Low-Density Lipoprotein Receptor (LDLR) Family Orchestrates Cholesterol Homeostasis (original) (raw)
. 2012 Mar 29;85(1):19–28.
Abstract
The LDLR family of proteins is involved in lipoproteins trafficking. While the role of LDLR in cardiovascular disease has been widely studied, only recently the role of other members of the LDLR proteins in lipoprotein homeostasis and atherosclerosis has emerged. LDLR, VLDLR, and LRPs bind and internalize apoE- and apoB-containing lipoprotein, including LDL and VLDL, and regulate their cellular uptake. LRP6 is a unique member of this family for its function as a co-receptor for Wnt signal transduction. The work in our laboratory has shown that LRP6 also plays a key role in lipoprotein and TG clearance, glucose homoeostasis, and atherosclerosis. The role of these receptor proteins in pathogenesis of diverse metabolic risk factors is emerging, rendering them targets of novel therapeutics for metabolic syndrome and atherosclerosis. This manuscript reviews the physiological role of the LDLR family of proteins and describes its involvement in pathogenesis of hyperlipidemia and atherosclerosis.
Keywords: cardiovascular disease, LDL clearance, LDLR family, lipoprotein trafficking
Introduction
Lipoprotein transport of cholesterol in plasma plays a physiological role for essential energy production, cell membrane, and hormone synthesis. Cells readily utilize cholesterol by internalizing lipoprotein ligands containing chylomicron, low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), or very low-density lipoprotein (VLDL) mediated by the LDL receptor (LDLR) family of membrane receptors. Internalized lipoprotein particles undergo an endocytic process that includes clustering of lipoprotein receptors in coated pits, transport to early and late endosomes followed by hydrolysis in lysosomes, release of the lipid to cytoplasm, and recycling of receptors back to cell surface. LDLR-mediated endocytosis has provided much of our understanding of lipoprotein clearance, and its defect is a major cause of familial hyperlipidemia and a fundamental risk factor for cardiovascular disease.
Elevated LDL is a prevalent risk factor for coronary artery disease and other atherosclerosis diseases, which constitute the largest cause of all mortalities in the United States [1]. Defect in LDLR is the most common cause of familial hypercholesterolemia (FH) and premature coronary artery diseases. The role of LDLR in cardiovascular diseases has been extensively studied. However, only recently the role of other members of the LDLR family of proteins in lipoprotein homeostasis and atherosclerosis has emerged [2-5]. While significant advances have been made in understanding the balance between cholesterol synthesis and transport, the mechanisms surrounding cholesterol homeostasis and hypertriglyceridemia remain incompletely understood. Therefore, this review will provide basic information on the role of the LDLR family in lipid homeostasis and pathogenesis of cardiovascular disease.
LDLR family
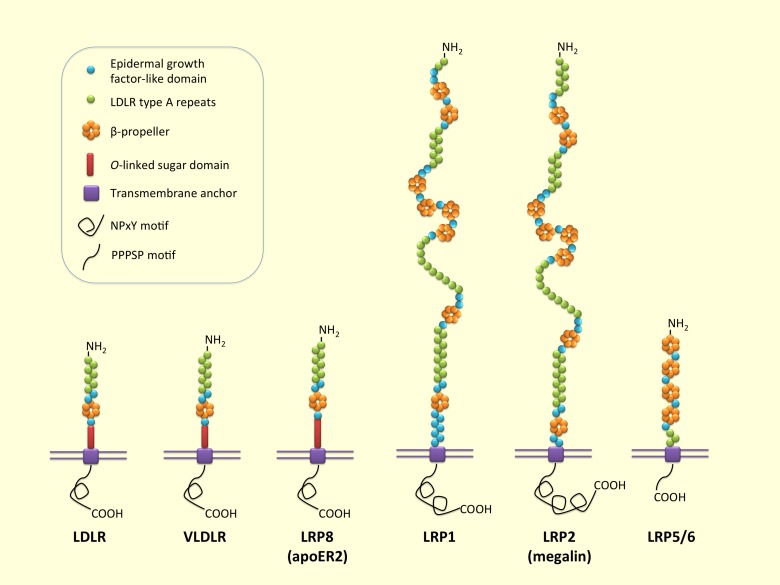
The LDLR family comprises a group of endocytic receptors on the cell surface, which bind and internalize extracellular ligands, including lipoproteins, exotoxins, and lipid-carrier complexes [6]. Members of the LDLR family are structurally and functionally related to LDLR, which is the patriarch of the entire family. Proteins of the LDLR family share structurally common motifs (Figure 1): LDLR type A repeats, epidermal growth factor (EGF)-like domain, transmembrane anchor, and, in certain instances, cytoplasmic domain. LDLR and VLDLR additionally contain o-link sugar domain, located just outside of the plasma membrane. LRP1 and LRP2 (megalin) have relatively large extracellular domains [7]. LDLR type A repeats are localized to a region at NH2-terminal and responsible for binding of ligands, including apoB-100- and apoE-containing lipoprotein. EGF-like precursor contains multiple EGF repeats along with a β-propeller domain and is involved in pH-dependent dissociation of ligand-receptor complex. Transmembrane domain helps anchor the receptors to the membrane. Cytoplasmic domain contains NPxY (Asn-Pro-any amino acid (x)-Tyr)-containing domain or PPPSP motif (Pro-Pro-Pro-Ser-Pro) [7] and is involved in the targeting of receptors to coated pits and signal transduction. Differences in position and number of each domain create the diversity in LDLR family members. To date, numerous members of the LDLR family are reported that participate in a wide range of physiological processes [8]. In particular, LDLR, VLDLR, LRP5/6, LRP1, and LRP2 play a pivotal role in cholesterol homeostasis and lipid metabolism [9-13].
