Prevalence of Prediabetes and Diabetes and Metabolic Profile of Patients With Nonalcoholic Fatty Liver Disease (NAFLD) (original) (raw)
Abstract
OBJECTIVE
Prediabetes and type 2 diabetes mellitus (T2DM) are believed to be common and associated with a worse metabolic profile in patients with nonalcoholic fatty liver disease (NAFLD). However, no previous study has systematically screened this population.
RESEARCH DESIGN AND METHODS
We studied the prevalence and the metabolic impact of prediabetes and T2DM in 118 patients with NAFLD. The control group comprised 20 subjects without NAFLD matched for age, sex, and adiposity. We measured 1) plasma glucose, insulin, and free fatty acid (FFA) concentration during an oral glucose tolerance test; 2) liver fat by magnetic resonance spectroscopy (MRS); 3) liver and muscle insulin sensitivity (euglycemic insulin clamp with 3-[3H]glucose); and 4) indexes of insulin resistance (IR) at the level of the liver (HIRi= endogenous glucose production × fasting plasma insulin [FPI]) and adipose tissue (Adipo-IRi= fasting FFA × FPI).
RESULTS
Prediabetes and T2DM was present in 85% versus 30% in controls (P < 0.0001), all unaware of having abnormal glucose metabolism. NAFLD patients were IR at the level of the adipose tissue, liver, and muscle (all P < 0.01–0.001). Muscle and liver insulin sensitivity were impaired in patients with NAFLD to a similar degree, whether they had prediabetes or T2DM. Only adipose tissue IR worsened in T2DM and correlated with the severity of muscle (r = 0.34; P < 0.001) and hepatic (r = 0.57; P < 0.0001) IR and steatosis by MRS (r = 0.35; P < 0.0001).
CONCLUSIONS
Patients with NAFLD may benefit from early screening for T2DM, because the prevalence of abnormal glucose metabolism is much higher than previously appreciated. Regardless of glucose tolerance status, severe IR is common. In patients with T2DM, adipose tissue IR appears to play a major role in the severity of NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is believed to be the most common chronic liver disease in industrialized countries (1). NAFLD is strongly correlated with insulin-resistant states such as obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM). Cross-sectional studies have associated T2DM with worse histology in NAFLD (2) and possibly with a greater risk of progression and more aggressive disease. For instance, patients with diabetes have a higher risk of developing fibrosis and cirrhosis (2–5), although the natural history of the disease in patients with T2DM remains unclear. Compared with nondiabetic subjects, subjects with T2DM are believed to have an increased risk of developing NAFLD (3,6), but the true prevalence of prediabetes and T2DM has never been systematically assessed by means of an oral glucose tolerance test (OGTT) among patients with NAFLD. The magnitude of the problem is large: an estimated 25.8 million people, or 8.3% of the U.S. population, have type 1 and type 2 diabetes (7). Even more worrisome is that 35% of adults and ∼50% of those aged >60 years have prediabetes, a condition that puts them at higher risk for developing T2DM. Whether NAFLD, a condition associated with insulin resistance (IR), increases the risk of developing T2DM remains unclear.
The purpose of our study was to determine the prevalence of abnormal glucose metabolism and understand how hyperglycemia and NAFLD impact the metabolic profile of these subjects.
RESEARCH DESIGN AND METHODS
Subjects
The study recruited 118 healthy overweight or obese subjects from the San Antonio, Texas, area. Patients were identified from responses to local newspaper advertisements or from referrals from medical school clinics to be screened for liver fat by magnetic resonance spectroscopy (MRS). An additional 20 overweight or obese subjects without fatty liver by MRS served as controls for the metabolic studies. Participants were in good general health, without evidence of any chronic diseases (other than NAFLD) as determined by history, examination, routine blood chemistry analysis, urinalysis, and electrocardiography. Volunteers were excluded if they had a history of heavy alcohol use (>12 to 15 g of alcohol per day, or >12 oz of beer, 5 oz of wine, or 1.5 oz of distilled spirits); if they had a fasting glucose level of ≥240 mg/dL (13.3 mmol/L); if they had type 1 diabetes, heart disease (congestive heart failure, New York Heart Association functional class ≥II), hepatic disease other than nonalcoholic steatohepatitis (hepatitis B or C, autoimmune hepatitis, hemochromatosis, Wilson disease, drug-induced disease, other), or renal disease; or if they were receiving metformin, thiazolidinediones, or insulin (patients with any type of diabetes were excluded). The study was approved by the local institutional review board, and informed written consent was obtained from each patient before participation.
