Chimpanzee Adenovirus Antibodies in Humans, Sub-Saharan Africa (original) (raw)
Abstract
Human sera from the United States, Thailand, and sub-Saharan Africa and chimpanzee sera were tested for neutralizing antibodies to 3 chimpanzee adenoviruses. Antibodies were more common in humans residing in sub-Saharan Africa than in humans living in the United States or Thailand. This finding suggests cross-species transmission of chimpanzee adenoviruses.
Keywords: Neutralizing antibodies, prevalence, chimpanzee adenovirus, chimpanzee-to-human transmission, Africa, vaccine, HIV-1, dispatch
Vaccines to HIV-1 are needed to stem further spread of the HIV pandemic. One vaccine modality that has shown promise is based on adenovirus vectors of human serotype 5 (AdHu5) (1–3). However, immune responses induced by AdHu5 vectors are reduced by preexisting AdHu5-neutralizing antibodies found in humans in the United States (4–7).
The Study
To circumvent the negative effect of preexisting immunity to common human serotypes of adenoviruses on the efficacy of adenovirus vaccine carriers, we developed vectors based on chimpanzee-derived adenoviruses C68, C6, and C1 (8). We previously showed that neutralizing antibodies to chimpanzee adenoviruses are rarely found in US residents (6). Because vaccines to HIV-1 are most urgently needed in sub-Saharan Africa, we evaluated the prevalence of neutralizing antibodies to chimpanzee adenoviruses in sera from humans residing in 3 sub-Saharan countries with natural habitats for chimpanzees: Nigeria, Cameroon, and Côte d'Ivoire. Sera from captive chimpanzees in the United States and human sera from Thailand and the United States, including samples from persons with known exposures to chimpanzees, were tested for comparison.
Neutralizing antibodies to AdHu5 were found in most serum samples from Africa and Thailand. Percentages of antibodies to AdHu5 were higher in serum samples from Africa and Thailand than in serum samples from the United States (Table 1). Titers to AdHu5 were comparable in serum samples from the United States and sub-Saharan Africa but were higher in the control group from Thailand (Table 2).
Table 1. Sera with neutralizing activity to different human and chimpanzee adenoviruses.
| Origin | % positive samples (p values)*† | |||
|---|---|---|---|---|
| AdHu5 | AdC68 | AdC6 | AdC1 | |
| Human controls, United States (n = 50) | 34.0 | 2.0 | 4.0 | 2.0 |
| Human zoo workers, United States (n = 50) | 28.0 | 0 | 0 | 0 |
| Humans, Thailand (n = 200) | 76.5 | 1.5 | 3.0 | 4.0 |
| Humans, Cameroon (n = 405) | 55.8 | 1.7 (0.6764) | 7.9 (0.0045) | 5.4 (0.1248) |
| Humans, Côte d'Ivoire (n = 169) | 95.8 | 9.5 (0.0003) | 10.7 (0.0008) | 3.0 (0.9796) |
| Humans, Nigeria (n = 182) | 89.0 | 4.9 (0.0267) | 18.7 (<0.0001) | 9.3 (0.0045) |
| Chimpanzees, United States (n = 50) | 44.0 | 86.0 (<0.0001) | 92.0 (<0.0001) | 46.0 (<0.0001) |
Table 2. Mean adenovirus neutralizing antibody titers for positive samples.
| Origin | Mean VNA* titer ± standard deviation† | |||
|---|---|---|---|---|
| AdHu5 | AdC68 | AdC6 | AdC1 | |
| Humans, United States | 116 ± 111 | 40 ± 0 | 20 ± 0 | 20 ± 0 |
| Humans, Thailand | 303 ± 353‡ | 20 ± 0 | 80 ± 44 | 33 ± 25 |
| Humans, Cameroon | 125 ± 114 | 109 ± 145 | 82 ± 52‡ | 58 ± 41 |
| Humans, Côte d'Ivoire | 162 ± 212 | 60 ± 51‡ | 37 ± 34‡ | 148 ± 275 |
| Humans, Nigeria | 165 ± 206 | 29 ± 20 | 80 ± 115‡ | 42 ± 23 |
| Chimpanzees, United States | 164 ± 233 | 201 ± 204 | 137 ± 160 | 64 ± 72 |
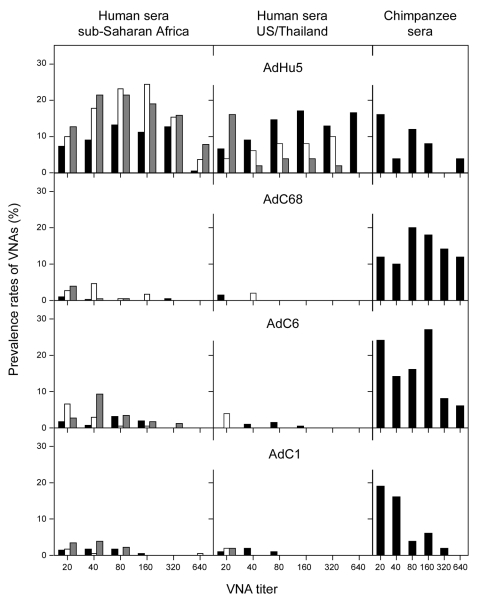
Neutralizing antibodies to AdC68, AdC6, and AdC1 were rare in sera from the United States and Thailand, with prevalence rates from 1.5% to 4% (Figure). Most positive samples had titers <80. Of 200 samples from Thailand, only 6 had high titers: 4 to AdC6 and 2 to AdC1. None of the samples from 50 US zoo workers, including those who reported regular contact with primates, had detectable antibodies to chimpanzee adenoviruses.
