SIRT1: new avenues of discovery for disorders of oxidative stress (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 11.
Published in final edited form as: Expert Opin Ther Targets. 2012 Jan 10;16(2):167–178. doi: 10.1517/14728222.2012.648926
Abstract
Introduction
The sirtuin SIRT1 is expressed throughout the body, has broad biological effects and can significantly affect both cellular survival and longevity during acute and long-term injuries, which involve both oxidative stress and cell metabolism.
Areas covered
SIRT1 has an intricate role in the pathology, progression, and treatment of several disease entities, including neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease, tumorigenesis, cardiovascular disease with myocardial injury and atherosclerosis, metabolic disease, and aging-related disease. New areas of study in these disciplines, with discussion of the cellular biology, are highlighted.
Expert opinion
Novel signaling pathways for SIRT1, which can be targeted to enhance cellular protection and potentially extend lifespan, continue to emerge. Investigations that can further determine the intracellular signaling, trafficking and post-translational modifications that occur with SIRT1 in a variety of cell systems and environments will allow us to further translate this knowledge into effective therapeutic strategies that will be applicable to multiple systems of the body.
Keywords: akt, Alzheimer's disease, AMP-activated protein kinase (AMPK), apoptosis, autophagy, cancer, cardiovascular disease; diabetes mellitus, erythropoietin, forkhead transcription factor, FoxO, insulin, neurodegenerative disease, NF-κB, nicotinamide adenine dinucleotide (NAD+), oxidative stress, Parkinson's disease, peroxisome proliferators activated receptor (PPAR), peroxisome proliferators-activated receptor-γ coactivator (PGC), protein tyrosine phosphatase, resveratrol, SIRT1, sirtuin
1. Introduction
The yeast silent information regulator-2 (Sir2), a NAD+-dependent protein deacetylase, was first identified in the yeast Saccharomyces cerevisiae and plays a role in chromatin silencing, life span extension and aging processes. Sirtuins are the mammalian homologues of Sir2 and are class III histone deacetylases, which are NAD+-dependent protein deacetylases. In general, histone deacetylases are enzymes that transfer acetyl groups from _ε_-N-acetyl lysine amino acids that exist on the histones of DNA to regulate transcription. It is important to note that although histone deacetylases primarily oversee DNA transcription, they may be involved in post-translational changes of proteins as well such as the ability of SIRT1 to control the post-translational phosphorylation of forkhead transcription factors [1,2]. During deacetylase reactions, sirtuins transfer the acetyl residue from the acetyllysine residue of histone to the ADP-ribose moiety of NAD+, resulting in the production of nicotinamide, 2′-O-acetyl ADP ribose, and deacetylated proteins. Seven mammalian homologues of Sir2 have been identified as SIRT1 through SIRT7. Of these, SIRT1 was the first identified and has been extensively investigated. SIRT1 is expressed in the brain, heart, liver, pancreas, skeletal muscle, spleen and adipose tissues. At the cellular level, SIRT1 is present in both the nucleus and cytoplasm with dominant expression in the nucleus. As a result, SIRT1 has a significant role in multiple biological processes that include oxidative stress, metabolism, cellular proliferation and genomic stability (Figure 1). Interestingly, SIRT1 has been demonstrated to regulate cellular protection against oxidative stress in many disease states that involve neurodegeneration, metabolic disorders and cardiovascular disease.
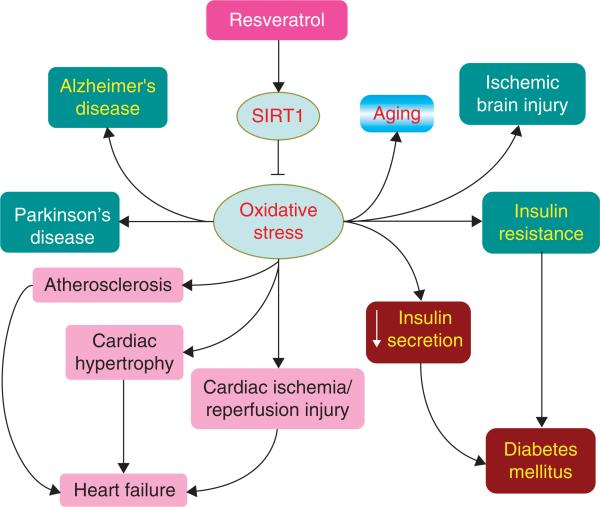
Figure 1. Activation of SIRT1 protects against oxidative-stress-associated diseases in multiple systems.
Oxidative stress has been linked to the progression of neurodegenerative diseases (Alzheimer's disease, Parkinson's disease, acute ischemic stroke), heart failure associated with atherosclerosis, cardiac hypertrophy and ischemic/reperfusion injury, diabetes mellitus as the result of insulin resistance and decreased secretion and aging. SIRT1 functions as an antioxidant to protect cells of these systems and increase insulin sensitivity to benefit the body metabolic homeostasis. Resveratrol can function similarly through activating SIRT1.
2. Signal transduction of the SIRT1 pathway
SIRT1 expression and activation can be influenced by several cellular conditions, such as calorie restriction, exercise and oxidative stress. In the cell, SIRT1 uses NAD+ as a substrate, but the level of NAD+ also can control the deacetylating activity of SIRT1. In the salvage pathway of NAD+ synthesis, nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of nicotinamide to nicotinamide mononucleotide, which is then converted to NAD+ by nicotinamide/nicotinic acid mono-nucleotide adenylyltransferase (NMNAT). NAMPT functions as the rate-limiting enzyme in the mammalian NAD+ biosynthesis pathway. Increased NAMPT activity raises the total cellular NAD level and subsequent transcriptional regulatory activity of SIRT1. In addition, NMNAT can regulate the deacetylating activity of SIRT1 at its target gene promoters [3].
