Second-line therapies in HCC: emergence of resistance to sorafenib (original) (raw)
. Author manuscript; available in PMC: 2013 Apr 1.
SUMMARY
There is a need for second line therapies in patients with advanced hepatocellular carcinoma who progress after sorafenib. Positive signals of brivanib in phase 2 studies reported herein have yet to be confirmed in phase 3 trials. Identification of the molecular mechanisms driving sorafenib resistance should guide drug development strategies in this setting.
In this issue of Clinical Cancer Research, Finn et al1 report the results of the first clinical trial evaluating a molecular targeted agent -brivanib- in second line for patients with advanced hepatocellular carcinoma (HCC) who progressed after receiving sorafenib.
The landscape of systemic therapy of HCC has significantly changed in the last 5 years. Following the approval of sorafenib after demonstrating its survival benefits in patients with advanced HCC in the landmark SHARP trial2, a number of molecular agents have entered different phases of clinical development covering the whole spectrum of the disease. Currently, there are more than 250 ongoing clinical trials assessing molecular targeting agents in the adjuvant setting after resection or ablation, in combination with loco-regional therapies, as first line treatment in combination (or competition) with sorafenib, or as second line therapy after progression on sorafenib3. However, only a subset (approximately ten) represent phase 3 studies designed to proceed to regulatory approval. Drugs tested include agents that block EGFR (erlotinib), mTOR (everolimus) and VEGFR, PDGFR and FGFR (brivanib, Fig. 1). As described below, other mutikinase inhibitors recently failed to meet the end point, such as sunitinib.
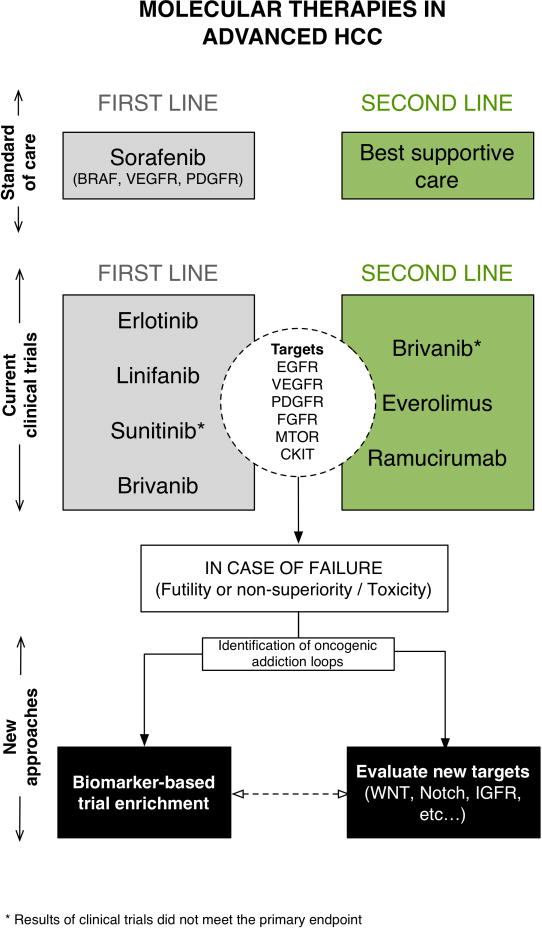
Figure 1.
Molecular targets and therapies in the management of patients with advanced HCC. Three approaches have already failed to meet the primary endpoint, and since there is certain degree of target overlap, it is possible that additional approaches will not succeed. New strategies should prime the development of new targets based on the identification of oncogenic addiction loops. Also, and similarly to other solid tumors, trial design for targeted therapies should consider enrichment strategies for patients with such molecular addiction events.
The study by Finn et al1 evaluated brivanib in 48 patients with advanced HCC as a second line option after progression/intolerance to sorafenib, using a single-arm, phase 2 design. Results include a median survival of 9.7 months, similar to what was reported for this agent as first-line treatment, 10 months4. The disease control rate was 70% (10% objective response; 60% stable disease) as assessed by mRECIST5. The toxicity profile of brivanib was different from sorafenib, with significantly less hand-foot syndrome and higher rates of arterial hypertension and fatigue. Similarly to sorafenib, brivanib did not induce liver dysfunction in enrolled patients with well-preserved liver function (90% were Child-Pugh A class). A phase 3 randomized trial comparing brivanib vs placebo as second line treatment in approximately 400 patients is ongoing. Just recently, a press release from Bristol-Myers-Squibb, the manufacturer of brivanib, revealed that in the phase 3 trial brivanib [sic] “did not meet the primary endpoint of improving overall survival versus placebo”. Until a full report of the phase 3 results is released, the exact response rate and overall survival are not fully known. Data regarding subgroup analyses could also highlight patients with a better response profile, that could be confirmed in future prospective studies.