Study design
All studies were performed at the research unit. Metabolic measurements included 1) fasting plasma glucose, A1C, lipid profile, liver function tests, insulin, free fatty acid (FFA); 2) total body fat by dual energy X-ray absorptiometry (DXA; Hologic Inc, Waltham, MA); 3) hepatic fat content by MRS; 4) a 75-g OGTT to establish the diagnosis of normal glucose tolerance or T2DM according to American Diabetes Association criteria (subjects were provided with general dietary advice and were asked to eat an unrestricted diet rich in carbohydrates [150–200 g/day] 3 days before the OGTT), with studies performed after an overnight fast (8); 5) euglycemic hyperinsulinemic clamp with 3-[3H]glucose to measure endogenous (primarily hepatic) and whole-body (largely muscle) insulin sensitivity (see below) (9); 6) ultrasound-guided liver biopsy was offered to all volunteers with NAFLD by MRS to establish the presence of nonalcoholic steatohepatitis (NASH) and grade of the disease.
Total body and liver fat content measurements.
For the measurement of hepatic fat content, localized 1H nuclear MRS images of the liver were acquired on a Siemens TIM TRIO 3.0T magnetic resonance imaging whole-body scanner and using methodology previously described (10). In brief, two areas of interest were taken using a echo time/repetition time/angle of 30 ms/2,000 ms/90°, and two liver areas with a volume of 30 × 30 × 30 mm were used. A liver fat content of >5.5% was considered diagnostic of NAFLD (11).
Euglycemic hyperinsulinemic clamp (9).
Subjects were admitted to the research unit at 6:30 a.m., after a 12-h overnight fast, and the study was performed as reported previously by our group (12,13). In brief, a polyethylene catheter was inserted into an antecubital vein for infusion of all test substances. A second catheter was inserted retrogradely into an ipsilateral wrist vein on the dorsum of the hand for collection of arterialized blood samples, and the hand was kept in a heated box at 65°C. A primed (25 μCi × [fasting glucose/100]) − continuous (0.25 μCi/min) infusion of 3-[3H]glucose [DuPont-NEN, Boston, MA]) was initiated and continued until study end. During the last 30 min of the basal equilibration period (150–180 min), plasma samples were taken at 5- to 10-min intervals for determination of plasma glucose, insulin concentrations, and 3-[3H]glucose-specific activity.
After the basal equilibration period, insulin was administered as a primed-continuous infusion at 10 μU/m2 ⋅ min for 120 min to assess suppression of endogenous (hepatic) glucose production, followed by another 2 h at an infusion rate of 80 μU/m2 ⋅ min for 120 min to assess whole-body insulin-stimulated glucose disposal (_R_d). The plasma glucose level was measured every 5 min after the start of insulin, and a variable infusion of 20% glucose was adjusted based on the negative feedback principle to maintain the plasma glucose concentration at ∼90 to 100 mg/dL, with a coefficient of variation of less than 5%. Plasma samples were collected every 5 to 10 min for determination of the plasma glucose, insulin, and FFA concentrations, and 3-[3H]glucose-specific activity.
Liver biopsy.
An ultrasound-guided liver biopsy was performed in patients with elevated liver aminotransferase levels when all other causes of liver disease were ruled out, or normal liver aminotransferase levels with NAFLD by MRS and well-known risk factors for NASH such as T2DM, MetS, and/or IR as established during a euglycemic insulin clamp. Histopathologic characteristics for the diagnosis of NASH were determined using standard criteria (14).
Analytic methods
Plasma glucose was measured by the glucose oxidase method (Analox Glucose Analyzer; Analox Instruments, Lunenburg, MA), plasma insulin by radioimmunoassay, and plasma FFA by standard colorimetric methods. A1C was measured by high-performance liquid chromatography (TOSOH G-7). The 3-[3H]glucose-specific activity was measured on barium hydroxide/zinc sulfate–deproteinized plasma samples as reported before (12,13).
