Trehalose 6-Phosphate Is Required for the Onset of Leaf Senescence Associated with High Carbon Availability (original) (raw)
Abstract
Trehalose 6-phosphate (T6P) is an important regulator of plant metabolism and development. T6P content increases when carbon availability is high, and in young growing tissue, T6P inhibits the activity of Snf1-related protein kinase (SnRK1). Here, strong accumulation of T6P was found in senescing leaves of Arabidopsis (Arabidopsis thaliana), in parallel with a rise in sugar contents. To determine the role of T6P in senescence, T6P content was altered by expressing the bacterial T6P synthase gene, otsA (to increase T6P), or the T6P phosphatase gene, otsB (to decrease T6P). In _otsB_-expressing plants, T6P accumulated less strongly during senescence than in wild-type plants, while _otsA_-expressing plants contained more T6P throughout. Mature _otsB_-expressing plants showed a similar phenotype as described for plants overexpressing the SnRK1 gene, KIN10, including reduced anthocyanin accumulation and delayed senescence. This was confirmed by quantitative reverse transcription-polymerase chain reaction analysis of senescence-associated genes and genes involved in anthocyanin synthesis. To analyze if the senescence phenotype was due to decreased sugar sensitivity, the response to sugars was determined. In combination with low nitrogen supply, metabolizable sugars (glucose, fructose, or sucrose) induced senescence in wild-type and _otsA_-expressing plants but to a smaller extent in _otsB_-expressing plants. The sugar analog 3-_O_-methyl glucose, on the other hand, did not induce senescence in any of the lines. Transfer of plants to and from glucose-containing medium suggested that glucose determines senescence during late development but that the effects of T6P on senescence are established by the sugar response of young plants.
In plants, the disaccharide trehalose is synthesized by the conversion of UDP-Glc and Glc-6-P to trehalose 6-phosphate (T6P) in a reaction catalyzed by T6P synthase (TPS), followed by hydrolysis of T6P to trehalose in a reaction catalyzed by T6P phosphatase (TPP). Since the identification of functional TPS and TPP genes in Arabidopsis (Arabidopsis thaliana; Blázquez et al., 1998; Vogel et al., 1998), the role of trehalose metabolism in plants has received increasing attention. Evidence has accumulated suggesting a role for the precursor of trehalose, T6P, as a signal for the regulation of plant metabolism and development (for review, see Eastmond and Graham, 2003; Paul et al., 2008; Schluepmann and Paul, 2009; Schluepmann et al., 2011).
T6P is considered to be a signal for high carbon availability: when carbon availability is increased by feeding of Suc, T6P content rises (Schluepmann et al., 2004; Lunn et al., 2006). Recently, a mechanism by which T6P signals in young tissues has been identified. In seedlings, young leaves, and other meristematic tissues, such as cauliflower (Brassica oleracea) florets, T6P inhibits the catalytic activity of the SNF1-related protein kinase SnRK1 (Zhang et al., 2009). SnRK1 is a central regulator of plant stress and starvation signaling (Baena-González et al., 2007). Changes in gene expression in Arabidopsis seedlings with increased or decreased T6P are consistent with the inhibition of SnRK1 by T6P in vivo (Zhang et al., 2009). This regulation is also in agreement with a role of T6P as a “feast” signal when carbon supply is high. However, an additional intermediary protein, which is not found or is unstable in extracts from mature leaves, is required for the inhibition of SnRK1 by T6P. The interaction of T6P with SnRK1 was confirmed by showing that Arabidopsis seedlings overexpressing the SnRK1 gene KIN10 are resistant to growth on high concentrations of trehalose, which results in T6P accumulation (Delatte et al., 2011). The T6P inhibition of SnRK1 has recently also been suggested to play a role in wheat (Triticum aestivum) grain development (Martínez-Barajas et al., 2011) and the growth of potato (Solanum tuberosum) tubers (Debast et al., 2011).
Based on the central role of T6P in carbon metabolism, it is not surprising that alteration of T6P metabolism results in a range of developmental phenotypes. For example, the Arabidopsis tps1 mutant is embryo lethal (Eastmond et al., 2002). However, the mutant can be complemented by expression of the bacterial TPS gene, otsA (Schluepmann et al., 2003), or rescued by expression of the Arabidopsis TPS1 gene behind a seed-specific promoter (Gómez et al., 2010). In addition, the altered sugar response of Arabidopsis seedlings with increased or decreased T6P through the expression of otsA or the TPP gene, otsB, respectively, demonstrated that T6P is required for carbon utilization and growth (Schluepmann et al., 2003). Furthermore, the Arabidopsis TPS1 gene was shown to be required throughout vegetative development and for the transition to flowering (van Dijken et al., 2004; Gómez et al., 2010). In tobacco (Nicotiana tabacum), increased T6P content resulted in higher photosynthetic capacity expressed on a leaf area basis, but a smaller leaf area, whereas the opposite was the case in plants with reduced T6P (Pellny et al., 2004). This suggests a role of T6P in photosynthetic development and leaf expansion.
The function of T6P as a signal for high carbon availability could also have consequences for the regulation of leaf senescence. In annual plants, senescence is required for the recycling of nutrients, such as nitrogen, from the old leaves to the seeds. Sugars accumulate during leaf senescence, as shown for Arabidopsis (Pourtau et al., 2006; Wingler et al., 2006) and other plant species, including tobacco, wheat, and maize (Zea mays; Noodén et al., 1997). In addition, external supply of Glc has been shown to induce leaf senescence when nitrogen availability is low (Wingler et al., 2004), probably because of a stronger accumulation of endogenous sugars than at high nitrogen supply (Pourtau et al., 2004). Global patterns of gene expression demonstrate that the senescence response to sugar supply is comparable to the changes that occur during developmental senescence (Wingler and Roitsch, 2008; Wingler et al., 2009). In contrast, dark incubation and starvation cause changes in gene expression that are different from those during developmental senescence (Wingler et al., 2009). These findings suggest that annual plants with determinate growth are sink limited once they stop growing and that the resulting sugar accumulation serves as a signal for senescence-induced nitrogen recycling.
