Constitutive type I interferon modulates homeostatic balance through tonic signaling (original) (raw)
. Author manuscript; available in PMC: 2013 Feb 24.
Abstract
Interferons (IFNs) were discovered nearly 60 years ago as a family of cytokines induced during and protecting from viral infection. They have been documented to play essential roles in numerous physiological processes beyond innate antiviral defense, including immunomodulation, regulation of the cell cycle, cell survival and differentiation, and the host response to microbial pathogens. Recent data have also uncovered a potentially darker side to the functions of IFN, including roles in autoimmunity and diabetes. Many IFN effects occur in the absence of acute viral infection, highlighting a physiologic role for constitutively produced IFN. Type I IFNs are constitutively produced at vanishingly low quantities and yet exert profound effects, mediated at least in part through modulation of signaling intermediates required for diverse cytokine response pathways. We review evidence for a yin-yang of IFN function through its role in modulating crosstalk between multiple cytokines by both feed-forward and feed-back regulation of common signaling intermediates and postulate that a similar mechanism underlies a homeostatic role for IFN through tonic signaling in the absence of acute infection.
Three types of interferon (IFN) have been identified in mammals. The type I IFN family encodes 7 subtypes, including 13 IFNα isoforms, IFN-ε, IFN-κ, IFN-ω and IFNβ. There is also a single type II IFN (IFNγ) and the type III IFNs comprised of IFNλ 1, 2 and 3 (also named IL29, IL28a, and IL28b, respectively) (Levy et al., 2011; Li et al., 2009; Pestka et al., 2004). Type I IFNs are secreted in abundance in response to viral infection, acting early during the immune response to potentiate antiviral responses and prime and maintain adaptive immunity (Durbin et al., 2000; Huber and Farrar, 2011; Muller et al., 1994). Interestingly, low amounts of IFNβ accumulate in the tissue milieu in the absence of infection. Until recently, the purpose of this constitutive IFNβ has been unclear, in part because the quantities of constitutive IFN are close to the threshold of detection of even the most sensitive assays (Hamilton et al., 1996; Vogel and Fertsch, 1984). It has been proposed that small quantities of IFNβ prime cells for an efficient subsequent response to other cytokines, and are also necessary for immune homeostasis, maintenance of bone density and antiviral and anti-tumor immunity. Similarly, constitutive IFN may play detrimental roles, particularly when improperly regulated, by augmenting an autoimmune-prone condition or by enhancing potentially debilitating inflammation that could lead to cancer (de Visser et al., 2006). It is important to build an understanding of the molecular events that underpin constitutive IFN production and action and how defects in this process impact disease.
Constitutive type I IFN secretion
In the early 1980s, Velio Bocci proposed a biological role for constitutive IFN signaling based on observations that uninfected tissue preparations produced an antiviral activity akin to IFN. It was proposed that IFN was induced by ongoing low-grade exposure of the mucosa to external pathogens, tissue remodeling or damage (Bocci, 1980). Whilst the constitutive production of type I IFN has not been addressed under germ free conditions to assess a potential role for commensal microorganisms, type I IFN mRNA and protein have subsequently been detected in tissues of healthy mice maintained in specific pathogen free environments (Gresser, 1990; Tovey et al., 1987; Viti et al., 1985; Yaar et al., 1986). The generation of neutralizing type I IFN antibodies and later animals lacking the type I IFN receptor (Ifnar) or the Ifnβ gene provided essential tools to further substantiate the existence and importance of constitutive type I IFN. It is now clear that constitutive IFNβ occurs in healthy animals and is necessary for a diverse array of biological effects, including maintenance of the hematopoietic stem cell (HSC) niche, immune cell function and bone remodeling. Moreover, perturbations of IFN contribute to the pathology of autoimmune disease, antiviral immunity and cancer.
Mechanism of constitutive type I IFN production
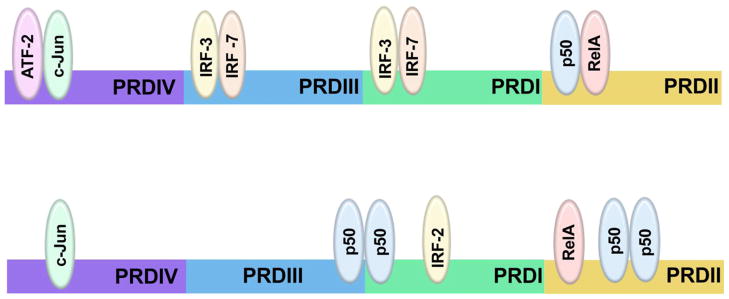
Although type I IFNs are encoded by multiple genes, they are co-regulated in a complex fashion involving a positive feed-forward regulatory loop (Marie et al., 1998; Sato et al., 1998). In the absence of priming amounts of IFNβ, mouse embryo fibroblasts (MEFs) do not produce other type I IFNs, suggesting that IFNβ is a master regulator and highlighting the importance of IFNβ secretion in regulating all type I IFN activities and presumably those mediated by type III IFN (Erlandsson et al., 1998; Takaoka et al., 2000). IFNβ mRNA expression increases dramatically in response to viral infection; in this context the occupancy of the IFNβ promoter and enhancer has been extensively characterized. The IFNβ promoter contains four positive regulatory domains (PRDI-IV), which are occupied by overlapping transcription factor complexes. Interferon regulatory factor (IRF)-3 and IRF7 bind PRDI and III; the AP-1 heterodimer of ATF-2 and c-Jun binds PRDIV; and the NF-κB p50-RelA heterodimer binds PRDII (Fig. 1a). In addition, roles for the architectural protein HMGA1 and for a positioned nucleosome have been defined (Ford and Thanos, 2010; Maniatis et al., 1998). The binding of each of these components in the correct orientation and location results in activation of the IFNβ promoter in response to viral infection. For virus induced IFNβ expression, IRF3 and IRF7 appear to be essential (Hata et al., 2001; Sato et al., 2000).
