Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study (original) (raw)
Summary
Background
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease of upper and lower motor neurons, associated with frontotemporal dementia (FTD) in about 14% of incident cases. We assessed the frequency of the recently identified C9orf72 repeat expansion in familial and apparently sporadic cases of ALS and characterised the cognitive and clinical phenotype of patients with this expansion.
Methods
A population-based register of patients with ALS has been in operation in Ireland since 1995, and an associated DNA bank has been in place since 1999. 435 representative DNA samples from the bank were screened using repeat-primed PCR for the presence of a GGGGCC repeat expansion in C9orf72. We assessed clinical, cognitive, behavioural, MRI, and survival data from 191 (44%) of these patients, who comprised a population-based incident group and had previously participated in a longitudinal study of cognitive and behavioural changes in ALS.
Findings
Samples from the DNA bank included 49 cases of known familial ALS and 386 apparently sporadic cases. Of these samples, 20 (41%) cases of familial ALS and 19 (5%) cases of apparently sporadic ALS had the C9orf72 repeat expansion. Of the 191 patients for whom phenotype data were available, 21 (11%) had the repeat expansion. Age at disease onset was lower in patients with the repeat expansion (mean 56·3 [SD 8·3] years) than in those without (61·3 [10·6] years; p=0·043). A family history of ALS or FTD was present in 18 (86%) of those with the repeat expansion. Patients with the repeat expansion had significantly more co-morbid FTD than patients without the repeat (50% vs 12%), and a distinct pattern of non-motor cortex changes on high-resolution 3 T magnetic resonance structural neuroimaging. Age-matched univariate analysis showed shorter survival (20 months vs 26 months) in patients with the repeat expansion. Multivariable analysis showed an increased hazard rate of 1·9 (95% 1·1–3·7; p=0·035) in those patients with the repeat expansion compared with patients without the expansion
Interpretation
Patients with ALS and the C9orf72 repeat expansion seem to present a recognisable phenotype characterised by earlier disease onset, the presence of cognitive and behavioural impairment, specific neuroimaging changes, a family history of neurodegeneration with autosomal dominant inheritance, and reduced survival. Recognition of patients with ALS who carry an expanded repeat is likely to be important in the context of appropriate disease management, stratification in clinical trials, and in recognition of other related phenotypes in family members.
Funding
Health Seventh Framework Programme, Health Research Board, Research Motor Neuron, Irish Motor Neuron Disease Association, The Motor Neurone Disease Association of Great Britain and Northern Ireland, ALS Association.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease of upper and lower motor neurons. Cognitive impairment occurs in up to 50% of cases, and one in seven patients develops frank frontotemporal dementia (FTD).1 The existence of families with pure ALS, pure FTD, and ALS with co-morbid FTD (ALS-FTD) has been long recognised.2 A combination of clinical, neuroimaging, and neuropathological data suggest that ALS and FTD might form part of a disease continuum, with pure ALS at one extreme and pure FTD at the other. Detailed genetic studies including conventional linkage2 and genome-wide association studies of families with ALS and FTD have identified a reproducible locus on chromosome 9p21,3–5 and a disease-segregating expanded hexanucleotide repeat in the C9orf72 gene in that locus accounts for up to 60% of familial ALS and up to 10% of sporadic ALS.6,7 Preliminary data suggest that hexanucleotide expansions of more than 23 are pathological, although further population-based control studies are warranted.6 Detailed phenotyping of patients with this pathological expansion has yet to be reported.
In this study, we characterised the clinical features, demographics, survival, neurocognitive profile, family history, and neuroimaging findings in a population-based cohort of Irish patients carrying the C9orf72 hexanucleotide repeat expansion.
Methods
Participants and study design
A population-based register of patients with ALS has been in operation in Ireland since 1995,8,9 and an associated bank of DNA extracted from venous leucocytes has been in place since 1999. 435 representative samples were selected for screening from the DNA bank on the basis of the following criteria: Irish origin; both incident and prevalent cases; sufficiently high-quality and quantity to permit subsequent Southern blotting; and proportionate representation of the familial and sporadic ALS contained in the DNA bank. 188 age-matched, sex-matched, and geographically matched controls were specifically selected through the patients' primary care provider for this study.
Of these 435 samples, 191 belonged to population-based incident patients diagnosed with ALS from November, 2006, to May, 2011, who were selected through the ALS register and enrolled in a prospective longitudinal case-control study of cognitive and behavioural function (webappendix p 2).1,10 Detailed longitudinal clinical, neurocognitive, and behavioural data, structural MRI, and survival data have been gathered on this cohort, and DNA has been banked for genomic analysis. Patient enrolment to the ALS register was achieved by direct referral by all neurologists and neurophysiologists practising in Ireland and by close and regular interaction with community-based primary-care and disability services. For inclusion in the register, extensive confirmatory measures such as clinical examination by a specialist, direct chart review, and assessment by a neurophysiologist are required. Clinical progression was tracked by regular telephone contact between the register coordinator, health-care professionals, patients, and carers, and by home visits by members of the Beaumont Hospital ALS research group run by OH. Further details of enrolment to the Irish register are published elsewhere.8,9
Ethics approval for all aspects of the study was obtained from Beaumont Hospital Research Ethics Committee. For patients unable to provide full informed written consent, a protocol of assent was used.
