Physical Exercise as a Preventive or Disease-Modifying Treatment of Dementia and Brain Aging (original) (raw)
Abstract
A rapidly growing literature strongly suggests that exercise, specifically aerobic exercise, may attenuate cognitive impairment and reduce dementia risk. We used PubMed (keywords exercise and cognition) and manuscript bibliographies to examine the published evidence of a cognitive neuroprotective effect of exercise. Meta-analyses of prospective studies documented a significantly reduced risk of dementia associated with midlife exercise; similarly, midlife exercise significantly reduced later risks of mild cognitive impairment in several studies. Among patients with dementia or mild cognitive impairment, randomized controlled trials (RCTs) documented better cognitive scores after 6 to 12 months of exercise compared with sedentary controls. Meta-analyses of RCTs of aerobic exercise in healthy adults were also associated with significantly improved cognitive scores. One year of aerobic exercise in a large RCT of seniors was associated with significantly larger hippocampal volumes and better spatial memory; other RCTs in seniors documented attenuation of age-related gray matter volume loss with aerobic exercise. Cross-sectional studies similarly reported significantly larger hippocampal or gray matter volumes among physically fit seniors compared with unfit seniors. Brain cognitive networks studied with functional magnetic resonance imaging display improved connectivity after 6 to 12 months of exercise. Animal studies indicate that exercise facilitates neuroplasticity via a variety of biomechanisms, with improved learning outcomes. Induction of brain neurotrophic factors by exercise has been confirmed in multiple animal studies, with indirect evidence for this process in humans. Besides a brain neuroprotective effect, physical exercise may also attenuate cognitive decline via mitigation of cerebrovascular risk, including the contribution of small vessel disease to dementia. Exercise should not be overlooked as an important therapeutic strategy.
AD = Alzheimer disease; BDNF = brain-derived neurotrophic factor; fMRI = functional brain magnetic resonance imaging; IGF-1 = insulin-like growth factor 1; MCI = mild cognitive impairment; MRI = magnetic resonance imaging; RCT = randomized controlled trial; 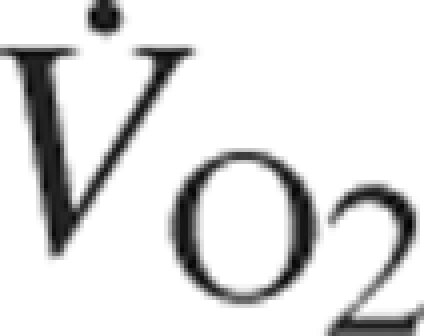 = oxygen consumption per unit time
= oxygen consumption per unit time
Dementia is a major threat to our aging population. Besides destroying life quality of affected patients, it affects immediate family, turning spouses or children into caregivers and often straining family finances. Alzheimer disease (AD) accounts for most dementia cases,1 with contributions from dementia with Lewy bodies, vascular disease, frontotemporal degeneration syndromes, and various other less common disorders. Less devastating but also disrupting life quality is mild cognitive impairment (MCI), documented in more than 10% of seniors older than 70 years, with more than 20% affected after the age of 80 years.2 Often MCI is a prelude to subsequent dementia.3
Notable also is the subtle loss of cognitive skills often accompanying normal aging. Seniors frequently experience reduced memory for names and telephone numbers. Whether the substrate is the progressive loss of gray matter routinely seen with brain magnetic resonance imaging (MRI) of seniors is debatable; indeed, normal brain aging is accompanied by loss of synaptic connections and attenuated neuropil.4,5
The neurodegenerative dementias are presumed to be proteinopathies, characterized by aggregation of a specific protein within the brain, such as β-amyloid and microtubule-associated protein tau in AD or α-synuclein in dementia with Lewy bodies. Despite intensive research directed at these and other neurodegenerative diseases, no drug effectively targets the pathogenic substrates. No medication has been proven to reduce the subsequent risk of dementia or age-related cognitive impairment.
REGULAR EXERCISE AS NEUROPROTECTIVE THERAPY
Although medications have no proven neuroprotective effect on dementia, an evolving literature documents significant benefit of long-term, regular exercise on cognition, dementia risk, and perhaps dementia progression. These studies suggest an attenuating effect on brain aging and resilience to dementing neurodegenerative mechanisms.
Exercise also favors brain health via the well-known attenuating influences on atherosclerotic cerebrovascular disease. Thus, primary vascular dementia is common and, moreover, cerebrovascular small vessel disease (eg, leukoaraiosis and lacunar disease) appears additive with neurodegenerative processes to cause dementia.6 These atherosclerotic cerebrovascular mechanisms are distinctive from neurodegeneration and age-related loss of neuropil and synapses. Because the benefit of exercise on atherosclerotic (cerebrovascular) risk seems well established, this contribution to the subject will not be a focus of this article.
Our focus is on the scientific basis for advocating regular exercise as a prophylactic and perhaps disease-slowing treatment of neurodegenerative and age-related dementia and MCI. Although certain studies in humans make it difficult to separate vascular contributions, the literature in the aggregate suggests that exercise may have more direct favorable effects on brain neuroplasticity and resilience to brain aging and neurodegeneration.
A recent National Institutes of Health State-of-the-Science Statement took a nihilistic view of exercise as a disease-modifying influence on cognition or dementing illness.7,8 However, as pointed out in a subsequent critique of this statement, the conclusions were based on a narrow scope of data.9
We present evidence that argues for the benefit of exercise on cognition and the forestalling of later-life cognitive decline. In contrast to the recent National Institutes of Health consensus statement,7,8 we considered a broad expanse of both animal and human studies relevant to this topic. In an attempt to capture the relevant literature, we reviewed all publications identified by a PubMed search using the keyword cognition cross-referenced with exercise (identifying 1603 publications, without date limitations) and identified additional relevant articles via review of bibliographies from these and other publications.
