HER-2 Pulsed Dendritic Cell Vaccine Can Eliminate HER-2 Expression and Impact DCIS (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 1.
Published in final edited form as: Cancer. 2012 Jan 17;118(17):4354–4362. doi: 10.1002/cncr.26734
Abstract
Background
HER-2/neu over-expression plays a critical role in breast cancer development and its expression in ductal carcinoma in situ (DCIS) is associated with development of invasive breast cancer. A vaccine targeting HER-2/neu expression in DCIS may initiate immunity against invasive cancer.
Methods
A HER-2/neu dendritic cell (DC) vaccine was administered to 27 patients with HER-2/neu over-expressing DCIS. The HER-2/neu vaccine was administered prior to surgical resection and pre- and post-vaccination analysis was conducted to assess clinical results.
Results
At surgery, 5 of 27 (18.5%) vaccinated subjects had no evidence of remaining disease, while among 22 subjects with residual DCIS, HER-2/neu expression was eradicated in 11 (50%). When comparing ERneg with ERpos DCIS lesions, vaccination was more effective in hormone-independent DCIS. Following vaccination, no residual DCIS was found in 40% of ERneg subjects compared to 5.9% in ERpos subject. Sustained HER-2/neu expression was found in 10% of ERneg subjects compared to 47.1% in ERpos subjects (p=0.04). Post-vaccination phenotypes were significantly different between ERpos and ERneg subjects (p=0.01), with 7 of 16 (43.8%) initially presenting with ERpos HER-2/neupos Luminal B phenotype finishing with the ERpos HER-2/neuneg Luminal A phenotype, and 3 of 6 (50%) with the ERneg HER-2/neupos phenotype changing to the ERneg HER-2/neuneg phenotype.
Conclusions
Results suggest vaccination against HER-2/neu is safe, well-tolerated and induces decline and or eradication of HER-2/neu expression. These findings warrant further exploration of HER-2/neu vaccination in estrogen-independent breast cancer and highlight the need to target additional tumor associated antigens and pathways.
Keywords: DCIS, Breast Cancer, Cancer Vaccine, DC Vaccine, HER-2/neu
BACKGROUND
Cancer vaccines have had limited success largely because previous cancer vaccine trials have focused on later-stages of disease when tumor burden is at its maximum and when even standard therapies, at times, have minimal benefit (1,2). Analyzing the history of vaccination strategies clearly demonstrates the prophylactic use of vaccination prior to the progression of disease. Thus, if cancer vaccines were to target early stages of disease, vaccination may prove to be more effective. Furthermore, most experimental cancer vaccines have targeted tumor-associated antigens that are not critical to tumor survival and disease progression therefore tumor cells can readily escape immunity and persist or metastasize. Finally, most cancer vaccines have not been utilized to target cancer stem cells or molecular pathways involved in specific cancer genotypes and phenotypes. A vaccination strategy that provides an integrated solution to each of these obstacles is therefore likely to meet with increased success.
The use of anti-estrogen therapy in both and primary and secondary prevention has resulted in significant benefits to patients developing estrogen dependent breast cancers (3, 4, 5). However, prevention for estrogen-independent breast cancer remains a significant issue (5). When considering breast cancer in particular, novel treatment or prevention strategies must take into account that breast cancer develops from several different progenitors, each leading to distinct tumor phenotypes which may vary characteristically in the ultimate course of disease and susceptibility to various therapies. These phenotypes include the Luminal A and B subtypes, HER-2, and basal phenotypes (6, 7). Although overlaps clearly do exist, each of these subtypes generally possesses a set of distinguishing features that allows for their convenient identification. The luminal subtypes express estrogen receptors while the basal and HER-2 and luminal HER-2 phenotypes often over-express, HER family members including EGFR (HER-1) and HER-2/neu (6,8,9). While anti-estrogen therapy can be utilized for primary and secondary prevention of the luminal tumors, there are currently no such therapies available for prevention of the high grade HER-2 luminal, HER-2 and basal tumors. Thus, targeting the HER family might be an ideal target for a breast cancer prevention.
With respect to breast cancer and potential intervention with a cancer vaccine, DCIS (ductal carcinoma in situ) is an ideal stage during which to test cancer vaccination and potentially halt disease progression. DCIS is an early pre-invasive malignancy of the breast and represents an intermediary between normal breast tissue and invasive breast cancer, thus, implementation of a breast cancer vaccine, in the early stages of disease, such as DCIS, would allow for a setting in which the tumor burden is quite low. Furthermore, the impact of treatment in a neoadjuvant setting can be rapidly assessed on tumors scheduled for timely surgical resection, rather than by waiting for disease development or progression in asymptomatic patients (10). Such an approach not only speeds development of novel therapies, but also offers a window into the biology of breast cancer development.