There is also evidence from free-air CO2 enrichment studies that increased carbon supply can accelerate senescence in plants with determinate growth, in particular in cereals (Nie et al., 1995; Zhu et al., 2009). However, the response of trees with indeterminate growth (e.g. poplar [Populus spp.]) is opposite, showing delayed autumnal senescence in response to elevated CO2 (Tricker et al., 2004; Taylor et al., 2008). Both in annual plants and in trees, senescence can be accompanied by anthocyanin accumulation. The anthocyanin biosynthetic pathway is regulated by carbon availability: Suc has been shown to stimulate anthocyanin synthesis in Arabidopsis (Solfanelli et al., 2006), and stem girdling of maple (Acer saccharum) trees leads to sugar and anthocyanin accumulation (Murakami et al., 2008). In poplar, growth in elevated CO2 led to increased Suc accumulation and the activation of the anthocyanin biosynthetic pathway. However, in this case, anthocyanin accumulation was not indicative of senescence, and it was proposed that anthocyanins play a role in extending leaf longevity (Tallis et al., 2010).
Whether T6P is involved in signaling sugar availability for senescence regulation and anthocyanin accumulation was not known. To analyze the role of T6P during leaf senescence, T6P content was determined and modified by expression of the bacterial TPS gene, otsA, or the TPP gene, otsB. Phenotypic differences in plants with altered T6P were visible throughout leaf development but became more obvious during later developmental stages, when the plants started to senesce. The function of T6P in senescence regulation, therefore, was investigated in more detail.
RESULTS
T6P Content during Developmental Senescence of Leaves
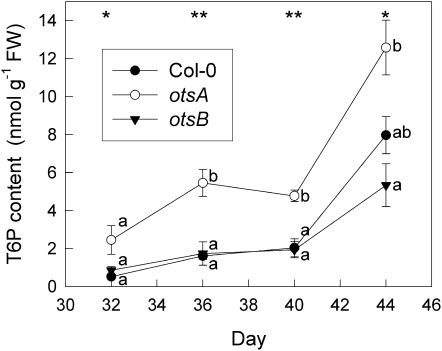
T6P content was measured during senescence in leaves 9 and 10 by HPLC/mass spectrometry (Delatte et al., 2009). After day 40, when leaves started to senesce, T6P content in wild-type (ecotype Columbia [Col-0]) plants accumulated strongly (Fig. 1). This trend was also seen in transgenic plants expressing the bacterial TPS gene, otsA, or the TPP gene, otsB. _otsA_-expressing plants generally contained more T6P than wild-type plants. _otsB_-expressing plants, which have decreased T6P content at the seedling stage (Zhang et al., 2009), did not show reduced T6P content compared with wild-type plants in mature leaves before the start of senescence. However, T6P increased only 6-fold between days 32 and 44, compared with 15-fold in wild-type plants. Plant material from the same experiment was also used to determine the effect of altered T6P on the senescence phenotype (Fig. 2), chlorophyll content and maximum quantum yield of PSII (_F_v/_F_m) during senescence (Fig. 3), gene expression (Fig. 4) , and sugar contents (Fig. 5).
Figure 1.
Senescence-dependent changes in T6P content in leaves 9 and 10 of wild-type Col-0 (black circles) and transgenic plants expressing the TPS gene, otsA (white circles), or the TPP gene, otsB (black triangles). Data are means ± se of three plants. Asterisks indicate significant differences (ANOVA) between the three genotypes for each time point: * P ≤ 0.05, ** P ≤ 0.01. Different letters indicate significant differences between the genotypes (P ≤ 0.05; Tukey’s pairwise comparison). FW, Fresh weight.
Figure 2.
Phenotypes of wild-type Col-0 and transgenic plants expressing the TPS gene, otsA, or the TPP gene, otsB. A, Rosettes of plants grown for 55 d. B, Individual leaves of plants grown for 52 d.
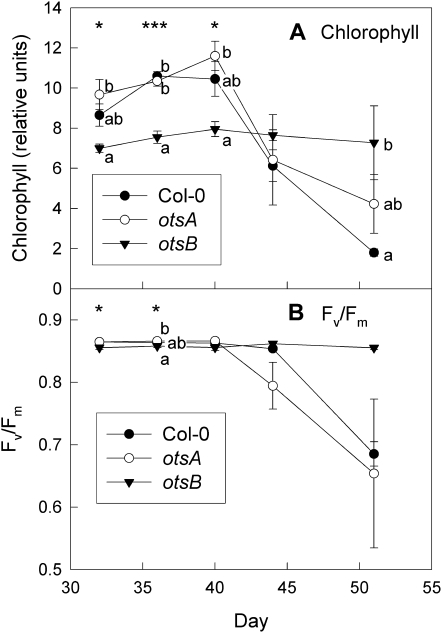
Figure 3.
Senescence-dependent changes in leaves 9 and 10 of wild-type Col-0 (black circles) and transgenic plants expressing the TPS gene, otsA (white circles), or the TPP gene, otsB (black triangles). A, Chlorophyll content. B, _F_v/_F_m. Data are means of four plants ± se. Asterisks indicate significant differences (ANOVA) between the three genotypes for each time point: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. Different letters indicate significant differences between the genotypes (P ≤ 0.05; Tukey’s pairwise comparison).
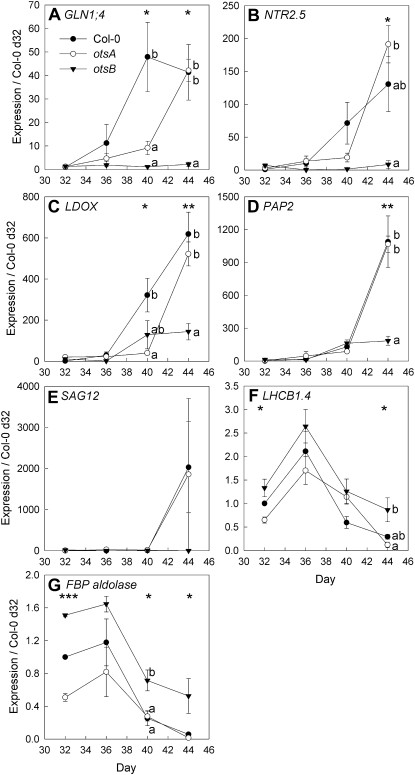
Figure 4.