DEFINING REGULAR EXERCISE
The literature on this subject, including animal studies, implies that potential benefits accrue with long-term, regular exercise. The exercise parameters cannot be precisely defined, but the connotation is aerobic physical exercise that is sufficient to increase the heart rate and the need for oxygen. Presumably, this must be sustained (eg, for at least 20-30 minutes per session) and ongoing. Ultimately, this translates into what physiologists characterize as cardiovascular fitness, objectively assessed with measurement of oxygen uptake during peak exercise (such as on a treadmill); this is reported as peak oxygen consumption per unit time (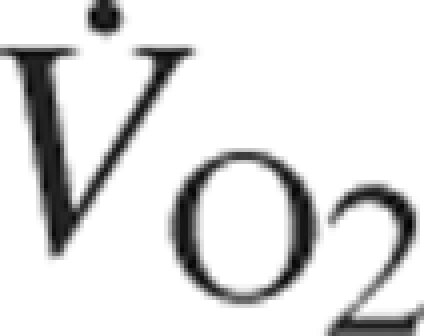 ), with higher values indicative of better fitness.
), with higher values indicative of better fitness.
Limited studies have also specifically addressed resistance exercise (effort against weighting or resistance) and cognition; however, this literature is currently insufficient to draw conclusions. Hence, we primarily focus on aerobic-type exercise that potentially leads to physical fitness.
EXERCISE MODALITIES
Although other medical conditions may limit the extent of exercise, modalities should be available for all people, except perhaps those with major cardiopulmonary disease or major organ failure. There is a wide variety of such aerobic exercise options, including walking, gym or health club routines, driveway basketball, and home activities, such as shoveling snow, raking leaves, or other yard work. Impaired ambulation does not preclude certain sitting exercises, such as use of rowing machines, exercise bicycles, or other gym machines.
IMPROVEMENT IN COGNITIVE SCORES IN HEALTHY ADULTS
Recent meta-analyses of 29 randomized controlled trials (RCTs) documented significant cognitive benefits from sustained exercise in adults without dementia (although 3 of the 29 trials enrolled patients with MCI).10 Significantly improved scores were noted in memory, attention, processing speed, and executive function, albeit with only modest improvement. Because the benefits accrued during 1 to 12 months of exercise (except for one 18-month trial), these findings are less easily explained by the secondary influence of exercise on cerebrovascular disease (eg, leukoaraiosis, lacune, or stroke risk).
FUNCTIONAL MRI COGNITIVE NETWORKS IN HEALTHY SENIORS
Functional brain MRI (fMRI) during cognitive tasks has also documented significantly improved cognitive networks with exercise or fitness. In one 6-month RCT among seniors, aerobic exercise translated into significantly improved cortical connectivity and activation, compared with controls.11 In a 12-month RCT, aerobic exercise likewise improved cognitive fMRI network connectivity; however, the control group undergoing nonaerobic stretching and toning also had improved fMRI outcomes.12
In cross-sectional analyses, physically fit seniors had fMRI evidence of significantly better cortical connectivity and activation during cognitive tasks than unfit seniors (assessed by peak 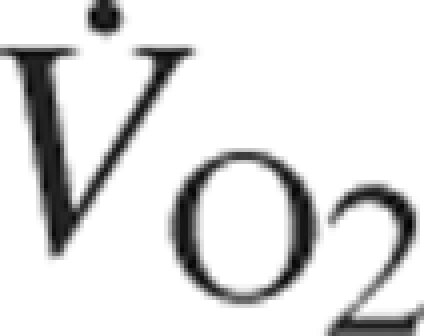 during exercise).11,13 Physically fit seniors also performed significantly better on cognitive tasks than unfit seniors in these cross-sectional studies.11,13,14
during exercise).11,13 Physically fit seniors also performed significantly better on cognitive tasks than unfit seniors in these cross-sectional studies.11,13,14
MRI GRAY MATTER VOLUME LOSS IN SENIORS
Brain gray matter volumes decrease with advancing age, as routinely seen in the clinic with brain MRI. In contrast to neurodegenerative disorders, which are associated with neuronal loss, the reductions of gray matter volumes seen in normal aging primarily reflect loss of neuropil and synapses.4,5
A recent RCT in a large cohort of seniors documented significantly larger hippocampal volumes after 1 year of aerobic exercise, compared with the control intervention of simple stretching and toning.15 This finding was associated with significant improvement in the primary cognitive outcome measure of spatial memory. Similar exercise outcomes have been documented in the neocortex. Thus, two 6-month RCTs of aerobic exercise in seniors without dementia were associated with increased cortical volumes compared with sedentary interventions.16,17 In a long-term, prospective cohort study, the usual weekly walking distances reported by healthy adults at baseline were positively associated with neocortical and hippocampal MRI volumes 9 years later.18
In a large, cross-sectional study of seniors without dementia, physical fitness, assessed by treadmill exercise testing (peak 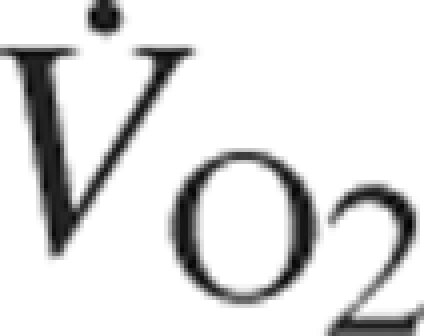 ), was highly and significantly associated with hippocampal volumes on MRI (controlling for age, sex, and educational level).19 In other cross-sectional studies, physical fitness (measured by peak