Calculations
Hepatic endogenous glucose production (EGP) and insulin-stimulated (muscle) glucose disposal (_R_d) were calculated as previously reported by our group (12,13). We also calculated an index of hepatic IR (HIRi) and of adipose tissue IR (Adipo-IRi) as previously described (5,15,16). The rationale for both indexes is based on the linear relationship between the rise in the fasting plasma insulin (FPI) level and the decline in the rate of basal (fasting) EGP in healthy subjects. The higher the rate of EGP and the level of FPI, the greater the severity of hepatic IR. Therefore, a HIRi was calculated as the product of fasting EGP and FPI concentration (HIRi = EGP × FPI [mg ⋅ kg−1 ⋅ min−1 ⋅ µU/mL]). Insulin is also a strong inhibitor of lipolysis, and a similar relationship exists in healthy subjects between the FPI concentration and fasting plasma FFA levels (17). Thus, the Adipo-IRi was calculated as the product of the fasting plasma FFA and insulin concentration (Adipo-IRi = FFA × FPI [mmol/L ⋅ µU/mL]). Our group has published experimental validation for both indexes previously (5,15–17).
Statistical analysis
All values are reported as the mean ± standard error of the mean for continuous variables and the number (percent) for categoric variables. Comparison of groups (i.e., with versus without NAFLD or by glucose tolerance status) was performed using ANOVA or Kruskal-Wallis for continuous variables and the Pearson χ2 or Fisher exact tests for categoric variables. Adjusted P values were calculated using fixed-effect models. Statistical significance was set at P < 0.05. All statistical calculations were performed using JMP 8.0 software (SAS Institute, Cary NC).
RESULTS
Patient characteristics
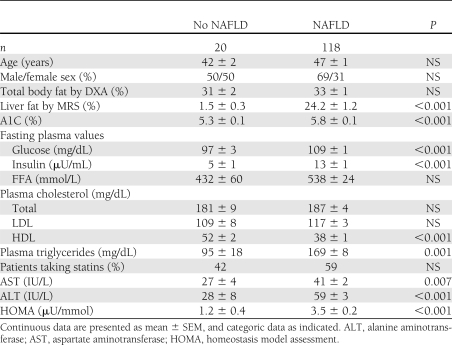
The patient characteristics of the study population are summarized in Table 1. Patients with versus without NAFLD were well matched for the major variables of age, sex, and adiposity. Although the patients without NAFLD had a lower BMI (29.6 ± 1.0 vs. 34.1 ± 0.4 kg/m2), the degree of adiposity measured more accurately by DXA was well matched between groups (31 ± 2 vs. 33 ± 1%). Ethnicity was similar in both groups. The population distribution followed that of the San Antonio area (18). Most NAFLD patients were of Hispanic ancestry, followed by Caucasians, African Americans, and other (59, 27, 11, and 3%). As can be observed in Table 1, fasting plasma glucose and A1C were slightly but significantly elevated among patients with NAFLD (P < 0.001). FPI and homeostasis model assessment were twofold higher in patients with NAFLD versus without NAFLD, indicative of IR. When comparing the lipid profile of both groups, neither total cholesterol nor LDL cholesterol were different, but patients with NAFLD had significantly higher plasma triglycerides and lower HDL cholesterol concentration. Despite similar total body fat, liver aminotransferases were within normal reference ranges in subjects without NAFLD and were elevated in patients with NAFLD in the typical pattern of higher alanine aminotransferase over aspartate aminotransferase (NAFLD 39 ± 2 and 59 ± 3 IU/L, respectively; both P < 0.01). All patients with NAFLD were invited to have an ultrasound-guided liver biopsy to establish the presence of NASH and the degree of disease, and 79% accepted. There were no significant clinical differences between patients who did and did not undergo liver biopsy.
Table 1.