Assuming that the median survival for brivanib treated patients is similar in both phase 2 and 3 studies, the main question is what should be the expected median survival of the control arm in second line that prevented a positive result in the phase 3 setting? This figure has not yet been reported, and expected outcomes derived from patients alive after sorafenib failure based on the SHARP trial (natural history of 4–6 months), might have been underestimated. One can speculate that a selection bias with enrichment of patients with indolent HCC, those with good ECOG status after sorafenib failure, might have contributed to these conflicting findings. In addition the treatment migration effect, by which patients are currently receiving sorafenib at intermediate stage after failure of chemoembolization, might also play a role6. In any case, the results of the phase 3 study further emphasize the recommendation of conducting randomized phase 2 studies to accurately capture signals of efficacy and provide reliable assumptions for trial design7. There are still two ongoing phase 3 trials in the second line setting, testing either everolimus (mTOR inhibitor) or ramucirumab (VEGFR2 monocolonal antibody), which will be informative. The negative result reported here adds to the recent failures of sunitinib investigations in first line investigations6, and further highlights the complexity of advancing the field of systemic treatment for patients with advanced HCC
In 2003, the Food and Drug Administration approved the first molecular targeted agent for a solid tumor (i.e., EGFR-inhibitor gefitinib in lung cancer). The development of this new family of drugs has dominated translational research in oncology during the last decade. Previous results of imatinib in chronic myeloid leukemia (CML) set high expectations for targeted therapies for solid tumors8. Imatinib was able to induce remarkable clinical remissions by blocking the activity of the BCR-ABL fusion protein, the known molecular substrate of the disease. As a conceptual consequence, the rationale behind molecular therapies was not to target the molecular aberrations present in tumors, but to preferentially antagonize alterations implicated in tumor progression (‘oncogene addiction’). Some recent successes using this approach include vemurafenib in BRAF mutated melanomas9 or c rizotinib in lung tumors with ALK rearrangements10. Unfortunately, the distinction between driver and passenger events in solid tumors is only beginning to be translated into clinical medicine. To date, potential drivers of oncogenic addiction have not been explored in the HCC clinical setting by trial enrichment either due to a lack of reliable biomarkers, or because of a marginal interest in expensive explorations of small niches of patients with good responses.
The high molecular heterogeneity in HCC favors de-regulation of multiple drivers, increasing the odds that a drug with a wider kinase blockade spectrum may be effective; such is the case of sorafenib. Our limited understanding of the mechanism of action of sorafenib in advanced HCC, however, makes it even more difficult to determine the possible resistance mechanisms. Preclinical models suggested that phenotypic resistance to VEGFR inhibition could induce activation of VEGF-independent angiogenic signals, by members of the FGF family11. In addition, genomic and functional studies indicated that FGF19 could act as an oncogenic driver in HCC12–13. Altogether, these data provided sufficient rationale to test FGFR inhibition in patients with sorafenib-resistant HCC. Increased understanding of the final results of the phase 3 trial will determine the role of brivanib as second line therapy. However, it seems clear that mechanisms of resistance to sorafenib include both FGFR-dependent and independent pathways.
In the last two years, two studies testing molecular therapies did not reach the primary end-point in first and second line settings for HCC. There are several drugs with new targets under evaluation in phase 2 and 3 trials3. All of these agents need to balance efficacy with the true bottleneck of trial success in cirrhotic patients, which is toxicity. In fact, safety profiles were the main issue jeopardizing trial success in one of the two cases in which results did not meet the primary end-point (i.e., sunitinib). This suggests that the management and trial design of HCC may be unique in oncology. Alternatively, other back-up strategies in drug development have to be taken into account. This requires a better understanding of the molecular pathogenesis of disease progression and resistance to sorafenib (Fig. 1). The development of targets based on their ability to behave as oncogenic addiction loops, and the implementation of clinical trials designed to enrich populations based on molecular biomarkers of these events should move the field forward. The success of trial enrichment based on molecular biomarkers in other solid tumors encourages this type of approaches12,13.
Footnotes
Conflict of interests: AV has nothing to disclose. JML has received research support from Bayer Pharmaceuticals and Bristol Myers Squibb; and has consultancy agreements with Bayer Pharmaceuticals, Bristol Myers Squibb, Imclone and Biocompatibles.
References
- 1.Finn SR, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, et al. Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1991. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JW, Finn RS, Kim JS, Karwal M, Li RK, Ismail F, et al. Phase II, open-label sutdy of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973–1983. doi: 10.1158/1078-0432.CCR-10-2011. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL-EORTC Guidelines for the management of hepatocellular carcinoma. J Hepatol. 2012 doi: 10.1016/j.jhep.2011.12.001. (in press) [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 8.Sawyers CL. Shifting paradigms: the seeds of oncogene addiction. Nat Med. 2009;15:1158–1161. doi: 10.1038/nm1009-1158. [DOI] [PubMed] [Google Scholar]
- 9.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011 doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, et al. Identification of a Therapeutic Strategy Targeting Amplified FGF19 in Liver Cancer by Oncogenomic Screening. Cancer Cell. 2011;19:347–358. doi: 10.1016/j.ccr.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]