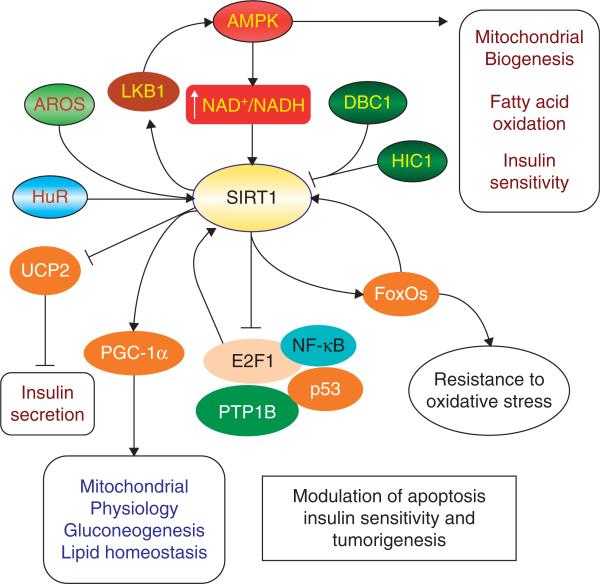
A number of feedback mechanisms exist that lead to the activation of SIRT1 (described below), such as the interactions of SIRT1 and forkhead transcription factors and the SIRT1–AMP-activated protein kinase (AMPK) pathway. In addition, Hu antigen R (HuR), a RNA binding protein, regulates the stability of many target mRNAs and can bind the 3′ non-translated region of the mRNA of SIRT1, leading to the stabilization of the SIRT1 mRNA and upregulation of SIRT1 expression [4]. During oxidative stress, HuR is phosphorylated, resulting in the dissociation of the HuR–SIRT1 mRNA complex and subsequent SIRT1 mRNA decay. A nuclear protein active regulator of SIRT1 (AROS) also can enhance SIRT1-mediated deacetylation of p53 both in vitro and in vivo models and prevents p53-mediated transcriptional activity [5]. Hypermethylated in cancer 1 (HIC1) and deleted in breast cancer 1 (DBC1) have been identified as negative regulators of SIRT1. HIC1, a transcriptional repressor, binds to the SIRT1 promoter and represses its transcription. Loss of HIC1 increases SIRT1 expression in normal or cancer cells, resulting in the deacetylation and inactivation of p53 and enhanced tumorigenesis [6]. Deleted in Breast Cancer 1 (DBC1) also directly interacts with SIRT1 to inhibit the activity of SIRT1. Loss of DBC1 expression can potentiate SIRT1-dependent inhibition of apoptosis (Figure 2) [6].
Figure 2. SIRT1 cell signaling pathways.
AMP activated protein kinase (AMPK) can activate SIRT1 through regulating the NAD+:NADH ratio, conversely, SIRT1 activates AMPK by deacetylation of the serine-threonine liver kinase B1 (LKB1), constituting a positive feedback system. Similarly, there is another positive feedback mechanism between SIRT1 and transcriptional factor FoxOs. FoxOs can directly bind to SIRT1 promoter to induce FoxOs-dependent SIRT1 transcription. Other SIRT1 substrates include PPAR-γ coactivator-1α (PGC-1α), protein tyrosine phosphatase 1B (PTP1B), p53, NF-κB and apoptotic transcription regulator E2F1. Interestingly, although SIRT1 inhibit E2F1 transcriptional activity, E2F1 can upregulate SIRT1 expression. In addition, SIRT1 can stimulate glucose-dependent insulin secretion from pancreatic β cells via repressing the uncoupling protein gene UCP2. The RNA binding protein HuR and A nuclear protein active regulator of SIRT1 (AROS) also enhances the SIRT1 activity. In contrast, hypermethylated in cancer 1 (HIC1) and deleted in breast cancer 1 (DBC1) have emerged as negative regulators of SIRT1. Through regulating these cell signals, SIRT1 can increase the resistance to oxidative stress and mediate metabolism.
3. SIRT1 and oxidative stress
Oxidative stress can result from the excessive generation of oxygen free radicals and other associated chemical species. Oxygen free radicals, consisting of superoxide free radicals, hydrogen peroxide, singlet oxygen, NO and peroxynitrite can be generated in elevated quantities during the reduction of oxygen and lead to cellular injury [7]. During normal physiological conditions, reactive oxygen species are produced at low levels and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase and small-molecule substances such as vitamins C and E [8].
When the production of oxygen free radicals overrides the capability of the endogenous antioxidant system, oxidative stress occurs followed by cellular injury. Oxidative stress has a significant role in the pathology of a wide range of diseases that involve metabolic disorders, cognitive impairment, cardiac disease, psychiatric disorders and hepatic disease (Figure 1) [9-11]. In cells, oxygen free radicals can result in cellular membrane lipid peroxidation and protein oxidation leading to the disruption of cellular integrity [11]. In addition, apoptosis and autophagy as a result of oxidative stress represent important mechanisms that lead to the destruction of cells in many cell systems including non-neuronal cells, neurons, vascular cells and inflammatory cells [12-16].
During oxidative stress, apoptosis consists of both the early exposure of membrane phosphatidylserine (PS) residues and the later destruction of genomic DNA [10,12]. Apoptotic membrane PS exposure is present during conditions such as low oxygen levels and β-amyloid (Aβ) exposure [17,18]. Membrane PS exposure can function as an ‘identity tag’ for the phagocytosis of cells as well as alter vascular system coagulation. The loss of membrane phospholipid asymmetry leads to the exposure of membrane PS residues on the cell surface and attracts microglia to target cells for phagocytosis [19-21].
SIRT1 provides cells with tolerance against oxidative stress (Figure 1). In some cells, SIRT1 may offer protection against oxidative stress through the modulation of forkhead transcription factors [22,23]. SIRT1 also protects cells against oxidative stress by increasing the activity of catalase [24]. SIRT1 overexpression enhances the tolerance against free radical toxicity in neuronal cells [25,26]. SIRT1 can block p53-induced apoptosis through p53 deacetylation and induction of manganese SOD (MnSOD) [27,28]. In many experimental paradigms, resveratrol (trans-3,5,4′-trihydroxystibene), a naturally occurring phytoalexin polyphenol in grapes and red wine, is used to increase SIRT1 activity (Figure 1). Resveratrol treatment prevents apoptotic injury in vascular endothelial cells during models of experimental diabetes with elevated glucose [2,29]. In contrast, inhibition of SIRT1, such as with nicotinamide, can block proliferation and lead to apoptosis in leukemic cells, possibly through p53-dependent and independent mechanisms [30]. Furthermore, agents such as sirtinol that inhibit SIRT1 activity can be detrimental to neurons during oxidative stress [2,26,31] while the use of the specific small-molecule inhibitor of SIRT1 EX527 [32] can block HDAC activity and increase vascular injury during oxidative stress, suggesting that an endogenous level of SIRT1 is required for vascular protection [2,29].
