T Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis (original) (raw)
. Author manuscript; available in PMC: 2013 May 1.
Abstract
Regulatory T cells (Tregs) are found infiltrating tumors in a vast array of tumor types, and tumor-infiltrating Tregs are often associated with a poor clinical outcome. Tregs are potent immunosuppressive cells of the immune system that promote progression of cancer through their ability to limit antitumor immunity and promote angiogenesis. Here, we discuss the ways in which Tregs suppress the antitumor immune response, and elaborate on our recent discovery that Tregs make significant direct contributions to tumor angiogenesis. Further, we highlight several current therapies aimed at the elimination of Tregs within cancer patients. Given the multifaceted role of Tregs in cancer, a greater understanding of their functions will ultimately strengthen future therapies.
Introduction
The tumor microenvironment is characterized by a multitude of mechanisms supporting angiogenesis and immune suppression (1). Many of the immune suppressive regulatory circuits operating in tumors are part of the physiologic regulatory mechanisms used by the immune system to maintain homeostasis in order to prevent autoimmunity and temper inflammation after infection or injury (1). Regulatory T cells (Tregs) are considered to be pivotal mediators of peripheral tolerance and immune suppression. Tregs are comprised of natural Tregs (nTregs), which are thymically-derived cells of FoxP3 lineage, and inducible Tregs (iTregs) that upregulate FoxP3 expression, and are derived in the periphery from naïve CD4+ T cell precursors under tolerogenic conditions (2). Tregs are highly enriched in the tumor microenvironment and are well known for their roles in tumor progression. They are considered to be significant in limiting antitumor immune responses and promoting immunological ignorance (peripheral tolerance) of cancer cells. Recently, we have expanded upon the roles of Tregs beyond immune suppression in tumors, and have demonstrated that Tregs are directly involved in promoting angiogenic reprogramming of the tumor microenvironment (3), highlighting a multifaceted role for Tregs in promoting cancer through tumor immune escape and angiogenesis. Thus, we assert that successful future cancer therapy strategies have to take into consideration either the elimination or the functional suppression of Tregs, as they play an important role in the establishment of aggressive tumor phenotypes.
Tregs are increased in tumors and are correlated with a poor prognosis
June and colleagues were the first to report an increase in Tregs in cancer patients (4). They demonstrated that regulatory CD4+CD25+ T cells were increased at tumor sites in non-small-cell lung and ovarian cancers, and these cells, now appreciated a s Tregs, secreted large amounts of transforming growth factor beta (TGFβ) that inhibited CD8+ effector T cell functions in vitro (4). An increase in Tregs in cancer has been demonstrated in a multitude of cancers including, but not limited to ovarian, breast, colorectal, lung, pancreatic cancers and melanoma [(5) and references therein]. In ovarian cancer patients, Tregs that were isolated from the tumor site, ascites, or peripheral blood were equally able to suppress tumor-antigen specific immune responses, suggesting that Tregs contribute to promotion of ovarian cancer, likely due to their enhanced recruitment or local expansion rather than enhanced suppressive capacity acquired in the tumor microenvironment (6).
Increased numbers of Treg in tumors have been associated with poor survival in many solid tumors including in breast cancer (7), gastric cancer (8), and ovarian cancer (6, 9). In ovarian cancer, a low abundance of tumor-infiltrating Tregs can translate into years of added survival, highlighting the importance of these cells to tumor progression (6). However, some groups have identified Treg infiltration to be a biomarker of good clinical outcome, e.g. in colon (10) or in ovarian carcinoma (11), highlighting the complexity of Tregs as biomarker. We have observed that Treg infiltration increases proportionally to the effector T cells in cancer, thus Treg could be associated with improved outcome, if considered as an isolated parameter, possibly reflecting the overall T cell infiltration which also predicts improved outcome in colon cancer (12–13) and ovarian cancer (14). Particularly important therefore, is the ratio of Tregs to CD8+ effector cells, with a high CD8:Treg ratio representing the best indicator of prolonged survival (9). Mouse models further support the role for Tregs in tumor progression, where depletion of Tregs facilitates tumor rejection and induction of antitumor immunity (15–16) that is associated with a fundamental shift in the tumor microenvironment cytokine milieu (17). Importantly, while transfer of tumor-reactive CD8+ T cells is known to result in tumor elimination experimentally, co-transfer of Tregs with CD8+ cells abrogates their efficacy in both ovarian cancer and melanoma models (6, 18). Furthermore, Treg depletion in vitro allowed for the expansion of NYESO-1-reactive Th1 cells derived from cancer patients (19). Thus, Tregs suppress tumor-specific immunity and significantly impact the course of tumor progression across multiple tumor types.
Mechanisms of immune suppression by Tregs
Much of what is known about Tregs in tumor progression is related to their ability to limit anti-tumor immune responses, resulting in immunological tolerance and ignorance of the tumor. The four best known mechanisms of immune regulation by Tregs include: a) secretion of soluble or membrane-tethered immunosuppressive molecules, b) direct cytolytic activity, c) metabolic disruption, and d) suppression of dendritic cells (DCs) [for an extensive review see (20)].
