A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer (original) (raw)
. Author manuscript; available in PMC: 2013 Jan 1.
Abstract
Twenty-seven subjects with HER-2/neu over-expressing ductal carcinoma in situ of the breast were enrolled in a neoadjuvant immunization trial for safety and immunogenicity of DC1-polarized dendritic cells (DC1) pulsed with six HER-2/neu promiscuous MHC class II-binding peptides, plus two additional HLA-A2.1 class I-binding peptides. DC1 were generated with IFN-γ plus a special clinical-grade bacterial endotoxin (LPS) and administered directly into groin lymph nodes four times at weekly intervals prior to scheduled surgical resection of DCIS. Subjects were monitored for the induction of new or enhanced anti-peptide reactivity by IFN-γ ELIspot and ELISA assays performed on Th cells obtained from peripheral blood or excised sentinel lymph nodes. Responses by CTL against HLA-A2.1-binding peptides were measured using peptide-pulsed T2 target cells or HER-2/neu-expressing or non-expressing tumor cell lines. DC1 showed surface phenotype indistinct from “gold standard” inflammatory cocktail-activated DC, but displayed a number of distinguishing functional characteristics including the secretion of soluble factors and enhanced “killer DC” capacity against tumor cells in vitro. Post-immunization, we observed sensitization of Th cells to at least 1 class II peptide in 22 of 25 (88%, 95% exact CI 68.8 – 97.5%) evaluable subjects, while eleven of 13 (84.6%, 95% exact CI 64 – 99.8%) HLA-A2.1 subjects were successfully sensitized to class I peptides. Perhaps most importantly, anti-HER-2/neu peptide responses were observed up to 52 months post-immunization. These data show even in the presence of early breast cancer such DC1 are potent inducers of durable type I-polarized immunity, suggesting potential clinical value for development of cancer immunotherapy.
Keywords: breast cancer, dendritic cell, vaccine, HER-2/neu
Introduction
The immune system has traditionally been divided into two parts; the innate and the adaptive. The innate immune system’s components include monocytes, macrophages, granulocytes, NK and dendritic cells (DC). The adaptive immune system is composed of antibody-producing B lymphocytes, as well as CD4pos helper T cells and CD8pos cytotoxic T cells. These cells work together to sense, control and eliminate infection. Agents of innate immunity identify microbes through special pattern recognition receptors that sense biochemical structures (usually non-proteins) common to broad classes of potential pathogens (1). On the other hand, T and B lymphocytes specialize in responding against antigens (usually proteins) specific to the individual species of microbe. DCs have a unique role in that they form a critical bridge between innate and adaptive immunity. Pattern recognition proteins belonging to the Toll-like family of transmembrane receptors (2) induce a maturation and migration program whereby various DC populations, including monocyte-derived DCs, convey peripherally-acquired proteins to T cells located in the regional draining lymph nodes (3). The DCs “present” the microbial antigens to T cells in the form of processed peptides complexed with self major histocompatibility proteins (4). This supplies an important signal (signal 1) to T cells that, along with maturation-enhanced co-stimulatory molecule (CD80, CD86) expression (signal 2), can fully activate T cells (5). DCs and some other accessory cells can supply so-called “third signals” (6) that often are expressed in the form of soluble factors, for example, IL-12, IL-23, IL-6 and TGF-beta. Such signals can profoundly influence helper T cell development toward discrete functional phenotypes that include IFN-γ-secreting Th1, IL-17- secreting Th17, as well as anti-inflammatory Treg that produce TGF-beta and IL-10 (7–10). In many instances, the precise combination of activation signals received by DC dictates whether individual 3rd signal agents will be produced, and hence which Th phenotypes will be selectively induced by the DC (11).
Although the immune system evolved primarily to deal with infections, it may be possible to direct it against malignancies. An ideal strategy for inducing anti-tumor immunity must successfully accomplish several goals-some of which are overlapping with traditional anti-microbial vaccines, but others unique to the particular requirements of effective anti-tumor immunity. For example, an effective anti-tumor vaccine must overcome the immune system’s natural tendency to resist the development of strong immunity against self-proteins (i.e. tolerance). It must also generate immunity of a quality and intensity likely to reduce or eliminate tumor burdens. In the case of therapeutic vaccines, immunity must be effectively induced when disease is already firmly established. Finally, such induced immunity should be durable, so that possible tumor recurrences can be suppressed for long periods post-immunization.
We formulated a novel, integrated DC-based immunization system designed to overcome some of the known obstacles facing anti-cancer vaccines. We call this strategy ICAIT (Immune Conditioning via Activated Innate (autologous) Transfer). Here, CD14pos peripheral blood monocytes (which represent a precursor pool for activated DC) were rapidly activated into fully-functional DC in vitro with IFN-γ and a special clinical-grade bacterial endotoxin (LPS). LPS signals through the Toll-like pattern recognition receptor TLR4 (12), mimicking infection and inducing a mature phenotype coincident with the production of a unique battery of secreted products, not seen with most commonly-employed DC activation regimens, including high levels of the Th1-polarizing third-signal cytokine IL-12 p70. These DC were also pulsed with tumor peptide antigens based on the HER-2/neu sequence (representing HLA-A2 (13–14) and promiscuous HLA-Class II-restricted epitopes (15)), so that both Th and CTL could potentially be activated. The ICAIT DCs were then administered directly to the lymph nodes of subjects with early HER-2/neupos breast cancer (ductal carcinoma in situ). Recall responses to HER-2 peptides were monitored for up to 52 months post immunization. We report high rates of sensitization and durable immune responses with the ICAIT immunization approach.
METHODS
Clinical trial design
We conducted a pilot trial of a HER-2/neu-based DC immunization strategy for patients with HER-2/neu over-expressing DCIS or DCIS with microinvasion. The primary objectives were to evaluate the feasibility, safety and immunogenecity (i.e., CD4pos and CD8pos T cells sensitized to HER-2/neu) of the immunization regimen. ICAIT DC were administered intranodally once per week for 4 weeks followed by definitive surgical resection of remaining tumor. A maximum of 30 patients were to be enrolled in order to establish safety and have adequate power to evaluate pre- versus post-ICAIT T cell sensitization status by McNemar’s test. If no occurrence of unacceptable toxicity was observed in the 30 patients, then the rate of unacceptable toxicity was no higher than 7% based on the upper-bound of the one-sided exact 90% confidence interval.
