Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response (original) (raw)
Abstract
Implantation of biomaterials and devices into soft tissues leads to the development of the foreign body response (FBR), which can interfere with implant function and eventually lead to failure. The FBR consists of overlapping acute and persistent inflammatory phases coupled with collagenous encapsulation and currently there are no therapeutic options. Initiation of the FBR involves macrophage activation, proceeding to giant cell formation, fibroblast activation, and collagen matrix deposition. Despite the recognition of this sequence of events, the molecular pathways required for the FBR have not been elucidated. We have identified that the acute inflammatory response to biomaterials requires nucleotide-binding domain and leucine-rich repeat-containing 3 (Nlrp3), apoptosis-associated speck-like protein containing CARD (Asc), and caspase-1, as well as plasma membrane cholesterol, and Syk signaling. Full development of the FBR is dependent on Asc and caspase-1, but not Nlrp3. The common antiinflammatory drug aspirin can reduce inflammasome activation and significantly reduce the FBR. Taken together, these findings expand the role of the inflammasome from one of sensing damage associated molecular patterns (DAMPs) to sensing all particulate matter irrespective of size. In addition, implication of the inflammasome in biomaterial recognition identifies key pathways, which can be targeted to limit the FBR.
The use of biomaterials is an established part of medical practice and such materials range from a single material such as silicone for breast implants to combinations of materials such as in sensors for measuring glucose concentration (1). The utility of implants and devices using biomaterials is limited, due to the development of the foreign body reaction (FBR), which is initially an acute sterile inflammatory response, subsequently overlapping with a chronic fibrotic response (2). Hallmarks of the FBR include accumulation of macrophages at the tissue–implant interface, formation of foreign body giant cells (FBGCs), and deposition of a dense layer of collagenous matrix that isolates the implant. The clinical consequences of the FBR include pain, scarring, and for some devices such as glucose sensors, device failure due the development of fibrous encapsulation. Macrophage activation and fusion have been identified as critical cellular events in the FBR and recent studies have identified key molecular events in the formation of FBGCs, including induction of E-cadherin, Rac1 activation, and secretion of matrix metalloproteinase-9 (MMP-9) (3). However, the initial critical events in macrophage–biomaterial interactions and the elicited downstream intracellular events have not been identified. Our current understanding of this process involves surface adsorption of proteins present in edematous interstitial fluid, such as fibrinogen, which causes their denaturation and renders them adhesive for inflammatory cells (4). For example, exposure of the cryptic integrin-specific epitopes P1 and P2 in fibrinogen has been shown to influence the accumulation of inflammatory cells in short-term in vivo studies (5). In addition, surface-induced activation of complement has been shown to occur and enhance biomaterial–inflammatory cell interactions (6, 7). However, modulation of these interactions has not been shown to lead to long-lasting attenuation of the FBR.
In addition to macrophages, dendritic cells (DCs) have been implicated in the foreign body response, primarily due to a combination of products that present antigenic stimuli (8). Moreover, an in vitro study has shown that DC–biomaterial interactions can occur via engagement of multiple Toll-like receptors (TLRs) and lead to significant induction of IL-6 and regulated upon activation, normal T-cell expressed, and secreted (RANTES) and mild induction of IL-1β and TNF-α (9). These observations raise the possibility that DCs can also participate in the recognition of biomaterials and serve as stimulators of the foreign body response. However, to date the presence of DCs at the tissue–biomaterial interface in vivo has not been documented (8, 9). Nevertheless, it is possible that DCs play a critical role in modulating cross-talk between innate and adaptive immunity, especially when engineered constructs contain immunogenic signals.
There has been rapid recognition of the role of a set of cytosolic proteins termed the inflammasomes in initiation of the inflammatory response to crystalline materials naturally found in vivo, including uric acid and cholesterol (10–12). In addition to these biologically formed materials, other small particulates such as alum, silica, asbestos, and nanoparticles have been shown to result in inflammasome activation (13–15). These materials have very different physical characteristics but are small enough to be phagocytosed, and subsequent phagosome rupture has been shown to result in inflammasome activation and production of IL-1β and IL-18 (16). An alternative mechanism of inflammasome activation by particulate matter has recently been demonstrated. This mechanism depends on reorganization of cholesterol rafts by interaction of the particulate matter with the plasma membrane, resulting in Syk activation. The presence of an inflammasome-activating pathway independent of phagocytosis prompted us to test the ability of biomaterials to activate the inflammasome (17). In addition, we investigated the dependence of the FBR on individual components of the inflammasome. Using biomaterials that are too large to be phagocytosed, we investigated the role of the inflammasome in phagocytosis-independent, biomaterial-induced inflammation and the FBR.
