TRPM7 Regulates Cell Adhesion by Controlling the Calcium-dependent Protease Calpain (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 28.
Published in final edited form as: J Biol Chem. 2006 Jan 25;281(16):11260–11270. doi: 10.1074/jbc.M512885200
Abstract
m-Calpain is a protease implicated in the control of cell adhesion through focal adhesion disassembly. The mechanism by which the enzyme is spatially and temporally controlled is not well understood, particularly because the dependence of calpain on calcium exceeds the submicromolar concentrations normally observed in cells. Here we show that the channel kinase TRPM7 localizes to peripheral adhesion complexes with m-calpain, where it regulates cell adhesion by controlling the activity of the protease. Our research revealed that overexpression of TRPM7 in cells caused cell rounding with a concomitant loss of cell adhesion that is dependent upon the channel of the protein but not its kinase activities. Knockdown of m-calpain blocked TRPM7-induced cell rounding and cell detachment. Silencing of TRPM7 by RNA interference, however, strengthened cell adhesion and increased the number of peripheral adhesion complexes in the cells. Together, our results suggest that the ion channel TRPM7 regulates cell adhesion through m-calpain by mediating the local influx of calcium into peripheral adhesion complexes.
TRPM7 is one of only two ion channels to possess its own kinase domain (1). It is a member of the transient receptor potential ion channel family with the closest similarity to its bifunctional homologue TRPM6 as well as to melastatin (TRPM1), whose reduced expression has been used as a prognosis marker for metastasis in patients with localized melanoma (2-6). TRPM7 is also distinctive in its ion permeability, allowing Ca2+ as well as Mg2+ and other cations to compose its inward current (7, 8). The channel kinase is a member of the recently discovered _α_-kinase family (9, 10). Annexin I has been identified as a substrate for the kinase, but the functional significance of annexin I phosphorylation by TRPM7 is not yet understood (11). Autophosphorylation of the channel does not alter channel activity (12). However, phospholipase C inactivates TRPM7 channel activity through hydrolysis of phosphatidylinositol 4,5-bisphosphate, which is presumably gating the channel (13, 14). Magnesium ions block channel activity (8, 15-17), and, more recently, TRPM7 current has been shown to be potentiated by protons (18). Despite these recent advances in understanding TRPM7 channel regulation, the physiological role of this unique bifunctional protein still remains unclear.
The passage of Mg2+ by TRPM7 has linked it to the regulation of magnesium homeostasis in mammalian cells (19). Its capacity to carry calcium, in contrast, has been associated with calcium overload during anoxic cell death (20), calcium-dependent regulation of the cell cycle (21), and most recently, skeletogenesis and kidney stone formation in zebrafish (22). An early study by Nadler et al. (8) showed that overexpression of TRPM7 caused HEK-293 cells to detach and die, suggesting that the channel may have a role in controlling cell adhesion.
Here we present evidence that TRPM7 is a potent regulator of m-calpain. Fourteen distinct members of the mammalian calpain family have been identified, but only two are well characterized: _μ_-calpain, which is activated by _μ_m calcium concentrations (in vitro), and m-calpain, which is activated by millimolar concentrations of Ca2+ (in vitro) (23). Both isoforms are thought to play significant roles in the regulation of cell adhesion (23-26). _μ_-Calpain is involved in the activation of Rac during focal complex formation during cell spreading (27, 28), whereas m-calpain has been implicated in adhesion complex disassembly and deadhesion (29-31). We found that expression of TRPM7 in HEK-293 cells produced cell rounding and a loss of cell adhesion that was dependent upon m-calpain. TRPM7-dependent cell rounding occurred without significantly elevating cytosolic calcium concentrations, suggesting that calcium influx through the channel was creating local calcium gradients to activate the protease. Indeed, TRPM7 colocalized with m-calpain at peripheral vinculin-containing adhesion complexes, where presumably, as one of its physiological roles, TRPM7 controls the activity of m-calpain to regulate cell adhesion. Recently, the bifunctional channel has been shown to play a key role in anoxic cell death. The control of m-calpain by TRPM7 may also contribute to some of the cellular events that occur during ischemia, as well as to other pathologies associated with the calcium-dependent protease (32, 33).
EXPERIMENTAL PROCEDURES
Materials
All of the chemicals, unless otherwise stated, were from Sigma. The calpain inhibitor ALLM,2 the caspase 3 inhibitor Z-DEVD-FMK, and the Rho kinase inhibitor Y27632 were from Calbiochem.
TRPM7-expressing Cell Lines
TRPM7, kinase mutants, and small hairpin RNA-expressing cell lines were made using the Flp-In system (Invitrogen) and the commercially available Flp-In T-Rex 293 cells following the manufacturer’s instructions. The Flp-In T-Rex 293 cell line expresses the tetracycline repressor protein (TetR), which in the absence of tetracycline blocks transcription from the cytomegalovirus promoter containing control elements from the bacterial tetracycline resistance operon. The Flp-In T-Rex 293 cell line contains a single, stably integrated FRT site at a transcriptionally active genomic locus, so that target integration of a Flp-In expression vector ensures reproducible isogenic high level gene expression.
To make the cell lines, an expression vector (pcDNA5/FRT/TO) containing amino-terminal hemagglutinin (HA)-tagged murine TRPM7 (GenBank™ accession number AF376052), TRPM7-K1645A, TRPM7-G1618D, TRPM7ΔKIN, GFP-KIN, or GFP-CTKIN was cotransfected into the parental cell line with the pOG44 plasmid that expresses Flp recombinase. The respective coding sequences were then integrated into the genome via Flp recombinase-mediated DNA recombination at the specific genomic location. To test the function of the kinase domain, we created cell lines expressing a version of TRPM7 in which the catalytic kinase domain has been removed (293-TRPM7ΔKIN) or one in which the kinase domain has been rendered catalytically inactive (293-TRPM7-K1645A, 293-TRPM7-G1618D). The truncation of the kinase domain was made by changing the TCG codon encoding serine 1501 into a stop codon. The K1645A substitution renders the kinase inactive by mutating a critical invariant lysine to alanine. The G1618D mutation disrupts kinase activity by blocking ATP binding to the P-loop in the catalytic domain. Control experiments have shown that a GST fusion of the kinase domain harboring either the K1645A or G1618D substitutions was soluble but lacked catalytic activity (data not shown). TRPM7-K1645A, TRPM7-G1618D, and TRPMΔKIN were generated using QuikChange (Stratagene) with the following primers: TRPM7-K1645A, 5′-CCT GAA GTC AGG GCA TCT CTA TAT CAT TGC GTC ATT TCT TCC TGA GGT G-3′ and 5′-CAC CTC AGG AAG AAA TGA CGC AAT GAT ATA GAG ATG CCC TGA CTT CAG G-3′; TRPM7-G1618D, 5′-GTA AAG AGG AAA TGG GAG ATG GTT TAC GAA GAG CAG-3′ and 5′-CTG CTC TTC GTA AAC CAT CTC CCA TTT CCT CTT TAC-3′; and TRPM7ΔKIN, 5′-CTG TAG TAG AAG AGC GTA GAC GGA AGA CTCT CCA G-3′ and 5′-CTG GAG AGT CTT CCG TCT ACG CTC TTC TAC TAC AG-3′.