), was highly and significantly associated with hippocampal volumes on MRI (controlling for age, sex, and educational level).19 In other cross-sectional studies, physical fitness (measured by peak 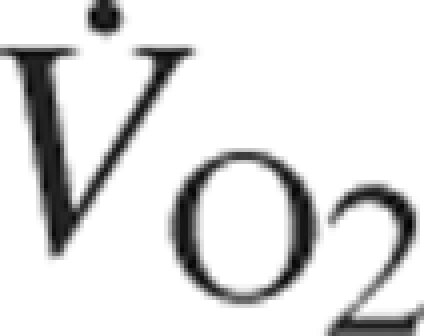 ) was associated with better preservation of gray matter volumes among both cognitively normal seniors14,20 and patients with early AD.21,22 The control groups in these latter 2 studies, however, did not generate expected results; in these seniors without dementia, there was no association of cardiorespiratory fitness (peak
) was associated with better preservation of gray matter volumes among both cognitively normal seniors14,20 and patients with early AD.21,22 The control groups in these latter 2 studies, however, did not generate expected results; in these seniors without dementia, there was no association of cardiorespiratory fitness (peak 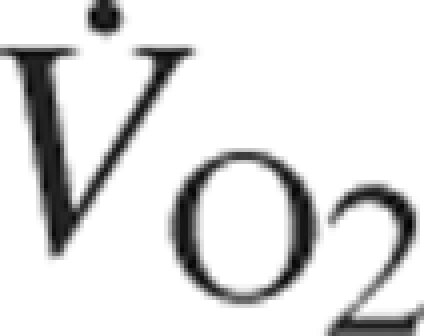 ) with gray matter volumes.21,22
) with gray matter volumes.21,22
MIDLIFE EXERCISE AND REDUCED RISKS OF LATER DEMENTIA AND MCI
Adults who routinely engaged in physical activities, sports, or regular exercise in midlife carried a significantly lower risk of dementia years later, based on a recent meta-analysis of prospective cohort studies.23 Thus, reduction of dementia risk was documented in 10 of 11 studies, with an estimated relative risk of 0.72 (P<.001).23
Several prospective cohort investigations have reported significantly reduced subsequent risks of MCI associated with midlife exercise.24-26 A population-based, case-control study similarly found that moderate exercise retrospectively reported for midlife was associated with a significantly reduced risk of MCI.27 Reduction of MCI risk with retrospectively reported earlier life exercise was also documented in a cross-sectional study of a large female cohort.28
One caveat: the association of midlife exercise with later cognitive preservation could be explained by reverse causality. In other words, those with very early, preclinical neurodegenerative disease might be disinclined to exercise.
INFLUENCE OF PHYSICAL ACTIVITY ON MORTALITY IN AD PATIENTS
A population-based, prospective cohort study of incident AD patients revealed that those with maintained physical activity had a significantly reduced risk of mortality.29 This was true even after statistically adjusting for APOE genotype, medical comorbidities, and cognitive performance. Again, however, reverse causality cannot be excluded.
SHORT-TERM COGNITIVE BENEFIT AMONG THOSE WITH MCI OR DEMENTIA
Reverse causality would not explain improved cognitive scores in short-term RCTs. A meta-analysis30 of RCTs in seniors with MCI or dementia tabulated outcomes with exercise durations spanning 2 to 112 weeks. Among the 12 trials, significant cognitive benefits were documented compared with control outcomes.
Several more recent studies have added to this literature. Most compelling was the Australian trial randomizing 170 subjects with “memory problems” to 6 months of moderate-intensity exercise vs a sedentary routine.31 The exercise group had significantly better scores on the primary outcome measure after the 6 months, the Alzheimer Disease Assessment Scale–Cognitive Subscale; this benefit persisted at 12 and 18 months. Interestingly, they noted that the extent of improvement on the Alzheimer Disease Assessment Scale–Cognitive Subscale compared favorably to the effect of donepezil documented in another large clinical trial.32 A similar outcome in seniors with MCI was documented in one smaller 6-month RCT of “high-intensity aerobic exercise” vs sedentary controls (stretching); however, the improvement was predominantly in women.33 One additional RCT in seniors with MCI identified similar but not statistically significant trends after 1 year of exercise; the investigators commented that the analysis was compromised by suboptimal adherence to the exercise program.34 In women with dementia, a small RCT of regular exercise for 1 year significantly improved the Mini-Mental State Examination score compared with slight (nonsignificant) worsening in the sedentary control group.35 Of note, 2 of these trials31,34 were included in the meta-analysis by Smith et al.10
PLAUSIBILITY FROM ANIMAL STUDIES
The studies in humans suggest that exercise may improve cognition in the short term, reduce risks of dementia or MCI in the long term, and reduce the age-associated progressive loss of brain volume. This issue lends itself to assessment in animal models, in which it is also possible to study putative biological mechanisms.
