated peritoneal and/or pleural effusions (for review, see Davidson4). In fact, 70% of new patients with ovarian cancer are diagnosed at stages III and IV according to the International Federation of Gynecology and Obstetrics (FIGO).3
Effusions are driven by the disruption of the homeostatic forces that control the flow into and out of serosal cavities, and are triggered by tumors. The mechanisms underlying the formation of effusions include lymphatic obstruction by tumor cells5 (which impairs normal drainage), vascular invasion and increased permeability driven by secretion of vascular endothelial growth factor (VEGF)6 and cytokines.7 Malignant cells, reactive mesothelial cells, which display features that mimic those of neoplastic transformation, and inflammatory cells (macrophages, tumor-infiltrating T cells) are the primary cellular components of effusions.8 Although effusions are found in a hypoxic microenvironment with reduced access to nutrients, the tumor cells can proliferate and further metastasize. Furthermore, these cells have the capability to overcome anoikis, the cell death that occurs due to insufficient cell-matrix interactions (for review, see Simpson et al.9). Because the malignant cells found in effusions are frequently resistant to standard chemotherapy, we postulate that they may express multidrug resistance (MDR) genes.
To test this hypothesis, we have extensively reviewed the literature of the past thirty years to select genes whose expression is associated with multidrug resistance. The expression profiles of these genes were assessed using a Taqman-based qRT-PCR assay in 32 unpaired ovarian serous carcinoma effusion samples obtained at diagnosis or at disease recurrence following chemotherapy. We recently showed that the Taqman low density array (TLDA), a high-throughput qRT-PCR assay, provides the highest sensitivity and specificity in measuring ABC transporter gene-expression profiles, a superfamily of 48 highly homologous members, initially studied in the NCI-60 cancer cell line panel.10 This is particularly important, as MDR is mediated by families of highly homologous genes encoding drug uptake transporters (solute carriers), efflux transporters (both ATP and non-ATP dependent transporters), DNA repair proteins and phase I and II metabolism enzymes. These mechanisms, along with evasion of drug-induced apoptosis, alteration of target proteins, and drug sequestration, can act individually or synergistically, leading to multidrug resistance (MDR), in which cells become resistant to a variety of structurally and mechanistically unrelated drugs (for review, see Gillet and Gottesman11). Here, we report a pilot study evaluating the power of MDR genes to predict overall and progression-free survival, which may help to categorize patients and individualize treatment.
Materials and Methods
Tumor Samples
Specimens and clinical data were obtained from the Department of Gynecologic Oncology at the Norwegian Radium Hospital. Fresh, non-fixed effusions (n=32; 27 peritoneal, 5 pleural) were obtained from 32 patients diagnosed with serous ovarian carcinoma (n=25), primary peritoneal serous carcinoma (n=6) or tubal serous carcinoma (n=1). All will be referred to as ovarian carcinoma henceforth due to their closely linked histogenesis and phenotype. Effusions were submitted for routine diagnostic purposes to the Division of Pathology at the Norwegian Radium Hospital from 2000–2006, and processed immediately after tapping. Cell blocks were prepared using the thrombin clot method. The remaining material was fresh-frozen at −70°C in RPMI1640 medium supplemented with 50% FCS and 20% DMSO at a ratio of 1:1 (Invitrogen, Carlsbad, CA, USA). Diagnoses were established by morphology and immunohistochemistry.12 Clinicopathologic data are detailed in Table 1. The Regional Committee for Medical Research Ethics in Norway approved the study.
Table 1.
Clinicopathologic data of the study cohort (32 patients).
| Parameter | Number | |
|---|---|---|
| Age | (Mean; Range) | 64; 45–83 |
| FIGO stage | III | 18 |
| IV | 12 | |
| NAa | 2 | |
| Grade | I | 1 |
| II | 5 | |
| III | 20 | |
| NAb | 6 | |
| Residual disease | ≤1 cm | 17 |
| >1 cm | 9 | |
| NAb | 6 | |
| Chemotherapy prior to tapping | No | 15 |
| Yes | 16 | |
| NAa | 1 |
Preparation of Total RNA
Total RNA was prepared using the Trizol method (Invitrogen, Carlsbad, CA, USA). RNA was quantitated using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). The integrity of the RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA, USA) and then stored at −80°C.
Reverse Transcription
Synthesis of cDNA from 1 µg total RNA in a 20 µl reaction volume was carried out using the High Capacity cDNA kit with RNAse inhibitor (Applied Biosystems, Foster City, CA, USA) as per the manufacturer’s instructions. The reverse transcription conditions were as follows: 10 minutes at 25°C, 120 minutes at 37°C, 5 seconds at 85°C. Following reverse transcription, cDNA was stored at 4°C.
Taqman Low Density Arrays (TLDAs)
Expression levels of 380 MDR-associated genes were measured using custom-made Taqman Low Density Arrays (Applied Biosystems, Foster City, CA, USA) (Supplementary Table 1). cDNA was mixed with 2X Taqman Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), loaded on the TLDA card, and run on an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) as per the manufacturer’s instructions.
Data Analysis
Data from TaqMan Low Density Arrays was collected for each of the 32 samples of ovarian carcinomas in SDS (Sequence Detection System) files, which were then uploaded into RQ (Relative Quantification) Manager software. This program provides the expression level in log2 value for each gene. These values are known as the cycle threshold and range from 1 to 40. Unexpressed genes are labeled as undetermined. Data sets are accessible from the Gene Expression Omnibus (GEO) repository, accession number GSE29702, at the following website: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29702. A total of 381 genes were analyzed; the ribosomal RNA 18s, used for quality control, was the only gene arrayed in four replicates. The median expression of each sample was subtracted from all gene expression data for that sample. The expression data from 18s probes were averaged together.