Patient characteristics
Prevalence of prediabetes and T2DM in overweight or obese patients with and without NAFLD
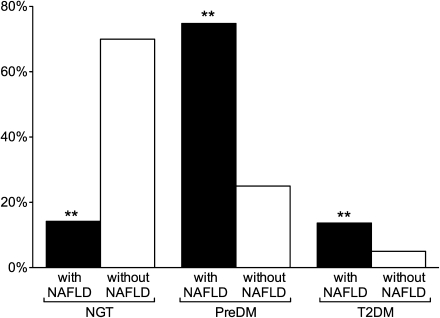
To establish the prevalence of prediabetes and T2DM, patients with and without NAFLD underwent a 75-g OGTT. The results of this testing were used to divide patients by glucose metabolism status as having normal glucose tolerance (NGT), prediabetes (impaired fasting glucose [IFG] and/or impaired glucose tolerance [IGT]) or T2DM. The prevalence of NGT was significantly lower in patients with versus without NAFLD (15% vs. 70%, respectively, P < 0.001; Fig. 1). Consistent with this finding, newly diagnosed prediabetes was considerably more common in patients with NAFLD than in those without NAFLD (75% vs. 25%, P < 0.001). Of note, in an apparently healthy population, T2DM was diagnosed in 14% of the patients. This was almost threefold higher than in patients without NAFLD (vs. 5%, P < 0.001).
Figure 1.
Prevalence of NGT, prediabetes (PreDM), and T2DM in patients with and without NAFLD. **P < 0.001 vs. without NAFLD.
Role of hyperglycemia on liver, adipose tissue, and muscle insulin sensitivity in overweight or obese patients with NAFLD
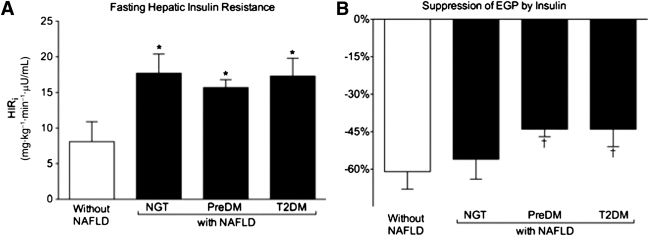
We examined in this population insulin sensitivity across different target tissues and the impact of abnormal glucose metabolism. Figure 2_A_ represents hepatic insulin sensitivity expressed as the HIRi (HIRi = fasting EGP [largely hepatic glucose production] × FPI concentration), a validated index in the fasting state (5,15,16). Patients with NAFLD had severe hepatic IR compared with patients without NAFLD (Fig. 2_A_), although there was no further worsening in the presence of deteriorating glucose tolerance (NGT: 17.7 ± 2.7; prediabetes: 15.7 ± 1.1; T2DM: 17.3 ± 2.5 [all P < 0.01] vs. without NAFLD: 8.1 ± 2.8 mg ⋅ kg−1 ⋅ min−1 ⋅ µU/mL). When insulin sensitivity at the level of the liver was measured as the suppression of EGP by low-dose insulin infusion during the euglycemic insulin clamp (Fig. 2_B_), only patients with NAFLD and prediabetes (−44 ± 3%) or T2DM (−44 ± 7%) had significantly worse IR compared with patients without NAFLD (−61 ± 7%, both P < 0.05). Consistent with these results, there was a near complete suppression of EGP by high-dose insulin in patients with NGT and NAFLD (99 ± 1%) and in those without NAFLD (vs. 98 ± 2%), but this was impaired in patients with NAFLD and prediabetes (−88 ± 2%) or T2DM (86 ± 4% [both P < 0.05] vs. patients without NAFLD).
Figure 2.
Role of hyperglycemia on hepatic insulin sensitivity. A: HIRi (HIRi = fasting EGP [hepatic] × FPI concentration). B: Percentage suppression of hepatic EGP by low-dose insulin infusion. Results are mean ± SEM.*P < 0.1 vs. without NAFLD. †P < 0.05 vs. without NAFLD.