DCs were activated with cytokines and a clinical-grade bacterial LPS (a TLR4 ligand) to induce a unique battery of chemokines and cytokines, including IL-12, which can “condition” T cells for superior anti-tumor immunity and memory (11–13). The DCs are pulsed with synthetic peptides based on the HER-2/neu sequence that are capable of sensitizing Th in most individuals (14, 15). HER-2/neu was selected as target since its over-expression in breast cancer is associated with enhanced invasiveness (16), metastatic potential, greater probability of local and systemic recurrence, and resistance to chemotherapy (17–20). It is also a defining phenotypic feature of some breast cancer stem cells associated with the capacity for self-renewal (21) and critically expressed in at least one non-ER dependent breast cancer phenotype (22). Vaccines were administered prior to surgical therapy so that the effects of immunization could be assessed on residual tumor at the time of surgical resection. We have previously reported some of the early results from this study (14). This manuscript represents the final analysis of trial. The primary objectives were safety and feasibility and the secondary objectives were immune response and clinical response to vaccination including the assessment of HER-2 expression in response to vaccination. We report the final analysis of the trial, exclusive of the immune response which is reported elsewhere (23). The trial suggests that vaccination in this early disease setting is safe, effective in inducing long term stable immune responses (23) and eliminating HER-2/neu-expressing tumor cells in residual DCIS lesions, and furthermore hints at possible new therapy options for patients with phenotypes underserved by currently-available therapies.
METHODS
Clinical trial design
We conducted a pilot trial of a HER-2/neu-based DC vaccination strategy for patients with HER-2/neu over-expressing DCIS (NCT001070211). The primary objectives were to evaluate the feasibility, safety and efficacy of HER-2/neu vaccination. The secondary objectives were to assess immune response to vaccination and response to vaccine including assessment of expression of HER-2 in remaining DCIS. HER-2/neu peptide loaded DC were administered intra-nodally once per week for 4 weeks and followed by surgical resection. A maximum of 30 patients were to be enrolled in order to establish safety and have adequate power to evaluate pre- versus post-vaccination T cell sensitization status by McNemar’s test. If no occurrence of unacceptable toxicity was observed in the 30 patients, then the rate of unacceptable toxicity was no higher than 7% based on the upper-bound of the one-sided exact 90% confidence interval.
Feasibility, defined as the rate of patients who received all 4 immunizations on schedule. Toxicities were graded using the NCI Common Toxicity Grading Scale Version 3.0. Immunogenicity was based on tetramer, ELISPOT, and in vitro sensitization assays. CD4pos and CD8pos sensitization of T cells were assessed in pre-and post-vaccination peripheral blood samples and in sentinel lymph nodes where available. HER-2/neu protein expression was evaluated in pre- and post-vaccination tissue specimens by a dedicated pathologist that oversees Immunohistochemistry Lab.
Eligible patients were greater than 18 years old, signed Informed Consent, had ECOG performance status 0 or 1, biopsy-proven DCIS who had not yet received definitive treatment. Patients whose DCIS was eliminated by excisional biopsy at diagnosis were not eligible. HER-2/neu positivity was determined as >5% cells expressing 2+ or 3+ intensity of the HER-2/neu protein. These criteria were used since the majority of DCIS studies considered 2+ HER-2/neu expression as positive and since there were no other current pathologic standards for HER-2 expression in DCIS when this trial was conceived, 2+ staining was considered HER-2/neu positive. These were all secondarily reviewed by our Pathologist PJZ for eligibility. Response to vaccination was defined as any decline in HER-2/neu protein expression equal to or greater than 20% on post-vaccination immunohistochemical staining. These were secondarily reviewed blinded for vaccine by our pathologist PJZ. Patients with areas suspicious of invasive disease were evaluated by MRI. All subjects underwent cardiac evaluation with MUGA scan or echocardiography to document adequate baseline cardiac function. Scans were performed prior to immunization and within 2 weeks of the final vaccine treatment. All patients underwent HLA Class I tissue typing pre-enrollment and had routine history, physical exams, EKG, blood work, and urinalysis prior to immunization. After informed consent, all subjects underwent pre-vaccination leukapheresis to obtain sufficient monocytes for vaccine preparation; in a few cases a second apheresis was required for additional monocytes. Post-vaccination leukapheresis was performed usually within two weeks of the final immunization. All patients underwent post-vaccination mammogram, MRI and surgical resection of DCIS with either lumpectomy or mastectomy. Patients were followed for 30 days following vaccination in order to assess safety and to undergo second leukapheresis. Long term immunologic surveillance (i.e., serial blood sampling) was not mandatory and was conducted on a voluntary basis only.
Materials and Reagents
HER-2/neu peptides were purchased from American Peptide Corporation (Sunnyvale, CA), Monocyte-Macrophage Medium (SFM) and Iscove’s Medium from Invitrogen (Carlsbad, CA), Lymphocyte separation Medium (LSM) from ICN Biomedical Inc. (Aurora, OH), Human AB serum and fetal calf serum from Sigma Chemical (St. Louis, MO) and GM-CSF from Amgen (Newbury Park, CA). Reagents for ELISA assays were obtained from Pharmingen, San Jose, CA, and Clinical grade IFN-γ from Intermune (Brisbane, CA). Clinical grade LPS was a generous gift from Dr. Anthony Suffredini at the NIH.