Results
Inflammasome Components Nlrp3, Asc, and Caspase-1 Are Required for the Acute Inflammatory Response to Biomaterials.
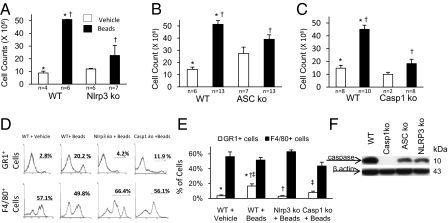
To investigate the acute inflammatory response to biomaterials we injected sterile microspheres of poly(methyl methacrylate) (PMMA) with a mean diameter of 153 μm into the peritoneal cavity of wild-type mice and quantified the inflammatory infiltrate 24 h later. The cell count in the lavage fluid of mice injected with microspheres was 5.09 × 107, which is significantly greater (P < 0.05) than of control mice injected with vehicle only (8.79 × 106 cells), demonstrating an acute inflammatory response (Fig. 1_A_). The same experiment was repeated in mice with genetic deletion of nucleotide-binding domain and leucine-rich repeat-containing 3 (Nlrp3), apoptosis-associated speck-like protein containing CARD (Asc), and caspase-1. In comparison with wild-type mice, mice lacking Nlrp3, Asc, or caspase-1 displayed reduced cell numbers that were similar to control mice injected with vehicle (Fig. 1 A_–_C). Findings in mice deficient in Nlrp3, Asc, or caspase-1 suggest that inflammasome activation plays a role in the initial phases of the inflammatory response to biomaterials. Flow cytometric analysis of the peritoneal lavage fluid showed that in wild-type mice injected with microspheres there was a relative increase in Gr1+ neutrophils, in comparison with F4/80 macrophages, although both cell populations had an absolute increase in numbers (Fig. 1_D_). In mice lacking Nlrp3 or caspase-1, the relative increase in Gr1+ neutrophils was significantly reduced (Fig. 1_E_). To directly confirm the occurrence of caspase-1 activation in response to biomaterials, we performed Western blot analysis of peritoneal cell lysates from wild-type and caspase-1 KO mice injected with microspheres, which showed cleavage of caspase-1 in the former (Fig. 1_F_). We further tested caspase-1 activation in Nlrp3 and Asc KO mice and found that caspase-1 cleavage did occur, although to a much lesser degree than in wild-type mice. This suggests that although Nlrp3 and Asc play a required role, additional molecules proximal to caspase-1 can respond to biomaterials and participate in the partial activation of caspase-1.
Fig. 1.
PMMA microspheres cause an acute inflammatory infiltrate, which is dependent on Nlrp3, Asc, and caspase-1. (A_–_C) Reduction in peritoneal infiltrate in Nlrp3, Asc, and caspase-1 KO mice compared with wild-type (WT) controls. (D) Relative increase in neutrophils in WT mice in response to microspheres. (E) Reduced neutrophil infiltrate in Nlrp3 KO and caspase-1 KO mice compared with WT. (F) Western blot showing specific caspase-1 activation in protein lysates from the inflammatory infiltrate elicited by microspheres from wild-type mice, with reduction in Nlrp3 and Asc KO mice. *†‡P ≤ 0.05.
Inflammasome Activation by the Microspheres Can Be Mediated Through Clustering of Lipid Rafts.