Suppressive cytokines and secreted molecules
Chief among the mechanisms of T cell suppression is the secretion of soluble or membrane-tethered mediators that inhibit effector T cell functions through cell contact-dependent, and independent mechanisms. The primary established Treg-derived cytokines that are responsible for this are IL-10, TGFβ, and IL-35 that function by inhibiting the activities of effector T cells (20). Important for tumor development, both TGFβ and IL-10 derived from Tregs have been shown to be key mediators that contribute to tumor progression by limiting antitumor immunity (21–22). These cytokines prevent the expansion, cytokine elaboration (e.g., IFNγ, TNFα), and effector functions (cytolysis) of effector cells that are critically important for the control of tumor growth, but also polarize DCs towards tolerogenic phenotypes. Our recent discovery that Tregs secrete VEGF (3), a known immunosuppressive molecule, adds to the panel of paracrine mechanisms through which Treg can exert suppression and affect the differentiation and function of DCs.
Cytolysis
An additional mechanism of regulation is the killing of effector T cells or possibly tumor antigen-presenting DCs. Tregs have been demonstrated to exert cytolytic functions, using a variety of mediators like granzyme B (23–24), the TRAIL pathway (25), and galectin-1 (26). The activation of these pathways by Tregs induces apoptosis on target effector cells. Importantly, Cao and colleagues were able to demonstrate that Treg-derived granzyme-B and perforin are responsible for the suppression of NK and cytotoxic CD8+ cells’ ability to eliminate tumors in multiple models (27).
Metabolic disruption
There are several proposed mechanisms of how Tregs could inhibit the functions of effector T cells by inhibiting them metabolically. Although controversial, it has been suggested that Tregs can essentially “starve” effector cells by depleting local resources of IL-2 that leads to effector cell apoptosis (28). Additionally, Tregs have been shown to catalyze ATP to adenosine through expression of CD39 and CD73, and in turn adenosine suppresses effector T cell functions (29). Finally, Tregs have been suggested to inhibit effector T cell function by the physical transfer of cAMP through membrane gap junctions (30). The contribution of these mechanisms to tumor immune escape is unknown.
DC interactions
There is evidence suggesting that Tregs may mediate immune suppression through secondary cells types, with the largest body of evidence supporting deleterious interactions with DCs. Tregs induce DCs, through cell-cell mediated reverse signaling by cytotoxic T-lymphocyte antigen 4 (CTLA-4), expressed on Tregs, and CD80 and/or CD86, expressed on DCs, to upregulate indoleamine 2,3-dioxygenase (IDO) in DCs (31). IDO expression is responsible for the catabolism of tryptophan that suppresses effector T cells function by simultaneously depleting essential tryptophan while generating immunosuppressive tryptophan metabolites. Further, Tregs have been shown to reduce the capacity for DCs to activate effector T cells through inhibition of costimulatory molecules, or through suppression of DC maturation via IL-10/TGFβ signaling or through Treg-DC interactions mediated by lymphocyte-activation gene 3 (LAG3) (32–33).
Recruitment of Tregs to the tumor microenvironment
The reason for increased numbers of Tregs at tumor sites is probably due to a number of factors. In tumor environments like ovarian cancer (6) and Hodgkin lymphoma (34), there are large amounts of CC-chemokine ligand 22 (CCL22) that are likely derived from both tumor cells and tumor macrophages. CCL22 can recruit Tregs through CCR4, and Treg migration can be abrogated through CCR4 blockade in vitro. Recently, we identified a novel immunosuppressive and angiogenic circuit that establishes a direct role for tumor hypoxia in the recruitment of Tregs in ovarian cancer (3). We showed that hypoxia, a key promoter of tumor angiogenesis and also linked to the infiltration of Tregs (35), increased the expression of CCL28 in ovarian cancer cells, which recruited CD4+CD25+Foxp3+ Treg cells through ligation of the cognate receptor CCR10 expressed on Tregs. In ovarian cancer patients, CCL28 expression was correlated with hypoxia-inducible factor 1 alpha (HIF1α) expression, which a poor prognosis biomarker, and importantly, high CCL28 expression in patient tumors was also an indicator of poor survival. Artificial overexpression of CCL28 in mouse ovarian cancer cells led to enhanced growth of intraperitoneal tumors, which were characterized by increased Treg infiltration and increased IL-10 production in the peritoneal ascites (3). Importantly, in the CCL28-overexpressing mouse tumor model, depletion of CD25+ or CCR10+ cells eliminated Tregs from the tumor and abrogated the tumor growth advantage conferred by CCL28 overexpression. It is possible that numerous additional chemokines regulate Treg recruitment in cancer [see ref (36) for a potential list of important chemokine receptors], and they may have non-redundant roles in recruiting as yet unidentified Treg subsets. In the case of CCL28-CCR10 interactions, recruitment of Tregs to the specific hypoxic environment may serve to enhance their immunosuppressive capacity as part of a biological program (1, 37), as hypoxia has been shown to increase the potency of Tregs, and hypoxia exposed Tregs were more effective in suppressing the proliferation of effector cells (1, 37).
