Polarity-regulating Kinase Partitioning-defective 1b (PAR1b) Phosphorylates Guanine Nucleotide Exchange Factor H1 (GEF-H1) to Regulate RhoA-dependent Actin Cytoskeletal Reorganization (original) (raw)
Background: Polarity-regulating kinase PAR1b is also involved in regulation of the actin cytoskeleton.
Results: PAR1b inhibits RhoA activator GEF-H1 by inducing phosphorylation on S885 and S959.
Conclusion: PAR1b controls RhoA activity through phosphorylation-dependent regulation of GEF-H1.
Significance: PAR1b coordinates the microtubule- and actin-cytoskeletal systems in cell regulation.
Keywords: Actin, Cytoskeleton, Microtubules, Phosphorylation, Rho, GEF-H1, MARK2, PAR1b
Abstract
Partitioning-defective 1b (PAR1b), also known as microtubule affinity-regulating kinase 2 (MARK2), is a member of evolutionally conserved PAR1/MARK serine/threonine kinase family, which plays a key role in the establishment and maintenance of cell polarity at least partly by phosphorylating microtubule-associated proteins (MAPs) that regulate microtubule stability. PAR1b has also been reported to influence actin cytoskeletal organization, raising the possibility that PAR1b functionally interacts with the Rho family of small GTPases, central regulators of the actin cytoskeletal system. Consistent with this notion, PAR1 was recently found to be physically associated with a RhoA-specific guanine nucleotide exchange factor H1 (GEF-H1). This observation suggests a functional link between PAR1b and GEF-H1. Here we show that PAR1b induces phosphorylation of GEF-H1 on serine 885 and serine 959. We also show that PAR1b-induced serine 885/serine 959 phosphorylation inhibits RhoA-specific GEF activity of GEF-H1. As a consequence, GEF-H1 phosphorylated on both of the serine residues loses the ability to stimulate RhoA and thereby fails to induce RhoA-dependent stress fiber formation. These findings indicate that PAR1b not only regulates microtubule stability through phosphorylation of MAPs but also influences actin stress fiber formation by inducing GEF-H1 phosphorylation. The dual function of PAR1b in the microtubule-based cytoskeletal system and the actin-based cytoskeletal system in the coordinated regulation of cell polarity, cell morphology, and cell movement.
Introduction
Partitioning-defective 1 (PAR1)2 is a serine/threonine kinase that is highly conserved from yeast to mammals. PAR1 was originally isolated as one of the partitioning defective genes in Caenorhabditis elegans (1–3). In mammals, PAR1 was first identified as a microtubule affinity-regulating kinase (MARK) that phosphorylates microtubule-associated proteins (MAPs) and thereby destabilizes microtubules (4, 5). Mammalian PAR1/MARK comprises four isoforms, PAR1a/MARK3, PAR1b/MARK2, PAR1c/MARK1, and PAR1d/MARK4. As in the case of C. elegans and Drosophila, the mammalian PAR1 isoforms, especially PAR1b, act as key regulators for the development and maintenance of cell polarity in various cell systems (6, 7). During epithelial polarization, PAR1b, a major PAR1 isoform, specifically localizes to the baso-lateral membrane, whereas the atypical protein kinase C (aPKC)/PAR3/PAR6 complex localizes specifically to the apical membrane. The asymmetric distribution of these two kinases, PAR1b and aPKC/PAR3/PAR6 complex, ensures the apical-basal polarization of epithelial cells (7–9). Phosphorylation-dependent regulation of microtubule stability by PAR1 is thought to underlie the asymmetric distribution of molecules that mediates the establishment and maintenance of epithelial cell polarity (4, 5, 7, 8).
Actin filaments are key structures of the cytoskeleton in cells (10). The actin cytoskeleton is involved in many cellular processes, including cell integrity, cell mobility, cell shape, cell division, and contraction. Actin filaments consist of polarized ATP-bound globular actin monomers. These filamentous actin (F-actin) molecules can associate into bundles or networks through actin cross-linking molecules (11). The best characterized F-actin bundles and networks are filopodia, lamellipodia, and actin stress fibers. Formation of the different actin bundles and networks is regulated by signal transduction pathways that depend on Rho family GTPases, most notably RhoA, Rac, and Cdc42 (12–15). Constitutively activated Rac and Cdc42 induce surface protrusion (lamellipodia) with membrane ruffling and finger-like membrane extension (filopodia), respectively. In contrast, RhoA promotes the assembly of contractile actin and myosin filaments (stress fiber) and formation of cortical actin.
PAR1b has recently been reported to influence stress fiber formation (16). In human gastric epithelial cells, actin filaments are primarily organized into cortical F-actin bundles anchored to focal adhesions. Upon ectopic expression of PAR1b, however, cells display a marked decrease in cortical actin (16). This observation raises the possibility that PAR1b modulates the function of RhoA, which is positively regulated by guanine nucleotide exchange factors (GEFs) and negatively regulated by GTPase-activation proteins (GAPs) (17, 18). Among the various RhoA-specific GEFs, GEF-H1 is of particular interest because GEF activity is not only regulated by a polarity-dependent mechanism but also by GEF-H1-microtubule interaction in non-polarized cells (19–21). More recently, it has been shown that PAR1b forms a physical complex with GEF-H1 (22, 23). This finding indicates that there is a functional interplay between PAR1b and GEF-H1.
In this study, we investigated the role of PAR1b in the GEF-H1/RhoA signaling pathway and found that PAR1b inhibits RhoA-specific GEF activity of GEF-H1 through inducing phosphorylation. We also obtained evidence supporting the idea that PAR1b influences RhoA-mediated regulation of the actin cytoskeletal system via GEF-H1 phosphorylation.
EXPERIMENTAL PROCEDURES
Expression Vectors
T7-tagged human PAR1b and its kinase-dead mutant, PAR1b-K49R, were described previously (16, 24). To generate an siRNA-resistant PAR1b (siR-PAR1b) cDNA, 7 silent mutations were introduced using PCR-based site-directed mutagenesis, and the cDNA fragment encoding the mutant was cloned into pEF-His-A mammalian expression vector. Flag-tagged human GEF-H1 was described previously (22). Phosphorylation-resistant GEF-H1 mutants (GEF-H1-S885A, GEF-H1-S959A, GEF-H1-S885A/S959A), phospho-mimetic GEF-H1 mutants (GEF-H1-S885D, GEF-H1-S959D, GEF-H1-S885D/S959D) were generated from wild type GEF-H1 by using Chameleon site-directed mutagenesis (Stratagene), and cDNA fragments encoding the mutants were cloned into p3xFLAG-CMV10 mammalian expression vector. The pSP65SRα-derived mammalian expression vector for Myc-tagged RhoA-G17A was described previously (22). A pCS4-derived mammalian expression vector for Myc-tagged 14-3-3ζ was a kind gift from Dr. Yukiko Gotoh (University of Tokyo, Japan) (25).