Feasibility, defined as the rate of patients who received all 4 immunizations on schedule, was 100%. Toxicities were graded using the NCI Common Toxicity Grading Scale Version 3.0. Sensitization of CD4pos and CD8pos T cells was assessed in pre- and post-ICAIT peripheral blood samples and in sentinel lymph nodes where available. Eligible patients were greater than 18 years old, signed Informed Consent, had ECOG performance status 0 or 1, and presented with biopsy-proven DCIS or DCIS with microinvasion but had not yet received definitive treatment. Patients whose DCIS was eliminated by excisional biopsy at diagnosis were not eligible. HER-2/neu positivity was determined as >10% cells expressing 2+ or 3+ intensity of the HER-2/neu protein. Patients with areas suspicious of invasive disease were evaluated by MRI. All subjects underwent cardiac evaluation with MUGA scan or echocardiography to document adequate baseline cardiac function. Scans were performed prior to immunization and within 2 weeks of the final ICAIT treatment. All patients underwent HLA Class I tissue typing pre-enrollment and had routine history, physical exams, EKG, blood work, and urinalysis prior to immunization. After informed consent, all subjects underwent pre-ICAIT leukapheresis to obtain sufficient monocytes for ICAIT preparation; in a few cases a second apheresis was required for additional monocytes. Post-ICAIT leukapheresis was performed usually within two weeks of the final immunization. All patients underwent post-ICAIT mammogram, MRI and surgical resection of DCIS with either lumpectomy or mastectomy. Patients could be candidates for either lumpectomy or mastectomy. Patients were followed for 30 days post-ICAIT treatment in order to assess safety and to undergo second leukapheresis. Long term immunologic surveillance (i.e., serial blood sampling) was not mandatory and was conducted on a voluntary basis only.
Materials and Reagents
HER-2/neu peptides were purchased from American Peptide Corporation (Sunnyvale, CA), Monocyte-Macrophage Medium (SFM) and Iscove’s Medium from Invitrogen (Carlsbad, CA), Lymphocyte separation Medium (LSM) from ICN Biomedical Inc. (Aurora, OH), Human AB serum and fetal calf serum from Sigma Chemical (St. Louis, MO) and GM-CSF from Amgen (Newbury Park, CA). Reagents for ELISA assays were obtained from Pharmingen, San Jose, CA, and R& D, (Minneapolis, MN) Clinical grade IFN-γ was obtained from Intermune (Brisbane, CA). Clinical grade LPS was a generous gift from Dr. Anthony Suffredini at the NIH.
Immunization Procedure
ICAIT immunizations were administered in the NIH-designated General Clinical Research Center at the Hospital of the University of Pennsylvania. They consisted of 10–20 million HER-2/neu-pulsed ICAIT DCs suspended in 1ml sterile saline. ICAIT DCs were administered (aided by ultrasound guidance) into a single lymph node in each groin as previously described (16). Half of each ICAIT DC dose was placed into each node with a 22g needle. The first 9 subjects were observed for 2 hours post-immunization with routine vital signs obtained at 15-minute intervals. Subsequent subjects were observed for one hour. Immunizations were administered once weekly for four weeks. All subjects completed all 4 scheduled ICAIT immunizations.
DC Immature and mature DC preparation
DC precursors (CD14pos peripheral blood monocytes) were obtained from subjects via tandem leukapheresis/countercurrent centrifugal elutriation (17). All DC cultures were incubated overnight at 37° C in Macrophage Serum-free Medium (SFM-Gibco Life Technologies, Carlsbad, CA) with GM-CSF 250 IU/ml (Berlex, Richmond, CA) and IL-4 1000 u/ml (R&D Systems, Minneapolis, MN). These cells were considered immature DC (iDC). For maturation, DC were further activated with IFN-γ (1000 U/ml) for at least 12 hours, followed by NIH reference standard LPS (10 ng/ml), with DCs harvested 6h later (ICAIT DC). Immature DC were also matured into “gold standard” DC (CMC-DC), similar to those commonly used in previous clinical trials (18–20), by treatment with a cytokine maturation cocktail (CMC) consisting of IL-1β (10 ng/ml), IL-6 (1000 U/ml), TNF-α (10 ng/ml), and PGE2 (1 μM).
Preparation of Clinical ICAIT DC
ICAIT DCs were produced as described previously (16). The DC vaccines were prepared under IND BB110843. Briefly, monocytic DC precursors were obtained from subjects via tandem leukapheresis/countercurrent centrifugal elutriation. Cells were cultured overnight at 37° C in SFM with GM-CSF and IL-4. The next day, DCs were pulsed with 6 HER-2/neu MHC class II promiscuous-binding peptides (42–56, 98–114, 328–345, 776–790, 927–941, 1166–1180) (15). After 8–12 hours incubation, IFN-γ (1000 U/ml) was added, and the next day, 6h prior to harvest, NIH reference standard LPS was added (10 ng/ml) to achieve full DC activation. Harvest was timed to recover the cells prior to their maximum secretion of IL-12 and other cytokines. For HLA-A2.1pos subjects, cells were also pulsed with MHC class I binding peptides 369–377 and 689–697 A2 (13–14). Harvested cells were washed and assessed by lot release criteria of >70% viability, negative Gram’s stain and endotoxin <5 EU/Kg. Only preparations meeting these criteria were released for administration to subjects.
FACS analysis
DCs were analyzed by multicolor flow cytometry using a Becton Dickenson FACScan cytometer running CellQuest analysis software (Becton-Dickinson, Mountain View, CA) as described previously (16). Propidium iodide-staining (nonviable) cells were excluded from analysis
ELISA assays
Capture and biotinylated detection antibodies and standards for IFN-γ, IL-4, IL-5, IL-6, RANTES, and IL-12p70 (PharMingen), or kits for macrophage-derived chemokine (MDC), thymus and activating-regulated chemokine (TARC), and migration inhibitory protein 1β (MIP-1α, R&D) were used according to the manufacturer’s recommendations and protocols. Supernatants from activated DC generally 8–24 hours after the addition of LPS were harvested and used to measure cytokine and chemokine production.
Immune monitoring
CD4pos T cell sensitization was evaluated from peripheral blood or sentinel nodes by determining IFN-γ production via ELISPOT (without prior in vitro antigen-driven expansion, described below) and also using in vitro sensitization (IVS) followed by standard ELISA to measure cytokine output for _in vitro_-expanded and re-stimulated cells. ICAIT sensitization of CD8pos T cells was also measured by examining IFN-γ output after a single round of in vitro antigen-driven expansion followed by testing against peptide-pulsed T2 cells or HER-2-neu-expressing cancer line targets. Experimental details have been published previously (16). For long-term assessments PBL were obtained from patients at various time points post-treatment and directly tested in ELISPOT with HER-2/neu MHC Class II peptides and testing for IFN-γ as described below.
ICAIT Cytotoxicity Assay
Breast cancer cell line T47D was labeled with CFSE and then co-cultured with DCs. Twenty-four hours later, cells were harvested, stained with 7–AAD and subjected to FACS analysis. CFSE positive cells were gated and analyzed. % apoptotic cells were calculated as 7AAD+/(7AAD+ and 7AAD−) × 100.