Immunization Procedure
Vaccine treatments were administered in the NIH-designated General Clinical Research Center at the Hospital of the University of Pennsylvania. They consisted of 10–20 million HER-2/neu-pulsed DCs suspended in 1ml sterile saline. DC were administered under ultrasound guidance into a single lymph node in each groin as previously described (23, 24). Half of each DC vaccine dose was placed into each node with a 22g needle. The first 9 subjects were observed for 2 hours post-immunization with routine vital signs obtained at 15-minute intervals. Subsequent subjects were observed for one hour. Immunizations were administered once weekly for four weeks. All subjects completed all 4 scheduled vaccine treatments.
Preparation of HER-2/neu Vaccine
IDCs were produced as described previously (12,14,23). Briefly, monocytic DC precursors were obtained from subjects via tandem leukapheresis/countercurrent centrifugal elutriation. Cells were cultured overnight at 37°C in SFM with GM-CSF and IL-4. The next day, DCs were pulsed with 6 HER-2/neu MHC class II promiscuous-binding peptides (42–56, 98–114, 328–345, 776–790, 927–941, 1166–1180) (25,26). After 8–12 hours incubation, IFN-γ (1000 U/ml) was added, and the next day, 6h prior to harvest, NIH reference standard LPS was added (10 ng/ml) to achieve full DC activation. For HLA-A2.1pos subjects, cells were also pulsed with MHC class I binding peptides 369–377 and 689–697 (2526–2728). Harvested cells were washed and lot release criteria of >70% viability, negative Gram’s stain and endotoxin <5 EU/Kg confirmed.
Immunohistochemical staining of DCIS lesions
Formalin-fixed, paraffin-embedded tissue blocks were sectioned and stained via HERcepTest (Dako, Carpinteria, CA), as well as with anti-CD4, anti-CD8 T lymphocyte, anti-CD20 (B cell), anti-CD56 (NK cell) anti-Fox-P3 (Treg) markers as described previously (14).
Statistical Methods
Event rates and exact 95% confidence intervals (CI) were calculated. Percent change in HER-2/neu expressing cells pre- and post-vaccine treatment was defined as {(percentage of HER-2/neu expressing cells post-treatment minus the percentage pre-treatment) divided by the percentage pre-treatment} multiplied by 100. Post-vaccine HER-2/neu expression was defined as negative if the percentage of cells staining HER-2/neu 2+ to 3+ was less than 5%. Clinical response to immunization was defined as either no residual DCIS at surgery or greater than 20% decrease in the percentage of HER-2/neu expressing cells following vaccination. The percentage of HER-2/neu expressing cells pre- and post-immunization was compared within patients by nonparametric Wilcoxon signed ranks test. The percent change in HER-2/neu expressing cells was compared between the study population (i.e., pre-versus post-vaccine change) and concurrent untreated controls (i.e., diagnosis versus surgical specimen) by nonparametric Wilcoxon rank sum test. Association between the pre-vaccine percentage of HER-2/neu expressing cells (a continuous predictor) and likelihood of response to immunization was evaluated by logistic regression analysis. Associations between pre-vaccine phenotype (e.g., ERposHER2pos or ERnegHER2pos) and both the post-vaccine response status (e.g., no residual DCIS at surgery, responder, non-responder) and phenotype (e.g., Luminal A, Luminal B, HER-2/neu-driven, Basal) were tested by Fisher’s exact test. All significance values were two-sided. Statistical analyses were performed either with SPSS 15.0 software (SPSS Inc., Chicago, IL) or StatXact software (Cytel Inc., Cambridge, MA).
RESULTS
Patient Characteristics
Between September 2003 and May 2008, 38 eligible patients were identified, of which 29 patients gave written informed consent and enrolled on the IRB-approved trial. Of the 29 patients who initially enrolled, two were deemed ineligible on re-review of HER-2 staining after consent and a third underwent leukapheresis and insufficient cells were obtained and the patient was withdrawn (Figure 1). The mean age was 53 (range 38 to 87 years). A listing of the patient and tumor characteristics is shown in Table 1. Feasibility was 100% since all patients that initiated immunization completed the 4 weekly vaccine treatments. Eighty five percent of patients demonstrated evidence of anti-HER-2/neu CD4 and CD8 T cell responses and are reported elsewhere (14,23). Seventeen Patients were ERpos HER-2pos and 10 patients were ERneg HER-2pos.
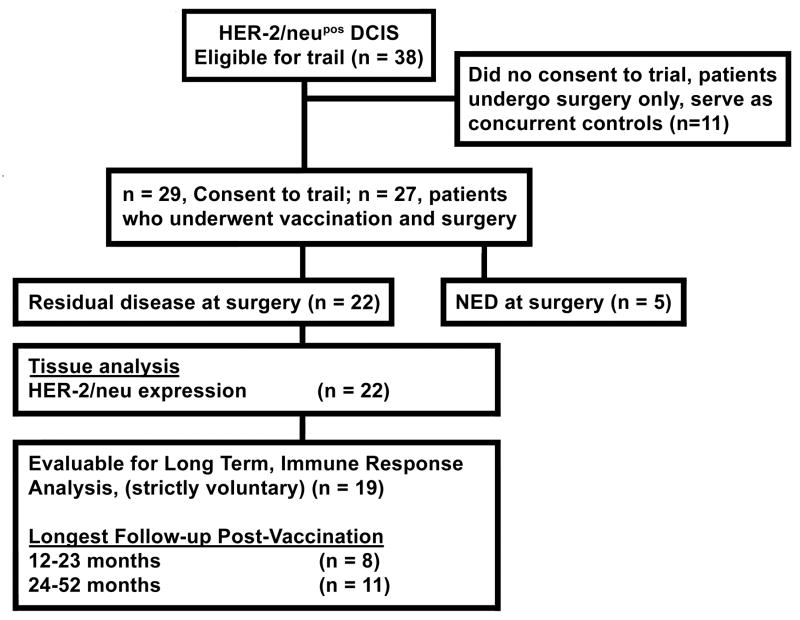
FIGURE 1.