Senescence-dependent changes in gene expression determined by quantitative RT-PCR in leaves 9 and 10 of wild-type Col-0 (black circles) and transgenic plants expressing the TPS gene, otsA (white circles), or the TPP gene, otsB (black triangles). A, Cytosolic glutamine synthetase 1;4 (GLN1;4; At5g16570). B, High-affinity nitrate transporter 2.5 (NTR2.5; At1g12940). C, Leucoanthocyanidin dioxygenase (LDOX; At4g22880). D, Anthocyanin pigment 2 protein (PAP2 = MYB90; At1g66390). E, Senescence-associated gene 12 (SAG12; At5g45890). F, Chlorophyll a/_b_-binding protein (LHCB1;4; At2g34430). G, Fructose-bisphosphate aldolase (FBP aldolase; At4g26530). Expression ratios are presented relative to Col-0 values on day 32. Data are means of three plants ± se. Asterisks indicate significant differences (ANOVA) between the three genotypes for each time point: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. Different letters indicate significant differences between the genotypes (P ≤ 0.05; Tukey’s pairwise comparison).
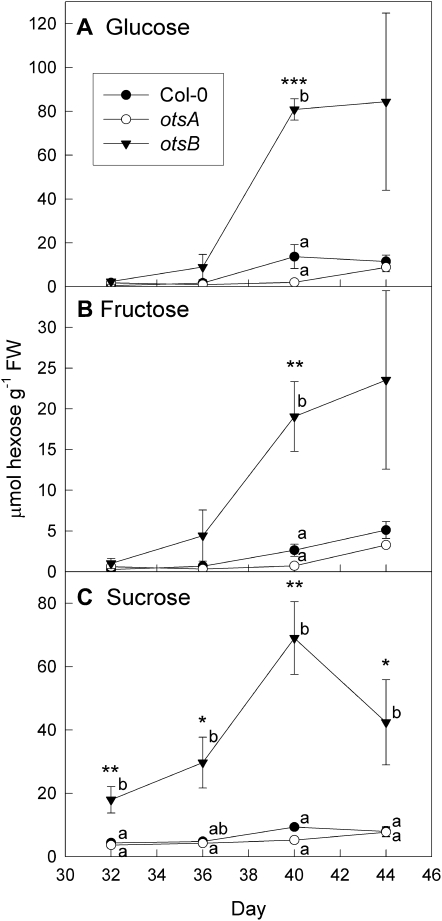
Figure 5.
Sugar contents during senescence in wild-type Col-0 (black circles) and transgenic plants expressing the TPS gene, otsA (white circles), or the TPP gene, otsB (black triangles). A, Glc. B, Fru. C, Suc. Data are means ± se of three plants. Asterisks indicate significant differences (ANOVA) between the three genotypes for each time point: * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. Different letters indicate significant differences between the genotypes (P ≤ 0.05; Tukey’s pairwise comparison). FW, Fresh weight.
Senescence in Plants with Altered T6P Metabolism
Considering the strong increase in T6P during leaf senescence, we determined if altered T6P metabolism had an effect on the course of senescence. The phenotype of mature plants expressing the TPS gene, otsA, or the TPP gene, otsB, was different from wild-type plants. _otsA_-expressing plants had a high anthocyanin content, as indicated by the red coloration (Fig. 2A), and leaves had a mottled appearance (Fig. 2B). When grown under 12-h days, _otsB_-expressing plants flowered later and the leaves were paler and accumulated less anthocyanin than wild-type plants, although some localized anthocyanin accumulation was found between the veins.
Chlorophyll content declined in _otsA_-expressing and wild-type plants after day 40 (Fig. 3A). _otsB_-expressing plants contained less chlorophyll during early development but remained green longer. However, chlorotic regions appeared around the edges of the leaves (Fig. 2B). Similar to chlorophyll content, _F_v/_F_m, a parameter that declines as leaves senesce, was maintained longer in _otsB_-expressing plants (Fig. 3B).
Experiments were repeated including an independent otsB transformant. Under 16-h days, the effect of otsB expression on flowering time was marginal, but _otsB_-expressing lines flowered after the formation of two additional leaves (Supplemental Fig. S1). The second line used (otsB9.6) had even paler green leaves before the onset of senescence (Supplemental Fig. S2) than line otsB12.1, which was used for the experiments presented in Figures 1 to 9. Reduced anthocyanin accumulation (Supplemental Fig. S3) and a delay in the senescence-dependent decline in _F_v/_F_m and chlorophyll content (Supplemental Fig. S2) were confirmed for the independent otsB transformant.
Figure 6.
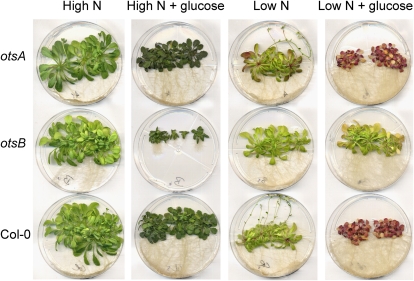
Response of wild-type Col-0 and transgenic plants expressing the TPS gene, otsA, or the TPP gene, otsB, to growth for 49 d on low-nitrogen (4.7 mm; Low N) or high-nitrogen (30 mm; High N) medium without or with the addition of 111 mm Glc.
Figure 7.
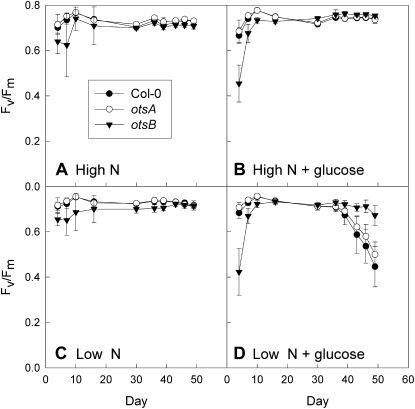
Whole-rosette _F_v/_F_m values in wild-type Col-0 (black circles) and transgenic plants expressing the TPS gene, otsA (white circles), or the TPP gene, otsB (black triangles), during growth on low-nitrogen (4.7 mm; Low N; C and D) or high-nitrogen (30 mm; High N; A and B) medium without (A and C) or with (B and D) the addition of 111 mm Glc. Data are means of at least 16 plants ± sd.
Figure 8.
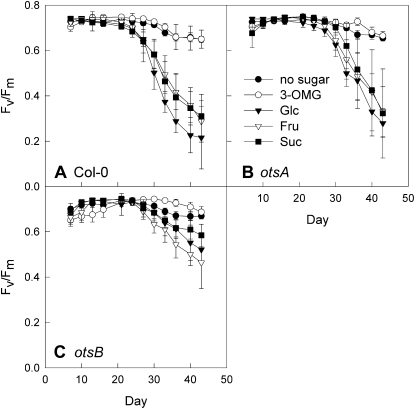
Whole-rosette _F_v/_F_m values during growth on low-nitrogen medium without the addition of sugar (black circles) or with the addition of 111 mm 3-OMG (white circles), Glc (black triangles), Fru (white triangles), or 55.5 mm Suc (black squares). A, Wild-type plants (Col-0). B, Transgenic plants expressing the TPS gene, otsA. C, Transgenic plants expressing the TPP gene, otsB. Data are means of at least 12 plants ± sd.