ELISPOT Assay
Anti-IFN-γ Ab was purchased from Mabtech Inc.(Mariemont, OH) and the ELISPOT assay was performed according the manufacturer’s instructions. Multiscreen filter plates (MAIPSWU10) were purchased from Millipore. Substrate solution (TMB-H) was purchased from Moss (Pasadena, MD). Coating Ab (1-D1K) was diluted to 12μg/ml in sterile PBS. The ELISPOT plates were pre-wet with 70% ethanol for 1 minute at RT and washed 5 times with sterile water. After addition of the coating Ab (100μl/well), the plates were incubated overnight at 4–8 °C. The plates were washed 5 times with sterile PBS prior to the addition of 200μl/well of culture medium. After blocking for > 30 min, the medium was removed. CD4pos cells or sentinel lymph node cells were added with either immature or mature dendritic cells, pulsed with the ICD and ECD peptides (total of 150ul/well) at a ratio of 1×105 to 2× 104. Tetanus was used as control recall antigen. The cells were incubated at 37° C for 20 hrs. The plates were then washed 5 times with 200μl of PBS. 100μl of detection Ab (7 B6-1-biotin) diluted to 1μg/ml in PBS with 0.5% fetal calf serum was added to each well. The plates were incubated at RT for 2 hrs. After washing 5 times, 1:1000 diluted Streptavidin-HRP in PBS with 0.5% fetal calf serum was added prior to incubation for an additional hour. The plates were washed and 100μl of substrate solution was added to each well. After color development, wells were rigorously washed with tap water. The plates were dried at RT and read in an ELISPOT reader (Immunospot CTL, Cleveland, OH). At routine intervals the relative coefficient of variance was determined. The maximum value was 23% (1029+− 63.34 = 0.448%; 993+−71= 3.18%; 635+−1.52 = 0.09%; 596+−35.2= 2.4%; 99+−23.8 = 12% and 120+−55.25 = 23%). Tetanus toxoid control antigen served as reference standard since all subjects had been vaccinated against this antigen at some point in the past. For all subjects the difference in ratio of pre-to post vaccine anti-tetanus toxoid ELISPOT reactivity was less than 2-fold.
CD8pos T cell sensitization
Autologous DCs were pulsed with HER-2/neu p369-377 or p689-697 at 10μg/ml 2h prior to harvest. DCs were then co-cultured with column purified pre- or post-vaccination CD8pos T cells at a ratio of 10:1 in 48-well plates. 30IU/ml of IL-2 was added to the cultures on day 2. After 7–10 days of sensitization (single stimulation), the CD8pos T cells were harvested and re-stimulated with T2 cells pulsed with either relevant or irrelevant peptides, or were tested against breast cancer cell lines MDA-MB-231 (HER-2/neu pos, HLA-A2 pos), MDA-MB435S (HER-2/neu pos, HLA-A2 neg), and ovarian cancer cell lines SKOV3 (HER-2/neu pos, HLA-0201pos/neg (transfected or non-transfected cells), a kind gift of Dr. Mary Disis, University of Washington). Supernatants were harvested after 24h and analyzed by ELISA.
Immunohistochemical staining of DCIS lesions
Formalin-fixed, paraffin embedded tissue blocks were sectioned at 5μm on plus slides (Fisher Scientific). Sections were heated for 1 hour @ 60°C to remove excess paraffin, cooled for 10 minutes and subsequently deparaffinized and rehydrated in a series of xylenes and alcohols. Immunohistochemistry was performed using the Dako Autostainer (Dako, Carpinteria, CA). All tissues were stained for HercepTest (Dako, Carpinteria, CA), CD3 (Novocastra Labs), CD4 (Biocare Medical, Walnut Creek, CA), CD8 (Dako), CD20 (Dako). Biopsy site reactions were easily distinguished and these regions excluded for assessing immune infiltration.
Statistical Methods
Event rates and exact 90% or exact 95% confidence intervals (CI) were calculated. Continuous variables were described by means and standard errors. Statistical analyses were performed with StatXact software (Cytel Inc., Cambridge, MA).
RESULTS
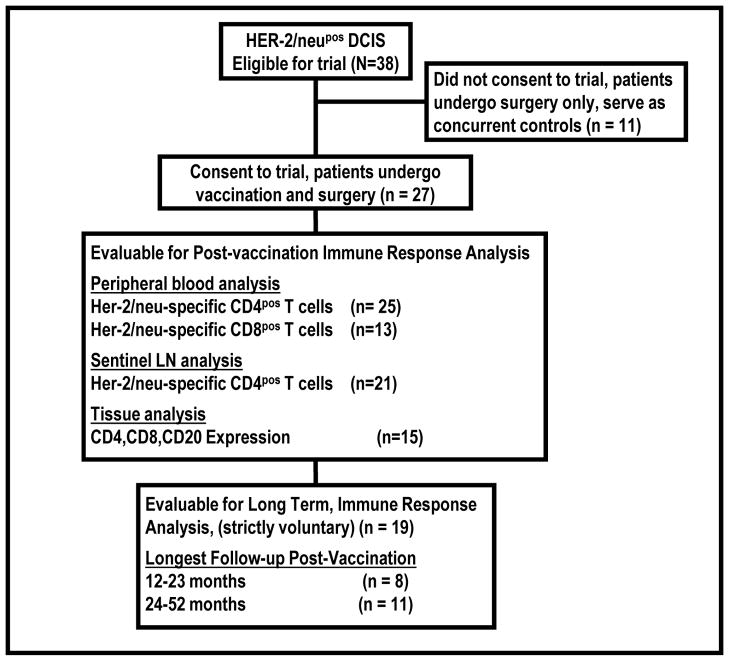
Between September 2003 and May 2008, 38 patients eligible for the trial were identified, of which 27 patients gave written informed consent and enrolled on the trial. No cases of unacceptable toxicity were observed, such that the upper bound of the one-sided exact 90% confidence interval for the rate of toxicity is 8.2%, which indicated that the vaccine was safe with a population toxicity rate no higher than 8%. Numbers of patients with specimens evaluable for post-vaccine immune analyses are displayed in the CONSORT diagram (Figure 1).
Figure 1.
CONSORT diagram of trial charting subject recruitment, and analysis of various available lymphocytes and tissues.
ICAIT DC Characteristics
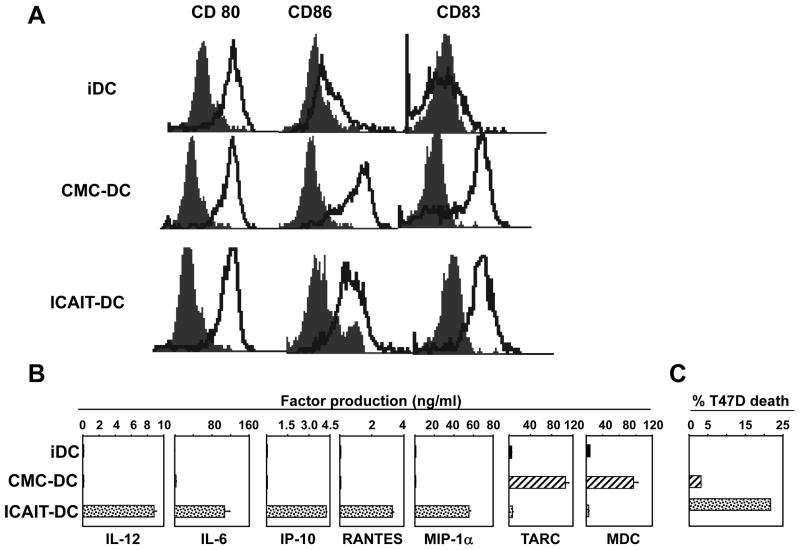
ICAIT DC were compared to “gold standard” inflammatory cytokine maturation cocktail-induced DC (CMC-DC) (21) as well as immature cells (iDC) for surface markers and production of cytokines and chemokines. Despite similar mature surface phenotypes (Fig 2A) including enhanced expression of co-stimulatory molecules and CD83, ICAIT DC produced disproportionately large quantities of IL-12, IL-6, IP-10, MIP-1α, and RANTES (Fig 2B). In contrast, CMC-DC specialized in the secretion of TARC and MDC, two factors associated with Th2 immunity (22–23). The range of IL-12 production across all vaccines was average 11,500 pg/ml with range 264 – 71,000 pg/ml. The iDC produced no detectable levels of the tested cytokines or chemokines. Interestingly, ICAIT-DC also seemed to possess a “killer DC” function as evidenced by induced apoptosis of breast cancer tumor cell line T47D (Figure 2C), while CMC-DC showed much weaker activity.