Figure 1. Transcription factors for inducible and constitutive IFNβ promoter activity.
The _IFN_β promoter contains four positive regulatory domains (PRDI-IV). (Upper) Stimulation-dependent induction of IFNβ expression requires cooperative recruitment of a number of transcription factor complexes to its promoter: IRF-3 and/or IRF-7, ATF-2 and c-Jun, p50 and RelA, and the architectural protein HMGA1 (not pictured). The binding of these factors to their specific regions (IRF-3 and IRF-7 bind PRDI and III; ATF-2 and c-Jun bind PRDIV; p50 and RelA bind PRDII) leads to rapid and robust induction of IFNβ expression. (Lower) In the absence of stimulation the IFNβ promoter is occupied by c-Jun at PRDIV and RelA at PRDII, which promote basal IFNβ expression; and IRF-2 at PRDI and p50 homodimers at PRDI, PRDII and III, which negatively regulate IFNβ production. Involvement of additional transcription factors, particularly partners for c-Jun and RelA in unstimulated cells, remain to be identified.
Less is known regarding the transcriptional regulation of constitutive IFNβ. The most notable distinction from acutely induced IFN expression is a switch from dependency on IRF3 and IRF7 to c-Jun and NF-κB components (Fig. 1b). In contrast to pathogen-induced IFNβ production, deletion of IRF3 does not prevent constitutive IFNβ expression (Hata et al., 2001). In addition, deletion of IRF9 resulting in undetectable amounts of IRF7 has no impact on constitutive IFNβ (Hata et al., 2001). However, AP-1 and NF-κB components appear essential. AP-1 and NF-κB are dimeric transcription factors, activated by a diverse array of cytokines and growth factors. AP-1 is comprised of Jun, Fos or ATF proteins; NF-κB is comprised of a dimer of p50, p52, p65 (RelA), RelB or c-Rel. Although AP-1 and NF-κB play important but non-essential roles in viral induction of IFNβ (Balachandran and Beg, 2011), both are essential for constitutive IFNβ production. c-Jun occupies PRDIV on the IFNβ promoter in uninfected cells, and its deletion decreases constitutive IFNβ expression by half (Gough et al., 2010). It is likely that the NF-κB subunit RelA also maintains constitutive expression of IFNβ, since RelA is bound to PRDII in unstimluated cells and loss of RelA causes gene expression defects in unstimulated cells resembling loss of IFN (Basagoudanavar et al., 2011; Wang et al., 2010). However, constitutive IFNβ expression by _Rela_−/− MEFs has not been directly measured.
Threshold expression of IFNβ in unstimulated cells is governed by IRF2 and p50 homodimers, both of which are constitutively expressed and bind the IFNβ promoter as repressors (Fig. 1b) (Cheng et al., 2011; Senger et al., 2000; Thanos and Maniatis, 1995); Harada et al., 1989). IRF2-deficient cells have heightened expression of IFNα and β and IFN target genes and _Irf2_−/− mice develop a psoriasis-like skin disease that is ameliorated by removing IFN signaling, such as ablation of IFNAR1 (Arakura et al., 2007; Honda et al., 2003). The abundance of constitutive IFNβ following loss of p50 has not been measured; however p50-deficient animals are developmentally normal with no signs of the skin disease caused by IRF2 loss, suggesting a lesser role for p50 as a repressor of constitutive IFNβ (Sha et al., 1995). Together, these reports document that the IFNβ promoter is occupied by both activators (c-Jun and RelA) and suppressors (IRF2 and p50 homodimers), the balance of which maintain tight control of constitutive IFNβ in absence of overt stimuli.
Regulation of cytokine signal transduction by constitutive IFNβ secretion
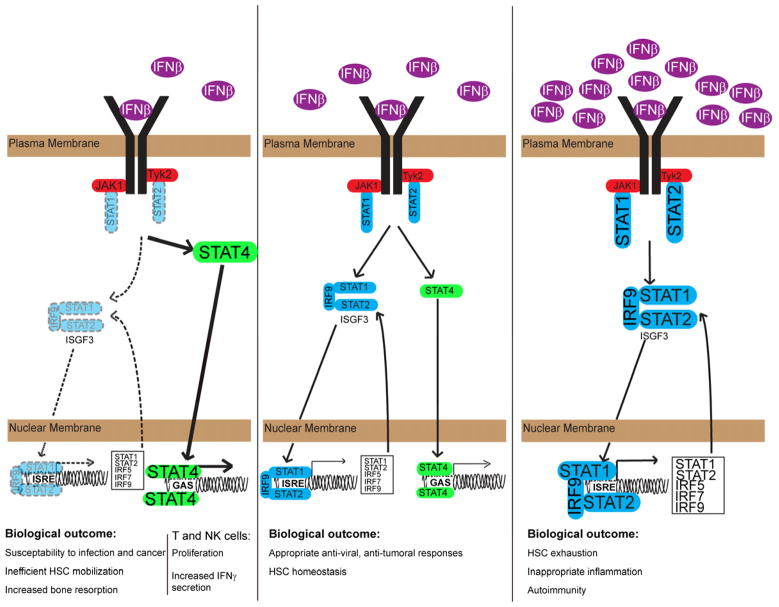
Binding of type I IFNs to IFNAR (Pestka et al., 2004) activates receptor-associated Janus kinases (JAKs) (Bach et al., 1997), which phosphorylate the receptor and activate latent Signal Transducers and Activators of Transcription (STATs) by receptor recruitment and phosphorylation on a caboxy-terminal tyrosine residue (Levy and Darnell, 2002; Stark et al., 1998). Activated STATs form higher order transcription factor complexes (Schroder et al., 2004), which translocate to the nucleus and bind to promoter elements to induce target gene expression (Fig. 2). These transcription factor complexes include STAT1 homodimers and a heterotrimer of STAT1, STAT2 and IRF9, known as ISGF3 (Levy et al., 1988). STATs 3, 4, 5 and 6 can also be phosphorylated in response to type I IFNs, forming both homodimers and heterodimers (Platanias, 2005).