Expansion of Tregs in the tumor microenvironment
Treg cells can be divided largely into natural Tregs (nTreg), which are derived from the thymus and maintained peripherally by TGFβ, or inducible Tregs (iTreg), which are induced from naïve CD4+ T cell precursors and exert similar suppressive characteristics to nTregs; both of these Treg subtypes express FoxP3 [a more detailed discussion of this concept can be found in (2)]. Beyond recruitment of nTregs through chemokines, the tumor microenvironment promotes continued expansion of nTregs (38) as well as the generation of iTregs (39) due to a tumor microenvironment rich in cytokines like IL-10 (40), TGFβ (41), and adenosine (42) derived from either the tumor cells or from tumor-resident immunosuppressive DCs (43) and TIE-2+ monocytes (39, 44). These circuits are a reflection of physiologic homeostatic mechanisms, which tumors co-opt in tissue-specific and anatomic compartment-restricted manners. For example, naïve CD4+ cells are converted into iTregs by CD103+ DCs in the mesenteric lymph nodes, a mechanism helping to maintain gut homeostasis in a Toll-like receptor agonist rich environment (45).
Tregs in tumor angiogenesis
Tumor Angiogenesis
Angiogenesis is defined as the sprouting of new blood vessels from preexisting ones. Under physiological conditions, such as development, angiogenesis occurs step-wise with vessel destabilization, endothelial cell migration and proliferation, sprouting, and resolution with vessel stabilization (46). Tumor angiogenesis differs in that there is generally a failure of the resolution phase and the vessel network is highly disordered, but blood vessel development is critical to tumor growth, providing essential nutrients and growth factors while providing a conduit for wastes and sustained angiogenesis has long been considered a “hallmark of cancer” (47).
The accumulation of Tregs at tumor sites has been correlated with biomarkers of accelerated angiogenesis such as VEGF overexpression and increased microvessel density in endometrial (48) and breast cancers (49), providing clinical cues for an association between Tregs and angiogenesis. Tregs can actually contribute to tumor angiogenesis through both indirect and direct mechanisms. Tregs promote angiogenesis indirectly, by suppressing the activities of Th1 effector T cells releasing angiostatic cytokines like TNFα and IFNγ, as well as interferon-induced chemokines such as CXCL9, 10 and 11 (50–51). Indeed, Tregs have been shown to promote tumor angiogenesis by specifically inhibiting tumor-reactive T cells (52). However, we have also demonstrated that Tregs can make significant contributions to the direct promotion of tumor angiogenesis [(3) and Figure 1]. We showed that tumor hypoxia in ovarian cancer leads to the recruitment of Tregs via CCL28 upregulation (3). Forced expression of CCL28 in mouse ovarian cancer cell lines caused robust Treg accumulation, but also resulted in increased VEGF levels and significantly increased blood vessel development, which was associated with rapid tumor growth (3). Importantly, depletion of CD25+ or CCR10+ cells eliminated Treg cells from the tumour microenvironment and significantly suppressed VEGF expression and angiogenesis at these sites (3). We demonstrated that CD4+CD25+ Treg cells secreted higher amounts of VEGF at the steady state as well as under hypoxic conditions when compared with CD4+CD25− T cells, while media conditioned by Tregs in hypoxia promoted capillary tube formation in vitro, an effect dependent on VEGF signaling. Further, using an entirely cell-free Matrigel implant, we showed that supernatants of hypoxic Tregs were able to significantly promote angiogenesis in vivo (3). Our results are supported by early observations that T cells exposed to hypoxia express VEGF, and T cells within tumors express VEGF (53). Thus, we established a new mechanism whereby tumor hypoxia recruits Tregs to tumor sites that leads to significant direct contributions to the proangiogenic tumor microenvironment.