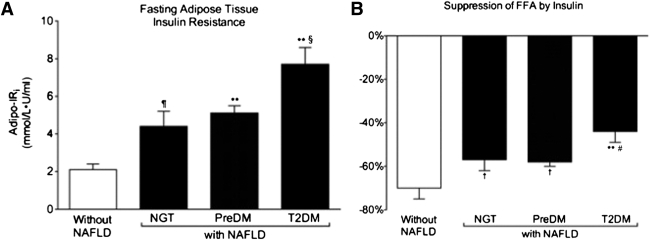
Given the important role that adipose tissue IR plays in the pathogenesis of NAFLD (19–21), we examined its impact by using the validated Adipo-IRi (5,15,16) derived from the product of the fasting plasma FFA and insulin concentration (Fig. 3_A_). Patients without NAFLD had preserved adipose tissue insulin sensitivity in contrast to patients with NAFLD-NGT (2.1 ± 0.3 vs. 4.4 ± 0.8 mmol/L ⋅ µU/mL, P < 0.04) and those with prediabetes and T2DM (5.1 ± 0.4 and 7.7 ± 0.9 mmol/L ⋅ µU/mL, respectively, both P < 0.001) that had significant adipose tissue IR. Of note within the NAFLD group, the degree of IR worsened as glucose metabolism deteriorated from prediabetes to T2DM by 51% (P < 0.01). Adipose tissue IR worsened in T2DM and correlated with the severity of muscle IR (r = 0.34; P < 0.001) and hepatic insulin resistance (r = 0.57; P < 0.0001), and hepatic steatosis measured by MRS (r = 0.35; P < 0.0001).
Figure 3.
Role of hyperglycemia on adipose tissue insulin sensitivity. A: Adipo-IRi (Adipo-IRi = fasting plasma FFA × FPI concentration). B: Percentage suppression of plasma FFA concentration by low-dose insulin infusion. Results are mean ± SEM. ¶P < 0.04 and P < 0.001 vs. without NAFLD. §P < 0.001 vs. NGT. †P < 0.05 and **P < 0.001 vs. without NAFLD. #P < 0.001 vs. without NGT.
We also examined directly the suppression of plasma FFA concentration by a low-dose insulin infusion (Fig. 3_B_). Consistent with the Adipo-IRi results, patients with NAFLD demonstrated again a diminished adipose tissue response to insulin compared with patients without NAFLD (−56 ± 2% vs. −70 ± 6%, respectively, P = 0.02). Among the NAFLD group, NGT and prediabetes patients had a similar response to insulin (−57 ± 5% vs. −58 ± 2%, respectively, NS), whereas T2DM patients had a significantly lower response to insulin compared with the NGT group (−44 ± 5% vs. −57 ± 5%, P < 0.02). We also analyzed liver fat content in relation to glucose status. Patients without NAFLD had a liver fat content by MRS of 1.5% compared with 20 ± 2% in NAFLD with NGT, 23.2 ± 1.6% in NAFLD with prediabetes, and 33 ± 2% in NAFLD with T2DM (all P < 0.0001 vs. without NAFLD; P < 0.01 NGT and prediabetes vs. T2DM).
Finally, we examined the differences between patients with NAFLD who agreed to have a liver biopsy (79%) and those who did not. There were no clinical differences between the two groups. Similarly, all parameters of insulin sensitivity (i.e., the hepatic IR index or HIRi; suppression of EGP by low-dose and high-dose insulin; Adipo-IRi and insulin-stimulated glucose disposal during the euglycemic insulin clamp) were not significantly different.
CONCLUSIONS
Because of the increasing prevalence of T2DM in the U.S. and its close relation with NAFLD, we felt compelled to examine the prevalence of prediabetes and T2DM in this population. This was also important because fatty liver may carry serious metabolic and liver-related complications (i.e., cirrhosis, hepatocellular carcinoma). Moreover, this has never been systematically assessed in the past by means of an OGTT. In addition, we wanted to study the relationship between IR in different target tissues (i.e., muscle, liver, and adipose tissue) and the development of prediabetes and T2DM in this population.
The prevalence of abnormal glucose metabolism in overweight and obese subjects with NAFLD (86% vs. 30%, P < 0.0001) was higher than expected. This can be attributed to a number of factors:
First, this analysis was limited to overweight and obese subjects, known to be normally at higher risk of prediabetes and T2DM.
Second, individuals of Hispanic ancestry comprise 63% of the San Antonio population (18) and are at higher risk of developing T2DM, which may have increased their chance of T2DM upon routine screening. In prior studies done in San Antonio (22) and in other Latino populations (23,24), the prevalence of prediabetes and T2DM in Hispanic individuals in the general population was somewhat lower than that of our NAFLD patients but included lean and obese subjects and likely included patients with and without NAFLD, which was not assessed by MRS in these reports.