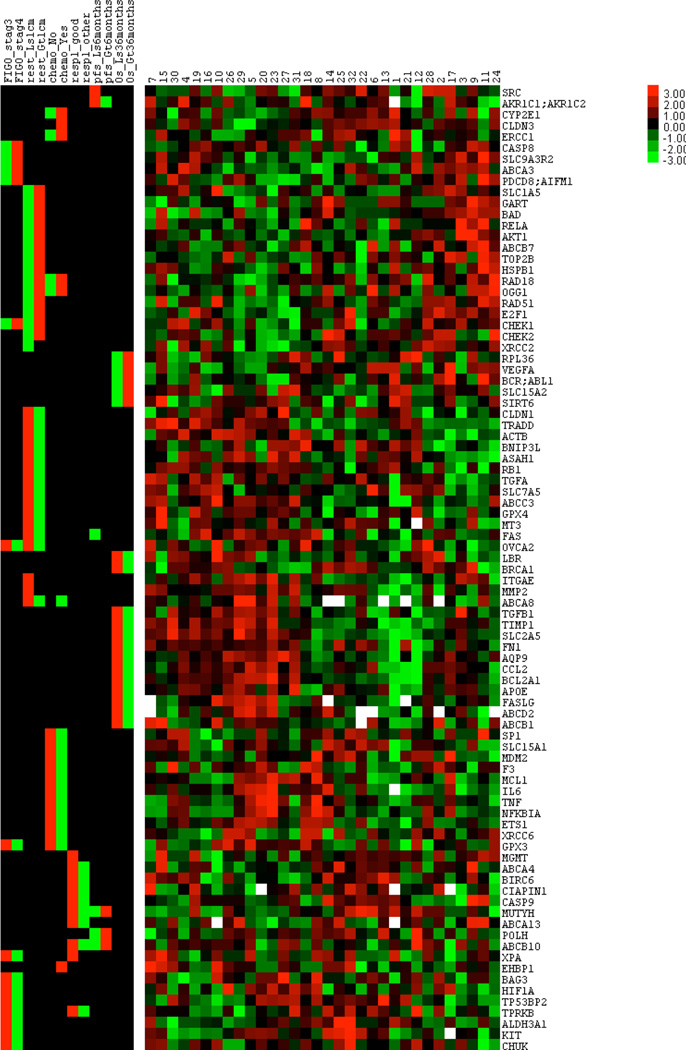
We first removed genes with more than 20% missing values across all samples, resulting in 350 genes for further data analysis. Based on these 350 genes, we then changed the sign of each value (positive to negative and vice versa) and transformed all values to Z-scores (i.e., gene expression values have mean = 0 and variance = 1 across all samples). Z-scores of the 350 genes were then fitted into a linear regression model with random sampling strategy in order to find the possible cause-and-effect between the gene expression level and the corresponding clinical parameters (i.e., survival time and FIGO stage). This was done using a P<0.05 cutoff for the linear model. For genes selected by this cutoff, we applied hierarchical clustering on the Z-scores, and then visualized both the Z-score clusters and the corresponding T-values of the enriched clinical parameters (e.g., median p-values of ten times random sampling in linear regression fitting <0.05) in a color-coded heatmap. In the heatmap, green and red colors in the left panel represent negative and positive correlation between clinical parameters and the corresponding gene expression levels, respectively; on the right panel, the green and red colors represent low and high gene expression levels, respectively; white color represents missing values.13–15 For the regression model with random sampling strategy, ten times leave-one-out sampling was used, a gene with median p-value smaller than 0.05 was selected as a potential marker gene. Based on the previous analysis, we classified samples into two groups according to the expression signature of the marker genes for each parameter. The ten times leave-one-out sampling approach was also applied to the classification as well as to the follow-up survival analysis (log-rank test).
Boxplot
MATLAB software (MathWorks, Natick, MA, USA) was used to perform the notched-box plot. All default parameters were applied in Matlab boxplot function. In this plot, the red line represents the medium of data center, and the outliers are larger than _q_3 + w(_q_3 − _q_1)_q_3 + w(_q_3 − _q_1) or smaller than _q_1 − w(_q_3 − _q_1), where _q_1 and _q_3 are the 25th and 75th percentiles, respectively. Maximum whisker length w, the default is a w of 1.5.
Results
Correlation of 380 MDR-linked Gene Expression Profiles with Clinical Covariates
We investigated the expression profiles of 380 multidrug resistance-associated genes in 32 unpaired ovarian serous carcinoma effusion samples obtained at diagnosis or at disease recurrence following chemotherapy (Table 1). These genes, selected from the literature published in the past 30 years, were reported to have a role in multidrug resistance, based mainly on in vitro studies.16 The correlation of the gene expression profiles was then assessed with six clinical parameters (Fig. 1). The genes were selected based on their p-value using a regression model with random sampling strategy (leave-one-out cross-validation model17). One sample at a time was excluded, and the remaining samples were analyzed to find significant correlations, based on our criterion of p<0.05 in the linear regression model. The model is then used to predict the excluded sample. This method yields an unbiased estimate of the prediction accuracy. All the selected genes had a median p-value <0.05. We found three gene signatures with a statistically significant correlation with overall survival (OS), response to treatment (complete response - CR vs. other), and progression free survival (PFS) (Table 2). The median log-rank p-values for the signatures were 0.023, 0.034, and 0.008, respectively. No correlation was found with residual tumor status after cytoreductive surgery (Supplementary Table 2), treatment (Supplementary Table 3) and stage defined according to the International Federation of Gynecology and Obstetrics (FIGO) (Supplementary Table 4).
Figure 1.