Figure 9.
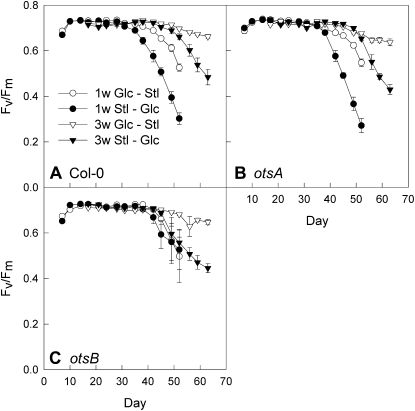
Effect of transfer to and from Glc-containing medium on whole-rosette _F_v/_F_m values. A, Wild-type plants (Col-0). B, Transgenic plants expressing the TPS gene, otsA. C, Transgenic plants expressing the TPP gene, otsB. Plants were transferred from low-nitrogen medium containing 111 mm Glc to medium containing 111 mm sorbitol (Glc-Stl; white symbols) or vice versa (Stl-Glc; black symbols) after 1 week (1w; circles) or 3 weeks (3w; triangles). Data are means of plants from five plates ± se.
Changes in Gene Expression during Senescence
To test if visible differences in leaf senescence were reflected in gene expression, changes in gene expression were analyzed by quantitative reverse transcription (RT)-PCR during development from mature leaves (day 32) to early senescence (day 44). The genes analyzed included known senescence-induced genes (Buchanan-Wollaston et al., 2005; Pourtau et al., 2006): GLN1;4 (At5g16570), NTR2.5 (At1g12940), LDOX (At4g22880), PAP2 (=MYB90; At1g66390), and SAG12 (At5g45890). The senescence-repressed genes LHCB1;4 (At2g34430) and FBP aldolase (At4g26530) were also used to monitor the progress of senescence.
The expression of known senescence marker genes increased as leaves of _otsA_-expressing and wild-type plants started to senesce (Fig. 4, A–E). At the final time point, the expression of these genes was lower in _otsB_-expressing plants than in _otsA_-expressing or wild-type plants. For the late senescence marker SAG12, however, this difference was not statistically significant due to the high variability of expression in _otsA_-transgenic and wild-type plants. Expression of LHCB1;4 and FBP aldolase declined during senescence (Fig. 4, F and G). In _otsB_-expressing plants, the decline in LHCB1;4 expression was less pronounced and FBP aldolase was more strongly expressed throughout. Overall, the changes in gene expression support the view that senescence was delayed in _otsB_-expressing plants.
Lower expression of LDOX and Production of Anthocyanin Pigment2 (PAP2) at the final time point is in agreement with reduced anthocyanin accumulation in _otsB_-expressing plants. While LDOX encodes a leucoanthocyanidin dioxygenase of the anthocyanin biosynthetic pathway, PAP2 encodes a sugar-inducible myb transcription factor that is involved in anthocyanin biosynthesis. Since anthocyanin synthesis and the expression of PAP2 and LDOX are induced by sugars (Lloyd and Zakhleniuk, 2004; Solfanelli et al., 2006; Loreti et al., 2008), reduced activation of the anthocyanin biosynthetic pathway during senescence in _otsB_-expressing plants could either be due to decreased sugar content or impaired sugar signaling.
Sugar Accumulation during Senescence
To determine if the delayed senescence and reduced anthocyanin accumulation in _otsB_-expressing plants were caused by reduced sugar content or by impaired sugar signaling, sugars were measured (Fig. 5). In all three genotypes tested (Col-0, otsA, and otsB), Glc and Fru, and to a lesser extent Suc, accumulated during senescence. However, hexose accumulation was much stronger in _otsB_-expressing plants, which also contained increased Suc throughout development. These results suggest that leaf senescence was delayed in _otsB_-expressing plants in spite of increased sugar accumulation. Therefore, sugar accumulation alone may not suffice to initiate the onset of senescence, and T6P is required.
Effect of T6P on SnRK1 Activity in Senescing Leaves
T6P inhibits SnRK1 activity in young leaves but not to the same extent in mature leaves, which lack a protein factor that is required for the inhibitory effect (Zhang et al., 2009). To determine if T6P inhibits SnRK1 in senescing leaves, T6P was incubated with desalted extracts from senescing leaves of soil-grown 44-d-old Arabidopsis plants. In the presence of high (1 mm) T6P, SnRK1 activity was 90% ± 0.8% of the value without T6P, which is comparable to mature leaves. Physiological T6P concentrations, therefore, are unlikely to have a significant effect on SnRK1 activity in senescing leaves, whereas T6P inhibited SnRK1 from seedlings in the low micromolar range and down to 20% of original activity at 1 mm T6P (Zhang et al., 2009).
Sugar Response in Plants with Altered T6P Metabolism
Since the majority of senescence-associated genes are sugar inducible (Pourtau et al., 2006; Wingler and Roitsch, 2008), it was possible that the delayed induction of these genes in _otsB_-expressing plants was due to decreased sensitivity to sugar. Senescence can be induced by the supply of 2% Glc in combination with low nitrogen supply (Wingler et al., 2004; Pourtau et al., 2006), which results in global changes in gene expression that are comparable to gene expression changes during developmental senescence (Wingler et al., 2009). This treatment led to anthocyanin accumulation and senescence in otsA transgenics and wild-type plants, while _otsB_-expressing plants stayed green (Fig. 6). Differences were also found on other media (e.g. anthocyanin accumulation in the _otsA_-expressing plants on low-nitrogen medium without sugar and reduced growth of _otsB_-expressing plants on high-nitrogen medium with Glc). Imaging of _F_v/_F_m to determine the course of senescence confirmed that growth on the low-nitrogen-plus-Glc medium induced senescence in wild-type and _otsA_-expressing plants, but not to the same extent in _otsB_-expressing plants (Fig. 7). Early photosynthetic development of _otsB_-expressing plants was delayed on Glc-containing medium, both at high and at low nitrogen supply, as indicated by the delayed rise in _F_v/_F_m. This is in agreement with the growth inhibition in otsB seedlings in response to sugar supply (Schluepmann et al., 2003).