Third, none of the previous screening studies in NAFLD performed an OGTT to evaluate for the presence of T2DM, so reports of T2DM ranging from 9 to 31% (2,25,26) likely grossly underestimated the true rate of abnormal glucose metabolism.
Fourth, obesity in NAFLD is associated with dysfunctional adipose tissue and lipotoxicity that promotes IR (19,21,27) and pancreatic β-cell dysfunction (28,29). Chronic hyperinsulinemia, per se (30–32), may worsen hepatic steatosis and peripheral (muscle) IR (33).
The combination of these factors places these subjects at a very high risk of developing T2DM, although clinicians are largely unaware. The clinical implication is that the presence of NAFLD, diagnosed by elevated liver aminotransferases and/or liver ultrasound imaging, should prompt health care providers to consider early screening for T2DM in such patients.
The presence of NAFLD and prediabetes or T2DM was associated with significant hepatic IR compared with subjects matched for adiposity without a fatty liver. Although NAFLD has been reported to be associated with liver IR (10,31,34,35), the specific effect of diabetes status has never been carefully examined before. In the fasting state, the HIRi was approximately twofold increased (worse) in the presence of NAFLD, but T2DM status itself did not appear to be a major factor (Fig. 2_A_). Consistent with this, there was a clear reduction in the suppression of EGP to insulin in patients with prediabetes and T2DM and NAFLD (Fig. 2_B_). Moreover, this impairment remained even at the high-dose insulin infusion during the euglycemic insulin clamp in patients with prediabetes and T2DM compared with patients with NGT with and without NAFLD. From these results, one may speculate that the presence of fatty liver may be an important factor in the progression of abnormal glucose tolerance in the natural history of T2DM.
Defects in adipose tissue are well established in obesity and T2DM (19,36). In the current study, the presence of NAFLD was clearly associated with dysfunctional fat (Fig. 3). When this finding was examined more closely in relation to glucose tolerance status, we observed that the presence of adipose tissue IR worsens with the progression of glucose intolerance. Dysregulated adipose tissue may alter glucose metabolism by multiple pathways that include subclinical inflammation (37) and lipotoxicity (19–21). Although dissecting the independent contribution of each is impossible, it is clear that worsening adipose tissue IR plays a role in the development of T2DM (Fig. 3_A_). Worsening adipose tissue IR may promote lipotoxicity by increasing the flux of FFA to the liver and other target tissues. In NAFLD, we have recently reported that the more dysfunctional the adipose tissue, the worse are the metabolic abnormalities in patients with NAFLD (38). The impact of adipose tissue IR in T2DM can be highlighted by the observation that its severity correlated very strongly with hepatic IR (r = 0.57; P < 0.0001) and the magnitude of liver steatosis assessed by MRS (r = 0.35; P < 0.0001). Taken together, they point toward the importance of the cross talk between the adipose tissue and the liver in NAFLD.
In conclusion, the current study brings to our attention that abnormal glucose metabolism is much more common than previously appreciated in patients with NAFLD. These patients are insulin-resistant in all target tissues, with adipose tissue IR playing a major role in the severity of NAFLD. The clinical implication is that some patients with NAFLD may benefit from early screening for T2DM to prevent the long-term complications of hyperglycemia and the progression to steatohepatitis (NASH) and cirrhosis.
Acknowledgments
This work was supported by the Burroughs Wellcome Fund (K.C.), by the American Diabetes Association (1-08-CR-08; K.C.), by the Veterans Affairs Medical Research Fund, and by Award Number UL 1RR025767 from the National Center for Research Resources.
No potential conflicts of interest relevant to this article were reported.