Color-coded heatmap of enriched clinical parameters with corresponding gene expression levels (median p-values of ten times random sampling in linear regression fitting <0.05). The left section of the heatmap shows the correlation of the 88 selected genes to various clinical parameters, whereas the right section shows the differential expression of these genes in 32 effusion samples. Green and red colors in the left section represent negative and positive correlation between clinical parameters and the corresponding gene expression levels, respectively. The green and red colors in the right section represent low and high gene expression levels, respectively, while white represents missing values. FIGO: International Federation of Gynecology and Obstetrics
Rest: residual tumor, Ls: less, Gt: greater, Chemo: chemotherapy, Resp1_good: complete response, Resp1_other: partial response/stable disease/progression/allergic or adverse reaction, PFS: progression-free survival, OS: overall survival
Table 2.
Correlation of gene expression profiles with clinical covariates1.
| Median P-values2 | 0.023 | 0.027 |
|---|---|---|
| Gene ID | OS Ls 36 months3 | OS Gt 36 months4 |
| VEGFA | −3.5 | 3.6 |
| BCR-ABL | −2.9 | 2.8 |
| RPL36 | −2.2 | 2.3 |
| SIRT6 | −2.2 | 2.2 |
| SLC15A2 | −2.1 | 2.1 |
| LBR | 2.1 | −2.2 |
| ABCB1 | 2.1 | −2.1 |
| FASLG | 2.3 | −2.2 |
| TIMP1 | 2.3 | −2.2 |
| BCL2A1 | 2.3 | −2.3 |
| ABCD2 | 2.4 | −2.3 |
| APOE | 2.4 | −2.3 |
| AQP9 | 2.7 | −2.7 |
| FN1 | 2.7 | −2.7 |
| TGFB1 | 2.8 | −3.0 |
| SLC2A5 | 3.0 | −3.0 |
| CCL2 | 3.3 | −3.2 |
| BRCA1 | 3.3 | −3.3 |
| Median P-values | 0.034 | 0.14 |
| Gene ID | Resp1, good5 | Resp1, other6 |
| XPA | 2.1 | 0.0 |
| MGMT | 2.1 | 0.0 |
| MUTYH | 2.1 | −2.2 |
| ABCA4 | 2.1 | −2.2 |
| TPRKB | 2.2 | −2.2 |
| ABCB10 | 2.2 | −2.2 |
| BIRC6 | 2.3 | −2.2 |
| ABCA13 | 2.4 | −2.4 |
| CIAPIN1 | 3.0 | −2.8 |
| CASP9 | 3.1 | −3.0 |
| Median P-values | 0.008 | 0.043 |
| Gene ID | PFS Ls 6 months7 | PFS Gt 6 months8 |
| POLH | −2.8 | 2.7 |
| ABCB10 | −2.2 | 2.1 |
| FAS | −2.2 | 0.0 |
| MUTYH | −2.1 | 2.2 |
| SRC | 2.1 | 0.0 |
| AKR1C1/AKR1C2 | 2.4 | −2.4 |
Survival Risk Prediction and Gene Pathway Analysis
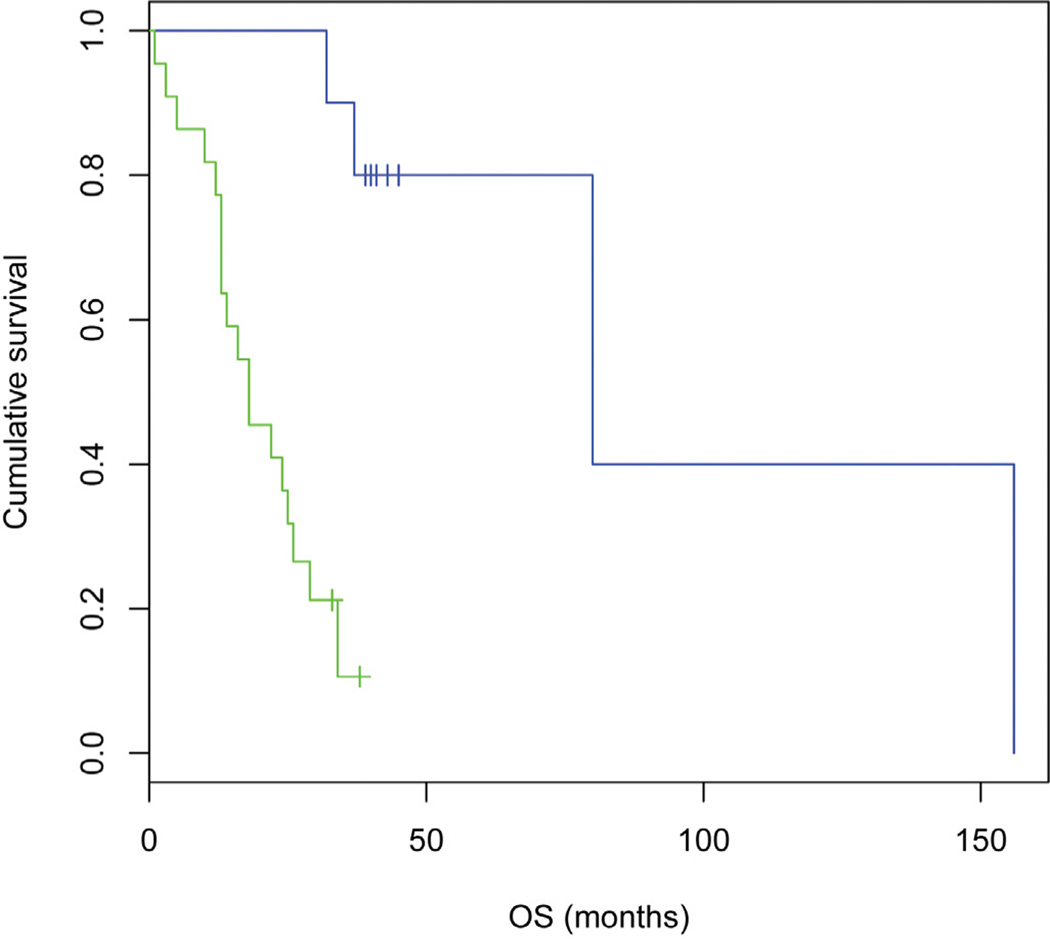
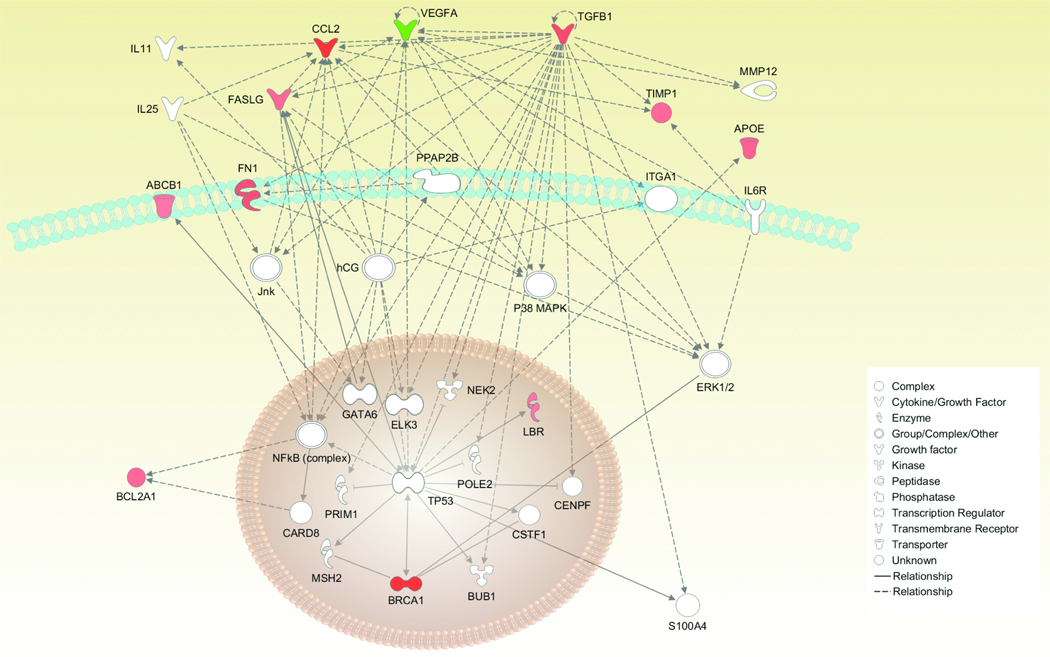
Our studies demonstrated that gene expression alone could effectively predict the survival outcome of women with ovarian serous carcinoma. As noted above, analysis of effusion samples led us to an 18-gene signature that predicts OS (log-rank p=0.0000) (Table 1 and Fig. 2). Thirteen genes were found to be positively correlated with poor OS, while five genes were found to be negatively correlated with poor OS. Further analysis of the 18-gene signature highlighted one pathway linking 11 genes (Fig. 3) that promote tumor growth and survival.
Figure 2.