Figure 2.
Dendritic cell characteristics. (A) Flow cytometry analysis of surface marker expression. ICAIT DC were compared to immature monocyte-derived DC (iDC), and “gold standard” inflammatory cytokine-matured DC (CMC-DC) for the expression of CD80, CD86 and CD83. Open traces indicate specific antibody-stained cells and filled traces represent isotype-matched controls. (B) Secreted factors. Production of IL-12, IL-6, IP-10, RANTES, MIP-1α, TARC and MDC were measured in 8–24h post-activation supernatants by ELISA. Data shown are the average of 4 representative experiments including standard error of mean. (C) Assessment of killer DC function. ICAIT-DC were compared with cytokine matured DC for their ability to induce apoptosis of breast cancer tumor cell line T47D. Results shown are representative of 7 different experiments with similar results.
ICAIT treatment induces anti-HER-2/neu CD8pos immunity
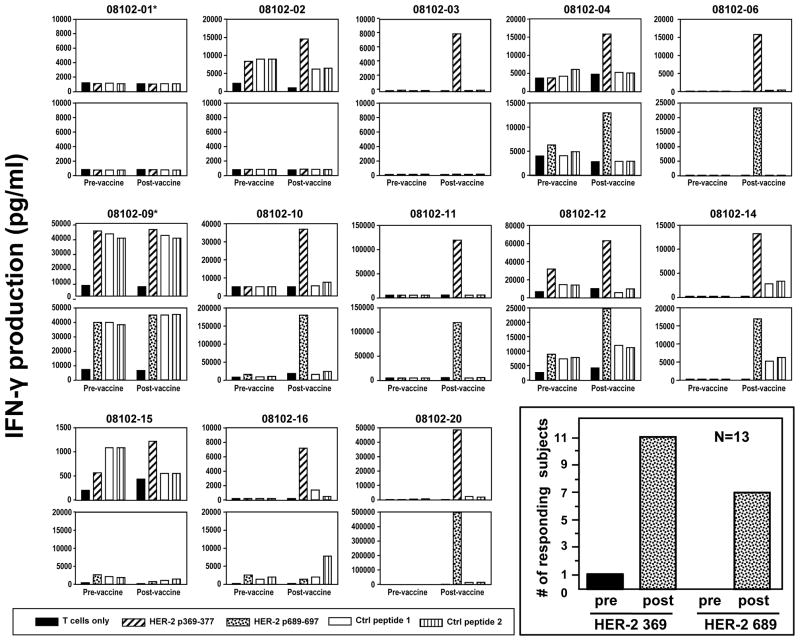
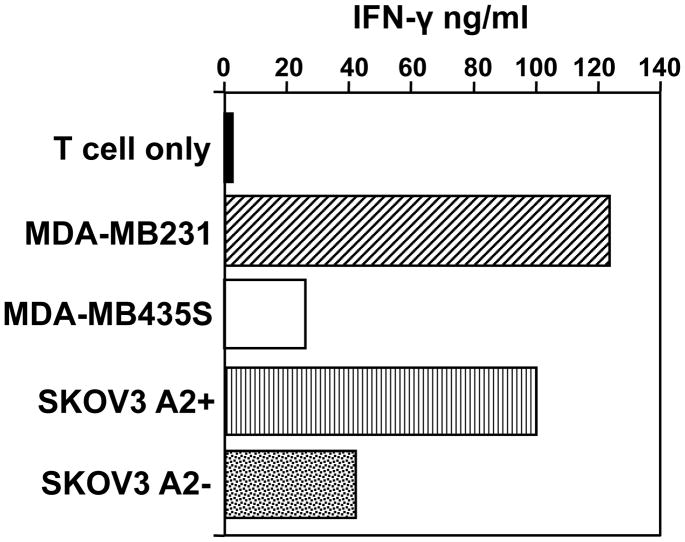
There were 13 HLA A2pos subjects that received ICAIT DC pulsed with additional HLA-A2 binding peptides (369–377 and 689–697) (13–14), each potentially capable of sensitizing CD8pos T cells. The capacity of each HLA-A2.1pos ICAIT-immunized subject’s CD8pos T cells to produce IFN-γ in response to T2 target cells pulsed either with HER-2 peptide, or irrelevant control peptide is displayed in Figure 3, with a summary of numbers of responders also provided (Figure 3 inset). Eleven of 13 (84.6%, 95% exact CI 64 – 99.8%) subjects with sufficient available blood CD8pos T cells to test demonstrated evidence of peptide reactivity to 369–377, and seven of 13 (54%) also developed reactivity to 689–697. It should be noted that there was only a single subject that demonstrated reactivity to one of the class I peptides (369–377) prior to ICAIT immunization. Interestingly, these subjects’ CD8pos T cells also showed, after ICAIT treatment, a tendency to directly recognized HLA-A2pos breast and ovarian cancer cell lines that both over-express HER-2/neu plus the appropriate HLA-A2.1 restriction element (Figure 4). A representative subject is shown in Figure 4. In fact, 11/13 (84.6%) subjects displayed recognition (assessed by IFN-γ secretion), of HER-2/neu-expressing tumor cells, but not those that were HLA A2neg or those that did not express HER-2/neu (Table 1). One of these subjects had evidence of tumor recognition prior to vaccination 080102-09 (Table 1). These data confirm that ICAIT therapy resulted in CD8pos T cell sensitization against HER-2/neu peptides in a large proportion of immunized subjects.
Figure 3.
Assessment of CD8pos T cell sensitization to peptide following ICAIT immunization. There were 13 HLA A2pos subjects that received ICAIT DC pulsed with additional HLA-A2 binding peptides (369–377 and 689–697). CD8pos T cells obtained before and after ICAIT-DC immunization were separately co-cultured for one week with immature autologous DC pulsed with either HER-2/neu 369 or 689 peptides prior to testing against monocyte pulsed with either HER-2/neu peptides or irrelevant controls. *****Denotes those 2 subjects that did not have evidence of 369 or 689 peptide specificity. A summary of the number of subjects displaying positive peptide reactivity is displayed in the figure inset, with positive responses defined as at least 2-fold greater specific IFN-γ secretion post-vaccination compared to pre-vaccination. Note the single subject demonstrating pre-existing peptide reactivity.