A flow diagram outlining the patient population participating in the clinical trial. Of the 38 patients eligible for the trial, 27 patients gave written informed consent and enrolled on the IRB-approved trial. Five patients had no residual disease following vaccination while 22 patients had residual disease thus allowing for analysis of post-vaccine immune response.
TABLE 1.
Tabulated data summary displaying characteristics of ALL (n=29) eligible patients as well as tumor characteristics.
| Subject # | Age | HLA | DCIS extent* | Biopsy# | Surgery+ | Pre-Vaccination, % cells staining 2+ to 3+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ER | PR | HER-2/neu | |||||||||
| 01 | 44 | A2 | 3 x 3 x 3 | S | MAST | − | 0 | − | 0 | + | 30 |
| 02 | 64 | A2 | 22 x 12 x 20 | C | LUMP | + | 90 | + | 70 | + | 65 |
| 03 | 48 | A2 | 19 x 50 x 44 | S | MAST | − | 0 | − | 0 | + | 60 |
| 04 | 67 | A2 | none | C | MAST | − | 0 | − | 0 | + | 30 |
| 05 | 45 | 15 x 20 x 13 | C | LUMP | + | 90 | + | 90 | + | 20 | |
| 06 | 45 | A2 | 5 x 5 x 5 | C | LUMP | − | 0 | − | 0 | + | 100 |
| 08 | 55 | 22 x 4 x 6 | C | LUMP | + | 5 | − | 0 | + | 100 | |
| 09 | 53 | A2 | 28 x 45 x 35 | C | MAST | − | 0 | − | 0 | + | 100 |
| 10 | 48 | A2 | none (palpable) | S | LUMP | + | 10 | + | 10 | + | 100 |
| 11 | 51 | A2 | 1 x 1 x 2 | C | LUMP | + | 90 | + | 40 | + | 50 |
| 12 | 58 | A2 | 39 x 38 x 18 | C | LUMP | − | 0 | − | 0 | + | 10 |
| 13 | 43 | 34 x 75 x 68 | C | MAST | + | 80 | + | 35 | + | 100 | |
| 14 | 38 | A2 | none | C, S | MAST | + | 50 | − | 0 | + | 50 |
| 15 | 59 | A2 | No significant calcs | S | MAST | − | 0 | − | 0 | + | 10 |
| 16 | 69 | A2 | none | C | MAST | − | 0 | − | 0 | + | 90 |
| 18 | 49 | 2.5 x 1.7 x 1.1 | C | MAST | + | 30 | − | 0 | + | 100 | |
| 19 | 87 | 18 x 18 x 14 | C | MAST | + | 90 | + | 30 | + | 20 | |
| 20 | 44 | A2 | 12 mm cluster | S | LUMP | + | 95 | + | 10 | + | 10 |
| 21 | 57 | 24 x 20 x 33 | C | LUMP | + | 10 | − | 0 | + | 90 | |
| 22 | 43 | No study | C | MAST | − | 0 | − | 0 | + | 10 | |
| 23 | 57 | No study | C | MAST | + | 90 | + | 5 | + | 80 | |
| 24 | 64 | 17 x 12 x18 | C | LUMP | + | 30 | − | 0 | + | 30 | |
| 25 | 64 | 47 x 32 x 22 | C | MAST | − | 0 | − | 0 | + | 30 | |
| 26 | 40 | small cluster of calcs | C | MAST | + | 90 | + | 85 | + | 25 | |
| 27 | 57 | ap 14.9, si 19.8, ml 24.0 | C | MAST | + | 95 | + | 90 | + | 60 | |
| 28 | 47 | A2 | ml 3.4, si 5.8, ap 11.7 | C | LUMP | + | 40 | + | 10 | + | 90 |
| 29 | 45 | scattered benign calcs | C | LUMP | + | 100 | + | 25 | + | 80 |
HER-2/neu Vaccine Safety and Toxicity
HER-2- neu vaccination treatments were well-tolerated with only grade 1 and 2 toxicities observed. The most common toxicities encountered include general malaise (72.4 %, 21/29), injection site soreness (58.6%, 17/29), chills/rigors (37.9%, 11/29), fever (27.5%, 8/29), and headaches (24%, 7/29). Three patients demonstrated an asymptomatic depression in cardiac ejection with 15–18% declines of grades 1 and 2 (29). None of the ejection fractions were below 50%. In one of the patients, a repeat MUGA scan returned to baseline within 30 days. Since no cases of unacceptable toxicity were observed in the final 27 patients that were evaluated in the trial, the upper bound of the one-sided exact 90% confidence interval for the toxicity rate is 8.2%, indicating that the vaccine is safe with a population toxicity rate no greater than 8%.