Kaplan-Meier curve for overall survival. An 18-gene signature alone can effectively predict the overall survival of women with ovarian serous carcinoma. (log-rank p=0.0000). Blue curve: 10 patients, mean OS 55 months, median 41 months. Green curve: 22 patients, mean OS 19 months, median 18 months.
Figure 3.
Ingenuity pathway analysis of the 18 genes predicting poor OS for patients presenting with ovarian serous carcinoma effusions. The 18-gene signature was analyzed by Ingenuity Pathways Analysis software (Ingenuity Systems, Redwood City, CA) to determine whether biological relationships exist between the genes present in this signature. A numerical value was determined by the software to rank networks according to relevance to the genes in the input dataset. The highest ranked pathway is presented. Green represents a decrease in expression, while red is an increase in expression of these genes. White represents the genes that are suggested by the software to be part of the proposed pathway.
Our study also revealed a 6-gene signature predicting progression-free survival (log-rank p=0.002) (Fig. 4). The signature is composed of 4 genes negatively correlated with poor PFS; they encode the base-excision repair enzyme MUTYH, the low fidelity polymerase POLH, the death receptor FAS, and the ABC transporter ABCB10. Two genes, the proto-oncogene SRC encoding a non receptor tyrosine kinase and AKR1C1, which encodes an enzyme involved in phase 1 metabolism, were positively correlated with poor PFS, therefore linking their upregulation with poor PFS. The assay used to detect AKR1C1 is also able to detect the transcript AKR1C2. Therefore, further study is required to determine which form is predominant in these samples. Subsequent gene network analysis did not identify a meaningful pathway linking these six genes.
Figure 4.
Kaplan-Meier curve for progression free survival. Log rank p-value = 0.002. 6-gene signature alone can effectively predict the progression free survival of women with ovarian serous carcinoma. (log-rank p = 0.002). Blue curve: 19 patients, mean PFS 4 months, median 2 months. Green curve: 13 patients, mean PFS 17 months, median 10 months.
Discussion
This pilot study reveals two different signatures able to predict overall and progression free survival, respectively, in ovarian serous carcinoma effusion samples. The gene signatures were validated using a leave-one-out cross-validation model. The results suggest strongly that the analyzed genes are involved in multidrug resistance, as we found a 6-gene signature for the prediction of PFS. Moreover, the data indicate that the genes we have selected promote cell proliferation, highlighted by an 18-gene signature predicting OS. Given that many primary ovarian tumors are drug-resistant, our study provides potential therapeutic targets in effusions that may be investigated in order to improve response to therapy.
Driver mutations are a class of somatic mutations that are often involved in cancer development. For example, C:G to A:T transversion mutations were identified to be among the most predominant type of these mutations in many cancers, including ovarian cancer.18 This highlights the importance of enzymes mediating DNA repair that preserve genomic integrity by counteracting the accumulation of mutation events. We found two DNA repair genes to be down-regulated in the 6-gene signature for PFS. Besides POLH, for which a role in ovarian cancer had not yet been studied, we identified MUTYH, an adenine DNA glycosylase that mediates removal of A paired with G or C.19
Two other genes, ABCB10 and FAS (CD95) were found to predict short PFS when they are down-regulated. ABCB10 is a mitochondrial ATP-binding cassette (ABC) transporter that may be involved in heme biosynthesis.20 To the best of our knowledge, this transporter has only been linked to cancer and MDR through one in vitro study showing an increase in DNA-copy numbers of this gene in a camptothecin-resistant colon cancer cell line (HT-29).21 As for FAS, several studies have reported its association with aggressive ovarian cancer.22–24 The overexpression of two genes, SRC and AKR1C1, was found to be associated with poor prognosis for PFS. SRC, a non-receptor tyrosine kinase, is a key mediator of multiple signaling pathways that regulate critical cellular functions, and is aberrantly expressed in many cancers including ovarian cancer.25 It was shown that SRC inhibition has potent anti-angiogenic effects.26 A strategy that is now being evaluated in a phase II trial (NCT00610714, OVERT1) uses the highly selective inhibitor of SRC, Saracatinib (AZD0530), with or without a combination of carboplatin and paclitaxel. Reports on the role of SRC in MDR are difficult to reconcile, as its overexpression (and activation) was shown in one study to promote drug resistance and tumor survival in a mouse ovarian cancer cell line,27 while another study revealed that its inhibition enhanced the cytotoxicity of paclitaxel and cisplatin in both mouse and human ovarian cancer cells.28 The aldo-keto reductase 1C also known as dihydrodiol dehydrogenase (DDH) mediates the metabolism of steroid hormones and xenobiotics.29 Expression of AKR1C1 was correlated with poor prognosis in non-small cell lung cancer, and with disease progression in esophageal cancer, whereas AKR1C2 was found to be correlated with disease progression in patients with prostatic cancer.30 Multiple studies have shown the role of AKR1C1 in MDR,31, 32 and specifically its role in cisplatin resistance in ovarian cancer cells.33–35
Interestingly, we found that ABCB1 (MDR1, P-gp), the most studied drug efflux transporter and a common cause of resistance to paclitaxel, which is uniformly used in the treatment of ovarian cancer, positively correlates with poor OS but not with PFS, as one could have hypothesized. We can therefore speculate that ABCB1 has a role in tumor biology in contrast to its expected role in conferring MDR. Research has shown that tumor cells activate diverse nonspecific stress response pathways in response to hypoxic, acidic, and oxidative stresses. Numerous studies have shown that one of the responses to these stresses is the upregulation of ABCB1 (for review, see Callaghan et al.36). Also, the immune system, through macrophages, can induce ABCB1 expression. However, whether ABCB1 expression has a role in tumor biology has yet to be substantiated.
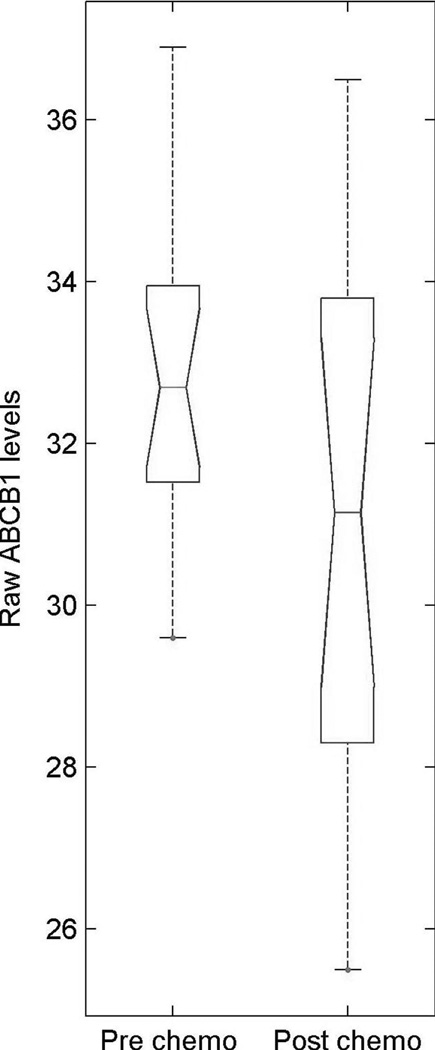
However, closer analysis of the dataset indicates some variability in the expression of ABCB1 among the patients profiled. Four patients expressed ABCB1 at a relatively high level (all had received chemotherapy, two had short PFS, one medium, and one long PFS), but this information is not revealed by the current analytical methods, which are based upon mean values to find differentially expressed genes (Fig. 5). This observation highlights a recurrent issue related to the analysis of high-throughput gene expression profiling. In addition to identifying a gene signature that defines a trend among patients, it is critical to pinpoint individual patients presenting any specific marker (e.g. ABCB1) that could lead to treatment failure. Although such precise analysis can be performed using a system of classifiers in which the system decision is based on a Boolean rule combining individual classifier decisions, this would require the analysis of several hundred samples to avoid overfitting problems.37, 38 Such subtle gene expression differences among patients might be the key to ending the long-lasting debate on intrinsic and acquired drug resistance signatures. Indeed, a standard analysis leads to the discrimination of patients in two groups based upon a pattern of gene expression representing an average state of resistance or sensitivity as might be seen in intrinsic resistance. Then, the individual response to chemotherapy rather than the statistical analysis would lead to the identification of a very small group of patients overexpressing poor survival markers, which are undetectable using current statistical analysis. The concept that chemotherapy resistance as manifested by recurrence after treatment (acquired resistance) results from the action of a small number (≥1) of MDR genes which differ from patient to patient is corroborated in this study, as we were not able to identify a specific gene signature correlating with samples taken post-chemotherapy. However, the small size of the dataset limits the strength of such a statement.