Cells and Transfection
COS-7 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. AGS human gastric epithelial cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. COS-7 cells were transfected with expression vectors by calcium phosphate method as described previously (26). AGS cells were transfected with expression vectors or siRNA using Lipofectamine 2000 reagent (Invitrogen).
Antibodies
Anti-FLAG M2 monoclonal antibody (SIGMA) was used for immunoprecipitation, immunoblotting, and immunostaining. Anti-Myc antibody (9E10, Santa Cruz Biotechnology) and anti-T7 antibody (D-8) were used for immunoprecipitation and immunoblotting. Anti-PAR1b antibody (a kind gift from Dr. Shigeo Ohno at Yokohama City University, Japan), anti-T7 antibody (M-21, Santa Cruz Biotechnology), anti-actin antibody (C11, Santa Cruz Biotechnology), anti-RhoA antibody (clone 3L74, Upstate), and anti-14-3-3ζ antibody (C-16, Santa Cruz Biotechnology) were used for immunoblotting. Specificity of anti-PAR1b antibody and anti-14-3-3ζ antibody was shown in supplemental Fig. S1.
RNA Interference
PAR1b-specific siRNA #709 was used to knockdown human PAR1b as described previously (16). For control siRNA, luciferase siRNA (16) or PAR1b siRNA with two mutations (5′-GCGGAGAGUCAUUUGAUUACC-3′; mutations underlined, synthesized by Operon, Japan) was used.
Immunostaining
At 24 h after transfection, AGS cells were fixed with PHEM buffer (60 mm PIPES, 25 mm HEPES, 10 mm EGTA, 2 mm MgCl2, 4% paraformaldehyde, 0.15% glutaraldehyde, and 0.2% Tween 20) as previously described (16). Fixed samples were then treated with primary antibodies and were visualized with Alexa Fluor-conjugated secondary antibodies (Invitrogen). F-actin was stained with Alexa Fluor 546-phalloidin (Invitrogen). All samples were observed under a confocal microscope (TCS-SPE, Leica).
Immunoblotting and Immunoprecipitation
Cells were lysed in lysis buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5 mm EDTA, 1% Triton-X100, 10% glycerol) containing 2 mm Na3VO4, 10 mm NaF, 10 mm β-glycerophosphate, 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml trypsin inhibitor. Immunoprecipitation and immunoblotting were performed as described previously (26). Proteins were visualized by using Western blot chemiluminescence reagent (PerkinElmer Life Sciences).
RhoA Pull-down Assay
RhoA activity was determined by using Rho Assay Reagent kit according to the manufacturer's protocol (Millipore). Briefly, AGS cells were lysed in lysis buffer (25 mm HEPES, pH 7.5, 150 mm NaCl, 10 mm MgCl2, 1 mm EDTA, 1% Nonidet P-40, and 10% glycerol) containing 25 mm NaF, 1 mm Na3VO4, 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml trypsin inhibitor. Total cell lysates were incubated with GST-Rhotekin-RBD beads for 45 min and then subjected to SDS-PAGE and immunoblotting.
14-3-3 Binding Assay
14-3-3 binding assay was performed as previously described (27). Briefly, AGS cells were harvested at 24 h after transfection and lysed in lysis buffer (25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 1 mm EDTA, 0.1 mm EGTA, 1 mm dithiothreitol, 1% Nonidet P-40, and 10% glycerol) containing 2 mm Na3VO4, 25 mm NaF, 25 mm β-glycerophosphate, 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml trypsin inhibitor. Cell lysates were incubated with DYKDDDDK tag antibody beads (WAKO) for 3 h. The beads were incubated with DYKDDDDK peptide (final concentration of 150 μg/ml) (WAKO) for 30 min to elute Flag-tagged protein following the manufacturer's protocol. Samples were subjected to SDS-PAGE and immunoblotting.
RhoA G17A Affinity Binding Assay
RhoA G17A affinity binding assay was performed as previously described (28). COS-7 cells or AGS cells transfected with indicated vectors were harvested and lysed in lysis buffer (20 mm HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 1% Triton X-100, 1 mm dithiothreitol) containing 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml trypsin inhibitor. Total cell lysates were pulled down with RhoA G17A and were subjected to SDS-PAGE and immunoblotting.
In Vitro Kinase Assay
As a substrate of PAR1b, GEF-H1-derived peptide that contains amino acids surrounding serine 885 (RRPVDPRRRSLPAGDALYL) was synthesized (Operon, Japan). As a negative control, GEF-H1 peptide in which serine 885 was substituted to alanine was synthesized. PAR1b was purified from COS-7 cells transfected with T7-tagged PAR1b expression vector by immunoprecipitation with anti-T7 antibody. Kinase reaction was performed as previously described (24).
Quantification of Stress Fiber Formation
Stress fiber formation was quantified by using Image J software (NIH). Images obtained from F-actin staining were thresholded and converted to binary images. The percentage of pixels within transfected cells was calculated to obtain the ratio of F-actin-positive area to whole cell area. The threshold value was kept constant throughout the analysis of all cells.
Statistical Analysis
All data were evaluated by Student's t test. p < 0.05 was considered to be statistically significant.