Figure 1.
Low-density lipoprotein receptor family. LDLR is the patriarch of the LDLR family. Members of the LDLR family share common structural motifs: LDLR type A repeats (responsible for binding of ligands), epidermal growth factor (EGF)-like domain (involved in pH-dependent release of ligands in endosome), transmembrane anchor, and cytoplasmic domain (binding of NPxY and ARH mediates clustering of the receptors into clathrin coated pit). LDLR, VLDLR, and LRP8 (ApoER2) additionally contain o-link sugar domain outside the plasma membrane and NPxY motif in the cytoplasmic domain. LRP1 and LRP2 have relatively large extracellular domains. LRP5/6 has PPPSP motif in cytoplasmic domain.
LDLR
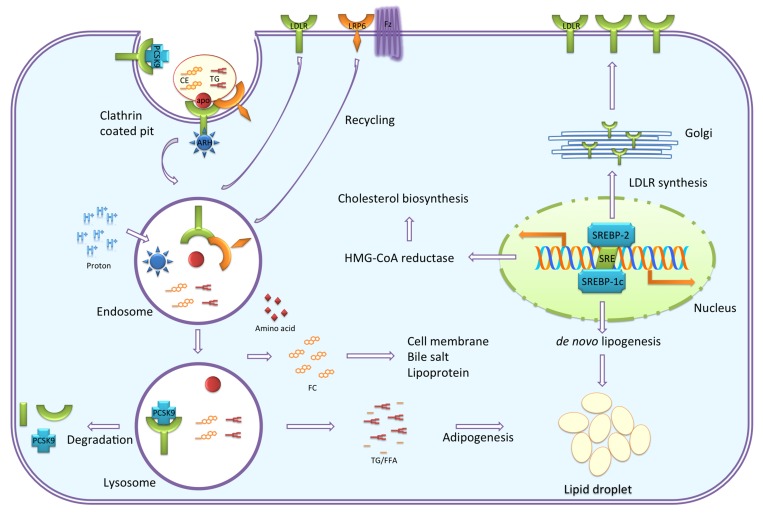
LDLR is a cell membrane glycoprotein that functions in the binding and internalizing of circulating cholesterol-containing lipoprotein particles. LDLR is ubiquitously expressed and is a key receptor for maintaining cholesterol homeostasis in mammals. LDLR-mediated endocytosis is essential for lipoprotein and lipid metabolism [14] (Figure 2). The clearance of LDL, a major carrier of cholesterol in human, by LDLR is extensively studied [14-19]. Recognition of apoB-100 of LDL particles occurs with a stoichiometry of a single copy of apoB-100 per one LDL particle per receptor monomer [20]. VLDL, IDL, high-density lipoprotein (HDL), and chylomicron remnant are also recognized by LDLR at neutral pH [21,22]. Receptor-ligand complexes undergo endocytosis via clathrin-coated pits. Coated vehicle dispenses to endosomes with LRP6 and autosomal recessive hypercholesterolemia protein (ARH, also known as LDLR adaptor protein), connecting the LDLR family protein and the endocytic machinery; thereby, acidic condition activates dissociation of internalized ligands. Released ligand particles further travel to the lysosome, in which the ligand is degraded by enzyme, while the receptors recycle back to the cell surface. LDLR contains seven LDLR type A repeats in binding domain, immediately followed by EGF-like modules, transmembrane anchor, and NPxY-repeats containing cytoplasmic domain. LDL particles trigger three steps after internalization: 1) reducing gene expression of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) to suppress cholesterol biosynthesis; 2) enhancing activity of acyl-CoA cholesteryl acyl transferase (ACAT) to reduce toxic free cholesterol; and 3) suppressing LDLR synthesis to reduce LDL uptake via SREBPs [23-25].
Figure 2.
Cellular cholesterol homeostasis. Vesicular uptake of lipoproteins is essential for lipoprotein and lipid metabolism. This process is regulated by the LDLR family of proteins. Recognition of apolipoproteins by the receptor at neutral pH initiates the internalization, followed by ARH (also known as LDLR adaptor protein) binding of the cytoplasmic NPxY motif and clustering of receptor-ligand complexes into clathrin-coated pits. Coated vehicle dispenses to endosomes, in which acidic condition activates the release of internalized ligands from the receptor. Released ligand particles travel further to lysosome, in which ligand is degraded by enzyme. The receptors recycle back to the cell surface. Internalized cholesterol reduces cholesterol biosynthesis and LDLR transcription by inhibiting SREBP-2. PCSK9 binds to LDLR, which is targeting LDLR to lysosome for degradation. De novo lipogenesis is also reduced by inhibition of SREBP-1c. TG undergoes adipogenesis to form lipid droplet.
Impaired LDLR function by genetic mutations results in a condition with extremely elevated serum LDL levels and early onset atherosclerosis known as familial hypercholesterolemia (FH) [26]. The prevalence is 1:500 for heterozygote and 1 in a million for homozygote FH [27]. While normal fibroblasts have high cell surface binding affinity for LDL via LDLR, cultured fibroblasts from FH patient fail to clear serum LDL, thus causing elevated serum LDL [16,19]. Patients homozygote for _LDLR_mutation have serum LDL levels that are as high as 800 mg/dL and show widespread accumulation of cholesterol-mediated atheromatous plaques in their coronary arteries, aorta, and aortic valves [18]. Heterozygote LDLR mutation carriers have typically twice-elevated plasma LDL concentration and two-fold increase risk for coronary artery disease compared to the general population. Most guidelines suggest adjustment of LDL levels to less than 100 mg/dL in individuals with two or more risk factors for coronary artery disease and 70 mg/dL for patients with established coronary artery disease. Approximately 50 percent of heterozygote FH patients develop various forms of cardiovascular disease in the fourth or fifth decades [17].LDLR mutations can be classified into five groups based on the functional characteristics of the encoded proteins: 1) null alleles causing receptor synthesis-defect; 2) transport-defective alleles with defect in targeting receptor to cell surface; 3) binding-defective alleles that encode proteins that fail to bind ligands; 4) internalization-defective alleles that encode proteins that fail to interact with clathrin coated pit; and 5) recycling-defective alleles, which remain undissociated in the acidic pH of lysosomes [28].
Ldlr-/- mice fed a 1.5 percent high cholesterol diet not only exhibit enhanced hyperlipidemia and increased generation of reactive oxygen species, but also alter vascular structure along with increased collagen content [29,30]. LDLR gene transfer by intravenous injection of adenovirus to Ldlr-/- mice reduces plasma LDL and VLDL cholesterol [10]. Autosomal recessive hypercholesterolemia clinically resembles FH but is caused by mutations in an LDLR adaptor protein, which is named after the disorder (ARH). LDLR adaptor protein deficient mice (Arh-/-) have impaired LDLR internalization in a variety of tissues such as the liver and exhibit a major defect in LDL clearance [31,32]. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a protein secreted from the hepatocytes, which undergo protein-protein interaction with LDLR, resulting in their degradation [33]. Gain of function in humans causes hypercholesterolemia, and loss of function is associated with low LDL cholesterol and protection against atherosclerosis. RNAi targets PCSK9, specifically lower plasma LDL cholesterol, by preventing LDLR degradation in vivo and in vitro [34]. Similarly, inhibition of PCSK9 effectively increases hepatic LDLR expression and reduces LDL cholesterol in plasma in mice [35]. In sum, LDLR is a pivotal receptor for endocytic machinery and plays a pivotal role in maintaining cholesterol homeostasis. Defect in LDLR function or expression triggers elevated LDL cholesterol and results in major atherosclerotic diseases.
VLDLR
VLDLR, along with LDLR and LRP1, is the main endocytic receptor recognizing apoE-containing lipoproteins. VLDLR is widely expressed in adipose tissues, skeletal muscle, heart, and endothelial cells of capillaries and small arterioles but not in hepatocytes in which LDLR is widely distributed [36]. In contrast, LRP8 (ApoER2), which has 50 percent homology with VLDLR, is mainly expressed in brain, testis, and placenta [37]. VLDLR primarily modulates the extra-hepatic metabolism of TG-rich lipoprotein. The structure of VLDLR is highly homologous to that of LDLR (Figure 1). VLDLR contains eight cysteine-rich LDLR type A repeat domains compared to seven in LDLR. ApoE-containing VLDL not only binds to VLDLR, but to LDLR, LRP8, LRP1, LRP2, and likely LRP6 as well. Unlike LDLR, VLDLR is not linked to a feedback mechanism for its expression in response to intracellular VLDL [38]. VLDLR expression is directly regulated by PPAR-γ. Accordingly, pioglitazone (agonist of PPAR-γ) increases Vldlr mRNA expression and protein level in 3T3-L1 preadipocytes (mouse fibroblasts). Mice treated with pioglitazone exhibit enhanced conversion of plasma TG to epididymal fat. This response is absent in VLDLR deficient mice, which implies that VLDLR plays a key role in fat deposition [39].