Elimination of HER-2/neu-expression following HER-2/neu vaccination
HER-2/neu expression between initial biopsy and surgical resection specimens is relatively constant (14,30). For 5 of 27 (18.5%, exact 95% CI 6.3 – 38.1%) immunized subjects, no residual DCIS could be identified at the time of surgical resection and were considered complete responses. In the remaining 22 patients, the median percent change in HER-2/neu expression post-DC vaccine was minus 88% (i.e., a decline of 88%) with a range of −100% to 700%. This pre- to post-vaccine difference was statistically significant (Wilcoxon signed ranks test, p=0.023). Furthermore, 13 of 22 (59.1%, exact 95% CI 36.3 – 79.2%) subjects demonstrated greater than 20% decline in HER-2/neu expression following vaccination and 11 (50%, exact 95% CI 28.2 – 71.8%) subjects actually achieved complete loss of detectable HER-2/neu expression as determined by HercepTest (Table 2). By logistic regression analysis we also found that subjects with higher pre-vaccine HER-2/neu expression appeared less likely to respond to immunization (estimated odds ratio 0.97, exact 95% CI 0.94 – 1.00, p=0.05), with a 3% lower risk of response for each percent increase in HER-2/neu expression pre-vaccine treatment (14,23,26). Eleven DCIS patients who were contemporaneously screened with those in the trial, but declined to participate, served as untreated controls. Percentages of HER-2/neu-expressing cells in diagnosis and surgical resection specimens ranged from 20 to 100%. Percent change in HER-2/neu expression between initial biopsy and surgical specimens was −10%, −10%, 0%, 0%, 0%, 0%, +21%, + 25%, +29%, +29%, +88%. The percent change in HER-2/neu expression was statistically significantly different between the 11 untreated controls (median 0%, range −10% to 88%) and 22 treated patients who had their tumor evaluable following vaccination (median −88%, range −100% to 700%, Wilcoxon rank sum test p=0.005, Table 2A, B). Therefore, these data suggest that HER-2/neu vaccination treatment results either in the destruction of HER-2/neu-expressing cells or suppression of their capacity to express HER-2/neu.
TABLE 2.
A and B. Tabulated data summary displaying percentage of HER-2/neu over-expressing cells enumerated before and after vaccination with percent change in expression noted. 2A depicts change in HER-2/neu expression pre and post vaccination among trial participants. 2B depicts HER-2/neu expression status among the control population.
| TABLE 2A - Vaccinated DCIS Study Patients, N = 27 | ||
|---|---|---|
| % Pre-vaccine | % Post-vaccine | Percent Change |
| 10 | NED | – |
| 30 | NED | – |
| 30 | NED | – |
| 90 | NED | – |
| 90 | NED | – |
| 10 | 0 | −100%* |
| 10 | 0 | −100%* |
| 20 | 0 | −100%* |
| 20 | 0 | −100%* |
| 25 | 0 | −100%* |
| 30 | 0 | −100%* |
| 30 | 0 | −100%* |
| 60 | 0 | −100%* |
| 65 | 0 | −100%* |
| 80 | 0 | −100%* |
| 50 | 2 | −96%* |
| 100 | 20 | −80% |
| 100 | 60 | −40% |
| 100 | 90 | −10% |
| 80 | 80 | 0% |
| 90 | 90 | 0% |
| 100 | >90 | 0% |
| 100 | 100 | 0% |
| 100 | 100 | 0% |
| 60 | 70 | +17% |
| 50 | 95 | +90% |
| 10 | 80 | +700% |
| TABLE 2B – DCIS Controls, N = 11 | ||
|---|---|---|
| % Diagnosis Specimen | % Surgical Specimen | Percent Change |
| 100 | 90 | −10% |
| 100 | 90 | −10% |
| 90 | 90 | 0% |
| 90 | 90 | 0% |
| 100 | 100 | 0% |
| 100 | 100 | 0% |
| 70 | 85 | +21% |
| 20 | 25 | +25% |
| 70 | 90 | +29% |
| 70 | 90 | +29% |
| 40 | 75 | +88% |
HER-2/neu vaccination is more effective in hormone-independent DCIS
Ten of the 27 patients were of the hormone-independent ERneg HER-2/neupos phenotype, and of these, 9 (90%) demonstrated clinical response to HER-2/neu vaccination. In fact, 4 of these patients (40%) had no detectable residual disease at the time of surgery, while 5 others demonstrated the requisite declines in HER-2/neu expression following HER-2/neu vaccination. Of the seventeen ERpos subjects, only 5.9% had no detectable disease at surgery. However 47.1% of those ERpos subjects had a decline in HER-2/neu expression following vaccination. The difference in response rates between subjects with ERneg versus ERpos tumors was statistically significant (Fisher’s exact test p=0.04, Table 3). Even patients who had significant HER-2/neu expression on 100% of tumor cells prior to vaccination with the ER negative phenotype demonstrated a response following vaccination with significant declines in HER-2/neu expression. In contrast, in the same subgroup within ERpos subjects, comparable declines were not observed. Interestingly, the fraction of subjects who did not demonstrate a decline in HER-2/neu expression following vaccination was greater among ERpos subjects compared to ERneg subjects, 47.1% vs 10%. Thus it appears that tumors with hormone-independent phenotypes are more susceptible to this immunization regimen than their ERpos counterparts.