An experiment with an independent otsB transformant (otsB9.6) was performed to confirm that the delayed senescence in response to Glc supply was due to reduced T6P content (Supplemental Fig. S4). The addition of 2% Glc to the growth medium resulted in reduced growth of this line (data not shown), and lower _F_v/_F_m values before the onset of senescence, but higher _F_v/_F_m values than in wild-type plants, were maintained during later stages, indicating a delayed senescence response to Glc.
Effect of the Sugar Source
To determine whether the effect of altered T6P on senescence was Glc specific, other sugars were tested (Fig. 8). In addition to Glc, the metabolizable sugars Fru and Suc induced senescence in wild-type and _otsA_-expressing plants, and this effect was delayed in otsB transgenics. The comparable effect of Glc, Fru, and Suc is not surprising, considering that these three sugars can easily be interconverted. Addition of 3-_O_-methyl glucose (3-OMG), on the other hand, did not induce senescence in any of the lines. Since 3-OMG is taken up by plant cells but only metabolized slowly, our results rule out that sugars induced senescence because of their osmotic effect.
Timing of Sugar Sensitivity
To analyze the developmental stage during which the senescence response to sugar treatment is determined, plants were transferred between low-nitrogen medium containing either Glc or sorbitol, which was used as an osmotic control that does not induce senescence in Arabidopsis (Wingler et al., 2004). In wild-type plants, the senescence-dependent decline in _F_v/_F_m was induced by transfer from sorbitol onto Glc-containing medium after 1 or 3 weeks (Fig. 9A), thus confirming that the senescence response is not determined by Glc availability during the first 3 weeks of development but induced later. Plants transferred after 3 weeks started senescing about 2 weeks later than those transferred after 1 week. This suggests that the plants need to be exposed to Glc for a minimum period of time, which is independent of plant age. Plants transferred from Glc to sorbitol after 3 weeks also senesced later than those transferred after 1 week, showing that transfer to fresh agar medium at a later stage, probably when nutrients such as nitrogen had started to become limiting, can extend plant longevity.
While the response of _otsA_-expressing plants was similar to that of wild-type plants (Fig. 9B), _otsB_-expressing plants showed a different response. In agreement with the less pronounced response to continuous growth on sugar-containing medium (Figs. 7D and 8C), transfer of _otsB_-expressing plants after 1 week onto Glc-containing medium had little effect compared with plants that were transferred onto sorbitol (Fig. 9C). However, _otsB_-expressing plants did show a response to Glc when they were transferred after 3 weeks, thus suggesting that the difference in the sugar response between these plants and the wild type was established before this time point.
DISCUSSION
We have shown here that T6P accumulates in senescing leaves to up to 15 times the contents found in mature leaves. T6P was further demonstrated to be required for the timely onset of senescence in response to high carbon supply.
T6P Content Is High When Sugars Accumulate during Developmental Senescence in Leaves
T6P content in plants is typically very low but changes rapidly in response to carbon supply. After resupply of Suc to starved Arabidopsis seedlings, T6P rose from about 0.02 to 0.5 nmol g−1 fresh weight within 3 h (Lunn et al., 2006). We found that T6P content in Col-0 plants also increased during leaf expansion, from 0.25 ± 0.05 nmol g−1 fresh weight at growth stage 1.06 (according to Boyes et al., 2001) to 0.36 ± 0.03 nmol g−1 fresh weight at growth stage 1.10 and to 0.47 ± 0.04 nmol g−1 fresh weight at growth stage 3.90. T6P content then accumulated even more strongly to 8 nmol g−1 fresh weight (Fig. 1) as leaves started to senesce (Fig. 3). This is much higher than previously determined in Arabidopsis tissues, but values up to 78 nmol g−1 fresh weight were recently reported for wheat grain (Martínez-Barajas et al., 2011).
Arabidopsis has 11 putative TPS genes and 10 putative TPP genes. While several of the Arabidopsis TPS genes show differential expression dependent on growth conditions and carbon availability (summarized by Paul et al., 2008), not all of these genes encode enzymatically active proteins. TPS1 has demonstrated enzymatic activity (Blázquez et al., 1998), while TPS11 was recently reported to have TPS and TPP activity (Singh et al., 2011). However, Ramon et al. (2009) argue that TPS5 to -11 are unlikely to have TPS or TPP activity. During leaf senescence, TPS1 expression declines (Supplemental Fig. S5), suggesting that the regulation of TPS1 expression is probably not responsible for T6P accumulation. Increased expression of TPS5 in senescing leaves is in agreement with its sugar inducibility (Schluepmann et al., 2004) and sugar accumulation during senescence (Fig. 5). Of the TPP genes, TPPA and TPPB encode enzymatically active proteins (Vogel et al., 1998). Expression of the more strongly expressed TPPA gene is high in mature leaves and declines during senescence (Supplemental Fig. S6). TPPA is also repressed when senescence is induced by Glc treatment (Pourtau et al., 2006). Regulation of TPPA expression thus could be responsible for the rise in T6P content during senescence. While T6P measurements confirmed increased T6P contents for _otsA_-expressing plants, there was no effect of expressing otsB on T6P in mature leaves. However, T6P accumulated less strongly (6-fold) than in wild-type plants (15-fold) during senescence (Fig. 1). The already very high expression of the endogenous Arabidopsis TPPA gene in mature leaves could be responsible for the lack of an additional effect of otsB activity on T6P content at this developmental stage.
Decreased T6P Delays the Onset of Leaf Senescence
Our results indicate that senescence is delayed in plants expressing the bacterial TPP gene, otsB, as indicated by the delayed senescence-dependent decline in chlorophyll content and _F_v/_F_m and by changes in gene expression (Figs. 3 and 4; Supplemental Fig. S2). The delayed senescence in otsB transgenics despite increased Glc, Fru, and Suc contents (Fig. 5) suggests that the sugar signaling pathway regulating senescence requires T6P. This is supported by the delayed senescence response to externally supplied sugar (Figs. 6–8; Supplemental Fig. S4). While anthocyanins accumulated in _otsA_-expressing plants (Figs. 2 and 6; Supplemental Fig. S3), less anthocyanin was present in _otsB_-expressing plants during developmental senescence on soil as well as during sugar-induced senescence on agar medium. In addition, expression of the LDOX gene of the anthocyanin biosynthetic pathway and of a transcription factor gene that is involved in anthocyanin synthesis, PAP2, was reduced in senescing leaves of _otsB_-expressing plants (Fig. 4). Since LDOX and PAP2 are both induced by sugars, in particular Suc (Lloyd and Zakhleniuk, 2004; Solfanelli et al., 2006; Loreti et al., 2008), it was surprising that anthocyanin synthesis was reduced in the otsB transgenics despite the high sugar content. This indicates that T6P is required for anthocyanin synthesis in response to Suc. The higher sugar contents are in agreement with increased hexose-phosphate contents found in _otsB_-expressing plants (Schluepmann et al., 2003) and consistent with a role of T6P in carbon utilization (Zhang et al., 2009; Paul et al., 2010).