EXERCISE IMPROVES COGNITION IN ANIMALS
Exercised rats or mice (eg, treadmills and running wheels) have significantly better scores on memory tests or object recognition compared with their more sedentary counterparts.36-42 Conversely, immobilization had the opposite effect, with reduced cognitive scores.43 These findings have been extended to primates; monkeys with scheduled exercise for 5 months had significantly better cognitive scores than sedentary animals.44
EVIDENCE FOR ENHANCED NEUROPLASTICITY INDUCED BY EXERCISE IN ANIMALS
Brain neuroplasticity is a fundamental mechanism for learning, memory, and general cognition. A voluminous literature in rats and mice has documented multiple mechanisms by which exercise may facilitate such neuroplasticity. Thus, exercise has been shown to increase expression of synaptic plasticity genes,45 gene products such as synapsin I and synaptophysin,46,47 and various neuroplasticity-related transcription factors such as cyclic adenosine monophosphate response element binding and intracellular kinases.42,48,49 Hippocampal dendritic length and dendritic spine complexity are enhanced with exercise.50,51 Neurogenesis within the hippocampal dentate gyrus is also induced by exercise.50,52-54 Finally, long-term potentiation, which is thought to be a primary neurophysiologic substrate in learning, is potentiated by exercise,38,41,54 although this effect was confined to male animals in one study.55
BRAIN EXPRESSION OF NEUROTROPHIC FACTORS INDUCED BY EXERCISE IN ANIMALS
Neurotrophic factors appear to be especially involved in learning and neuroplasticity. Brain-derived neurotrophic factor (BDNF) has been most extensively investigated and, in vitro, modulates brain plasticity, including increasing neuritic outgrowth and synaptic function. It also promotes in vitro survival of a vast array of neurons affected by neurodegenerative conditions, including AD.56 Numerous investigations in mice or rats have found elevated brain BDNF concentrations and expression with exercise,36,37,41,42,46,47,49,54,57-60 although with one exception.55 Insulin-like growth factor 1 (IGF-1) interacts with BDNF and is likewise elevated in the rat brain by exercise.40,61 Rat brain concentrations of glial-derived neurotrophic factor are similarly upregulated by exercise.59,62
EXERCISE AND HIPPOCAMPAL NEUROGENESIS IN HUMANS
The hippocampus is crucial for memory and progressively degenerates in patients with AD, an effect already apparent in the earliest stages of dementia (MCI).63 The hippocampal dentate gyrus is also the region most vulnerable to aging.64 However, this region is one of the few brain regions that supports neurogenesis, and dentate gyrus neurogenesis is significantly facilitated by exercise in animal studies, as previously mentioned.50,52-54,65
Regional hippocampal dentate gyrus blood volume can be measured with brain MRI, and this was shown to be a neurogenesis biomarker in mice.65 Extending this to humans in a small, prospective, uncontrolled trial of young adults, 3 months of aerobic exercise resulted in significantly increased hippocampal dentate gyrus blood volume over baseline; other hippocampal regions were unchanged.65 This was interpreted as reflective of dentate gyrus angiogenesis and hence neurogenesis. It was associated with mildly improved cognitive scores. Fitness, as measured by peak 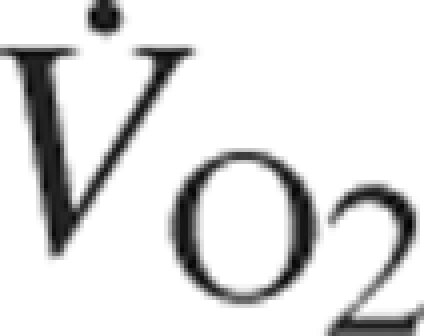 , significantly correlated with individual differences in dentate gyrus blood volume.65
, significantly correlated with individual differences in dentate gyrus blood volume.65
NEUROTROPHIC FACTORS, COGNITION, AND EXERCISE IN HUMANS
Theoretically, neurotrophic factors may be important in combating age-related brain atrophy and perhaps neurodegenerative disease. In contrast to laboratory animals, however, brain concentrations of neurotrophic factors cannot easily be studied in humans. Human investigations have focused on circulating levels, which may or may not reflect what is going on within the brain.
BDNF is widely expressed throughout the human adult brain,56 whereas levels are significantly reduced in the brains of AD patients.66-69 BDNF is rapidly transported in both directions across the blood-brain barrier,70,71 and hence measurement of circulating levels could be relevant to the brain. Thus, circulating BDNF levels are reduced in patients with AD72,73; moreover, AD patients whose condition is rapidly declining have significantly lower serum BDNF concentrations than those whose condition is slowly declining.74 Note also that in healthy young adults, BDNF appears to be released from the human brain by both short-term vigorous exercise75 and long-term endurance training76 on the basis of arterial and venous measurements.
In cross-sectional studies of seniors, circulating BDNF levels have been significantly associated with cognitive test scores after adjusting for multiple covariables,77,78 although confined to women in one study.77 In fact, fitness (peak 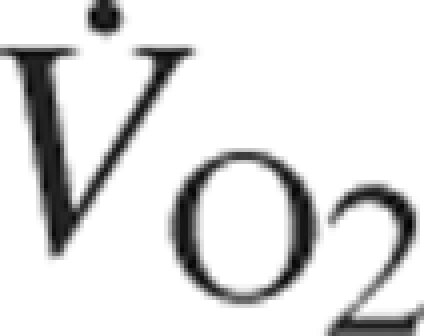 ) was significantly correlated with both BDNF and cognitive improvement in one of these studies.78 Moreover, in a 1-year RCT of exercise among seniors, increased serum BDNF level was associated with increased hippocampal volume.15
) was significantly correlated with both BDNF and cognitive improvement in one of these studies.78 Moreover, in a 1-year RCT of exercise among seniors, increased serum BDNF level was associated with increased hippocampal volume.15
The study of aerobic exercise on plasma or serum BDNF levels has generated complex findings. Most investigations in young adults have documented significant transient increases of circulating BDNF with short-term aerobic exercise,79-82 with one exception.83 Prospective studies of long-term aerobic exercise, however, have generated negative or inconsistent results. Thus, 5 weeks of chronic aerobic exercise in young adults was associated with increased levels of circulating BDNF in one uncontrolled trial,84 whereas two trials were negative (8-12 weeks; one controlled).83,85 In one RCT of patients with MCI, 6 months of “high-intensity aerobic exercise” resulted in a nonsignificant trend toward increased plasma BDNF levels in men but reduced in women (compared with control values).33 Somewhat paradoxically, 2 cross-sectional studies documented an inverse relationship between physical fitness and serum BDNF concentrations.86,87
The few prospective trials of resistance (not aerobic) exercise influences on plasma or serum BDNF levels have generated primarily negative results. In contrast to aerobic exercise, strength and resistance exercise did not elevate circulating BDNF concentrations.88-90 A prospective, controlled trial of strength training (10 weeks) failed to increase serum BDNF levels.90 As an exception, 5 weeks of resistance exercise raised serum BDNF levels in one other prospective, uncontrolled trial in young men.91