Procedures
DNA samples were screened with repeat-primed PCR for the presence of a GGGGCC hexanucleotide repeat expansion in C9orf72. PCR products were analysed on an Applied Biosystems (Foster City, CA, USA) 3130xl genetic analyser and visualised using GeneMapper software (version 4.0). Patients with the characteristic appearance of the expanded hexanucleotide repeat on repeat-primed PCR, consisting of a decaying series of more than 23 peaks, were classed as having the expansion; this cutoff was chosen because DeJesus-Hernandez and colleagues6 reported no more than 23 repeats in healthy individuals. The PCR assay was validated using Southern blotting in samples from 12 unrelated individuals who carry the C9orf72 expansion but were not included in our main analyses. Insertions between 9·3 Kb and 16·8 Kb were detected, confirming that the assay identifies the expansion. Southern blotting was also used on non-pathological repeat expansions to quantify the size in a validation process.
For screening of known mutations, target-enriched sequencing libraries were prepared from genomic DNA with standard protocols (Agilent SureSelect, Santa Clare, CA, USA) and sequenced on an Illumina Genome Analyzer II (San Diego, CA, USA). Sequence data were aligned and processed using BWA (version 0.5.9), SAMtools (version 0.1.18), Picard (version 1.49), and GATK (version 1.3.14) to generate variant calls that were filtered to exclude dbSNP132 polymorphisms. For haplotype analyses, chromosome 9 single nucleotide polymorphisms (SNPs) were phased using BEAGLE (version 3.3.2).11
Of the 191 patients who participated in the prospective longitudinal case-control study, cognitive and behavioural testing was done at 6-month intervals in patients' homes. Exclusion criteria for the prospective longitudinal case-control study included a history of traumatic brain injury, learning disability, alcohol dependence, type 1 or uncontrolled type 2 diabetes mellitus, and serious active mental illness. A neuropsychological battery was used to assess cognitive domains, including executive function (Stroop, Brixton, Verbal Fluency, Category Fluency, Backwards Digit Span), memory function (Logical Memory, Rey Osterrith Figure Test Immediate, and Delayed, Visual Paired Associates, California Verbal Learning Test), language (Boston Naming Test), visuospatial domains (Rey Osterrith Figure Test Copy), and behaviour (Frontal Systems Behavior Scale).1,10 Matched controls were recruited for comparative purposes. Controls for the neuropsychological arm of the study were recruited through a network of volunteers obtained via advertisement and through the patients' primary care providers. Details of the methods and categorisation have been described previously.1,10 Patients completed a validated family history questionnaire,12 and were subsequently contacted by telephone and interviewed. Family history was further corroborated by another relative. All reported deaths within each extended kindred were verified by death certification.
For those with a family history of neurodegeneration, a posthumous classification was made on the basis of all clinical details available including age of onset, rate of disease progression, and type of dementia. A family history of FTD was defined when a first-degree or second-degree relative had any of the following criteria: a clinical diagnosis of FTD while under the care of a neurologist or physician, confirmed by review of clinical notes; a clinical diagnosis of Pick's disease confirmed by death certification; and documentation of onset of dementia before the age of 65 years and with striking personality changes. Relatives with clinical features suggestive of Alzheimer's disease or multi-infarct dementia were not recorded as having FTD. Family history was classed as strong when three or more first-degree or second-degree relatives were affected in each pedigree.
3 T magnetic resonance grey-matter voxel-based morphometry analysis was done in ten patients with the repeat expansion and 30 without (webappendix p 1). The demographic details (age, disease duration, and clinical phenotype) of the two groups were matched. A study-specific template was created, to which the grey-matter images from each patient were non-linearly co-registered. A voxel-wise generalised linear model was used to test for differences between the groups using permutation-based non-parametric testing. A minimum cluster size of 800 μL was applied to the results of the analysis to show only significant regions of differences. Significance was set at p<0·01 (voxel level) and corrected for multiple comparisons at p<0·05 (family-wise error).13
Statistical analysis
Demographic and clinical characteristics of the participants are reported as percentages for categorical variables and means (SD) and medians (IQR) for continuous variables. Comparisons were made with χ2, Fisher's exact, or two-sample t tests as appropriate. For group analysis, when the sample size was small, the p value was calculated with the Montecarlo method.14 When variables were normally distributed, a mean was reported. When variables were not normally distributed, a median was reported and non-parametric tests were used. Univariate ANCOVA was done to adjust for confounding factors. Spearman's rho test was used when assessing the degree of correlation strength between variables with a non-parametric distribution. Disease progression in patients who had undergone second neuropsychological assessments was calculated using the ALS functional rating scale15 by dividing the decline in functional scores by the time between the two assessments. Survival time was defined as time from diagnosis to death, co-varied for time from onset. Time from symptom onset to diagnosis was compared between groups to ensure lack of lead-time bias. Patients who were alive at the time of analysis were censored. Univariate assessment of the survival effect of categorical variables was done with Kaplain-Meier survival analysis and equality of outcome was assessed with the log-rank test. Cox proportional hazards method was used for multivariable survival analysis with backward elimination (Wald test), estimation of hazard ratio, and 95% CIs. Predictive modelling with direct logistic binary regression was used to ascertain predictive factors associated with patients testing positive for the repeat expansion. All tests were two-tailed and significance was set at p<0·05. Statistical analyses were done using SPSS (version 18).
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
Of 435 banked DNA samples derived from the Irish ALS Register, 39 (9%) had the characteristic appearance of a GGGGCC hexanucleotide expansion, consisting of a decaying series of more than 23 peaks on a PCR electropherogram, and were therefore classed as having the C9orf72 repeat expansion. All samples from carriers of the expansion had 30 or more peaks that showed exponential decay, whereas the electropherograms of samples without the repeat expansion had 23 or fewer repeats and showed an abrupt cutoff. 20 (41%) of 49 patients with familial ALS and 19 (5%) of 386 with apparently sporadic ALS carried the expansion. No expansions above 23 repeats were noted in 188 healthy controls (webappendix p 3).
Of the 39 patients with the repeat expansion, 19 had previously been genotyped using Illumina 550k or 610k genome-wide SNP arrays (San Diego, CA, USA). Analysis of chromosome-9 SNPs showed that 16 of these patients carried the 20-SNP consensus haplotype reported by Mok and colleagues,16 whereas the remaining three carried a truncated 18-SNP version containing the T allele for rs1822723 at the telomeric end of the 20-SNP haplotype.
Phenotype data were available for 191 (44%) of the 435 samples from the DNA bank, comprising the population-based incident group recruited to participate in the longitudinal case-control study of cognition and behaviour in ALS.1,10 Analysis of sequence data generated for SOD1, TARDBP, and FUS showed no known or potentially new pathogenic variants. 21 (11%) of the 191 patients in this cohort carried the C9orf72 repeat expansion. In total, 18 of 21 patients with the repeat expansion had a verified first-degree or second-degree relative with either ALS (12 patients) or FTD (six patients). Of those who did not carry the C9orf72 repeat (170 patients) only three (2%) described a family history of FTD (p<0·0001).
Of the three remaining patients carrying an expanded repeat, who apparently had sporadic ALS, one had a strong family history of depressive illness with suicide, one had a family history of parkinsonism in two paternal siblings and unspecified dementia and mobility problems in a paternal grandmother, and one was unable to provide a family history because both parents had died prematurely. Construction of multiple generation pedigrees was possible in kindreds from 15 of 21 patients with the repeat expansion. A dominant inheritance pattern was apparent in all cases, although six of 15 exhibited a pattern of apparently incomplete penetrance in which obligate carriers survived into the eighth or ninth decade of life.
Age at disease onset and diagnosis was lower in patients with the repeat expansion than in those without, and time from symptom onset to diagnosis did not differ between those with the repeat expansion and those without (14·7 months for carriers vs 13·2 months for non-carriers; p=0·541; table 1). Sex, site of disease onset, and ALS functional rating score did not differ at first assessment between the two groups.
Table 1.
Demographic and clinical information for patients with amyotrophic lateral sclerosis from the population-based cohort
| Patients without the C9orf72 repeat expansion (n=170) | Patients with the C9orf72 repeat expansion (n=21) | p value | |
|---|---|---|---|
| Age at onset (years) | |||
| Mean | 61·3 (10·6) | 56·3 (8·3) | 0·043 |
| Median | 61·9 (56·4–67·9) | 57·5 (50·5–63·5) | 0·019 |
| Age at diagnosis (years) | |||
| Mean | 62·5 (10·6) | 57·5 (8·1) | 0·041 |
| Median | 62·8 (57·1–69·1) | 59·6 (51·9–64·2) | 0·015 |
| Site of onset | |||
| Bulbar | 57 (34%) | 7 (33%) | 0·822 |
| Spinal | 113 (66%) | 14 (67%) | .. |
| Sex | |||
| Male | 101 (59%) | 10 (48%) | 0·353 |
| Female | 69 (41%) | 11 (53%) | .. |
| Mean ALS functional rating scale score at first assessment | 36·4 (8·1) | 33·8 (6·7) | 0·181 |
All 191 patients in the population-based incident cohort had previously undergone detailed neuropsychological testing and 186 (97%) had been classified into one of four cognitive categories:1 ALS-FTD, executive impairment, non-executive impairment, and normal cognition. Five (3%) of 191 patients were too unwell to complete the full cognitive battery and were therefore not classified according to cognitive criteria. However, behavioural data were available from all five, and these data were included in the analysis. Of the 186 patients who completed full cognitive testing at the time of first assessment, 91 (49%) underwent further testing after 6 months and 34 (18%) had a third assessment 1 year after initial assessment. Detailed behavioural data from the Frontal Systems Behavioural Scale were available for 130 (68%) of 191 patients.17
C9orf72 repeat expansion carriers exhibited a characteristic profile, with a significantly higher frequency of co-morbid FTD (50% vs 12%) in the group with the expanded repeat (table 2). In a univariate ANCOVA analysis comparing performance on executive tasks (category fluency, verbal fluency index, Brixton spatial anticipation task, Stroop interference task, and backward digit span tests) between patients with the repeat expansion and those without, after adjustment for differences in age at time of assessment, patients with the repeat expansion had significantly more impairment according to the Brixton scaled score than did those without the repeat (table 3).