shRNA Cell Lines
To fully test the hypothesis that TRPM7 is involved in regulating cell adhesion, we created 293 cells in which native TRPM7 protein levels were lowered by expression of shRNAs that target TRPM7 (34). We designed six variant shRNAs to target either human, mouse, or rat TRPM7 from the following sequences: M7shRNA1, 5′-GCA AAT GGA GTT ACC CAA AC-3′; M7shRNA2, 5′-GCA TAA ATT CCT TAC CAT TC-3′; M7shRNA3, 5′-GGT TGG ATC CTT GGA ACA AGC-3′; M7shRNA4, 5′-GGA ACA AGC TAT GCT TGA TGC-3′; M7shRNA5, 5′-GGA AAT CTT CCT CCA GGA TAT-3′; and M7shRNA6, 5′-GCA CTC CTC AGT TGC GAA AGA-3′. Cell lines were constructed by first cloning double-stranded oligonucleotides that encoded the shRNAs into the pENTR/H1/TO vector (Invitrogen). Expression from the pENTR/H1/TO vector is driven by RNA polymerase III off a H1 promoter modified to contain two tetracycline operator 2 (TetO2) sites. We screened the pENTR/H1/TO TRPM7 shRNA clones by transfecting them into 293-TRPM7ΔKIN-expressing cells and then evaluated their ability to attenuate TRPM7 expression (supplemental Fig. S1). Four of the best constructs were then used to make TRPM7-shRNA-expressing cell lines. First, pcDNA5/FRT/TO/GATEWAY was created using the Gateway Vector Conversion System (Invitrogen) by blunt end ligation of the Gateway cassette into the NruI and EcoRV sites of pcDNA5/FRT/TO (which removed the cytomegalovirus promotor). A Gateway LR recombination reaction (Invitrogen) was then performed to introduce the shRNA expression cassette into pcDNA5/FRT/TO/GATEWAY. The final GATEWAY vectors expressing shRNAs against TRPM7 (pcDNA5/FRT/TO/GATEWAY-M7shRNA-2,M7shRNA-3, M7shRNA-5, and M7shRNA-6) were used to generate stable cell lines employing the Flp-In system (Invitrogen). A nonsilencing sequence (5′-AAT TCT CCG AAC GTG TCA CGT-3′) was used to make the control cell line 293-shRNA-C (20).
Anti-TRPM7 Antibodies
The TRPM7-specific PLIKC47 antibody (_α_-C47), which recognizes residues 1816–1863 from rat TRPM7, has been previously described (14). A second rabbit polyclonal TRPM7 (_α_-CTERM) was generated using a GST fusion protein with residues 1384–1506 of murine TRPM7 as the antigen. The _α_-CTERM antibody was purified following protocols that have been described (14). Both _α_-C47 and _α_-CTERM are specific and suitable for immunocytochemistry, Western blotting, and immunoprecipitation experiments (supplemental Fig. S2).
Western Blotting and Immunoprecipitation Experiments
293-TRPM7 cells expressing recombinant HA-tagged TRPM7 or channel and kinase variants were lysed after a 24-h treatment with tetracycline using 2 ml of ice-cold radioimmune precipitation assay buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Igepal CA-630, 0.5% (w/v) deoxycholate, 0.1% (w/v) SDS, and 10 mm iodoacetamide). The proteins were immunoprecipitated overnight from cell lysates from a 60-mm dish with an anti-HA affinity matrix. In the case of GFP-KIN and GFP-CTKIN, expressed proteins were immunoprecipitated using a monoclonal anti-GFP antibody (Roche Applied Science) bound to protein G-agarose. The samples were washed three times in TBST (50 mm Tris-Cl, pH 7.6, 150 mm NaCl, 0.05% Tween 20), eluted by boiling in 2× SDS-PAGE sample buffer, and then resolved by SDS-PAGE and Western blotting following standard protocols. The monoclonal 12CA5 anti-HA antibody or the monoclonal 7.1 and 13.1 anti-GFP antibodies were used as the primary antibodies (Roche Applied Science). The SuperSignal West Dura maximum sensitive substrate (Pierce) was used for immunochemiluminescence detection.
To detect native TRPM7 in the HEK-293 line, cells were lysed from a 10-cm dish using 2 ml of ice-cold radioimmune precipitation assay buffer. TRPM7 was immunoprecipitated overnight from cell lysates using 10 _μ_g of _α_-C47 antibody absorbed to protein A-agarose (Santa Cruz Biotechnology). The samples were washed three times in TBST buffer, eluted in 2× SDS-PAGE sample buffer, and then resolved by SDS-PAGE and Western blotting using the second anti-TRPM7 antibody (_α_-CTERM). The SuperSignal West Dura maximum sensitive substrate (Pierce) was used for immunochemiluminescence detection.
Detection of Talin Cleavage
Talin was detected using a primary monoclonal antibody from Upstate Biotechnology, Inc. (clone TA205), which recognizes an epitope within the head domain of human talin (amino acids 139–433).
Immunokinase Assay
The immunokinase assay was performed as follows. Briefly, 10-cm dishes of cells were grown to confluence and allowed to express the individual proteins by the addition of tetracycline (1 _μ_g/ml) to the medium. After 24 h, the cells were lysed in radioimmune precipitation assay buffer, and the specific proteins were immunoprecipitated as described above. The immunocaptured proteins were washed four times with ice-cold phosphate-buffered saline containing 0.1% polyoxyethylenesorbitan monolaurate (Tween 20). The wash buffer was replaced by ice-cold reaction buffer (KIN-DET) containing 50 mm HEPES (pH 7.0), 50 mm NaCl, 5 mm MgCl2, 3.5 mm MnCl2, 0.1% Tween 20, 0.5 mm ATP, and 2 _μ_Ci of [_γ_-32P]ATP (specific activity of 3000 Ci/mmol). The samples were then incubated at 37 °C for 30 min in a 50-_μ_l reaction, before being terminated by the addition of 10 _μ_l of 6× SDS sample buffer. The samples were then resolved on a 6% SDS-PAGE gel. The gels were dried, and _γ_-32P incorporation was visualized by autoradiography.
Immunofluorescence and Confocal Microscopy
293-TRPM7 cells were plated onto polylysine coated coverslips and treated with tetracycline for 20 h to analyze the cellular distribution of HA-tagged TRPM7. The cells were fixed at room temperature for 10 min in phosphate-buffered saline (pH 7.4) with 4% paraformaldehyde (Electron Microscopy Sciences) and permeabilized in phosphate-buffered saline with 0.1% Saponin (Sigma). Primary antibodies against TRPM7 (described above) or the monoclonal 12CA5 anti-HA (Roche Applied Science) were used to visualize TRPM7 by immunofluorescence. A monoclonal antibody against vinculin (HVIN-1 clone; Sigma) was used to image peripheral adhesion complexes. A rabbit polyclonal antibody against m-calpain (Triple Point Biologics, Inc.) was used to visualize the cellular distribution of the protease. A 1:2000 dilution of Alexa Fluor 488 or Alexa Fluor 568 goat antibody to rabbit or mouse (Molecular Probes) was used as the secondary antibody. The images were obtained from a Zeiss LSM 410 confocal microscope using a 488-nm excitation wavelength and a 512-nm band pass emission filter. The pinhole size used was 30 Airy Units, and the contrast/brightness settings were kept the same for each image.
Electrophysiological Recordings and Calcium Imaging
The voltage clamp technique was used to evaluate the whole cell currents of TRPM7 expressed in HEK-293 cells as described (14). Briefly, whole cell current recordings of TRPM7-expressing cells were elicited by voltage stimuli lasting 250 ms delivered every 1 s using voltage ramps from −120 to +100 mV. The data were digitized at 2 or 5 kHz and digitally filtered off-line at 1 kHz. The internal pipette solution for macroscopic current recordings contained 145 mm cesium methanesulfonate, 8 mm NaCl, 10 mm EGTA, and 10 mm HEPES, pH adjusted to 7.2 with CsOH. The extracellular solution for whole cell recordings contained 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 20 mm HEPES, and 10 mm glucose, pH adjusted to 7.4 (NaOH).