TABLE 3.
Tabulated data summary displaying vaccine response based on pre-vaccination and post-vaccination hormone receptor status. Patients who were ER negative and HER-2/neu+ had a greater response to vaccination than those who were ER positive and HER-2/neu+.
| Post-vaccination Response Status by Pre-vaccination ER and HER2/neu Phenotype | |||||
|---|---|---|---|---|---|
| Pre-vaccine Phenotype | N | No Tumor at Surgery | HER2/neu Responder* | HER2/neu Non-responder | Fisher’s Exact P |
| ERpos HER2pos | 17 | 1 (5.9%) | 8 (47.1%) | 8 (47.1%) | 0.04 |
| ERneg HER2pos | 10 | 4 (40.0%) | 5 (50.0%) | 1 (10.0%) |
HER-2/Neu vaccination alters the ultimate phenotype of DCIS lesions
The selection of DCIS to test the effectiveness of DC-pulsed HER-2neu vaccination offered the opportunity to rapidly assess effects on tumors in an early disease setting. In this regard, HER-2/neu vaccination appeared to alter tumor phenotype, causing ERpos HER-2/neupos DCIS to become ERpos HER-2neg in 43.8% of patients, and ERneg HER-2/neupos DCIS to become triple-negative, (6.3%), as shown in Table 4. A depiction of the post-vaccination HER-2/neu and the hormone receptor staining is demonstrated in Figure 2. In total, 4/22 (18.2%) of the patients with residual DCIS became triple-negative; an unusually high proportion because this phenotype tends to account for only 3–5% of DCIS (31). It can be reasonably concluded that the observed losses of HER-2 expression in about half of the patients are a result of vaccination since similar alterations were not observed in non-immunized controls. These clinical alterations in tumor protein expression suggest that the vaccination treatment rapidly induced an immune response that may have ultimately impacted the overall phenotype of the tumor.
TABLE 4.
Data summary comparing pre-vaccine phenotype to post-vaccine phenotype of DCIS lesions. Of the patients who initially started with the ER positive, HER-2/neu positive phenotype, 43.8% of those patients were converted to the ER positive, HER-2/neu negative phenotype due to the loss of HER-2/neu following vaccination. Interestingly, 50% of the patients with an initial HER-2/neu positive, ER negative phenotype were transformed into the triple negative phenotype by the loss of HER-2/neu antigen following vaccination.
| Post-vaccination ER and HER2/neu Phenotype by Pre-vaccination ER and HER2/neu Phenotype | ||||||
|---|---|---|---|---|---|---|
| Pre-vaccine Phenotype | N | Post-vaccination Phenotype | ||||
| ERposHER2neg | ERposHER2pos | ERnegHER2pos | ERnegHER2neg | Fisher’s Exact P | ||
| ERpos HER2pos | 16 | 7 (43.8%) | 5 (31.3%)* | 3 (18.8%) | 1 (6.3%) | 0.01 |
| ERneg HER2pos | 6 | 0 (0.0%) | 0 (0.0%) | 3 (50.0%)* | 3 (50.0%) |
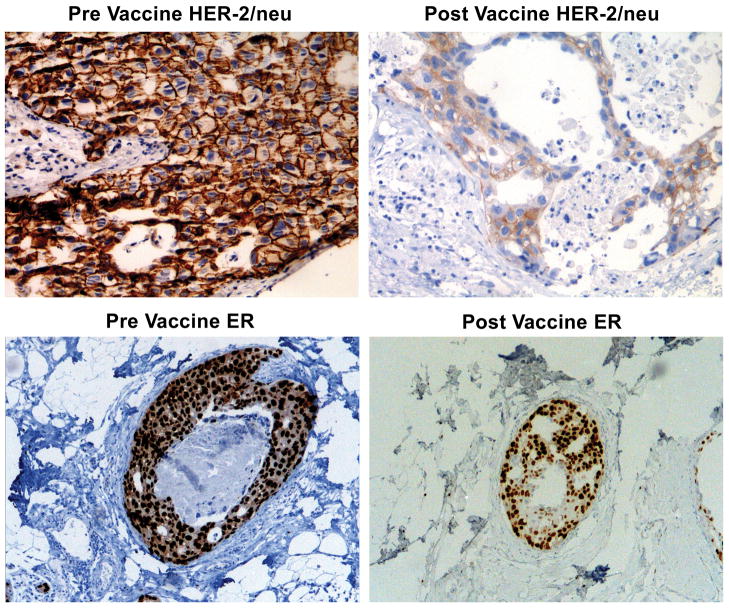
FIGURE 2.
Vaccination induces decline in HER-2/neu expression. Photographic depiction of slides from representative subject stained for HER-2/neu (HercepTest) and estrogen receptor (ER). Note dramatic reduction in HER-2/neu staining intensity in post-vaccination tissue sample and the decline in estrogen receptor staining following vaccination.