In consideration of these studies, SIRT1 not only may offer significant protection for cell survival in a number of disorders during oxidative stress, but also should be considered as an important therapeutic avenue for the development of new treatment modalities. As one example, the cytokine and growth factor erythropoietin (EPO) employs SIRT1 to function as a mediator of cellular protection during oxidative stress. EPO is a 30.4 kDa glycoprotein with four glycosylated chains that include three N-linked and one O-linked acidic oligosaccharide side chains [33,34]. EPO is not only important for erythropoiesis, but also plays an important role in other organ systems such as the brain, heart and vascular system [35-45]. New studies have shown that EPO increases endogenous SIRT1 activity in endothelial cells and fosters the subcellular trafficking of SIRT1 to the nucleus which is necessary for EPO to foster vascular protection [29]. Downstream from SIRT1, activation of protein kinase B (Akt1) is required and SIRT1 has been associated with enhanced activity of Akt [2,29,46-48].
One family of targets to consider for SIRT1 are the mammalian forkhead transcription factors of the O class (FoxO1, FoxO3, FoxO4 and FoxO6) are involved in cell metabolism, insulin sensitivity, aging and oxidative stress (Figure 2). SIRT1 employs Akt1 to modulate the phosphorylation and subcellular trafficking of FoxOs [29]. Phosphorylation of FoxOs results in the retention of these proteins in the cytoplasm and the inhibition of transcription activity [22,23]. Acetylation of FoxOs can also modulate their transcriptional activity through facilitating their phosphorylation and nuclear translocation. Nuclear localization of FOXO is induced by deacetylation and is inhibited by phosphorylation [22,23].
SIRT1 attenuates oxidative stress through the control of nuclear shuttling and transcriptional activity of forkhead transcription factors. In response to oxidative stress, FoxOs translocate to the nucleus and interact with SIRT1, resulting in the deacetylation of FoxOs. Dependent upon the post-translational changes on FoxOs, SIRT1 can inhibit FoxO activity and protect cells from oxidative stress [2,29] or increase the activity of FoxOs to lead to gene activation [22,23,49]. For example, loss of SIRT1 results in the impairment of FoxO4 nuclear translocation and the expression of growth arrest and DNA damage 45 (GADD45) [50].
This modulation by SIRT1 over FoxO function occurs via NAD-dependent deacetylation in response to oxidative stress. SIRT1 also can target FoxOs such as FoxO1 to bind and deacetylate FoxO1 at residues that are acetylated by cAMP-response element-binding protein [51]. Studies with overexpression of SIRT1 demonstrate protection of cardiomyocytes from oxidative stress through a FoxO1-dependent pathway [52]. It should be noted that SIRT1 can enhance expression of FoxO targets that are involved in stress resistance, such as MnSOD and GADD45, but diminishes the expression of pro-apoptotic FoxO targets (Fas ligand and B cell leukemia-2-interacting mediator of cell death (Bim)), suggesting that SIRT1 may modulate the balance between stress resistance and cell death within cells [22,49]. Deacetylation of FoxOs by SIRT1 also can regulate autophagy. SIRT1-mediated deacetylation of FoxO1 has been associated with increases in autophagic flux, which may be required to maintain cardiac function during glucose deprivation and starvation [53]. FoxO1 also increases the expression of Rab7, a small GTP-binding protein that mediates late autophagosome–lysosome fusion, which is both necessary and sufficient for mediating FoxO1-induced increases in autophagic process [53].
FoxOs also exert a positive feedback mechanism regulating SIRT1 expression. FoxO1 can directly bind to the SIRT1 promoter region containing a cluster of five putative FoxO1 core binding repeat motifs (insulin receptor substrate 1 (IRS-1)) and a forkhead-like consensus-binding site (FKHD-L). This leads to a FoxO1 modulating SIRT1 transcription and leads to an increase in the expression of SIRT1 [54]. FoxO3a also can regulate the expression of SIRT1 through binding to two p53 binding sites within the SIRT1 promoter to induce SIRT1 transcription during acute nutrient withdrawal [55].
Given the ability of FoxO proteins to promote apoptotic cell death and cell cycle progression, FoxO proteins are ideal candidates to regulate tumor growth. FoxO3a and FoxO4 can promote cell cycle arrest in mouse myoblastic cell lines through modulation of growth-arrest and DNA-damage-response protein 45 [22,23]. The transcription factor E2F1 that controls the induction of the cell cycle can increase the endogenous expression of FoxO1 and FoxO3a to lead to cell cycle arrest [56]. Yet, loss of FoxO3a activity in association with c-myc, p27, and NF-κB can result in cell cycle induction and malignant transformation of mouse cells in the presence of oncogene activation [22,23]. SIRT1 may oversee FoxO transcription pathways to enhance anti-tumor activity. For example, resveratrol can block the phosphorylation of forkhead in rhabdomyosarcoma-like 1 (FKHRL1) leading to increased activity and enhancement of TNF-related apoptosis-inducing ligand (TRAIL) pathways to block tumor growth in prostate cancer [57]. In addition, SIRT1 can interact with E2F1 during cell cycle regulation and apoptosis [58]. As an important regulator in response to DNA-damage induced stress, E2F1 can positively regulate SIRT1 expression at the transcriptional level. SIRT1 binds to E2F1 and deacetylates it, resulting in the inhibition of E2F1 transcriptional activity (Figure 2).
4. SIRT1 and neurodegenerative disease
Enhancing the activity of SIRT1 through the application of resveratrol has been demonstrated to protect against cerebral ischemia. Resveratrol protects neuronal cells against oxidative stress that is dependent on SIRT1 activation [59]. Resveratrol also can mimic ischemic preconditioning in the brain to reduce ischemic brain injury. In contrast, inactivation of SIRT1 by the SIRT1 inhibitor sirtinol abolishes neuroprotection by resveratrol [26,31]. Treatment with resveratrol reduces brain infarct volume, decreases neurological deficits, and increases regional brain blood flow after cerebral ischemia [60]. Recent studies indicate that resveratrol also exerts neuroprotection in both transient focal and global cerebral ischemia via the reduction of the generation of oxygen free radicals and prevention of lipid peroxidation [61].
The activity of SIRT1 has also been linked to chronic neurodegenerative diseases, such as Alzheimer's disease (AD) (Figure 1). SIRT1 is essential in maintaining normal learning, memory and synaptic plasticity [62]. In AD patients, a decrease in SIRT1 is present in the parietal cortex, which is closely associated with the accumulation of amyloid-beta (Aβ) and tau protein with cognitive impairment [63]. In animal models of AD, application of SIRT1 and resveratrol reduces neurodegeneration in the hippocampus and prevents learning impairment [64]. Over-expression of SIRT1 in a mouse model of AD reduces the accumulation of Aβ, while gene silencing of SIRT1 in the brain results in increased production of Aβ [65]. The SIRT1 activator resveratrol also has been reported to protect neuronal cells against Aβ induced oxidative toxicity [59].