Insulin-like growth factor 1 is widely expressed in the human brain, and IGF-1 insufficiency has been proposed as a risk factor for AD.92 Patients with AD had significantly lower circulating IGF-1 levels than controls in one small cross-sectional study, and these levels were inversely correlated with the degree of cognitive impairment.93 Meta-analysis of the predominantly cross-sectional studies assessing circulating IGF-1 levels and cognition in seniors revealed a highly significant positive association.94 In healthy young adults, circulating IGF-1 is increased by exercise in most88,95-97 but not all studies.98 In young adults, long-term aerobic exercise failed to elevate circulating IGF-1 levels in 2 RCTs (12-16 weeks).85,99 In contrast to aerobic exercise, long-term resistance training elevated serum IGF-1 concentrations in 2 prospective, controlled trials100,101 but not in another.102
EXERCISE IMPACT ON BRAIN β-AMYLOID AND TAU PROTEIN
Alzheimer disease is the most common neurodegenerative dementia and is neuropathologically marked by the accumulation of neuritic plaques, as well as neurofibrillary tangles containing hyperphosphorylated tau protein. Perhaps a crucial inciting factor for AD development is the brain deposition of β-amyloid, the primary component of neuritic plaques. Brain accumulation of β-amyloid can be assessed in vivo using Pittsburgh compound B positron emission tomography. One recent investigation (cross-sectional design) documented an inverse relationship between long-term exercise levels and brain Pittsburgh compound B imaging density in a large cohort of cognitively normal seniors.103 These brain imaging findings were mirrored by spinal fluid tau protein and β-amyloid42 biomarkers. Again, however, reverse causality cannot be excluded. A small RCT of 6 months of exercise in patients with MCI documented a nonsignificant trend toward reduced plasma concentrations of β-amyloid42 compared with sedentary controls.33
Most investigations of exercise and brain β-amyloid deposition, however, have been performed using transgenic mice overexpressing pathogenic amyloid precursor protein (or presenilin 2104). The findings have been mixed, with a reduction of brain pathogenic β-amyloid deposition or amyloid plaques in most104-109 but not all studies.110-112
Neurofibrillary tangles are marked by hyperphosphorylated tau protein and are one of the pathological hallmarks of AD. In transgenic mice expressing a human pathogenic tau gene, 9 months of exercise prevented both the development of hippocampal tau disease and memory impairment, which were present in the sedentary control group.113 In 2 other studies, 12 weeks of exercise significantly reduced tau phosphorylation in transgenic mice expressing pathogenic tau114 or presenilin 2104 genes (compared with sedentary controls).
ATTENUATION OF VASCULAR CONTRIBUTIONS TO NEURODEGENERATIVE DEMENTIA BY EXERCISE
There is a striking overlap of the risk factors for AD and vascular dementia. Glucose intolerance and diabetes mellitus, hypertension, hyperlipidemia, and obesity contribute to not only vascular dementia but also to the risk of neurodegenerative dementia.6 Intuitively, the influence of these vascular factors may be indirect via superimposed small vessel disease (eg, leukoaraiosis, lacunar strokes, and microbleeds). The added burden of cerebrovascular brain damage may simply superimpose on neurodegeneration. However, a more direct effect of these vascular risk factors on neurodegenerative processes is plausible. Regardless, long-term exercise is well known to attenuate each of these risk factors.115-117
OTHER BENEFICIAL EFFECTS OF EXERCISE
Numerous noncognitive, nonvascular benefits additionally benefit from exercise, which may be especially relevant to an aging population. This includes reduction of osteoporosis and fracture risk,118 age-related sarcopenia,119 and benefits directed at depression120 and anxiety.121 An exercise program may improve behavioral management in seniors with dementia122 and fall risk.123 Importantly, long-term physical activity and fitness reduce mortality risk in the general population.117,124
ADVERSE EFFECTS OF EXERCISE
Advocating for an intervention (in this case, exercise) should be balanced against possible adverse effects. Exercise may result in orthopedic injuries, increase fall risk, and provoke acute coronary syndromes. Thus, physicians should help patients select exercise programs compatible with their capabilities and cardiopulmonary status. In general, people who have been sedentary for an extended time should begin an exercise program with modest exercise targets, but escalating as fitness is progressively achieved.
RESISTANCE TRAINING
Although the focus of this article has been on aerobic fitness, limited studies have suggested that regular resistance exercise (pushing or pulling against fixed weighting) may also improve cognition. Indeed, improved cognitive scores were documented in RCTs conducted for 2,125 6,101 and 12126 months. An additional 1-year follow-up in this last study revealed that cognitive benefits were sustained in the exercise group compared with the sedentary group.127 However, whole-brain volumes were inexplicably reduced in this 12-month resistance training trial126; this is in contrast to previously cited aerobic exercise trials in which cortical and hippocampal volumes were increased.15-19
Obviously, resistance training may contribute to aerobic fitness if the focus is on lighter weights (lesser resistance), more repetitions, and brief rest periods. However, the effect of resistance training on cognition has been inadequately studied to date and is difficult to assess in animal studies.
IS MORE EXERCISE BETTER?
The literature cited herein suggests cognitive benefits from aerobic exercise, but it remains unclear whether there are threshold effects or whether exercise duration and intensity are important variables. In mice, longer durations of exercise were more effective than shorter durations in attenuating the neuropathologic and clinical effects of the dopaminergic neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydro pyri dine.128,129 In a single human study, serum BDNF levels increased with exercise in proportion to the degree of lactate elevation.80
Human clinical trials assessing exercise duration or intensity, however, have been confined to resistance training. In a 6-month RCT in seniors, 2 intensities of resistance exercises (moderate and high) resulted in similar degrees of cognitive benefit.101 In another RCT, once-weekly resistance exercise significantly improved cognitive scores similar to twice-weekly exercise.126 However, duration was important in this latter trial in that the cognitive benefit was only documented at 12 but not 6 months. These 2 trials, however, assessed resistance training, not aerobic exercise, per se.