RESULTS
PAR1b Inhibits RhoA-mediated Stress Fiber Formation
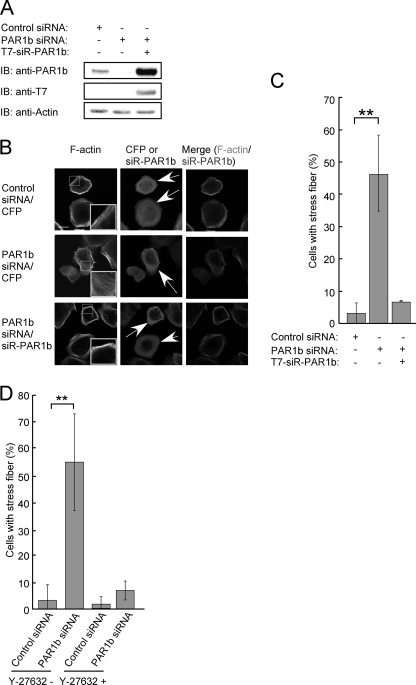
RhoA activation is critically involved in actin cytoskeleton reorganization (12, 14, 15). To investigate the functional interaction between PAR1b and RhoA, AGS human gastric epithelial cells were treated with control or PAR1b-specific siRNA. At 36 h after siRNA treatment, the efficiency of PAR1b knockdown was more than 80% as described previously (Fig. 1A and supplemental Fig. S2) (16). At this time point, siRNA-treated AGS cells were fixed and stained with phalloidin to visualize F-actin. In AGS cells treated with control siRNA, cortical actin was observed along the cell periphery (Fig. 1B). Upon PAR1b knockdown, AGS cells showed extensive stress fiber formation in the cytoplasm (Fig. 1B). To quantitate the effect of PAR1b knockdown on stress fiber formation, AGS cells were treated with control siRNA or PAR1b-specific siRNA and the number of cells showing stress fiber formation was counted. Inhibition of PAR1b expression substantially increased the number of stress fiber-positive cells (Fig. 1C). This increase in stress fiber formation by PAR1b-specific siRNA was reverted by ectopic expression of PAR1b, which is encoded by an siRNA-resistant PAR1b cDNA (siR-PAR1b) (Fig. 1, A/C), or by treatment of cells with Y-27632, a chemical reagent that inhibits ROCK, the downstream target of RhoA (Fig. 1D and supplemental Fig. S3). These observations indicated that PAR1b negatively regulates the activity of RhoA and thus inhibits RhoA-mediated stress fiber formation.
FIGURE 1.
PAR1b knockdown induces non-peripheral stress fiber formation. A, AGS cells were transfected with control or PAR1b siRNA in the presence or absence of a T7-tagged siRNA-resistant PAR1b (T7-siR-PAR1b) vector. At 36 h after transfection, cells were harvested and lysed. Total cell lysates (TCLs) were subjected to immunoblotting (IB) with indicated antibodies. B, AGS cells were co-transfected with a PAR1b-specific siRNA and a cyan fluorescent protein (CFP) vector or siR-PAR1b vector. For negative control, AGS cells were transfected with a control siRNA together with a CFP expression vector. At 36 h after transfection, stress fiber formation was analyzed. Confocal x-y plane views of F-actin and PAR1b staining. Arrows indicate CFP- or PAR1b-positive cells. The boxed regions are shown at higher magnification in the insets. Scale bar, 20 μm. C, percentage of cells showing stress fiber formation, which was calculated from images presented in Fig. 1B. Error bars represent mean ± S.D., n = 3. **, p < 0.01, Student's t test. D, AGS cells were co-transfected with control siRNA or PAR1b siRNA together with CFP vector. At 36 h after transfection, cells were treated with 3 μm Y-27632 or solvent control, water, for 15 min, before the analysis of stress fiber formation. Percentage of cells showing stress fiber formation was calculated from images presented in supplemental Fig. S2. Scale bar, 10 μm. Error bars represent mean ± S.D., n = 3. **, p < 0.01, Student's t test.
PAR1b Suppresses Stress Fiber Formation by Inhibiting GEF-H1
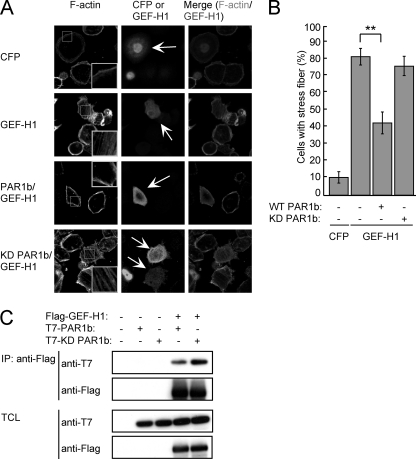
We previously reported that PAR1b physically interacts with the RhoA-specific guanine nucleotide exchange factor GEF-H1 (22). This observation raised the possibility that PAR1b influences RhoA via GEF-H1. To address this possibility, we investigated the effect of PAR1b on the ability of GEF-H1 to induce formation of stress fibers. To this end, AGS cells were transiently transfected with a control cyan fluorescent protein (CFP) vector or a Flag-tagged GEF-H1 vector together with a T7-tagged wild-type (WT) or a kinase-dead (KD) PAR1b vector (Fig. 2, A/B). Counting the number of cells showing stress fiber formation in each transfection group revealed that expression of GEF-H1 stimulated stress fiber formation, which was partially reverted by co-expression of wild-type PAR1b but not by co-expression of kinase-dead PAR1b. The possibility that PAR1b inhibited GEF-H1 activity as a result of physical complex formation was excluded by the observation that kinase-dead PAR1b, which failed to inhibit GEF-H1-dependent stress fiber formation, was still capable of binding with GEF-H1 (Fig. 2C). From these observations, we concluded that PAR1b suppresses the RhoA GEF activity of GEF-H1 and thereby impairs RhoA-dependent actin stress fiber formation.
FIGURE 2.
PAR1b inhibits stress fiber formation through GEF-H1. A, AGS cells were transfected with CFP or GEF-H1 vectors together with wild-type or kinase-dead (KD) PAR1b vector. At 24 h after transfection, stress fiber formation was analyzed. Confocal x-y plane views of F-actin and GEF-H1 staining. Arrows indicate CFP- or GEF-H1-positive cells. The boxed regions are shown at higher magnification in the insets. Scale bar, 10 μm. B, percentage of cells showing stress fiber formation, which was calculated from images presented in Fig. 2A. Error bars represent mean ± S.D., n = 3. **, p < 0.01, Student's t test. C, COS-7 cells were transfected with a Flag-tagged GEF-H1 or control vector together with a T7-tagged wild-type or kinase-dead PAR1b vector. At 24 h after transfection, Total cell lysates (TCLs) were prepared and subjected to immunoprecipitation (IP) with an anti-Flag antibody. The anti-Flag immunoprecipitates and TCLs were subjected to immunoblotting (IB) with the indicated antibodies.