Given that oxidative stress is one of the pathological processes that can lead to Parkinson's disease (PD) [66], it is conceivable that SIRT1 plays a role in PD. Administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin and oxidant that mimics experimental PD, significantly induces motor coordination impairment in mice with resulting neuronal loss in the substantia nigra. Application of the SIRT1 activator resveratrol significantly reduces MPTP-induced motor coordination impairment, hydroxyl radical formation and neuronal loss [67]. In other experimental models of PD caused by 6-hydroxydopamine (6-OHDA), resveratrol was shown to increase the activity of antioxidant enzymes and lower dopamine loss in the substantia nigra [68]. The findings suggest that SIRT1 can be a potential target to treat PD as a result of its anti-oxidant cellular effects.
Since neurodegenerative disorders may be the result of pathology that occurs in multiple cells or in multiple systems of the body, knowledge of oxidative stress pathways that SIRT1 oversees may provide further insight into the etiology and treatment of neurodegenerative disorders. As a result, NF-κB, in conjunction with SIRT1, has been identified as one transcription factor that may play a significant role in the protection against oxidative stress. Once activated, NF-κB is translocated to the cell nucleus and activates several anti-apoptotic genes, such as the inhibitors of apoptotic protein (IAPs), Gadd45b, and B-cell leukemia/lymphoma x (Bcl-xL) to prevent apoptosis against oxidative stress. Yet, it should be recognized that NF-κB at times also can lead to pro-inflammatory gene induction that promotes inflammation, cell death and tumorigenesis [21,69,70].
SIRT1 has been shown to interact with and deacetylate the RelA/p65 subunit of NF-κB and inhibit its transcriptional activity [71]. In addition, use of resveratrol potentiates chromatin-associated SIRT1 protein on the cellular inhibitor of apoptosis protein 2 (cIAP-2) promoter region and inhibits NF-κB-regulated gene expression. This leads to an increase in the sensitization of cells to TNF-α-induced apoptosis [71]. With regards to AD, activation of SIRT1 reduces Aβ toxicity and decreases NF-κB transcriptional activity, suggesting that SIRT1 promotes cell protection through inhibiting NF-κB transcriptional activity (Figure 2) [72].
5. SIRT1 and cardiac disorders
At the cellular level, SIRT1 is expressed in both the cytoplasm and the nucleus. Once in the nucleus, SIRT1 controls gene transcription to prevent apoptosis. During events that incite apoptosis, SIRT1 translocates from the cytoplasm to the nucleus. Resveratrol enhances SIRT1 translocation to the nucleus to protect cells from oxidative stress [2,29]. In cardiomyocytes, nuclear SIRT1 blocks myoblast injury from oxidative stress by enhancing the expression of MnSOD while loss of MnSOD negates protection by SIRT1 to illustrate the role SIRT1 plays during oxidative stress [27]. Prevention of SIRT1 nuclear translocation also abolishes protection by SIRT1 and resveratrol, suggesting that translocation of SIRT1 to the nucleus is necessary as well to protect against vascular injury [2,8,29].
Interestingly, SIRT1 also may serve to block atherosclerosis. High-fat diets with the release of fatty acid anion and lipoprotein lipase products can activate vascular endothelial cells and impair the integrity of endothelium providing the foundation for atherosclerotic plaque [8,73]. Injured endothelial cells also are known to lead to platelet activation and form vessel thrombi [34]. SIRT1 can protect endothelial cells from oxidative stress and oxidized low-density-lipoprotein (LDL)-induced apoptosis [2,29,74]. Over-expression of SIRT1 in HUVECs prevents cell injury from oxidized LDLs. In endothelial-cell-specific SIRT1 transgenic mice, impairment of vasorelaxation during high fat exposure was reduced with atherosclerotic lesions [74], suggesting that SIRT1 improves endothelial function to prevent atherosclerosis. Activation of SIRT1 also improves endothelium relaxation through upregulating eNOS expression and production of NO [75]. However, it is important to note in studies that use resveratrol, the anti-platelet properties of resveratrol may also contribute to its ability to prevent atherosclerosis.
Activation of SIRT1 also can inhibit vascular smooth muscle cell (VSMC) hypertrophy, which can contribute to atherosclerosis. Overexpression of SIRT1 prevents angiotensin-II-induced VSMC hypertrophy. Treatment with resveratrol prevents oxidative stress induced human coronary smooth muscle cell proliferation through the inhibition of extracellular-signal-regulated kinase (ERK) activation [76]. SIRT1 in VSMCs also may regulate atherosclerosis through enhancing the activity of tissue inhibitor of metalloproteinase 3 (TIMP3). TIMP3 is responsible for preventing metalloproteinase 3 to digest vascular intracellular matrix [77]. Downregulation of TIMP3 has been tied to atherosclerosis in diabetic patients, since TIMP3 is significantly reduced in human carotid atherosclerotic plaques with decreased levels of SIRT1 [78]. In contrast, SIRT1 overexpression in VSMCs promotes TIMP3 expression and may, as a result, prevent atherosclerotic plaque [77,78].
In cases of ischemic/reperfusion injury, SIRT1 can be cardioprotective. Treatment with resveratrol during myocardial ischemia/reperfusion in rats can reduce rhythm disturbances, cardiac infarct size and plasma levels of lactate dehydrogenase and creatine kinase [79]. Cell protection in the cardiovascular system may be determined by SIRT1 fostering protection against cellular inflammation and apoptosis [2,29]. SIRT1 also has been demonstrated to promote the transcriptional activity of FoxO1 to upregulate MnSOD, suppress oxidative stress in cardiac myocytes, reduce cardiac infarct volume, and improve functional recovery after ischemic/reperfusion in murine models [80]. Activation of SIRT1 through resveratrol can assist cardiac recovery following global ischemia that may be tied to new vessel growth [81]. Loss of SIRT1 in endothelial cells leads to impairment of new blood vessel formation in ischemic tissues. The effect of SIRT1 on angiogenesis may be dependent on NF-κB and induction of NOS in endothelial cells [82]. Resveratrol also acts in animal models of cardiac infarction to increase the expression of VEGF and its tyrosine kinase receptor fetal liver kinase-1 (Flk-1).