Figure 4.
Assessment of direct tumor recognition by immune CTL. Purified peripheral CD8pos T cells were expanded by co-culture with autologous DCs pulsed with HER-2/neu 369 peptide. Seven -10 days later the cells were harvested and re-cultured with a variety of tumor lines selected for their divergent expression of HER-2/neu and the HLA-A2.1 restriction element (see materials and methods). Culture supernatants were harvested 24h later and analyzed by ELISA for IFN-γ production. Data shown is from one representative subject.
Table 1.
CD8 T cell recognition of breast cancer cell lines
| IFN-g secretion (pg/ml) | HLA-A2+, HER2+ cell line | * Control cell lines | ||
|---|---|---|---|---|
| T cell only | ||||
| 08102- 01 | pre | 1096 | 881 | 672 |
| post | 977 | 807 | 670 | |
| 08102-02 | pre | 2193 | 5826 | 4942 |
| post | 1800 | 10819 | 3323 | |
| 08102-03 | pre | 10 | 10 | 10 |
| post | 10 | 173 | 10 | |
| 08102-04 | pre | 50 | 2909 | 2531 |
| post | 50 | 5105 | 1570 | |
| 08102-06 | pre | 50 | 50 | 50 |
| post | 2526 | 123236 | 2396 | |
| 08102-09 | pre | 7539 | 19966 | 7976 |
| post | 6540 | 14679 | 7461 | |
| 08102-10 | pre | 500 | 1858 | 4201 |
| post | 3000 | 135125 | 14678 | |
| 08102-11 | pre | 100 | 9783 | 7821 |
| post | 100 | 31761 | 10175 | |
| 08102-12 | pre | 617 | 4848 | 4376 |
| post | 1015 | 7835 | 3747 | |
| 08102-14 | pre | 100 | 525 | 421 |
| post | 100 | 2398 | 317 | |
| 08102-15 | pre | 4468 | 8000 | 4468 |
| post | 282 | 956 | 216 | |
| 08102-16 | pre | 0 | 0 | 0 |
| post | 0 | 0 | 0 | |
| 08102-20 | pre | 100 | 796 | 623 |
| post | 100 | 2437 | 709 |
ICAIT treatment induces anti-HER-2/neu CD4pos immunity
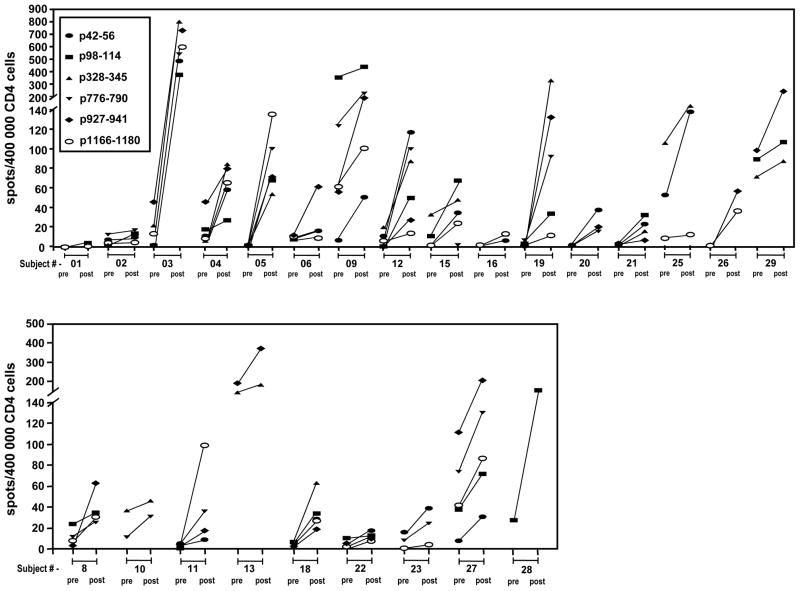
ELISPOT assays were performed directly on unexpanded patient PBMC. There were three subjects with insufficient cells to perform ELISPOT and in vitro sensitization leaving 22 for evaluation. These showed that 22 of 25 (88%, 95% exact CI 68.8 – 97.5%) subjects (CD4pos T cells) experienced at least two-fold increases in the ratio of HER-2/neu peptide-specific IFN-γ secreting cells (post-ICAIT immunization compared to pre-ICAIT) for at least one of the six tested peptides (Figure 5). Nineteen of these 25 (76%, 95% exact CI 54.9 – 90.6%) subjects demonstrated such ICAIT-enhanced responses to more than one peptide. ELISA results from in vitro sensitization assays confirmed the anti-HER-2/neu responses in these patients (data not shown). There was no evidence of IL-5 or IL-4 production by these cells using ELISPOT or IVS (data not shown). The pre-post ratio of tetanus spots was always less that 2, suggesting the sensitization seen of CD4pos T cells to HER-2/neu was specific to vaccination not just non-specific immune activation.
Figure 5.
ELISPOT analysis of peripheral blood CD4pos T cells pre- and post- ICAIT immunization. Unexpanded purified CD4pos T cells were co-cultured overnight with individual HER-2/neu-pulsed immature dendritic cells. Analysis enumerated IFN-γ secreting lymphocytes per 400,000 total cells. Solid lines connect pre- and post-vaccination IFN-γ spots for individual peptides.
Identification of lymphocytes trafficking to sites of DCIS and analysis of anti-HER-2/neu CD4pos T cells in tumor-draining lymph nodes
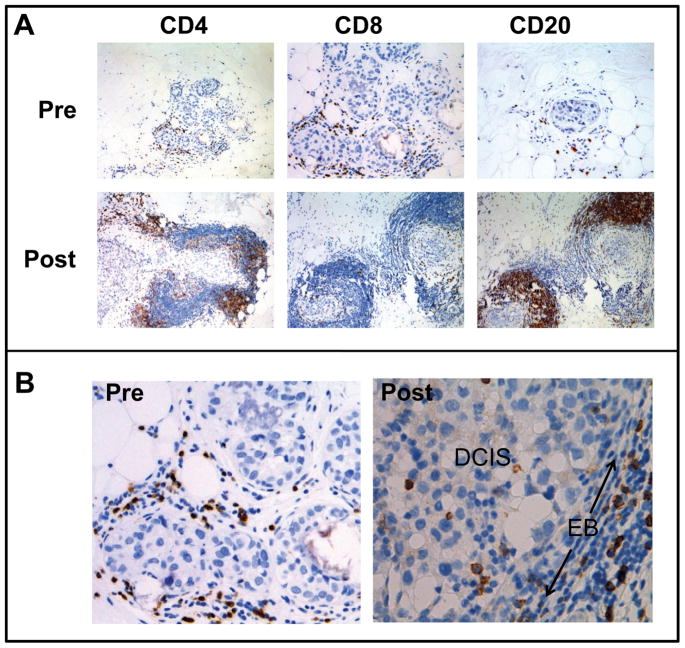
ICAIT DCs were administered in distant groin nodes with the expectation that they would efficiently sensitize lymphocytes within these secondary lymphoid tissues. However, to be effective, node-sensitized lymphocytes must traffic to sites of disease. We therefore performed immunohistochemical analysis comparing pre-ICAIT breast biopsies and post-ICAIT surgical specimens to determine whether increased levels of lymphocytes could be observed around sites of DCIS as an apparent consequence of ICAIT immunization (Fig 6A). In pre-treatment biopsy specimens, it was common to observe low to moderate levels of lymphocytes scattered in the stromal regions outside the DCIS-containing ducts. However, for about 50% of subjects assessed, we observed post-ICAIT increases in CD4pos (Th) and CD8pos (CTL) T cells, as well as CD20pos cells (which were probably B lymphocytes). Not only were the lymphocytes more numerous, their distribution within the breast tissues was greatly altered, with the infiltrating lymphocytes now crowding close around the DCIS-containing ducts, forming pronounced “collars”. In addition, we noted that prior to vaccination no lymphocytes could be routinely observed within the DCIS lesions. However, after ICAIT treatment, CD8pos cells were detected among the tumor cells (Figure 6B). It should be noted that the subject depicted in Figure 6 was not an HLA-A2.1pos individual, and thus received DCs pulsed with the promiscuous class II peptides, but not the 369 or 689 HLA-A2.1-binding CTL epitopes. In addition, we did not observe any changes consistent with increases in CD56pos NK or DCs (not shown).