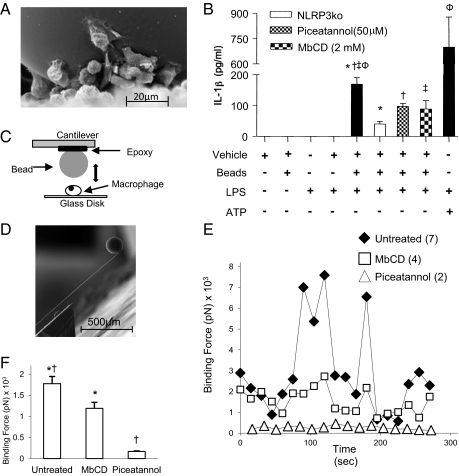
Internalization of crystals has been demonstrated as one mechanism of inflammasome activation, however the microspheres used in the present study were too large to be phagocytosed (Fig. 2_A_). Inflammasome activation can also occur by membrane affinity triggered signaling (MATS), which involves clustering of lipid rafts at the site of contact, resulting in nonspecific cytosolic aggregation of immunoreceptor tyrosine-based activation motif (ITAM) signaling domains and activation of a Syk kinase pathway (17). The involvement of this mechanism can be examined by depleting plasma cholesterol with methyl-β-cyclodextrin (MβCD) or by inhibiting Syk activation with piceatannol. To do this an in vitro system was established, using peritoneal cells isolated from thioglycolate-primed mice. Inflammasome activation was assayed by quantifying IL-1β in the supernatant by ELISA. IL-1β production was not induced in cells treated with the vehicle, microspheres, or LPS alone. Microspheres in addition to LPS resulted in a significant rise in IL-1β levels, which was reduced in Nlrp3 KO macrophages and by MbCD or piceatannol (Fig. 2_B_). These results suggest that the physical contact of the microspheres with the plasma membrane is the vital interaction for inflammasome activation. Confirmation for this suggestion was obtained by measuring the binding force of a single cell to a single microsphere using atomic force microscopy (AFM). The experimental design of the physical interaction between the microsphere and the cell (Fig. 2_C_) and an actual microsphere on a cantilever (Fig. 2_D_) is shown. After ∼50 s of oscillating contact, there was a dramatic increase in the binding force between the cell and the microsphere, which fluctuated over the duration of the experiment (Fig. 2 E and F). As predicted by the ELISA data (Fig. 2_B_), there was a significant decrease in the binding force of the cells to the microsphere in the presence of MbCD or piceatannol.
Fig. 2.
PMMA microspheres induce IL-1β production from peritoneal macrophages in a membrane lipid- and Syk-dependent manner. (A) Single microsphere, which is much larger than attached macrophages and cannot be phagocytosed. (B) Microspheres induce IL-1β, which is significantly less in the absence of Nlrp3 and can be reduced in wild-type macrophages by the cholesterol inhibitor MbCD (50 μm), and the Syk inhibitor piceatannol (2 mM). (C) Experimental design for measuring the binding force between a single microsphere and a single macrophage using an AFM. (D) Single microsphere on the cantilever of the AFM. (E) Binding force between a single microsphere and cell over a period in the presence and absence of MbCD or piceatannol. (F) Readings of binding affinity in all experiments depicted in the E are averaged. *†‡φ_P_ ≤ 0.05.
Inflammasome Components Asc and Caspase-1, but Not Nlrp3 or NLRC4, Are Required for the Development of the Foreign Body Reaction.
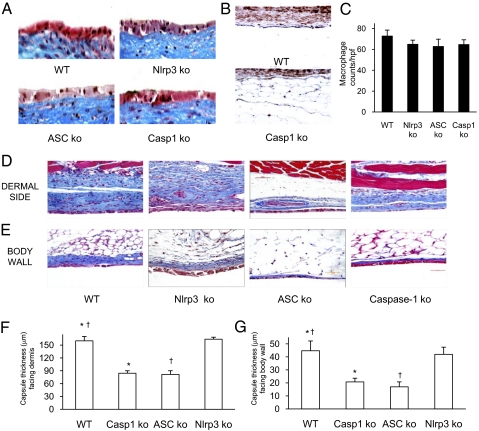
To confirm that the role of the inflammasome was not limited to one biomaterial, and to test the role of inflammasome components in the development of the FBR, we used the well-established model of s.c. silicone implantation. Silicone disks (6 mm diameter) were implanted for 4 wk, excised en bloc, and processed for histological analysis. A central feature of the FBR is the accumulation of macrophages and fusion into FBGCs that surround the foreign body. Formation of FBGCs adjacent to the silicone implants was similar in wild-type, Nlrp3, Asc, or caspase-1 KO mice (Fig. 3_A_). Immunohistochemical detection of macrophages revealed that their presence was not reduced in the Nlrp3, Asc, or caspase-1 KO mice (Fig. 3 B and C). These findings demonstrate that the absence of these inflammasome components does not inhibit macrophage localization and FBGC formation in the FBR.