Figure 2. Constitutive IFNβ signaling sets the balance of signaling intermediaries.
In a healthy organism, low amounts of IFNβ are constitutively secreted, which maintains appropriate expression of IFN-inducible signaling intermediaries, including the transcription factors STAT1, STAT2, IRF5, IRF7, and IRF9 (center). Appropriate expression allows a balance between STAT1 or ISGF3 signaling and signaling through other STAT proteins (e.g., STAT4). Diminished constitutive IFNβ secretion (left) results in decreased expression of ISGF3 subunit proteins, altering the balance of signaling (e.g., between ISGF3 and STAT4) and culminating in susceptibility to infection and cancer, inefficient hematopoietic stem cell (HSC) mobilization, and increased bone resorption. Excessive expression of IFNβ (right) drives over-expression of ISGF3 proteins, initiating an inflammatory environment that can result in autoimmunity and exhaustion of the HSC niche.
Somewhat paradoxically, loss of constitutive IFNβ or loss of IFN responsiveness (e.g., as observed in Ifnar1−/− animals) attenuates cellular responses not only to type I IFNs but also to unrelated cytokines, such as IFNγ and IL-6. Several hypotheses have been advanced to explain this phenomenon. In one model, IFN augments other cytokine responses by direct interaction of heterologous receptors, allowing co-recruitment of downstream effector molecules. To date there have been two such hybrid receptors proposed; the association of IFNAR1 with IFNγR2 (Takaoka et al., 2000) and the association of IFNAR1 with the common gp130 chain of the IL-6 receptor (Mitani et al., 2001). It was proposed that constitutive IFNβ secretion leads to the assembly of these multimeric receptors, and cytokine-dependent receptor crosstalk augments tyrosine phosphorylation of IFNAR1, providing a docking site for latent STAT1 or STAT3, transcription factors necessary for IFNγ and IL-6 responses. This model could account for cooperation between type I and II IFN and IL-6; however, this model would also suggest far greater overlap in the transcriptional response and biological outcome to stimulation with these cytokines than has actually been observed (Der et al., 1998; Weihua et al., 2000).
An alternative model posits regulation of signaling intermediaries by priming amounts of IFNβ, rather than direct receptor crosstalk. In this model, low level IFNβ signaling is required to maintain adequate expression of STAT1 and 2, and likely a host of other signaling components, all of which are STAT target genes. In the absence of constitutive IFNβ or its receptor, basal STAT expression is diminished, compromising STAT-dependent biological responses, such as those induced by IFNγ and IL6 (Fig. 2). Cells with defects in IFNβ signaling have decreased basal expression of STAT1 and STAT2 compared to wild type cells (Fleetwood et al., 2009; Gough et al., 2010). Furthermore, gene expression studies demonstrated that basal expression of other signaling molecules is affected by loss of constitutive IFNβ signaling, including reduced IRF1, IRF7 and possibly STAT3 (Fleetwood et al., 2009; Gough et al., 2010; Thomas et al., 2006). STAT6 is also IFN- responsive and therefore is likely diminished in the absence of constitutive IFNβ (de Veer et al., 2001). Given the many intermediary cytokine signaling molecules that are themselves induced by JAK-STAT activation, loss of basal signaling likely leads to a paucity of intermediary proteins required for an array of diverse responses. This model would explain the attenuated signaling and biological activity of other cytokines that engage the STAT pathways, such as reduced IFNγ, IL-6 and CSF-1 signaling observed in Ifnar1−/− cells (Gough et al., 2010; Karaghiosoff et al., 2000; Park et al., 2000; Thomas et al., 2006). This hypothesis of a direct priming role for constitutive IFN would also account for the observation that treatment of Ifnar1−/− macrophages with sub-threshold expression of IFNγ increases both basal STAT1 expression and subsequent IFNγ-induced STAT1 phosphorylation (Hu et al., 2002). If reduced responsiveness is due to limited STAT1 abundance, increasing STAT1 expression can rescue signaling, regardless of the receptor complex involved. However, recovery of IFNγ responsiveness by IFNγ priming in the absence of IFNAR would not be predicted by a receptor cross-talk model.
An extension to the IFN priming hypothesis is that the ratio of specific STAT proteins shapes the biological response to a stimulus. In the case of Ifnar1−/− or _Ifnb1_−/− cells, STAT1 and STAT2 expression is diminished providing a stoichiometric advantage to other STAT proteins for receptor binding. An example of the biological relevance of STAT protein ratios is the IL-6 to IFNγ switch caused by reduced STAT3 expression. IL-6 signaling in the absence of STAT3 exhibits a transcriptional and biological profile similar to that of IFNγ by preferentially engaging STAT1 (Costa-Pereira et al., 2002). The inverse happens in response to IFN, leading to enhanced antiviral signaling due to absence of the tonic effect of STAT1-STAT3 competition (Wang et al., 2011). Because constitutive IFNβ is a critical regulator of STAT protein expression, it is capable of sculpting subsequent responses to diverse stimuli of the JAK-STAT pathway by shifting the ratio of available STATs. Taken together, these findings suggest that the precise set-point of STAT and other signaling protein abundance, maintained by basal IFN signaling, is a critical regulator of the quantity and quality of subsequent responses.