Figure.
Prevalence of neutralizing antibody titers to chimpanzee adenoviruses. Percentages of negative samples are not shown. Left column: Cameroon, black bars; Côte d'Ivoire, white bars; Nigeria: gray bars. Middle column: Thailand, black bars; US controls, white bars; US zoo keepers or animal handlers, gray bars. VNAs, virus neutralizing antibodies. Coded human serum samples that had been collected for other studies were obtained under an institutional review board exemption.
In contrast, serum samples from sub-Saharan African cohorts had higher prevalences of neutralizing antibodies to chimpanzee adenoviruses (Figure, Table 1). Samples from persons living in Cameroon and Nigeria showed a higher prevalence of neutralizing antibodies to AdC6 and AdC1. Titers of neutralizing antibodies to AdC6 and AdC1 were high, and samples were positive at dilutions >1:80. When compared with sera from Nigeria and Cameroon, sera from Côte d'Ivoire had a different pattern of antibodies reactive to the chimpanzee adenoviruses; prevalence rates were low to AdC1 but higher to AdC68 and AdC6. Although most samples had low to moderate titers, several samples had titers >80. No association was found between neutralizing antibodies to AdC68, AdC6, and AdC1 in any of the human samples tested, and only a few human serum samples had neutralizing antibodies to >1 chimpanzee adenovirus (data not shown). One serum sample from Cameroon neutralized all 3 chimpanzee adenoviruses and had titers of 20 to AdC68 and 160 to AdC6 and AdC1.
Circulating neutralizing antibodies to AdHu5 were found in 44% of captive US chimpanzees; titers in chimpanzee samples were comparable to those in human sera, suggesting that AdHu5 can readily cause an infection in captive chimpanzees. The prevalence of antibodies to AdC6 and AdC68 was high and exceeded that of antibodies to AdC1 (Table 1). Titers to AdC68 and AdC6 were higher in chimpanzees than in humans, but titers to AdC1 in both species were similar (Table 2).
Conclusions
Our data show that as expected, neutralizing antibodies to chimpanzee adenoviruses are rarely found in humans residing in the United States or Thailand. In contrast, their prevalence is higher in human sera from sub-Saharan Africa, where hunting and butchering of nonhuman primates for food are widespread and eating bush meat is common (11). Different prevalence rates of neutralizing antibodies to the 3 chimpanzee adenoviruses in human samples from the 3 African countries tested may reflect different infection rates in chimpanzees residing in these areas.
Cameroon, Gabon, and Republic of Congo are home to most Central African common chimpanzees (Pan troglodytes troglodytes). Most Western common chimpanzees (P. t. verus) inhabit Côte d'Ivoire and Guinea. The rare Nigeria chimpanzee (P. t. vellerosus) is found only in eastern Nigeria and western Cameroon, and Eastern African common chimpanzees (P. t. schweinfurthii) reside in a range from Central African Republic and the Democratic Republic of the Congo through western Uganda and Tanzania. No clear association was found between chimpanzee subspecies and neutralizing antibodies to different chimpanzee adenoviruses. Nevertheless, most samples were from P. t. verus, and only a limited number of samples were from P. t. troglodytes (n = 4) and P. t. schweinfurthii (n = 3). No samples from P. t. vellerosus were tested. In addition, the chimpanzee samples evaluated in the study were from animals kept in captivity and thus may not provide information on distribution of these viruses in free-ranging animals.
Increased prevalence of neutralizing antibodies to chimpanzee adenoviruses in sub-Saharan Africa may reflect cross-species transmission of these viruses from chimpanzees to humans. If transmission occurs, human-to-human spread of chimpanzee adenoviruses might further contribute to the comparatively high seroprevalence seen in equatorial Africa. Although we have no direct proof for viral cross-species transmission, this process has been previously described for other chimpanzee viruses. For example, the AIDS epidemic is believed to have originated from simian immunodeficiency virus–infected chimpanzees (12). Another chimpanzee retrovirus, simian foamy virus, has been reported to infect persons exposed to primates at zoos and research centers (13) and to infect Bantus in Cameroon (14). Chimpanzee adenoviruses do not appear to spread easily to humans through occupational contact with primates because none of the 23 persons who had routine exposure to primates, including chimpanzees, had serologic evidence of exposure despite the high prevalence of antibodies to chimpanzee adenoviruses in captive US chimpanzees.