VLDLR has an important function in postprandial chylomicron and VLDL clearance by up-regulating lipoprotein lipase (LPL)-mediated TG hydrolysis and direct uptake of TG-rich lipoproteins in endothelial cell [3]. VLDL and LPL have similar distribution patterns in peripheral tissues. Vldlr-/- mice have diminished LPL expression, which causes increased plasma TG; _Vldlr-/-_mice exhibit 250 percent higher TG levels and 60 percent lower chylomicron uptake compared to those in wildtype [3]. Therefore, VLDLR actively functions as a lipoprotein receptor by uptake of TG-rich VLDL (but not LDL) and provides sufficient energy substrates in peripheral tissues. Vldlr-/- mice also exhibit low adipose tissue mass without significant change in their plasma lipoprotein profiles. This latter finding is due to the fact that HDL has the greatest contribution to lipoproteins in mice [11]. Conversely, adenovirus transfer of Vldlr cDNA to the liver increases cholesterol clearance by binding apoE-containing lipoprotein in mice model [40,41]. Expression of VLDLR increases with fasting, which also increases fatty acid binding protein and acetyl CoA synthase to provide a sufficient energy source for vital organs such as the heart and brain [42]. The exact molecular mechanisms of VLDLR function in lipoprotein metabolism are not fully understood.
Low-density lipoprotein-related protein 6 (LRP6)
LRP6 is a member of the LDLR family, which together with LRP5 has the unique structure and function as an essential co-receptor for Wnt/β-catenin signaling [43]. In particular, extracellular domain of LRP6 has three types of subdomains, including LDLR type A repeats, EGF-like domain, and YWTD-type β-propeller domain (Figure 1). LRP6 is comprised of three large clusters of ligand binding repeats, each with ligand binding functions with Wnt, DKK1, and lipoprotein particles, whereas LDLR and VLDLR contain only a single ligand-binding repeat cluster. Upon binding of Wnt to cell surface receptor Frizzled and its co-receptors LRP5/6 complex, the cytoplasmic tail of LRP6 is phosphorylated by CK1 and glycogen synthase kinase 3β (GSK-3β), leading to Dv1 recruitment and binding of Axin complex. This results in stabilization of β-catenin and its translocation onto the nucleus, where it binds to transcription factors such as T cell factor (TCF)/lymphoid enhancer factor (LEF) and stimulates various gene expressions [44].
A missense mutation in LRP6 at a highly conserved residue of an EGF domain has been linked to autosomal-dominant early onset of cardiovascular disease and metabolic syndrome traits, including hyperlipidemia, diabetes, osteoporosis, and hypertension [2]. Patients who carry the LRP6R611C mutation exhibit elevated serum LDL cholesterols, TG, and fasting glucose level, which together constitute the metabolic syndrome, a major risk factor for atherosclerosis and myocardial infarction. These associations strongly suggest a pluripotent effect of this gene.
Common genetic variations within LRP6 also have been associated with plasma LDL levels, implicating LRP6 as a potential regulator of lipid metabolism and a novel target for pharmacological interventions [45]. Liu et al. [46] have demonstrated that an intact LRP6 function is essential for normal LDL clearance, whereas the LRP6R611C mutation results in reduced LRP6 membrane expression and impaired LDL clearance. Splenic macrophages of LRP6+/- mice display reduced LDL uptake compared to wild-type mice. Peripheral B-lymphocytes of_LRP6R611C_ mutation carriers exhibit significantly impaired LDL internalization despite similar affinities for apoB at neutral pH. LRP6 augments cellular LDL binding and uptake, both in LDLR dependent and independent manner. According to Ye et al. [9], LRP6 co-localizes with LDLR and regulates its clathrin-dependent internalization (Figure 2). LRP6 forms a complex with clathrin and ARH and is required for LDL clearance via clathrin-mediated internalization. Human fibroblast of_LRP6R611C_ mutation carriers show impaired function of the LDLR-dependent endocytosis. Preliminary work in our laboratory has shown that LRP6 not only regulates LDL and TG clearance, but it is also involved in synthesis of TG and fatty acids.