Biological significance of constitutive IFN secretion
Constitutive type I IFN is important for the maintenance and mobilization of the hematopoietic stem cell niche (Essers et al., 2009; Sato et al., 2009). Treatment of mice with poly(I:C), a strong inducer of type I IFNs, or with IFNα itself, causes HSC exit from G0 and temporarily induces proliferation of dormant HSCs (Essers et al., 2009; Sato et al., 2009). Mice lacking IFNAR1 are refractory to type I IFN and therefore their HSC compartment remains quiescent in response to either poly(I:C) or IFNα treatment (Essers et al., 2009; Sato et al., 2009). These studies documented a role for induced IFN in the regulation of hematopoietic expansion, but analysis of the bone marrow of un-manipulated _Ifnar1_−/− animals revealed a role for constitutive IFN in HSC homeostasis. _Ifnar_−/− mice display a reduction in the total number of HSCs compared to wild type animals (Essers et al., 2009), suggesting that tonic IFN signaling maintains the HSC compartment. Because bone marrow from _Ifnar1_−/− mice is capable of reconstituting the hematopoietic compartment of lethally irradiated wild-type hosts (Diamond et al., 2011; Dunn et al., 2005), some of the tonic effects of IFN likely act on the bone marrow stroma in the HSC niche. Mice lacking STAT1 have a defective response to poly(I:C) similar to Ifnar1−/− suggesting that IFNα induced HSC proliferation is STAT1 dependent (Essers et al., 2009).
IRF2, as a negative regulator of IFNβ expression, modulates the concentration of constitutively secreted IFNβ (Arakura et al., 2007; Honda et al., 2003; Senger et al., 2000). Increased IFNβ secretion in _Irf2_−/− mice leads to the exhaustion of the HSC niche, similar to that observed following prolonged IFN treatment. This depletion of HSCs can be averted by ablation of Ifnar1 (Sato et al., 2009). These data can be explained by positing that constitutive type I IFN is normally at a concentration below the threshold necessary to mobilize HSCs but at a level sufficient to maintain the HSC niche. Therefore, either the absence of constitutive IFN or prolonged elevated IFN depletes the HSC niche, although due to opposing underlying mechanisms.
Immune cell homeostasis and activity
The importance of constitutive IFNβ in maintaining immune homeostasis has been revealed by studies examining the aberrant phenotype of mice lacking type I IFN receptors. IFNAR-deficient mice have decreased splenic NK cells and B220 positive B lymphocytes and increased Gr1+CD11c+ myeloid cells (Hwang et al., 1995; Swann et al., 2007). In the absence of constitutive IFNβ signaling, murine hematopoietic cells exhibit enhanced proliferative responses to low doses of CSF-1 and increased expression of the activation markers CD11c and CD11b (Hamilton et al., 1996; Honda et al., 2003; Hwang et al., 1995; Teige et al., 2003).
Constitutive IFNβ augments myeloid cell function and macrophage homeostasis, as shown by analysis of macrophages from C3H-HeJ mice, which are incapable of inducing IFN in response to LPS due to a defect in the Tlr4 gene (Poltorak et al., 1998). Culturing C3H-HeJ macrophages with supernatants from wildtype C3H-HeN macrophages that express constitutive IFNβ enhanced their phagocytic potential. A similar effect was obtained by adding low ‘priming’ concentrations of IFN to C3H-HeJ macrophages (Vogel and Fertsch, 1984). Conversely, phagocytic potential was attenuated when C3H/HeN macrophages were incubated with IFNα and IFNβ neutralizing antibodies (Vogel and Fertsch, 1984), documenting the requirement for constitutive IFN in maintaining macrophage function. The physiologic consequence of the importance of constitutive IFN for macrophage function may be reflected by the influence of the gut microbiota on hematopoietic homeostasis through basal TLR signaling (Musso et al., 2011).
Given the role of constitutive IFNβ in regulating macrophage activity, effects on other myeloid lineages could be expected. For instance, osteoclasts are required for bone remodeling and therefore critical for maintaining bone homeostasis. Constitutive IFNβ impairs bone marrow-derived macrophage differentiation into osteoclasts, resulting in reduced bone resorption. This is seen most clearly in vivo by comparing wild type, Ifnβ −/− and _Ifnar1_−/− mice; the mutant strains exhibit decreased bone density and increased numbers of osteoclasts (Takayanagi et al., 2002). Therefore constitutive IFNβ probably maintains the entire myeloid lineage at correct homeostatic numbers, which has implications for both the regulation of the innate immune system and maintenance of the skeletal system.
Type I IFN has complex effects on T cells. It is a potent suppressor of proliferation in most cell types, but it enhances the survival and proliferation of CD8+ blasts (Marrack and Kappler, 2004). Unexpectedly, _Ifnar1_- and _Ifnb_-deficient animals do not have altered CD8+ T cell numbers; however, following peptide or DNA-based immunization these mice accumulate twice the number of antigen specific CD8+ T cells compared to wild type (Dikopoulos et al., 2005). One explanation for this apparent contradiction is the relative ratios of STAT proteins expressed. IFNAR-deficient cells express lower amounts of STAT1, enabling JAK-STAT signaling to favor STAT4 over STAT1 (Fig. 2). STAT4 stimulates T-cell proliferation, production of the Th1 subset of CD4+ cells, and is a master regulator of IFNγ secretion from NK cells. In contrast STAT1 activation is typically growth inhibitory and impedes IFNγ secretion from NK cells (Miyagi et al., 2007; Nguyen et al., 2002). In addition, CD8+ T cells have a less robust induction of STAT1 expression than CD4+ T cells (Gil et al., 2006), which would compound the STAT1 to STAT4 switch and lead to reduction in IL-10 producing CD4+ T regulatory cells (Dikopoulos et al., 2005).