Fig. 3.
Macrophage recruitment and foreign body giant cell formation is intact in the absence of Nlrp3, Asc, or caspase-1, but the FBR is reduced. (A) Formation of foreign body giant cells stained “reddish” with dark nuclei adjacent to the silicone is normal in Nlrp3 KO, Asc KO, and caspase-1 KO mice. (B and C) The density of tissue macrophages is unchanged in Nlrp3 KO, Asc KO, and caspase-1 KO mice compared with WT. (D_–_G) Capsule thickness and collagen of the FBR stained blue is significantly reduced in Asc KO and caspase-1 KO but not Nlrp3 KO mice compared with WT. *†P ≤ 0.05.
The thickness of the fibrotic reaction in the FBR is a clinically relevant parameter as it can limit the longevity of biomaterials and complicate the function of medical devices. In contrast to the preservation of FBGC formation and macrophage density, the development of the FBR was significantly reduced in mice deficient in Asc or caspase-1 (Fig. 3 D_–_F). Histological analysis showed significantly reduced collagen deposition in Asc or caspase-1 KO mice, but not in Nlrp3 KO mice. The mean capsule thickness in wild-type mice on the dermal side was 160 ± 10.2 μm, and 84.3 ± 5.7 μm in caspase-1 KO, 81.5 ± 8.6 μm in Asc KO and 163.7 ± 4.7 μm in Nlrp3 KO mice. For the side facing the body wall (Fig. 3_D_), the thickness was 44.6 ± 7.5 μm in wild-type mice, and 20.8 ± 2.8 μm in caspase-1 KO, 17 ± 3.8 μm in Asc KO, and 42 ± 5.5 μm in Nlrp3 KO mice. It is interesting to note that although Nlrp3 was required for the early inflammatory response to foreign materials, for the full chronic FBR either Nlrp3 has no role, or there is redundancy with other NLRs that can compensate in the absence of Nlrp3. Because NLRC4 is known to be proximal to Asc, we investigated the FBR in NLRC4 KO mice and found that it was normal (Fig. S1).
Aspirin Can Reduce the Acute Inflammation and the Foreign Body Response.
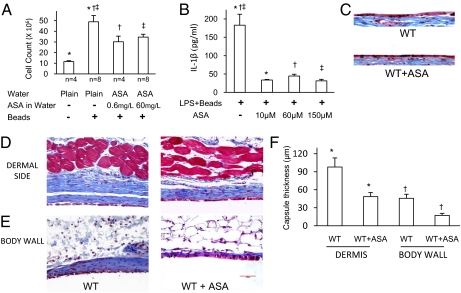
Aspirin is widely recognized to have antiinflammatory properties. In addition to antiplatelet effects, and its ability to inhibit production of prostaglandins by Cox-1 and prostanoids by Cox-2, aspirin also increases production of the potent antiinflammatory molecules lipoxins (18, 19). We have previously shown that aspirin can inhibit the inflammasome pathway and reduce production of IL-1β. In view of this inhibition by aspirin, and the above demonstration that acute inflammation to foreign materials and the FBR are dependent on the inflammasome pathway, we tested the ability of aspirin to modulate these responses. Aspirin at a concentration of 60 mg/L or 0.6 mg/L in the drinking water was able to almost completely inhibit the acute peritoneal infiltrate 24 h after injection of microspheres (Fig. 4_A_). We then sought to determine whether aspirin had a direct effect on macrophages. At 60 mg/L aspirin in the drinking water the average serum concentration was 62 μM. Using LPS/bead-activated peritoneal macrophages, we evaluated the ability of 10–150 μM aspirin to inhibit IL-1β production and found that, even at 10 μM, aspirin was effective (Fig. 4_B_). To further test whether aspirin can limit the FBR, mice were continuously placed on drinking water containing aspirin at a concentration of 60 mg/L immediately after implantation of the silicone discs. Consistent with our observations in mice lacking inflammasome components, the formation of FBGCs was not impaired by aspirin (Fig. 4_C_). However, collagen deposition, measured as capsule thickness, was significantly reduced by aspirin (Fig. 4 D_–_F). The mean FBR in WT mice on the dermal side was 98 ± 15.1 μm, and 48.6 ± 6.7 μm in mice on aspirin. In juxtaposition to the body wall the thickness was 45.8 ± 6.7 μm in WT and 17.3 ± 3.3 μm in mice on aspirin. This was comparable to the reduction of the FBR in mice lacking Asc or caspase-1.