SIRT1 appears to play an important role during cardiac hypertrophy. Overexpression of SIRT1 in cardiac tissue has been shown to reduce cardiac hypertrophy, cellular apoptosis, cardiac dysfunction and expression of senescence markers [52]. Yet, high expression of SIRT1 by 12.5-fold results in oxidative stress, apoptosis and increased cardiac hypertrophy, possibly as a result of mitochondrial dysfunction and depletion of NAD+ [52]. Treatment with resveratrol to increase SIRT1 activity prevents cardiac hypertrophy and cardiac cell dysfunction through the reduction of oxidative stress without lowering the blood pressure [83], can suppress pressure overload induced cardiac hypertrophy in rats [84] and can inhibit angiotensin II induced cardiomyocyte hypertrophy [85]. SIRT1 also prevents phenylephrine-induced neonatal cardiomyocyte hypertrophy and inhibits phenylephrine induced downregulation of fatty acid oxidation genes. These observations with SIRT1 have been associated with the activation of PPAR-α [86]. The anti-hypertrophic effects of SIRT1 and resveratrol treatment also may function via AMPK and the inhibition of Akt (protein kinase B) with subsequent suppression of protein synthesis and gene transcription [87]. Akt is a central path way for cell growth and protection. In some cell pathways, Akt activation in conjunction with SIRT1 may be necessary to foster cell survival [2,29,88].
6. SIRT1, cellular metabolism, and diabetes mellitus
Diabetes mellitus (DM) is a significant health concern for both young and older populations. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions [42,89]. Insulin resistance and the complications of DM can be the result of cellular oxidative stress. Hyperglycemia leads to increased production of reactive oxygen species in endothelial cells, liver cells, and pancreatic β-cells. As a result, patients with DM can develop immune dysfunction, cognitive disorders, hepatic dysfunction, renal disease, hematological disease, neurodegenerative disorders and cardiovascular disease [42,89].
SIRT1 activity has been associated with insulin sensitivity. In rats placed on a high-fat diet, SIRT1 expression is decreased in the pancreas and liver and may be associated with insulin resistance [90]. In insulin-resistant cells, SIRT1 protein is significantly decreased and the reduction of SIRT1 levels in gastrocnemius muscle in mice results in the impairment of glucose tolerance [91]. Knockdown or inhibition of SIRT1 impairs the insulin signaling by interfering with insulin stimulated insulin receptor phosphorylation and glycogen synthase [91]. In contrast, overexpression of SIRT1 in the liver serves to attenuate hepatic steatosis and improves insulin sensitivity, resulting in improved glucose homeostasis [92]. The ability of SIRT1 to improve insulin sensitivity may occur through several factors such as modulation of fat mobilization [93], gluconeogenesis [94] and inflammation [95]. SIRT1 also can function as a positive modulator of insulin signaling in insulin-sensitive organs and activate the insulin downstream target Akt through PI3K [96]. SIRT1 has been reported to stimulate glucose-dependent insulin secretion from pancreatic β cells via repressing the uncoupling protein (UCP) gene UCP2 [97]. In addition, the SIRT1 activator resveratrol promotes glucose-stimulated insulin secretion in insolinoma INS-1E cells and human islets that is dependent on active SIRT1 [98].
SIRT1 not only has a role with insulin sensitivity, but also can affect fat metabolism and obesity. In obese mice, SIRT1 expression is low in adipose tissue and loss of SIRT1 in white adipose cells results in the impairment of fatty acid mobilization. In contrast, overexpression of SIRT1 in adipose tissue suppresses the transcriptional activity of PPAR-γ to inhibit adipogenesis and activation of lipolysis during fasting [93]. Treatment with resveratrol can mimic calorie restriction to prevent obesity as a result of a high-calorie diet in mice [99]. SIRT1 has been shown to be expressed in anorexigenic proopiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus and to regulate food intake and cellular metabolism [100]. For example, overexpression of SIRT1 in the hypothalamus prevents FoxO1 from promoting hyperphagia and body weight gain [100]. In contrast, lack of SIRT1 in POMC neurons leads to obesity due to reduced energy expenditure [101].
SIRT1 may regulate insulin sensitivity and metabolism through the phosphorylation of AMPK. SIRT1 regulates AMPK through the AMPK kinase, serine-threonine liver kinase B1 (LKB1). Overexpression of SIRT1 results in the deacetylation of LKB1, leading to its translocation from the nucleus to the cytoplasm, where LKB1 activates AMPK [102]. AMPK, in turn, can mediate the activation of SIRT1. Activation of AMPK occurs during periods of decreased energy with a subsequent increase in the AMP:ATP ratio. Activation of AMPK through phosphorylation functions to promote insulin sensitivity, fatty acid oxidation and mitochondrial biogenesis. This leads to the generation of ATP and reduction in oxidative stress (Figure 2).
AMPK-mediated impairment of muscle differentiation during glucose restriction and PPAR-γ coactivator (PGC)-1α mediated gene expression is SIRT1-dependent. However, AMPK cannot directly activate SIRT1, but may enhance SIRT1 activity. AMPK activation enhances SIRT1 activity either by increasing cellular NAD+:NADH ratio, resulting in the deacetylation and modulation of the activity of downstream SIRT1 targets that include PGC-1a, FoxO1 and FoxO3a [103] or by upregulating nicotinamide phosphoribosyltransferase (Nampt) during glucose restriction, leading to increased NAD+ and decreased nicotinamide, an inhibitor of SIRT1 [104]. The SIRT1 activator resveratrol also has been demonstrated to activate AMPK through SIRT1 dependent or independent mechanisms [103,105]. Resveratrol increases AMPK phosphorylation to protect cells against elevated glucose concentration, improve insulin sensitivity and stimulate glucose transport. For example, increased AMPK activation reduces myocardial infarct size in both non-diabetic and diabetic rat hearts following ischemia/reperfusion, which may be mediated through the inhibition of mitochondrial permeability transition pore opening in cardiomyocytes [106]. Mice expressing dominant-negative AMPK or loss of AMPK have glucose uptake inhibition and increased infarct volume following cardiac ischemia (Figure 2) [107].
SIRT1 also controls insulin sensitivity through targeting protein tyrosine phosphatase (PTP). In the PTP family, PTP1B has been found to negatively regulate insulin signal transduction via targeting the insulin receptor. PTP1B deficiency or inhibition leads to improved insulin sensitivity and glycemic control. Lowering the PTP1B level in the liver decreases blood glucose in diabetic mice. SIRT1 overexpression or SIRT1 activation can reduce both the PTP1B mRNA and protein levels during insulin resistance. In contrast, an increase in PTP1B expression prevents SIRT1-mediated glucose uptake and insulin receptor phosphorylation in response to insulin stimulation, suggesting that SIRT1 improves insulin sensitivity through the repression of PTP1B [91].