Phosphorylation of GEF-H1 by PAR1b
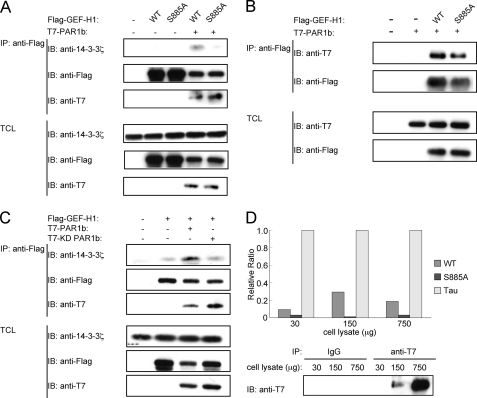
To elucidate the mechanism by which PAR1b suppresses GEF-H1-mediated RhoA activation, we hypothesized that PAR1b phosphorylates GEF-H1 upon complex formation and that this phosphorylation inhibits the RhoA-GEF activity of GEF-H1. To find a clue to the possible sites of GEF-H1 phosphorylation by PAR1b, we searched PhosphoSite (29), a protein phosphorylation data base, and found 36 reported phosphorylation sites in human GEF-H1 (supplemental Fig. S4). Among these sites, serine 885 (S885) and serine 959 (S959) have been shown to inhibit RhoA-GEF activity of GEF-H1 upon phosphorylation (30). To test if S885 undergoes phosphorylation in cells expressing PAR1, we made use of 14-3-3ζ, which specifically binds to GEF-H1 phosphorylated on S885 (27). AGS cells were transfected with a Flag-tagged wild-type GEF-H1 or GEF-H1-S885A vector with or without a T7-tagged wild-type PAR1b vector, and cell lysates prepared were immunoprecipitated with an anti-Flag antibody (Fig. 3A). The immunoprecipitates were then immunoblotted with an anti-14-3-3ζ antibody. The results of the experiment revealed that 14-3-3ζ bound to wild-type GEF-H1, but not to GEF-H1-S885A, when PAR1b was co-expressed (Fig. 3A). Of note, PAR1b formed a complex with GEF-H1-S885A as efficiently as did wild-type GEF-H1, indicating that PAR1b-GEF-H1 interaction is independent of the phosphorylation status of GEF-H1 on S885 (Fig. 3B). AGS cells were then transfected with the Flag-tagged GEF-H1 vector together with the T7-tagged wild-type PAR1b vector or a T7-tagged kinase-dead PAR1b vector. The cell lysates were immunoprecipitated with an anti-Flag antibody and the anti-Flag immunoprecipitates were immunoblotted with an anti-14-3-3ζ antibody. The results of the experiment showed that wild-type PAR1b potentiated the interaction of GEF-H1 with 14-3-3ζ, whereas kinase-dead PAR1b failed to do so (Fig. 3C). These observations collectively indicated that PAR1b induces phosphorylation of GEF-H1 on S885, which enables the interaction of GEF-H1 with 14-3-3ζ. It should be noted that although there is a commercially available anti-phospho S885 GEF-H1, the antibody did not specifically recognize GEF-H1 that was phosphorylated on S885 (data not shown). To investigate the possibility that S885 of GEF-H1 is directly phosphorylated by PAR1b, we performed an in vitro kinase assay. As shown in Fig. 3D, purified PAR1b was capable of phosphorylating a GEF-H1-derived 19-mer peptide containing S885 but not a peptide having S885A mutation. These results support the idea that PAR1b directly phosphorylates GEF-H1 on S885.
FIGURE 3.
PAR1b phosphorylates GEF-H1 at serine 885 in vitro. A, and B, AGS cells were transfected with a Flag-tagged wild-type (WT) GEF-H1 or GEF-H1-S885A vector with or without a T7-tagged PAR1b vector. At 24 h after transfection, Total cell lysates (TCLs) were prepared and subjected to immunoprecipitation (IP) with an anti-Flag antibody. The anti-Flag immunoprecipitates (IP) and TCLs were immunoblotted with indicated antibodies. C, AGS cells were transfected with a Flag-WT-GEF-H1 together with T7-WT-PAR1b or kinase-dead (KD) PAR1b vector. At 24 h after transfection, TCLs were prepared and subjected to immunoprecipitation (IP) with an anti-Flag antibody. The anti-Flag immunoprecipitates (IP) and TCLs were immunoblotted with the indicated antibodies. D, GEF-H1-derived peptides were subjected to an in vitro kinase assay with PAR1b immunopurified from COS-7 cells transfected with a T7-WT-PAR1b vector (upper). The anti-T7 immunoprecipitates were immunoblotted (IB) with anti-T7 antibody (lower).