Calcium imaging was conducted using an IonOptix ratio calcium imaging system. In brief, the cells were plated on 25-mm glass coverslips and incubated with Fura 2/acetoxymethyl (5 _μ_m) for 50 min. After the extracellular Fura 2/acetoxymethyl was washed away, the cells were incubated for an additional 30 min. Fluorescence intensity at 510 nm with 340- and 380-nm excitation was collected at a rate of 1 Hz using a intensified CCD camera (Ionoptix), and the data were analyzed using Ionwizard (Ionoptix). 1 _μ_m ionomycin was applied to each cell and was used as a reference to normalize the changes in the _F_340/_F_380 ratio. For Ca2+ imaging the extracellular Tyrode’s solution contained 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 20 mm HEPES, and 10 mm glucose, pH adjusted to 7.4 (NaOH).
Cell Rounding Assay
Changes in 293 cell morphology were scored manually employing the following criteria. The cells that had a fully round cell body with no membrane extension processes were given 1 point. Partially rounded cells with one or two membrane extensions were assigned half a point. Nonrounded cells having three or four membrane extension processes, with a cell morphology similar to wild type HEK-293 cells, were given 0 points.
Adhesion Assay
Cell adhesion was measured using a trypsinization assay (36). Briefly, ~2.5×106 cells were plated onto a 60-mm Falcon tissue culture dish for 24 h and then treated with 0.5 ml of 0.05% trypsin-EDTA for 4 min to stimulate cell detachment. Detached cells in the trypsin-EDTA solution were collected, and the trypsinization was terminated by the addition of 3 ml of culture medium. The number of detached cells was then manually counted using a hemocytometer and expressed as a percentage of the total number of cells on the plate.
Rho Activity Assay
Detection of activated Rho was accomplished using a modified GST pull-down purification assay. To detect activated Rho, the pull-down was performed using GST fused to the Rhotekin Rho-binding domain (37). The assay was performed by incubating cell lysates with partially purified GST fused to the Rhotekin Rho-binding domain bound to glutathione beads for 1 h (Amersham Biosciences), washing the beads, and then resolving bound Rho by SDS-PAGE and Western blotting as described (38). The cells transfected with ephexin, a Rho-GEF, were used as a positive control. The ephexin expression vector was a gift of Michael Greenberg (Division of Neuroscience, Children’s Hospital, Boston, MA).
Calpain 2 shRNA Blockade in LTRPC7 Cells
RNA interference of m-calpain (calpain 2) expression was achieved by expression of a shRNA in the pSUPERretro vector specific to human m-calpain. To make the pSUPERretro-CAPN2 vector, the following oligonucleotides were cloned into the BglII-HindIII sites of pSUPERretro (sense, 5′-GAT CCC CGG CAT ACG CCA AGA TCA ACT TCA AGA GAG TTG ATC TTG GCG TAT GCC TTT TTG GAA A-3′; antisense, 5′-AGC TTT TCC AAA AAG GCA TAC GCC AAG ATC AAC TCT CTT GAA GTT GAT CTT GGC GTA TGC CGG G-3′), in which the m-calpain target sequence is 5′-AAG GCA UAC GCC AAG AUC AAC-3′. pSUPERretro-CAPN2 was transiently transfected using Lipofectamine 2000 (Invitrogen) into LTRPC7 cells (generously provided by Dr. Andrew Scharenberg, University of Washington) (8). Cells expressing the shRNA targeting m-calpain were selected using 5 _μ_g/ml puromycin for 3 days prior to expression of TRPM7 by the addition of tetracycline (1 _μ_g/ml) to the media. Control experiments showed that nontransfected cells treated with puromycin died within 3 days. Silencing of m-calpain was assessed by SDS-PAGE and Western blotting of cell lysates, using a rabbit polyclonal antibody against m-calpain (Triple Point Biologics, Inc.). A monoclonal antibody against _μ_-calpain (Alexis Biochemicals) was used to show that pSUPERretro-CAPN2 did not affect _μ_-calpain protein levels.
RESULTS
TRPM7 Regulates Cell Adhesion
A previous study had showed that overexpression of TRPM7 induces cell detachment and subsequent cell death (8). We therefore sought to determine whether TRPM7 is involved in the control of cell adhesion. To study the cellular function of TRPM7, we employed the Flp-In system and Flp-In T-Rex cells (Invitrogen) to generate a HEK-293 cell line that could inducibly overexpress TRPM7 with the addition of tetracycline to the growth medium (293-TRPM7). 293-TRPM7 cells expressed high levels of channel activity and produced whole cell currents with activation kinetics and current-voltage relationships similar to those described earlier (see Fig. 2_A_) (1, 8). As previously reported, HEK-293 cells that express TRPM7 rounded up within 18–24 h and became loosely attached to tissue culture plates (Fig. 1_A_) (8, 20). We tested whether expression of TRPM7 in 293-TRPM7 cells was toxic, because an earlier study by Nadler et al. (8) found that overexpression of TRPM7 in their LTRPC7 cell line led to cell rounding, detachment, and subsequent cell death. Nonexpressing 293-TRPM7 cells had comparable amounts of cell death (less than 10%) to cells expressing TRPM7 for at least 72 h, as assessed by trypan blue exclusion analysis (data not shown). However, expression of TRPM7 in the original LTRPC7 cell line, which expresses two or three times more channel activity than 293-TRPM7 cells, did cause significant cell death (~25%) (data not shown). In addition, the effect of TRPM7 on cell morphology and adhesion is specific. Expression of TRPM1, TRPC5, lymphocyte _α_-kinase, and TRPM6 (a second TRPM family member with a kinase domain) in HEK-293 cells failed to produce the morphological changes that were visible when TRPM7 was expressed (data not shown). This finding is consistent with a recent report showing that TRPM6 and TRPM7 are functionally nonredundant (39).
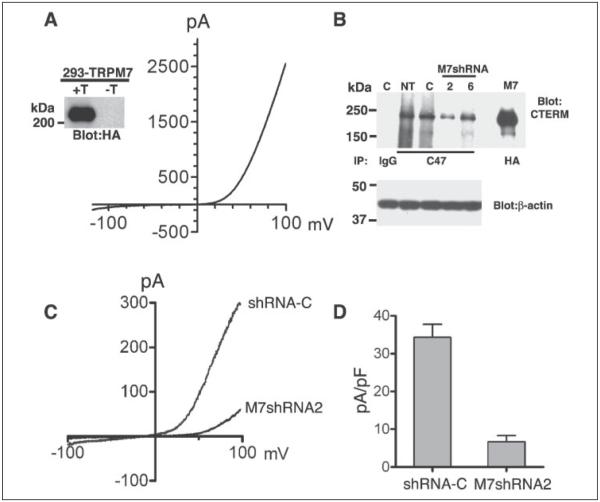
FIGURE 2. Characterization of 293-TRPM7 and 293-M7shRNA2 cells.
A, expression of HA-tagged TRPM7 in the 293-TRPM7 cell line produced a whole cell current with a large outwardly rectifying conductance and small inward current at negative potentials. The inset shows tetracycline-induced (+T) expression of the 220-kDa channel by immunoprecipitation, SDS-PAGE, and Western blotting. −T, no tetracycline. B, cells stably expressing shRNAs targeting TRPM7 (shRNA-2 and -6; lanes 2 and 6, respectively) reduced TRPM7 expression compared with nontransfected (lane NT) cells or cells expressing a control shRNA (shRNA-C; lane C). Native TRPM7 was immunoprecipated with the anti-C47 antibody and detected with the anti-CTERM antibody. Rabbit IgG was used as an immunoprecipitation control. HA-tagged TRPM7 (lane M7) was immunoprecipitated with HA-agarose and detected with the anti-CTERM antibody as a migration control. Equivalent amounts of lysates were used for the immunoprecipitation study. A blot of _β_-actin is shown as a loading control. C, 293-M7shRNA2 cells express reduced amount of endogenous MagNUM/MIC current. D, the current-density of MagNUM/MIC current at +100 mV in 293-M7sRNA2 cells was reduced by 80% compared with 293-shRNA-C cells.