Fig. 4.
Oral administration of aspirin reduces the acute inflammatory response to biomaterials and the FBR. (A) Aspirin reduces the peritoneal acute inflammatory infiltrate elicited by microspheres. (B) Aspirin reduces IL-1β production by macrophages in response to LPS/beads. (C) Aspirin does not inhibit foreign body giant cell formation in response to s.c. silicone implants. (D_–_F) Aspirin reduces the FBR in response to s.c. silicone implants. *†‡P ≤ 0.05.
To determine whether either of the two signaling pathways required for the production of IL-1β is reduced by aspirin, the degree of caspase-1 activation and pro–IL-1β up-regulation induced by PMMA microspheres in vivo was determined. As shown in Fig. S2, there was significantly less activation of caspase-1 by PMMA microspheres in the peritoneal infiltrate of mice treated with aspirin in the drinking water (60 mg/mL). In contrast, there was no reduction in the up-regulation of pro–IL-1β expression. The use of selective Cox-1 and Cox-2 inhibitors individually, or in combination, did not reduce IL-1β production by microsphere-stimulated macrophages (Fig. S3).
Discussion
Foreign bodies that come in contact with vascularized tissues elicit a FBR that progresses through a stereotypical sequence of events involving activation of complement and deposition of plasma proteins, followed by a recruitment of neutrophils and macrophages. The latter adhere to the surfaces of foreign bodies and form FBGCs (2). Over a period of weeks to months, the response progresses to recruitment of fibroblasts that deposit collagen, leading to encapsulation of the foreign body. Despite the established sequence of events outlined above, the molecular components required for sensing the foreign material and initiating the FBR have not been identified. In the present study we show that biomaterials trigger activation of the inflammasome leading to generation of active caspase-1 and secretion of IL-1β. Our findings are consistent with previous demonstrations showing that the inflammasome is required for the sterile inflammatory response to a wide range of particulate matter including uric acid, silica, and alum. These particulates have a very different structure from each other, but have in common the fact that they are small enough to be phagocytosed. In fact an important mechanism of inflammasome activation is phagocytosis with subsequent rupture of the phagosome (16). These findings, and the proposed phagosome-dependent model, have resulted in the inflammasome being identified as a sensor of microscopic particulate matter. However, our data clearly show that 150-μM microspheres can activate the inflammasome and induce an inflammatory infiltrate. Thus, we presume that the microspheres trigger activation in the absence of phagosomal lysis.
Recently, a study using atomic force microscopy described a complementary model where extracellular particles activate the inflammasome via physical interaction with the plasma membrane, resulting in reconfiguration of plasma membrane cholesterol and colocalization and activation of cytoplasmic ITAMS and Syk activation. We considered this MATS model relevant for biomaterials because in most applications biomaterials are too large to be phagocytosed. Consistent with this hypothesis, we observed that microspheres can activate the inflammasome and induce an inflammatory infiltrate that is dependent on the inflammasome components Nlrp3, Asc, and caspase-1. These are the same components that are required for initiation of inflammation to microscopic particulate matter, and the inflammasome appears to be a common pathway for initiating inflammation in response to physical materials irrespective of their size. To confirm that the described mechanism of perturbation of plasma membrane cholesterol was involved in this process we show that IL-1β production can be inhibited by MbCD and also by the Syk inhibitor piceatannol. These results suggested these agents should ameliorate the binding force between microspheres and the plasma membrane. This was tested by atomic force microscopy and both MbCD and piceatannol significantly reduced the binding force. More importantly, these studies were performed in serum-free media suggesting that the microspheres could not acquire a proteinaceous coat. Thus, in this model we envision direct interaction of macrophages with the biomaterial surface. Interestingly, microparticles like alum can also induce syk activation in DCs, which raises the possibility that these cells have a similar ability to respond to biomaterials (20). However, the DC response to alum was shown to be caspase-1 independent, implicating the involvement of a separate pathway.