Inhibition of RhoA GEF Activity of GEF-H1 through PAR1b-induced GEF-H1 Phosphorylation
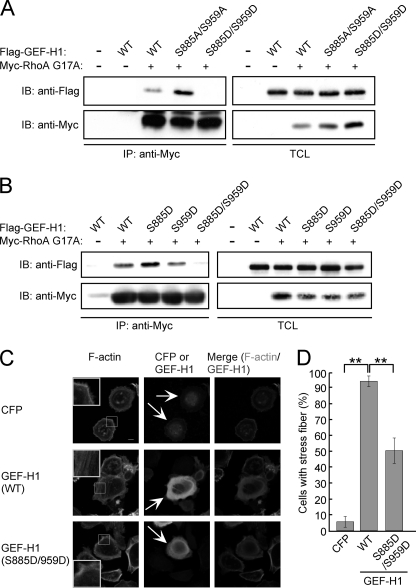
We next investigated whether phosphorylation status of GEF-H1 influences GEF-H1 activity toward RhoA. To this end, we generated a Flag-tagged GEF-H1 mutant in which S885 and S959 were replaced by non-phosphorylatable alanine residues (S885A/S959A). We also generated another GEF-H1 mutant in which S885 and S959 were replaced by phosphomimetic aspartic acid residues (S885D/S959D). COS-7 cells were transiently transfected with an expression vector for Flag-tagged GEF-H1-S885A/S959A or a Flag-tagged GEF-H1-S885D/S959D together with a Myc-tagged RhoA G17A vector. RhoA G17A is a nucleotide-free RhoA mutant that mimics the intermediate state of RhoA during nucleotide exchange. Because activated RhoA-GEF stably interacts with the nucleotide-free RhoA, the RhoA G17A mutant has high binding affinity toward activated form of RhoA-specific GEFs such as GEF-H1 (28). Cell lysates prepared from transfected COS-7 cells were immunoprecipitated with an anti-Myc antibody and were then subjected to immunoblotting with an anti-Flag antibody. The results of the experiment revealed that wild-type GEF-H1 interacted with RhoA G17A and this interaction was potentiated in the case of the GEF-H1-S885A/S959A mutant (Fig. 4A). On the other hand, the phosphomimetic GEF-H1-S885D/S959D mutant displayed a dramatic reduction in its binding-activity toward RhoA G17A, indicating that its RhoA-GEF function was substantially impaired (Fig. 4A). Next, to determine the site(s) of GEF-H1 phosphorylation that is required for suppressing its RhoA-GEF activity, COS-7 cells were transfected with the Myc-tagged RhoA G17A vector together with the Flag-tagged wild-type GEF-H1 vector, Flag-tagged GEF-H1-S885D vector, Flag-tagged GEF-H1-S959D vector, or Flag-tagged GEF-H1-S885D/S959D vector, and the cell lysates were immunoprecipitated with an anti-Myc antibody. The anti-Myc immunoprecipitates were then immunoblotted with an anti-Flag antibody. In contrast to the GEF-H1-S885D/S959D double mutant, the GEF-H1-S885D or GEF-H1-S959D single mutant retained the ability to bind to RhoA G17A (Fig. 4B). The results indicated that phosphorylation at both S885 and S959 is required for the inactivation of the RhoA-GEF activity of GEF-H1. We also investigated the role of GEF-H1 phosphorylation/dephosphorylation on S885 and S959 in the induction of actin stress fiber formation by transiently expressing wild-type GEF-H1 or the phosphomimetic GEF-H1-S885D/S959D mutant in AGS cells. Cells expressing wild-type GEF-H1 showed stress fiber formation, whereas cells expressing GEF-H1-S885D/959D showed much less activity to induce stress fiber formation (Fig. 4, C/D).
FIGURE 4.
Phosphorylation of both serine 885 and serine 959 in GEF-H1 inhibits RhoA-GEF activity of GEF-H1. A and B, RhoA-GEF activity was analyzed by RhoA G17A affinity binding assay. COS-7 cells were co-transfected with Myc-tagged RhoA G17A and the indicated GEF-H1 vectors. Total cell lysates (TCLs) were immunoprecipitated with an anti-Myc antibody. Immunoprecipitates (IP) and TCLs were immunoblotted with indicated antibodies. C, AGS cells were transfected with the indicated vectors. Confocal x-y plane views of F-actin and GEF-H1 staining. Arrows indicate CFP- or GEF-H1-positive cells. The boxed regions are shown at higher magnification in the insets. Scale bar, 10 μm. D, percentage of cells showing stress fiber formation was calculated from images presented in Fig. 4C. Error bars represent mean ± S.D., n = 3. **, p < 0.01, Student's t test.
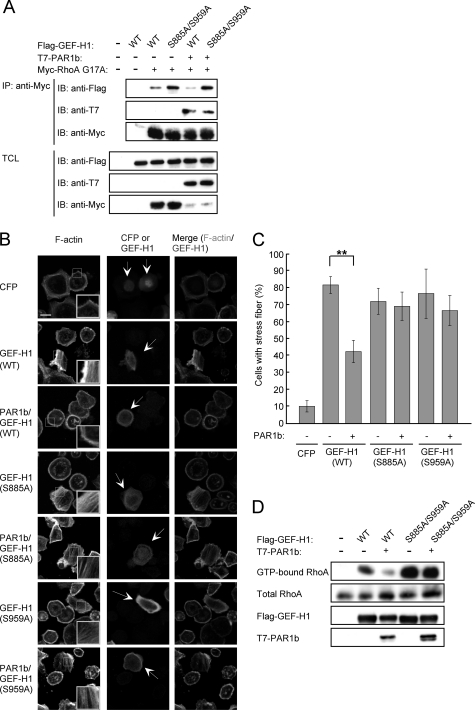
PAR1b-induced Phosphorylation of GEF-H1 on S885 and S959 Inhibits GEF-H1-mediated RhoA Activation
To substantiate the involvement of PAR1b in the suppression of RhoA-GEF activity of GEF-H1 through phosphorylation, COS-7 cells were co-transfected with the T7-tagged PAR1b vector and Myc-tagged RhoA G17A vector together with the Flag-tagged GEF-H1 vector or Flag-tagged GEF-H1-S885A/S959A vector. Cell lysates were immunoprecipitated with an anti-Myc antibody and subjected to immunoblotting with an anti-Flag antibody. The results of the experiment revealed that expression of PAR1b reduced wild-type GEF-H1 binding affinity toward RhoA G17A. On the other hand, PAR1b did not affect binding activity of GEF-H1-S885A/S959A toward RhoA G17A. Hence, PAR1b inhibits RhoA-GEF activity of GEF-H1 by inducing phosphorylation at both S885 and S959 (Fig. 5A). Given this, we next wished to know the effect of PAR1b-mediated GEF-H1 phosphorylation on stress fiber formation. To this end, AGS cells were transfected with wild-type GEF-H1, GEF-H1-S885A or GEF-H1-S959A vector together with a wild-type PAR1b vector. As described above, expression of GEF-H1 induced stress fiber formation, which was inhibited by co-expression of PAR1b. In contrast, induction of stress fibers by the expression of GEF-H1-S885A or GEF-H1-S959A mutant was not impaired by PAR1b co-expression (Fig. 5, B/C), consistent with the conclusion that PAR1b-mediated phosphorylation of both S885 and S959 is required for inactivation of GEF-H1. To confirm that the change in actin stress fiber formation was due to activation/inactivation of RhoA, we determined the levels of active RhoA by using a Rhotekin RBD pull-down assay. As expected, GEF-H1 expression increased the active form (GTP-bound form) of RhoA, which was significantly decreased upon co-expression of PAR1b. In contrast, the amount of active RhoA in GEF-H1-S885A/S959A-expressing cells was not affected by co-expressed PAR1b (Fig. 5D). These results collectively indicated that phosphorylation of GEF-H1 on both S885 and S959 is necessary for PAR1b to regulate RhoA-mediated stress fiber formation.