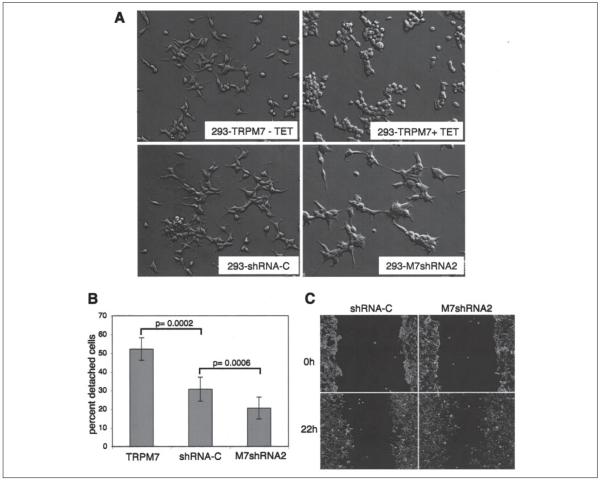
FIGURE 1. TRPM7 regulates cell adhesion in HEK-293 cells.
A, application of tetracycline (TET) induced expression of HA-TRPM7 and produced cell rounding of 293-TRPM7 cells. Knockdown of native TRPM7 in 293-M7shRNA2 cells produced cells that were more spread and had 50% longer extensions than a control cell line expressing a nonsilencing shRNA (293-shRNA-C). The same magnification was used in all four images. B, 293-M7shRNA2 cells adhered more strongly to the substratum than 293-shRNA-C or 293-TRPM7-expressing cells. Adhesion was measured using a trypsinization assay in which ~2.5 × 106 cells were plated onto 60-mm Falcon tissue culture dishes for 24 h and then treated with 0.05% trypsin-EDTA for 4 min to stimulate cell detachment. The number of detached cells was counted using a hemocytometer and expressed as a percentage of the total number of cells on the plate. The error bars represent the standard error of the mean from seven independent experiments. p = 0.0002 and p = 0.0006 were compared with shRNA-C using the one-sided pair t test (α = 0.05). C, 293-M7shRNA2 cells migrated 56% more efficiently than 293-shRNA-C cells in a wound healing assay. The cells were outlined with white to enhance their visibility.
If overexpression of TRPM7 causes cell rounding and loss of adhesion, than reducing TRPM7 protein levels should produce an opposite effect. To test this idea we created the 293-M7shRNA2 cell line, in which native TRPM7 levels have been reduced by the expression of a specific shRNA that targets TRPM7. Immunoprecipitation and Western blotting analysis of 293-M7shRNA2 cells showed that native TRPM7 levels were reduced by 60–80% compared with nontransfected cells or cells expressing a nonsilencing control shRNA (293-shRNA-C) (Fig. 2_B_). A comparison of the whole cell currents of 293-shRNA-C cells to 293-M7shRNA2 cells showed that a TRPM7-like current was reduced by 80%, giving further evidence that the current previously identified as MagNuM (8) or MIC (17) contains TRPM7 (Fig. 2, C and D). In a cell attachment assay 293-M7shRNA2 cells adhered more firmly to tissue culture plates compared with wild type HEK-293 cells or 293-shRNA-C cells (Fig. 1_B_). As expected 293-TRPM7 cells that overexpressed TRPM7 attached more weakly than controls. We also found that when 293-M7shRNA2 cells were plated at a low density, they were more spread than wild type cells and produced protrusions that were ~50% longer, which is the converse of the morphology of HEK-293 cells overexpressing the channel (Fig. 1_A_). A wound healing assay was performed to assess how silencing the channel kinase affects cell migration.
Reducing TRPM7 levels significantly enhanced the motility of HEK-293 cells (Fig. 1_C_); 293-M7shRNA2 cells were able to migrate into the wound ~56% more efficiently than control cells. These results strongly indicate that TRPM7 regulates the adhesion and migration of HEK-293 cells.
TRPM7 Channel Activity Is Required for Changes in Cell Adhesion
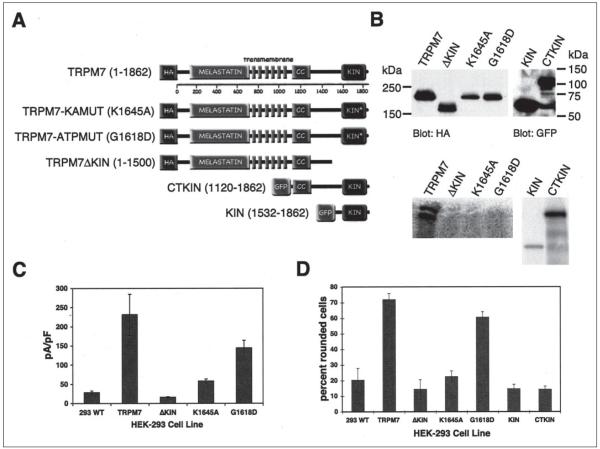
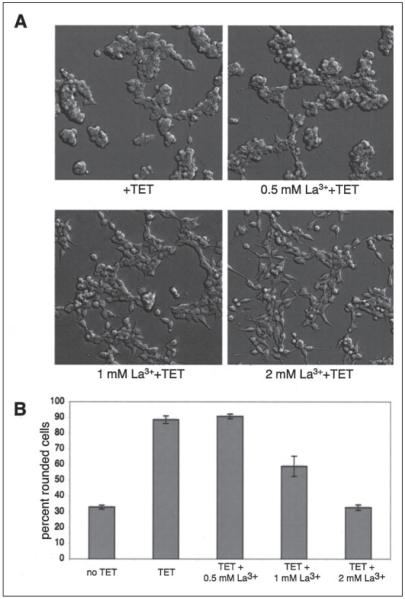
To investigate the role of the channel and kinase activities of TRPM7 in regulating cell adhesion, we applied the Flp-In system to create 293-TRPM7-G1618D and 293-TRPM7-K1645A cells, which expresses kinase-inactive mutants of TRPM7. We also constructed the 293-TRPM7ΔKIN cell line, which expresses TRPM7 without its kinase domain, and the 293-KIN and 293-CTKIN lines that express GFP fusion proteins of the kinase domain (293-KIN; amino acids 1532–1862) and the carboxyl terminus containing the kinase domain (293-CTKIN; amino acids 1120–1862). The K1645A substitution changes the invariant lysine that is required for catalytic activity in all protein kinases, whereas the G1618D substitution is predicted to disrupt kinase activity by blocking binding of ATP to the P-loop within the kinase domain. The advantage of the Flp-In system is that the parental Flp-In T-Rex 293 cell line has a unique FRT site integrated into its genome. This feature allows multiple cell lines to be constructed in which Flp recombinase-mediated DNA recombination of the introduced gene occurs at the same genomic location. Control in vitro kinase experiments revealed that a GST fusion of the kinase domain harboring either the K1645A or G1618D substitutions was soluble but lacked catalytic activity (data not shown). We also tested the ability of the immunoprecipitated TRPM7-K1645A, TRPM7-G1618D, TRPM7ΔKIN, TRPM7, GFP-KIN, and GFP-CTKIN to autophosphorylate in an immunokinase assay using [_γ_-32P]ATP (Fig. 3_B_). As expected, TRPM7, GFP-KIN, and GFP-CTKIN retained the ability to autophosphorylate, whereas the kinase mutants TRPM7-K1645A, TRPM7-G1618D, and TRPM7ΔKIN did not. Immunoprecipitation of expressed proteins from cell lysates followed by SDS-PAGE and Western blotting showed that 293-TRPM7-K1645A and 293-TRPM7-G1618D cells expressed lower amounts of recombinant protein than 293-TRPM7 cells with proportionally smaller current amplitudes (Fig. 3, B and C). The current-voltage relationships observed in 293-TRPM7-K1645A and 293-TRPM7-G1618D cells were virtually identical to the wild type current from 293-TRPM7 cells (data not shown). By contrast, although the 293-TRPM7ΔKIN cell line expressed similar levels of protein to TRPM7 with a similar cellular distribution (supplemental Fig. S3), whole cell recordings revealed very little channel activity (Fig. 3_C_). These results are similar to those observed by Schmitz et al. (19), who suggested that some functional coupling between the channel and kinase domains of TRPM7 may exist. Surprisingly, we found that the kinase domain of TRPM7 was not required for cell rounding and loss of adhesion. Expression of TRPM7-G1618D, which retained significant channel activity, caused