Constitutive type I IFN is involved in the maintenance of NK cell biology at several levels. The primary cytokine necessary for NK cell proliferation is IL-15, which is a type I IFN-regulated gene (Fehniger et al., 2001; Kennedy et al., 2000; Lodolce et al., 1998; Montoya et al., 2002). Although IL-2 can equivalently support STAT1-deficient NK cell growth, IL-15 is able not only to support growth but also to restore cytotoxicity to _Stat1_−/− NK cells (Lee et al., 2000). Due to the inability of _Ifnar1_−/− or _Ifnar2_−/− mice to respond to constitutively secreted IFNβ, it was unsurprising to find that the number of NK cells in these animals was lower than wild type animals and that these mice were highly susceptible to the outgrowth of NK-targeted tumor cells (Swann et al., 2007). Again, these effects occur in the absence of an overt pathogenic stimulation, suggesting that the mutant animal phenotype is caused by loss of tonic IFN signaling. In addition to a proliferative defect, NK cells derived from _Ifnar2_−/− mice are defective in IL-2 mediated killing of RMAS cells in vitro, although the cytotoxicity of naïve IFNAR-deficient NK cells to some targets is not affected (Lee et al., 2000; Swann et al., 2007). In contrast, complete absence rather than just reduced expression of STAT1 results in a profound defect in NK cell cytotoxicity (Lee et al., 2000). Interestingly, the dichotomy of STAT1 and STAT4 expression also influences the activity of NK cells. Basal expression of STAT4 is higher than that of STAT1 in NK cells (Mack et al., 2011), where it drives IFNγ production (Mack et al., 2011; Miyagi et al., 2007). IFN generated during infection increases STAT1 expression in NK cells, which displaces STAT4 on IFNAR leading to suppression of IFNγ production (Mack et al., 2011; Miyagi et al., 2007). NK cells from _Ifnar1_−/− animals have attenuated STAT1 expression (Miyagi et al., 2007). Abundant STAT1 and STAT2 leads to stimulation of NK cell mediated cytotoxicity, whereas reduced STAT1 but abundant STAT4 stimulates cytokine secretion at the expense of cytotoxicity (Nguyen et al., 2002). Because the ratios of STAT proteins are set by tonic IFN signaling, constitutive IFN is critical for modulating subsequent cellular responses.
These observations highlight another important consequence of constitutive IFN, to modulate relative expression of STAT proteins. STAT1, STAT2, and STAT6 are all IFN responsive genes and as such constitutive IFN governs their abundance (de Veer et al., 2001). We have recently confirmed that absence of constitutive IFN signaling, either due to genetic ablation of Ifnar1 or by treatment with blocking antibodies, markedly reduced STAT1 and STAT2 expression in a range of primary tissues, including dendritic cells, splenocytes, fibroblasts, heart, large intestine, small intestine, liver, lung and pancreas (Gough et al., 2010). This observation suggests that the ratio between STAT1 and other STATs is governed by constitutive IFNβ, establishing homeostatic regulation of multiple lineages. Taken together, these observations demonstrate that basal levels of STATs and other proteins is maintained through tonic IFN signaling
Antiviral and anti-tumor effects
Type I and type II IFNs bind to distinct receptors and therefore it was assumed that loss of IFNAR would have no effect on IFNγ signaling. Surprisingly, it was found that IFNγ dependent antiviral responses are compromised in the absence of IFNAR1 (Gough et al., 2010; Muller et al., 1994; Takaoka et al., 2000). The importance of constitutive IFNβ secretion in maintaining IFNγ-dependent antiviral activities has been highlighted in a number of experimental observations. Priming IFNβ-deficient MEFs with low dose IFNβ restored IFNγ-mediated antiviral responses and increased resistance to influenza virus by promoting activation of DCs (Phipps-Yonas et al., 2008; Takaoka et al., 2000). Since maintenance of STAT1 expression in MEFs is dependent on constitutive IFNβ, absence of IFN responsiveness (e.g., _Ifnar1_−/− or _Ifnb1_−/− mice) attenuated IFNγ-mediated antiviral responses. Ectopic restoration of STAT1 to physiologic expression in IFNAR-deficient cells rescued IFNγ-mediated antiviral protection without altering the response to type I IFN (Gough et al., 2010). Positive correlation between the abundance of constitutive IFNβ and subsequent antiviral responses has also been observed in physiologic situations. For example, cardiac myocytes have higher constitutive IFNβ expression than cardiac fibroblasts, and myocytes show increased resistance to viral infection (Zurney et al., 2007).
Type I IFN is used as a treatment for viral infection (hepatitis B and C) and cancer (including chronic myeloid leukemia and melanoma) and its efficacy is affected by constitutive IFN. Melanoma is often refractory to IFNα and IFNβ treatment relative to normal tissue. This loss of IFN responsiveness is associated with reduced STAT1 expression (Landolfo et al., 2000; Sun et al., 1998; Wong et al., 1997) and restoration of STAT1 expression to amounts seen in normal tissue via transduction or priming with IFNγ regained responsiveness to type I IFN treatment (Wong et al., 1998; Wong et al., 1997). Other studies have shown that low amounts of IFNβ repress tumor growth by preventing production of pro-angiogenic cytokines by tumor infiltrating neutrophils (Jablonska et al., 2010). Defects in type I IFN secretion and/or signaling have also been seen in other human malignancies, including the deletion of IFN genes in acute leukemia cells (Colamonici et al., 1992) and malignant T-cells (Heyman et al., 1994); down-regulation of IFNAR in hairy cell leukemia (Billard et al., 1986), gastric cancer (Chen et al., 2009) and lymphoblastoid cells (Pfeffer and Donner, 1990); loss of JAK1 in lung carcinoma (Kaplan et al., 1998); down regulation of IFN signaling components in melanoma (Wong et al., 1997) and chronic myeloid leukemia (Landolfo et al., 2000); and overexpression of negative regulatory SOCS proteins in cancer (Lesinski et al., 2010; Li et al., 2004). The number and variety of malignancies with mutations that prevent production of or alter responsiveness to IFN suggests that aberrations in IFN sensitivity and production provide a survival advantage for tumor cells and thus a potential target for therapeutic intervention.