Figure 6.
Immunohistochemical analysis of pre-ICAIT immunization biopsies and post-ICAIT surgical specimens. (A) Slides stained for CD4, CD8 (T lymphocytes) or CD20 (B lymphocytes). (B) Increased magnification of CD8-stained slides demonstrating in post-ICAIT immunization tissues the enhanced migration of T cells across the epithelial border (EB) and into ducts containing DCIS.
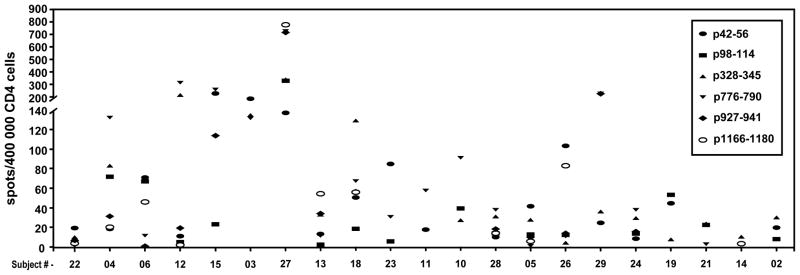
These immunohistochemical studies showed post-ICAIT increases in lymphocytic infiltration at areas of DCIS, but could not prove the actual antigen specificity of the trafficked cells. Because assessment of T cell antigen specificity even within fresh DCIS specimens is not practical, we instead recovered lymphocytes from the tumor-draining sentinel lymph nodes that were excised at the time of surgery to remove residual DCIS. We reasoned that if HER-2/neu-specific T cells were entering diseased breast tissues, some of them would, as part of their natural tendency to re-circulate, transit through the draining lymph nodes. Unexpanded CD4pos T cells obtained from draining sentinel nodes showed HER-2/neu-specific responses in 21 of 22 (95.5%, 95% CI 77.2 – 99.9%) subjects from whom such sentinel nodes were available (Fig 7). This high proportion suggests that T cells sensitized at remote locations (i.e. anatomically distal lymph nodes) via ICAIT do routinely traffic to the site of disease in the breast.
Figure 7.
Assessment of CD4pos anti-HER-2/neu T cells in draining sentinel lymph nodes. Single cell suspensions were made from surgically-excised axillary lymph nodes for all ICAIT immunized subjects from whom these tissues were available. Unexpanded cells were stimulated with the indicated individual HER-2/neu synthetic peptides pulsed onto immature dendritic cells and subjected to ELISPOT analysis to determine the number of IFN-γ-secreting T cells per 400,000 total cells.
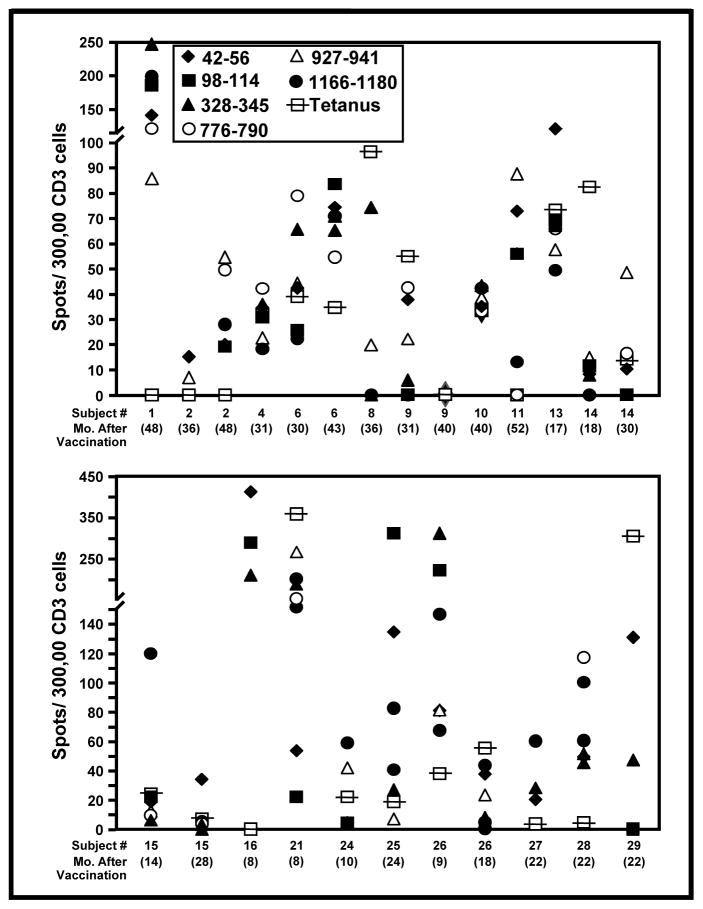
ICAIT treatment induces long-lasting immunity
Some patients voluntarily gave additional blood at various times ranging from 6 months through 52 months post-ICAIT immunization. Thirteen of 14 patients from whom PBL were obtained at least 6 months after completion of the study demonstrated reactivity to one or more HER-2/neu peptides by ELISPOT assay (Figure 8). The data shown are ELISPOT results obtained from PBL stimulated only once with recall peptide without any prior in vitro expansion. Even in follow-up beyond two years, there remained robust anti-HER-2/neu CD4pos reactivity for many subjects, including all three individuals who were assessed beyond 48 months. The long-term responses to HER-2 were of such intensity that they were in the range or sometimes exceeded even the magnitude of the recall response against tetanus toxoid in 22 of the 25 samples tested. These results are particularly encouraging since long-lasting immunity, though often difficult to achieve against tumor antigens, is probably key for preventing disease recurrence.
Figure 8.
ICAIT immunization induces long-lasting T cell immunity. Peripheral blood mononuclear cells (PBMC) were obtained from representative subjects ranging from 6 to 52 months post vaccination. Unstimulated PBL were tested against each of the six HLA Class II-restricted HER-2/neu peptides. ELISPOT analysis enumerated IFN-γ secreting T cells per 300,000 total cells with a minimum limit for positivity of 10 or more spots. Tetanus toxoid acted as the positive control.