Keramati et al. have reported increased expression and co-localization of LRP6 with PDGF receptor β in human atherosclerotic coronary arteries [47]. Wild-type LRP6 forms a complex with platelet-derived growth factor receptor (PDGFR)-β and triggers its lysosomal degradation. This effect reduces vascular smooth muscle cell proliferation and is considered protective against atherosclerosis. Conversely, the_LRP6R611C_ mutation significantly activates PDGF signaling and increases PDGF-dependent cell-cycle activity in smooth muscle cell. This causes increased smooth muscle proliferation, which is an important component of atherosclerosis development.
Low-density lipoprotein-related protein 1
LRP1 (also known as a2-macroglobulin receptor or CD91) is ubiquitously expressed in most tissues and abundantly exists in the liver and brain. LRP1 is composed of a large ligand binding subunit (515-kDa), which recognizes more than 40 ligands, and a relatively small transmembrane fragment (85-kDa) (Figure 1). LRP1 harbors ligands as a cargo transporter and modulates physiologic processes including inflammatory response in the lung [48], amyloid ß42 uptake in blood-brain barrier [49], and clearance of factor VIII in the blood coagulation process [50]. A number of proteases and protease inhibitor complexes are regulated by LRP1-mediated pathway [51]. LRP1 also functions as a receptor for removal of apoE-rich chylomicron remnant; i.e., it shuttles dietary lipid from intestine to liver. LRP1 is likewise involved in the clearance of apoE-rich lipoprotein, including VLDL in liver, smooth muscle cells, and macrophages.
LRP1 is essential for the maintenance of vascular integrity during embryonic development. In adult life, LRP1 plays an essential role in protecting against atherosclerosis by reducing vascular smooth muscle cell (VSMC) proliferation and regulating levels of PDGF receptor in the vessel wall [52]. Lrp1-/- mice increase susceptibility to development of atherosclerotic lesions by greater secretion of apoE, reduced uptake of remnant in macrophages, and reduced HDL plasma level [4]. LRP1 inactivation likewise increases postprandial dyslipidemia and atherosclerosis in_Lrp1-/-_ mice [53]. In addition to cholesterol homeostasis, LRP1 is involved in fatty acid uptake. Lrp1-/- fibroblasts decrease the uptake of fatty acid, resulting in an increase of free fatty acid and redistribution to the liver [12]. Statin intervention in rats has shown to up-regulate both LRP1 and LDLR via activated SREBP-2 related pathway, resulting in protection against atherosclerosis [54].
Low-density lipoprotein-related protein 2, megalin
LRP2, also named megalin for its huge molecular structure, is a member of the LDLR family that is abundantly expressed in different epithelial cell types [55]. LRP2 is an endocytic receptor for diverse ligands, including lipoprotein, steroid, and retinoid. Renal re-absorption of a variety of molecules, in particular vitamin B12 and HDL, is an important function of LRP2, which is positively regulated by PPAR-α/β [13]. LRP2 is composed of extracellular ligand binding domain, transmembrane fragment, and cytoplasmic tail containing three NPxY motifs (a total molecular weight of 517 kDa) (Figure 1). Similar to LRP1, receptor function of LRP2 is connected to proteolysis between the transmembrane anchor and cytoplasmic motif. Ligand binding to LRP2 triggers shedding of its ectodomain by protein kinase C (PKC)-induced matrix metalloproteinase. Cytoplasmic C-terminal fragment is cleaved by β-secretase, resulting in its release into the cytoplasm, whereby it presumably functions as a transcriptional regulator in the nucleus [56]. Similar to LRP5/6, LRP2 needs mesoderm development (MESD) chaperone, which prevents misfolding of LRP2 [57]. LRP2 is involved in embryonic renal development, including vitamin D homeostasis, sex hormone signaling, and holoprosencephaly [58-60]. Genetic variations in_LRP2_ have been associated with elevated total cholesterol and LDL in patients with dyslipidemia [61]. LRP2 harbors cubilin, which bind to HDL, thus providing an important role in endocytosis of HDL cholesterol [5]. The role of LRP2 in dyslipidemia and atherosclerosis is far from complete.
Low-density lipoprotein-related protein 5
LRP5 structure is analogous to that of the LRP6 and similarly functions as a co-receptor for Wnt/β-catenin signaling. LRP5 is well known for its affect on bone mass density and mineralization. Loss of function mutations are associated with osteoporosis pseudoglioma [62], and gain of function mutations are associated with high bone density [63]. LRP5 may be involved in mineralization of the atherosclerotic lesion [64].Apoe-/- mice fed with western diet (42 percent kcal from fat and 0.2 percent cholesterol) show increased LRP5 expression. Interestingly,Apoe-/- mice on a Western diet develop calcified plaque, while Lrp5-/- or_Apoe-/-_, Lrp5-/- double knockouts have no or minimal calcified lesions [65]. Accordingly, _Ldlr-/-_mice fed either chow (18 percent kcal from fat) or Western diet have high expression levels of Lrp5 in aortic tissue [66]. Lrp5-/- mice exhibit decreased hepatic clearance of chylomicron remnants, plasma cholesterol levels, and impaired insulin secretion [67]. Further investigation is necessary to clarify the role of LRP5 in calcification of atheromatous plaque and lipoprotein endocytosis.