cell rounding, but cells expressing the fragments of TRPM7 with only the kinase domain (GFP-KIN, GFP-CTKIN) did not (Fig. 3_D_). We also found that cells expressing TRPM7 variants that produced diminished channel activity (TRPMΔKIN, TRPM7-K1645A) were not as effective in producing cell rounding as wild type TRPM7 or TRPM7-G1618D (Fig. 3_D_). This result suggests that a threshold of channel activity must be overcome for TRPM7 to produce the observed changes in cell morphology and adhesion. To confirm that TRPM7 channel activity was required for cell rounding, we applied increasing concentrations of LaCl3 into the culture medium of tetracycline-treated cells. La3+ blocks TRPM7 inward current by 97% at 2 mm (1). As expected, supplementation of the medium with increasing concentrations of La3+ progressively inhibited the rounding and detachment of TRPM7-expressing cells, with full blockade observed at 2 mm (Fig. 4). We therefore conclude that TRPM7 channel activity is required for the above changes in cell adhesion.
FIGURE 3. TRPM7 kinase activity is not required for changes in cell adhesion.
A, one-dimensional structure of TRPM7 showing conserved melastatin domain followed by pore-forming transmembrane _α_-helices and a coiled-coil (CC) region that precedes the carboxyl-terminal kinase (KIN) domain. Substitution of K1645A or G1618D within the kinase domain rendered the kinase inactive. The TRPM7 variants shown were used to make individual 293 cell lines. B, top panel, Western blot showing expression levels of TRPM7 variants described in A. Bottom panel, autoradiograph showing immunokinase reactions demonstrate the capacity of the described TRPM7 variants to autophosphorylate. C, average current density at +100 mV from whole cell recordings of TRPM7 variant cell lines after 24 h of treatment with tetracycline. D, the capacity of 293 cells, 293-TRPM7, 293-TRPM7ΔKIN, 293-TRPM7-K1645A, 293-TRPM7-G1618D, 293-KIN, and 293-CTKIN to produce cell rounding upon induction of expression with tetracycline was scored manually (see “Experimental Procedures”). 293-TRPM7-G1618D cells, which express a kinase-inactive form of TRPM7 with high channel activity, produced cell rounding, whereas expression of the kinase domain alone (KIN) or the COOH terminus containing the kinase domain (CTKIN) did not. WT, wild type.
FIGURE 4. TRPM7 channel activity is required for cell rounding.
A, application of La3+ to LTRPC7 cells treated with tetracycline (TET) inhibited cell rounding in a concentration-dependent manner. Full blockade was observed at 2 mm, a concentration that had been shown block TRPM7 inward current by 97% (1). B, quantization of the degree of cell rounding under the conditions depicted in A.
TRPM7 Activates m-Calpain
Several signaling molecules have been shown to be involved in cell rounding and the loss of adhesion in HEK-293 cells. Activation of ephrin and sphingosine 1-phosphate receptors coupled to Rho GTPase elicits cell rounding in neuronal and other cell types (40-43). Activation of caspase 3 during apoptosis produces similar changes in cell morphology through the proteolytic activation of Rho Kinase (ROCK I) (44). In addition to the Rho pathway, activation of m-calpain can also cause cell rounding (31).
We tested whether overexpression of TRPM7 in the 293-TRPM7 cell line stimulates Rho using a Rho activity assay (37). For this procedure cell lysates from TRPM7-expressing and control cells were subjected to a pull-down purification assay using GST fused to the Rhotekin Rho-binding domain, which is a fusion protein of GST and the Rho-binding domain of Rhotekin that binds to active Rho-GTP. Although there was a slight increase in the amount of Rho-GTP captured from cell lysates from TRPM7-expressing cells compared with control cells (Fig. 5_B_), expression of dominant-negative isoforms of Rho (RhoT19N), Rac (RacT17N), and Cdc42 (Cdc42T17N) failed to prevent TRPM7-dependent cell rounding by expression of TRPM7 (data not shown). We therefore conclude that the change in cell morphology and the loss in adhesion that occurred in HEK-293 cells overexpressing TRPM7 were not dependent upon Rho, Rac, or Cdc42.
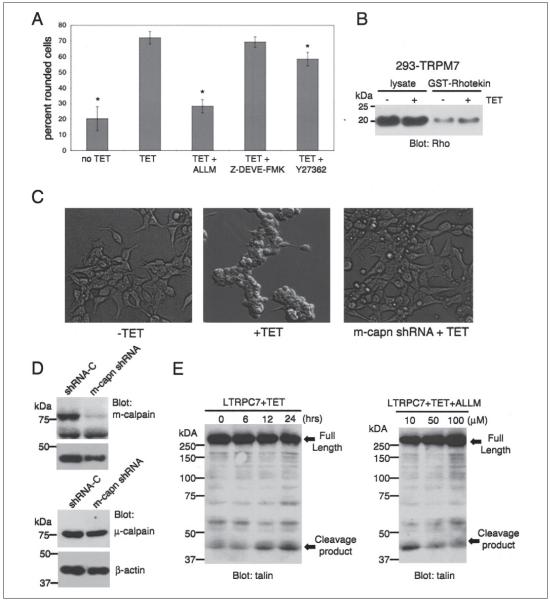
FIGURE 5. Calpain is required for TRPM7-mediated loss of cell adhesion.
A, application of the calpain inhibitor ALLM (100 _μ_m) to 293-TRPM7 cells treated with tetracycline (TET) for 24 h reduced TRPM7-induced cell rounding to a level similar to cells grown in the absence of tetracycline, as assessed by the scoring system described in the legend to Fig. 3. In contrast, treatment of cells with the caspase 3 inhibitor Z-DEVE-FMK (10 _μ_m) or the Rho Kinase inhibitor (10 _μ_m) had little effect. A _χ_2 test was employed to test differences in cell rounding between 293-TRPM7 cells treated with the different inhibitors. An asterisk indicates treatments that produced a decrease in cell rounding that was significantly different from 293-TRPM7 cells grown in tetracycline. B, a Rho activity assay shows that expression of TRPM7 (+TET) caused only a modest increase in Rho activity. C, expression of a shRNA targeting m-calpain blocked cell rounding and loss of adhesion in the LTRPC7 cell line. D, Western blot exhibiting the capacity of an shRNA targeting m-calpain to specifically reduce the protein levels of m-calpain in LTRPC7 cells. E, a Western blot using a monoclonal talin antibody that recognizes the head domain of talin was used to probe cell lysates from LTRPM7 cells treated with tetracycline for different durations. Expression of TRPM7 caused the cleavage of the focal adhesion protein talin into its head and rod domains. The head domain cleavage product migrated at 47 kDa. Application of increasing concentrations of the calpain inhibitor ALLM attenuated proteolysis of talin in response to TRPM7 expression.