AEROBIC EXERCISE PRESCRIPTION
Aerobic exercise implies training that elevates heart rate and increases 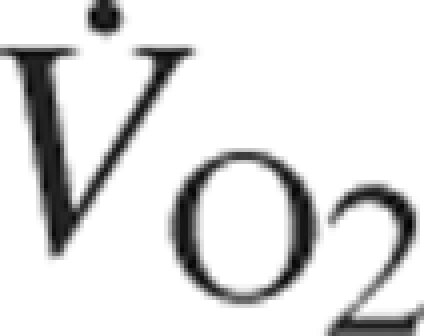 , but the exercise parameters to recommend are not well delineated. The human trials summarized herein have primarily used moderate aerobic exercise, which typically implies exercise sufficient to elevate heart rate or
, but the exercise parameters to recommend are not well delineated. The human trials summarized herein have primarily used moderate aerobic exercise, which typically implies exercise sufficient to elevate heart rate or 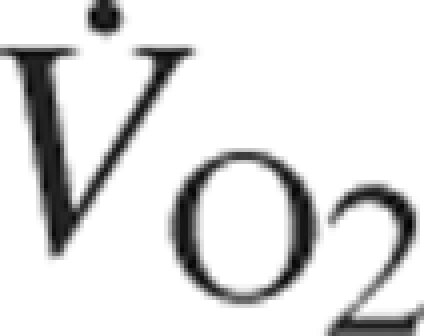 to approximately 60% of the maximum. For example, in 2 RCTs, the dose of 150 minutes of moderate aerobic exercise per week was sufficient to be cognitively protective31 and associated with increased hippocampal volume plus improved spatial memory.15 Such moderate intensity is a practical exercise target, recognizing that greater exercise intensity might not be tolerated and lead to greater numbers of study dropouts or nonadherence, at least initially.
to approximately 60% of the maximum. For example, in 2 RCTs, the dose of 150 minutes of moderate aerobic exercise per week was sufficient to be cognitively protective31 and associated with increased hippocampal volume plus improved spatial memory.15 Such moderate intensity is a practical exercise target, recognizing that greater exercise intensity might not be tolerated and lead to greater numbers of study dropouts or nonadherence, at least initially.
Regular aerobic exercise gradually increased to achieve 60% of maximal heart rate or 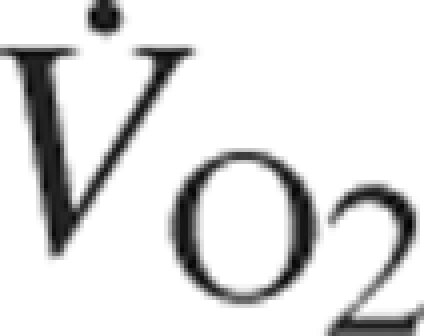 and performed at least 150 minutes weekly seems reasonable as an initial regimen. This is similar to the recommendation of the American Heart Association, which advises “...moderate-intensity aerobic physical activity for a minimum of 30 minutes on five days each week or vigorous-intensity aerobic activity for a minimum of 20 minutes on three days each week”; parenthetically, they also recommended resistance exercises “for a minimum of two days each week.”130 Future research should investigate exercise parameters to better determine the optimal recommendations for preservation of cognition and brain health.
and performed at least 150 minutes weekly seems reasonable as an initial regimen. This is similar to the recommendation of the American Heart Association, which advises “...moderate-intensity aerobic physical activity for a minimum of 30 minutes on five days each week or vigorous-intensity aerobic activity for a minimum of 20 minutes on three days each week”; parenthetically, they also recommended resistance exercises “for a minimum of two days each week.”130 Future research should investigate exercise parameters to better determine the optimal recommendations for preservation of cognition and brain health.
Choice of exercise routines needs to be guided by patients' capabilities. Those with imbalance or lower limb arthritis may take advantage of health facilities that provide exercise machines used in the sitting position. The choice of exercise should also be consonant with patient interests because if too onerous it is likely to be abandoned. For very sedentary individuals, a therapist or knowledgeable trainer may be advisable to gradually introduce and escalate exercise routines and further reinforce patient effort.
CONCLUSION
These data suggest that aerobic exercise is associated with a reduced risk of cognitive impairment and dementia; it may slow dementing illness. A compelling argument can be made for this via 2 plausible biologic pathways. First, a convergence of evidence from both animal and human studies suggests that aerobic exercise may attenuate progression of neurodegenerative processes and age-related loss of synapses and neuropil. This may occur via a direct influence on neurodegenerative disease mechanisms or facilitation of neuroprotective neurotrophic factors and neuroplasticity. Not to be overlooked, however, is a second pathway, cerebrovascular disease. Cerebrovascular burden contributes to dementia risk, especially via small vessel disease (eg, lacunes and leukoaraiosis). Vascular risk factors are well known to be reduced by aerobic exercise. Thus, ongoing, moderate-intensity physical exercise should be considered as a prescription for lowering cognitive risks and slowing cognitive decline across the age spectrum.