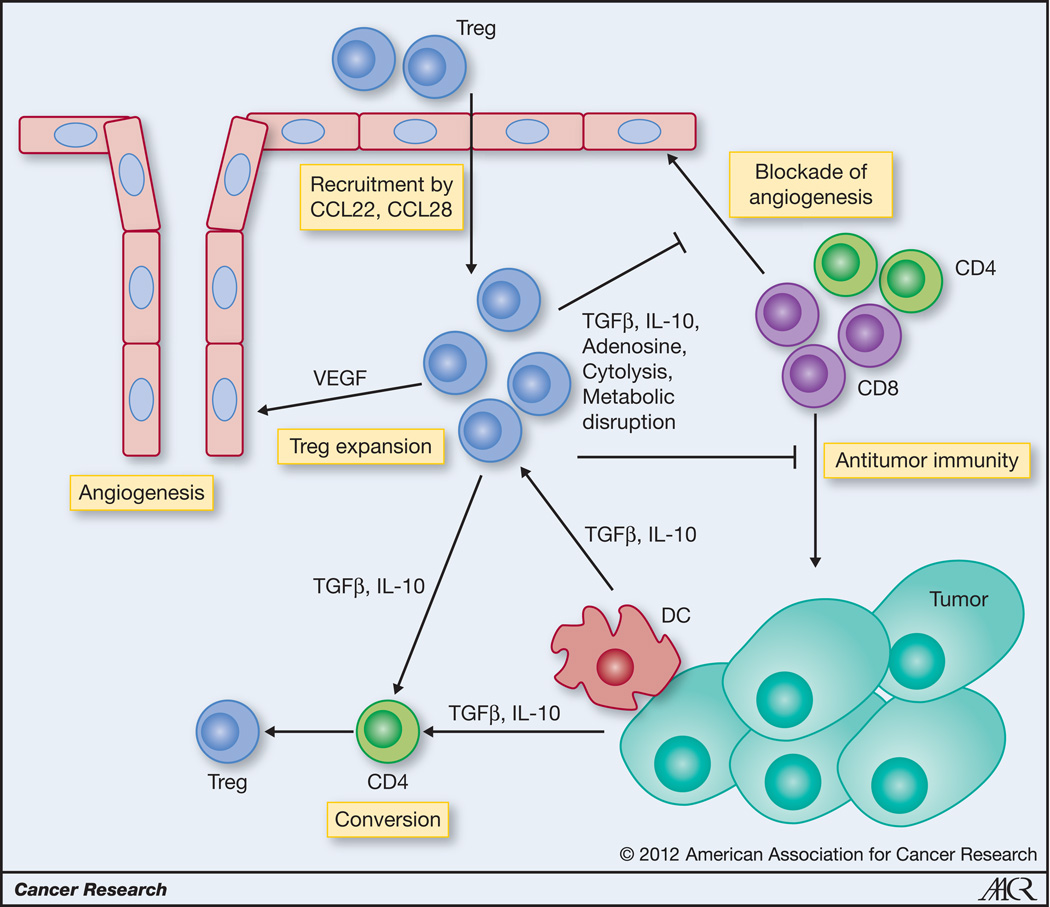
Figure 1. Role of Tregs in tumor progression.
Tregs are recruited to tumors from the periphery by tumor-derived, hypoxia-induced CCL28, but also DC and tumor derived CCL22. Within the tumor microenvironment Tregs can be expanded by TGFβ and possibly IL-10, that can also convert CD4+ naïve precursors into induced Tregs. Tregs promote tumor progression by direct inhibition of antitumor effector CD4+ and CD8+ T cells through inhibitory cytokines, cytolysis and metabolic disruption. Further, Tregs recruited to hypoxic areas directly stimulate angiogenesis through production of VEGF. Tregs also indirectly encourage angiogenesis by blocking effector cell-derived angiostatic cytokines like IFNγ and CXCL-10.
Tregs as targets for cancer immunotherapy
Based on the information provided above, it’s apparent that Tregs make significant contributions to tumor ‘immune’ escape, as well as having newly described functions in angiogenesis. Therefore, elimination of Tregs in cancer patients, and particularly within the tumor microenvironment, should be considered to be an essential component of any successful cancer therapy. There are several available therapeutics that either disrupt Treg functions or reduce their numbers. Interestingly, several chemotherapeutic drugs that interfere with Tregs, like methotrexate and cyclophosphamide, have well-described immunostimulatory and anti-angiogenic effects in cancer patients.
Nonspecific targeting of Tregs
A number of commonly used chemotherapeutics have been demonstrated to either reduce Tregs, or reduce their immunosuppressive capacity. These drugs include antimitotics such as cyclophosphamide, gemcitabine, mitoxantrone and fludarabine, as well as thalidomide analogs and cyclooxygenase 2 (COX-2) inhibitors. Thus, it is intriguing to speculate that these drugs have some off-target anti-tumor effects mediated through modulation of Tregs.
Cyclophosphamide (CY) has been shown to preferentially deplete CD4+CD25+ Tregs in rats, and only a single injection prior to tumor challenge with a rat colon cancer line was sufficient to delay tumor growth (54). Cyclophosphamide alkylates DNA, resulting in crosslinks between (inter-strand) and within (intra-strand) DNA strands, which leads to cell death, and it has been suggested that Treg are more sensitive to CY-induced apoptosis (55). For example, Jaffee and colleagues have shown that low-dose CY given to HER-2/neu transgenic mice with HER-2/neu-expressing mammary tumors selectively depleted Tregs that were progressing through the cell cycle. Interestingly, in untreated tumor-bearing mice, Tregs were shown to be the predominant cycling T-cell population, although less than half of the Foxp3+CD4+CD25+ Treg cells were cycling and thus susceptible to depletion by CY (56). Sherman and colleagues reported that effector cells that are in the process of being tolerized or deleted are also cycling and proliferating, indicating that depletion of cycling cells with CY may be beneficial in clearing out tolerizing cells (57). Further, low-dose CY also disrupts Treg homeostatic proliferation, and decreases their immunosuppressive functionality by decreasing FoxP3 and GITR (55), and recent evidence also suggests that inhibition of Treg function by lowdose CY may be the result of selective depletion of intracellular stores of ATP caused by increase surface expression of CD39 (an ATP-to-adenosine conversion enzyme) (58). However, in one study CY was shown to also deplete effector T cells in addition to Tregs (59). Therefore, CY disrupts Tregs using a multitude of mechanisms, but may also have unintended effects on tumor-reactive effector T cells.
