Tissue deformation spatially modulates VEGF signaling and angiogenesis (original) (raw)
Abstract
Physical forces play a major role in the organization of developing tissues. During vascular development, physical forces originating from a fluid phase or from cells pulling on their environment can alter cellular signaling and the behavior of cells. Here, we observe how tissue deformation spatially modulates angiogenic signals and angiogenesis. Using soft lithographic templates, we assemble three-dimensional, geometric tissues. The tissues contract autonomously, change shape stereotypically and form patterns of vascular structures in regions of high deformations. We show that this emergence correlates with the formation of a long-range gradient of Vascular Endothelial Growth Factor (VEGF) in interstitial cells, the local overexpression of the corresponding receptor VEGF receptor 2 (VEGFR-2) and local differences in endothelial cells proliferation. We suggest that tissue contractility and deformation can induce the formation of gradients of angiogenic microenvironments which could contribute to the long-range patterning of the vascular system.
Keywords: vascular patterning, blood vessels, morphogenesis, mechanical forces
Tissue deformation influences the development of the vasculature in the embryo (1) and in the contracting wound (2). Current models suggest that physical forces originating from the blood (3), from cells pulling on neighboring cells (4), and on the Extra Cellular Matrix (ECM) (5) distort cellular membrane receptors and cytoskeletal elements, modulate biochemical signaling pathways and the behavior of endothelial and smooth muscle cells (6). However it is not known whether physical forces can spatially modulates angiogenic signals and contribute to vascular patterning.
Angiogenic molecules orchestrate cellular behaviors (growth, apoptosis, mobility) whose local differentials underlie morphogenesis and the formation of vascular patterns. The Vascular Endothelial Growth Factor (VEGF) is a highly vascular-specific signaling molecule. The principal component of the VEGF molecules is VEGF-A, it binds the Receptor Tyrosine Kinase VEGF Receptor 2 (VEGFR-2). VEGFR-2 activates multiple downstream signaling molecules including Mitogen-Activated Protein Kinases (MAPKs), Phosphoinositide 3-Kinases, PKB (Protein Kinase B) or small GTPases (7). As a result, endothelial cells (EC) proliferate, migrate, degrade the ECM, and modulate endothelium permeability (7). The tight spatial regulation of the VEGF-A/VEGFR-2 system is thus essential to control vascular development. Interestingly, the growth and apoptosis of EC (8, 9) and the expression of VEGFR-2 (3, 10) are modulated by physical forces. This regulation might occur via the Rho inhibitor p190RhoGAP and the balance between antagonist transcriptions factors TFII-I (also known as GTF2I) and GATA-2 (5).
Vascular patterning is governed by oxygen deficiency (hypoxia) which controls the local production of VEGF via Hypoxia-Inducible Factor [HIF1α, (11)]. A gradient of VEGF overlays a gradient of hypoxia which orientates, attracts, and induces proliferation of new blood vessels (12). Upon irrigation of the tissue, hypoxia decreases and so does the production of VEGF. This patterning mechanism does not imply a role for the physical deformation of tissues.
Microfabricated platforms are powerful tools to recapitulate and systematically manipulate simplified cases of tissue morphogenesis with an intermediate complexity between 2D cell culture and model organisms (13). In this manuscript, we show a simple, cost-effective, widely applicable platform to assemble 3D, free-standing tissues. We manipulate tissue deformation and observe its impact on the formation of gradients of angiogenic microenvironments and angiogenesis.
Results
Self-Remodeling Microfabricated Tissues.
We mimicked the early events of angiogenesis using a coculture of two cell types: human Umbilical Vein Endothelial Cells (hUVEC) as a well characterized EC type (14) and human Mesenchymal Stem Cells (hMSC) which can generate forces (15), produce VEGF-A (16) thus mimicking some aspects of interstitial stromal cells. Our microarray data, along with others (17), show that hMSC do not significantly express VEGFR-2. Indeed, in this coculture, immunofluorescence of VEGFR-2 was restricted to hUVEC (Fig. S1). We previously showed that this coculture supports the formation of a 3D network of PECAM-1+ vascular structures (VS) (18).