FIGURE 5.
The phosphorylation at both serine 885 and serine 959 in GEF-H1 is required for the PAR1b-dependent inhibition of GEF-H1 activity. A, RhoA-GEF activity was analyzed by RhoA G17A affinity binding assay. COS-7 cells were co-transfected with Myc-RhoA G17A, PAR1b, and the indicated GEF-H1 vectors. Total cell lysates (TCLs) were immunoprecipitated with anti-Myc antibody. Immunoprecipitates (IP) and TCLs were immunoblotted (IB) with the indicated antibodies. B, AGS cells were transfected with a CFP, wild-type (WT) GEF-H1, GEF-H1-S885A, or GEF-H1-S959A vector together with or without a PAR1b vector. Stress fiber formation was analyzed at 24 h after transfection. Confocal x-y plane views of F-actin and GEF-H1 staining. Arrows indicate CFP- or GEF-H1-positive cells. The boxed regions are shown at higher magnification in the insets. Scale bar, 20 μm. C, percentage of cells showing stress fiber formation was calculated from Fig. 5B. Error bars represent mean ± S.D., n = 3. **, p < 0.01, Student's t test. D, RhoA activity was analyzed by a GST-RBD-Rhotekin pull down assay. AGS cells co-transfected with PAR1b and the indicated GEF-H1 vectors were lysed and subjected to precipitation with GST-RBD-Rhotekin beads. Precipitates and TCLs were immunoblotted with the indicated antibodies.
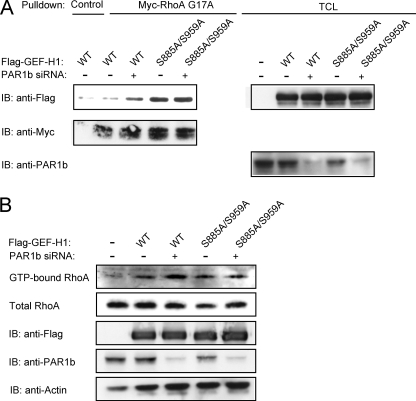
Endogenous PAR1b Inhibits GEF-H1 through Phosphorylation
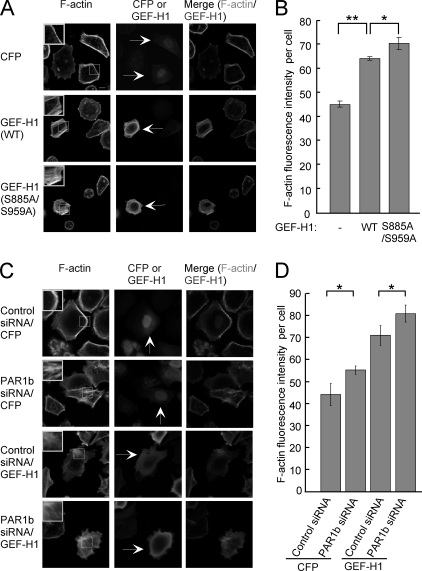
Given the above-described observations, we wished to know if endogenous PAR1b regulates RhoA activity through phosphorylation of GEF-H1. To this end, we compared the activation status of GEF-H1/RhoA signaling in AGS cells transfected with wild-type GEF-H1 or with GEF-H1-S885A/S959A together with PAR1b-specific siRNA (Fig. 6A). The RhoA G17A binding assay showed that the amount of active GEF-H1 was increased when PAR1b was knocked down in wild-type GEF-H1-expressing cells. In contrast, the amount of active GEF-H1 in cells expressing GEF-H1-S885A/S959A was not affected by PAR1b knockdown. In this experiment, we also determined the amount of active RhoA by a Rhotekin RBD pull-down assay and found that the level of active RhoA was increased when PAR1b was knocked down in cells expressing wild-type GEF-H1 but not in cells expressing GEF-H1-S885A/S959A (Fig. 6B). Additionally, we compared the intensity of stress fiber formation in AGS cells expressing wild-type GEF-H1 and that in AGS cells expressing the GEF-H1-S885A/S959A mutant. The number of wild-type GEF-H1-expressing cells and that of GEF-H1-S885A/S959A-expressing cells showing stress fiber formation were not significantly different (data not shown). However, the intensity of stress fiber formation, which was determined by F-actin fluorescence intensity in each cell, was significantly greater in cells expressing GEF-H1-S885A/S959A than in cells expressing wild-type GEF-H1 (Fig. 7, A/B). To know if the difference was due to differential degree of GEF-H1 phosphorylation on S885 and S959 by endogenous PAR1b, we next compared the magnitude of stress fiber assembly in AGS cells expressing wild-type GEF-H1 in the presence or absence of PAR1b-specific siRNA (Fig. 7, C/D). Hence, stress fiber formation was substantially increased following inhibition of endogenous PAR1b expression. From these observations, we concluded that endogenous PAR1b-mediated phosphorylation of GEF-H1 on S885 and S959 represses RhoA-dependent stress fiber formation in cells.
FIGURE 6.
Endogenous PAR1b suppresses RhoA activity through GEF-H1 phosphorylation. A, RhoA-GEF activity was analyzed by RhoA G17A affinity binding assay. AGS cells co-transfected with PAR1b siRNA and indicated GEF-H1 vectors were lysed, and Total cell lysates (TCLs) were pulled down with Myc-RhoA G17A. Precipitates and TCLs were immunoblotted (IB) with indicated antibodies. B, RhoA activity was determined by a GST-RBD-Rhotekin pull down assay. AGS cells co-transfected with PAR1b siRNA and the indicated GEF-H1 vector were lysed and subjected to precipitation with GST-RBD-Rhotekin beads. Precipitates and TCLs were immunoblotted (IB) with indicated antibodies.
FIGURE 7.