Two drugs inhibiting DNA synthesis, fludarabine and gemcitabine have also been demonstrated to disrupt Tregs. Fludarabine as a standard five-day course administration induces lymphopenia that has proved favorable for chronic lymphocytic leukemia (CLL) and other hematological malignancy patients. In a clinical setting fludarabine treatment of CLL resulted in surprising increases of Treg apoptosis and decrease of Treg inhibitory functions (60). Further, fludarabine blocked the expansion of IL-10-producing CD4+ Tregs in vitro, which was associated with higher number of antigen-specific CTLs (61). In a phase I study in non-small cell lung cancer, gemcitabine administration induced lymphopenia with a decrease in effector T cell populations (62), but another phase I study of colon cancer patients showed that gemcitabine caused an increase in CTLs with a concomitant decrease in CD25+CD4+ T cells in clinical responders (63). Although both studies demonstrated positive results, the direct effects on lymphocyte populations is unknown. Gemcitabine affects a variety of immunosuppressive cells including myeloid-derived suppressor cells (MDSCs). Given at a clinically equivalent dose, gemcitabine resulted in a dramatically reduced number of MDSCs found in spleens of animals, accompanied by an increase in the antitumor activity of CD8+ T cells and activated NK cells (64). In light of the observation that MDSCs are capable of converting naïve CD4 cells to Tregs, it is entirely plausible that gemcitabine limits Tregs through its effects on MDSCs (65). Although both of these drugs exert little-to-no direct specificity for Tregs, there may be a particular dosing regimen that could provide optimal disruption of Tregs, while concomitantly inhibiting tumor growth.
Thalidomide and thalidomide derivatives have been utilized for treatment of nonmalignant diseases, including cutaneous and systemic inflammatory disorders (66–68). Lenalidomide (CC-5013, Revlimid; Celgene Corp., NJ, USA) is an FDA approved oral thalidomide analog used for treatment of multiple myeloma and low to intermediate risk myelodysplastic syndrome (MDS) caused by deletion of chromosome 5q (5q-syndrome). Lenalidomide induces myeloma cell apoptosis directly and indirectly by inhibition of bone marrow stromal cell support, by anti-angiogenic and anti-osteoclastogenic effects, and through immunomodulatory activity. Lenalidomide has a broad range of immunomodulatory properties that can be exploited to treat many hematologic and solid cancers. Lenalidomide inhibits human Treg cell proliferation in response to IL-2 and downregulates FoxP3 expression (69). It was also shown to significantly reduce Treg cells in mouse lymph nodes (69). In a recent clinical trial in chronic leukocyte leukemia, the administration of lenalidomide resulted in a decrease of Treg and increase of Th17 cells in peripheral blood (70), supporting a potential role for thalidomide analogs for the elimination of Tregs in patients (69). Importantly lenalidomide has also been shown to exert costimulatory effects on T cells and enhance T cell proliferation, effector function (71–73), and Th1 reprogramming (74), and in combination therapy it has augmented tumor lysate vaccines (75).
Cyclooxygenase (COX) enzymes, and particularly COX-2, are known to contribute to many facets of tumor progression. Patients taking non-steroidal anti-inflammatory drugs (NSAIDs) like aspirin, are significantly less likely to develop colorectal cancer (76–77), and several investigators have demonstrated an adjuvant property of COX-2 inhibitors in combination with cancer vaccines (78–80). Experimentally, COX-2 inhibition reduced Treg cell frequency and suppressive activity, attenuated FoxP3 expression in tumor-infiltrating lymphocytes, and decreased tumor burden in vivo (81). In patients with colon cancer, treatment with an oral NSAID significant increased CD8+ tumor-infiltrating T cells and decreased expression of FoxP3 and IL-10 (82). In these patients with colorectal cancer there are increased concentrations of prostaglandin E2 (PGE2) in peripheral blood, and although tumors express large amounts of PGE2, it has been demonstrated that Treg cells also express COX-2 and produce PGE2 in a manner that suppresses effector T cells. A role for Treg-derived PGE2 in immune suppression is supported by the demonstration that indomethacin (a COX-2 inhibitor) reverses Treg-mediated anti-tumor suppression in vitro (83).
Specific targeting of Tregs
Because of the recognized important role played by Tregs in different kinds of tumors and other pathology, several compounds (often depletion antibodies) have been developed to target directly Tregs, through recognition of Treg markers like CD25, CTLA-4, and GITR.