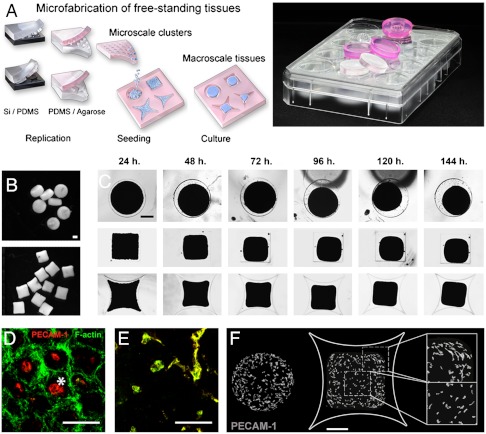
We used conventional soft lithography and micromolding to form nonadherent agarose templates (Fig. 1A). On these templates, we assembled tissues by sequentially aggregating cells into microscale clusters and millimeter-scale tissues (Fig. 1A). Resulting tissues are free-standing and biomaterial-free and can easily be manipulated (Fig. 1B). Tissues have an initial projection area of 1 mm2 and a height of 250 μm. Over five days of culture, tissues contracted autonomously, deformed stereotypically, and finally stabilized their shapes (Fig. 1C, Fig. S2). Disc-shaped tissues deformed isotropically (Fig. 1C, first row) whereas square-shaped tissues deformed anisotropically. Corners displaced and deformed more as compared to sides (Fig. 1C, second row, Fig. S2, p = 0.0015, n = 10). To compensate, we designed star-shaped tissues which progressively deformed into a square (Fig. 1C, third row). This anisotropic deformation suggested a heterogeneous distribution of internal forces. It was previously modeled that boundaries and sharp geometries generate greater forces as compared to regions away from these boundaries (9, 19, 20). Based on macroscopic behavior and cellular density, we estimated the local strain to be twofold higher in tissue corners as compared to tissue centers (Fig. S3).
Fig. 1.
Microfabricated tissues deform autonomously and form vascular structures in regions of high deformations. (A) Arrays of 3D millimeter-scale tissues are assembled by sequential aggregation of cells and microscale cell clusters in nonadherent micromolded wells. Arrays are replicated from PDMS stamps (transparent arrays) into agarose (pink arrays) and inserted in standard tissue culture plates. (B) Resulting tissues are 3D, millimeter-scale, free-standing and biomaterial-free (scale bar, 500 μm). (C) Microfabricated tissues contract, deform isotropically (top row) or anisotropically (second/third rows), and finally stabilize their shape (scale bar, 500 μm). (D_–_E) PECAM-1 is depicted in red and F-actin in green. Microscale cell clusters of hUVEC and hMSC first spontaneously form a PECAM-1+ core [D, asterisk, (scale bar, 100 μm)] and a PECAM-1- external layer. Microscale cell clusters generate an F-actin intense interface upon fusion with neighbors (24 h). (E) After 24 h, PECAM-1+ cells are F-actin+ and start sprouting [E, (scale bar, 100 μm)]. (F) Upon tissue deformation, PECAM-1 immunostaining reveals a preferential formation of VS in regions of high deformations [tissue periphery and corners, (scale bar, 500 μm)].
Endothelial Cell Sprouting and the Formation of Patterns of Vascular Structures.