Supplementary Material
Author Interview
REFERENCES
- 1.Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78(10):1290-1308 [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology. 2010;75(10):889-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133-1142 [DOI] [PubMed] [Google Scholar]
- 4.Terry RD, Katzman R. Life span and synapses: will there be a primary senile dementia? Neurobiol Aging. 2001;22(3):347-348 [DOI] [PubMed] [Google Scholar]
- 5.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607-613 [DOI] [PubMed] [Google Scholar]
- 6.Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daviglus ML, Bell CC, Berrettini W, et al. NIH State-of-the-Science Conference Statement: preventing Alzheimer's disease and cognitive decline [published online ahead of print April 28, 2010]. NIH Consens State Sci Statements. 2010;27(4). [PubMed] [Google Scholar]
- 8.Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182-193 [DOI] [PubMed] [Google Scholar]
- 9.Flicker L, Liu-Ambrose T, Kramer AF. Why so negative about preventing cognitive decline and dementia? The jury has already come to the verdict for physical activity and smoking cessation [editorial]. Br J Sports Med. 2011;45:465-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316-3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voss MW, Prakash RS, Erickson KI, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2.pii.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss MW, Erickson KI, Prakash RS, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394-1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon BA, Rykhlevskaia EI, Brumback CR, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017-3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166-1170 [DOI] [PubMed] [Google Scholar]
- 17.Ruscheweyh R, Willemer C, Kruger K, et al. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2009;32(7):1304-1319 [DOI] [PubMed] [Google Scholar]
- 18.Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75:1415-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176-180 [DOI] [PubMed] [Google Scholar]
- 21.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honea RA, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3-11 [DOI] [PubMed] [Google Scholar]
- 24.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498-504 [DOI] [PubMed] [Google Scholar]
- 25.Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II Prospective Cohort Study. Am J Public Health. 2005;95:2252-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jedrziewski MK, Ewbank DC, Wang H, Trojanowski JQ. Exercise and cognition: results from the National Long Term Care Survey. Alzheimers Dement. 2010;6:448-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geda YE, Roberts RO, Knopman DS, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton LE, Barnes DE, Lui LY, Yaffe K. Physical activity over the life course and its association with cognitive performance and impairment in old age. J Am Geriatr Soc. 2010;58:1322-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarmeas N, Luchsinger JA, Brickman AM, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19(5):471-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85:1694-1704 [DOI] [PubMed] [Google Scholar]
- 31.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027-1037 [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379-2388 [DOI] [PubMed] [Google Scholar]
- 33.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42:344-351 [DOI] [PubMed] [Google Scholar]
- 35.Kwak YS, Um SY, Son TG, Kim DJ. Effect of regular exercise on senile dementia patients. Int J Sports Med. 2008;29:471-474 [DOI] [PubMed] [Google Scholar]
- 36.Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The time-course of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43-48 [DOI] [PubMed] [Google Scholar]
- 37.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580-2590 [DOI] [PubMed] [Google Scholar]
- 38.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427-13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680-8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823-833 [DOI] [PubMed] [Google Scholar]
- 41.O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: a comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362-366 [DOI] [PubMed] [Google Scholar]
- 42.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588-597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasuhara T, Hara K, Maki M, et al. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007;149:182-191 [DOI] [PubMed] [Google Scholar]
- 44.Rhyu IJ, Bytheway JA, Kohler SJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stranahan AM, Lee K, Becker KG, et al. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31:1937-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356-362 [DOI] [PubMed] [Google Scholar]
- 47.Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124-130 [DOI] [PubMed] [Google Scholar]
- 48.Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219-229 [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278-2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39-47 [DOI] [PubMed] [Google Scholar]
- 51.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299-1307 [DOI] [PubMed] [Google Scholar]
- 52.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628-1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabel K, Fabel K, Tam B, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803-2812 [DOI] [PubMed] [Google Scholar]
- 54.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71-79 [DOI] [PubMed] [Google Scholar]
- 55.Titterness AK, Wiebe E, Kwasnica A, Keyes G, Christie BR. Voluntary exercise does not enhance long-term potentiation in the adolescent female dentate gyrus. Neuroscience. 2011;183:25-31 [DOI] [PubMed] [Google Scholar]
- 56.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71-124 [DOI] [PubMed] [Google Scholar]
- 57.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49-56 [PubMed] [Google Scholar]
- 58.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853-861 [DOI] [PubMed] [Google Scholar]
- 59.Tajiri N, Yasuhara T, Shingo T, et al. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 2010;1310:200-207 [DOI] [PubMed] [Google Scholar]
- 60.Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25(1):135-146 [DOI] [PubMed] [Google Scholar]
- 61.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926-2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299-305 [DOI] [PubMed] [Google Scholar]
- 63.Morra JH, Tu Z, Apostolova LG, et al. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45:S3-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181-7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638-5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695-702 [DOI] [PubMed] [Google Scholar]
- 67.Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res Mol Brain Res. 1997;49:71-81 [DOI] [PubMed] [Google Scholar]
- 68.Holsinger RMD, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res Mol Brain Res. 2000;76:347-354 [DOI] [PubMed] [Google Scholar]
- 69.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412-1421 [DOI] [PubMed] [Google Scholar]
- 70.Poduslo JF, Curran GL. Permeability at the blood-brain and bloodnerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36:280-286 [DOI] [PubMed] [Google Scholar]
- 71.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553-1561 [DOI] [PubMed] [Google Scholar]
- 72.Yasutake C, Kuroda K, Yanagawa T, Okamura T, Yoneda H. Serum BDNF, TNF-α and IL-1Î2 levels in dementia patients. Eur Arch Psychiatry Clin Neurosci. 2006;256:402-406 [DOI] [PubMed] [Google Scholar]
- 73.Laske C, Stransky E, Leyhe T, et al. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387-394 [DOI] [PubMed] [Google Scholar]
- 74.Laske C, Stellos K, Hoffmann N, et al. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 2011;14(3):399-404 [DOI] [PubMed] [Google Scholar]
- 75.Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062-1069 [DOI] [PubMed] [Google Scholar]