A large number of Treg targeting strategies rely on specific recognition of CD25. In several mouse tumor models, CD4+CD25+ Treg depletion using antibodies targeting CD25 produced significant anti-tumor activity, although often associated with an increased incidence of autoimmunity. Combinatorial approaches using monoclonal antibodies and vaccines have been investigated in murine models, and the positive results of these preclinical studies clearly highlight the potential of Treg depletion approach in cancer immunotherapy (84). In an early phase I clinical trial in patients with metastatic breast cancer, the anti-CD25 antibody daclizumab significantly depleted Treg cells, and enhanced the immunogenicity of a cancer vaccine. From the 10 patients who received the vaccine, 5 had stable disease over several months (85–86). However, another study using daclizumab in combination with a dendritic cell vaccine noted a detrimental role of daclizumab treatment, which may be due to timing of administration (87).
Denileukin diftitox (Ontak®; Esai, Inc., Woodcliff Lake, NJ) is a fusion protein of human IL-2 and diphtheria toxin. The IL-2 portion of the fusion protein binds preferentially to cells expressing intermediate to high affinity IL-2 receptors (IL-2Rs) comprised of IL-2Rα(CD25)/β(CD122)/γ(CD132) subunits or IL-2Rβ/γ subunits and results in cell death by interfering with protein synthesis following endocytosis. Ontak has proven efficacious in advanced chronic T-cell lymphoma (CTCL) with high CD25 expression, where high CD25 expression is associated with clinical response to Ontak therapy (88). In melanoma patients, the application of recombinant Ontak significantly but transiently reduced the frequency of Treg in peripheral blood. However, another study evaluating the treatment of melanoma patients with Ontak failed to show any Treg depletion or clinical benefit (89).
LMB-2 is a fusion protein obtained fusing a single-chain variable fragment antibody (scFv) against CD25 to Pseudomonas exotoxin A. In vitro, treatment of human PBMCs with LMB-2 resulted in specific CD4+CD25+Tregs depletion (90). In a phase I clinical trial, treatment of CD25+ T-cell malignancies with a dose over 60μg/kg of LMB-2 showed encouraging results, with 8 objective responses in a cohort of 20 patients indicating that LMB-2 is efficacious in patients (91). However, in melanoma patients, LMB-2 administration in combination with peptide vaccination showed a significant, yet transient, decrease of FoxP3+CD4+CD25+ Tregs in peripheral blood that returned to pretreatment levels within days. As might be expected, there was no objective clinical response (92). Thus, the utility of LMB-2 is not yet clear.
After CTLA-4 was first cloned in 1987, it was not clear whether CTLA-4 was involved in stimulatory or inhibitory pathways in T cells. The generation of CTLA-4 knockout mice solved this riddle; knockout mice developed a progressive and uncontrolled accumulation of activated T cells and died of lymphoproliferative disease (93). The seminal study by Leach and colleagues, showed that CTLA-4 blockade could attenuate the growth of several implanted murine tumors (94), and the mechanism of inhibition was immune mediated. CTLA-4 is expressed on the surface of Tregs, but blockade could actually expand functionally suppressive Tregs (95). Although various CTLA-4 blockade therapies reduce tumor-infiltrating Treg (84), this effect may be due entirely to the ability of CTLA-4 blockade to promote the generation of memory and promote effector T cell functions (96).
So far, two humanized anti-human CTLA-4 neutralizing antibodies have been developed, MDX-010 (Ipilimumab) and CP-675206 (Tremelimumab), which have been tested in phase I through III trials. The first phase I clinical trial with anti-CTLA-4 blocking antibody was carried out in 2002 at UCLA and M.D. Anderson Cancer Center. The majority of enrolled patients had measurable metastatic melanoma. This trial tested doses from 0.01–15 mg/kg within seven cohorts. Objective tumor responses were noted in a subset of patients starting at a dose of 3 mg/kg and becoming more frequent at 15 mg/kg (97). Interestingly, supporting the immune modulatory effects of CTLA-4, treatment of metastatic melanoma patients with ipilimumab resulted in tumor regression in 36% of patients, and was associated with autoimmune toxicity, but patients without autoimmune toxicity were less likely to experience tumor regression (98). A further trial combining high-dose IL-2 and varied doses of ipilimumab showed synergy compared with earlier studies evaluating IL-2 alone in metastatic melanoma (99). Further analysis of the peripheral blood mononuclear cells in patients undergoing anti-CTLA-4 treatment for stage IV metastatic melanoma and RCC has highlighted, by in vitro co-culture, that there is no inhibition of the suppressive activity of CD4+CD25+ T cells per se, but a probable enhancement of effector T-cell function. The results of a phase III clinical trial including 502 untreated metastatic melanoma patients were recently reported (100). Ipilimumab (10 mg/kg) in combination with dacarbazine, as compared with dacarbazine plus placebo, improved overall survival in patients, leading to FDA approval for the treatment of metastatic melanoma (100). Tremelimumab has been shown not only able to suppress Treg activity, but also to induce expansion of effector and memory CD4+ and CD8+ T-cells, with anti-tumor efficacy (101). Thus, it has been suggested that depletion of Tregs may be secondary in importance to modulating the ratio of CD8+ effector cells to Tregs, which may be mediated through direct interactions of anti-CTLA-4 antibody with effector cells.