Table 2.
Cognitive categories according to neuropsychological test results
| Patients without the C9orf72 repeat expansion (n=166) | Patients with the C9orf72 repeat expansion (n=20) | |
|---|---|---|
| ALS-FTD | 20 (12%) | 10 (50%) |
| Executive impairment | 46 (28%) | 4 (20%) |
| Non-executive cognitive impairment | 27 (16%) | 0 (0%) |
| Normal | 73 (44%) | 6 (30%) |
Table 3.
Executive function test results
| Patients without the C9orf72 repeat expansion | Patients with the C9orf72 repeat expansion | p value | |
|---|---|---|---|
| Category fluency* | 16·5 (7·9; 126) | 15·1 (7·9; 14) | 0·100 |
| Combined verbal fluency index† | 13·8 (7·7–27·0; 149) | 17·8 (11·3–48·7; 19) | 0·056 |
| Brixton scaled score* | 5·1 (2·3; 154) | 4·6 (2·8; 19) | 0·043 |
| Stroop score | |||
| CW score* | 69·3 (33·3; 119) | 60·3 (32·5; 14) | 0·067 |
| SEF score† | 37·1 (25·9; 119) | 44·5 (25·6; 14) | 0·054 |
| Backward digit span* | 10·6 (3·5; 117) | 9·7 (3·7; 12) | 0·221 |
Further analysis of the subgroup of patients with ALS-FTD (30 patients) showed that a third of patients carried the repeat expansion (table 4). Patients with ALS-FTD and a repeat expansion were significantly younger when symptoms of ALS appeared. Other differences noted in patients with ALS-FTD carrying the repeat expansion included increased rate of spinal onset, predominance of behavioural variant FTD, and younger age at diagnosis, although these differences were not significant (table 4). The mean language score of patients with the C9orf72 repeat on the Boston naming test was higher (20·1 [SD 7·5] carriers vs 13·5 [8·9] in non-carriers, p=0·043).
Table 4.
Demographic and clinical information for patients with amyotrophic lateral sclerosis with co-morbid frontotemporal dementia
| Patients without the C9orf72 repeat expansion (n=20) | Patients with the C9orf72 repeat expansion (n=10) | p value | |
|---|---|---|---|
| Age at onset (years) | |||
| Mean | 64·8 (9·5) | 57·4 (8·1) | 0·045 |
| Median | 65·6 (56·7–72·5) | 58·2 (55·9–62·8) | 0·100 |
| Age at diagnosis (years) | |||
| Mean | 65·9 (9·7) | 58·7 (7·9) | 0·053 |
| Median | 66·9 (57·5–74·2) | 59·8 (56·9–63·9) | 0·100 |
| Site of onset | |||
| Bulbar | 10 (50%) | 1 (10%) | 0·049 |
| Spinal | 10 (50%) | 9 (90%) | .. |
| Sex | |||
| Male | 13 (65%) | 7 (70%) | 1·000 |
| Female | 7 (35%) | 3 (30%) | .. |
| Type of frontotemporal dementia | |||
| Behavioural | 15 (75%) | 10 (100%) | 0·143 |
| Language variant | 5 (25%) | 0 (0%) | .. |
Of the patients who had been classified as having normal cognition in our previous report,1 patients with the repeat expansion exhibited a higher rate of behavioural impairment than did patients without the repeat expansion (four of six vs 11 of 50; p=0·038). Patients with the repeat expansion had significantly higher apathy scores (18·0 [IQR 12·5–35·5]; p=0·032) and dysexecutive behaviour (8·0 [IQR 2·5–27·0]; p=0·023; webappendix p 1).
Of the 21 patients with the repeat expansion, only two were classified as having neither cognitive impairment nor behavioural impairment at the time of their first assessment. Follow-up testing was not available for either patient; one patient had a strong family history of ALS and the other had a strong family history of FTD. None of the 63 patients who had previously been classed as behaviourally normal and had a negative family history had the repeat expansion.
Compared with patients without the repeat expansion (82 patients), those with the expansion (nine) had a significantly faster rate of motor progression according to total ALS functional rating scale (revised) scores (median decline of 1·54 [1·07–2·08] points per month in carriers vs 0·83 [IQR 0·34–1·43] points per month in non-carriers; p=0·009). The spinal subscore of the ALS functional rating scale showed that this difference was driven by a higher rate of decline in spinal function (median decline of 1·2 [IQR 0·77–1·53] points in carriers vs 0·5 [0·17–0·83] points per month in non-carriers; p=0·016).