DISCUSSION
Because the term “dendritic cell” encompasses a fairly diverse set of cell types with unique lineages and functions, there is presently no clear consensus which type of DC or DC precursor, or which activation regimen, is best suited for use in vaccine development. A seminal discovery in DC research was that peripheral blood monocytes could be made to adopt the characteristics of immature DC if cultured for a week or more in cytokines such as GM-CSF and/or IL-4 (24–26), with mature DC phenotypical features obtainable through further culture with inflammatory cytokine combinations or other activating agents (18, 27–28). These findings were of great practical importance because unlike most DC populations, CD14pos monocytes are relatively abundant in peripheral blood (about 10% of total WBC), making them a highly convenient source of DC for in vitro studies and clinical use. However, it remained uncertain whether the monocyte-derived cells were “authentic” DC rather than merely DC-like cells. Also at issue was whether monocytes represented a biologically-relevant precursor pool for DCs in vivo, or if the previous observations were merely an artifact of in vitro culture and activation. Part of this doubt stemmed from an inability to define an anatomical compartment where monocytes would be exposed to cytokines long periods (29).
The question as to whether monocytes can become functionally authentic DC seems now largely put to rest with the report that mouse monocytes can be mobilized to develop into DCs in vivo in a TLR4- and Trif-dependent manner when driven by bacterial LPS or gram-negative bacteria (living or killed). This transformation is rapid, leads to monocyte-derived DC trafficking to T-dependent areas of draining lymph nodes, and results in acquisition of antigen presenting function equal or in some cases superior to “authentic” non monocyte-derived DCs (3). These studies not only establish monocytes as a bona fide precursor to authentic DCs in vivo, but also reinforce the notion that simulating actual infection (with agents such as LPS), rather than aseptic inflammation (using cytokine cocktails), induces cells with exquisitely-developed DC function.
Our group has pioneered the use of mimics of infection to drive the rapid in vitro differentiation of monocytes into DCs (30–32) with the ultimate goal of using such cells as platforms for immunization. To this end, we have also formulated a novel, integrated DC-based immunization strategy to maximize vaccine effectiveness. This approach harvests CD14pos monocytes from the peripheral blood, but places them, after antigen loading and LPS-activated DC differentiation, directly into lymph nodes, which serve as the cradles for T cell-dependent immune responses (16, 33). Historically, the removal of a tissue and its replacement at a new location within the donor is termed an “autologous transfer”. This vaccine strategy also relies uniquely upon the specialized cytokine/chemokine environment created by the LPS-activated DCs in the lymph node. These soluble products have the potential to exert a critical influence over many aspects of T lymphocytes functional capacity (discussed below). The tandem use of cytokine-secreting DCs, and their placement directly into lymph nodes rather than injecting them at peripheral sites, (where they may needlessly expend these factors) means that the lymph node T cells are not merely sensitized to antigen, but also “conditioned” by the soluble factors that drive the acquisition of specialized and desirable functional characteristics. Because of these innovative features, we refer to this vaccination system as ICAIT (Immune Conditioning by Activated Innate (autologous) Transfer.
There were several reasons to predict that combining IFN-γ with LPS would induce vaccine DCs that would serve as unusually active vehicles for generating strong, long-lasting Th1-polarized immunity against tumors and tumor antigens. For example, this combination induces extremely high levels of many soluble products from maturing DCs not seen with conventional activation regimens (Fig 2). Among these factors, IL-12 in particular possesses a number of activities well-documented in vitro and in animal models, which are highly supportive of anti-tumor immunity. For example, IL-12 is known to polarize CD4pos Th cells into the IFN-γ-secreting Th1 phenotype (34), and IFN-γ has been shown to be a critical feature in effective anti-tumor immunity (35). In addition, IL-12 contributes to avoiding tolerance, enhancing granzyme B production (improving cytotoxicity), and also prolongs T cell survival through a Bcl-3-mediated mechanism (36–38). Finally, its presence during CD8pos CTL sensitization has been shown in vitro to enhance functional avidity (i.e. antigen sensitivity), making the T cells better at recognizing targets with low levels of specific peptide:MHC complexes (39). Considering these precedents, it was therefore not unreasonable that DCs pulsed with HER-2/neu peptides and activated with LPS and IFN-γ should be capable either of inducing de novo or enhancing anti-HER-2/neu CD4pos T cell responses in well over 80% of immunized subjects. As might be expected, the cytokine secretion profiles for all subjects tested demonstrated strong Th1 polarization (high IFN-γ with low or absent production of other cytokines such as IL-4, IL-5 and IL-10). Also, 11 of 13 HLA-A2.1pos immunized subjects produced CTL that directly recognized tumor lines that naturally over-express HER-2/neu. This finding is of particular interest because it has proven notoriously difficult to achieve such direct recognition of tumors (40, 41), despite the fact that such direct recognition is probably critical for anti-tumor CTL activity in vivo. Our earlier in vitro studies suggested that IL-12 produced by DC at the time of CTL sensitization endowed the T cells with the capacity to recognize both peptide-pulsed T2 targets and antigen-expressing tumor cells in 85% of the healthy donors tested, owing to enhancements in T cell functional avidity (39). These original in vitro sensitization studies using T cells and DC from healthy donors almost precisely anticipated the rates of sensitization leading to direct tumor recognition observed in this in vivo, neoadjuvant immunization trial using similar IL-12-secreting DCs.
One particularly interesting feature that could not be anticipated from our earlier in vitro work was the tendency of ICAIT-sensitized T cells to traffic to areas of disease. Although prior to ICAIT treatment, lymphocytes could be seen scattered about the breast tissues, they were rarely, if ever, observed within the DCIS lesions themselves. After ICAIT treatment, however, the number of lymphocytes in breast tissues greatly increased for many subjects. These cells tended to congregated densely about the DCIS-containing ducts, with CD8pos CTL even crossing the epithelial border and mingling with the actual tumor cells. Moreover, we observed CD8pos infiltrates even in cases where the treated subjects were HLA-A2.1neg, and therefore received neither the 369 nor the 689 peptide CTL epitopes as part of their immunization. Thus the presence of CTL within these tumors after immunization suggests either that stimulation of Th cells is somehow recruiting endogenously-sensitized CTL, or there are nested CTL epitopes within the promiscuous class II peptides that can be processed and cross-presented to CD8pos cells in the ICAIT-treated individual. In either case this bodes well for the sensitization of critical CTL even in instances where we cannot know and provide precise HLA class I-binding CTL epitopes as part of the ICAIT treatment administered to HLA-A2.1neg individuals.
