Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 1.
Published in final edited form as: Trends Neurosci. 2011 Jun 21;34(8):430–442. doi: 10.1016/j.tins.2011.05.005
Abstract
Alzheimer’s disease (AD) is a progressive age-related neurodegenerative disease. At the time of clinical manifestation of dementia, significant irreversible brain damage is already present, rendering the diagnosis of AD at early stages of the disease an urgent prerequisite for therapeutic treatment to halt, or at least slow, disease progression. In this Review, we discuss various neuroimaging measures that are proving to have potential value as biomarkers of AD pathology for the detection and prediction of AD before the onset of dementia. Recent studies that have identified AD-like structural and functional brain changes in elderly people who are cognitively within the normal range or who have mild cognitive impairment (MCI) are discussed. A dynamic sequence model of changes that occur in neuroimaging markers during the different disease stages is presented and the predictive value of multimodal neuroimaging for AD dementia is considered.
Introduction
About 35.6 million people are affected world-wide today by a mild to severe clinical dementia syndrome, associated with costs of about US$ 604 billion (World Alzheimer Report 2010, www.alz.org). The number of affected people is predicted to dramatically increase to 115 million by 2050 (World Alzheimer Report 2010, www.alz.org). Dementia of the Alzheimer’s disease (AD) type is the most frequent form of age-related dementia [1–2].
Clinically, initial progressive memory deficits that are eventually accompanied by more global cognitive and attention deficits are typical in AD dementia. Major pathologies in the brain associated with AD dementia have been identified, but the initial causes of such pathological changes are largely unknown. Causative factors include autosomal-dominant inheritable mutations in the genes of presenilin 1 (PS1) and presenilin 2 (PS2) and the amyloid precursor protein (APP). Familial AD (an inheritable form of AD) is typically associated with an early onset of the disease before the age of 65 years, but accounts only for about 1% of all AD cases [5]. For AD without the presence of such known genetic causes (sporadic AD), a late onset of dementia (age > 65 yrs) is typical. The most important risk factor for sporadic late-onset AD is age, with the annual incidence of clinically diagnosed AD dementia being < 1% of adults between 65–69 years old, but > 8% in adults 85 years and older [6]. Presence of the apolipoprotein E (ApoE) ε4 allele is the strongest genetic risk factor of sporadic AD [3]. Presence of at least one ApoE ε4 allele advances the age of clinical onset of AD dementia significantly (from age 84 years to 68 years) and increases the risk of AD dementia by a factor of 4 (for regularly updated meta-analyses, see http://www.alzgene.org/, last update Jan 29, 2010).
Core neuropathologies in AD include the accumulation of the protein amyloid-beta (Aβ) and the development of neurofibrillary tangles, which have been associated with neuronal degeneration and clinical symptoms of dementia [7] Such brain changes occur decades before the onset of dementia. The detection of Aβ in the brain of living subjects has been made possible by recent developments in positron emission tomography (PET) using radiotracers such as [C-11]-labeled Pittsburgh Compound-B (PiB), which label Aβ deposits [8]. Such studies have shown that substantial levels of Aβ deposits are present in subjects before the onset of dementia or even before any overt signs of cognitive impairment (as discussed later in the review). Other early brain changes include a decline in synaptic function, as assessed by [F-18]-fluorodeoxyglucose positron emission tomography (FDG-PET), gross neuronal loss causing atrophy, as measured by volumetric magnetic resonance imaging (MRI), and white matter changes within axonal projections as detected by diffusion tensor imaging (DTI)(Box 1). These different types of changes may evolve sequentially and relate to the development of cognitive impairment within the clinical course of AD [9].
Box 1. Neuroimaging methods commonly used to detect in vivo brain changes associated with neurodegeneration and cognitive decline in human subjects.
- PET, in combination with in vivo amyloid imaging agents that sbind to fibrillar Aβ deposits in the brain, has been a valuable technique for visualizing and quantifying the deposition of Aβ throughout the brain in living subjects. Current ligands include the [C-11]-labeled radiotracer PiB, or [F-18]-labelled tracers such as [F-18]-florbetaben, [F-18]-florbetapir (also called [F-18]-AV-45) and [F-18]-Flutemetamol.
- FDG-PET uses [F-18]-fluorodeoxyglucose to detect changes in glucose metabolism and blood flow in the brain. Unlike fMRI (see below), FDG-PET is mostly acquired during resting state of the subjects, due to its relatively poor temporal resolution.
- Structural MRI detects tissue changes in the grey matter, white matter and CSF. This technique is especially sensitive for grey matter volume changes due to gross neuronal loss and atrophy.
- DTI is an MRI technique that is used to assess microstructural brain changes within the white matter fiber tracts of the brain. The most commonly used index is fractional anisotropy (FA) that is determined by the degree of directionality (anisotropy) of the movement of the water molecules. A reduced FA value is reflective of axonal degradation and myelin damage in the brain.
- In fMRI, the blood oxygenation dependent (BOLD) signal is used to measure blood flow and blood oxygenation, which is believed to correlate with changes in neuronal activity at a time scale of a few seconds. fMRI has been used to assess cognitive task-related changes in brain activity and basal brain activity during resting state.
Mild cognitive impairment (MCI, see Glossary) is a well defined clinical syndrome which includes deficits in memory or other cognitive abilities that may herald AD dementia [10]. Subjects with MCI have a higher risk to progress to AD dementia [11] but a substantial proportion of MCI subjects remain stable for years or revert to normal, indicating that clinical MCI symptoms can also stem from non-AD related etiologies. This notion is substantiated by the finding that only a subset of of patients with MCI display measurable amounts of Aβ on PiB-PET scans (for a review, see [12]). Therefore, the combination of cognitive deficits (as present at the MCI stage) with abnormal values for biomarkers indicative of AD pathology (e.g. Aβ levels as measured by PiB-PET) has been proposed as the research diagnostic criteria of predementia AD, called “MCI due to AD” or “prodromal AD” [3–4, 13]. Arevision of the clinical diagnostic criteria of AD by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer's Disease and Related Disorders Association (ADRDA) [14] has been recently proposed [3, 13, 15]. The National Institute of Aging (NIA) and Alzheimer’s Association (AA) work group- still recommended purely clinical and neuropsychological criteria for routine clinical diagnosis of AD dementia and MCI [13, 15]. For the use as research diagnostic criteria of AD and MCI, however, the dual clinico-pathological definition based on clinical testing and biomarker measurements has been adopted [13, 15]. . In this Review, we use the term “MCI due to AD” in reference to subjects who show abnormalities in neuroimaging biomarkers of AD in the presence of progressive cognitive deficits such as the presence of MCI. It should be mentioned that MCI due to AD is a diagnostic label in view of clinical and biomarker evidence, but neither does this diagnosis mean that AD pathology has necessarily caused the clinical symptoms nor that subjects with MCI due to AD necessarily progress to AD dementia. Rather it is a research concept that has not yet been fully validated.
The question of the clinical fate of subjects with AD-like brain changes becomes even more urgent at an earlier time point, i.e. the preclinical phase of AD [16], when such brain changes are present without any overt cognitive deficits. The extent to which subjects with preclinical AD progress to dementia is currently not known. Of note, it has not been established that all subjects with AD-like brain changes inevitably progress to AD dementia, and many subjects with preclinical AD die of natural age-related causes before any signs of dementia are ever observed.
In the current article, we review recent imaging studies that have examined brain changes in both the preclinical and MCI phase, and evaluate the utility of neuroimaging markers for the prediction of clinical progression in these two distinct phases. The association between different imaging modalities is shown for each of the stages, and stage-specific changes in neuroimaging markers are discussed with respect to the predictive value for cognitive decline and the progression from MCI to AD dementia.
Multimodal neuroimaging changes in AD dementia
Before discussing neuroimaging changes of relevance for the preclinical and MCI due to AD stages, it is helpful to begin by briefly overviewing the typical neuroimaging findings that have been observed over the past several years in patients with clinically manifest AD dementia. Numerous studies have determined that the pattern of Aβ deposits detected in the brains of living subjects diagnosed with AD dementia using [C-11]-PiB PET closely matches the pattern predicted by the histochemical detection of Aβ in postmortem brain tissue from subjects with clinical AD [8, 12] (Figure 1). Other amyloid PET imaging tracers that have been recently developed, such as the [F-18]-labeled radioligand florbetapir (also known as [F-18]-AV-45) have shown similar results of a good correspondence between the distribution of amyloid PET and histochemically detected Aβ in the brain [11–13]. Clinical utility is expected, especially from these[F-18]-labeled ligands, which possess a radiotracer half-life that is sufficiently long enough to be produced off-site and shipped to clinics. However, compared to [C-11]PiB-PET, such novel amyloid PET ligands are currently less well characterized in clinical populations of MCI and AD patients [12].
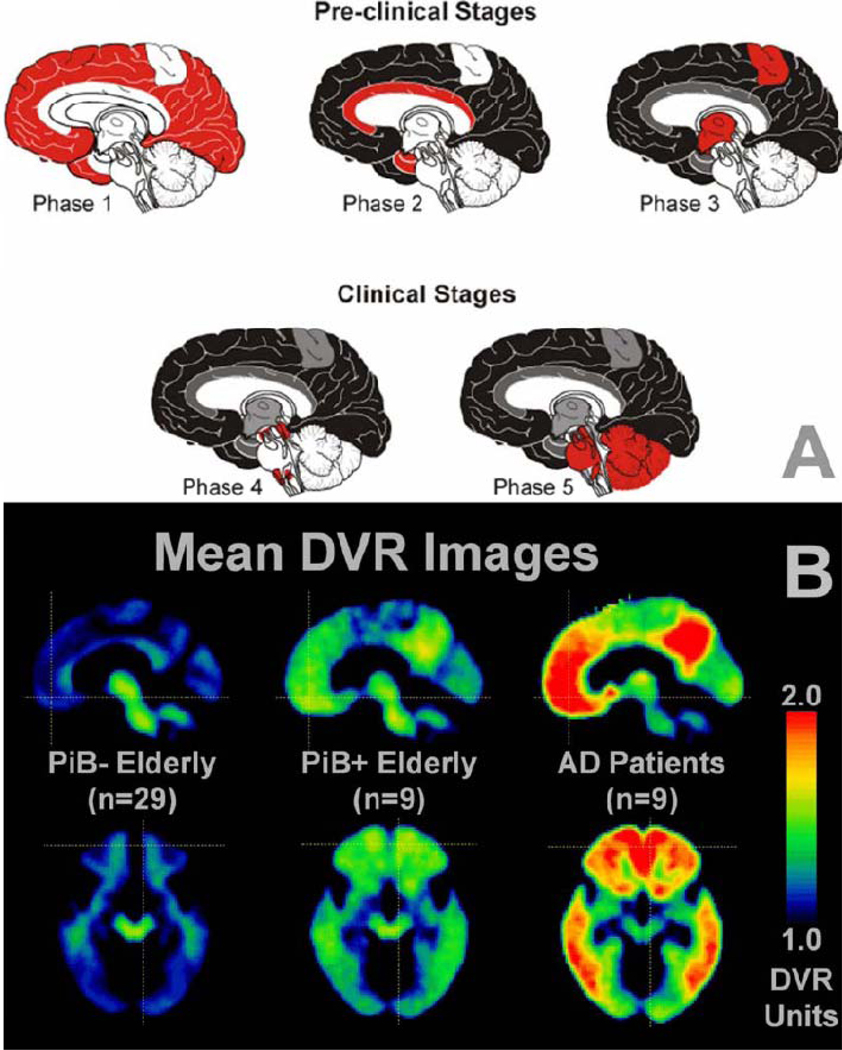
Figure 1. The progression of amyloid deposits as assessed at different stages of AD in (A) post-mortem brain tissue using immunohistochemical techniques against Aβ and (B) in vivo amyloid-PET scans.
(A) Phases (1–5) of postmortem histological appearance of Aβ in clinical AD, as based on [125]. Note that the stages refer to Aβ brain deposition and not necessarily to clinical severity. In Phase 1, Aβ is largely restricted to neocortical brain areas. In phases 2 and 3, when clinical signs of cognitive decline have yet to appear, Aβ deposits are localized throughout the neocortex, allortex, including brain areas such as the medial temporal lobe, and also begin to occur in the motor cortex and subcortical brain structures. In the clinical stages of AD (phase 4 & 5), Aβ deposits are observed globally in the brain including the brain stem and the cerebellum in the final stage of the disease (phase 5). Key: white, no Aβ deposits; red, novel Aβ deposition; grey, Aβ deposits that were missing in phase 1; black, Aβ deposits that were present already in phase 1 (B) A similar spatial pattern of progression of Aβ deposition is observed when using PiB-PET in cognitively normal elderly controls who appear to be Aβ-free (PiB−), cognitively normal elderly controls who appear to be in the early stages of Aβ deposition (PiB+) and in symptomatic AD patients [18]. Mean sagittal distribution volume ratio (DVR; cerebellum as reference) images are at the top and transaxial images at the bottom. Reproduced, with permission, from [125] (A) and [18] (B).
During the pre-clinical stages of AD, the pattern of Aβ in the brain, as derived from histochemical post-mortem brain examinations [17], shows an initial diffuse distribution within the neocortex and progresses towards the temporal allocortex, including the hippocampus, as well as subcortical brain structures during the course of the disease (Figure 1A). In the clinical stages of cognitive impairment, Aβ deposits are increased in both the amount and spatial extent throughout the brain [17] (Figure 1A). At the stage of AD, Aβ deposits are most frequent in neocortical brain regions, hippocampus, amygdala, and subcortical brain regions (e.g. striatum and basal ganglia), but only weakly distributed within the cerebellum and some brain stem areas [17]. . Consistent with the histochemical post-mortem findings on Aβ deposition in the brain, a distribution of amyloid PET uptake can be already seen widely within the brain in the preclinical stage [18]. A significant increase in Aβ deposition in predilection brain areas of AD can be observed in AD dementia patients (Figure 1B). Based on global brain PiB-PET uptake, subjects are often dichotomized into groups with high and low PiB-PET values, i.e. PiB-PET(+) and PiB-PET(−) groups [18]. Data from 15 research groups have shown that 96% of 341 clinically diagnosed AD patients and 24% of 651 cognitively normal elderly controls were PiB-PET(+) [18–27]. This finding points out the diagnostic sensitivity of PiB-PET imaging, but also demonstrates that Aβ pathology is not specific for the dementia phase of AD, similar to what has been known from brain autopsy studies [28].
Longitudinal studies including serial scans provide an assessment of individual trajectories of brain changes and give insight into variability of brain changes between different persons. A recent longitudinal study with serial PiB-PET scans has reported a mean annual increase of 4% in global PiB-PET uptake over a period of 2 years in elderly subjects with clinically diagnosed AD [29]. However, such changes are relatively small, and show substantial variability between AD patients [30]. Furthermore, some studies do not report a significant increase in global PiB-PET uptake with time, at least when assessed over a few years [31–33], suggesting that Aβ deposition may increase only slowly or has already reached a plateau in patients with AD dementia [32]. However, it should be noted that most longitudinal studies have so far included only small numbers of subjects (ie. usually <20 subjects), and due to the substantial variability between subjects, such studies may have lacked sufficient statistical power to detect a significant annual increase in PiB-PET [31–32, 34].
FDG-PET-derived measures of brain glucose metabolism and cerebral blood flow are markers of synaptic dysfunction, typically obtained during resting state (Box 1). A characteristic pattern of hypometabolism in the temporo-parietal region of the cortex, which is involved in episodic memory function, is present at the AD dementia stage [35–36]. Joint assessment of FDG-PET and PiB-PET shows the expected inverse association between both modalities within the temporoparietal region, although not frontal regions [37–38], suggesting that hypometabolism in core brain regions is associated with Aβ pathology.
A well-established finding using functional MRI (fMRI; Box 1) to examine AD-associated differences in brain activation is decreased activation in the hippocampus during episodic memory tasks [39–40]. Such results are consistent with clinical findings of early episodic memory deficits in AD subjects [41][Ref]. Abnormal fMRI-assessed brain activation can also be observed in AD subjects without engaging subjects in a cognitive task. In healthy subjects, the default network of brain regions is active during resting periods (probably a reflection of cognitive processes such as introspection) but becomes deactivated during cognitive processes that are focused on external stimulation, such as performance of a cognitive task [42]. The intrinsic functional connectivity (i.e. the coordinated co-activation of the default network’s brain regions measured with resting state fMRI) is impaired in AD dementia subjects during the resting state as compared to cognitively healthy elderly subjects [43–44]. Furthermore, the default mode network of AD subjects do not show the beneficial deactivations that healthy subjects show when assessed during memory tasks [45].The abnormal fMRI activation and connectivity within the default model network brain regions overlaps spatially with the temporo-parietal regions of FDG-PET hypometabolism in AD subjects, suggesting that both modalities exhibit a converging pattern of functional brain impairment in AD.
Structural MRI is another imaging technique that has been used to assess brain changes in AD subjects, specifically with respect to grey matter volume changes. A recent meta-analysis that included 826 patients with AD and 1027 elderly cognitively normal subjects found that the medial temporal lobe showed the strongest changes in AD dementia [46], with a volume loss of 20% in the hippocampus already present at a mild stage of AD dementia[46]. Other brain areas including the lateral temporal lobe, parietal and prefrontal lobes were found to be negatively affected as well, but changes in brain regions outside of the medial temporal lobe were smaller and more variable across studies [47].
An assessment of white matter fibers using the imaging technique of DTI has revealed that the fibers connecting the hippocampus and posterior cingulate gyrus are impaired in AD subjects to a significantly greater degree as compared to control subjects [43]. This suggests that white matter damage may relate to grey matter atrophy within the temporo-parietal brain network in AD. A recent meta-analysis of DTI studies of AD subjects confirmed a large effect size of white matter damage in the posterior cingulum, but also within major white matter bundles connecting the prefrontal cortex with the medial temporal lobe or the parietal cortex [48], suggesting that white matter damage affects large-scale networks in AD.
Taking such diverse imaging modality findings together, it is clear that gross changes in the living brain of AD dementia subjects can be visualized and measured. A hypothetical model of sequential neuroimaging changes in AD has recently been proposed [9]and Box 2 provides an overview of disease-stage specific changes for different neuroimaging markers throughout the course of the disease. The neuroimaging changes in the early stages of disease preceding dementia are discussed in the following sections.
Box 2: Biomarkers commonly used to detect the development of AD dementia.
Neuroimaging biomarkers
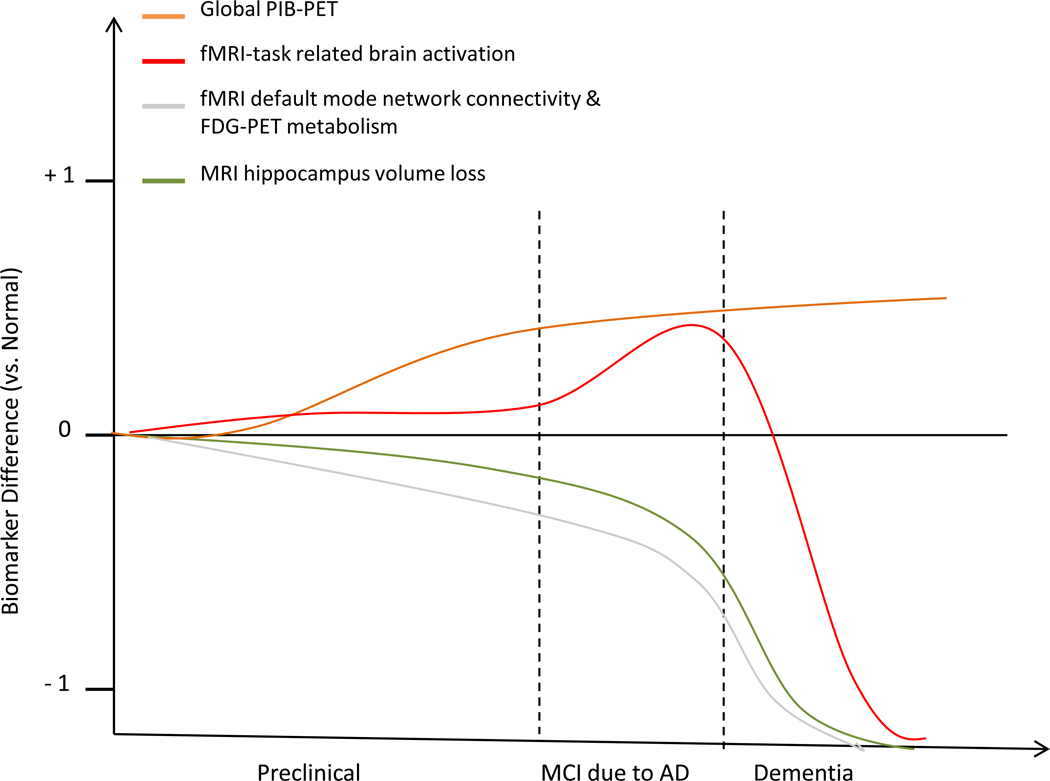
There are a variety of neuroimaging biomarkers which are in use for the prediction and validation of AD. Some of these techniques detect core AD pathology such as amyloid deposition (as measured by amyloid PET), while other imaging modalities detect neurodegeneration such as FDG-PET and MRI assessed functional and structural changes that may occur in a specific temporal order as recently proposed [9], Some neuroimaging detected changes occur already at the preclinical stage, others at the MCI stage and all of them are useful for detecting changes at the AD dementia stage (Figure I). The dynamic changes in neuroimaging markers are known to occur non-linearly throughout the stages of the disease [111–112]. However, the exact temporal sequence of structural and functional brain changes, and how changes in these various different imaging modalities are related, remains to be determined. The model sequence model displayed presents a hypothetic framework based upon currently available neuroimaging data.
- PiB-PET imaging (orange line) – studies have observed that 10–30% of elderly cognitively normal subjects have significant Aβ deposition (ie. already at the preclinical phase) [12].
- fMRI-task related brain activation (red line) - Increased PiB-PET is correlated with abnormally increased hippocampus activity during memory task performance. Such functional changes begin to be observed in the late preclinical stage and decline in late stages of MCI [62, 92]. Abnormally decreased hippocampal activity is observed in these later stages [41, 45].
- fMRI assessed default Mode Network (grey line) - begins to decline in activation levels during the preclinical stage, in correlation with PiB-PET [22, 62, 113]. Resting state FDG-PET metabolism (grey line) is reduced in ApoE e4 carriers without cognitive impairment that may, however, not be accounted for by preclinical deposition of Aβ pathology [126].
- Volumetric MRI (green line) - MRI-detected grey matter atrophy starts primarily, though not exclusively, in the hippocampus, in inverse correlation with PiB-PET levels, and continues to decline throughout the progression of the disease [70, 112]. Widespread cortical and subcortical atrophy is observed at the dementia stage [114].
Other biological-based biomarkers
Another major category of primary biomarker candidates for AD consists of CSF biomarkers (for review, see [115]). CSF samples can be obtained from subjects by lumbar puncture and analyzed in standard laboratory tests. Major CSF-derived markers include:
- Soluble Aβ1–42, which correlates inversely with Aβ plaque deposition in the brain [116] and global PiB-PET scores [117].
- Phospho-tau, which correlates positively with neurofibrillary pathology in the brain [118]
- Total tau, which is thought to be associated with neuronal loss in the brain, since it is elevated in neurodegenerative diseases such as Creutzfeldt-Jakob disease, or stroke, that show large neuronal loss but are inconspicuous of AD-like neurofibrillary pathology (for reviews, see [127–128].
Multimodal neuroimaging in preclinical AD
In this section, we will provide an overview of neuroimaging studies that have assessed changes in subjects with preclinical AD, i.e. in elderly subjects who show normal cognitive abilities but have already AD-like brain abnormalities such as discussed above. Findings from amyloid PET studies will be discussed first and compared to findings on amyloid PET in AD dementia. Functional and structural brain changes (as assessed by FDG-PET and MRI methods) will be discussed subsequently, especially in relation to early Aβ pathology, taking into account the influence of ApoE genotype as a potential modulating factor of such brain changes at this early disease stage.
Amyloid-PET
As discussed above, PET imaging studies using the PiB ligand have revealed that about 10–30% of elderly cognitively normal subjects are PiB(+) [12]. ApoE genotype is known to modulate the level of Aβ deposition in the brain [49], and homozygous ApoE ε4 carriers show the highest proportion of PIB(+) [50]. A recent study that imaged amyloid in the brains of subjects within the age range of 18–50 years using [F-18]-AV-45-labeled PET revealed an absence of Aβ deposits, regardless of ApoE genotype [11]. This suggests that substantial elevations in Aβ occur only after 50 years of age [51]. An increase in Aβ levels, as assessed by PiB-PET, has been associated with more rapid memory and global cognitive decline [52]; whether cognitively normal subjects with increased PiB-PET uptake will show higher likelihood to progress to dementia is, however, unknown and studies have so far not included sufficient follow-up durations to adequately assess this question. Vulnerability factors (such as sub-clinical cerebrovascular disease) and brain/cognitive reserve factors are likely to play a modulatory role in the process of Aβ deposition [26, 53–55]. However, the strongest determining factor at this stage remains the ApoE4 allele, therefore this genetic information is important to take into account when examining functional and structural brain changes in subjects.
FDG-PET
A recent study of cognitively normal subjects who were characterized as PiB-PET(+) did not find any evidence of FDG-PET hypometabolism in the prefrontal cortex and parietal lobe during resting state [56]. However, additional studies are needed to replicate this finding and to assess whether such a conclusion is common across different experimental groups. Other studies which have assessed FDG-PET hypometabolism in a subset of cognitively normal subjects that carry the ApoE ε4 allelle have detected changes in a subset of regions affected in AD [57–58], and these changes predict progression to MCI [59]. Since ApoE ε4 increases the deposition of Aβ already in the preclinical stage [60], it is possible that such genetic effects on FDG-PET are related to the presence of Aβ pathology. However, results from FDG-PET data obtained in ApoE ε4 carriers within the 20–39 year age range suggest that a fundamental metabolic abnormality is present already at an age when substantial fibrillar Aβ deposition is unlikely to have developed [61]. This suggests that ApoE genotype may exert an influence on FDG-PET metabolism beyond its influence on Aβ in the preclinical phase.
FMRI
FMRI studies have uncovered several AD-like alterations in brain activity that are associated with Aβ or ApoE ε4 genotype at an early preclinical phase. Although memory task-induced brain activation within the medial temporal memory system does not appear to be strongly affected in cognitively normal PiB-PET(+)subjects, the default-network shows an AD-characteristic lack of deactivation, and even reverts into paradoxical increases in some of the network’s brain areas [62–63]. In further parallel to the findings in AD dementia, the functional connectivity between brain regions of the default mode network is disrupted in elderly PiB-PET(+) adults [22, 64–65]. These findings suggest that Aβ is associated with abnormal regulation of large-scale networks, especially within the default mode brain regions, even at this early preclinical stage [62]. However, disruption of default mode network connectivity has recently been reported in elderly adults who carry the ApoE ε4 allele but who are PiB-PET(−)[66] and in young ApoE ε4 carriers who are unlikely to have substantial Aβ deposition [53]. Furthermore, the presence of the ApoE e4 allele has also been associated with increased cortical activation during a visual learning task [67] and disruption of functional connectivity of default mode network [68]. Thus, similar to the findings of an ApoE effect on FDG-PET at the preclinical stage, ApoE ε4 genotype and elevation of Aβ may both influence - to some extent independently - brain activation [66].
Structural MRI
There is now accumulating evidence that Aβ deposition in the brain is associated with grey matter atrophy in the preclinical stage of AD. In the so far largest combined cross-sectional MRI & PiB-PET study that included 135 elderly cognitively normal subjects, PiB(+) subjects showed higher hippocampus and cingulate cortex atrophy compared to PiB(−) subjects [69]. Furthermore, clinical longitudinal assessment revealed that PiB(+) subjects, but not PiB(−) subjects, showed decline in episodic memory and working memory over a time span of 16 years that preceded or succeeded the PET scan [69]. Consistent with these findings, other cross-sectional studies have shown an association between PiB-PET and hippocampus volume loss [70–71] and posterior cingulate gyrus atrophy [72]. A major question is what is the threshold level of Aβ deposition that is associated with atrophy? Some researchers argue that even small levels of elevation in Aβ, including at levels that are currently defined as PIB(−), may already be associated with increased brain atrophy [70]. On the other hand, the observation in PiB-PET studies that cognitively normal subjects show a bimodal segregation into groups of high and low levels of PIB binding [i.e. PIB(+) and PIB(−)] [34] suggests the existence of a distinct group of elderly adults with abnormal and potentially disease-specific elevations of Aβ. At this stage, any differences in the sensitivity of using absolute levels of Ab-labeled, versus the identification of two distinct groups of PIB(+) vs. PIB(−), for the detection of brain atrophy and prediction of dementia have yet to be validated. Furthermore, other pathological events such as the formation of soluble Aβ oligomers [73] ,or the development of tau-related neuropathologies [74], are likely to be critical factors in Aβ-related neurodegeneration, and will need to be taken into consideration in future assessments of Aβ-associated brain atrophy.
ApoE ε4 genotype may also be an important determinant of brain atrophy at the preclinical stage. Similar to cross-sectional findings in AD dementia subjects [75], elderly cognitively normal subjects who carry the ApoE ε4 allele have greater hippocampus volume loss when compared to elderly cognitive normal adults without that allele [76]. A large longitudinal population-based study with serial MRI scans in 1186 healthy subjects, showed that cognitively normal subjects with two ApoE ε4 alleles (i.e. homozygous) had a faster atrophy rate for the hippocampus and whole brain over a 4 year interval as compared to heterozyguous ApoE ε4 carriers and non-carriers [77]. . Thus, both elevated Aβ levels and ApoE genetic risk are associated with grey matter atrophy already in subjects without cognitive impairment.
Microstructural white matter changes, such as detected by DTI, have not been assessed in preclinical subjects in association with PiB-PET so far, and only few studies have investigated the impact of ApoE genotype on white matter changes in this subject group. cognitively normal adults with at least one copy of ApoE ε4 show reduced white matter integrity (as measured by a reduced FA value, Box 1) in the posterior cingulum, corpus callosum, and other major white matter bundles that connect different lobes of the brain and support the default mode network [76]. These changes were observed in young adults (20–35 years) [78], middle aged (49–65 years) [79] and elderly cognitively normal subjects (> 65 years) [78]. Such changes are reminiscent of the early functional brain differences that were observed in ApoE ε4 carriers at both a young and old age, suggesting that these changes may occur independently of Aβ, as discussed above.
In summary, the amyloid PET findings are in accordance with histochemical autopsy studies demonstrating substantial deposition in the neocortex in elderly subjects without cognitive deficits, who are in the preclinical stages of AD. These PiB-PET(+) subjects already already a number of structural and functional brain alterations including reduced functional connectivity between brain regions of the default mode network and accelerated medial temporal lobe atrophy that is associated with episodic memory impairment. Such functional and structural brain changes in PiB-PET(+) subjects are reminiscent of brain changes seen in AD dementia. Whether these early brain changes at the preclinical stage herald clinical progression to AD dementia is, however, currently known. In the next section, we review neuroimaging findings in subjects who are at a clinically more progressed stage, i.e. MCI.
Multimodal neuroimaging changes in MCI
In the following section, we review neuroimaging findings in subjects with clinically manifest MCI without full-blown dementia. The same imaging modalities as discussed previously are reviewed to allow for a direct comparison with findings in the preclinical AD and AD dementia stages discussed above.
Amyloid-PET
About 33 – 61% of subjects diagnosed with MCI show AD pathophysiology by virtue of being PiB-PET(+) [80]. The proportion of PiB-PET(+) subjects is intermediate compared to the proportion of PiB-PET(+) subjects found at the preclinical stage and the AD dementia stage (see above), suggesting an increase in the frequency of PiB-PET(+) from preclinical to clinical AD dementia stages. Clinical longitudinal studies show that subjects with MCI who convert to AD dementia within 2–3 years have higher baseline PiB-PET levels than MCI subjects who do not clinically worsen within that timeframe [80–81]. The evidence for the utility of PiB-PET in identifying AD pathophysiology in the setting of clinical MCI is becoming increasingly convincing. When pooling data from nine PiB-PET studies that examined the PIB-PET status in MCI [20, 23, 27, 81–82], overall 161 of 272 MCI subjects were amyloid-positive (ie. 59%). Five of these studies (155 MCI patients) included longitudinal clinical follow-up assessments, which showed that between 20–50% of subjects progressed to clinical AD over 1–3 years; the large majority of these MCI-AD converters were PiB-PET(+) at baseline [20, 23, 81–83]. This number will almost certainly increase as the follow-up time is increased to at least five years for all subjects. In contrast, only a relatively small proportion of PiB-PET(−) MCI patients (~8–15%) progressed to clinical AD in these studies [20, 23, 81–83]. Amongst PiB-PET(+) subjects with MCI, it is known that the ApoE ε4 genotype accelerates the time to progression from MCI to AD [81]. Thus, the combination of amyloid-imaging together with genetic determination of ApoE alleles is valuable for predicting higher risk of progressing to AD at the MCI stage.
FDG-PET
The AD-like hypometabolic pattern (e.g., decreased signals in the precuneus and temporo-parietal cortex) has likewise been observed in MCI subjects (for a review, see [84]; Figure 2). Indeed, several studies have shown that abnormalities in FDG PET predict progression from MCI to AD [85–87]. Based upon FDG-PET metabolism within the temporo-parietal brain regions and posterior cingulate gyrus, the test accuracy to predict conversion from MCI to AD dementia within 1 – 1.5 years ranged between 80–90% among these three studies, and both the sensitivity and specificity to predict AD dementia were above 80% in each study [85–87]. The predictive accuracy was even higher in ApoE ε4 carriers [87], suggesting that ApoE genotype may provide additional clinically relevant information for the prediction of AD dementia by FDG-PET hypometabolism. Whether FDG-PET hypometabolism in MCI is linked to deposition of Aβ is not clear at this stage. Only few studies have examined the association between FDG-PET and amyloid PET at the MCI stage, reporting weak negative associations between FDG-PET and PIB-PE [88], which is consistent with the observation that FDG-PET and PIB-PET provide complimentary information for the prediction of conversion from MCI to AD [89]. Factors such as cognitive reserve may modulate the association between FDG-PET assessed brain metabolism and amyloid PET uptake [56].l conversion to AD.
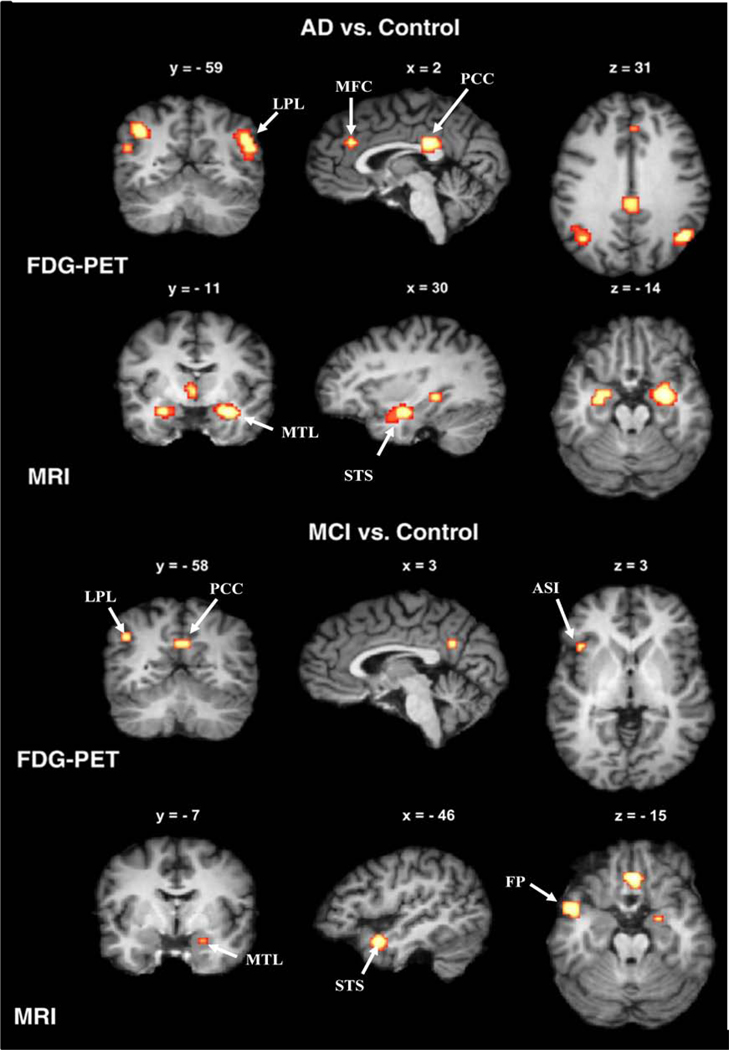
Figure 2. Brain regions of functional and structural brain abnormalities that show strongest effects for the diagnosis and prognosis of AD dementia.
Yellow-to-red labeled clusters show significantly decreased activity, as measured by FDG-PET (first and third row), and reduced grey matter volume, as measured by structural MRI (second and last row) in AD subjects (top half) and MCI subjects (bottom half) as compared to elderly cognitively normalcontrol subjects in a meta-analysis of voxel-based MRI and FDG-PET studies [47]. Note that in AD subjects, reduced FDG-PET is present in clusters of the posterior cingulum cortex (PCC), lateral parietal lobe (LPL), and medial frontal cortex (MFC), (top row), and is already present in the inferior PLP and PCC at the stage of MCI, in addition to a small cluster in the anterior superior insula (ASI) The majority of the grey matter volume decreases in AD appear in the medial temporal lobe (MTL) extending towards the superior temporal sulcus (STS), and these volume reductions are already present to a smaller extent in MCI subjects. Clusters of significant group differences were projected onto standardized MRI images of brain slices in coronal view (left column), sagittal view (middle column), and axial view (right column). The x, y, and z values label the slice coordinates within the standard Talairach atlas space. Adapted, with permission, from [47].
FMRI
During resting state, disrupted connectivity of the default mode network is present in MCI [90], similar to what was reported in the preclinical stage in PiB-PET(+) subjects and subjects with AD dementia (as discussed above). Reduced default mode network connectivity has been associated with a higher risk of converting to AD dementia within a 3–4 year follow up interval [91], suggesting that impaired default mode activity is predictive of impending clinical progression to dementia. Cognitive–task associated changes in fMRI have also shown to be predictive of cognitive decline in MCI [92]. Specifically, hyperactivation within the medial temporal lobe has been observed in the mild stages of MCI [45, 93] in association with subsequent cognitive decline [92]. Such hippocampus hyperactivation during memory performance is already present in PIB-PET(+) subjects with a clinical dementia rating (CDR) of 0.5, who are in the transition phase between preclinical AD and MCI due to AD [62]. This suggests that memory-related hyperactivation of specific brain regions becomes abnormal early in the course of the disease. Interestingly, at clinically more advanced stages of MCI and in AD dementia subjects, this memory task-induced hyperactivation of the temporal-parietal memory network, however, turns into an activation deficit [45]. Specifically what this initial hyperactivation is due to and how that turns into an activation deficit remains unknown at this time, however, it may represent a transient phase of impending breakdown of neuronal networks. Possibly, the hyperactivation of particular brain areas reflects mechanisms of compensation within neural circuits. Alternatively, it may be more directly related to Aβ-pathology, either as a consequence of Aβ-induced hyperexcitability of neurons [94–95] or by increasing vulnerability to Aβ toxicity [96], but the exact mechanism remains to be determined in future studies. Whatever the underlying reason, initial evidence suggests that hyperactivity may reflect impending neuronal dysfunction and clinical decline [92].
Structural MRI
Several studies have shown that increased brain levels of Aβ (as assessed by PiB-PET), or decreased cerebral spinal fluid (CSF)-Aβ levels, are associated with atrophy of the hippocampus and other brain structures [70, 97] in subjects with MCI, as assessed by volumetric MRI. These findings are consistent with those observed in preclinical AD. Multivariate statistical analyses have shown that an AD-typical pattern of MRI-assessed brain atrophy is present in a subgroup of MCI subjects [98–99]. Specifically, an atrophy pattern including the medial temporal lobe, posterior cingulate and orbitofrontal cortex is predictive of the development of AD dementia in MCI [99].
Apart from grey matter volume changes, increased white matter abnormalities have been observed in subjects with increased risk of AD. DTI abnormalities within the medial temporo-parietal network associated with episodic memory impairment have been repeatedly reported in the literature (for a review, see [100]) and are among the brain regions that show the strongest predictive value for the discrimination between MCI and cognitively normal subjects [48, 101–102]. Currently, there are only a few clinical longitudinal studies available for DTI [103–105], however, a recent study has shown that DTI-assessed diffusivity of the left hippocampus is predictive of the conversion from MCI to AD within 1.5 years [106].
In summary, AD-typical structural and functional changes at the MCI stage are reflected by changes in a variety of measures. Such measures include significant levels of amyloid PET binding in the brain, fMRI-assessed task-related medial temporal lobe hyperactivation and default network dysfunction, FDG-PET assessed temporo-parietal hypometabolism, MRI-assessed medial temporal lobe atrophy and diffusion changes. In the next section, we review data in MCI subjects of whether the combination of such markers is beneficial for predict the risk of developing AD dementia within a few years.
Combining neuroimaging markers for the prediction of clinical progression to AD dementia
There is now accumulating evidence that a combination of neuroimaging markers shows additive effects of different modalities for the prediction of progression from MCI to AD dementia. Cross-sectional studies suggest that PiB-PET and hippocampus volume provide complementary information for the diagnostic classification of AD dementia [34]. Within MCI subjects, the combination of structural MRI to assess hippocampus volume and DTI in the posterior parietal lobe contributes to the prediction of the severity of the memory deficits [107]. Neuroimaging studies with longitudinal clinical follow-up have shown that the combination of MRI-assessed grey matter atrophy and FDG-PET hypometabolism within the posterior cingulate is associated with increased risk of clinical progression within 2 years when compared to either predictor alone [108]. When neuropsychological measures are included as predictors as well, MCI subjects who are abnormal on both FDG-PET and episodic memory are ~12 times more likely to progress to AD than subjects who were normal on these measures, which is significantly higher when compared to an increase in risk by ~4 times based on episodic memory deficits alone [109]. In that study, the hippocampus volume did not add to the predictive accuracy, suggesting redundancies between the different predictors tested. In fact, the prediction accuracy of some of the best single predictors including right entorhinal cortical thickness, right hippocampus volume, or trail making test B (TMT-B) may only be marginally improved by combining multiple markers [110]. Thus, although the risk prediction of AD may be increased by combining markers, case-by-case decisions of classifying subjects into MCI to AD converters may not necessarily be improved by the use multiple neuroimaging markers. For the assessment of the utility of neuropsychological predictors as discussed above, it should be taken into account that neuropsychological performance is part of the definition of AD dementia, and thus neuropsychological predictors may correlate with progression to dementia, especially in studies that use only short clinical follow-up time intervals.
Eventually, any gain in predictive accuracy of a biomarker or test for the detection of AD needs to be weighed against costs in terms of side effects (e.g. radioactivity of PET tracers), potential invasiveness of methods (eg. lumbar puncture in the case of CSF-based tests; Box 2), and availability of the technology (e.g. the imaging scanners and/or radiotracers may only be available at major hospitals and research centers). Notably, there are also psychological costs for the individual consequent to learning about the presence of AD-like brain pathology and possible clinical prognosis based on such biomarker test results. Such considerations are especially important to carefully weigh in light of the fact that no cure for AD or treatment to effectively slow or halt disease progression currently exists. At this stage, the main utility of biomarker-based early detection of AD is in identifying subjects with high likelihood of progression to AD dementia in clinical trials for the testing of novel drugs for disease prevention or treatment. Large-scale cost effectiveness studies are needed to evaluate the utility of biomarker-aided prediction of AD in clinical practice, and additional studies to better understand the underlying neurobiological changes that are occurring during disease progression in AD are needed (Box 3).
Box 3. Outstanding questions.
- Can early functional outcome measures or surrogate biomarkers (eg. based on fMRI ) be developed for use at early stages of disease progression stages prior to established neurodegeneration? If so, is it possible to fully reverse such early-changes, or at least halt disease progression, using disease-modifying treatments?
- What are reliable predictors of progression to AD dementia in cognitively normal subjects with high levels of Aβ in the brain?
- Are fMRI hyperactivation within the medial temporal lobe and disrupted functional connectivity within the default mode network reliable predictors of AD dementia and what is the underlying pathophysiological mechanism?
- What are reliable threshold values (i.e. quantitative decision criteria) for each of the different neuroimaging biomarker methods that can be used by clinicians to estimate the risk of AD in non-demented subjects and to diagnose AD dementia?
- What is the most cost-effective multi-biomarker model for the prediction of AD dementia?
- Is there a distinctive multi-modal neuroimaging marker signature from the first adaptational and functional brain changes to fully established neurodegeneration and dementia in AD subjects?
Summary and Perspectives
Neuroimaging methods are capable of detecting substantial brain changes not only in subjects with AD dementia, but also in subjects in the mildly symptomatic MCI due to AD stage and even in cognitively normal subjects who may be in the preclinical stage of AD (Box 2). There are imaging modality-specific changes within these clinical/preclinical stages. In the preclinical stage, Aβ deposits are already present in a substantial number of subjects. Such changes are associated with increased grey matter brain atrophy, especially within the hippocampus, a key region affected in AD dementia. FMRI-assessed resting state functional connectivity and reduced deactivation of the default network is already impaired in association with Aβ deposition in the preclinical phase. Hyperactivation within the hippocampus memory network during memory performance occurs early in the MCI phase but reduced hippocampus activation is visible shortly before progressing to dementia, suggesting that such hippocampus hyperactivation is a transient sign impending clinical worsening. In contrast to fMRI detected changes in brain activity, FDG-PET abnormalities have not yet been associated with Aβ deposition in the preclinical phase and only become detectable in the MCI phase. Therefore, resting state fMRI may be a more sensitive measure than FDG PET to detect early changes associated with AD pathology. DTI lacks a clear characterisation at the preclinical stage so far, but similarly to the other neuroimaging markers shows specific patterns of alteration in association with ApoE genotype. Across the various imaging modalities, ApoE genotype is an important modulating factor. The presence of the ApoE ε4 allele is associated with accelerating Aβ deposition, hippocampus atrophy and default mode network dysfunction, beginning in the preclinical stage.
At the stage of MCI, AD-like patterns of brain changes are observed. These include a high proportion of amyloid deposits, medial temporal and parietal brain atrophy, and temporo-parietal FDG-PET hypometabolism, all of which are predictive of short-term conversion to AD. Although imaging modalities show independent contribution towards the prediction of AD, there are considerable redundancies among the neuroimaging modalities for the prediction of AD, and additional costs of combining different imaging acquisitions need to be weighed against the actual gain in prediction accuracy. Accessibility to imaging equipment can also be a limiting factor. The [11-C]PiB-PET radioligand, with a relatively short half-life of decay, can be manufactured most likely only at major clinical centers where the imaging equipment is located on-site. However, the development of other 18F-labelled tracers of amyloid with properties more amenable for wider distribution may supplement PiB-PET for clinical use. Of note, the Food and Drug Administration (FDA) has given given conditional support for the use of the [F-18]-AV-45 PET radiotracer, which should significantly enhance the use of amyloid-imaging during the clinical and preclinical stages of AD.
Already many in the research community appear ready to accept biomarker-aided diagnostic criteria for research purposes, such as been proposed for the revision of the NINCDS-ADRDA criteria, in part because they provide useful hypotheses for future verification. However, in view of the current evidence, further studies concerning the specific approaches to implement these criteria need to be conducted, including the establishment of quantitative criteria for different biomarkers and their extensive validation (by observing conversion to dementia of the AD type and pathological verification). Only after the clinical utility of such biomarker-aided criteria for the prediction of dementia are firmly established, can such measures be fully adopted for standardized clinical trials and routine clinical use. Finally, almost all results are reported in carefully selected cohorts, not epidemiological community-based populations; thus generalizability of these results to the population as a whole needs to be established. Such a validation process will likely take many years. This is especially true when one considers that new biomarkers, including blood and CSF assays of compounds not currently known (ie. evolving from exploratory proteomic approaches), and new imaging radiotracers and techniques (such as PET measures of tau or inflammation, and high-field MRI methods to detect brain amyloid) will likely emerge. Nevertheless, such efforts to develop effective, reliable and robust biomarkers for AD are critical given the significant number of people who will continue to be diagnosed with AD dementia in the coming decades.
Box 2 Figure I.
Hypothetical model of various different neuroimaging biomarkers and their predicted utility during disease progression, as based on a variety of studies discussed in this review.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R01AG10897, P41RR023953, and U01AG024904 (to MW), which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs (VA) Medical Center, San Francisco, California. Additional support came from by NIH grants: P50 AG005133, R37 AG025516, P01 AG025204 (to BK), NIA: P01 AG036694; R01AG027435 (to RS), the Science Foundation Ireland (SFI) investigator neuroimaging program award 08/IN.1/B1846 (to HH), the “Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz” (LOEWE) neuroimaging-neurophysiology research program grant „Neuronale Koordination Forschungsschwerpunkt Frankfurt“ (NeFF), Neuronal Coordination, Neurodegeneration & Alzheimer’s disease project (to HH).
Glossary
Amyloid-beta (Aβ)
A peptide that is the main component of amyloid deposits in forms of plaques in the brain, such as detected by amyloid PET tracers. The Aβ peptide is cleaved at different lengths of amino acid chains (isoforms) from the amyloid precursor protein (APP), and the isoform Aβ1–42 shows increased tendency to aggregate into plaques. Although plaques are abundant in the AD brain, the exact mechanism of neurotoxicity of Aβ has not been established. Recent evidence suggests a role of the formation of soluble Aβ oligomers in neurodegeneration [73].
Alzheimer’s disease (AD) dementia
According to the traditional NINCDS-ADRDA criteria, AD dementia includes gradual onset of cognitive impairment in episodic memory and at least one other domain [14]. The recent NIA-AA revision of the diagnostic criteria does not make impairment in the episodic memory domain mandatory anymore, but requires impairment in amnestic or nonamnestic cognitive abilities [15]. Presence of impaired social functioning and instrumental activities of daily living is considered as the crucial difference for the clinical diagnosis of AD, as opposed to MCI.
AD according to the Dubois criteria [3–4]
Presence of episodic memory impairment and abnormality on at least one of the core MRI, PET, or CSF based biomarkers of AD pathology. Encompasses both subjects with abnormal biomarkers who show clinical AD dementia and those who only show mild memory impairment (i.e. prodromal AD).
Apolipoprotein E (ApoE) gene
The gene encodes, through three different alleles (ε2, ε3, ε4), three major isoforms of the ApoE protein, which are known to be involved in lipid metabolism. The ApoE ε3/3 genotype is the most common form (prevalent in ~50–70% of the population). The ApoE ε4 allele, which occurs only in ~10–15% of the population, accounts for the majority of AD cases [119] is also associated with increased risk of other forms of dementia, such as vascular dementia and Lewy body dementia [120]. ApoE ε4 shows a gene dose effect in that presence of two ApoE ε4 alleles is associated with a higher risk compared to a single ApoE ε4 allele [120]. In AD, the ApoE ε4 isoform has been associated with increased Aβ deposition, although Aβ-independent mechanisms of ApoE, such as reduced adult neurogenesis in the hippocampus or phosphorylation of the protein tau (associated with increased tau pathology in AD) have also been demonstrated [119].
Biomarker for AD
A quantifiable surrogate measure of brain pathologies underlying AD dementia [121]. Neuroimaging-derived measures have been proposed as core biomarkers for the early detection of AD. Other types of biomarker candidates that are considered as core biological indicators of AD pathology, include CSF-derived measures of Aβ1–42, or the protein tau as a measure of neurofibrillary tangles (reviewed in [115]).
Clinical dementia rating (CDR)
A clinical rating scale where the physician assesses performance in five different cognitive domains based on a semi-structured interview of both the patient and the informant [122]. The global CDR score ranges from 1 to 5, where a score of 0 means no cognitive impairment and a score of 5 corresponds to severe dementia. A score of 0.5 is often used in the diagnosis of MCI in clinical research studies [123].
Default mode network
During resting state, i.e. when a person is not challenged by external stimulation (e.g. a memory task), a brain circuit called the “default mode network” including the medial frontal, temporal and parietal brain regions are activated in healthy subjects. This network typically demonstrates beneficial deactivations during memory encoding in healthy subjects. Alterations in the activation pattern of the default network are also observed in numerous neurological disorders, including in AD and schizophrenia, as well as in aged subjects [42].
Mild cognitive impairment (MCI) (core clinical criteria)
clinical diagnosis that includes progressive cognitive decline in memory (amnestic MCI) or other cognitive domains (non-amnestic MCI). Subjective cognitive impairment reported by subjects themselves or their relatives in addition to cognitive impairment as assessed by neuropsychological tests, without affecting social functioning and instrumental activities of daily living [124]. According to the core clinical criteria of MCI proposed by the NIA-AA work group [13], other potential causes of cognitive decline - including vascular, traumatic or medical causes - or presence of symptoms of neurodegenerative diseases other than AD should be sought to be excluded by the clinician [13].
MCI due to AD (research criteria)
Subjects in this group fulfil the core clinical criteria of MCI, but show also pathophysiology of AD as confirmed by biomarkers [13]. This dual clinic-pathological definition of MCI due to AD is similar to prodromal AD (see below), with the difference that only progressive episodic memory impairment in addition to abnormal biomarkers are required to meet the criteria for prodromal AD.
Preclinical AD
Term that is used to refer to clinically normal subjects with evidence of AD pathology as detected by biomarkers. Subjects may show subjective memory impairment or very subtle cognitive decline, but would not demonstrate impairment of cognitive ability as detectable by standard neuropsychological tests. Such a definition also includes subjects with genetic mutations that cause AD but with an absence of cognitive impairment (presymptomatic AD) [4]. A classification scheme of progressive accumulation of AD brain changes has recently been proposed by the NIA-AA work group [16]. Preclinical AD is a research concept and not recommended for clinical routine diagnosis. It is currently unknown what proportion of patients with preclinical AD go on to develop AD dementia.
Prodromal AD
Diagnostic classification for research purposes that includes the presence of episodic memory impairment (without affecting normal social functioning and instrumental activities of daily living) and, in addition, abnormalities as assessed with one or more biomarkers [4].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB-PET imaging technology described in this manuscript. W.E.K. is a co-inventor of PiB and, as such, has a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. R.A.S. has served as a consultant for Elan, Janssen, Pfizer, Link and Bristol-Myers Squibb (BMS), and receives research support from Elan, Janssen, and BMS. H.H. has received research support from Novartis and Janssen-Cilag. He has served as a consultant for BMS, Pfizer, Novartis, Janssen-Cilag and Merz Pharmaceuticals. M.W.W. has been on Advisory boards for Lilly, Araclon and Institut Catala de Neurociencies Aplicades, Gulf War Veterans Illnesses Advisory Committee, Veterans Affairs Central Office, Biogen Idec, Elan/Wyeth Alzheimer’s Immunotherapy Program North American Advisory Board, Novartis Misfolded Protein Scientific Advisory Board Meeting, and the Banner Alzheimer’s Institute Alzheimer’s Prevention Initiative Advisory Board Meeting. He has been a consultant for Elan/Wyeth, Novartis, Forest, Ipsen, Daiichi Sankyo, Inc., Astra Zeneca, Araclon, Medivation/Pfizer, TauRx Therapeutics LTD, Bayer Healthcare, Biogen Idec, Exonhit Therapeutics, Servier, and Synarc. He has received research/ travel support from Merck, Avid, Elan/Wyeth, Forest, Innogenetics, NeuroVigil, Inc., Siemens, AstraZeneca, Lilly, Pfizer, and Novartis, and holds stock options with Synarc and Elan.
References
- 1.Ott A, et al. Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol. 1998;147:574–580. doi: 10.1093/oxfordjournals.aje.a009489. [DOI] [PubMed] [Google Scholar]
- 2.Seshadri S, et al. Operationalizing diagnostic criteria for Alzheimer's disease and other age-related cognitive impairment-Part 2. Alzheimers Dement. 2011;7:35–52. doi: 10.1016/j.jalz.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet neurology. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 5.Pastor P, and Goate AM. Molecular genetics of Alzheimer's disease. Curr Psychiatry Rep. 2004;6:125–133. doi: 10.1007/s11920-004-0052-6. [DOI] [PubMed] [Google Scholar]
- 6.Hebert LE, et al. Age-specific incidence of Alzheimer's disease in a community population. Journal of American Medical Association. 1995;273:1354–1359. [PubMed] [Google Scholar]
- 7.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 8.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell J, et al. Outcome in subgroups of mild cognitive impairment (MCI) is highly predictable using a simple algorithm. J Neurol. 2009;256:1500–1509. doi: 10.1007/s00415-009-5152-0. [DOI] [PubMed] [Google Scholar]
- 12.Quigley H, et al. PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry. 2010 doi: 10.1002/gps.2640. [DOI] [PubMed] [Google Scholar]
- 13.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.McKhann GM, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thal DR, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 18.Aizenstein HJ, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edison P, et al. Microglia, amyloid, and cognition in Alzheimer's disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiology of disease. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Wolk DA, et al. Amyloid imaging in mild cognitive impairment subtypes. Annals of neurology. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drzezga A, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 22.Hedden T, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagust WJ, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forsberg A, et al. High PIB retention in Alzheimer's disease is an early event with complex relationship with CSF biomarkers and functional parameters. Current Alzheimer research. 2010;7:56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 25.Rabinovici GD, et al. Increased metabolic vulnerability in early-onset alzheimer's disease is not related to amyloid burden. Brain. 2010;133:512–528. doi: 10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roe CM, et al. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010;75:42–48. doi: 10.1212/WNL.0b013e3181e620f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe CC, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Price JL, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimmer T, et al. Progression of cerebral amyloid load is associated with the apolipoprotein E epsilon4 genotype in Alzheimer's disease. Biol Psychiatry. 2010;68:879–884. doi: 10.1016/j.biopsych.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinin NM, et al. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology. 2009;73:1186–1192. doi: 10.1212/WNL.0b013e3181bacf1b. [DOI] [PubMed] [Google Scholar]
- 31.Engler H, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 32.Kadir A, et al. Dynamic changes in PET amyloid and FDG imaging at different stages of Alzheimer's disease. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman DH. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J Nucl Med. 2004;45:594–607. [PubMed] [Google Scholar]
- 36.Herholz K, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 37.Edison P, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease. An [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 38.Klunk WE, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 39.Rombouts SA, et al. Functional MR imaging in Alzheimer's disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 40.Sperling RA, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remy F, et al. Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. Neuroimage. 2005;25:253–266. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 42.Buckner RL, et al. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 43.Greicius MD, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, et al. Altered default mode network connectivity in alzheimer's disease-A resting functional MRI and bayesian network study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celone KA, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karow DS, et al. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology. 2010;256:932–942. doi: 10.1148/radiol.10091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeter ML, et al. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. Neuroimage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sexton CE, et al. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Drzezga A, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 50.Reiman EM, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark CM, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. Jama. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Resnick SM, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen AD, et al. Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemppainen NM, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Annals of neurology. 2008;63:112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 55.Rentz DM, et al. Cognition, reserve, and amyloid deposition in normal aging. Annals of neurology. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen AD, et al. Basal Cerebral Metabolism May Modulate the Cognitive Effects of A{beta} in Mild Cognitive Impairment: An Example of Brain Reserve. J Neurosci. 2009;29:14770–14778. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Small GW, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. Jama. 1995;273:942–947. [PubMed] [Google Scholar]
- 58.Reiman EM, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 59.de Leon MJ, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 60.Reiman EM, et al. Fibrillar amyloid-{beta} burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sperling RA, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vannini P, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheline YI, et al. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biological Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mormino EC, et al. Relationships between Beta-Amyloid and Functional Connectivity in Different Components of the Default Mode Network in Aging. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheline YI, et al. APOE4 Allele Disrupts Resting State fMRI Connectivity in the Absence of Amyloid Plaques or Decreased CSF A{beta}42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bondi MW, et al. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bookheimer SY, et al. Patterns of brain activation in people at risk for Alzheimer's disease. The New England journal of medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Storandt M, et al. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mormino EC, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bourgeat P, et al. {beta}-Amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 72.Oh H, et al. beta-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 74.Ittner LM, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 75.Wolk DA, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Honea RA, et al. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J Alzheimers Dis. 2009;18:553–564. doi: 10.3233/JAD-2009-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crivello F, et al. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53:1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- 78.Heise V, et al. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.90. [DOI] [PubMed] [Google Scholar]
- 79.Persson J, et al. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- 80.Koivunen J, et al. Amyloid PET imaging in patients with mild cognitive impairment: A 2-year follow-up study. Neurology. 2011;76:1085–1090. doi: 10.1212/WNL.0b013e318212015e. [DOI] [PubMed] [Google Scholar]
- 81.Okello A, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forsberg A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 83.Koivunen J, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid beta-amyloid in mild cognitive impairment. Dementia and geriatric cognitive disorders. 2008;26:378–383. doi: 10.1159/000163927. [DOI] [PubMed] [Google Scholar]
- 84.Mosconi L, et al. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anchisi D, et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005;62:1728–1733. doi: 10.1001/archneur.62.11.1728. [DOI] [PubMed] [Google Scholar]
- 86.Drzezga A, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 87.Mosconi L, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 88.Devanand DP, et al. Pittsburgh compound B (11C-PIB) and fluorodeoxyglucose (18 F-FDG) PET in patients with Alzheimer disease, mild cognitive impairment, and healthy controls. J Geriatr Psychiatry Neurol. 2010;23:185–198. doi: 10.1177/0891988710363715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorg C, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Petrella JR, et al. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76:511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller SL, et al. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickerson BC, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palop JJ, et al. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 95.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuff N, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan Y, et al. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39:1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Misra C, et al. Baseline and longitudinal patterns of brain atrophy in MCI patients, and their use in prediction of short-term conversion to AD: results from ADNI. Neuroimage. 2009;44:1415–1422. doi: 10.1016/j.neuroimage.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chua TC, et al. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- 102.Rose SE, et al. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mielke MM, et al. Regionally-specific diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2009;46:47–55. doi: 10.1016/j.neuroimage.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walhovd KB, et al. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage. 2009;45:215–223. doi: 10.1016/j.neuroimage.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 105.Teipel SJ, et al. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 2010;22:507–522. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- 106.Fellgiebel A, et al. Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res. 2006;146:283–287. doi: 10.1016/j.pscychresns.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 107.Walhovd KB, et al. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage. 2009;45:215–223. doi: 10.1016/j.neuroimage.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 108.Walhovd KB, et al. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Landau SM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ewers M, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caroli A, Frisoni GB. The dynamics of Alzheimer's disease biomarkers in the Alzheimer's Disease Neuroimaging Initiative cohort. Neurobiol Aging. 2010;31:1263–1274. doi: 10.1016/j.neurobiolaging.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schuff N, et al. Nonlinear time course of brain volume loss in cognitively normal and impaired elders. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheline YI, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thompson PM, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blennow K, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 116.Strozyk D, et al. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 117.Fagan AM, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 118.Buerger K, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr Opin Lipidol. 2010;21:337–345. doi: 10.1097/MOL.0b013e32833af368. [DOI] [PubMed] [Google Scholar]
- 120.Verghese PB, et al. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hampel H, et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 122.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 123.Petersen RC, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Petersen RC, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 125.Thal DR, et al. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;23:26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- 126.Reiman EM, et al. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olsson B, et al. Biomarker-based dissection of neurodegenerative diseases. Prog Neurobiol. 2011 doi: 10.1016/j.pneurobio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 128.Riemenschneider M, et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]