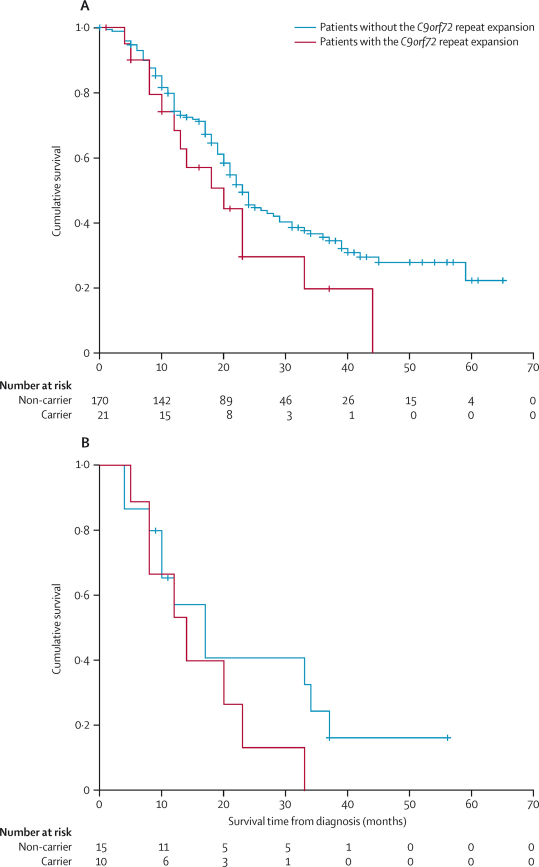
Age-matched univariate analysis showed significantly shorter survival in patients with the repeat expansion (21 patients; median survival 20 months, 95% CI 8·9–31·1) than in patients without the expansion (170; 26 months, 21·1–30·9; p=0·017; figure 1). Presence of the repeat expansion generated a significant hazard ratio (1·9, 95% CI 1·1–3·7; p=0·035) on multivariable analysis after adjusting for age of symptom onset, sex, time from onset to diagnosis, and site of onset. Multivariable analysis of patients with ALS and behavioural variant FTD showed that the presence of the repeat expansion was associated with a hazard ratio of 3·7 (95% CI 1·1–12·3; p=0·034).
Figure 1.
Kaplan-Meier survival probabilities for patients with amyotrophic lateral sclerosis stratified for the presence of the C9orf72 hexanucleotide repeat expansion
Kaplan-Meier survival probabilities for all patients with amyotrophic lateral sclerosis (ALS) in the population-based cohort (191 patients; A), and for the subgroup of patients with behavioural variant frontotemporal dementia (25 patients; B).
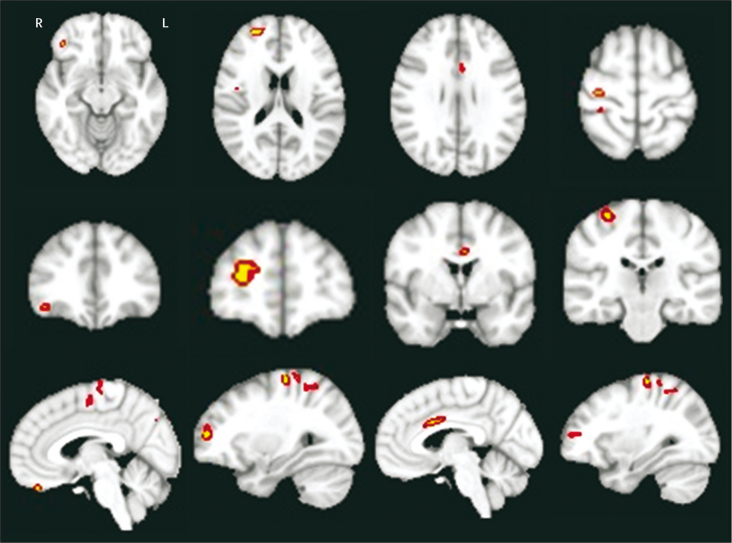
3 T magnetic resonance grey-matter voxel-based morphometry was assessed on a cohort of ten patients with ALS and the repeat expansion and 30 age-matched and disease-duration-matched patients with ALS without the repeat expansion (webappendix p 1). Significant differences in grey-matter atrophy were identified in the cohort with the repeat expansion in the following brain regions: right inferior frontal gyrus, right superior frontal gyrus, left anterior cingulated gyrus, and the right precentral gyrus (figure 2).
Figure 2.
Voxel-based morphometry analysis
Clusters of significant grey-matter atrophy in a cohort of ten patients with amyotrophic lateral sclerosis (ALS) and the pathological expanded C9orf72 hexanucleotide repeat compared with 30 matched patients with ALS without the repeat expansion. The four columns show the four clusters (from left to right: in the right inferior frontal gyrus, right superior frontal gyrus, left anterior cingulate gyrus, and the right precentral gyrus) in the three main radiological planes in each row (top to bottom: axial, coronal, saggital). R=right hemisphere. L=left hemisphere.