Conclusions
Members of the LDLR family of proteins dynamically bind and internalize apoE- and apoB-containing lipoprotein, including LDL, VLDL, and chylomicron remnant. Defect in these receptors is associated with dyslipidemia and atherosclerosis in mammals. LDL defect is the most common cause of familial hypercholesterolemia, which is associated with very high plasma LDL levels, accounted for by impaired LDL clearance and enhanced cholesterol biosynthesis. VLDLR modulates the binding and uptake of apoE-containing lipoprotein, including chylomicron and VLDL, and regulates TG level in plasma. LRP6, well known as co-receptor for Wnt signal pathway, is a unique member of this receptor family. Recent work in our laboratory and by others has shown that LRP6 also functions as a regulator of cholesterol and lipid homeostasis, glucose metabolism, and blood pressure. Preliminary work in our laboratory has shown that this receptor not only regulates LDL and TG uptake, but it is also involved in synthesis of TG and fatty acids. In addition, LRP6 plays a major role in vascular integrity and protection against atherosclerosis, in part by its anti-proliferative effects. Therefore, LDLR family members are principal targets of novel therapeutics for metabolic risk factors and diseases. While current lipid lowering drugs such as statins have been very effective in reducing serum LDL levels in the general population, there remain a significant number of patients with extremely high LDL levels that show no or modest responses. Furthermore, these drugs have no or small effects on serum TG and HDL. Inflammatory processes and vascular smooth muscle cell proliferation also play a pivotal role in cardiovascular diseases. Therefore, other members of the LDLR family may be targets of novel therapeutic for the most common form of hyperlipidemia known as combined familial hyperlipidemia. For instance, targeting LRP6 may reverse diverse pathophysiological processes in atherosclerotic diseases, including increased TG/VLDL synthesis, elevated lipolysis, decreased LDL clearance, and enhanced VSMC proliferation.
Abbreviations
ACAT
acyl-CoA cholesteryl acyl transferase
ARH
autosomal recessive hypercholesterolemia protein
EGF
epidermal growth factor
FH
familial hypercholesterolemia
GSK-3
glycogen synthase kinase-3
HDL
high-density lipoprotein
HMGCR
3-hydroxy-3-methylglutaryl-CoA reductase
IDL
intermediate-density lipoprotein
LEF
lymphoid enhancer factor
LDL
low-density lipoprotein
LDLR
low-density lipoprotein receptor
LPL
lipoprotein lipase
LRP
low-density lipoprotein related protein
MESD
mesoderm development
PCSK9
proprotein convertase subtilisin/kexin type 9
PDGF
platelet-derived growth factor
PKC
protein kinase C
PPAR
peroxisome proliferator-activated receptor
SREBP
sterol regulatory element-binding protein
TCF
T cell factor
TG
triglycerides
VLDL
very low-density lipoprotein
VLDLR
very low-density lipoprotein receptor
VSMC
vascular smooth muscle cells
References
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM. et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani M-A, Nelson-Williams C. et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan JR, Espirito Santo SMS, Voshol PJ, Teusink B, van Dijk KW, van Vlijmen BMJ. et al. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res. 2004;45(8):1475–1481. doi: 10.1194/jlr.M400009-JLR200. [DOI] [PubMed] [Google Scholar]
- Basford JE, Wancata L, Hofmann SM, Silva RAGD, Davidson WS, Howles PN. et al. Hepatic deficiency of low density lipoprotein receptor-related protein-1 reduces high density lipoprotein secretion and plasma levels in mice. J Biol Chem. 2011;286(15):13079–13087. doi: 10.1074/jbc.M111.229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad SM, Barth JL, Knaak C, Argraves WS. Megalin acts in concert with cubilin to mediate endocytosis of high density lipoproteins. J Biol Chem. 2000;275(16):12003–12008. doi: 10.1074/jbc.275.16.12003. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol. 1999;1(6):E157–E162. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- Jeon H, Blacklow SC. Structure and Physiologic Function of the Low-density Lipoprotein Receptor. Ann Rev Biochem. 2005;74(1):535–562. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol. 2001;23(1):53–67. doi: 10.1385/MN:23:1:53. [DOI] [PubMed] [Google Scholar]
- Ye ZJ, Go GW, Singh R, Liu W, Keramati AR, Mani A. LRP6 regulates LDLR-mediated LDL uptake. J Biol Chem. 2012;287(2):1335–1344. doi: 10.1074/jbc.M111.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY. et al. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem. 2002;277(12):10037–10043. doi: 10.1074/jbc.M109966200. [DOI] [PubMed] [Google Scholar]
- Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P. et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284(1):381–388. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas F, Lagos J, Céspedes C, Vio CP, Bronfman M, Marzolo M-P. Megalin/LRP2 expression is induced by peroxisome proliferator-activated receptor -alpha and -gamma: implications for PPARs’ roles in renal function. PloS one. 2011;6(2):e16794. doi: 10.1371/journal.pone.0016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein JL, Brown MS, Stone NJ. Genetics of the LDL receptor: evidence that the mutations affecting binding and internalization are allelic. Cell. 1977;12(3):629–641. doi: 10.1016/0092-8674(77)90263-x. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Dana SE, Brown MS. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci USA. 1974;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci USA. 1973;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci USA. 1974;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974;185(4145):61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Wiklund O, Dyer CA, Tsao BP, Curtiss LK. Stoichiometric binding of apolipoprotein B-specific monoclonal antibodies to low density lipoproteins. J Biol Chem. 1985;260(20):10956–10960. [PubMed] [Google Scholar]
- Innerarity TL, Mahley RW, Weisgraber KH, Bersot TP. Apoprotein (E--A-II) complex of human plasma lipoproteins. II. Receptor binding activity of a high density lipoprotein subfraction modulated by the apo(E--A-II) complex. J Biol Chem. 1978;253(17):6289–6295. [PubMed] [Google Scholar]
- Innerarity TL, Mahley RW. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1139–1142. doi: 10.1172/JCI24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Shimano H, Horton JD, Goldstein JL, Brown MS. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci USA. 1997;94(23):12354–12359. doi: 10.1073/pnas.94.23.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Dana SE, Goldstein JL. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249(3):789–796. [PubMed] [Google Scholar]
- Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1(6):445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet. 1990;24:133–170. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- Lauzier B, Delemasure S, Collin B, Duvillard L, Menetrier F, Vergely C. et al. Effect of a chronic cholesterol-rich diet on vascular structure and oxidative stress in LDLR-/- mice. International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2011;27(1):31–36. doi: 10.1159/000325211. [DOI] [PubMed] [Google Scholar]
- Kypreos KE, Zannis VI. LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J Lipid Res. 2006;47(3):521–529. doi: 10.1194/jlr.M500322-JLR200. [DOI] [PubMed] [Google Scholar]
- Jones C, Garuti R, Michaely P, Li W-P, Maeda N, Cohen JC. et al. Disruption of LDL but not VLDL clearance in autosomal recessive hypercholesterolemia. J Clin Invest. 2007;117(1):165–174. doi: 10.1172/JCI29415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Hammer RE, Li W-P, Cohen JC, Hobbs HH, Herz J. Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J Biol Chem. 2003;278(31):29024–29030. doi: 10.1074/jbc.M304855200. [DOI] [PubMed] [Google Scholar]
- Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N. et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283(4):2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A. et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105(33):11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST. et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48(4):763–767. doi: 10.1194/jlr.C600025-JLR200. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci USA. 1992;89(19):9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SS, Connor TE, Weeber EJ, Rebeck W. Similarities and differences in structure, expression, and functions of VLDLR and ApoER2. Molecular Neurodegeneration. 2011;6:30. doi: 10.1186/1750-1326-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J. et al. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb. 2004;11(4):200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- Tao H, Aakula S, Abumrad NN, Hajri T. Peroxisome proliferator-activated receptor-gamma regulates the expression and function of very-low-density lipoprotein receptor. Am J Physiol Endocrinol Metab. 2010;298(1):E68–E79. doi: 10.1152/ajpendo.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Liu Y, Sun T, Xu Y, Zhang J, Yang Y. et al. The therapeutic role of very low-density lipoprotein receptor gene in hyperlipidemia in type 2 diabetic rats. Hum Gene Ther. 2011;22(3):302–312. doi: 10.1089/hum.2010.038. [DOI] [PubMed] [Google Scholar]
- Jacobs F, Van Craeyveld E, Feng Y, Snoeys J, De Geest B. Adenoviral low density lipoprotein receptor attenuates progression of atherosclerosis and decreases tissue cholesterol levels in a murine model of familial hypercholesterolemia. Atherosclerosis. 2008;201(2):289–297. doi: 10.1016/j.atherosclerosis.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Kwok S, Singh-Bist A, Natu V, Kraemer FB. Dietary regulation of the very low density lipoprotein receptor in mouse heart and fat. Horm Metab Res. 1997;29(10):524–529. doi: 10.1055/s-2007-979094. [DOI] [PubMed] [Google Scholar]