- 76.Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372-R377 [DOI] [PubMed] [Google Scholar]
- 77.Komulainen P, Pedersen M, Hanninen T, et al. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem. 2008;90:596-603 [DOI] [PubMed] [Google Scholar]
- 78.Swardfager W, Herrmann N, Marzolini S, et al. Brain derived neurotrophic factor, cardiopulmonary fitness and cognition in patients with coronary artery disease [published online ahead of print April 30, 2011]. Brain Behav Immun. doi:10.1016/j.bbi.2011.04.017 [DOI] [PMC free article] [PubMed]
- 79.Gold SM, Schulz KH, Hartmann S, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138:99-105 [DOI] [PubMed] [Google Scholar]
- 80.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728-734 [DOI] [PubMed] [Google Scholar]
- 81.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008;431:62-65 [DOI] [PubMed] [Google Scholar]
- 82.Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59-65 [DOI] [PubMed] [Google Scholar]
- 83.Castellano V, White LJ. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci. 2008;269:85-91 [DOI] [PubMed] [Google Scholar]
- 84.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59(suppl 7):119-132 [PubMed] [Google Scholar]
- 85.Schiffer T, Schulte S, Hollmann W, Bloch W, Struder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res. 2009;41:250-254 [DOI] [PubMed] [Google Scholar]
- 86.Nofuji Y, Suwa M, Moriyama Y, et al. Decreased serum brain-derived neurotrophic factor in trained men. Neurosci Lett. 2008;437:29-32 [DOI] [PubMed] [Google Scholar]
- 87.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardiorespiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451:152-155 [DOI] [PubMed] [Google Scholar]
- 88.Rojas Vega S, Knicker A, Hollmann W, Bloch W, Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm Metab Res. 2010;42:982-986 [DOI] [PubMed] [Google Scholar]
- 89.Correia PR, Pansani A, Machado F, et al. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics (Sao Paulo). 2010;65(11):1123-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goekint M, De Pauw K, Roelands B, et al. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol. 2010;110:285-293 [DOI] [PubMed] [Google Scholar]
- 91.Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett. 2010;479:161-165 [DOI] [PubMed] [Google Scholar]
- 92.Torres-Aleman I. Mouse models of Alzheimer's dementia: current concepts and new trends. Endocrinology. 2008;149:5952-5957 [DOI] [PubMed] [Google Scholar]
- 93.Murialdo G, Barreca A, Nobili F, et al. Relationships between cortisol, dehydroepiandrosterone sulphate and insulin-like growth factor-I system in dementia. J Endocrinol Invest. 2001;24:139-146 [DOI] [PubMed] [Google Scholar]
- 94.Arwert LI, Deijen JB, Drent ML. The relation between insulin-like growth factor I levels and cognition in healthy elderly: a meta-analysis. Growth Horm IGF Res. 2005;15:416-422 [DOI] [PubMed] [Google Scholar]
- 95.Bang P, Brandt J, Degerblad M, et al. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur J Clin Invest. 1990;20:285-292 [DOI] [PubMed] [Google Scholar]
- 96.Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492-3497 [DOI] [PubMed] [Google Scholar]
- 97.Copeland JL, Heggie L. IGF-I and IGFBP-3 during continuous and interval exercise. Int J Sports Med. 2008;29:182-187 [DOI] [PubMed] [Google Scholar]
- 98.Stokes KA, Sykes D, Gilbert KL, Chen JW, Frystyk J. Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity. Growth Horm IGF Res. 2010;20:289-294 [DOI] [PubMed] [Google Scholar]
- 99.Arikawa AY, Kurzer MS, Thomas W, Schmitz KH. No effect of exercise on insulin-like growth factor-I, insulin, and glucose in young women participating in a 16-week randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2010;19:2987-2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Borst SE, De Hoyos DV, Garzarella L, et al. Effects of resistance training on insulin-like growth factor-I and IGF binding proteins. Med Sci Sports Exerc. 2001;33:648-653 [DOI] [PubMed] [Google Scholar]
- 101.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401-1407 [DOI] [PubMed] [Google Scholar]
- 102.Borst SE, Vincent KR, Lowenthal DT, Braith RW. Effects of resistance training on insulin-like growth factor and its binding proteins in men and women aged 60 to 85. J Am Geriatr Soc. 2002;50:884-888 [DOI] [PubMed] [Google Scholar]
- 103.Liang KY, Mintun MA, Fagan AM, et al. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Um HS, Kang EB, Koo JH, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res. 2011;69:161-173 [DOI] [PubMed] [Google Scholar]
- 105.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217-4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J Neuroinflammation. 2008;5:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529-539 [PubMed] [Google Scholar]
- 108.Yuede CM, Zimmerman SD, Dong H, et al. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:426-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19:1008-1018 [DOI] [PubMed] [Google Scholar]
- 110.Wolf SA, Kronenberg G, Lehmann K, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314-1323 [DOI] [PubMed] [Google Scholar]
- 111.Parachikova A, Nichol KE, Cotman CW. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol Dis. 2008;30:121-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Richter H, Ambree O, Lewejohann L, et al. Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav Brain Res. 2008;190:74-84 [DOI] [PubMed] [Google Scholar]
- 113.Belarbi K, Burnouf S, Fernandez-Gomez FJ, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease-like Tau pathology. Neurobiol Dis. 2011;43(2):486-494 [DOI] [PubMed] [Google Scholar]
- 114.Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561-2570 [DOI] [PubMed] [Google Scholar]
- 115.Smith JK. Exercise and atherogenesis. Exerc Sport Sci Rev. 2001;29:49-53 [DOI] [PubMed] [Google Scholar]
- 116.Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise and the metabolic syndrome. Rev Diabet Stud. 2006;3:118-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kokkinos P, Sheriff H, Kheirbek R. Physical inactivity and mortality risk. Cardiol Res Pract. 2011;2011:924945 doi: 10.4061/2011/924945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rizzoli R, Bruyere O, Cannata-Andia JB, et al. Management of osteoporosis in the elderly. Curr Med Res Opin. 2009;25:2373-2387 [DOI] [PubMed] [Google Scholar]
- 119.Thomas DR. Sarcopenia. Clin Geriatr Med. 2010;26(2):331-346 [DOI] [PubMed] [Google Scholar]
- 120.Conn VS. Depressive symptom outcomes of physical activity interventions: meta-analysis findings. Ann Behav Med. 2010;39(2):128-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dunn AL. Review: exercise programmes reduce anxiety symptoms in sedentary patients with chronic illnesses. Evid Based Ment Health. 2010;13:95 [DOI] [PubMed] [Google Scholar]
- 122.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290:2015-2022 [DOI] [PubMed] [Google Scholar]
- 123.Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS ONE. 2009;4:e5521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4 suppl):27-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perrig-Chiello P, Perrig WJ, Ehrsam R, Staehelin HB, Krings F. The effects of resistance training on well-being and memory in elderly volunteers. Age Ageing. 1998;27:469-475 [DOI] [PubMed] [Google Scholar]
- 126.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis JC, Marra CA, Beattie BL, et al. Sustained cognitive and economic benefits of resistance training among community-dwelling senior women: a 1-year follow-up study of the Brain Power study. Arch Intern Med. 2010;170:2036-2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ahmad SO, Park JH, Stenho-Bittel L, Lau YS. Effects of endurance exercise on ventral tegmental area neurons in the chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid-treated mice. Neurosci Lett. 2009;450:102-105 [DOI] [PubMed] [Google Scholar]
- 129.Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094-1105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview