Administration of Pigment Epithelium-Derived Factor Inhibits Left Ventricular Remodeling and Improves Cardiac Function in Rats with Acute Myocardial Infarction (original) (raw)
Abstract
Oxidative stress and inflammation are involved in cardiac remodeling after acute myocardial infarction (AMI). We have found that pigment epithelium-derived factor (PEDF) inhibits vascular inflammation through its anti-oxidative properties. However, effects of PEDF on cardiac remodeling after AMI remain unknown. We investigated whether PEDF could inhibit left ventricular remodeling and improve cardiac function in rats with AMI. AMI was induced in 8-week-old Sprague-Dawley rats by ligation of the left ascending coronary artery. Rats were treated intravenously with vehicle or 10 μg PEDF/100 g b.wt. every day for up to 2 weeks after AMI. Each rat was followed until 16 weeks of age. PEDF levels in infarcted areas and serum were significantly decreased at 1 week after AMI and remained low during the observational periods. PEDF administration inhibited apoptotic cell death and oxidative stress generation around the infarcted areas at 2 and 8 weeks after AMI. Further, PEDF injection suppressed cardiac fibrosis by reducing transforming growth factor-β and type III collagen expression, improved left ventricular ejection fraction, ameliorated diastolic dysfunction, and inhibited the increase in left ventricular mass index at 8 weeks after AMI. The present study demonstrated that PEDF could inhibit tissue remodeling and improve cardiac function in AMI rats. Substitution of PEDF may be a novel therapeutic strategy for cardiac remodeling after AMI.
Left ventricular (LV) remodeling following acute myocardial infarction (AMI) is a dynamic process.1–3 Early remodeling is LV dilatation, which likely occurs because of wall thinning of the infarcted areas and is one of the processes to maintain systemic hemodynamics.1–3 During the chronic phase of AMI, LV dilatation progresses, and the process is associated with LV hypertrophy and fibrosis of the noninfarcted areas.1–3 These structural alterations lead to cardiac dysfunction and overt heart failure in patients with AMI, and ventricular remodeling is thought to be one of the markers for late mortality and other serious clinical events after AMI.4,5 Recently, oxidative stress and inflammation have been shown to contribute to LV remodeling after AMI.6–8 Indeed, oxidative stress and inflammation are involved in apoptotic cell death of myocytes and myocardial fibrosis, thus promoting LV remodeling after AMI.6–8 These observations suggest that oxidative stress generation and inflammatory reactions are novel therapeutic targets for LV remodeling in patients with AMI.
Pigment epithelium-derived factor (PEDF) is a glycoprotein that belongs to the superfamily of serine protease inhibitors.9 It was first purified from the conditioned media of human retinal pigment epithelial cells as a factor with potent neuronal differentiating activity.9 Recently, PEDF has been shown to be produced from a variety of tissues, including vascular cells, inflammatory cells, and adipocytes.10–13 Furthermore, we and others have found that PEDF blocks cytokine- or growth factor-induced endothelial cell damage, platelet aggregation, and T-cell activation in vitro through its anti-oxidative and anti-inflammatory properties.14–21 PEDF also has been shown to reduce vascular inflammation and hyperpermeability in diabetic rats, suppress thrombosis in a rat model of arterial occlusive thrombus formation, and prevent balloon injury-induced neointimal hyperplasia in rats.22–25 These observations led us to speculate that PEDF could exert beneficial effects on LV remodeling by suppressing oxidative stress generation and inflammatory reactions in AMI. However, the protective role of PEDF against LV remodeling after AMI remains unknown. Therefore, in this study we have investigated whether and how administration of PEDF could inhibit LV remodeling and improve cardiac function in rats with AMI.
Materials and Methods
Preparation of PEDF Proteins
PEDF proteins were purified as described previously.26 In brief, 293T cells (American Type Culture Collection, Manassas, VA) were transfected with the recombinant vector pBK-CMV-C-terminally hexahistidine-tagged PEDF using FuGENE 6 transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Hexahistidine-tagged PEDF proteins were purified from conditioned media by an Ni-NTA spin kit (Qiagen GmbH, Hilden, Germany) according to the supplier's instructions. SDS-polyacrylamide gel electrophoresis analysis of purified PEDF proteins revealed a single band with a molecular weight of about 50 kDa, which showed positive reactivity with monoclonal antibodies against human PEDF (Transgenic, Kumamoto, Japan).
Rat AMI Model
AMI was introduced by ligation of the left anterior-descending (LAD) artery as described previously.27 Briefly, male Sprague-Dawley rats (weighting about 300 g at 8 weeks of age; Charles River Breeding Laboratories, Yokohama, Japan) were anesthetized with intraperitoneal injections of ketamine hydrochloride (100 mg/kg b.wt.) and xylazine (5 mg/kg b.wt.). Left thoracotomy through the fourth intercostal space was performed under sterile conditions on a volume-cycled ventilator SAR-830 (Bio Research Center Inc., Nagoya, Japan). Then the LAD artery was ligated approximately 2 mm distal from its origin with a 6.0 polypropylene suture. Proximal LAD artery ligation creates a reproducibly large lateral wall infarction in rats. From the next day after the procedure, rats with AMI were given injections via the tail vein with vehicle or 10 μg PEDF/100 g b.wt. every day for up to 2 weeks. At 1, 2, 4, or 8 weeks after LAD artery ligation, infarcted and noninfarcted LV areas were excised for Western blot and immunohistochemical analysis. Rats that underwent a sham operation underwent the same procedure, excluding coronary artery ligation. All animal experiments were conducted according to the guidelines provided by the Kurume University Institutional Animal Care and Use Committee under an approved protocol.
Western Blot Analysis
Fifteen μg of protein extracted from infarcted and noninfarcted LV areas were subjected to SDS-polyacrylamide gel electrophoresis and Western blotting with specific primary antibodies against PEDF (Santa Cruz Biotechnology, Santa Cruz, CA) and glyceraldehyde-3-phosphate dehydrogenase (Chemicon International, Inc., Temecula, CA), and a horseradish peroxidase-conjugated secondary antibody (Promega, Madison, WI). Detection was performed by enhanced chemiluminescence (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Immunohistochemical Analysis
Specimens of LV tissues were embedded in paraffin, sectioned at 4-μm intervals, and mounted on glass slides. The sections were incubated in 0.3% hydrogen peroxide methanol for 30 minutes to block endogenous peroxidase activity and incubated overnight at 4°C with anti-PEDF polyclonal antibodies (Santa Cruz Biotechnology), anti-hexahistidine tag antibodies (Abcam K.K., Tokyo, Japan), anti-cardiac myosin type 2 mouse monoclonal antibodies (Acris Antibodies, GmbH, Herford, Germany), anti-ED-1 (anti-macrophage) mouse monoclonal antibodies (Serotec, Oxford, UK), and anti-platelet/endothelial cell adhesion molecule-1 (PECAM-1) polyclonal antibodies (Santa Cruz Biotechnology). Then the sections were incubated with MAX-PO(MULTI) (Nichirei Co., Tokyo, Japan) or anti-goat IgG-horseradish peroxidase (Santa Cruz Biotechnology) for 120 minutes and visualized with 3,3′-diaminobenzidine solution (Nichirei, Tokyo, Japan). Five different fields of each sample were analyzed. ED-1 positive cells (%) were shown as the ratio of number of ED-1 positive cells to number of total cells. Number of vessels was shown as number of PECAM-1 positive cells per field.
Measurements of Serum PEDF Levels
Serum PEDF levels in rats were measured with an enzyme-linked immunosorbent assay as previously described.28,29
Quantitation of Infarction Size with Triphenyltetrazolium Chloride Staining
Three hours after LAD artery ligation, hearts were cut at the basal, mid-papillary and apical level, and 2-mm thick slices of LV tissues were incubated in 1% triphenyltetrazolium chloride (TTC) (Sigma, St. Louis, MO) at 37°C for 20 minutes as described previously.30 The infarcted area was identified as an unstained area by TTC.30 The tissues were analyzed by microcomputer-assisted Image-Pro Plus (Media Cybermetics Inc., Bethesda, MD). Infarction size was expressed as a percentage of whole LV area at each level.
Dihydroethidium Staining
After excision, the specimens of noninfarcted LV were embedded in OCT compound (Tissue-Tek, Sakura Finetechnical Co., Ltd., Tokyo, Japan) to freeze on dry ice and were cut into 6-μm sections. The frozen sections were incubated with 2 μmol/L dihydroethidium (Molecular Probe Inc., Eugene, OR) at 37°C for 30 minutes in the dark. Images were obtained by a confocal laser-scanning fluorescence microscopy. Fluorescence intensity in five different fields of each sample was analyzed by microcomputer-assisted Image-Pro Plus).
TUNEL Assay
The specimens of noninfarcted LV were embedded in paraffin, sectioned at 4-μm intervals, and mounted on glass slides. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed with an in situ Apoptosis Detection Kit (Takara Bio Inc., Siga, Japan) according to the manufacturer's instructions. Counterstaining with methyl green was performed to visualize normal nuclei. The apoptotic index was shown as the ratio of number of apoptotic nuclei to number of total nuclei (%). Four measurements were obtained from the border zone area of each sample.
Sirius Red Staining
The specimens of infarcted and noninfarcted LV were embedded in paraffin, sectioned at 4-μm intervals, and stained with 1% Sirius red (Muto Pure Chemicals Co., Ltd., Tokyo, Japan). Fibrotic areas in 10 different fields of each sample were analyzed by microcomputer-assisted Image-Pro Plus. Fibrotic areas were shown as the percentages of the areas of Sirius red-stained tissue within a given field (%).
Real-Time RT-PCR
Total RNA was extracted from noninfarcted LV areas with TRIzol Plus RNA Purification Kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Quantitative real-time RT-PCR was performed using SYBR green reagent (Applied Biosystems, Foster city, CA) according to the manufacturer's recommendation. Identifications of primers for rat transforming growth factor-β (TGF-β), type III collagen, and β-actin gene were RA010073, RA022228, and RA015375, respectively (Takara Bio Inc.).
Measurements of Hemodynamic and Echocardiographic Parameters
Heart rate and blood pressure were measured by a tail-cuff sphygmomanometer using an automated system with a photoelectric sensor (BP-98A; Softron, Tokyo, Japan). Then the rats were anesthetized with ketamine and xylazine. The chests were shaved, and the rats were placed in the supine position on a table. A two-dimensional M-mode echocardiogram (VeVo 770 High-Resolution Imaging System, Visual Sonics, Toronto, Canada) was obtained along the short-axis view of the LV at the level of the papillary muscles. The anterior and posterior wall thickness of LV, LV end-diastolic and end-systolic dimension, ejection fraction, ratio of _trans-_mitral early filling velocity to atrial filling, and LV mass index were measured by M-mode and pulsed-wave Doppler echocardiography over three consecutive cardiac cycles according to the American Society of Echocardiography leading-edge method.31
Measurements of Matrix Metalloproteinase-2 Expression and Activity
Pro- and active matrix metalloproteinase-2 (MMP-2) levels in noninfarcted areas of LV were measured by gelatin zymography, using 10% SDS-polyacrylamide gels containing 1 mg/ml gelatin (Invitrogen Co., Carlsbad, CA) as described previously.32
Statistical Analysis
All values were presented as means ± SE. One-way analysis of variance followed by the Scheffé F test was performed for statistical comparisons; P < 0.05 was considered significant.
Results
PEDF Expression Levels
Of the 90 rats entered into the present experiment, 36 died within 24 hours of surgery. Thus, 54 rats were available for morphometric and hemodynamic analyses and randomized into PEDF or vehicle treatment groups (PEDF-treated groups: n = 4 at 1 week, n = 9 at 2 weeks, n = 3 at 4 weeks, and n = 10 at 8 weeks; vehicle-treated groups: n = 4 at 1 week, n = 10 at 2 weeks, n = 3 at 4 weeks, and n = 11 at 8 weeks). Eleven rats underwent surgery without coronary artery ligation and were used as a sham group.
We first examined the time course of PEDF expression levels in LV and serum after AMI. As shown in Figure 1, A and D, PEDF levels in the infarcted areas of LV and the serum were significantly decreased at 1 week after AMI compared with those of the sham group and remained low during the observational periods. In contrast, PEDF levels in noninfarcted areas of LV began to increase at 2 weeks and reached a maximum at 4 weeks after LAD artery ligation; the peak value was 1.5-fold higher than the basal level (Figure 1B). As shown in Figure 1C, most of the PEDF proteins were expressed in cardiomyocytes, and their levels in noninfarcted areas were increased at 4 weeks after AMI.
Figure 1.
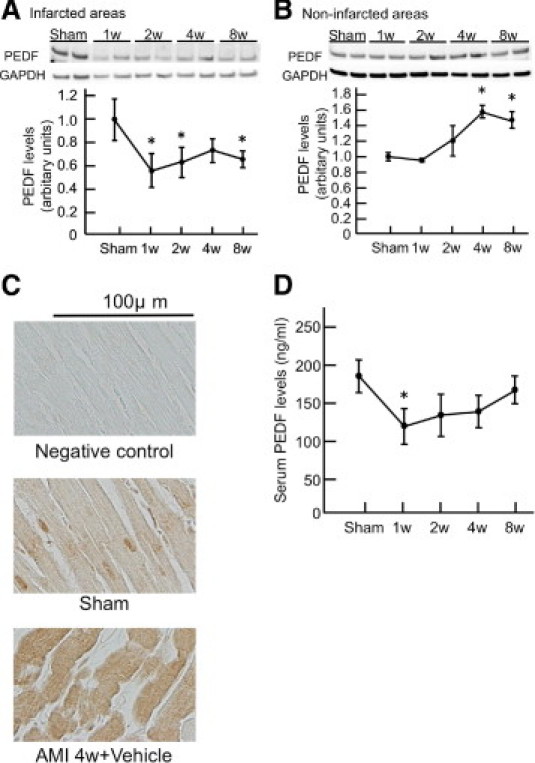
PEDF levels in infarcted (A), non-infarcted areas of LV (B) and cardiomyocytes (C), and serum (D) in rats with AMI. A and B: Upper panel shows representative results of Western blots. Lower panel shows the quantitative data. C: Representative results of PEDF staining in noninfarcted areas. n = 3 per group for each time point. w indicates weeks; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05 compared with the value of the sham group.
Morphometric Analysis
Before examining the effects of PEDF treatment on infarct size, we investigated whether exogenously administered PEDF proteins were actually delivered to LV areas. As shown in Figure 2A, immunohistochemical staining with hexahistidine tag antibodies revealed that administered PEDF proteins were delivered to cardiomyocytes and vessels in noninfarcted areas of LV at 1 week after AMI.
Figure 2.
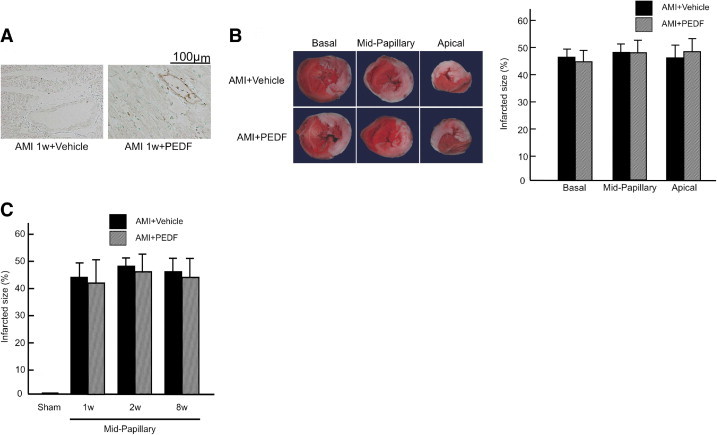
Immunostaining of exogeneously administered PEDF (A), TTC staining of LV tissues (B), and effects of PEDF treatment on infarcted size (C). A: Representative results of immunostaining of exogeneously administered PEDF proteins in noninfarcted areas. n = 6 per group. B: Upper panel shows representative cross-sections of rat hearts at basal, mid-papillary, and apical level. Viable myocardium was stained red, while necrotic areas remained unstained by TTC. Lower panel shows the quantitative results. Infarction size was expressed as a percentage of whole LV area at each level. n = 6 per group. C: Infarcted size of PEDF- and vehicle-treated group at 1, 2, and 8 weeks after AMI. n = 5 for sham group, n = 4 for each group at 1 week, n = 10 for vehicle group at 2 weeks, n = 9 for PEDF-treated group at 2 weeks, n = 11 for vehicle group at 8 weeks, n = 10 for PEDF-treated group at 8 weeks. w indicates weeks.
TTC staining revealed that acute infarction size at the basal, mid-papillary, and apical level of LV tissues was identical in rats assigned to vehicle-treated or PEDF-treated groups (Figure 2B). Infarct size at the mid-papillary level of heart of the PEDF- and vehicle-treated group at 1, 2, and 8 weeks after treatments was similar and was about 40% (Figure 2C) when it was determined using the following equation: infarct size (%) = [(infarct endocardial length/total endocardial length)/2 + (infarct epicardial length/total epicardial length)/2] × 100.33
PEDF Treatment Inhibits Oxidative Stress Generation in Noninfarcted LV Areas
We next examined the effects of PEDF on superoxide generation in noninfarcted LV areas. As shown in Figure 3, dihydroethidium staining, which is a specific for superoxide generation,34 showed a high-intensity fluorescence signal in the noninfarcted areas of LV at 2 and 8 weeks after AMI. PEDF treatment significantly attenuated the fluorescence intensity in these areas (Figure 3).
Figure 3.
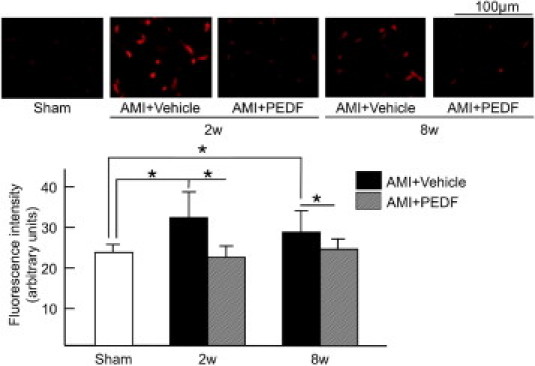
Effects of PEDF on oxidative stress generation in noninfarcted areas of LV. Upper panel shows representative results of DHE staining. Lower panel shows the quantitative data. n = 6 for sham group, n = 6 for each group at 2 weeks and 8 weeks. w indicates weeks. *P < 0.01.
PEDF Treatment Inhibits Apoptotic Cell Death in Noninfarcted LV Areas
Then we investigated whether PEDF treatment inhibited apoptotic cell death in the border zone areas surrounding the infarction. As shown in Figure 4A, apoptotic cell death was markedly induced at 2 weeks and modestly at 8 weeks after AMI, both of which were prevented by the treatments with PEDF; TUNEL assay analysis revealed that PEDF treatments had a <50% reduction in the percentage of apoptotic cells in the border zone at 2 weeks after LAD artery ligation compared with vehicle-treated rats. Further, most of the apoptotic cells were identified as cardiomyocytes because TUNEL-positive cells were found to be cardiac myosin-positive myocytes (Figure 4B).
Figure 4.
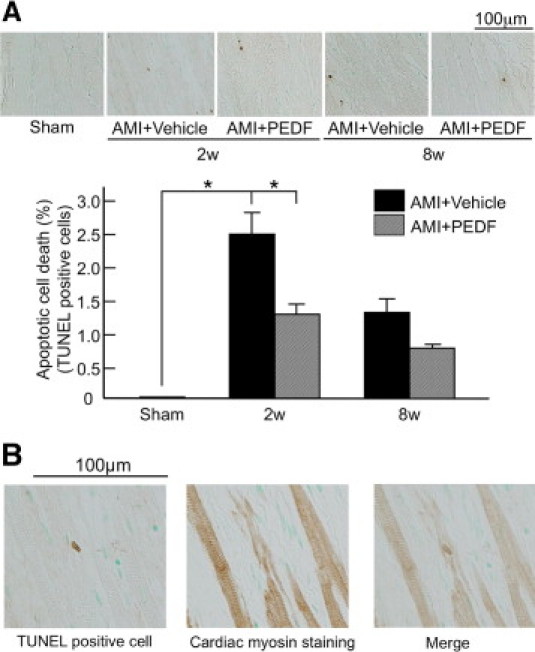
Effects of PEDF on apoptotic cell death in noninfarcted areas of LV. A: Upper panel shows representative results of TUNEL staining. Lower panel shows the quantitative data. n = 5 for Sham group, n = 5 for each group at 2 weeks and 8 weeks. B: Representative images of TUNEL-positive cells and cardiac myosin-positive cells. n = 6 per group. w indicates weeks. *P < 0.01.
Effects of PEDF Treatment on Macrophage Infiltration and Angiogenesis in Noninfarcted LV Areas
As shown in Figure 5, A and B, immunostaining of ED-1 and PECAM-1, markers of macrophage and endothelial cells, respectively, revealed that macrophage infiltration and angiogenesis were enhanced in noninfarcted areas of LV, which were not affected by the treatment of PEDF.
Figure 5.
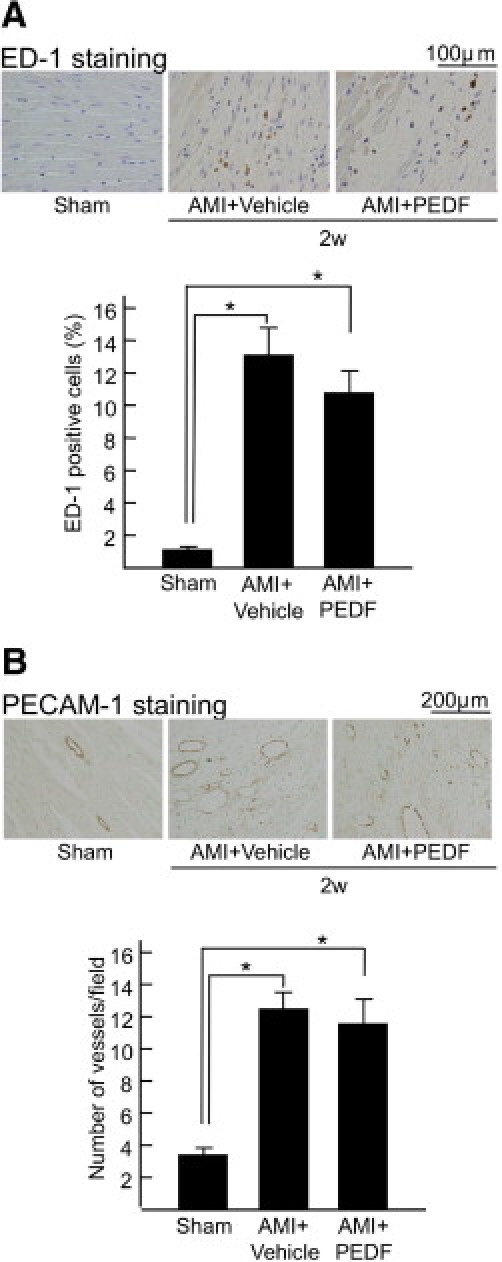
Effects of PEDF on macrophage infiltration (A) and angiogenesis (B) in noninfarcted areas of LV. Upper panels show representative results of ED-1 and PECAM-1 immunostaining. Lower panels show the quantitative data of ED-1 (A) and PECAM-1 (B). n = 5 per group. w indicates weeks, *P < 0.01.
PEDF Treatment Inhibits Cardiac Fibrosis in Noninfarcted LV Areas
We next examined the effects of PEDF on cardiac fibrosis in infarcted and noninfarcted areas of LV. Sirius red staining of LV cross-sections revealed that fibrotic areas in infarcted lesions were not affected by the treatment of PEDF (Figure 6A). However, PEDF treatment significantly blocked the increase in fibrotic areas of noninfarcted LV lesions at 8 weeks after LAD artery ligation (Figure 6B). In addition, TGF-β and type III collagen gene expression was significantly enhanced in noninfarcted LV, which were prevented by the treatments with PEDF (Figure 6, C and D).
Figure 6.
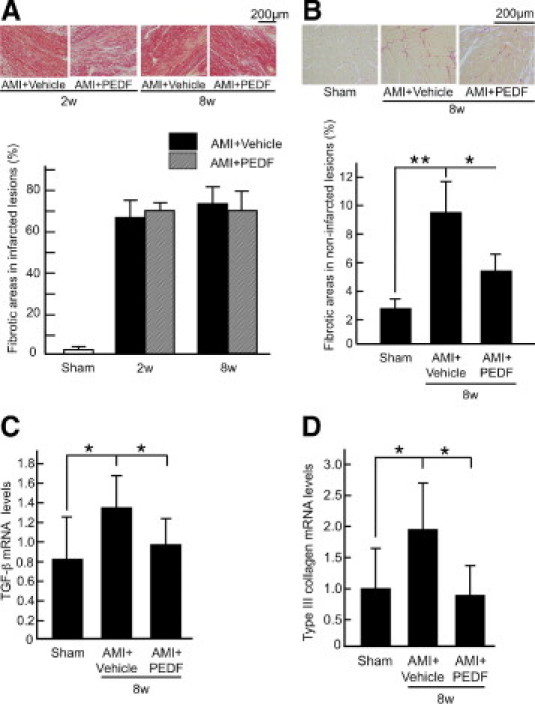
Effects of PEDF on cardiac fibrosis (A and B) and fibrosis-related gene expression (C and D) in infarcted heart. A and B: Each upper panel shows representative results of Sirius red staining of infarcted (A) and noninfarcted (B) LV lesions**.** Each lower panel shows the quantitative data. n = 10 for the sham group, n = 10 for the vehicle group at 2 weeks, n = 9 for the PEDF-treated group at 2 weeks, n = 11 for vehicle group at 8 weeks, n = 10 for PEDF-treated group at 8 weeks. C and D: Quantitative real-time RT-PCR was performed. Data were normalized by the intensity of β-actin mRNA-derived signals and related to the value of the sham group. n = 6 per group. w indicates weeks. *P < 0.05, **P < 0.01.
PEDF Treatment Decreases MMP-2 Expression and Activity in Noninfarcted LV Areas
We investigated the effects of PEDF on MMP-2 activity in noninfarcted areas of LV. Gelatin zymography revealed that PEDF treatment significantly decreased both pro- and active MMP-2 levels in remodeling myocardium at 1 week after AMI (Figure 7).
Figure 7.
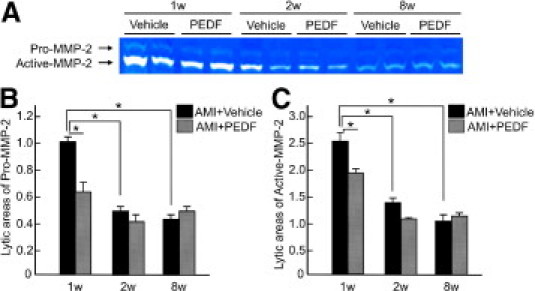
Effects of PEDF on pro-MMP-2 and active MMP-2 levels in noninfarcted heart. A: Representative results of zymograms. B-C shows the quantitative data. Lytic area of pro-MMP-2 in AMI + vehicle rats at 1 week is indicated as 1. n = 4 per group. w indicates weeks. *P < 0.01.
PEDF Treatment Improves Cardiac Function in AMI Rats
PEDF treatment significantly decreased heart rate in AMI rats, although it did not affect b.wt. or systolic blood pressure in these animals (Table 1). Further, echocardiographic studies at 8 weeks after AMI revealed that PEDF administration significantly blocked the increase in LV end-diastolic and end-systolic dimension, ratio of _trans-_mitral early filling velocity to atrial filling, and LV mass index, as well as the decrease in anterior wall thickness and LV ejection fraction (Table 1).
Table 1.
Hemodynamic and Echocardiographic Parameters
| 8 weeks | |||
|---|---|---|---|
| Sham | AMI + vehicle | AMI + PEDF | |
| b.wt. (g) | 520.4 ± 8.48 | 485.3 ± 6.83⁎ | 490.5 ± 13.16⁎ |
| SBP (mmHg) | 110.0 ± 2.8 | 108.2 ± 5.13 | 111.3 ± 3.91 |
| HR (bpm) | 357.2 ± 2 | 367.6 ± 8⁎ | 351.9 ± 10† |
| AWT (mm) | 1.66 ± 0.02 | 0.68 ± 0.05⁎ | 0.71 ± 0.04⁎ |
| PWT (mm) | 1.69 ± 0.03 | 1.66 ± 0.05 | 1.76 ± 0.04 |
| LVDD (mm) | 9.05 ± 0.25 | 12.77 ± 0.19⁎ | 11.66 ± 0.16⁎† |
| LVSD (mm) | 5.80 ± 0.28 | 11.49 ± 0.19⁎ | 9.89 ± 0.20⁎† |
| LVEF (%) | 63.12 ± 1.81 | 20.24 ± 1.00⁎ | 30.05 ± 1.60⁎† |
| E/A | 1.65 ± 0.05 | 6.95 ± 0.96⁎ | 3.75 ± 0.68⁎† |
| LVMI (mg/g) | 2.26 ± 0.07 | 2.82 ± 0.04⁎ | 2.49 ± 0.06⁎† |
Discussion
In the present study, we demonstrated for the first time that PEDF levels in the infarcted LV areas and the serum were decreased at the early phase of AMI and that administration of PEDF could inhibit LV remodeling and cardiac dysfunction in the border zone areas surrounding the infarction in a rat model of AMI.
To examine the effects of PEDF on LV remodeling and cardiac function, we first introduced AMI to rats by ligating the LAD artery because this method was technically feasible and reproducible for inducing transmural myocardial infarction in rats.27 With this procedure, we obtained a rat model of AMI, whose mean infarct size was about 40% to 50% of the whole left ventricle (Figure 2B). In this study, we found that PEDF was normally expressed in cardiomyocytes of LV tissues (Figure 1C, sham) and that its levels in infarcted areas were decreased after LAD artery ligation (Figure 1A). These findings suggest that cardiomyocytes expressed large amounts of PEDF and that their replacement could result in the reduction of PEDF levels in infarcted areas. The results were consistent with our previous observations showing that PEDF levels were decreased in damaged vascular tissues such as balloon-injured arteries, diabetic retinas, and glomeruli with Adriamycin-associated nephrotic syndrome in rats.23,25,29 In contrast to the infarcted LV areas, we found that PEDF levels were significantly increased in cardiomyocytes of noninfarcted LV areas 4 and 8 weeks after AMI (Figure 1, B and C). Although we did not clarify here the molecular mechanism of PEDF up regulation in noninfarcted areas, given the anti-oxidative and anti-inflammatory properties of PEDF,14–25 elevation of PEDF in this area may be a protective mechanism against LV damage in AMI. In the present study, we also found that rat serum levels of PEDF were decreased after AMI (Figure 1D). Because we have previously shown that PEDF levels in the coronary circulation in patients with acute coronary syndromes are significantly decreased compared with those of control subjects,24 serum PEDF level may be a marker that could reflect tissue damage after AMI. It may be interesting to examine whether serum PEDF level at early phase of AMI is a prognostic marker in these patients.
In this study, to clarify the pathophysiological role of PEDF in AMI, we evaluated the effects of PEDF on oxidative stress generation, LV remodeling, and cardiac dysfunction in rats with AMI. To do so, we chose the treatment condition with intravenous administration of 10 μg PEDF/100 g b.wt. once daily in our model because we have previously found that (1) intravenous administration of 3.3 to 10 μg PEDF/100 g b.wt. once a day exerts antithrombogenic, antipermeability, and anti-inflammatory effects in rats16,23,24,29; (2) the peak concentration of circulating PEDF level is obtained at 2 hours after the 10 μg PEDF injection/100 g b.wt.; and (3) its level is increased to about 2-fold of basal level (from about 200 ng/ml to 400 ng/ml) in SD rats at 8 weeks of age.29
In the present study, we found that oxidative stress generation and apoptotic cell death were significantly induced in noninfarcted LV areas at the early phase of AMI, and then the levels were gradually decreased at 8 weeks after AMI, both of which were inhibited by administration of PEDF, especially at the early phase of AMI (Figures 3 and 4). Further, PEDF treatment also was found to suppress cardiac fibrosis in noninfarcted LV areas at 8 weeks after AMI (Figure 6B). Several articles have shown the active involvement of oxidative stress generation in myocardial apoptotic cell death, cardiac fibrosis, and LV remodeling in AMI.6–8,35,36 In addition, apoptotic cell death in and around the region of myocardial infarction is reported to contribute to the process of cardiac fibrosis and LV remodeling in AMI.37,38 These observations suggest that PEDF could inhibit cardiac fibrosis and LV remodeling after AMI partly by inhibiting apoptotic cell death of cardiomyocytes via suppression of oxidative stress generation in noninfarcted LV areas. In this study, PEDF administration inhibited TGF-β and type III collagen gene expression in noninfarcted LV areas at 8 weeks after AMI (Figure 6, C and D). Several pieces of evidence have implicated the TGF-β system as a major etiological agent in the pathogenesis of cardiac fibrosis, including type III collagen overexpression and LV remodeling in AMI.8,35,36 Because latent TGF-β can be activated in vitro on oxidative stress generation,39 anti-oxidative properties of PEDF could contribute directly to its antifibrotic effects in our AMI model as well.
LV remodeling with chamber dilatation and wall thinning is a key component of cardiac dysfunction after AMI.37,38 In the present study, PEDF administration not only inhibited LV dilatation and anterior wall thickness thinning but also suppressed LV remodeling evaluated by LV mass index and improved cardiac systolic and diastolic function at 8 weeks after AMI (Table 1). Attenuated collagen deposition in the remodeling myocardium could explain decreased diastolic dysfunction but is not necessarily responsible for reduced dilative remodeling in PEDF-treated animals. In this study, PEDF treatment decreased pro- and active MMP-2 levels in remodeling myocardium (Figure 7). Therefore, PEDF treatment may attenuate adverse ventricular remodeling partly by reducing MMP-2 expression and activity. Given the fact that PEDF levels were decreased in both infarcted areas and serum of early phase of AMI, our present study provides a novel therapeutic potential of substitution of PEDF for cardiac remodeling and dysfunction in AMI.
In the present study, it is unlikely that anti-angiogenic properties of PEDF could impair the repair process of infarcted heart because PEDF treatment did not affect angiogenesis in noninfarcted areas (Figure 5B). Furthermore, the findings that PEDF treatment did not affect infarct size (Figure 2C) or fibrotic areas in infarcted LV lesions (Figure 6A) suggest that infarcted areas themselves may not be a target of beneficial effects of PEDF.
Limitations
Although the number of rats used for the experiments (n = 54) was relatively small, we did independent experiments repeatedly for each assay and obtained the same statistical results. Further, we chose the rats of the treatment groups at random for each experiment. Time course evaluation showed that PEDF levels in infarcted areas of LV and in serum were decreased, while those in noninfarcted areas were increased. However, the sample size for assessment of PEDF levels in the myocardium and in the serum was very small (n = 3). In this study, PEDF treatment did not affect macrophage infiltration in noninfarcted areas (Figure 5A). However, we cannot totally exclude the possibility that PEDF could inhibit LV remodeling and ameliorate cardiac function in AMI rats by reducing early expression of pro-inflammatory cytokines. Lastly, although PEDF treatment did not affect survival rate during the observational periods, we did not know whether PEDF could improve the long-term mortality in AMI rats. Further longitudinal study is needed to address the issue.
Footnotes
Supported in part by grants of Venture Research and Development Centers from the Ministry of Education, Culture, Sports, Science and Technology, Japan (S.Y.).
References
- 1.Jugdutt B.I. Aging and remodeling during healing of the wounded heart: current therapies and novel drug targets. Curr Drug Targets. 2008;9:325–344. doi: 10.2174/138945008783954934. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications: Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.White H.D., Norris R.M., Brown M.A., Brandt P.W., Whitlock R.M., Wild C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 4.Iraqi W., Rossignol P., Angioi M., Fay R., Nuée J., Ketelslegers J.M., Vincent J., Pitt B., Zannad F. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119:2471–2479. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 5.Barthélémy O., Beygui F., Vicaut E., Rouanet S., Van Belle E., Baulac C., Degrandsart A., Dallongeville J., Montalescot G., OPERA Investigators Relation of high concentrations of plasma carboxy-terminal telopeptide of collagen type I with outcome in acute myocardial infarction. Am J Cardiol. 2009;104:904–909. doi: 10.1016/j.amjcard.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Hori M., Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2009;81:457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 7.Qin F., Liang M.C., Liang C.S. Progressive left ventricular remodeling, myocyte apoptosis, and protein signaling cascades after myocardial infarction in rabbits. Biochim Biophys Acta. 2005;1740:499–513. doi: 10.1016/j.bbadis.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Bujak M., Frangogiannis N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tombran-Tink J., Chader C.G., Johnson L.V. PEDF: pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 10.Yamagishi S., Matsui T., Nakamura K. Atheroprotective properties of pigment epithelium-derived factor (PEDF) in cardiometabolic disorders. Curr Pharm Des. 2009;15:1027–1033. doi: 10.2174/138161209787581940. [DOI] [PubMed] [Google Scholar]
- 11.Tombran-Tink J., Mazuruk K., Rodriguez I.R., Chung D., Linker T., Englander E., Chader G.J. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 12.Yamagishi S., Inagaki Y., Nakamura K., Abe R., Shimizu T., Yoshimura A., Imaizumi T. Pigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generation. J Mol Cell Cardiol. 2004;37:497–506. doi: 10.1016/j.yjmcc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura K., Yamagishi S., Adachi H., Kurita-Nakamura Y., Matsui T., Inoue H. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with visceral adiposity in Japanese patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:52–56. doi: 10.1002/dmrr.820. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki Y., Yamagishi S., Okamoto T., Takeuchi M., Amano S. Pigment epithelium-derived factor prevents advanced glycation end products-induced monocyte chemoattractant protein-1 production in microvascular endothelial cells by suppressing intracellular reactive oxygen species generation. Diabetologia. 2003;46:284–287. doi: 10.1007/s00125-002-1013-4. [DOI] [PubMed] [Google Scholar]
- 15.Jinnouchi Y., Yamagishi S., Matsui T., Takenaka K., Yoshida Y., Nakamura K., Ueda S., Imaizumi T. Administration of pigment epithelium-derived factor (PEDF) inhibits cold injury-induced brain edema in mice. Brain Res. 2007;1167:92–100. doi: 10.1016/j.brainres.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi S., Nakamura K., Matsui T., Inagaki Y., Takenaka K., Jinnouchi Y., Yoshida Y., Matsuura T., Narama I., Motomiya Y., Takeuchi M., Inoue H., Yoshimura A., Bucala R., Imaizumi T. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem. 2006;281:20213–20220. doi: 10.1074/jbc.M602110200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S.X., Wang J.J., Gao G., Shao C., Mott R., Ma J.X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi S., Nakamura K., Ueda S., Kato S., Imaizumi T. Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti-oxidative mechanism of PEDF. Cell Tissue Res. 2005;320:437–445. doi: 10.1007/s00441-005-1094-8. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi S.I., Matsui T., Nakamura K., Takenaka K. Administration of pigment epithelium-derived factor prolongs bleeding time by suppressing plasminogen activator inhibitor-1 activity and platelet aggregation in rats. Clin Exp Med. 2009;9:73–76. doi: 10.1007/s10238-008-0010-4. [DOI] [PubMed] [Google Scholar]