Figure 5.
Boxplot of ABCB1 expression in patients treated or not with chemotherapy. The y-axis indicates log2 cycle threshold (Ct) values. Smaller is the Ct value, greater is the expression. Post-chemo group = 17, pre-chemo group = 15. The median is indicated by the red line, while the first and third quartiles are the edges of the white area, known as the inter-quartile range (IQR). The extreme values are the ends of the dashed lines (whiskers) extending from the IQR (within 1.5 times the IQR from the upper or lower quartile), whereas the outliers are larger than the whiskers.
In addition to ABCB1, upregulation of 12 other genes was found to be correlated with poor overall survival. Among these genes, the roles of the peroxisomal ABC transporter ABCD2 (ALDRP - adrenoleukodystrophy related protein), which transports the coenzyme A esters of very-long-chain fatty acids,39 and SLC2A5 (GLUT5), a transporter involved in fructose uptake,40 have yet to be determined. Five genes were found to be negatively correlated with poor OS. The roles of the uptake transporter SLC15A2 (PEPT2), a proton-coupled oligopeptide transporter,41, 42 and the tyrosine kinase BCR-ABL in ovarian serous carcinoma, are yet to be unraveled.
Paradoxically, we found that down-regulation of VEGFA (VEGF) and the upregulation of TIMP1, a tissue inhibitor of metalloproteinases (MMPs), correlate with poor OS. In support of this paradoxical observation, multiple other studies have shown an association of TIMP1 expression with poor prognosis in several cancers including breast, renal, colorectal, and papillary thyroid cancers as well as non-Hodgkin lymphoma.43–45 Functional studies showed that TIMP1 has a role in the regulation of apoptosis, which may lead to cancer progression through apoptosis inhibition.46, 47 As for VEGF, numerous studies performed to assess its clinical significance in ovarian cancer (mainly in primary tumors) have shown an association between elevated VEGF and poor OS (for review, see Carpini et al.48). However, our finding is consistent with Davidson and colleagues, who characterized the mRNA and protein profiles of angiogenesis-related genes in effusion samples of patients with ovarian carcinoma.7 One should bear in mind that although the effusion samples analyzed in this study contained greater than 50% cancer cells, these samples also contained inflammatory cells that might promote antitumor signals. Another parameter that must not be neglected is the epithelial-mesenchymal transition that the cells analyzed in this study have undergone. Cells have lost their epithelial morphology, reorganized their cytoskeleton, and acquired a motile phenotype, which clearly affects their gene expression profile compared to the epithelial cells from the solid tumor of origin.49 A recent study investigating the same genes indicates that primary ovarian serous carcinoma has a different pattern of MDR gene expression with a different signature predicting overall survival (J-P Gillet et al., unpublished data).
One interesting observation is that the signature for overall survival is the same in patients at first presentation and those who had chemotherapy and relapsed. The simplest explanation for this observation is that the chemotherapy did not select in general for a new population of cancer cells, suggesting that the surviving cells were stochastic survivors of less than 100% effective chemotherapy and not a strongly selected subpopulation. This does not rule out a possible role for individual drug-resistance genes being expressed at higher levels when ovarian cancer effusions relapse after chemotherapy, but only suggest that the biology of the original tumor is unchanged.
This pilot study provides strong data on MDR-associated gene expression profiles in ovarian serous carcinoma effusions, using a high-throughput Taqman-based qRT-PCR assay. The findings warrant further analysis to validate these two survival signatures on a larger cohort of effusion samples. Although we and others have shown the superiority of Taqman-based qRT-PCR over high-density microarrays, especially for studying genes that belong to a highly homologous gene family,10, 50 the best analytical strategy remains to be determined. Deep sequencing arises as a promising accurate method of analysis of the whole transcriptome. However, the benefits of such an assay have to be carefully weighed in terms of data quality, efficacy, and cost compared with state-of-the-art Taqman-based qRT-PCR.