Endogenous PAR1b suppresses RhoA-mediated stress fiber formation via GEF-H1. AGS cells were transfected with the indicated vectors. A, confocal x-y plane views of F-actin and GEF-H1 staining. Arrows indicate CFP- or GEF-H1-positive cell. The boxed regions are shown at higher magnification in the insets. Scale bar, 10 μm. B, stress fiber formation was quantified by measuring F-actin fluorescence intensity per cell. Error bars represent mean ± S.D., n = 3. **, p < 0.01; *, p < 0.05, Student's t test. C, confocal x-y plane views of F-actin and GEF-H1 staining. Arrows indicate CFP-positive cells or GEF-H1-positive cells. The boxed regions are shown at higher magnification in the insets. Scale bar, 10 μm. D, stress fiber formation was quantified by measuring F-actin fluorescence intensity per cell. Error bars represent mean ± S.D., n = 3. *, p < 0.05, Student's t test.
DISCUSSION
In this work, we found that the polarity-regulating serine/threonine kinase PAR1b induces phosphorylation of RhoA activator GEF-H1 on S885 and S959. This PAR1b-induced GEF-H1 phosphorylation inhibits the RhoA-specific nucleotide exchange activity of GEF-H1, which results in the suppression of actin stress fiber formation by RhoA.
The molecular basis for the establishment and maintenance of cell polarity remains largely unknown. Nevertheless, it is reasonable to assume that the organization of actin and microtubule cytoskeletons and the direction of vesicular transport pathways are key events in this process. Our results support a role of PAR1b in the regulation of actin cytoskeleton via the GEF-H1/RhoA signaling pathway. Given that other PAR1 members also interact with GEF-H1 (22, 23), it is likely that all of the PAR1 isoforms redundantly regulate GEF-H1. However, the observation that treatment of cells with PAR1b-specific siRNA potentiates stress fiber assembly suggests that PAR1b is a major regulator of GEF-H1 among the PAR1 members in epithelial cells. The present study revealed that PAR1b phosphorylates GEF-H1 to restrict RhoA activity below a certain threshold, thereby ensuring an adequate level of stress fiber formation.
GEF-H1 activity is controlled by two distinct mechanisms (21). First, binding with microtubules in the cytoplasm inhibits GEF-H1 activity. Second, phosphorylation regulates the nucleotide exchange activity of GEF-H1. Surprisingly, GEF-H1 can undergo phosphorylation at 36 residues (including serine, threonine and tyrosine residues by PhosphoSite), indicating complicated regulation of GEF-H1 activity by differential phosphorylation (27, 30). For instance, PAK-1 phosphorylates GEF-H1 at S885 and this phosphorylation induces recruitment of GEF-H1 to microtubules through binding to 14-3-3, which may also inhibit RhoA GEF activity of GEF-H1 (27). Early in mitosis, Aurora A/B kinase and cyclin B1/Cdk1 phosphorylate S885 and S959, respectively, on GEF-H1 and thereby inhibit GEF-H1 catalytic activity (30). Dephosphorylation of GEF-H1 during telophase triggers RhoA activation, which provokes cleavage furrow formation and ingression during cytokinesis. These observations together with results of the present work suggest that S885 and S959 behave as inhibitory switches of GEF-H1, which is targeted by multiple distinct kinases in a context-dependent manner. In contrast, ERK1 and ERK2 phosphorylate GEF-H1 at Thr-678 and this phosphorylation stimulates the ability of GEF-H1 to activate RhoA (31).
How is GEF-H1 inhibited by PAR1b? In the present study, we obtained evidence that S885 is phosphorylated by PAR1b by demonstrating that co-expression with PAR1b induced interaction of GEF-H1 with 14-3-3ζ, which specifically binds to GEF-H1 that has been phosphorylated on S885. Because 14-3-3 binding causes conformational change of its target protein, modulates the activity of its target protein, and changes subcellular localization of its target protein (32), it is possible that the reduction of GEF-H1 catalytic activity by PAR1b may involve 14-3-3ζ binding induced by S885 phosphorylation. However, as shown in Fig. 4, we found that a phosphomimic mutant of GEF-H1 (S885D/S959D), which should not bind 14-3-3, lost RhoA-GEF activity. It therefore seems that S885/S959 phosphorylation on its own is sufficient to inactivate GEF-H1. Whereas PAR1b can directly phosphorylate GEF-H1 on S885, the kinase that phosphorylates S959 upon PAR1b expression is not known. At present, the possibility that PAR1b directly phosphorylates S959 as well remains.
The PAR1b-aPKC system is not only required for the establishment/maintenance of apical-basal polarity but also involved in front-rear polarity (33, 34). However, the actual role of PAR1b in front-rear polarity has yet to be elucidated. Our finding that PAR1b inhibits GEF-H1-mediated RhoA activation may provide clues to this question. Given that RhoA is transiently activated during rear retraction (35), PAR1b-mediated regulation of the GEF-H1/RhoA signaling pathway may indeed contribute to the regulation of rear retraction. Notably, we previously reported that H. pylori virulence factor CagA, which is delivered into gastric epithelial cells, causes deregulation of SHP2 phosphatase and at the same time inhibits the kinase activity of PAR1b, thereby inducing an extremely elongated cell shape termed the hummingbird phenotype. Given that the hummingbird phenotype is characterized by a rear retraction defect (16, 36), the morphological change may be due at least in part to the loss of “front-rear” polarity as a result of PAR1b inhibition by CagA. This notion is supported by the observation that induction of the hummingbird phenotype by CagA is counteracted by elevated PAR1b (16, 24).
While this report was in preparation, Yoshimura and Miki reported that PAR1b phosphorylates GEF-H1 on S143, S172, and S186 (37). This phosphorylation causes dissociation of GEF-H1 from microtubules, which could activate GEF-H1. Their observation is inconsistent with the results of the present study. However, we speculate that PAR1b has two distinct roles in the regulation of GEF-H1 activity: it alters subcellular localization of GEF-H1 via N-terminal phosphorylation (S143/S172/S186) and at the same time it regulates nucleotide exchange activity of RhoA-specific GEF-H1 via C-terminal phosphorylation (S885 and S959). As a result, phosphorylation of GEF-H1 at multiple sites by PAR1b has both positive and negative effects on GEF-H1 activity. We suspect that dissociation of GEF-H1 from microtubules, which is caused via its N-terminal phosphorylation by PAR1b, is required but not sufficient for the activation of GEF-H1 as long as both S885 and S959 are phosphorylated. In this scenario, stimulation of RhoA GEF activity of GEF-H1 additionally requires dephosphorylation of GEF-H1 on S885 or S959 by cellular phosphatases.