In microscale clusters (first step, Fig. 1A, top row), hUVEC and hMSC separated into a core of PECAM-1+ cells (asterisk, Fig. 1D) and an external layer of PECAM-1- cells. Sorting probably occurred according to differences in cellular affinity (strength of adhesion molecules) and cortical tension which determines cell association (21). When pooled (second step, Fig. 1A, bottom row), microscale clusters fused with neighbors via the transient and peripheral expression of filamentous actin (F-actin, Fig. 1D, 24 h, Fig. S4). After 24 h, PECAM-1+ cells became F-actin intense, sprouted into VS (Fig. 1E) which branched and formed cord-like structures including few lumens. VS did not undergo full tubulogenesis but rather form small, interspersed lumens as described previously (18). This model is relevant to the early stage of vascular morphogenesis (13, 18, 20, 22). Angiogenic factors were produced locally by cells cultured in serum-free medium without any growth factors. Tissues contracted and deformed autonomously (no external forces applied). This model allows to couple endogenous tissue contractility and deformation to the formation of VS.
Upon tissues contraction and deformation (day 5), VS had formed preferentially in peripheral regions (Fig. 1F). The preferential formation of VS was consistently observed in disc-, star-, and triangle-shaped tissues (Fig. 1F, Fig. S5). In star- and triangle-shaped tissues, VS formed at higher density in corners as compared to sides (p = 0.0026 and p = 0.002 respectively, Fig. S5). We concluded that VS formed preferentially in regions of higher deformation.
Actin-Myosin Regulates Tissue Deformation.
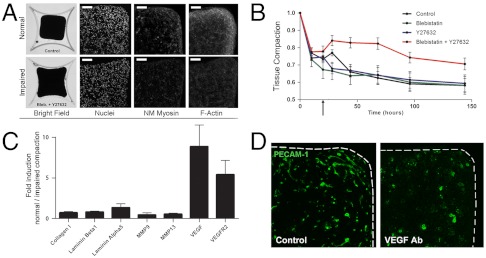
We impaired tissue contractility by blocking force-generating elements in cells. Non muscle Myosins II A, B, and C (NMM II) and F-actin are likely to be main generators of forces in hMSC (15). Blebistatin blocks the activation of NMM II ATPase and impairs its interaction with F-actin (23). Y27632 suppresses p160ROCK (Rho associated coiled-coil containing protein kinase) thus inhibiting the phosphorylation of Myosin Light Chain and the formation of F-actin stress fibers (23). Upon culture with Blebistatin or Y27632 alone, tissues contracted and deformed similarly to controls. However, upon exposure to the combination of the two inhibitors, tissues rapidly relaxed from 78% to 84% of their initial area (Fig. 2 A and B, see black arrow, p = 0.0002, n = 10). This relaxation demonstrated an internal tensional state generated by the actin-myosin complex. Upon inhibition of actin and myosin, tissue contraction was later impaired (Fig. 2 A and B, 144 h, 70% of the initial projected area vs. 58%, p = 6.10-7, n = 10). Tissue contraction was impaired but not stopped which implies a possible contribution of other cytoskeletal components or mechanisms. Based on cellular density and macroscopic behavior, we estimated the overall strain to be twofold lower when contraction was impaired (Fig. S3).
Fig. 2.
Tissue deformation is actin-myosin dependent and regulates VEGF-A and VEGFR-2 expression. (A, B) The combined inhibition of non muscle Myosin II (Blebistatin) and Rho associated protein kinase p160ROCK (Y27632) at 24 h (see black arrow in B) induces a relaxation of the internal tissue tension (28 h) and impairs tissue deformation (150 h). (A, top row) Tissue deformation results in local compaction (nuclear, DAPI staining) and a concentration of force-generating elements [nm Myosin II and F-actin immunofluorescence, (scale bar, 100 μm)] in regions of high deformations. (A, bottom row) This effect is impaired when deformation is impaired. (C) Upon impairment of tissue deformation, expression levels of VEGF-A and VEGFR-2 are lower. ECM related genes remain similar. Standard deviations, n = 3. (D) The formation of VS is strongly impaired when a VEGF scavenging antibody is added to the culture medium (PECAM-1 immunofluorescence).
Tissue Contraction Regulates the Gene Expression of VEGF-A and VEGFR-2.