Because TRPM7 activity has been linked to cell death, we next investigated whether the rounding of HEK-293 cells might be caused through channel-mediated initiation of apoptosis and caspase 3 activation (8, 20). Activated caspase 3 cleaves ROCK I within the conserved DETD113/G sequence, removing its carboxyl-terminal inhibitor domain and producing a constitutively active form that causes cell rounding (44). Treatment of the 293-TRPM7 cells with the caspase 3 inhibitor Z-DEVD-FMK or the ROCK inhibitor Y27632 had little to no effect on TRPM7-dependent morphological changes, supporting our conclusion that expression of TRPM7 does not cause cell rounding by initiating apoptosis or by activating ROCK I (Fig. 5_A_).
Our investigation then focused on the role of calpain in mediating TRPM7-dependent changes in cell adhesion. Calpains are intracellular, nonlysosomal, Ca2+-dependent cysteine proteases that are active at physiological pH (45). Treatment of 293-TRPM7-expressing cells with the calpain inhibitor ALLM blocked TRPM7-dependent changes in morphology and deadhesion (Fig. 5_A_), a finding strongly suggesting that TRPM7 is activating calpain to produce these cellular effects.
Recent work has revealed m-calpain as a key regulator of cell adhesion by cleaving specific substrates that are components of focal adhesions (for reviews, see Refs. 23 and 46). For example, it has been shown that m-calpain is solely responsible for the proteolysis of talin at glutamine 433 into its separate head and rod domains (47). We found that the m-calpain substrate and focal adhesion protein talin was cleaved into its head and rod domains in TRPM7-overexpressing cells (Fig. 5_E_), indicating that TRPM7 may be activating this protease (47, 48). Treatment of 293-TRPM7 cells with increasing concentrations of the calpain inhibitor ALLM reduced talin proteolysis, providing further evidence that TRPM7 causes cell rounding and loss of adhesion by stimulating the cleavage of key adhesion proteins through m-calpain (Fig. 5_E_). To test the role of m-calpain in mediating TRPM7-dependent cell rounding and the loss of cell adhesion, we reduced m-calpain protein levels by expression of a specific shRNA against the protease. Silencing of m-calpain by RNA interference blocked detachment of LTRPC7 cells (Fig. 5_C_), indicating that m-calpain is required for the dramatic loss of cell adhesion caused by overexpression of TRPM7.
Overexpression of TRPM7 Does Not Cause Calcium Overload
The half-maximal concentration range of calcium required for activation of m-calpain in vitro is 400–800 _μ_m (45). Autolysis of m-calpain can reduce the half-maximal concentration range of calcium required for activity to 50–150 _μ_m. However, because global increases in calcium to these concentrations are rarely observed in cells, there has been speculation that local transient increases of calcium mediated by calcium-permeant ion channels may be regulating calpain activation (23).
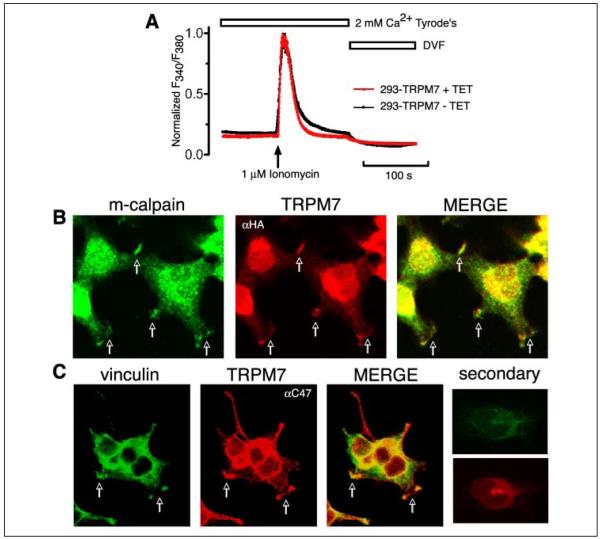
The inward current of TRPM7 is extremely small at the physiological membrane potentials observed in nonexcitable cells (−10 to −20 mV; see Fig. 2_A_) (7). For this reason it would be too difficult to measure fluxes of local calcium concentrations surrounding the channel. Instead, we asked whether overexpression of TRPM7 under conditions that normally elicit cell rounding and loss of adhesion raises cytosolic calcium levels above those observed in nonexpressing cells. As measured by the ratiometric indicator Fura-2 and fluorescence microscopy, when cells were constantly perfused with 2 mm Ca2+ Tyrode’s solution, the calcium concentrations represented by the normalized ratio of _F_340/_F_380 in TRPM7-expressing cells did not significantly differ from nonexpressing cells (Fig. 6_A_), indicating that TRPM7 was not activating calpain by raising global cytosolic calcium concentrations.
FIGURE 6. Overexpression of TRPM7 does not cause calcium overload.
A, normalized ratio of _F_340/_F_380 in 293-TRPM7 cells with or without tetracycline (TET) induction. The Ca2+ imaging traces were averaged from 24 cells for both groups. The experiment was repeated four times with similar results. No significant difference of the normalized ratio was observed when cells were constantly perfused with 2 mm Ca2+ Tyrode’s solution and divalent-free solution (DVF). The above results indicate that overexpression of TRPM7 does not cause calcium overload in 293-TRPM7 cells. B, heterologously expressed HA-TRPM7 colocalized with endogenous m-calpain to peripheral adhesion complexes (see arrows). Staining was performed on cells treated with tetracycline at concentrations below those required to cause full cell rounding. C, TRPM7 also colocalized with the focal adhesion protein vinculin to peripheral adhesion complexes. The nuclear fluorescence in the TRPM7 panels was from nonspecific secondary antibody staining, which was more easily detected because of the low expression level of TRPM7. The experiment was repeated four times with similar results.
TRPM7 Colocalizes with m-Calpain to Peripheral Vinculin-containing Adhesion Complexes
We found that when TRPM7 was heterologously expressed at concentrations below the threshold required to cause cell rounding, the channel colocalized with m-calpain into clusters at the tips or ends of cellular protrusions emanating from the cell body of HEK-293 cells (Fig. 6_B_). Because many of the substrates of m-calpain are components of focal adhesions, we decided to investigate whether TRPM7 localized to these cellular structures as well. We found that TRPM7 colocalized with vinculin to the same peripheral adhesion complexes (Fig. 6_B_). Unfortunately, we were unable to acquire evidence that TRPM7 and calpain coassemble into a macromolecular complex, because m-calpain did not coimmunopurify with HA-TRPM7 from 293-TRPM7-expressing cell lysates (data not shown). Nevertheless, we cannot rule out that such a complex exists, because the interaction of TRPM7 with m-calpain may be weak, transient, or indirect.