Use of chimpanzee adenovirus vectors as vaccines for HIV-1 would require that most persons at high risk for HIV-1 infection lack neutralizing antibodies to these adenoviruses because such antibodies impair induction of transgene product-specific immune responses (4,5). Even in countries where chimpanzees are endemic, neutralizing antibodies to chimpanzee adenoviruses are less common in humans than those directed to AdHu5, which is currently in clinical trials as a vaccine for HIV-1 antigens in a vectored and replication-defective form. An alternative human serotype is AdHu35, which is being developed as a potential vaccine against HIV-1 (15). Prevalence rates to AdHu35 are low in the United States and Europe (<5%); however, rates are markedly higher (<20%) in equatorial Africa (15).
Although neutralizing antibodies to chimpanzee adenoviruses are also relatively more common in human sera from sub-Saharan Africa, they are found less frequently than antibodies to AdHu5 or AdHu35. Nonetheless, increased prevalence of neutralizing antibodies to adenoviruses in countries that are hardest hit by the AIDS pandemic needs to be taken into account in the design of vaccines based on chimpanzee adenovirus vectors. Vectors derived from other species are being developed and may provide additional or alternative vaccine carriers.
Acknowledgments
We thank H. Wilde, P. Marx, and J. Nkengasong for providing human sera; the National Primate Centers for providing chimpanzee sera; and Colin Barth for help in preparation of this article.
This study was supported by grant 5P01AI052271-04 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Hildegund C.J. Ertl has a patent for the use of adenovirus of the human serotype 5 as a vaccine carrier for HIV-1 infections. She has a similar patent pending for the use of chimpanzee adenovirus vectors (AdC68).
Biography
Dr Xiang is a senior staff scientist at the Wistar Institute. His primary research interests are assessing immune responses to novel vaccine prototypes based on viral vectors and studying immune responses to vaccines expressing antigens of rabies virus or HIV-1.
Footnotes
Suggested citation for this article: Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis [serial on the Internet]. 2006 Oct [_date cited_]. http://dx.doi.org/10.3201/eid1210.060078
References
- 1.Xiang ZQ, Yang Y, Wilson JM, Ertl HC. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–7. 10.1006/viro.1996.0239 [DOI] [PubMed] [Google Scholar]
- 2.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. 10.1038/415331a [DOI] [PubMed] [Google Scholar]
- 3.Vinner L, Wee EG, Patel S, Corbet S, Gao GP, Nielsen C, et al. Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J Gen Virol. 2003;84:203–13. 10.1099/vir.0.18589-0 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–22. [DOI] [PubMed] [Google Scholar]
- 5.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:6305–13. 10.1128/JVI.77.11.6305-6313.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–13. 10.1128/JVI.75.23.11603-11613.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiver JW. Gene therapy: chimpanzee-origin adenovirus vectors as vaccine. Banff, Alberta, Canada: Keystone Symposia; 2003. [Google Scholar]
- 8.Basnight M Jr, Rogers NG, Gibbs CJ Jr, Gajdusek DC. Characterization of four new adenovirus serotypes isolated from chimpanzee tissue explants. Am J Epidemiol. 1971;94:166–71. [DOI] [PubMed] [Google Scholar]
- 9.The LOGISTIC procedure. SAS version 9.1.3. Cary (NC): SAS Institute, Inc.; 2005. [Google Scholar]
- 10.Reyes-Sandoval A, Fitzgerald JC, Grant R, Roy S, Xiang ZQ, Li Y, et al. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol. 2004;78:7392–9. 10.1128/JVI.78.14.7392-7399.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tutin CE. Ecology and social organization of African tropical forest primates: aid in understanding retrovirus transmission. Bull Soc Pathol Exot. 2000;93:157–61. [PubMed] [Google Scholar]
- 12.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–14. 10.1126/science.287.5453.607 [DOI] [PubMed] [Google Scholar]
- 13.Switzer WM, Bhullar V, Shanmugam V, Cong M, Parekh B, Lerche NW, et al. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J Virol. 2004;78:2780–9. 10.1128/JVI.78.6.2780-2789.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calattini S, Maudere P, Tortevoye P, Froment A, Saib A, Gessain A. Interspecies transmission of simian foamy virus from chimpanzees and gorillas to Bantus and Pygmy hunters in southern Cameroon [abstract]. Würzburg, Germany: Fifth International Foamy Virus Conference. 2004. Jul 9–1. [Google Scholar]
- 15.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–6. 10.1097/00002030-200405210-00019 [DOI] [PubMed] [Google Scholar]