To investigate the molecular changes underlying tissue contraction, we compared the expression of angiogenic genes in tissues undergoing normal and impaired contraction. Expression levels of ECM genes (collagen type I, laminin α1, laminin β5) and matrix-degrading metalloproteinase genes (MMP9, MMP13) remained unchanged. However, expression levels of VEGF-A and VEGFR-2 were higher in contracting tissues as compared to tissue with impaired contraction (day 3, Fig. 2C). We tested the contribution of VEGF to the formation of the VS by adding a scavenging antibody to the culture medium. Addition of the VEGF antibody dramatically reduced the formation of VS, demonstrating thus a critical dependence of VS upon VEGF (Fig. 2D). In a control assay, we showed, in cell pellets, that the VEGF antibody prevented the formation of VS by decreasing cellular proliferation (Fig. S6). We concluded that VEGF was necessary for the formation of VS and that tissue contraction did not directly induce the formation of VS.
Local Tissue Compaction Partially Contributes to the Formation of Vascular Patterns.
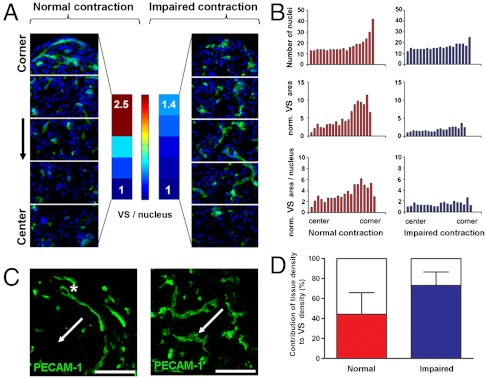
We then asked if the anisotropic deformation of tissues resulted in local changes in cellular density. Upon tissue contraction and deformation, cellular density gradually increased from the tissue center to the periphery (Fig. 2B, 3A). This gradient was impaired when contraction was impaired (Fig. 2B, 3A) and correlated with an increase of NMM II and F-actin (Fig. 2B).
Fig. 3.
Local tissue compaction partly contributes to the local formation of Vascular Structures. (A, B) VS form at a higher density in regions of high deformation (A, B, left, from the center to periphery: 2.5-fold increase in VS/nucleus). When tissue contraction and deformation are impaired, VS grow homogeneously throughout the tissue (A, B right). (B, C) Tissue deformation induces local tissue compaction (B, number of nuclei, top row) and the formation of thin VS including some lumens (asterisk, C left). VS preferentially elongate perpendicular to tissue displacement (A, C, arrows show the direction of displacement). This phenotype and directionality are impaired when contraction and deformation are impaired [A, C, right, (scale bars, are 100 μm)]. (D) Local tissue compaction partially contributes to the local formation of VS. The local increase in cellular density accounts for 48% of the local increase of VS (D, left, normal contraction). This contribution increases to 73% when contraction is impaired (D, right).
We then assessed the contribution of local tissue compaction on the local formation of VS. Cellular density was homogeneous in the tissue center but gradually increased in the peripheral regions (Fig. 3 A and B). In parallel, the density of VS increased by 4.8-fold and the density of VS/nucleus increased by 2.5-fold (Fig. 3 A and B). When tissue contractility was impaired, the gradient of cellular density was impaired and VS grew homogeneously throughout the tissues (Fig. 3 A and B). Higher local cellular density directly contributed to 48% of the increased density of VS (Fig. 3D). This measurement assessed that local tissue compaction induced more than a direct concentration of VS and suggested a more complex role for endogenous contractile forces. Indeed, upon impairment of tissue contraction, the contribution of cellular density increased to 73% (Fig. 3D).
Tissue Contraction Induces Changes in the Phenotype of Vascular Structures.
The phenotypes of VS changed across the tissue. In regions of high deformations, VS were thin, shaped and elongated perpendicular to the tissue displacement (Fig. 3C, left, white arrow shows the corner-to-center direction). This orientation of VS correlates with previous reports describing an elongation of EC perpendicular to externally applied mechanical stress (24). When endogenous contractility was impaired, VS grew homogeneously, were wider, tortuous, and randomly oriented (Fig. 3C right).