Knockdown of TRPM7 Increases Peripheral Adhesion Complexes in Cells
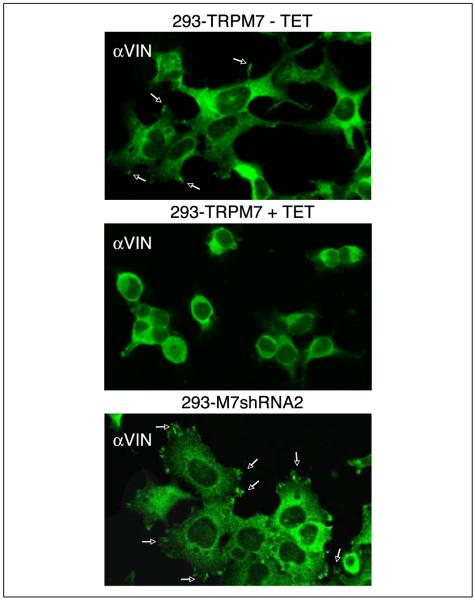
Because 293-M7shRNA2 cells adhere more tightly to the substratum (Fig. 1_B_), we tested whether these cells produce more peripheral adhesions. As expected, overexpression of TRPM7 in 293-TRPM7 cells greatly reduced the number of these cellular structures as compared with nonexpressing cells. In contrast, 293-M7shRNA2 cells were more elongated and spread than wild type cells and had more peripheral adhesion complexes (Fig. 7). These data suggest that 293-M7shRNA2 cells adhere more strongly to the substratum because they have more adhesive contacts.
FIGURE 7. TRPM7 regulates peripheral adhesion levels.
293-TRPM7 grown in the absence of tetracycline extended protrusions with vinculin (α_VIN_) containing peripheral adhesions (see arrows) at their tips (top panel). Expression of TRPM7 (+TET) caused these cells to round and reduced the number of these cellular structures (middle panel). 293-M7shRNA2 cells, which had reduced levels of native TRPM7, were more spread and contained more peripheral adhesions, suggesting that TRPM7 controls cell adhesion by stimulating peripheral adhesion turnover. The experiment was repeated three times with similar results.
DISCUSSION
To the best of our knowledge TRPM7 is the first ion channel shown to localize to adhesion complexes and to regulate cell adhesion and spreading. Calpain has also been implicated in these cellular processes, but there is limited understanding of what regulates its activity temporally and spatially. In this study we have identified m-calpain as a key effector of TRPM7 in regulating cell adhesion. We found that the channel activity of TRPM7 was solely regulating the calcium-dependent protease and that the kinase activity of the channel was not required to produce cell rounding and loss of adhesion. Although this study did not reveal a role for the kinase in regulating cell adhesion, we cannot rule out that the kinase, which displays significant homology to myosin heavy chain kinases from Dictyostelium, may serve specific cytoskeletal functions in other cell types. We also investigated whether TRPM7 controls m-calpain by raising cytosolic calcium levels. Our research indicates that overexpression of TRPM7 does not significantly elevate the basal calcium concentration in cells, a finding that is in agreement with other studies showing that depletion of intracellular ATP is required to significantly increase intracellular Ca2+ concentrations beyond basal levels in TRPM7-expressing LTRPC7 cells (7). These results are perhaps not surprising considering the small size of the inward current of TRPM7, especially at the physiological membrane potentials normally observed in nonexcitable cells (−10 to −20 mV; e.g. Fig. 2_A_). Thus, under physiological conditions, overexpression of TRPM7 does not overwhelm the ability of a HEK-293 cell to maintain normal calcium homeostasis.
Because expression of the channel did not raise cytosolic calcium concentrations to the levels required for activation of m-calpain (50–150 _μ_m), TRPM7 must have activated the protease by another mechanism. We found that TRPM7 colocalized with m-calpain to vinculin-containing peripheral adhesion complexes. These data strongly suggest a model in which TRPM7 regulates calcium concentrations within peripheral adhesion complexes to activate m-calpain and initiate the disassembly or turnover of peripheral adhesion complexes (Fig. 8). The discovery that 293-TRPM7 cells overexpressing the channel are rounded and lack these cellular structures, whereas cells that have reduced levels of the native channel are more spread and have more abundant peripheral adhesion complexes, supports this premise.
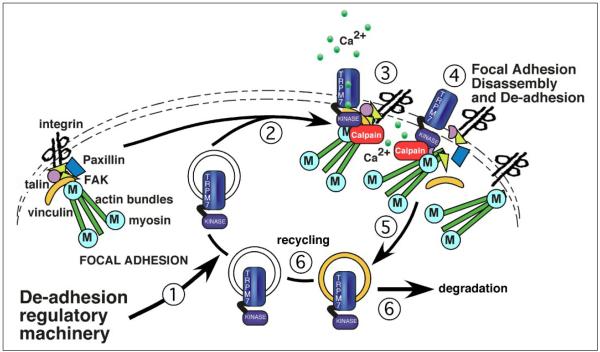
FIGURE 8. A working model of the role of TRPM7 in regulating cell adhesion.
We hypothesize that recruitment of TRPM7 to areas of focal adhesion remodeling and turnover is under signal control (Step 1). The entry of TRPM7 into these cytoskeletal structures (Step 2) raises local calcium levels by virtue of increased calcium influx through the channel (Step 3). Calcium entry stimulates key molecules, such as m-calpain, to regulate focal adhesion disassembly. The role of the kinase domain is unknown, but it may be regulating key cytoskeletal events as well. Once focal adhesion turnover is completed, TRPM7 undergoes endocytosis (Step 5) and is then either recycled or degraded (Step 6).
The specific signaling pathway(s) that control the entry into and exit from these adhesion complexes by TRPM7 are still unknown. Additional research is required to understand how TRPM7-dependent regulation of m-calpain fits into the elaborate orchestration of proteins that regulate cell adhesion and locomotion. Nonetheless, calpain has been implicated in a number of migratory processes, including neurite out-growth and growth cone motility (49-51), cell migration (23, 25, 52, 53), and cancer cell metastasis (24, 54-57). Calpain has also been associated with cell death during ischemia in various organs (35, 58-67). TRPM7 was originally shown to be activated by depletion of intracellular Mg·ATP levels, and the channel has been recently implicated in causing the death of cultured cortical neurons during anoxia (20). One cannot help but speculate whether anoxic-induced activation of TRPM7 causes the overstimulation of m-calpain that contributes to the demise of cells during anoxia. To better understand such cellular events, further investigation into how TRPM7-dependent regulation of m-calpain controls cell adhesion and migration in other cell types will be important to pursue.
Supplementary Material
suplementary data
Acknowledgments
We are grateful to Dr. Michael Greenberg (Children’s Hospital, Boston, MA) for the ephexin expression vector and Dr. Andrew Scharenberg (University of Washington, Seattle, WA) for the LTRPC7 cell line. We thank Dr. Alexey Ryazanov (UMDNJ-Robert Wood Johnson Medical School), Elizabeth Puccini, and Cindy Tong for constructive suggestions and comments.
Footnotes
2
The abbreviations used are: ALLM, _N_-acetyl-leucyl-leucyl-methioninal; Z, benzyloxycarbonyl; FMK, fluoromethyl ketone; HA, hemagglutinin; GST, glutathione _S_-transferase; FRT, Flp recombination target.