Discussion
In this trial, we activated peptide-pulsed DCs using a special clinical grade bacterial LPS that induced a unique set of soluble factors including high levels of IL-12 and Th1 chemokines not elicited through traditional DC vaccine production methods (12,14). Th1 responses are associated with good clinical outcomes in breast cancer (32). These DC’s were then pulsed with HER-2neu peptides and administered to patients with DCIS in the neoadjuvant setting in an effort to attack the early stage of disease. This clinical DC HER-2/neu vaccine was successful in inducing strong long-lasting immune responses (23) and either reducing or eliminating HER-2/neu expression in most vaccinated subjects. These results suggest that this strategy holds considerable promise, and could be further explored for treating early breast cancer, preventing recurrence, or for the outright prevention of primary disease in high-risk populations.
The fact that vaccination resulted in significantly more complete responses in ER independent compared to ER dependent tumors is interesting and continues to persist in our current study (unpublished observation). This may be biologically relevant as ERpos HER-2pos IBC seem to be less sensitive than ERneg HER-2pos tumors with fewer complete responses noted in patients treated with immune based trastuzumab and chemotherapy or chemotherapy alone (33, 34). We observed no difference in immune response development between ERpos HER-2pos compared with ERneg HER-2pos subjects (not shown) and it has been suggested ER signaling can result in other HER family members being activated (35), this may account for these biologic differences. Identifying response differences in ERpos HER-2pos DCIS to immune based therapies may shed light on these differences in response in IBC as well.
This DC based HER-2 vaccine therapy may prove a good adjunct for preventing both local regional or distant recurrence, both of which are more frequent for the HER-2 and basal phenotypes (36, 37). Notwithstanding, the emergence of HER-2/neuneg phenotypes after vaccination serves as a warning that a single target may not be sufficient to completely eliminate or prevent disease in all individuals. Since about 18% of the participants became triple negative after being originally HER-2pos this data provides evidence that at least some triple negative breast cancers can be prevented through targeting and eliminating HER-2pos clones prior to breast cancer development. It also suggests that future experimental vaccine formulations must take other phenotypes (such as estrogen-dependent and triple-negatives) into account to block potential escape variants. Refinements of this approach will therefore include pairing vaccine therapy with anti-estrogen therapy such as Tamoxifen to address hormone-dependent proliferating tumors, the inclusion of additional vaccine target antigens such as HER-1 (EGFR), HER-3 and survivin to block the emergence of escape variants with a triple-negative phenotype as well as immune modulation of the primary site.
Clearly, new primary and secondary prevention strategies, especially for estrogen-independent breast cancers, are needed to reduce risk of recurrence and eventual deaths caused by these aggressive breast cancer phenotypes. As recently suggested, younger patients with triple negative breast cancers may derive benefits in survival from contralateral prophylactic mastectomy (38). However, less radical alternative therapies would be preferable. Indeed, immune-based approaches targeting EGFR (HER-1), HER-2/neu, and HER-3 are of potential value because of the essential role these molecules play in the biology of both estrogen-dependent and estrogen-independent breast cancer phenotypes (39). Indeed targeted immune therapies are currently becoming mainstay for treatment of HER-2/neu expressing invasive breast cancer as the engineered monoclonal antibody, trastuzumab, (directed at HER-2) has demonstrated dramatic effects in diminishing distant recurrence and improving survival (40,41). Targeting the HER-2/neu pathway in early breast cancer and DCIS using DC1 pulsed with HER-2/neu peptides clearly impacted these lesions especially those with estrogen independent HER-2/neupos DCIS. It is conceivable that these vaccines may reduce secondary recurrence and could be developed for primary prevention as well. There combination with trastuzumab in patients with invasive breast cancer may also be worth pursuing as a few of the patients in this trial were found to have some invasive disease large enough to warrant chemotherapy and trastuzumab and completed this additional therapy without after vaccination without an increased morbidity. We are currently investigating DC1 vaccine therapy for HER-2/neupos IBC.
Acknowledgments
Supported by NIH R01 CA096997, the Harrington Foundation, Pennies-in-action.org, and the Mistler Foundation
The authors wish to acknowledge the assistance of Jeanne Schueller, and Vickie Sallee in the conduct of this trial. We acknowledge the support of the staff of the General Clinical Research Center and the Leukapheresis Unit at the Hospital of the University of Pennsylvania and the graphic arts support of Robin Noel.