- Mi K, Johnson GVW. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J Cell Biochem. 2005;95(2):328–338. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci. 2010;67(15):2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski M, Charchar FJ, Barnes T, Gawron-Kiszka M, Sedkowska A, Podolecka E. et al. A common variant in low-density lipoprotein receptor-related protein 6 gene (LRP6) is associated with LDL-cholesterol. Arterioscler Thromb Vasc Biol. 2009;29(9):1316–1321. doi: 10.1161/ATVBAHA.109.185355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Mani S, Davis NR, Sarrafzadegan N, Kavathas PB, Mani A. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. 2008;103(11):1280–1288. doi: 10.1161/CIRCRESAHA.108.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati AR, Singh R, Lin A, Faramarzi S, Ye Z-j, Mane S. et al. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2011;108(5):1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, Xiao Y-Q, Dickinson M, Nick JA, Voelker DR, Greene KE. et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115(1):13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Janko MC, André-Dohmen B, Reuss S, Weggen S, Roebroek AJM. et al. LRP1 mediates bidirectional transcytosis of amyloid-β across the blood-brain barrier. Neurobiol Aging. 2011;32(12):2323.e1-11. doi: 10.1016/j.neurobiolaging.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Franchini M, Montagnana M. Low-density lipoprotein receptor-related protein 1: new functions for an old molecule. Clin Chem Lab Med. 2011;49(6):967–970. doi: 10.1515/CCLM.2011.154. [DOI] [PubMed] [Google Scholar]
- Strickland DK, Ranganathan S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J Thromb Haemost. 2003;1(7):1663–1670. doi: 10.1046/j.1538-7836.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- Boucher P, Gotthardt M, Li W-P, Anderson RGW, Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300(5617):329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- Gordts PLSM, Reekmans S, Lauwers A, Van Dongen A, Verbeek L, Roebroek AJM. Inactivation of the LRP1 intracellular NPxYxxL motif in LDLR-deficient mice enhances postprandial dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(9):1258–1264. doi: 10.1161/ATVBAHA.109.192211. [DOI] [PubMed] [Google Scholar]
- Moon JH, Kang SB, Park JS, Lee BW, Kang ES, Ahn CW. et al. Up-regulation of hepatic low-density lipoprotein receptor-related protein 1: a possible novel mechanism of antiatherogenic activity of hydroxymethylglutaryl-coenzyme A reductase inhibitor Atorvastatin and hepatic LRP1 expression. Metabolism. 2011;60(7):930–940. doi: 10.1016/j.metabol.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Marzolo M-P, Farfán P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. 2011;44(1):89–105. doi: 10.4067/S0716-97602011000100012. [DOI] [PubMed] [Google Scholar]
- Pieper-Fürst U, Hall R, Huss S, Hochrath K, Fischer H-P, Tacke F. et al. Expression of the megalin C-terminal fragment by macrophages during liver fibrogenesis in mice. Biochim Biophys Acta. 2011;1812(12):1640–1648. doi: 10.1016/j.bbadis.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Lighthouse JK, Zhang L, Hsieh J-C, Rosenquist T, Holdener BC. MESD is essential for apical localization of megalin/LRP2 in the visceral endoderm. Dev Dyn. 2011;240(3):577–588. doi: 10.1002/dvdy.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK. et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA. 1996;93(16):8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J. et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H. et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122(5):751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Mii A, Nakajima T, Fujita Y, Iino Y, Kamimura K, Bujo H. et al. Genetic association of low-density lipoprotein receptor-related protein 2 (LRP2) with plasma lipid levels. J Atheroscler Thromb. 2007;14(6):310–316. doi: 10.5551/jat.e494. [DOI] [PubMed] [Google Scholar]
- Narumi S, Numakura C, Shiihara T, Seiwa C, Nozaki Y, Yamagata T. et al. Various types of LRP5 mutations in four patients with osteoporosis-pseudoglioma syndrome: identification of a 7.2-kb microdeletion using oligonucleotide tiling microarray. Am J Med Genet. 2010;152A(1):133–140. doi: 10.1002/ajmg.a.33177. [DOI] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR. et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE. et al. Osteopontin Is Expressed in Human Aortic Valvular Lesions. Circulation. 1995;92(8):2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: experimental hypercholesterolemia in the ApoE-/- /Lrp5-/- mice. J Biol Chem. 2011;112(10):2987–2991. doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan Z, Denis M, Bailey D, Giaid A, Prat A, Goltzman D. et al. The LDLR deficient mouse as a model for aortic calcification and quantification by micro-computed tomography. Atherosclerosis. 2011;219(2):455–462. doi: 10.1016/j.atherosclerosis.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang M-J, Ikeda Y, Sone H, Takada S. et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA. 2003;100(1):229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]