We find the involvement of syk signaling in biomaterial recognition by macrophages intriguing because the interaction with biomaterials leads to macrophage fusion and formation of FBGCs. In fact, syk has also been shown to be required for fusion as Syk KO macrophages do not fuse in response to IL-4/GM-CSF (21). Similarly, the fusion of monocytes/macrophages to form osteoclasts on bone surfaces involves Syk (22). Specifically, studies have linked Syk signaling in osteoclast maturation and it is conceivable that in addition to production of proinflammatory signals, activation of syk interfaces with the fusogenic machinery.
The inflammatory response to a foreign material examined by the above experiments with microspheres involved the early phase of the FBR. To study the long-term role of the inflammasome in the FBR, we used s.c. implantation of silicone discs for 4 wk. In the absence of Nlrp3, Asc, or caspase-1 the ability of macrophages to migrate to the site of the biomaterial and to form FBGC was not impaired. In addition, macrophage density in the tissue adjacent to the biomaterial was not decreased. In contrast to the macrophage response, there was a very significant reduction in the thickness of the FBR in the mice lacking Asc and caspase-1, but not in mice lacking Nlrp3 or NLRC4. These data lead to the question of why Nlrp3 is required for the acute inflammatory response to biomaterials, but not the chronic fibrotic response. We propose that this is due to the mechanism of MATS-induced activation, which by forming cholesterol rafts induces clustering of a variety of molecules with cytoplasmic ITAMS. These molecules will have varying degree of efficiency in activating Asc/caspase-1, with Nlrp3 being very efficient. In the setting of a few hours, such as acute inflammation, the low-efficiency molecules cannot make a significant contribution; however, over a period of several weeks, are allowed the low-efficiency molecules could provide a significant activation signal to Asc/caspase-1. In addition to Nlrp3 and NLR4C, AIM2 and NLRP1 are known to form Asc-dependent inflammasomes, and a number of other NLRs have not been fully characterized yet (23). Taken together, these findings suggest that the loss of inflammasome components hinders the progression of the FBR possibly through the attenuation of profibrotic signals. Consistent with this suggestion, the inability to form the inflammasome has been shown to be associated with reduced fibrosis in injury models of liver fibrosis, myocardial ischemia/reperfusion, unilateral ureteral obstruction, and bleomycin (BLM)-induced lung injury (24–27).
We had previously shown that continuous administration of aspirin could inhibit the archetypal inflammasome-dependent inflammation by uric acid crystals (28). Based on our findings regarding the requirement for Asc and caspase-1 in the FBR, we asked whether aspirin could inhibit the acute inflammatory response to microspheres. As we demonstrate here, administration of aspirin in the drinking water significantly reduced the peritoneal inflammatory infiltrate, and also reduced IL1β production by macrophages in vitro, indicating that the effect was specific for these cells. Of the two main pathways required for the production of IL-1β, we have demonstrated a reduction in caspase-1 activation by aspirin. Aspirin has a number of complex in vivo effects that include inhibition of NF-κβ, Cox-1, and platelets, as well as switching the catalytic activity of Cox-2, resulting in the generation of a family of molecules termed aspirin-triggered lipoxins (ATLs) (18). Pharmacological inhibition of Cox-1 and/or Cox-2 did not reproduce the inhibitory effect of aspirin on microsphere-induced inflammasome activation, and it is of great interest to examine the role of the family of ATLs in our system. We further tested whether continuous aspirin administration could influence the FBR to silicone implants and found a significant reduction in thickness of the FBR. Of potential clinical importance, the thickness of the FBR was reduced to less than 100 μM, which could allow for enhanced diffusion of small molecules (29).