Autoimmunity
The development and progression of autoimmune diseases is multifactorial; however, increased secretion of type I IFN or an IFN-stimulated gene signature is typical in patients with a number of diseases, such as systemic lupus erythematosus (SLE), Sjögren’s Syndrome or type I diabetes mellitus (DM), correlating with increased disease severity (Crow, 2010; Kirou et al., 2005; Lu et al., 2007). A subset of patients receiving IFN treatment for hepatitis C develop DM, arthritis or multiple sclerosis (MS) (Passos de Souza et al., 2001; Yamazaki et al., 2010), despite the fact that IFNβ is commonly used to treat MS (Buttmann and Rieckmann, 2007; Tak, 2004). Similarly, there is a subset of MS patients that do not respond to IFNβ treatment. Non-responders present with high circulating amounts of IFNβ, suggesting that a precise setpoint of IFNβ concentration must be maintained; both higher or lower amounts result in pathologic consequences (Comabella et al., 2009).
Elevated IFNα expression in SLE patients may be responsible for the generation of autoimmune antibodies by inducing monocyte differentiation and potentiating activation of CD4+ T cells in response to self antigens (Blanco et al., 2001). The pathologic consequence of elevated IFN in SLE is revealed by disease amelioration in mice by IFNAR deficiency. Moreover, IFN neutralizing antibodies have shown promise in clinical trials in SLE (Nacionales et al., 2007; Yao et al., 2009).
As noted above, IRF-2 deficiency leads to psoriasis due to elevated IFN (Arakura et al., 2007). The AP-1 and NF-κB subunits c-Jun and RelA that drive constitutive IFNβ expression are elevated in psoriatic plaques (Lizzul et al., 2005; Mehic et al., 2005; van der Fits et al., 2003). No discrete pathogens have been implicated in disease causation, suggesting that altered tonic type I IFN signalling contributes to the pathogenesis of psoriasis.
These data support a unified concept of IFN-mediated homeostasis. Appropriate amounts of basal IFN maintain the abundance of a wide variety of signalling molecules important for both immunity and tissue homeostasis. Neither reduced nor enhanced levels of IFN can be tolerated without adverse consequences of either impaired hematopoietic homeostasis and reduced immune responses on the one hand or increased inflammation and autoimmunity on the other. It will be of great interest to understand the contribution of the microbiome to both the appropriate and the inappropriate regulation of tonic IFN (Musso et al., 2011).
Conclusions
IFNβ and other type I IFNs are constitutively secreted at low amounts by many tissues of the body as a means of maintaining homeostasis and priming cells to maintain a rapid and robust innate and adaptive immune response to subsequent challenge. It will be important to determine whether there are other similar biological processes whereby constitutive low level autocrine or paracrine cytokine signaling maintains homeostatic balance. The range of influences of constitutive IFNβ is due, in large part, to its role in maintaining the expression of STAT proteins and other signaling intermediates, although the full range of signaling proteins whose basal abundance is determined by tonic IFN signaling remains to be documented. The loss of priming concentrations of IFNβ leads to compromised expression of STAT and other regulatory proteins, resulting in impaired cross-talk between cytokine signaling networks and a host of additional phenotypes, such as aberrant immune cell function, bone remodeling and deregulation of HSC homeostasis. Documenting the extent of innate and adaptive immune pathways influenced by IFN priming will be an important area of further investigation. Modulation of the abundance of signaling intermediates by constitutive IFN regulates both the quantity and the quality of subsequent responses, due to competitive interactions among related proteins. This mechanism potentially explains many situations of cross-talk between diverse cytokine families that signal through overlapping components subject to feed-back and feed-forward regulatory networks. Increased research into the regulation and function of tonic IFN signaling and identification of the relevant IFN-stimulated genes that contribute to homeostasis could have major implications for our understanding of how a healthy immune system is maintained. Of importance will be development of a more complete picture of the sources of tonic IFN, in particular determining the contribution of environmental signals, such as influences from microbial flora.
Acknowledgments
D.J.G is supported by a Special Fellow award from the Leukemia and Lymphoma society. N.L.M is supported by a Cancer Research Institute Predoctoral Emphasis in Tumor Immunology Scholarship. R.W.J. is a Principal Research Fellow of the National Health and Medical Research Council of Australia (NHMRC) and supported by NHMRC Program and Project Grants, the Susan G. Komen Breast Cancer Foundation, the Prostate Cancer Foundation of Australia, Cancer Council Victoria, The Leukemia Foundation of Australia, Victorian Breast Cancer Research Consortium, Victorian Cancer Agency and the Australian Rotary Health Foundation. D.E.L acknowledges funding from the National Institutes of Health (R01AI28900, U54AI057158).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakura F, Hida S, Ichikawa E, Yajima C, Nakajima S, Saida T, Taki S. Genetic control directed toward spontaneous IFN-alpha/IFN-beta responses and downstream IFN-gamma expression influences the pathogenesis of a murine psoriasis-like skin disease. J Immunol. 2007;179:3249–3257. doi: 10.4049/jimmunol.179.5.3249. [DOI] [PubMed] [Google Scholar]