GITR (glucocorticoid-induced tumor necrosis factor receptor) is a TNF receptor family member expressed at low levels on resting CD8+ and CD4+Foxp3− T cells, but constitutively expressed at high levels on CD4+CD25+Foxp3+ Tregs (102). Treg cells express even higher levels of GITR in tumors than elsewhere (103–104). Although it may not affect systemic Treg, GITR ligation specifically depletes Tregs in tumors, increasing tumor Teff:Treg ratios (105). DTA-1, a GITR agonistic Ab, may disable Treg, depletes intratumoral Treg, and enhances T cell immunity against tumors (106–109). Importantly, it also co-stimulates CD4+ and CD8+ T cell proliferation and effector functions, renders Teff cells resistant to Tregs, and enhances a variety of T cell responses (110–111).
However, additional studies have demonstrated that DTA-1 does not affect Tregs numbers or function. Rather, administration of the agonistic antibody prevented the infiltration of Tregs into the tumor microenvironment, promoting a high CD8/Treg ratio resulting in the control of tumor growth in mice (105). Thus, targeting Tregs through GITR is an interesting approach, but may require additional therapeutics to promote systemic antitumor immune responses.
Based on our recent work, we believe we have added a new possible target to this list, CCR10. Tumor hypoxia induced CCL28 expression, leading to the recruitment of CCR10+ Tregs, while the depletion of CCR10+ positive cells using an anti-CCR10 immunotoxin resulted in complete Treg depletion and loss of the tumor growth advantage conferred by CCL28 overexpression (3). Further, it appears that CCR10 expression on Tregs is associated with a peripheral homing phenotype, and is a marker of highly suppressive Tregs cells (112). Although CCR10 expression is not restricted entirely to Tregs, the benefit of CCR10+ cell depletion is beneficial in cancer (3). Thus, CCR10 is an attractive new target for disrupting Tregs in cancer.
Concluding Remarks
Based on the information presented above, it should be apparent that Tregs are instrumental in the establishment of tumor immune tolerance, and are significant cellular mediators of tumor progression in patients. Beyond immune suppression, our recent work has now expanded this view, as we have shown that Tregs can make significant contributions to the direct promotion of tumor angiogenesis. Thus, we believe that Tregs are key orchestrators of tumor development, linking immune suppression and angiogenesis in one biological program, highlighting the need to specifically target these cells to promote antitumor immunity and tumor regression. Indeed, reducing Treg functions and/or numbers in patients with cancer should allow more effective immunebased therapies, alone or in combination with traditional chemotherapeutics. Here, we have presented numerous preclinical and clinical data that supports the notion that elimination of Tregs should be considered crucial to many cancer therapies. A major therapeutic challenge however remains, that is the paucity of tools to target Tregs effectively in the clinic. Unraveling the complexity of Tregs is only just beginning, and further understanding of their biology and characterization of targets will undoubtedly enhance future therapeutic opportunities.
Acknowledgments
Supported by NIH R01-CA116779; NCI P01-CA83638 Ovarian SPORE; and the Ovarian Cancer Research Fund (OCRF)
References
- 1.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 2.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 4.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 5.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 8.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 9.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–441. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 15.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 16.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 17.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106:1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 20.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, et al. IL-10 controls ultraviolet-induced carcinogenesis in mice. J Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 22.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 23.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 24.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 25.Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076–2084. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 26.Garin MI, Chu CC, Golshayan D, Cernuda-Morollon E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 27.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 29.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 32.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 34.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 35.Yan M, Jene N, Byrne D, Millar EK, O'Toole SA, McNeil CM, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13:R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 38.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 39.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 40.Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 45.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 47.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 48.Giatromanolaki A, Bates GJ, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, et al. The presence of tumor-infiltrating FOXP3+ lymphocytes correlates with intratumoral angiogenesis in endometrial cancer. Gynecol Oncol. 2008;110:216–221. doi: 10.1016/j.ygyno.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Gupta S, Joshi K, Wig JD, Arora SK. Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 2007;46:792–797. doi: 10.1080/02841860701233443. [DOI] [PubMed] [Google Scholar]