References
- 1.Koski G, Cohen, Roses R. Reengineering dendritic cell based anti-cancer vaccines. Immunol Rev. 2008;222:256–67. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S, Yang J, Restifo N. Cancer Immunotherapy: Moving beyond current vaccines. Nature Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Hackshaw A, Roughton M, Forsyth S, et al. Long-Term Benefits of 5 Years of Tamoxifen: 10-Year Follow-Up of a Large Randomized Trial in Women at Least 50 Years of Age With Early Breast Cancer. Journal of Clinical Oncology. 2011;29:1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- 6.Perou C, Sorlie T, Eisen M, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie T, Perou C, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielson T, Hsu F, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Research. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 9.Cheang M, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Research. 2008;14:1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, DeVries S, Anderson J, et al. Pathologic and biologic response to preoperative endocrine therapy in patients with ER-positive ductal carcinoma in situ. BMC Cancer. 2009;18:285–294. doi: 10.1186/1471-2407-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu S, Koldovsky U, Xu M, et al. High avidity antitumor T cell generation by toll receptor 8-primed, myeloid-derived dendritic cells is mediated by IL-12 production. Surgery. 2006;140:170–8. doi: 10.1016/j.surg.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Xu S, Koski G, Faries M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12 dependent mechanism. J Immunol. 2003;171:2251–61. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt C, Mescher M. Peptide antigen priming of naïve, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;68:5521–29. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 14.Czerniecki B, Koski G, Koldovsky U, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Research. 2007;76:1842–52. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 15.Perez S, Sotiropoulou P, Sotiriadou N, et al. HER-2/neu-derived peptide 884–889 is expressed by human breast, colorectal and pancreatic adenocarcinomas and is recognized by in-vitro-induced specific CD4+ T cell clones. Cancer Immunol Immunother. 2002;50:615–24. doi: 10.1007/s002620100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roses R, Paulson E, Sharma A, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–9. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes N, Stern D. The biology of erb-2/neu/HER-2 and its role in cancer. Biochem Biophys Acta. 1994;1198:165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 18.Hynes N, Gerber H, Saurer S, et al. Overexpression of the c-erb-2 protein in human breast tumor cell lines. J Cell Biochem. 1989;39:167–73. doi: 10.1002/jcb.240390208. [DOI] [PubMed] [Google Scholar]
- 19.Hynes N. Amplification and overexpression of the erb-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin Cancer Biol. 1994;4:19–26. [PubMed] [Google Scholar]
- 20.Pegram M, Finn R, Arzoo K, et al. The effect of HER-2/neu overespression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene. 1997;15:537–47. doi: 10.1038/sj.onc.1201222. [DOI] [PubMed] [Google Scholar]
- 21.Kakarala M, Wicha M. Implications of the cancer stem cell hypothesis for breast cancer prevention and therapy. Journal of Clinical Oncol. 2008;26:2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korkaya H, Paulson A, Lovino F, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koski G, Koldovsky U, Xu S, et al. A novel dendritic cell based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. Journal of Immunotherapy. 2011 doi: 10.1097/CJI.0b013e318235f512. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedrosian I, Mick R, Xu S, et al. Intranodal administration of peptide pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–35. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Wood M, Song Y, et al. Defining promiscuous MHC class II helper T cell epitopes for the HER-2/neu tumor antigen. Cancer Res. 2000;60:5228–36. [PubMed] [Google Scholar]
- 26.Knutson K, Schiffman K, Cheever M, et al. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide specific immunity. Clin Cancer Res. 2002;8:1014–8. [PubMed] [Google Scholar]
- 27.Knutson K, Schiffman K, Disis M. Immunization with a HER-2/neu peptide vaccine generates HER-2/neu CD8 T cell immunity in cancer patients. J Clin Invest. 2001;107:477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray J, Gillogly M, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTL’s by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony stimulating factor in HLA-A2+ patients. Clin Cancer Res. 2002;8:3407–18. [PubMed] [Google Scholar]
- 29.Bahl S, Roses R, Sharma A, et al. Asymptomatic changes in cardiac function can occur in ductal-carcinoma-in-situ patients following treatment with HER-2/neu pulsed dendritic cell vaccine. American Journal of Surgery. 2009;198:488–94. doi: 10.1016/j.amjsurg.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burge C, Chang H, Apple S. Do the histologic features and results of breast cancer biomarker studies differ between core biopsy and surgical specimens? Breast. 2006;15:167–72. doi: 10.1016/j.breast.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Tamimi R, Baer H, Marotti J, et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Research. 2008;10(4):R67. doi: 10.1186/bcr2128. Epub August 5, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen V, Vaske C, Ursini-Siegel, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA. 2011 Sep 9; doi: 10.1073/pnas.1108781108. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horiquchi J, Oyama T, Takata D, et al. Pathological Complete Response and Prognosis in Patients Receiving Neoadjuvant Paclitaxel and Trastuzumab with and without Anthracyclines for Stage II and III, HER2-positive Operable Breast Cancer: A Single-institute Experience. Anticancer Res. 2011;31(9):3041–6. [PubMed] [Google Scholar]
- 34.Ring A, Smith I, Ashley S, et al. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arpino G, Wiechmann L, Osborne C, et al. Crosstalk between the estrogen receptor status and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29(2):217–33. doi: 10.1210/er.2006-0045. Epub 2008 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccart-Gebhart M, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-postive breast cancer. New England Journal of Medicine. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 37.Dent R, Clemons M. Trastuzumab after primary treatment for early stage HER2-positive breast cancer reduces recurrence. Cancer Treat Rev. 2006;32:144–8. doi: 10.1016/j.ctrv.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Bedrosian I, Hu C, Chang G. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. 2010;102:401–9. doi: 10.1093/jnci/djq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiff R, Massarweh S, Shou J, et al. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331s–336s. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 40.Nahta R, Esteva F. Herceptin:mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Haffy B, Yang Q, Reiss M, et al. Loco-regional relapse and distant metastasis in conservatively managed triple negative early stage breast cancer. J Clin Oncology. 2006;24:5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]