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Beg AA. Defining emerging roles for NF-kappaB in antivirus responses: revisiting the interferon-beta enhanceosome paradigm. PLoS Pathog. 2011;7:e1002165. doi: 10.1371/journal.ppat.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagoudanavar SH, Thapa RJ, Nogusa S, Wang J, Beg AA, Balachandran S. Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. J Virol. 2011;85:2599–2610. doi: 10.1128/JVI.02213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard C, Sigaux F, Castaigne S, Valensi F, Flandrin G, Degos L, Falcoff E, Aguet M. Treatment of hairy cell leukemia with recombinant alpha interferon: II. In vivo down-regulation of alpha interferon receptors on tumor cells. Blood. 1986;67:821–826. [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Bocci V. Is interferon produced in physiologic conditions? Med Hypotheses. 1980;6:735–745. doi: 10.1016/0306-9877(80)90091-2. [DOI] [PubMed] [Google Scholar]
- Buttmann M, Rieckmann P. Interferon-beta1b in multiple sclerosis. Expert Rev Neurother. 2007;7:227–239. doi: 10.1586/14737175.7.3.227. [DOI] [PubMed] [Google Scholar]
- Chen HM, Tanaka N, Mitani Y, Oda E, Nozawa H, Chen JZ, Yanai H, Negishi H, Choi MK, Iwasaki T, et al. Critical role for constitutive type I interferon signaling in the prevention of cellular transformation. Cancer Sci. 2009;100:449–456. doi: 10.1111/j.1349-7006.2008.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CS, Feldman KE, Lee J, Verma S, Huang DB, Huynh K, Chang M, Ponomarenko JV, Sun SC, Benedict CA, et al. The specificity of innate immune responses is enforced by repression of interferon response elements by NF-kappaB p50. Sci Signal. 2011;4:ra11. doi: 10.1126/scisignal.2001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Platanias LC, Diaz MO. Correlation between interferon (IFN) alpha resistance and deletion of the IFN alpha/beta genes in acute leukemia cell lines suggests selection against the IFN system. Blood. 1992;80:744–749. [PubMed] [Google Scholar]
- Comabella M, Lunemann JD, Rio J, Sanchez A, Lopez C, Julia E, Fernandez M, Nonell L, Camina-Tato M, Deisenhammer F, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–3365. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is’harc H, Gesualdo I, Newman SJ, Kerr IM, Poli V. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci USA. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res Ther. 2010;12(Suppl 1):S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikopoulos N, Bertoletti A, Kroger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8+ T cell responses through IL-10-producing CD4+ T regulatory 1 cells. J Immunol. 2005;174:99–109. doi: 10.4049/jimmunol.174.1.99. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Fernandez-Sesma A, Lee CK, Rao TD, Frey AB, Moran TM, Vukmanovic S, Garcia-Sastre A, Levy DE. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- Erlandsson L, Blumenthal R, Eloranta ML, Engel H, Alm G, Weiss S, Leanderson T. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr Biol. 1998;8:223–226. doi: 10.1016/s0960-9822(98)70086-7. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinson ML, Durbin J, Caligiuri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and MCSF-dependent macrophage phenotypes display differential dependence on Type I interferon signaling. J Leukoc Biol. 2009 doi: 10.1189/jlb.1108702. [DOI] [PubMed] [Google Scholar]
- Ford E, Thanos D. The transcriptional code of human IFN-beta gene expression. Biochim Biophys Acta. 2010;1799:328–336. doi: 10.1016/j.bbagrm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ, Messina NL, Hii L, Gould JA, Sabapathy K, Robertson AP, Trapani JA, Levy DE, Hertzog PJ, Clarke CJ, et al. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol. 2010;8:e1000361. doi: 10.1371/journal.pbio.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I. Biologic effects of interferons. J Invest Dermatol. 1990;95:66S–71S. doi: 10.1111/1523-1747.ep12874776. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Whitty GA, Kola I, Hertzog PJ. Endogenous IFN-alpha beta suppresses colony-stimulating factor (CSF)-1-stimulated macrophage DNA synthesis and mediates inhibitory effects of lipopolysaccharide and TNF-alpha. J Immunol. 1996;156:2553–2557. [PubMed] [Google Scholar]
- Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Hata N, Sato M, Takaoka A, Asagiri M, Tanaka N, Taniguchi T. Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem Biophys Res Commun. 2001;285:518–525. doi: 10.1006/bbrc.2001.5159. [DOI] [PubMed] [Google Scholar]
- Heyman M, Grander D, Brondum-Nielsen K, Cederblad B, Liu Y, Xu B, Einhorn S. Interferon system defects in malignant T-cells. Leukemia. 1994;8:425–434. [PubMed] [Google Scholar]
- Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, et al. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Herrero C, Li WP, Antoniv TT, Falck-Pedersen E, Koch AE, Woods JM, Haines GK, Ivashkiv LB. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132:466–474. doi: 10.1111/j.1365-2567.2011.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci USA. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, McCoy B, Bogdan C, Decker T, Brem G, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- Landolfo S, Guarini A, Riera L, Gariglio M, Gribaudo G, Cignetti A, Cordone I, Montefusco E, Mandelli F, Foa R. Chronic myeloid leukemia cells resistant to interferon-alpha lack STAT1 expression. Hematol J. 2000;1:7–14. doi: 10.1038/sj.thj.6200004. [DOI] [PubMed] [Google Scholar]
- Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- Lesinski GB, Zimmerer JM, Kreiner M, Trefry J, Bill MA, Young GS, Becknell B, Carson WE., 3rd Modulation of SOCS protein expression influences the interferon responsiveness of human melanoma cells. BMC Cancer. 2010;10:142. doi: 10.1186/1471-2407-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Darnell JE., Jr STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Levy DE, Kessler DS, Pine R, Reich N, Darnell JE. Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative control. Genes Dev. 1988;2:383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Levy DE, Marié IJ, Durbin JE. Induction and Function of Type I and III Interferon in Response to Viral Infection. Curr Opin Virol. 2011 doi: 10.1016/j.coviro.2011.11.001. in press ( http://dx.doi.org/10.1016/j.coviro.2011.11.001. [DOI] [PMC free article] [PubMed]
- Li M, Liu X, Zhou Y, Su SB. Interferon-{lambda}s: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009 doi: 10.1189/jlb.1208761. [DOI] [PubMed] [Google Scholar]