Predictive modelling determined which factors were associated with presence of a repeat expansion. Of the 191 patients for whom phenotypic data were available, 79 did not have complete behavioural data available from the first assessment and so 112 were included in the modelling analysis. The final model contained the following predictors associated with the presence of the repeat expansion in patients with ALS: a family history of FTD, yielding an odds ratio of 102·2 (95% CI 12·3–845·5; p<0·0001); a family history of ALS (defined as at least one first-degree or second-degree relative with ALS) with an odds ratio of 30·8 (95% CI 6·8–138·5; p<0·0001); and abnormal behaviour (behavioural impairment was defined as a t-score of 65 or more in at least two subscales of the Frontal System Behavioural Scale) at first assessment, which generated an odds ratio of 4·9 (95% CI 1·2–19·9; p=0·027).
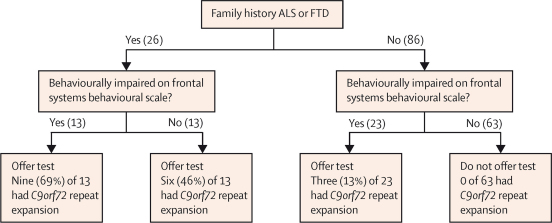
A clinical screening algorithm (figure 3) was developed using the predictive factors that had been generated. The algorithm was then applied to the clinical cohort of patients, for whom data was available, to calculate the sensitivity and specificity of the screening method. If genetic testing was offered to all patients with a family history of ALS or FTD, and also to those patients without a family history but with behavioural impairment, and not to those with no family history of ALS or FTD and no behavioural impairment, the sensitivity of this screening algorithm was 100% (95% CI 0·85–1·00) and the specificity was 67% (95% CI 0·57–0·76). The high sensitivity of the screening algorithm is due to the fact that no patients with normal cognition and behaviour, who had a negative family history, had the repeat expansion (63 patients).
Figure 3.
Screening algorithm
Numbers are numbers of patients from our population-based incident cohort. ALS=amyotrophic lateral sclerosis. FTD=frontotemporal dementia.
Discussion
We have shown that patients with ALS and the C9orf72 hexanucleotide repeat expansion represent a recognisable subphenotype characterised by a lower age of onset, presence of cognitive and behavioural impairment, specific neuroimaging changes, a strong family history of neurodegeneration, and reduced survival. In our cohort, the repeat expansion was not present in patients who had sporadic ALS and no behavioural abnormalities. Our findings show that detailed phenotyping and careful characterisation within a population-based cohort can rapidly generate an algorithm that could potentially be clinically useful in the context of new genetic discoveries (panel).
Panel. Research in Context.
Systematic review
We searched PubMed for articles published between January, 1995, and January, 2012, with the search terms “chromosome 9” AND “FTD”, “chromosome 9” AND “ALS”, “chromosome 9” AND “ALS” AND “phenotype”, “GWAS” AND “ALS”, and “repeat expansion”. We also searched in the reference lists of included papers.
Interpretation
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are known to occur together in some kindreds, and recent work has identified an expanded hexanucleotide repeat in chromosome 9 that segregates with both ALS and FTD.2–5 Our detailed analysis of a population-based incident cohort of patients with ALS has shown that the expanded hexanucleotide repeat in C9orf72 is associated with a subphenotype characterised by a lower age of onset, the presence of cognitive and behavioural impairment, specific neuroimaging changes, a strong family history of neurodegeneration including pure FTD, and reduced survival. The definition of familial ALS will require amendment to include a family history of FTD. Expanded repeats were not present in patients with sporadic ALS, normal cognition, and a negative family history of neurodegeneration, suggesting that genetic testing for the C9orf72 expansion in true sporadic cases of ALS with normal cognition and behaviour is not warranted.
All patients with the repeat expansion who had been genotyped carried a portion of the 20-SNP consensus haplotype reported by Mok and colleagues,16 confirming that the C9orf72 hexanucleotide expansion in the Irish population is probably derived from the same founder as other European populations. Many recent discussions of ALS and FTD have suggested that these disorders form two ends of a disease spectrum.18 However, detailed cognitive assessment of our 191 population-based incident cases1,10 suggests that patients with ALS are clinically heterogeneous, and can be divided into at least two broad categories: patients with pure sporadic ALS (no cognitive or behavioural impairment and no reported family history of ALS or FTD), and patients with a predominance of executive cognitive impairment and behavioural change. We have shown that a high proportion of the latter group carry the repeat expansion. Carriers had a younger age at disease onset, more rapid disease progression, and shorter survival than did non-carriers. This finding has important implications for stratification of patients in clinical trials; it has potential implications for drug efficacy, although our preliminary work has shown no difference in the survival effects of riluzole in patients with the expansion compared with those without (data not shown).