Overall, our findings constitute a unique demonstration of the participation of the inflammasome in cell–biomaterial interactions and in the development of the FBR. These results are also significant and unexpected because they extend the role of inflammasome components to biomaterials, irrespective of size, and show that inflammasome activation can be triggered by physical membrane contact. Due to the pervasive use of biomaterials, identification of the molecular components required for the FBR, and the demonstration of a clinically translatable method for reducing the FBR have broad applicability.
Materials and Methods
Animals.
Nlrp3, Asc, IPAF, and caspase-1 KO mice were on the C57BL/6 background and were maintained in specific pathogen-free facilities. Asc−/− and Nlrp3−/− mice were originally from Millennium Pharmaceuticals. Control C57BL/6 mice were purchased from NCI, and all mice were used at 8–12 wk of age. All procedures were performed in accordance with the regulations adopted by the National Institutes of Health and approved by the animal care and use committee of Yale University.
Biomaterial Induced Peritoneal Inflammation.
Ultrapure PMMA microspheres (Bang Labs; BB05N/5438) were obtained as dry powder and washed extensively in 70% ethanol and endotoxin-free water. A 0.5-mL suspension of 5 × 105 PMMA bead in PBS supplemented with 0.02% (vol/vol) ethanol and 0.05% (wt/vol) hydroxypropyl cellulose (6–10 cps; TCI) was injected i.p. using a 22-gauge needle and sterile techniques. At 24 h after i.p. injection, mice were euthanized and peritoneal fluid was lavaged, cell counts determined, and flow cytometric analysis performed using antibodies to Gr1 and F4/80 (BD Biosciences) using a FACS Calibur flow cytometer. Cellular protein lysates were also prepared for Western blot analysis of caspase-1 activation. The following antibody was used: caspase-1 p10 (M-20) from Santa Cruz (sc-514, rabbit polyclonal IgG).
In Vitro Macrophage Activation by PMMA Microspheres.
C57BL/6 mice were injected i.p. with 4% thioglycolate (Fluka; B2551) under aseptic conditions. After 3 d, mice were euthanized and inflammatory monocytes/macrophages were collected by peritoneal lavage. Cells were plated into 12-well plates at a density of 3 × 106 per well. After 3 h, the media (DMEM high glucose) was replaced with fresh media and cultured with 100 ng/mL of LPS (Sigma; L9764) for 16 h. The media was changed afterward. For positive control wells, 5 mM ATP (Sigma; A2383) was freshly added for exactly 20 min, and then the medium was changed. A ratio of one PMMA bead per 20 cells was used to add in the respective wells. A total of 2 mM MbCD (Sigma; C4555) and 50 μm piceatannol (Sigma; P0453) were added 40 min before addition of PMMA microspheres in the respective wells. The plates were then incubated for 5 h in the incubator, and the supernatant collected to assay IL-1β. Cox inhibitors sc-560 and sc-58125 were purchased from Cayman Chemicals.
ELISA.
High-binding 96-well ELISA plates (BD Biosciences; 353279) were used as per manufacturer instructions. The capture antibody was antimouse IL-1β (R&D; MAB401), and the detection antibody was polyclonal biotinylated antimouse IL-1β antibody (R&D; BAF401).
Atomic Force Microscopy.
Arrow TL-1 cantilevers (Nanoworld) were functionalized with PMMA tips under AFM control. A total of 5 × 105 lavaged macrophages were plated on 25-mm glass disks in six-well plates. After 60 min, media were removed and replaced with warm fresh media and incubated overnight at 37 °C. AFM was performed as described previously except for increasing hold time to 10 s in relative intermittent contact mode in a temperature (37 °C)- and CO2 (5%)-controlled humidified chamber (17). Force curves were obtained over 300 s and analyzed using JPK image processing software. For inhibitor studies, MbCD (10 mM) or piceatannol (50 μM) were present for 30 min before AFM reading.
SEM Analysis.