Our findings reveal a dual role of PAR1b in regulation of the microtubule-based cytoskeletal system and the actin-based cytoskeletal system. In particular, spatiotemporal regulation of PAR1b/GEF-H1/RhoA signaling may play an important role in the coordination of cell polarity, cell morphology, and cell movement.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Dr. Shigeo Ohno for providing anti-PAR1b antibody. We also thank Dr. Yukiko Gotoh for providing 14-3-3ζ vector.
*
This work was supported by Grants-in-Aid for the Scientific Research on Innovative Area from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
2
The abbreviations used are:
PAR1
partitioning-defective 1
MARK
microtubule affinity-regulating kinase
GEF
guanine nucleotide exchange factor.
REFERENCES
- 1.Kemphues K. J., Priess J. R., Morton D. G, Cheng N. S. (1988) Cell 52, 311–320 [DOI] [PubMed] [Google Scholar]
- 2.Watts J. L., Etemad-Moghadam B., Guo S., Boyd L., Draper B. W., Mello C. C., Priess J. R., Kemphues K. J. (1996) Development 122, 3133–3140 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein B., Macara I. G. (2007) Dev. Cell 13, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drewes G., Ebneth A., Preuss U., Mandelkow E. M., Mandelkow E. (1997) Cell 89, 297–308 [DOI] [PubMed] [Google Scholar]
- 5.Ebneth A., Drewes G., Mandelkow E. M., Mandelkow E. (1999) Cell Motil. Cytoskeleton 44, 209–224 [DOI] [PubMed] [Google Scholar]
- 6.Benton R., St. Johnston D. (2003) Cell 115, 691–704 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A., Hirata M., Kamimura K., Maniwa R., Yamanaka T., Mizuno K., Kishikawa M., Hirose H., Amano Y., Izumi N., Miwa Y., Ohno S. (2004) Curr. Biol. 14, 1425–1435 [DOI] [PubMed] [Google Scholar]
- 8.Hurov J. B., Watkins J. L., Piwnica-Worms H. (2004) Curr. Biol. 14, 736–741 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki A., Ohno S. (2006) J. Cell Sci. 119, 979–987 [DOI] [PubMed] [Google Scholar]
- 10.Pellegrin S., Mellor H. (2007) J. Cell Sci. 120, 3491–3499 [DOI] [PubMed] [Google Scholar]
- 11.Bugyi B., Carlier M. F. (2010) Annu. Rev. Biophys. 39, 449–470 [DOI] [PubMed] [Google Scholar]
- 12.Ridley A. J., Hall A. (1992) Cell 70, 389–399 [DOI] [PubMed] [Google Scholar]
- 13.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 14.Tapon N., Hall A. (1997) Curr. Opin. Cell Biol. 9, 86–92 [DOI] [PubMed] [Google Scholar]
- 15.Ridley A. J. (2006) Trends Cell Biol. 16, 522–529 [DOI] [PubMed] [Google Scholar]
- 16.Lu H. S., Murata-Kamiya N., Saito Y., Hatakeyama M. (2009) J. Biol. Chem. 284, 23024–23036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burridge K., Wennerberg K. (2004) Cell 116, 167–179 [DOI] [PubMed] [Google Scholar]
- 18.Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 19.Krendel M., Zenke F. T., Bokoch G. M. (2002) Nat. Cell Biol. 4, 294–301 [DOI] [PubMed] [Google Scholar]
- 20.Aijaz S., D'Atri F., Citi S., Balda M. S., Matter K. (2005) Dev. Cell 8, 777–786 [DOI] [PubMed] [Google Scholar]
- 21.Birkenfeld J., Nalbant P., Yoon S. H., Bokoch G. M. (2008) Trends Cell Biol. 18, 210–219 [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Murata-Kamiya N., Hirayama T., Ohba Y., Hatakeyama M. (2010) J. Exp. Med. 207, 2157–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brajenovic M., Joberty G., Küster B., Bouwmeester T., Drewes G. (2004) J. Biol. Chem. 279, 12804–12811 [DOI] [PubMed] [Google Scholar]
- 24.Saadat I., Higashi H., Obuse C., Umeda M., Murata-Kamiya N., Saito Y., Lu H., Ohnishi N., Azuma T., Suzuki A., Ohno S., Hatakeyama M. (2007) Nature 447, 330–333 [DOI] [PubMed] [Google Scholar]
- 25.Sunayama J., Tsuruta F., Masuyama N., Gotoh Y. (2005) J. Cell Biol. 170, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashi H., Tsutumi R., Muto S., Sugiyama T., Azuma T., Asaka M., Hatakeyama M. (2002) Science 295, 683–686 [DOI] [PubMed] [Google Scholar]
- 27.Zenke F. T., Krendel M., DerMardirossian C., King C. C., Bohl B. P., Bokoch G. M. (2004) J. Biol. Chem. 279, 18392–18400 [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Mata R., Winnerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., Burrige K. (2006) Methods Enzymol. 406, 425–437 [DOI] [PubMed] [Google Scholar]
- 29.Hornbeck P. V., Chabra I., Kornhauser J. M., Skrzypek E., Zhang B. (2004) Proteomics 4, 1551–1561 [DOI] [PubMed] [Google Scholar]
- 30.Birkenfeld J., Nalbant P., Bohl B. P., Pertz O., Hahn K. M., Bokoch G. M. (2007) Dev. Cell 12, 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujishiro S. H., Tanimura S., Mure S., Kashimoto Y., Watanabe K., Kohno M. (2008) Biochem. Biophys. Res. Commun. 368, 162–167 [DOI] [PubMed] [Google Scholar]
- 32.Bridges D., Moorhead G. B. (2005) Sci. STKE 296, re10. [DOI] [PubMed] [Google Scholar]
- 33.McCaffrey L. M., Macara I. G. (2009) Cold Spring Harb. Perspect. Biol. 1, a001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etienne-Manneville S., Hall A. (2003) Curr. Opin. Cell Biol. 15, 67–72 [DOI] [PubMed] [Google Scholar]
- 35.Pertz O., Hodgson L., Klemke R. L., Hahn K. M. (2006) Nature 440, 1069–1072 [DOI] [PubMed] [Google Scholar]
- 36.Bourzac K. M., Botham C. M., Guillemin K., (2007) Infect. Immun. 75, 1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura Y., Miki H. (2011) Biochem. Biophys. Res. Commun. 408, 322–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data