Patients with the repeat expansion also had substantial non-motor cortex changes on high-resolution 3 T structural MRI, and reduced grey-matter volume. These changes correlated with the extensive neuropsychological findings in patients with the repeat expansion, which included increased apathy, increased dysexecutive behaviour, and some evidence of worsened executive function, especially on the Stroop interference task, phonemic verbal fluency, and Brixton spatial anticipation task. Apathy has been consistently linked with the anterior cingulate gyri,19 as have the Stroop interference task and the verbal fluency task.20,21 Behavioural change was also a prominent feature in patients with the repeat expansion. More extensive imaging and autopsy studies will be needed to characterise in detail the structural and neuropathological differences between patients with and without the repeat expansion. Nevertheless, the prominence of cognitive and behavioural impairment in patients with the C9orf72 repeat expansion has implications in the development of clinical-care pathways and also for education and support of carers, because cognitive and behavioural changes affect patients' ability to adhere to symptomatic treatments, including non-invasive ventilation, and can increase carer burden.22
We have shown that a positive family history of ALS or FTD had the highest predictive values for the presence of the repeat expansion. This finding highlights the importance of an accurate and detailed family history. In our cohort, a small proportion of patients originally categorised as sporadic were subsequently shown to have familial ALS on the basis of extensive pedigree interrogation. Identification of FTD in preceding generations is challenging, as clinical recognition of the disorder is relatively recent.23 We sought to resolve this problem by detailed face-to-face semi-structured interviews with at least two family members, coupled with chart review and death certification, and applied stringent criteria for posthumous diagnosis of FTD. These findings have implications for the current operational definition of familial ALS.24 Although no consensus exists, most investigators judge familial ALS to include at least one first-degree or second-degree relative with ALS.25 Our data suggest that a family history of FTD should also be included in the revised definition of familial ALS.
Our detailed family studies suggest that ALS associated with the C9orf72 repeat expansion is probably an autosomal dominant disorder with an expanded phenotype of neurodegeneration and variable penetrance. Patients with a negative family history of neurodegeneration, who have normal cognition and behaviour, are extremely unlikely to harbour the repeat expansion. Validation of the clinical screening algorithm will be necessary in a larger cohort.
Testing for the presence of the repeat expansion in the at-risk group (ie, patients with evidence of cognitive and behavioural impairment or a family history of neurodegenerative disease) outside of a research setting should be undertaken with caution. Diagnostic testing demands a high degree of certainty, and because of the extensive implications for both patients and their family members, stringent quality control is needed. At present, the precise cutoff for a pathogenic expanded number of repeats remains unclear, and variability exists in the maximum repeat number identified in control populations.6,7,26 Moreover, because the maximum size of the pathological expansions cannot be determined using repeat-primed PCR, formal diagnostics will require definitive validation using Southern blotting.
Whether presymptomatic family members should be offered testing is also unclear. Insufficient data are available to predict the probability that an asymptomatic person with the expansion will develop disease, nor can the likely phenotype be predicted. While presymptomatic testing would be valuable in a research setting, underpinned by strict ethical guidelines, a larger body of research will be required to sufficiently address important issues such as the degree of penetrance, the probability of an expansion from one generation to the next, and patterns of inheritance.
Our study is limited by the small size of the cohort with the repeat expansion, although the larger size of the unaffected cohort provides statistical power. Detailed studies of cognitive and family history are time consuming for both the investigator and the patient, and attrition rates are high in longitudinal studies. Nevertheless, our findings provide strong evidence that the C9orf72 repeat expansion is almost exclusively autosomal dominant with variable penetrance, and is associated with a characteristic cognitive and behavioural phenotype and shorter survival. These findings will require verification both in larger cohorts and by detailed neuropsychological, imaging, and autopsy studies.27
Our study is also limited by the use of repeat-primed PCR rather than Southern blotting. We have not presented any data relating to maximum size of the pathological expansion because of the limited ability of reverse prime-PCR to accurately assess size beyond 60 repeats. Further studies correlating the size of the expansion with clinical phenotype are needed. Also, although we have shown evidence of incomplete disease penetrance, generation of an accurate estimate of the penetrance of the pathological variant has not been possible, because we do not have data relating to the C9orf72 status of unaffected family members. Further studies of larger kindreds, supported by genotyping, will be required to calculate the true penetrance of this variant.
Acknowledgments
Acknowledgments
We received funding from the Health Seventh Framework Programme (FP7/2007-2013) under grant agreement number 249867 (EUROMOTOR), the Health Research Board, Research Motor Neuron, and the Irish Motor Neuron Disease Association. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 259867. We thank the NIHR specialist Biomedical Research Centre for Mental Health, South London and Maudsley NHS Foundation Trust (SLaM), London, UK, and the Institute of Psychiatry, King's College, London, UK.