In contrast to mature leaves, the expression of otsB reduced T6P content in seedlings (Zhang et al., 2009). This could indicate that delayed senescence in _otsB_-expressing plants was a consequence of earlier developmental changes, possibly occurring in the seedling meristems and in young leaves. Transfer onto Glc-containing medium confirmed that the difference in the sugar response between _otsB_-expressing plants and the wild type was established before the age of 3 weeks (Fig. 9). _otsB_-expressing plants that were continuously grown on Glc or transferred to Glc-containing medium before this time point showed little sugar response, whereas Glc induced senescence in plants that were transferred after 3 weeks. Similarly, the dependence on T6P for the transition to flowering is likely due to altered floral induction processes known to occur at the seedling stage (Simon et al., 1996; van Dijken et al., 2004).
While the senescence effect of otsB expression was robust and confirmed at different daylengths, flowering was more strongly delayed in _otsB_-expressing plants grown under short days (12 h) than under long days (16 h). However, even under long days, both otsB transformants flowered after the formation of an additional two leaves (Supplemental Fig. S1). The observation that, on low-nitrogen medium with Glc, senescence in wild-type and _otsA_-expressing plants occurred before flowering (Fig. 6) shows that the delayed senescence due to otsB expression cannot solely be explained by a delay in flowering. Delayed flowering was also observed in an embryo-rescued Arabidopsis tps1 mutant without TPS1 expression after germination and in TILLING mutants with weak TPS1 alleles (Gómez et al., 2010). For these plants, senescence was observed in the absence of flowering, but the timing was not compared with wild-type plants. The observation that plants with decreased T6P senesce eventually is in agreement with an involvement of flowering-dependent and -independent pathways in senescence regulation (Wingler et al., 2010). While an effect of increased trehalose formation due to otsB expression on senescence cannot be fully excluded, this is unlikely, because plants expressing a functional Escherichia coli trehalase, TreF, flower and senesce at the same time as the wild type (Schluepmann et al., 2003). Accelerated senescence of an Arabidopsis mutant that contains increased amounts of T6P, trehalose, and other sugars (Veyres et al., 2008) supports the link between carbon availability, T6P, and senescence shown here.
Interaction with SnRK1 during Early Development May Determine Senescence Regulation during Later Stages
In growing tissues, T6P inhibits SnRK1 in vitro and in vivo (Zhang et al., 2009). If inhibition of SnRK1 by T6P has consequences for mature plants, one would expect _otsB_-expressing plants with reduced T6P to have a similar phenotype as SnRK1-overexpressing plants, whereas _otsA_-expressing plants would resemble plants with reduced SnRK1. Overexpression of the SnRK1 gene, KIN10, resulted in delayed flowering and senescence, whereas senescence was accelerated and anthocyanin accumulated in plants with reduced expression of both SnRK1 genes, KIN10 and KIN11 (Baena-González et al., 2007). This is consistent with the senescence and anthocyanin accumulation phenotypes described here. However, while SnRK1 from seedlings and growing leaves is inhibited by T6P, inhibition is minimal in mature leaves, probably because they lack an unknown protein factor that binds T6P and interacts with SnRK1 (Zhang et al., 2009). In extracts from senescing leaves, SnRK1 activity was not inhibited by T6P at physiological concentrations. Given the phenotypic similarities between mature plants with modified SnRK1 and plants with altered T6P, in addition to the normal T6P contents in mature leaves of _otsB_-expressing plants (Fig. 1), it is likely that the observed phenotypes are a consequence of changes caused by the inhibition of SnRK1 during early development. This is also supported by the finding that _otsB_-expressing plants only showed sugar insensitivity of senescence when they were exposed to sugar before 3 weeks of growth (Fig. 9).
Sugar Signaling Pathways That Determine Senescence during Late Development
Once growth has stopped, T6P may no longer be required for biosynthetic reactions in the leaves and sugars accumulate (Fig. 5). In addition to the early sugar response involving T6P, sugars regulate senescence during later development: when plants were transferred onto Glc-containing medium after 3 weeks of growth, all lines, including _otsB_-expressing plants, showed accelerated senescence compared with plants transferred to sorbitol as an osmotic control (Fig. 9), indicating that this effect is not caused by the presence of Glc during germination and early development.
Overall, the findings for plants grown on agar with the addition of a metabolizable sugar source are similar to those for plants grown on compost. The generally late senescence of plants grown on agar without an additional carbon source may suggest that these plants are starved. Glc, Fru, and Suc all triggered a senescence response (Fig. 8). Given that Glc, Fru, and Suc can be interconverted easily (Pourtau et al., 2004), this does not provide any information about whether this effect is hexose or Suc dependent. 3-OMG, in contrast, is taken up by plant cells and phosphorylated at a low rate, but it does not trigger sugar signaling responses involving hexokinase (Cortès et al., 2003; Villadsen and Smith, 2004). The lack of senescence induction by 3-OMG (Fig. 8) thus confirms that 2% (111 mm) Glc does not accelerate senescence due to an osmotic effect and is consistent with the proposed role of hexokinase in the regulation of senescence by sugars (Pourtau et al., 2006). Whether T6P is involved in hexokinase signaling is currently unclear. Whereas yeast hexokinase is inhibited by T6P, no such effect has been found for plants (Wiese et al., 1999; Eastmond et al., 2002; Kandel-Kfir et al., 2006), and the impact of T6P on gene expression in seedlings was considered to be largely independent of hexokinase signaling (Zhang et al., 2009). Nevertheless, effects of hexokinase-1 on TPS1 expression in Arabidopsis seedlings were demonstrated by Avonce et al. (2004), suggesting that there may be signaling interactions.