Supplementary Material
Supplementary data
Acknowledgments
We thank George Leiman for editorial assistance. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and by the Inger and John Fredriksen Foundation for Ovarian Cancer Research.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Supplementary Tables 1–4 including correlation of gene expression profiles with residual tumor status after cytoreductive surgery, with treatment, and with FIGO stage and multidrug resistance-linked gene profiles. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, Bowtell D, Brady M, Casado A, Cervantes A, Eisenhauer E, Friedlaender M, Fujiwara K, Grenman S, Guastalla JP, Harper P, Hogberg T, Kaye S, Kitchener H, Kristensen G, Mannel R, Meier W, Miller B, Neijt JP, Oza A, Ozols R, Parmar M, Pecorelli S, Pfisterer J, Poveda A, Provencher D, Pujade-Lauraine E, Randall M, Rochon J, Rustin G, Sagae S, Stehman F, Stuart G, Trimble E, Vasey P, Vergote I, Verheijen R, Wagner U. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann. Oncol. 2005;16 Suppl 8:viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N. Engl. J. Med. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 4.Davidson B. Biological characteristics of cancers involving the serosal cavities. Crit. Rev. Oncog. 2007;13(3):189–227. doi: 10.1615/critrevoncog.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Feldman GB, Knapp RC. Lymphatic drainage of the peritoneal cavity and its significance in ovarian cancer. Am. J. Obstet. Gynecol. 1974;119(7):991–994. doi: 10.1016/0002-9378(74)90021-0. [DOI] [PubMed] [Google Scholar]
- 6.Zebrowski BK, Yano S, Liu W, Shaheen RM, Hicklin DJ, Putnam JB, Jr, Ellis LM. Vascular endothelial growth factor levels and induction of permeability in malignant pleural effusions. Clin. Cancer Res. 1999;5(11):3364–3368. [PubMed] [Google Scholar]
- 7.Davidson B, Reich R, Kopolovic J, Berner A, Nesland JM, Kristensen GB, Trope CG, Bryne M, Risberg B, van de Putte G, Goldberg I. Interleukin-8 and vascular endothelial growth factor mRNA and protein levels are down-regulated in ovarian carcinoma cells in serous effusions. Clin. Exp. Metastasis. 2002;19(2):135–144. doi: 10.1023/a:1014582911680. [DOI] [PubMed] [Google Scholar]
- 8.Bedrossian CW. Diagnostic problems in serous effusions. Diagn. Cytopathol. 1998;19(2):131–137. doi: 10.1002/(sici)1097-0339(199808)19:2<131::aid-dc14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272(2):177–185. doi: 10.1016/j.canlet.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Orina JN, Calcagno AM, Wu CP, Varma S, Shih J, Lin M, Eichler G, Weinstein JN, Pommier Y, Ambudkar SV, Gottesman MM, Gillet JP. Evaluation of current methods used to analyze the expression profiles of ATP-binding cassette transporters yields an improved drug-discovery database. Mol. Cancer Ther. 2009;8(7):2057–2066. doi: 10.1158/1535-7163.MCT-09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 12.Davidson B, Nielsen S, Christensen J, Asschenfeldt P, Berner A, Risberg B, Johansen P. The role of desmin and N-cadherin in effusion cytology: a comparative study using established markers of mesothelial and epithelial cells. Am. J. Surg. Pathol. 2001;25(11):1405–1412. doi: 10.1097/00000478-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Wang J. A new framework for identifying combinatorial regulation of transcription factors: a case study of the yeast cell cycle. J. Biomed. Inform. 2007;40(6):707–725. doi: 10.1016/j.jbi.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Bo TH, Jonassen I, Myklebost O, Hovig E. Tumor classification and marker gene prediction by feature selection and fuzzy c-means clustering using microarray data. BMC Bioinformatics. 2003;4:60. doi: 10.1186/1471-2105-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Delabie J, Aasheim H, Smeland E, Myklebost O. Clustering of the SOM easily reveals distinct gene expression patterns: results of a reanalysis of lymphoma study. BMC Bioinformatics. 2002;3:36. doi: 10.1186/1471-2105-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, Ambudkar SV. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J. Natl. Cancer Inst. 2010;102(21):1637–1652. doi: 10.1093/jnci/djq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon R, Radmacher MD, Dobbin K, McShane LM. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J. Natl. Cancer Inst. 2003;95(1):14–18. doi: 10.1093/jnci/95.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, Edkins S, O'Meara S, Vastrik I, Schmidt EE, Avis T, Barthorpe S, Bhamra G, Buck G, Choudhury B, Clements J, Cole J, Dicks E, Forbes S, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jenkinson A, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, Webb T, West S, Widaa S, Yates A, Cahill DP, Louis DN, Goldstraw P, Nicholson AG, Brasseur F, Looijenga L, Weber BL, Chiew YE, DeFazio A, Greaves MF, Green AR, Campbell P, Birney E, Easton DF, Chenevix-Trench G, Tan MH, Khoo SK, Teh BT, Yuen ST, Leung SY, Wooster R, Futreal PA, Stratton MR. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manuel RC, Hitomi K, Arvai AS, House PG, Kurtz AJ, Dodson ML, McCullough AK, Tainer JA, Lloyd RS. Reaction intermediates in the catalytic mechanism of Escherichia coli MutY DNA glycosylase. J. Biol. Chem. 2004;279(45):46930–46939. doi: 10.1074/jbc.M403944200. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J, Paw BH. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. U S A. 2009;106(38):16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, Tsuruo T, Inazawa J. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64(4):1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhry P, Srinivasan R, Patel FD. Expression of the major fas family and Bcl-2 family of proteins in epithelial ovarian cancer (EOC) and their correlation to chemotherapeutic response and outcome. Oncol. Res. 2010;18(11–12):549–559. doi: 10.3727/096504010x12767359113884. [DOI] [PubMed] [Google Scholar]
- 23.Dong HP, Kleinberg L, Silins I, Florenes VA, Trope CG, Risberg B, Nesland JM, Davidson B. Death receptor expression is associated with poor response to chemotherapy and shorter survival in metastatic ovarian carcinoma. Cancer. 2008;112(1):84–93. doi: 10.1002/cncr.23140. [DOI] [PubMed] [Google Scholar]