- 20.Yamagishi S.I., Kikuchi S., Nakamura K., Matsui T., Makino T., Norisugi O., Shimizu T., Inoue H., Imaizumi T. Pigment epithelium-derived factor (PEDF) blocks angiotensin II-induced T cell adhesion to endothelial cells by suppressing intercellular adhesion molecule-1. Horm Metab Res. 2006;38:546–548. doi: 10.1055/s-2006-949529. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi S., Matsui T., Takenaka K., Nakamura K., Takeuchi M., Inoue H. Pigment epithelium-derived factor (PEDF) prevents platelet activation and aggregation in diabetic rats by blocking deleterious effects of advanced glycation end products (AGEs) Diabetes Metab Res Rev. 2009;25:266–271. doi: 10.1002/dmrr.906. [DOI] [PubMed] [Google Scholar]
- 22.Zamiri P., Masli S., Streilein J.W., Taylor A.W., Zamiri P., Masli S., Streilein J.W., Taylor A.W. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci. 2006;47:3912–3918. doi: 10.1167/iovs.05-1267. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida Y., Yamagishi S., Matsui T., Jinnouchi Y., Fukami K., Imaizumi T., Yamakawa R. Protective role of pigment epithelium-derived factor (PEDF) in early phase of experimental diabetic retinopathy. Diabetes Metab Res Rev. 2009;25:678–686. doi: 10.1002/dmrr.1007. [DOI] [PubMed] [Google Scholar]
- 24.Takenaka K., Yamagishi S., Matsui T., Nakamura K., Jinnouchi Y., Yoshida Y., Ueda S., Katsuki Y., Katsuda Y., Imaizumi T. Pigment epithelium-derived factor (PEDF) administration inhibits occlusive thrombus formation in rats: a possible participation of reduced intraplatelet PEDF in thrombosis of acute coronary syndromes. Atherosclerosis. 2008;197:25–33. doi: 10.1016/j.atherosclerosis.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura K., Yamagishi S., Matsui T., Yoshida T., Takenaka K., Jinnouchi Y., Yoshida Y., Ueda S., Adachi H., Imaizumi T. Pigment epithelium-derived factor inhibits neointimal hyperplasia after vascular injury by blocking NADPH oxidase-mediated reactive oxygen species generation. Am J Pathol. 2007;170:2159–2170. doi: 10.2353/ajpath.2007.060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamagishi S., Inagaki Y., Amano S., Okamoto T., Takeuchi M., Makita Z. Pigment epithelium-derived factor protects cultured retinal pericytes from advanced glycation end products-induced injury through its antioxidative properties. Biochem Biophys Res Commun. 2002;296:877–882. doi: 10.1016/s0006-291x(02)00940-3. [DOI] [PubMed] [Google Scholar]
- 27.Orenstein T.L., Parker T.G., Butany J.W., Goodman J.M., Dawood F., Wen W.H., Wee L., Martino T., McLaughlin P.R., Liu P.P. Favorable left ventricular remodeling following large myocardial infarction by exercise training. J Clin Invest. 1995;96:858–866. doi: 10.1172/JCI118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi S., Adachi H., Abe A., Yashiro T., Enomoto M., Furuki K., Hino A., Jinnouchi Y., Takenaka K., Matsui T., Nakamura K., Imaizumi T. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 29.Fujimura T., Yamagishi S., Ueda S., Fukami K., Shibata R., Matsumoto Y., Kaida Y., Hayashida A., Koike K., Matsui T., Nakamura K., Okuda S. Administration of pigment epithelium-derived factor (PEDF) reduces proteinuria by suppressing decreased nephrin and increased VEGF expression in the glomeruli of adriamycin-injected rats. Nephrol Dial Transplant. 2009;24:1397–1406. doi: 10.1093/ndt/gfn659. [DOI] [PubMed] [Google Scholar]
- 30.Okamura T., Miura T., Takemura G., Fujiwara H., Iwamoto H., Kawamura S., Kimura M., Ikeda Y., Iwatate M., Matsuzaki M. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res. 2000;45:642–650. doi: 10.1016/s0008-6363(99)00271-0. [DOI] [PubMed] [Google Scholar]
- 31.Litwin S.E., Katz S.E., Morgan J.P., Douglas P.S. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89:345–354. doi: 10.1161/01.cir.89.1.345. [DOI] [PubMed] [Google Scholar]
- 32.Lassila M., Fukami K., Jandeleit-Dahm K., Semple T., Carmeliet P., Cooper M.E., Kitching A.R. Plasminogen activator inhibitor-1 production is pathogenetic in experimental murine diabetic renal disease. Diabetologia. 2007;50:1315–1326. doi: 10.1007/s00125-007-0652-x. [DOI] [PubMed] [Google Scholar]
- 33.Alhaddad I.A., Tkaczevski L., Siddiqui F., Mir R., Brown E.J., Jr Aspirin enhances the benefits of late reperfusion on infarct shape. A possible mechanism of the beneficial effects of aspirin on survival after acute myocardial infarction: Circulation. 1995;91:2819–2823. doi: 10.1161/01.cir.91.11.2819. [DOI] [PubMed] [Google Scholar]
- 34.Yamagishi S., Abe R., Inagaki Y., Nakamura K., Sugawara H., Inokuma D., Nakamura H., Shimizu T., Takeuchi M., Yoshimura A., Bucala R., Shimizu H., Imaizumi T. Minodronate, a newly developed nitrogen-containing bisphosphonate, suppresses melanoma growth and improves survival in nude mice by blocking vascular endothelial growth factor signaling. Am J Pathol. 2004;165:1865–1874. doi: 10.1016/s0002-9440(10)63239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao W., Zhao D., Yan R., Sun Y. Cardiac oxidative stress and remodeling following infarction: role of NADPH oxidase. Cardiovasc Pathol. 2009;18:156–166. doi: 10.1016/j.carpath.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou S.Z., Zhou Y., Zhang Y.L., Lei J., Wang J.F. Antioxidant probucol attenuates myocardial oxidative stress and collagen expressions in post-myocardial infarction rats. J Cardiovasc Pharmacol. 2009;54:154–162. doi: 10.1097/FJC.0b013e3181af6d7f. [DOI] [PubMed] [Google Scholar]
- 37.Jayasankar V., Woo Y.J., Bish L.T., Pirolli T.J., Chatterjee S., Berry M.F., Burdick J., Gardner T.J., Sweeney H.L. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2008;108 doi: 10.1161/01.cir.0000087444.53354.66. II-230-236. [DOI] [PubMed] [Google Scholar]
- 38.Cheng W., Kajstura J., Nitahara J.A., Li B., Reiss K., Liu Y., Clark W.A., Krajewski S., Reed J.C., Olivetti G., Anversa P. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp Cell Res. 1996;226:316–327. doi: 10.1006/excr.1996.0232. [DOI] [PubMed] [Google Scholar]
- 39.Barcellos-Hoff M.H., Dix T.A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1079–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]