C.O.-L. contributed to the collection and analysis of research data, wrote the manuscript, and contributed to discussion. R.L., B.O., J.F., Z.C., V.G.K., and J.H. contributed to research data collection and analysis. K.C. participated in the discussion and reviewed and edited the manuscript. K.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank the study volunteers, the Clinical and Translational Science Award nursing staff (in particular, Norma Diaz and Rose Kaminski-Graham), and the nutrition and laboratory staff (University of Texas Health Science Center at San Antonio, San Antonio, TX) for their assistance in performing the described studies.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
References
- 1.Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis 2010;28:162–168 [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. ; NASH Clinical Research Network Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010;52:913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupte P, Amarapurkar D, Agal S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol 2004;19:854–858 [DOI] [PubMed] [Google Scholar]
- 4.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol 2011;7:456–465 [DOI] [PubMed] [Google Scholar]
- 5.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology 2011;54:837–845 [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National diabetes fact sheet, 2011: national estimates and general information on diabetes and prediabetes in the United States [article online]. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Accessed 24 September 2011
- 8.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 10.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307 [DOI] [PubMed] [Google Scholar]
- 11.Szczepaniak Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:E462–E468 [DOI] [PubMed] [Google Scholar]
- 12.Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1996;81:4059–4067 [DOI] [PubMed] [Google Scholar]
- 13.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000;105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Harrison SA, Belfort-Aguilar R, et al. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 2009;50:1087–1093 [DOI] [PubMed] [Google Scholar]
- 17.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Texas State Data Center. Census 2010 Summary File 1 [Internet] 2010. Available from: http://txsdc.utsa.edu Accessed 24 September 2011
- 19.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 2010;10:306–315 [DOI] [PubMed] [Google Scholar]
- 20.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 2010;375:2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774–788 [DOI] [PubMed] [Google Scholar]
- 22.Lorenzo C, Serrano-Ríos M, Martínez-Larrad MT, et al. Which obesity index best explains prevalence differences in type 2 diabetes mellitus? Obesity (Silver Spring) 2007;15:1294–1301 [DOI] [PubMed] [Google Scholar]
- 23.Pan JJ, Qu HQ, Rentfro A, McCormick JB, Fisher-Hoch SP, Fallon MB. Prevalence of metabolic syndrome and risks of abnormal serum alanine aminotransferase in Hispanics: a population-based study. PLoS ONE 2011;6:e21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobashi-Margáin RA, Gutiérrez-Grobe Y, Ponciano-Rodríguez G, Uribe M, Méndez-Sánchez N. Prevalence of type 2 diabetes mellitus and chronic liver disease: a retrospective study of the association of two increasingly common diseases in Mexico. Ann Hepatol 2010;9:282–288 [PubMed] [Google Scholar]
- 25.Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab 2011;96:1093–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura Y, Hyogo H, Ishitobi T, Nabeshima Y, Arihiro K, Chayama K. Postprandial insulin secretion pattern is associated with histological severity in non-alcoholic fatty liver disease patients without prior known diabetes mellitus. J Gastroenterol Hepatol 2011;26:517–522 [DOI] [PubMed] [Google Scholar]
- 27.Unger RH, Zhou YT. Lipotoxicity of β-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001;50(Suppl. 1):S118–S121 [DOI] [PubMed] [Google Scholar]
- 28.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 29.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- 30.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31(Suppl. 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 31.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis (NASH): pathophysiology and clinical implications. J Gastro. 8 February 2012 [Epub ahead of print] [DOI] [PubMed]
- 32.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 2009;119:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iozzo P, Pratipanawatr T, Pijl H, et al. Physiological hyperinsulinemia impairs insulin-stimulated glycogen synthase activity and glycogen synthesis. A J Physiol Endocrinol Metabolism 2001;280:E712–E719 [DOI] [PubMed] [Google Scholar]
- 34.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol 2005;16:421–427 [DOI] [PubMed] [Google Scholar]
- 35.Kotronen AM, Juurinen LM, Hakkarainen AB, et al. Liver fat is increased in type 2 diabetic patients and underestimated by serum alanine aminotransferase compared with equally obese nondiabetic subjects. Diabetes Care 2008;31:165–169 [DOI] [PubMed] [Google Scholar]
- 36.Bozzetto L, Prinster A, Mancini M, et al. Liver fat in obesity: role of type 2 diabetes mellitus and adipose tissue distribution. Eur J Clin Invest 2011;41:39–44 [DOI] [PubMed] [Google Scholar]
- 37.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with NAFLD. Hepatology. 20 December 2011 [Epub ahead of print] [DOI] [PubMed]