- 24.Duiker EW, van der Zee AG, de Graeff P, Boersma-van Ek W, Hollema H, de Bock GH, de Jong S, de Vries EG. The extrinsic apoptosis pathway and its prognostic impact in ovarian cancer. Gynecol. Oncol. 2010;116(3):549–555. doi: 10.1016/j.ygyno.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, Bast RC, Mills GB, Gallick GE. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol. Oncol. 2003;88(1):73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 26.Han LY, Landen CN, Trevino JG, Halder J, Lin YG, Kamat AA, Kim TJ, Merritt WM, Coleman RL, Gershenson DM, Shakespeare WC, Wang Y, Sundaramoorth R, Metcalf CA, 3rd, Dalgarno DC, Sawyer TK, Gallick GE, Sood AK. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66(17):8633–8639. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem. Biophys. Res. Commun. 2003;309(2):377–383. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol. Cancer Ther. 2005;4(2):217–224. [PubMed] [Google Scholar]
- 29.O'Connor T, Ireland LS, Harrison DJ, Hayes JD. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 1999;343(Pt 2):487–504. [PMC free article] [PubMed] [Google Scholar]
- 30.Huang KH, Chiou SH, Chow KC, Lin TY, Chang HW, Chiang IP, Lee MC. Overexpression of aldo-keto reductase 1C2 is associated with disease progression in patients with prostatic cancer. Histopathology. 2010;57(3):384–394. doi: 10.1111/j.1365-2559.2010.03647.x. [DOI] [PubMed] [Google Scholar]
- 31.Hsu NY, Ho HC, Chow KC, Lin TY, Shih CS, Wang LS, Tsai CM. Overexpression of dihydrodiol dehydrogenase as a prognostic marker of non-small cell lung cancer. Cancer Res. 2001;61(6):2727–2731. [PubMed] [Google Scholar]
- 32.Shen H, Kauvar L, Tew KD. Importance of glutathione and associated enzymes in drug response. Oncol. Res. 1997;9(6–7):295–302. [PubMed] [Google Scholar]
- 33.Chen YJ, Yuan CC, Chow KC, Wang PH, Lai CR, Yen MS, Wang LS. Overexpression of dihydrodiol dehydrogenase is associated with cisplatin-based chemotherapy resistance in ovarian cancer patients. Gynecol. Oncol. 2005;97(1):110–117. doi: 10.1016/j.ygyno.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Deng HB, Adikari M, Parekh HK, Simpkins H. Ubiquitous induction of resistance to platinum drugs in human ovarian, cervical, germ-cell and lung carcinoma tumor cells overexpressing isoforms 1 and 2 of dihydrodiol dehydrogenase. Cancer Chemother. Pharmacol. 2004;54(4):301–307. doi: 10.1007/s00280-004-0815-0. [DOI] [PubMed] [Google Scholar]
- 35.Deng HB, Parekh HK, Chow KC, Simpkins H. Increased expression of dihydrodiol dehydrogenase induces resistance to cisplatin in human ovarian carcinoma cells. J. Biol. Chem. 2002;277(17):15035–15043. doi: 10.1074/jbc.M112028200. [DOI] [PubMed] [Google Scholar]
- 36.Callaghan R, Crowley E, Potter S, Kerr ID. P-glycoprotein: so many ways to turn it on. J. Clin. Pharmacol. 2008;48(3):365–378. doi: 10.1177/0091270007311568. [DOI] [PubMed] [Google Scholar]
- 37.Fehrmann RS, Li XY, van der Zee AG, de Jong S, Te Meerman GJ, de Vries EG, Crijns AP. Profiling studies in ovarian cancer: a review. Oncologist. 2007;12(8):960–966. doi: 10.1634/theoncologist.12-8-960. [DOI] [PubMed] [Google Scholar]
- 38.Hickman GJ, Hodgman TC. Inference of gene regulatory networks using boolean-network inference methods. J. Bioinform. Comput. Biol. 2009;7(6):1013–1029. doi: 10.1142/s0219720009004448. [DOI] [PubMed] [Google Scholar]
- 39.Wanders RJ, Visser WF, van Roermund CW, Kemp S, Waterham HR. The peroxisomal ABC transporter family. Pflugers Arch. 2007;453(5):719–734. doi: 10.1007/s00424-006-0142-x. [DOI] [PubMed] [Google Scholar]
- 40.Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem. 2009;284(8):5056–5066. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandsch M. Transport of drugs by proton-coupled peptide transporters: pearls and pitfalls. Expert Opin. Drug Metab. Toxicol. 2009;5(8):887–905. doi: 10.1517/17425250903042292. [DOI] [PubMed] [Google Scholar]
- 42.Kamal MA, Keep RF, Smith DE. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab. Pharmacokinet. 2008;23(4):236–242. doi: 10.2133/dmpk.23.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 2001;7(10):3113–3119. [PubMed] [Google Scholar]
- 44.Nielsen HJ, Brunner N, Frederiksen C, Lomholt AF, King D, Jorgensen LN, Olsen J, Rahr HB, Thygesen K, Hoyer U, Laurberg S, Christensen IJ. Plasma tissue inhibitor of metalloproteinases-1 (TIMP-1): a novel biological marker in the detection of primary colorectal cancer. Protocol outlines of the Danish-Australian endoscopy study group on colorectal cancer detection. Scand. J. Gastroenterol. 2008;43(2):242–248. doi: 10.1080/00365520701523439. [DOI] [PubMed] [Google Scholar]
- 45.Wurtz SO, Schrohl AS, Mouridsen H, Brunner N. TIMP-1 as a tumor marker in breast cancer--an update. Acta Oncol. 2008;47(4):580–590. doi: 10.1080/02841860802022976. [DOI] [PubMed] [Google Scholar]
- 46.Djafarzadeh R, Noessner E, Engelmann H, Schendel DJ, Notohamiprodjo M, von Luettichau I, Nelson PJ. GPI-anchored TIMP-1 treatment renders renal cell carcinoma sensitive to FAS-meditated killing. Oncogene. 2006;25(10):1496–1508. doi: 10.1038/sj.onc.1209188. [DOI] [PubMed] [Google Scholar]
- 47.Lambert E, Boudot C, Kadri Z, Soula-Rothhut M, Sowa ML, Mayeux P, Hornebeck W, Haye B, Petitfrere E. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem. J. 2003;372(Pt 3):767–774. doi: 10.1042/BJ20030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 2010;13(1):43–58. doi: 10.1007/s10456-010-9163-3. [DOI] [PubMed] [Google Scholar]
- 49.Vergara D, Merlot B, Lucot JP, Collinet P, Vinatier D, Fournier I, Salzet M. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291(1):59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Langmann T, Mauerer R, Schmitz G. Human ATP-binding cassette transporter TaqMan low-density array: analysis of macrophage differentiation and foam cell formation. Clin. Chem. 2006;52(2):310–313. doi: 10.1373/clinchem.2005.059774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data