Vascular morphogenesis can be driven by local tissue hypoxia which regulates the production of multiple angiogenic factors (11). Tissues were designed with dimensions alleviating the formation of a hypoxic environment. However, we tested the possible formation of local hypoxic environments by looking at the distribution of HIF1α which translocates to the nuclei of hypoxic cells to activate angiogenic genes (11). Here, HIF1α was cytoplasmic and homogeneously expressed across the tissues. The cytoplasmic expression of HIF1a suggests that hypoxic levels are low and homogeneous across the tissues (Fig. S7).
Tissue Contractility Locally Regulates the Expression of VEGFR-2.
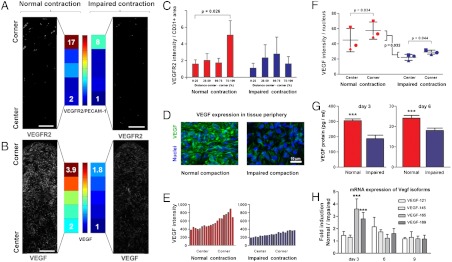
EC are mechanosensitive cells (10, 20) which can transcriptionally regulate the expression of the VEGFR-2 receptor based on the mechanical properties of the microenvironment (5, 10). VEGFR-2 mediates the major growth and permeability roles of VEGF-A (5). Based on the differences in VEGFR-2 gene expression level (Fig. 2C), we tested the possibility that EC respond to tissue deformation by spatially modulating the expression of VEGFR-2. VEGFR-2 expression was restricted to PECAM-1+ cells (Fig. S1), a cell-cell adhesion molecule which is thought to convey mechanical forces but whose expression is not regulated by mechanical forces (3). Upon tissue contraction, PECAM-1+ cells in regions of high deformation expressed a higher level of  area (8.5-fold increase) than cells in the central region (p = 0.026, n = 15, Fig. 4 A and C). When contraction was impaired, the expression of VEGFR-2/PECAM-1+ area decreased and became homogenous across the tissue (Fig. 4 A and C). This local increase in VEGFR-2 expression correlated with the formation of clusters of high fluorescent intensity at the cellular membrane (Fig. S1). This observation is consistent with previous reports describing the formation of VEGFR-2 complexes in response to mechanical shear stress (10). The region of VEGFR-2 overexpression was very localized (last 20% of the center to periphery distance). We concluded, based on the mRNA expression level (Fig. 2C) and on the differential in immunofluorescent intensity, that endogenous tissue contractility induced a quantitative and spatial regulation of the VEGFR-2 receptor.
area (8.5-fold increase) than cells in the central region (p = 0.026, n = 15, Fig. 4 A and C). When contraction was impaired, the expression of VEGFR-2/PECAM-1+ area decreased and became homogenous across the tissue (Fig. 4 A and C). This local increase in VEGFR-2 expression correlated with the formation of clusters of high fluorescent intensity at the cellular membrane (Fig. S1). This observation is consistent with previous reports describing the formation of VEGFR-2 complexes in response to mechanical shear stress (10). The region of VEGFR-2 overexpression was very localized (last 20% of the center to periphery distance). We concluded, based on the mRNA expression level (Fig. 2C) and on the differential in immunofluorescent intensity, that endogenous tissue contractility induced a quantitative and spatial regulation of the VEGFR-2 receptor.
Fig. 4.