CONCLUSION
Our results show that T6P accumulates in parallel with sugars during senescence and that T6P is required for the initiation of senescence in response to high carbon availability. However, this effect appears to be established during an earlier developmental phase, during which T6P is involved in regulating the utilization of available carbon for rapid growth.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) wild-type (Col-0) and transgenic plants expressing the Escherichia coli TPS gene otsA (otsA19.3) or the E. coli TPP gene otsB (otsB12.1) driven by the cauliflower mosaic virus 35S promoter (Schluepmann et al., 2003) were used. For growth on compost, seeds were stratified for 3 to 4 d at 4°C in 0.1% (w/v) agar and then pipetted onto Rothamsted Standard Compost Mix (Petersfield Products). The plants were grown under 12-h days with 150 μmol m−2 s−1 irradiance and 23°C/18°C day/night temperatures. Leaves 9 and 10 were marked with thread before determination of chlorophyll, _F_v/_F_m, gene expression, and T6P content. To confirm the effects on senescence in an independent otsB transformant, line otsB9.6 was included in a separate experiment. For this experiment, the plants were grown in Levington Multi-Purpose Compost (Scotts) under 16-h days with 125 μmol m−2 s−1 irradiance and temperatures of 22°C during the day and 18°C at night.
For growth on agar plates, seeds were sown onto high-nitrogen (10.3 mm NH4+ and 19.7 mm NO3−) or low-nitrogen (4.7 mm NO3−) agar medium as described by Wingler et al. (2004). Sugars (111 mm Glc, Fru, or 3-OMG or 55.5 mm Suc) were added by sterile filtration to the autoclaved medium. After stratification for 3 to 4 d at 4°C, the agar plates were placed vertically into growth chambers and incubated under 12-h days at 100 μmol m−2 s−1 irradiance and temperatures of 22°C during the day and 18°C at night. The experiment was repeated with an independent otsB transformant (otsB9.6). For this experiment, the plants were grown under 16-h days with 125 μmol m−2 s−1 irradiance.
To determine whether the sugar effect on senescence is a consequence of earlier developmental changes, plants were also transferred between low-nitrogen plates with 111 mm Glc or 111 mm sorbitol (control). Agar concentration was 1.2% to avoid penetration of the agar to the roots and thus damage to the plants. Daylength was set to 16 h at 100 μmol m−2 s−1 irradiance. About 10 plants per plate were transferred with tweezers.
Determination of T6P and Sugar Contents
All samples were harvested around midday. T6P was extracted and determined by the combination of liquid- and solid-phase extractions combined with HPLC/mass spectrometry according to Delatte et al. (2009). The method allowed baseline resolution of peaks surrounding the elution of T6P, Suc-6-P, and the internal control lactose 6-phosphate at the mass-to-charge ratio of T6P. Sugars (Glc, Fru, and Suc) were extracted in 80% ethanol at 80°C and determined spectrophotometrically in coupled enzymatic assays (Stitt et al., 1989).
Determination of Chlorophyll and _F_v/_F_m
Chlorophyll content was determined in leaves 9 and 10 using a CCM-200 chlorophyll content meter (Opti-Sciences), which measures the absorbance of red light by chlorophyll at 660 nm compared with the absorbance of infrared light at 940 nm. _F_v/_F_m in individual leaves of plants grown in compost was analyzed using an FMS-2 pulse-modulated fluorometer (Hansatech), and whole rosette _F_v/_F_m in plants grown on agar plates was analyzed with a FluorCam 700MF kinetic imaging fluorometer (Photon Systems Instruments) as described before (Wingler et al., 2004).
Quantitative RT-PCR
Leaves 9 and 10 were harvested at midday. RNA was isolated and gene expression was analyzed by quantitative RT-PCR as described by Zhang et al. (2009) with UBC9 (At4g27960) as a constitutive control (Czechowski et al., 2005). Primer sequences are given in Supplemental Table S1.
SnRK1 Assay
SnRK1 was assayed with and without 1 mm T6P by determining phosphorylation of the AMARA peptide as described by Zhang et al. (2009).
Supplemental Data
The following materials are available in the online version of this article.
- Supplemental Figure S1. Number of leaves at flowering.
- Supplemental Figure S2. Senescence-dependent changes in chlorophyll content and _F_v/_F_m.
- Supplemental Figure S3. Senescence phenotype.
- Supplemental Figure S4. Whole-rosette _F_v/_F_m values during growth on low nitrogen medium with or without Glc.
- Supplemental Figure S5. Expression of the TPS genes TPS1 to TPS11 during leaf development.
- Supplemental Figure S6. Expression of the TPP genes TPPA to TPPJ during leaf development.
- Supplemental Table S1. Primers used for quantitative RT-PCR.
Acknowledgments
We thank Thushyanthi Sivagnanam for excellent technical assistance.