During adipogenesis, SIRT1 in association with PPAR-γ plays an important role. PPAR-α increases free fatty acid uptake and decreases lipolysis. Under nutrient restriction, SIRT1 protein binds to and represses genes controlled by the fat regulator PPAR-γ. SIRT1 inhibits PPAR-γ and silencing mediator of retinoid and thyroid hormone receptors, resulting in the mobilization of fatty acids from white adipocytes upon fasting [93]. PPAR-γ also may directly interact with SIRT1 and form a negative feedback to regulate SIRT1 activity [108].
With regards to cellular metabolic regulation, SIRT1 regulates the activity of PGC-1α via deacetylation and it can interact with PGC-1α in the liver to induce gluconeogenic genes and hepatic glucose output. PGC-1α is a member of a family of transcriptional coactivators that includes PGC-1α, PGC-1β and PGC-1-related coactivator (PRC). PGC-1α interacts with transcription factors to activate transcription and increases the expression of genes that regulate mitochondrial functions and fatty acid oxidation [109]. As a result, enhanced PGC-1α activity may function to protect against some metabolic diseases and improve mitochondrial biogenesis. SIRT1 also can control the ability of PGC-1α to repress glycolytic genes in response to fasting and pyruvate [94]. Hepatic SIRT1 interacts with PPAR-α through activating PGC-1α to mediate lipid homeostasis. This has been supported by studies with SIRT1 deletion. SIRT1 deletion in the liver results in the loss of PGC-1α activity and the impairment of fatty acid oxidation, leading to a predisposition to develop hepatic steatosis when fed with a high-fat diet [95].
7. SIRT1 and aging
As previously described, enhanced expression and activity of SIRT1 can be protective during a number of disorders the involve neurodegeneration, the cardiovascular system and metabolic pathways. Yet, of equal importance is the ability of extending the lifespan of a cell in conjunction with protecting a cell against acute insults [7,88]. Recent studies suggest that acute cell protection by SIRT1 is closely linked to extending cell longevity. SIRT1 can promote increased lifespan in higher organisms above yeast and metazoans while also promoting the protection of neuronal cells during oxidant stress exposure [26,31]. SIRT1 during both acute injury and ageing processes may be necessary for DNA repair to block apoptotic cell death [30]. Furthermore, in clinical studies of aging in patients with Alzheimer's disease, loss of SIRT1 has been associated with the increased accumulation of β-amyloid and tau [63]. In the vascular system, increased SIRT1 activity is necessary to block endothelial death, extend cellular lifespan and decrease endothelial senescence in models of both DM and oxidative stress [2,29,46]. Moderate overexpression of SIRT1 in cardiac cells can lessen age-dependent increases in cardiac hypertrophy and reduce the expression of senescence markers [52].
8. Conclusion
SIRT1 is expressed throughout the body including cells of the CNS, cardiac system, gastrointestinal system and the musculoskeletal system. The biological effects of SIRT1 are broad and can alter cellular survival and longevity during acute and long-term injuries that involve both oxidative stress and cell metabolism. SIRT1 not only controls, but also is governed by multiple signal transduction pathways that include NAD+, AMPK, forkhead transcription factors, NF-κB, TIMP3, Akt, insulin, PPAR and PTP. As a result, SIRT1 has an intricate role in the pathology, progression and treatment of multiple disease entities involving neurodegenerative disorders such as AD and PD, tumorigenesis, cardiovascular disease with myocardial injury and atherosclerosis, metabolic disease and DM and aging-related disease. Given the current novel studies, SIRT1 has emerged as a critical target for consideration in the development of new treatment modalities for multiple disorders.
9. Expert opinion
SIRT1 can provide critical protection against a number of disorders that can affect the body through pathways that involve oxidative stress. As this field continues to advance, new signaling pathways for SIRT1 continue to emerge that can be targeted for new therapeutic strategies for disorders that can be either of acute onset or more long-term and progressive in nature. Of considerable importance is the ability to elucidate the pathways of SIRT1 that can differentiate between providing cellular protection during a toxic injury from those that can extend the longevity and lifespan of cells and organisms.
For example, the relationship between SIRT1 and forkhead transcription factors represents a fine balance to foster cell protection with cell longevity. Activation of SIRT1 can increase lifespan in higher organisms such as Drosophila while also protecting brain cells from oxidant stress [26,31]. With regards to cellular protection, SIRT1 may rely upon the regulation of FoxO transcription factors such as to protect against inflammation [110], to maintain cardiac function during starvation [53,111], to protect against cardiac ischemia [80] and to maintain vessel integrity during oxidative stress [2,29]. Furthermore, an increase in FoxO3a and SIRT1 activity can occur in the heart during exercise [112], suggesting that physical activity may be beneficial for the cardiovascular system through SIRT1 and FoxO proteins.
In contrast, the relationships between SIRT1 and pathways such as FoxO are not always straightforward. In some cell systems, such as in renal cells, the presence of FoxO3a may be necessary for SIRT1 to exert protection and maintain cell longevity during oxidative stress [24]. SIRT1 also may be dependent upon other forkhead transcription factors, such as FoxO1, to regulate the transcription of SIRT1 [54]. Under such circumstances, enhanced SIRT1 expression during increased FoxO1 activity may negate the protective ability of SIRT1 in the presence of active FoxO1 and ultimately lead to the demise of cells. In other scenarios, SIRT1 with combined FoxO activity may offer inhibition of cell cycle progression that can limit apoptosis and be beneficial in cardiac or neurodegenerative disorders [113,114], but in the setting of tumorigenesis, loss of cell cycle regulation could spell disaster for a patient.
It therefore becomes crucial as we gain further knowledge of SIRT1 to be able to fully comprehend its signaling pathways. The great potential for alleviating disability from a number of disorders must be carefully weighed with the biological consequences that may occur with targeting SIRT1. Investigations that can further determine the intracellular signaling, trafficking and post-translational modifications that occur with SIRT1 in a variety of cell systems and environments will allow us to further translate this knowledge into effective treatment strategies that will be applicable to multiple systems of the body. At another level, it is important to recognize that current agents used to activate or inhibit SIRT1 are useful as investigational tools, but may not offer the precision necessary to develop safe and efficacious drug treatment regimens. For example, resveratrol may function to simulate SIRT1 activity in models using oxidative stress, but resveratrol can activate a host of other pathways, such as protein kinases and the mammalian target of rapamycin (mTOR), that may confound the ability to determine the role of SIRT1 alone [96], 115. As a result, in the coming years, it will be critical to elucidate the multiple roles SIRT1 plays in cell biology in order to thoughtfully develop safe and effective new treatments for both acute and chronic disorders that are intimately tied to the detrimental effects of cellular oxidative stress.