- Li Z, Metze D, Nashan D, Muller-Tidow C, Serve HL, Poremba C, Luger TA, Bohm M. Expression of SOCS-1, suppressor of cytokine signalling-1, in human melanoma. J Invest Dermatol. 2004;123:737–745. doi: 10.1111/j.0022-202X.2004.23408.x. [DOI] [PubMed] [Google Scholar]
- Lizzul PF, Aphale A, Malaviya R, Sun Y, Masud S, Dombrovskiy V, Gottlieb AB. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005;124:1275–1283. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Lu Q, Shen N, Li XM, Chen SL. Genomic view of IFN-alpha response in pre-autoimmune NZB/W and MRL/lpr mice. Genes Immun. 2007;8:590–603. doi: 10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. mBio. 2011:2. doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Falvo JV, Kim TH, Kim TK, Lin CH, Parekh BS, Wathelet MG. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Mehic D, Bakiri L, Ghannadan M, Wagner EF, Tschachler E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J Invest Dermatol. 2005;124:212–220. doi: 10.1111/j.0022-202X.2004.23558.x. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Takaoka A, Kim SH, Kato Y, Yokochi T, Tanaka N, Taniguchi T. Cross talk of the interferon-alpha/beta signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells. 2001;6:631–640. doi: 10.1046/j.1365-2443.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–380. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- Park C, Li S, Cha E, Schindler C. Immune response in Stat2 knockout mice. Immunity. 2000;13:795–804. doi: 10.1016/s1074-7613(00)00077-7. [DOI] [PubMed] [Google Scholar]
- Passos de Souza E, Evangelista Segundo PT, Jose FF, Lemaire D, Santiago M. Rheumatoid arthritis induced by alpha-interferon therapy. Clin Rheumatol. 2001;20:297–299. doi: 10.1007/pl00011206. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer LM, Donner DB. The down-regulation of alpha-interferon receptors in human lymphoblastoid cells: relation to cellular responsiveness to the antiproliferative action of alpha-interferon. Cancer Res. 1990;50:2654–2657. [PubMed] [Google Scholar]
- Phipps-Yonas H, Seto J, Sealfon SC, Moran TM, Fernandez-Sesma A. Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog. 2008;4:e1000193. doi: 10.1371/journal.ppat.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Senger K, Merika M, Agalioti T, Yie J, Escalante CR, Chen G, Aggarwal AK, Thanos D. Gene repression by coactivator repulsion. Mol Cell. 2000;6:931–937. doi: 10.1016/s1097-2765(05)00081-x. [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Sun WH, Pabon C, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC, Rosen ST. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood. 1998;91:570–576. [PubMed] [Google Scholar]
- Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- Tak PP. IFN-beta in rheumatoid arthritis. Front Biosci. 2004;9:3242–3247. doi: 10.2741/1475. [DOI] [PubMed] [Google Scholar]
- Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S, Tanaka N, Taniguchi T. Cross talk between interferon-gamma and -alpha/beta signaling components in caveolar membrane domains. Science. 2000;288:2357–2360. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, Holmdahl R, Issazadeh-Navikas S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- Tovey MG, Streuli M, Gresser I, Gugenheim J, Blanchard B, Guymarho J, Vignaux F, Gigou M. Interferon messenger RNA is produced constitutively in the organs of normal individuals. Proc Natl Acad Sci USA. 1987;84:5038–5042. doi: 10.1073/pnas.84.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, van der Wel LI, Laman JD, Prens EP, Verschuren MC. Psoriatic lesional skin exhibits an aberrant expression pattern of interferon regulatory factor-2 (IRF-2) J Pathol. 2003;199:107–114. doi: 10.1002/path.1263. [DOI] [PubMed] [Google Scholar]
- Viti A, Muscettola M, Paulesu L, Bocci V, Almi A. Effect of exercise on plasma interferon levels. J Appl Physiol. 1985;59:426–428. doi: 10.1152/jappl.1985.59.2.426. [DOI] [PubMed] [Google Scholar]
- Vogel SN, Fertsch D. Endogenous interferon production by endotoxin-responsive macrophages provides an autostimulatory differentiation signal. Infect Immun. 1984;45:417–423. doi: 10.1128/iai.45.2.417-423.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, Garcia-Sastre A, Balachandran S, Beg AA. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J Immunol. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WB, Levy DE, Lee CK. STAT3 Negatively Regulates Type I IFN-Mediated Antiviral Response. J Immunol. 2011;187:2578–2585. doi: 10.4049/jimmunol.1004128. [DOI] [PubMed] [Google Scholar]
- Weihua X, Hu J, Roy SK, Mannino SB, Kalvakolanu DV. Interleukin-6 modulates interferon-regulated gene expression by inducing the ISGF3 gamma gene using CCAAT/enhancer binding protein-beta(C/EBP-beta) Biochim Biophys Acta. 2000;1492:163–171. doi: 10.1016/s0167-4781(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Wong LH, Hatzinisiriou I, Devenish RJ, Ralph SJ. IFN-gamma priming up-regulates IFN-stimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFN-resistant melanoma cells to type I IFNs. J Immunol. 1998;160:5475–5484. [PubMed] [Google Scholar]
- Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J Biol Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- Yaar M, Palleroni AV, Gilchrest BA. Normal human epidermis contains an interferon-like protein. J Cell Biol. 1986;103:1349–1354. doi: 10.1083/jcb.103.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Sato A, Takeda T, Komatsu M. Distinct clinical courses in type 1 diabetes mellitus induced by peg-interferon-alpha treatment for chronic hepatitis C. Intern Med. 2010;49:403–407. doi: 10.2169/internalmedicine.49.2656. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA, et al. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- Zurney J, Howard KE, Sherry B. Basal expression levels of IFNAR and Jak-STAT components are determinants of cell-type-specific differences in cardiac antiviral responses. J Virol. 2007;81:13668–13680. doi: 10.1128/JVI.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]