Another feature of the ICAIT DC that warrants comment is their demonstrated “killer DC” function, especially in relation to the “gold standard” CMC-DC. It had been shown previously by others that human monocyte-derived DCs are capable of adopting a somewhat unexpected effector function characterized by the ability to induce apoptotic death in diverse tumor cells. This capacity could be induced by such activating agents as type I interferons (42) and double-stranded RNA (43). Similar findings were also reported with bacterial LPS acting on pre-committed peripheral blood DC (44), with further augmentation observed in combination with IFN-γ. The ICAIT DC in our study showed that this latter combination also efficiently induced killer DC function in monocyte-derived DC. Although we have not yet determined whether the killing is mediated through TNF, TRAIL or some other mechanism, this observation suggests additional therapeutic approaches incorporating ICAIT DC. For example, in addition to injecting ICAIT DC into lymph nodes for T cell sensitization and conditioning, they could conceivably be introduced to the site of disease (tumors). There, the activated DCs could serve as effectors to promote direct tumor killing, thereby releasing additional tumor antigens to further drive T cell sensitization and expansion. In addition, the secreted cytokines and chemokines made by ICAIT DC could serve to enhance target homing of the T lymphocytes sensitized in the lymph nodes. The locally-secreted products could also conceivably alter the microenvironment of the tumor itself to favor acute inflammation and tumor destruction.
In summary, the ICAIT approach utilizing LPS and IFN-γ-activated DC resulted in high rates T cell sensitization to synthetic HER-2/neu class I- and class II-restricted peptides. The sensitizations were particularly unusual in their durability (extending up to 52 months), their tendency to induce T cells capable of direct tumor recognition, and the capacity of ICAIT-sensitized lymphocytes to traffic to and penetrate sites of disease. These results, coupled with recent findings that LPS-activated mouse monocytes acquire most potent DC function (3) strongly suggest that LPS-activated monocyte-derived DCs should be further explored as platforms for inducing enhanced anti-tumor immunity in humans.
Acknowledgments
Supported by NIH R01 CA096997, the Harrington Foundation, Pennies-in-action.org, and the Mistler Foundation
This manuscript is dedicated to our friend Ursula Koldovsky. We also acknowledge the support of Jeanne Schueller, Robin Noel, the Transfusion Medicine Department, and the Cell Processing Facility of the Abramson Cancer Center.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 2.Rock FL, Hardiman G, Timans JC, et al. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95(2):588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheong C, Matos I, Choi JH, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinkernagel RM. Restriction by H-2 gene complex of transfer of cell mediated immunity to Listeria monocytogenes. Nature. 1974;251:230–234. doi: 10.1038/251230a0. [DOI] [PubMed] [Google Scholar]
- 5.Linsley PS, et al. T cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/B1. PNAS. 1990;87:5031–5037. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaliński P, Hilkens CM, Wierenga EA, et al. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20(12):561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 7.Kapsenberg ML, Hilkens CM, Wierenga EA, et al. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy. 1999;29 (Suppl 2):33–36. [PubMed] [Google Scholar]
- 8.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Langrish CL, McKenzie B, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitani G, Rinaldi A, Bertoni F, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow JC, Young DW, Golenbock DT, et al. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 13.Fisk B, Blevins TL, Wharton JT, et al. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181(6):2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kono K, Rongcun Y, Charo J, et al. Identification of HER2/neu-derived peptide epitopes recognized by gastric cancer-specific cytotoxic T lymphocytes. Int J Cancer. 1998;78(2):202–208. doi: 10.1002/(sici)1097-0215(19981005)78:2<202::aid-ijc14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5(6):1289–1297. [PubMed] [Google Scholar]
- 16.Czerniecki BJ, Koski GK, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 17.Czerniecki BJ, Carter C, Rivoltini L, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997;159(8):3823–3837. [PubMed] [Google Scholar]
- 18.Jonuleit H, Giesecke-Tuettenberg A, Tüting T, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93(2):243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 19.Schuler-Thurner B, Schultz ES, Berger TG, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195(10):1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger TG, Haendle I, Schrama D, et al. Circulation and homing of melanoma-reactive T cells to both cutaneous and visceral metastases after vaccination with monocyte-derived dendritic cells. Int J Cancer. 2004;111(2):229–237. doi: 10.1002/ijc.20238. [DOI] [PubMed] [Google Scholar]
- 21.Jonuleit H, Kühn U, Müller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Lenig D, Mackay CR, et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187(6):875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrew DP, Chang MS, McNinch J, et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161(9):5027–5038. [PubMed] [Google Scholar]
- 24.Ruppert J, Schütt C, Ostermeier D, et al. Down-regulation and release of CD14 on human monocytes by IL-4 depends on the presence of serum or GM-CSF. Adv Exp Med Biol. 1993;329:281–286. doi: 10.1007/978-1-4615-2930-9_47. [DOI] [PubMed] [Google Scholar]
- 25.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiertscher SM, Roth MD. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59(2):208–218. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93(6):2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinman RM, Inaba K. Myeloid dendritic cells. Leukoc Biol. 1999;66(2):205–208. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 30.Lyakh LA, Koski GK, Young HA, et al. Adenovirus type 5 vectors induce dendritic cell differentiation in human CD14(+) monocytes cultured under serum-free conditions. Blood. 2002;99(2):600–608. doi: 10.1182/blood.v99.2.600. [DOI] [PubMed] [Google Scholar]
- 31.Koski GK, Lyakh LA, Rice NR. Rapid lipopolysaccharide-induced differentiation of CD14(+) monocytes into CD83(+) dendritic cells is modulated under serum-free conditions by exogenously added IFN-gamma and endogenously produced IL-10. Eur J Immunol. 2001;31(12):3773–3781. doi: 10.1002/1521-4141(200112)31:12<3773::aid-immu3773>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Lyakh LA, Koski GK, Telford W, et al. Bacterial lipopolysaccharide, TNF-alpha, and calcium ionophore under serum-free conditions promote rapid dendritic cell-like differentiation in CD14+ monocytes through distinct pathways that activate NK-kappa B. J Immunol. 2000;165(7):3647–3655. doi: 10.4049/jimmunol.165.7.3647. [DOI] [PubMed] [Google Scholar]
- 33.Bedrosian I, Mick R, Xu S. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21(20):3825–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CS, Macatonia SE, Tripp CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 35.Barth RJ, Jr, Mulé JJ, Spiess PJ, et al. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med. 1991;173(3):647–645. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filatenkov AA, Jacovetty EL, Fischer UB, et al. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol. 2005;174(11):6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 37.Curtsinger JM, Lins DC, Johnson CM, et al. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J Immunol. 2005;175(7):4392–4399. doi: 10.4049/jimmunol.175.7.4392. [DOI] [PubMed] [Google Scholar]
- 38.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol. 2005;174(2):600–604. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 39.Xu S, Koski GK, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171(5):2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 40.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369-377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58(21):4902–4908. [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Young JW, Chapman PB, et al. Paucity of functional T-cell memory to melanoma antigens in healthy donors and melanoma patients. Clin Cancer Res. 2000;6(12):4831–4838. [PubMed] [Google Scholar]
- 42.Santini SM, Lapenta C, Logozzi M, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191(10):1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidalain PO, Azocar O, Yagita H, et al. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167(7):3765–3772. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 44.Manna PP, Mohanakumar T. Human dendritic cell mediated cytotoxicity against breast carcinoma cells in vitro. J Leukoc Biol. 2002;72(2):312–320. [PubMed] [Google Scholar]