Article highlights.
- Sirtuin 1 (SIRT1) expression and activation can be influenced by several cellular conditions, such as calorie restriction, exercise and oxidative stress.
- Oxidative stress has a significant role in the pathology of a wide range of diseases that involve metabolic disorders, cognitive impairment, cardiac disease, psychiatric disorders and hepatic disease.
- SIRT1 has also been linked to chronic neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease.
- SIRT1 can modulate insulin resistance, fat metabolism, cardiac injury, atherosclerosis and obesity.
- Recent work suggests that SIRT1 can oversee not only cellular protection, but also long-term survival, that can be associated with age-related disorders.
- SIRT1 not only controls, but also is governed by multiple signal transduction pathways that include cytokines, NAD+, AMP-activated protein kinase (AMPK), forkhead transcription factors, NF-κB, tissue inhibitor of metalloprotease 3 (TIMP3), Akt, insulin, PPAR and protein tyrosine ohosphatase (PTP).
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The other authors state no conflict of interest and have received no payment in preparation of this manuscript. This research was supported by the following grants: to K Maiese American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS), NIH National Institute on Aging (NIA), NIH National Institute of Neurological Disorders and Stroke (NINDS), and NIH American Recovery & Reinvestment Act (ARRA).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol. 2010;321:194–206. doi: 10.1016/j.mce.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95–112. doi: 10.2174/156720210791184899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Berrocal JG, Frizzell KM, et al. Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem. 2009;284:20408–17. doi: 10.1074/jbc.M109.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelmohsen K, Pullmann R, Jr, Lal A, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–57. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–90. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 6••.Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–6. doi: 10.1038/nature06500. [This research demonstrates that SIRT1 may have a significant role the onset and progression of tumors.] [DOI] [PubMed] [Google Scholar]
- 7.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: biomarkers and novel therapeutic pathways. Exp Gerontol. 2010;45:217–34. doi: 10.1016/j.exger.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446–85. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Invest. 2009;89:1397–409. doi: 10.1038/labinvest.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tupe RS, Tupe SG, Agte VV. Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Br J Nutr. 2011;105:1741–9. doi: 10.1017/S0007114510005520. [DOI] [PubMed] [Google Scholar]
- 12.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22:1251–67. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3:153–65. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011;45:888–905. doi: 10.3109/10715762.2011.574290. [DOI] [PubMed] [Google Scholar]
- 15.Jourde-Chiche N, Dou L, Cerini C, et al. Vascular incompetence in dialysis patients-protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–37. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 16.Komandirov MA, Knyazeva EA, Fedorenko YP, et al. On the role of phosphatidylinositol 3-kinase, protein kinase b/akt, and glycogen synthase kinase-3beta in photodynamic injury of crayfish neurons and glial cells. J Mol Neurosci. 2011;45:229–35. doi: 10.1007/s12031-011-9499-1. [DOI] [PubMed] [Google Scholar]
- 17.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–62. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009;6:20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong ZZ, Hou J, Shang YC, et al. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–20. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15:1072–82. doi: 10.1007/s10495-010-0503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr Neurovasc Res. 2011;8:270–85. doi: 10.2174/156720211798120990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219–27. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009;29:395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa K, Wakino S, Yoshioka K, et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372:51–6. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- 25.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–85. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5:159–70. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Tanno M, Kuno A, Yano T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–82. doi: 10.1074/jbc.M109.090266. [This research demonstrates that SIRT1 increases MnSOD activity to preserve cardiac function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kume S, Haneda M, Kanasaki K, et al. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med. 2006;40:2175–82. doi: 10.1016/j.freeradbiomed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 29•.Hou J, Wang S, Shang YC, et al. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220–35. doi: 10.2174/156720211796558069. [This paper illustrates that the cytokine erythropoietin fosters nuclear translocation of SIRT1 to protect against oxidative stress.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Audrito V, Vaisitti T, Rossi D, et al. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011;71:4473–83. doi: 10.1158/0008-5472.CAN-10-4452. [DOI] [PubMed] [Google Scholar]
- 31.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–19. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon JM, Pasupuleti R, Xu L, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–55. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 36.Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22:503–14. doi: 10.1097/00004647-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Kaushal N, Hegde S, Lumadue J, et al. The regulation of erythropoiesis by selenium in mice. Antioxid Redox Signal. 2011;14:1403–12. doi: 10.1089/ars.2010.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumral A, Tuzun F, Oner MG, et al. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev. 2011;33:632–43. doi: 10.1016/j.braindev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Lanfranconi S, Locatelli F, Corti S, et al. Growth factors in ischemic stroke. J Cell Mol Med. 2011;15:1645–87. doi: 10.1111/j.1582-4934.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78:41–53. doi: 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- 41.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5:125–42. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009;9:1072–104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastromarino V, Volpe M, Musumeci MB, et al. Erythropoietin and the heart: facts and perspectives. Clin Sci (Lond) 2011;120:51–63. doi: 10.1042/CS20100305. [DOI] [PubMed] [Google Scholar]
- 44.Moore EM, Bellomo R, Nichol AD. Erythropoietin as a novel brain and kidney protective agent. Anaesth Intensive Care. 2011;39:356–72. doi: 10.1177/0310057X1103900306. [DOI] [PubMed] [Google Scholar]
- 45.Murua A, Orive G, Hernandez RM, Pedraz JL. Emerging technologies in the delivery of erythropoietin for therapeutics. Med Res Rev. 2011;31:284–309. doi: 10.1002/med.20184. [DOI] [PubMed] [Google Scholar]
- 46.Orimo M, Minamino T, Miyauchi H, et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–94. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 47.Sundaresan NR, Pillai VB, Wolfgeher D, et al. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizaki T, Milne JC, Imamura T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–74. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Storz P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal. 2011;14:593–605. doi: 10.1089/ars.2010.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi Y, Furukawa-Hibi Y, Chen C, et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–43. [PubMed] [Google Scholar]
- 51.Daitoku H, Hatta M, Matsuzaki H, et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 2004;101:10042–7. doi: 10.1073/pnas.0400593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–21. doi: 10.1161/01.RES.0000267723.65696.4a. [This research illustrates that specific levels of SIRT1 activity may be relevant to retarding aging.] [DOI] [PubMed] [Google Scholar]