References
- Avonce N, Leyman B, Mascorro-Gallardo JO, Van Dijck P, Thevelein JM, Iturriaga G. (2004) The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol 136: 3649–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Santos E, Flores CL, Martínez-Zapater JM, Salinas J, Gancedo C. (1998) Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 13: 685–689 [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, et al. (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42: 567–585 [DOI] [PubMed] [Google Scholar]
- Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM. (2003) In plants, _3-o-_methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131: 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Börnke F. (2011) Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiol 156: 1754–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Sedijani P, Kondou Y, Matsui M, de Jong GJ, Somsen GW, Wiese-Klinkenberg A, Primavesi LF, Paul MJ, Schluepmann H. (2011) Growth arrest by trehalose-6-phosphate: an astonishing case of primary metabolite control over growth by way of the SnRK1 signaling pathway. Plant Physiol 157: 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte TL, Selman MHJ, Schluepmann H, Somsen GW, Smeekens SCM, de Jong GJ. (2009) Determination of trehalose-6-phosphate in Arabidopsis seedlings by successive extractions followed by anion exchange chromatography-mass spectrometry. Anal Biochem 389: 12–17 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Graham IA. (2003) Trehalose metabolism: a regulatory role for trehalose-6-phosphate? Curr Opin Plant Biol 6: 231–235 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JD, Smeekens SC, Graham IA. (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29: 225–235 [DOI] [PubMed] [Google Scholar]
- Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA. (2010) _AtTPS1_-mediated trehalose 6-phosphate synthesis is essential for embryonic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J 64: 1–13 [DOI] [PubMed] [Google Scholar]
- Kandel-Kfir M, Damari-Weissler H, German MA, Gidoni D, Mett A, Belausov E, Petreikov M, Adir N, Granot D. (2006) Two newly identified membrane-associated and plastidic tomato HXKs: characteristics, predicted structure and intracellular localization. Planta 224: 1341–1352 [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Zakhleniuk OV. (2004) Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot 55: 1221–1230 [DOI] [PubMed] [Google Scholar]
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. (2008) Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179: 1004–1016 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M. (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RAC, Paul MJ. (2011) Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pregrain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiol 156: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami PF, Schaberg PG, Shane JB. (2008) Stem girdling manipulates leaf sugar concentrations and anthocyanin expression in sugar maple trees during autumn. Tree Physiol 28: 1467–1473 [DOI] [PubMed] [Google Scholar]
- Nie GY, Long SP, Garcia RL, Kimball BA, Lamorte RL, Pinter PJ, Wall GW, Webber AN. (1995) Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ 18: 855–864 [Google Scholar]
- Noodén LD, Guiamét JJ, John I. (1997) Senescence mechanisms. Physiol Plant 101: 746–753 [Google Scholar]
- Paul MJ, Jhurreea D, Zhang Y, Primavesi LF, Delatte T, Schluepmann H, Wingler A. (2010) Upregulation of biosynthetic processes associated with growth by trehalose 6-phosphate. Plant Signal Behav 5: 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y. (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59: 417–441 [DOI] [PubMed] [Google Scholar]
- Pellny TK, Ghannoum O, Conroy JP, Schluepmann H, Smeekens S, Andralojc J, Krause KP, Goddijn O, Paul MJ. (2004) Genetic modification of photosynthesis with E. coli genes for trehalose synthesis. Plant Biotechnol J 2: 71–82 [DOI] [PubMed] [Google Scholar]
- Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A. (2006) Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 224: 556–568 [DOI] [PubMed] [Google Scholar]
- Pourtau N, Marès M, Purdy S, Quentin N, Ruël A, Wingler A. (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219: 765–772 [DOI] [PubMed] [Google Scholar]
- Ramon M, De Smet I, Vandesteene L, Naudts M, Leyman B, Van Dijck P, Rolland F, Beeckman T, Thevelein JM. (2009) Extensive expression regulation and lack of heterologous enzymatic activity of the class II trehalose metabolism proteins from Arabidopsis thaliana. Plant Cell Environ 32: 1015–1032 [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Berke L, Sanchez-Perez GF. (2011) Metabolism control over growth: a case for trehalose-6-phosphate in plants. J Exp Bot (in press) [DOI] [PubMed] [Google Scholar]
- Schluepmann H, Paul M. (2009) Trehalose metabolites in Arabidopsis: elusive, active and central. The Arabidopsis Book 7: e0122, doi/10.1199/tab.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S. (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135: 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Igeño MI, Coupland G. (1996) Activation of floral meristem identity genes in Arabidopsis. Nature 384: 59–62 [DOI] [PubMed] [Google Scholar]
- Singh V, Louis J, Ayre BG, Reese JC, Shah J. (2011) _TREHALOSE PHOSPHATE SYNTHASE11_-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. Plant J 67: 94–104 [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140: 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RMC, Gerhardt R, Heldt HW. (1989) Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174: 518–552 [Google Scholar]
- Tallis MJ, Lin Y, Rogers A, Zhang J, Street NR, Miglietta F, Karnosky DF, De Angelis P, Calfapietra C, Taylor G. (2010) The transcriptome of Populus in elevated CO reveals increased anthocyanin biosynthesis during delayed autumnal senescence. New Phytol 186: 415–428 [DOI] [PubMed] [Google Scholar]
- Taylor G, Tallis MJ, Giardina CP, Percy KE, Miglietta F, Gupta PS, Gioli B, Calfapietra C, Gielen B, Kubiske ME, et al. (2008) Future atmospheric CO2 leads to delayed autumnal senescence. Glob Change Biol 14: 264–275 [Google Scholar]
- Tricker PJ, Calfapietra C, Kuzminsky E, Puleggi R, Ferris R, Nathoo M, Pleasants LJ, Alston V, de Angelis P, Taylor G. (2004) Long-term acclimation to leaf production, development, longevity and quality following 3 yr exposure to free-air CO2 enrichment during canopy closure in Populus. New Phytol 162: 413–426 [Google Scholar]
- van Dijken AJH, Schluepmann H, Smeekens SCM. (2004) Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol 135: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyres N, Danon A, Aono M, Galliot S, Karibasappa YB, Diet A, Grandmottet F, Tamaoki M, Lesur D, Pilard S, et al. (2008) The Arabidopsis sweetie mutant is affected in carbohydrate metabolism and defective in the control of growth, development and senescence. Plant J 55: 665–686 [DOI] [PubMed] [Google Scholar]
- Villadsen D, Smith SM. (2004) Identification of more than 200 glucose-responsive Arabidopsis genes none of which responds to 3-O-methylglucose or 6-deoxyglucose. Plant Mol Biol 55: 467–477 [DOI] [PubMed] [Google Scholar]
- Vogel G, Aeschbacher RA, Müller J, Boller T, Wiemken A. (1998) Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J 13: 673–683 [DOI] [PubMed] [Google Scholar]
- Wiese A, Gröner F, Sonnewald U, Deppner H, Lerchl J, Hebbeker U, Flügge U-I, Weber A. (1999) Spinach hexokinase I is located in the outer envelope membrane of plastids. FEBS Lett 461: 13–18 [DOI] [PubMed] [Google Scholar]
- Wingler A, Marès M, Pourtau N. (2004) Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol 161: 781–789 [DOI] [PubMed] [Google Scholar]
- Wingler A, Masclaux-Daubresse C, Fischer AM. (2009) Sugars, senescence, and ageing in plants and heterotrophic organisms. J Exp Bot 60: 1063–1066 [DOI] [PubMed] [Google Scholar]
- Wingler A, Purdy S, MacLean JA, Pourtau N. (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57: 391–399 [DOI] [PubMed] [Google Scholar]
- Wingler A, Purdy SJ, Edwards SA, Chardon F, Masclaux-Daubresse C. (2010) QTL analysis for sugar-regulated leaf senescence supports flowering-dependent and -independent senescence pathways. New Phytol 185: 420–433 [DOI] [PubMed] [Google Scholar]
- Wingler A, Roitsch T. (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol (Stuttg) (Suppl 1)10: 50–62 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Zhu J, Zeng Q, Liu G, Xie Z, Tang H, Cao J, Zhao X. (2009) Elevated CO2 accelerates flag leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Funct Plant Biol 36: 291–299 [DOI] [PubMed] [Google Scholar]