- 50.Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Qin Z, Blankenstein T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 52.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 53.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 54.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 55.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 56.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Redmond WL, Hernandez J, Sherman LA. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J Immunol. 2003;171:6349–6354. doi: 10.4049/jimmunol.171.12.6349. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850–4858. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- 59.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 61.Hegde U, Chhabra A, Chattopadhyay S, Das R, Ray S, Chakraborty NG. Presence of low dose of fludarabine in cultures blocks regulatory T cell expansion and maintains tumor-specific cytotoxic T lymphocyte activity generated with peripheral blood lymphocytes. Pathobiology. 2008;75:200–208. doi: 10.1159/000124981. [DOI] [PubMed] [Google Scholar]
- 62.Levitt ML, Kassem B, Gooding WE, Miketic LM, Landreneau RJ, Ferson PF, et al. Phase I study of gemcitabine given weekly as a short infusion for non-small cell lung cancer: results and possible immune system-related mechanisms. Lung Cancer. 2004;43:335–344. doi: 10.1016/j.lungcan.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, et al. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 65.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okafor MC. Thalidomide for erythema nodosum leprosum and other applications. Pharmacotherapy. 2003;23:481–493. doi: 10.1592/phco.23.4.481.32115. [DOI] [PubMed] [Google Scholar]
- 67.Lazzerini M, Martelossi S, Marchetti F, Scabar A, Bradaschia F, Ronfani L, et al. Efficacy and safety of thalidomide in children and young adults with intractable inflammatory bowel disease: long-term results. Aliment Pharmacol Ther. 2007;25:419–427. doi: 10.1111/j.1365-2036.2006.03211.x. [DOI] [PubMed] [Google Scholar]
- 68.Ossandon A, Cassara EA, Priori R, Valesini G. Thalidomide: focus on its employment in rheumatologic diseases. Clin Exp Rheumatol. 2002;20:709–718. [PubMed] [Google Scholar]
- 69.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Idler I, Giannopoulos K, Zenz T, Bhattacharya N, Nothing M, Dohner H, et al. Lenalidomide treatment of chronic lymphocytic leukaemia patients reduces regulatory T cells and induces Th17 T helper cells. Br J Haematol. 2010;148:948–950. doi: 10.1111/j.1365-2141.2009.08014.x. [DOI] [PubMed] [Google Scholar]
- 71.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 72.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 73.Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis. 2003;187:946–555. doi: 10.1086/368126. [DOI] [PubMed] [Google Scholar]
- 74.Xu W, Celeridad M, Sankar S, Webb DR, Bennett BL. CC-4047 promotes Th1 cell differentiation and reprograms polarized human Th2 cells by enhancing transcription factor T-bet. Clin Immunol. 2008;128:392–399. doi: 10.1016/j.clim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 75.Dredge K, Marriott JB, Todryk SM, Muller GW, Chen R, Stirling DI, et al. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. J Immunol. 2002;168:4914–4919. doi: 10.4049/jimmunol.168.10.4914. [DOI] [PubMed] [Google Scholar]
- 76.Benamouzig R, Uzzan B, Deyra J, Martin A, Girard B, Little J, et al. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 2011 doi: 10.1136/gutjnl-2011-300113. [DOI] [PubMed] [Google Scholar]
- 77.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 78.Toomey D, Conroy H, Jarnicki AG, Higgins SC, Sutton C, Mills KH. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine. 2008;26:3540–3549. doi: 10.1016/j.vaccine.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Mukherjee P, Basu GD, Tinder TL, Subramani DB, Bradley JM, Arefayene M, et al. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182:216–224. [PMC free article] [PubMed] [Google Scholar]
- 80.Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 81.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, et al. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 82.Lonnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, et al. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun. 2008;8:5. [PMC free article] [PubMed] [Google Scholar]
- 83.Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, et al. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813–821. doi: 10.1007/s00262-007-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99–106. doi: 10.1111/j.1749-6632.2009.04939.x. [DOI] [PubMed] [Google Scholar]
- 86.Rech AJ, Mick R, Recio A, DeMichele A, Tweed CK, Fox KR, et al. Phase I study of anti-CD25 mab daclizumab to deplete regulatory T cells prior to telomerase/survivin peptide vaccination in patients (pts) with metastatic breast cancer (MBC) ASCO. 2010 2010. [Google Scholar]
- 87.Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16:5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]
- 88.Talpur R, Jones DM, Alencar AJ, Apisarnthanarax N, Herne KL, Yang Y, et al. CD25 expression is correlated with histological grade and response to denileukin diftitox in cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:575–583. doi: 10.1038/sj.jid.5700122. [DOI] [PubMed] [Google Scholar]
- 89.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Attia P, Powell DJ, Jr, Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother. 2006;29:208–214. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 92.Powell DJ, Jr, Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 94.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 95.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci U S A. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 98.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 101.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–5249. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 102.Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- 103.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coe D, Begom S, Addey C, White M, Dyson J, Chai JG. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 109.Sharma S, Dominguez AL, Manrique SZ, Cavallo F, Sakaguchi S, Lustgarten J. Systemic targeting of CpG-ODN to the tumor microenvironment with anti-neu-CpG hybrid molecule and T regulatory cell depletion induces memory responses in BALB-neuT tolerant mice. Cancer Res. 2008;68:7530–7540. doi: 10.1158/0008-5472.CAN-08-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 111.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, et al. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. J Exp Med. 2004;200:149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol. 2006;177:593–603. doi: 10.4049/jimmunol.177.1.593. [DOI] [PubMed] [Google Scholar]