Contributors
SB and AS undertook the genetic analysis. SB gathered family data, and did the statistical analysis. SB and OH wrote the report. ME undertook the neurocognitive incident study and analysed cognitive data. ME, PB, and AA-C contributed to the writing of the report. PB undertook the MRI and MRI analysis. AS advised on methods for assessment of genetics. CW provided statistical input to the project. BC supported the neurocognitive incident study. MH gathered data on family history. NJ, CO'B, and BW gathered neuropsychological data. KK and RLM run the amyotrophic lateral sclerosis (ALS) bank. KK, RLM, and DB advised on aspects of genetics. CL maintains the ALS register. PMI contributed to data collection. JP contributed to a portion of the neurocognitive incident study and gathered some of neuropsychological data. ALB advised on aspects of MRI. NP advised on neuropsychological aspects. AA-C provided laboratory resources and expertise in genetic testing for expansions in C9orf72 and contributed to writing of the manuscript and to the interpretation and analysis of the data. OH is director of the Irish ALS register, designed all aspects of the study, and wrote and edited the manuscript. All authors reviewed the report before submission.
Conflicts of interest
We declare that we have no conflicts of interest.
Web Extra Material
Supplementary webappendix
References
- 1.Phukan J, Elamin M, Bede P. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2011;83:102–108. doi: 10.1136/jnnp-2011-300188. [DOI] [PubMed] [Google Scholar]
- 2.Morita M, Al-Chalabi A, Andersen PM. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- 3.Van Es MA, Veldink JH, Saris CG. Genome wide association study identifies 19p 13.3 (UNC13A) and 9p 21.2 as susceptibility loci for amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 4.Shatunov A, Mok K, Newhouse S. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;10:986–994. doi: 10.1016/S1474-4422(10)70197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laaksovirta H, Peuralinna T, Schymick JC. Chromosome (p21 in amyotrophic lateral sclerosis in Finland: a genome wide association study. Lancet Neurol. 2010;10:978–985. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, Mackenzie IR, Boeve BF. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–254. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renton AE, Majounie E, Waite A. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p-21 linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Toole O, Traynor BJ, Brennan P. Epidemiology and clinical features of amyotrophic lateral sclerosis in Ireland between 1995 and 2004. J Neurol Neurosurg Psychiatry. 2008;79:30–33. doi: 10.1136/jnnp.2007.117788. [DOI] [PubMed] [Google Scholar]
- 9.Traynor BJ, Codd MB, Corr B. Incidence and prevalence of ALS in Ireland, 1995–1997: a population-based study. Neurology. 1999;52:504–509. doi: 10.1212/wnl.52.3.504. [DOI] [PubMed] [Google Scholar]
- 10.Elamin M, Phukan J, Bede P. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology. 2011;76:1263–1269. doi: 10.1212/WNL.0b013e318214359f. [DOI] [PubMed] [Google Scholar]
- 11.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole J, Conneally M, Hodes ME. Genetic Family History Questionnaire. J Med Genet. 1978;1:10–18. doi: 10.1136/jmg.15.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 14.Hope ACA. A simplified Monte Carlo significance test procedure. J Roy Statist Soc. 1968;B30:582–598. [Google Scholar]
- 15.Cedarbaum JM, Stambler N, Malta E. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;31:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 16.Mok K, Traynor BJ, Schymick J. The chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol Aging. 2012;33:209. doi: 10.1016/j.neurobiolaging.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment. 1999;6:269–284. doi: 10.1177/107319119900600307. [DOI] [PubMed] [Google Scholar]
- 18.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;71:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 19.Royall DR, Lauterbach EC, Cummings JL. Executive control function: a review of its promise and challenges for clinical research. J Neurol Neurosurg Psychiatry. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams S, Goldstein LH, Simmons M. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507–1517. doi: 10.1093/brain/awh170. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann L, Koppelstaetter F, Delazer M. Neural correlates of distance and congruity effects in a numerical Stroop task: An event-related fMRI study. Neuroimage. 2005;15:888–898. doi: 10.1016/j.neuroimage.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Chio A, Vignola A, Mastro E. Neurobehavioural symptoms in ALS are negatively related to caregivers' burden and quality of life. Eur J Neurol. 2010;10:1298–1303. doi: 10.1111/j.1468-1331.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 23.Neary D, Snowdon JS, Gustafson L. Frontemporal lobar degeneration. A consensus clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 24.Byrne S, Bede P, Elamin M. Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:157–159. doi: 10.3109/17482968.2010.545420. [DOI] [PubMed] [Google Scholar]
- 25.Byrne S, Elamin M, Bede P. Absence of consensus in diagnostic criteria for familial neurodegenerative diseases. J Neurol Neurosurg Psychiatry. 2012 doi: 10.1136/jnnp-2011-301530. (in press). [DOI] [PubMed] [Google Scholar]
- 26.Gijselinck I, Van Langenhove T, van der Zee J. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol. 2012;11:54–65. doi: 10.1016/S1474-4422(11)70261-7. [DOI] [PubMed] [Google Scholar]
- 27.Cooper-Knock J, Hewitt C, Robin Highley J. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012 doi: 10.1093/brain/awr365. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary webappendix