SEM was performed by plating 2 × 106 cells per well in a 24-well plate containing a sterile 12-mm glass disk as described previously. After 60 min, medium was removed and replaced with warm fresh media. Microspheres were added and shaken by hand for 2–3 min and then allowed to settle. After 60 min incubation, media were removed, samples were washed twice with PBS, and 1% EM grade glutaraldehyde (Electron Microscopy Sciences) was added. After overnight incubation (4 °C), samples were dehydrated first by ethanol gradient followed by hexamethyldisilazane (HMDS; Electron Microscopy Sciences) gradient. Samples were sputter coated with gold-palladium (Techniques Hummer II Sputter Coater) and analyzed using SEM (environmental scanning electron microscope, XL30).
Foreign Body Reaction.
All procedures were performed in accordance with the regulations adopted by the National Institutes of Health and approved by the animal care and use committee of Yale University. Six-millimeter disks were punched out from sterile 100% medical grade silicone sheet with 1-mm thickness (Invotec) using a sterile Acu-Punch (Acuderm). Discs were implanted s.c. as described previously (30). After 4 wk, discs were removed and the tissue stained by Masson's trichrome stain according to standard protocols.
Administration of Aspirin.
For the acute experiments with PMMA microspheres, aspirin was provided in the drinking water for 3 d before bead injection. For the chronic FBR experiments, aspirin was provided in the drinking water immediately after implantation of silicone discs.
Supplementary Material
Supporting Information
Acknowledgments
We thank Eleni A. Skokos and Salma Kamal for technical assistance and Drs. Jordan Pober and Tarek Fahmy for a critical reading of the manuscript. This work was supported by National Institutes of Health Grant R01DK076674-01A2 and a Veterans Administration Merit Award (to W.Z.M.) and RO1 GM072194-01 (to T.R.K.), R21AI089963 (to Y.S.), and 1K08DK092281-01 (to R.H.). R.A.F. is an Investigator for the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: W.Z.M. and T.R.K. are co-inventors on a patent filed by Yale University, which is under license on the use of aspirin to reduce the foreign body reaction.
This article is a PNAS Direct Submission.
References
- 1.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010;12:188–196. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Luttikhuizen DT, Harmsen MC, Van Luyn MJ. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006;12:1955–1970. doi: 10.1089/ten.2006.12.1955. [DOI] [PubMed] [Google Scholar]
- 5.Hu WJ, Eaton JW, Ugarova TP, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001;98:1231–1238. doi: 10.1182/blood.v98.4.1231. [DOI] [PubMed] [Google Scholar]
- 6.Tang L, Wu Y, Timmons RB. Fibrinogen adsorption and host tissue responses to plasma functionalized surfaces. J Biomed Mater Res. 1998;42:156–163. doi: 10.1002/(sici)1097-4636(199810)42:1<156::aid-jbm19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Szott LM, Stein MJ, Ratner BD, Horbett TA. Complement activation on poly(ethylene oxide)-like radiofrequency glow discharge-deposited surfaces. J Biomed Mater Res A. 2011;96:150–161. doi: 10.1002/jbm.a.32954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kou PM, Babensee JE. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A. 2011;96:239–260. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 9.Shokouhi B, et al. The role of multiple toll-like receptor signalling cascades on interactions between biomedical polymers and dendritic cells. Biomaterials. 2010;31:5759–5771. doi: 10.1016/j.biomaterials.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 12.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demento SL, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng G, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang N, Serhan CN. New mechanism for an old drug: aspirin triggers anti-inflammatory lipid mediators with gender implications. Compr Ther. 2006;32:150–157. doi: 10.1007/s12019-006-0005-6. [DOI] [PubMed] [Google Scholar]
- 19.Flower R. What are all the things that aspirin does? BMJ. 2003;327:572–573. doi: 10.1136/bmj.327.7415.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flach TL, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 21.Helming L, et al. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal. 2008;1:ra11. doi: 10.1126/scisignal.1159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauernfeind F, et al. Inflammasomes: Current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi M, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 25.Vilaysane A, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasse P, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe A, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1248–G1257. doi: 10.1152/ajpgi.90223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaeda AB, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398:1695–1705. doi: 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyriakides TR, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–2166. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information