- 53.Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res. 2010;107:1470–82. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289–99. doi: 10.1074/jbc.M110.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 56.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769:244–52. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Ganapathy S, Chen Q, Singh KP, et al. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One. 2010;5:e15627. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang C, Chen L, Hou X, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–31. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 59.Albani D, Polito L, Batelli S, et al. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–56. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 60.Lu KT, Chiou RY, Chen LG, et al. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54:3126–31. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- 61.Simao F, Matte A, Matte C, et al. Resveratrol prevents oxidative stress and inhibition of Na+K+-ATPase activity induced by transient global cerebral ischemia in rats. J Nutr Biochem. 2011;22:921–8. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Michan S, Li Y, Chou MM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–32. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–46. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Lu KT, Ko MC, Chen BY, et al. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem. 2008;56:6910–13. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- 68.Khan MM, Ahmad A, Ishrat T, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. 2010;1328:139–51. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 69.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005;2:387–99. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19:263–72. [PMC free article] [PubMed] [Google Scholar]
- 71.Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng FY, Tang BL. NF-kappaB signaling in neurite growth and neuronal survival. Rev Neurosci. 2010;21:299–313. doi: 10.1515/revneuro.2010.21.4.299. [DOI] [PubMed] [Google Scholar]
- 73.Melnik BC, John SM, Schmitz G. Over-stimulation of insulin/IGF-1 signaling by western diet may promote diseases of civilization: lessons learnt from laron syndrome. Nutr Metab (Lond) 2011;8:41. doi: 10.1186/1743-7075-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang QJ, Wang Z, Chen HZ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–9. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–60. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Mowafy AM, Alkhalaf M, El-Kashef HA. Resveratrol reverses hydrogen peroxide-induced proliferative effects in human coronary smooth muscle cells: a novel signaling mechanism. Arch Med Res. 2008;39:155–61. doi: 10.1016/j.arcmed.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640–7. doi: 10.4161/cc.10.4.14863. [DOI] [PubMed] [Google Scholar]
- 78.Cardellini M, Menghini R, Martelli E, et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med. 2004;36:774–81. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 80.Hsu CP, Zhai P, Yamamoto T, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–82. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dernek S, Ikizler M, Erkasap N, et al. Cardioprotection with resveratrol pretreatment: improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand Cardiovasc J. 2004;38:245–54. doi: 10.1080/14017430410035476. [DOI] [PubMed] [Google Scholar]
- 82.Fukuda S, Kaga S, Zhan L, et al. Resveratrol ameliorates myocardial damage by inducing vascular endothelial growth factor-angiogenesis and tyrosine kinase receptor Flk-1. Cell Biochem Biophys. 2006;44:43–9. doi: 10.1385/CBB:44:1:043. [DOI] [PubMed] [Google Scholar]
- 83.Thandapilly SJ, Wojciechowski P, Behbahani J, et al. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–6. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- 84.Wojciechowski P, Juric D, Louis XL, et al. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J Nutr. 2010;140:962–8. doi: 10.3945/jn.109.115006. [DOI] [PubMed] [Google Scholar]
- 85.Cheng TH, Liu JC, Lin H, et al. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:239–44. doi: 10.1007/s00210-003-0849-6. [DOI] [PubMed] [Google Scholar]
- 86.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res. 2011;90:276–84. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 87.Chan AY, Dolinsky VW, Soltys CL, et al. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maiese K, Chong ZZ, Shang YC, Hou J. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51:128–52. doi: 10.1177/0091270010362904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maiese K, Shang YC, Chong ZZ, Hou J. Diabetes mellitus: channeling care through cellular discovery. Curr Neurovasc Res. 2010;7:59–64. doi: 10.2174/156720210790820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen YR, Fang SR, Fu YC, et al. Calorie restriction on insulin resistance and expression of SIRT1 and SIRT4 in rats. Biochem Cell Biol. 2010;88:715–22. doi: 10.1139/O10-010. [DOI] [PubMed] [Google Scholar]
- 91.Sun C, Zhang F, Ge X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Xu S, Giles A, et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. Faseb J. 2011;25:1664–79. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–18. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 95.Purushotham A, Schug TT, Xu Q, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frojdo S, Durand C, Molin L, et al. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011;335:166–76. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 97.Bordone L, Motta MC, Picard F, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vetterli L, Brun T, Giovannoni L, et al. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E beta-cells and human islets through Sirt1 dependent mechanism. J Biol Chem. 2011;286:6049–60. doi: 10.1074/jbc.M110.176842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sasaki T, Kitamura T. Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 2010;57:939–46. doi: 10.1507/endocrj.k10e-320. [DOI] [PubMed] [Google Scholar]
- 101.Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–35. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–31. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fulco M, Cen Y, Zhao P, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–73. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819–23. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paiva MA, Rutter-Locher Z, Goncalves LM, et al. Enhancing AMPK activation during ischemia protects the diabetic heart against reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;300:H2123–34. doi: 10.1152/ajpheart.00707.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carvajal K, Zarrinpashneh E, Szarszoi O, et al. Dual cardiac contractile effects of the alpha2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2007;292:H3136–47. doi: 10.1152/ajpheart.00683.2006. [DOI] [PubMed] [Google Scholar]
- 108.Han L, Zhou R, Niu J, et al. SIRT1 is regulated by a PPARgamma-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res. 2010;38:7458–71. doi: 10.1093/nar/gkq609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sugden MC, Caton PW, Holness MJ. PPAR control: it's SIRTainly as easy as PGC. J Endocrinol. 2010;204:93–104. doi: 10.1677/JOE-09-0359. [DOI] [PubMed] [Google Scholar]
- 110.Nerurkar PV, Johns LM, Buesa LM, et al. Momordica charantia (bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation. J Neuroinflammation. 2011;8:64. doi: 10.1186/1742-2094-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011;14:649–61. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferrara N, Rinaldi B, Corbi G, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–50. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 113.Chong ZZ, Li F, Maiese K. Attempted cell cycle induction in post-mitotic neurons occurs in early and late apoptotic programs through Rb, E2F1, and caspase 3. Curr Neurovasc Res. 2006;3:25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wohlschlaeger J, Schmitz KJ, Takeda A, et al. Reversible regulation of the retinoblastoma protein/E2F-1 pathway during “reverse cardiac remodelling” after ventricular unloading. J Heart Lung Transplant. 2010;29:117–24. doi: 10.1016/j.healun.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 115.Chong ZZ, Shang YC, Zhang L, et al. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid Med Cell Longev. 2010;3:374–91. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]