*
This work was supported by American Heart Association Grant 0335375T. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “_advertisement_” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Runnels LW, Yue L, Clapham DE. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 2.Fleig A, Penner R. Novartis Found. Symp. 2004;258:248–266. [PubMed] [Google Scholar]
- 3.Nilius B, Voets T. Novartis Found Symp. 2004;258:140–159. 263–266. [PubMed] [Google Scholar]
- 4.Clapham DE. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 5.Montell C, Birnbaumer L, Flockerzi V. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 6.Clapham DE, Runnels LW, Strubing C. Nat. Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 7.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. J. Gen. Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadler MJS, Hermosura MC, Inabe K, Perraud A-L, Zhu Q, Stokes AJ, Kurosaki T, Kine J-P, Penner R, Scharenberg AM, Fleig A. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 9.Ryazanov AG, Pavur KS, Dorovkov MV. Curr. Biol. 1999;9:R43–R45. doi: 10.1016/s0960-9822(99)80006-2. [DOI] [PubMed] [Google Scholar]
- 10.Ryazanov AG. FEBS Lett. 2002;514:26–29. doi: 10.1016/s0014-5793(02)02299-8. [DOI] [PubMed] [Google Scholar]
- 11.Dorovkov MV, Ryazanov AG. J. Biol. Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita M, Kozak JA, Shimizu Y, McLachlin DT, Yamaguchi H, Wei FY, Tomizawa K, Matsui H, Chait BT, Cahalan MD, Nairn AC. J. Biol. Chem. 2005;280:20793–20803. doi: 10.1074/jbc.M413671200. [DOI] [PubMed] [Google Scholar]
- 13.Kozak JA, Matsushita M, Nairn AC, Cahalan MD. J. Gen. Physiol. 2005;126:499–514. doi: 10.1085/jgp.200509324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Runnels LW, Yue L, Clapham DE. Nat. Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 15.Kerschbaum HH, Kozak JA, Cahalan MD. Biophys. J. 2003;84:2293–2305. doi: 10.1016/S0006-3495(03)75035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak JA, Cahalan MD. Biophys. J. 2003;84:922–927. doi: 10.1016/S0006-3495(03)74909-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prakriya M, Lewis RS. J. Gen. Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J, Li M, Yue L. J. Gen. Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 20.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 21.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. J. Pharmacol. Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- 22.Elizondo MR, Arduini BL, Paulsen J, Macdonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Curr. Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 23.Glading A, Lauffenburger DA, Wells A. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- 24.Carragher NO, Westhoff MA, Riley D, Potter DA, Dutt P, Elce JS, Greer PA, Frame MC. Mol. Cell. Biol. 2002;22:257–269. doi: 10.1128/MCB.22.1.257-269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco SJ, Huttenlocher A. J. Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 26.Wells A, Huttenlocher A, Lauffenburger DA. Int. Rev. Cytol. 2005;245:1–16. doi: 10.1016/S0074-7696(05)45001-9. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni S, Saido TC, Suzuki K, Fox JE. J. Biol. Chem. 1999;274:21265–21275. doi: 10.1074/jbc.274.30.21265. [DOI] [PubMed] [Google Scholar]
- 28.Bialkowska K, Kulkarni S, Du X, Goll DE, Saido TC, Fox JE. J. Cell Biol. 2000;151:685–696. doi: 10.1083/jcb.151.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glading A, Chang P, Lauffenburger DA, Wells A. J. Biol. Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- 30.Glading A, Uberall F, Keyse SM, Lauffenburger DA, Wells A. J. Biol. Chem. 2001;276:23341–23348. doi: 10.1074/jbc.M008847200. [DOI] [PubMed] [Google Scholar]
- 31.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Mol. Cell. Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starling A, de Paula F, Silva H, Vainzof M, Zatz M. J. Mol. Neurosci. 2003;21:233–236. doi: 10.1385/jmn:21:3:233. [DOI] [PubMed] [Google Scholar]
- 33.Zatz M, Starling A. N. Engl. J. Med. 2005;352:2413–2423. doi: 10.1056/NEJMra043361. [DOI] [PubMed] [Google Scholar]
- 34.Matsukura S, Jones PA, Takai D. Nucleic Acids Res. 2003;31:e77. doi: 10.1093/nar/gng077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashima T. Cell Calcium. 2004;36:285–293. doi: 10.1016/j.ceca.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz-Urda S, Garcia J, Green CL, Chen L, Lin Q, Veitch DP, Sakai LY, Lee H, Marinkovich MP, Khavari PA. Science. 2005;307:1773–1776. doi: 10.1126/science.1106209. [DOI] [PubMed] [Google Scholar]
- 37.Ren XD, Kiosses WB, Schwartz MA. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habas R, Kato Y, He X. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. J. Biol. Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 40.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. J. Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrenson ID, Wimmer-Kleikamp SH, Lock P, Schoenwaelder SM, Down M, Boyd AW, Alewood PF, Lackmann M. J. Cell Sci. 2002;115:1059–1072. doi: 10.1242/jcs.115.5.1059. [DOI] [PubMed] [Google Scholar]
- 42.Postma FR, Jalink K, Hengeveld T, Moolenaar WH. EMBO J. 1996;15:2388–2392. [PMC free article] [PubMed] [Google Scholar]
- 43.Van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S. J. Biol. Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 44.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Nat. Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 45.Goll DE, Thompson VF, Li H, Wei W, Cong J. Physiol. Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 46.Frame MC, Fincham VJ, Carragher NO, Wyke JA. Nat. Rev. Mol. Cell. Biol. 2002;3:233–245. doi: 10.1038/nrm779. [DOI] [PubMed] [Google Scholar]
- 47.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Nat. Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 48.Perrin BJ, Amann KJ, Huttenlocher A. Mol. Biol. Cell. 2005 doi: 10.1091/mbc.E05-06-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robles E, Huttenlocher A, Gomez TM. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 50.Song DK, Malmstrom T, Kater SB, Mykles DL. J. Neurosci. Res. 1994;39:474–481. doi: 10.1002/jnr.490390414. [DOI] [PubMed] [Google Scholar]
- 51.Spira ME, Oren R, Dormann A, Gitler D. J. Comp. Neurol. 2003;457:293–312. doi: 10.1002/cne.10569. [DOI] [PubMed] [Google Scholar]
- 52.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. J. Biol. Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 53.Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. J. Biol. Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- 54.Carragher NO, Fonseca BD, Frame MC. Neoplasia. 2004;6:53–73. doi: 10.1016/s1476-5586(04)80053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carragher NO, Frame MC. Int. J. Biochem. Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 56.Mamoune A, Luo JH, Lauffenburger DA, Wells A. Cancer Res. 2003;63:4632–4640. [PubMed] [Google Scholar]
- 57.Wells A, Kassis J, Solava J, Turner T, Lauffenburger DA. Acta Oncol. 2002;41:124–130. doi: 10.1080/028418602753669481. [DOI] [PubMed] [Google Scholar]
- 58.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 59.Chen M, Won DJ, Krajewski S, Gottlieb RA. J. Biol. Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 60.Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Circ. Res. 2005;97:465–473. doi: 10.1161/01.RES.0000181170.87738.f3. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Rainey JJ, Harriman JF, Schnellmann RG. Am. J. Physiol. 2001;281:F728–F738. doi: 10.1152/ajprenal.2001.281.4.F728. [DOI] [PubMed] [Google Scholar]
- 62.Marzocco S, Di Paola R, Autore G, Mazzon E, Pinto A, Caputi AP, Thiemermann C, Cuzzocrea S. Shock. 2004;21:38–44. doi: 10.1097/01.shk.0000095056.62263.b2. [DOI] [PubMed] [Google Scholar]
- 63.Mehendale HM, Limaye PB. Trends Pharmacol. Sci. 2005;26:232–236. doi: 10.1016/j.tips.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Nagy Z, Simon L. Adv. Exp. Med. Biol. 2004;541:39–54. [PubMed] [Google Scholar]
- 65.Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, Wang KK. Biochem. Biophys. Res. Commun. 2000;274:16–21. doi: 10.1006/bbrc.2000.3070. [DOI] [PubMed] [Google Scholar]
- 66.Patzke H, Tsai LH. 2002;277:8054–8060. doi: 10.1074/jbc.M109645200. [DOI] [PubMed] [Google Scholar]
- 67.Tamada Y, Fukiage C, Daibo S, Yoshida Y, Azuma M, Shearer TR. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2002;131:221–225. doi: 10.1016/s1096-4959(01)00489-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
suplementary data