Tissue contractility spatially regulates the expression of VEGF and VEGFR-2. (A, C) Upon tissue contraction and deformation, VEGFR-2 is expressed at higher levels in regions of high deformation (A, C, left, VEGFR-2 fluorescent intensity/PECAM-1+ area, 3.5-fold increase, n = 15, p = 0.026). This local increase in VEGFR-2 is impaired when tissue contractility is impaired [A, C right, (scale bars, are 100 μm)]. In regions of high deformation, VEGFR-2 forms clusters at the cellular membrane of EC (Fig. S1). (B, D, E, F) Upon normal contraction, a gradient of VEGF forms via local compaction and graded production. (B_–_E) Fluorescent intensity is twofold higher in regions of high deformation (B_–_E, left; C is a close up of corner regions). This observation is confirmed at a mRNA level (Fig. S8_A_). The gradient of VEGF is impaired when tissue contractility is impaired (B, E, right). (F) Upon normalization to the number of nuclei, the fluorescent intensity of VEGF/nucleus is higher in regions of high deformation (p = 0.034) and higher in contracting tissues than in tissues whose contraction is impaired (p = 0.032, see also Fig. S8_B_). (G) The production of VEGF (ELISA assay) is higher in cell pellets undergoing normal contraction than when contraction is impaired (p < 0.001). (H) Impaired contraction specifically affects the expression of VEGF isoforms 165 and 189 (ECM-binding isoforms). Expression levels of isoforms 121 and 145 (non-ECM-binding isoforms) remain unchanged (p < 0.001).
Tissue Contractility Regulates the Formation of a Gradient of VEGF.
Based on the differences in VEGF-A gene expression level (Fig. 2C) and because hMSC are mechanosensitive cells (15), we tested the possibility that tissue contractility spatially regulated the expression of VEGF-A proteins. We cut the tissue corners and observe that the expression of VEGF-A mRNA was 1.5-fold higher in corners as compared to centers (n = 3, p = 0.07, Fig. S8_A_). Immunofluorescent staining (all VEGF isoforms) revealed the formation of a gradient of VEGF: fluorescent intensity in tissue corners was twofold higher than in tissue centers (Fig. 4 B, D and E). When contraction was impaired, the gradient of VEGF fluorescent intensity was impaired (Fig. 4E) and the total fluorescent intensity expressed in the tissue decreased by twofold (p = 0.032, n = 3, Fig. 4F). Upon normalization to the number of nuclei, cells in the regions of high deformation had higher fluorescent intensity as compared to cells located in the center (p = 0.034, n = 15, Fig. 4F). We assessed the validity of the measurement of fluorescent intensity using a 2D assay: Deferoxamine [DFO, a HIF-1a inducer (25)] induces a twofold increase in VEGF imunofluorescent staining intensity. This increase correlated with a fivefold increase in the intracellular production of VEGF (ELISA assay for isoforms 165 and 121 on cell lysate, Fig. S9). We further assessed the role of cell-generated forces on the production of VEGF proteins in cell pellets (0.5 million cells, 92% hMSC, and 8% hUVEC). Pellets whose contraction was impaired produce 1.6-fold less VEGF proteins than pellets undergoing normal contraction (Fig. 4G, ELISA assay on culture medium, n = 3, p < 0.001). These observations suggest that tissue contraction regulates the expression of VEGF. We concluded that tissue deformation regulates the formation of a VEGF gradient formed via a local tissue compaction and a local regulation of VEGF expression.
Cell-Generated Forces Modulate the Production of ECM Binding Isoforms VEGF189 and VEGF189.
The production of VEGF is regulated via mRNA splicing which induces the formation of various isoforms (26). VEGF isoforms differ in heparin-binding domains (HBD) which affects their capacity to bind to cellular membranes and ECM. Such property could modulate their localization in the extracellular space (26) and is suited for the creation of molecular gradients (27). VEGF121, the shortest isoform, lacks HBD, is weakly acidic and freely diffusible upon secretion. VEGF189, a longer isoform, has two HBD and is mostly sequestrated in the ECM and at the cell surface. VEGF165 possesses intermediate properties, has a moderate affinity for heparin and is both soluble and bounded (26). Using isoform-specific polymerase chain reaction, we observed in cell pellets that VEGF165 and VEGF189 expression was 2.5-fold higher upon normal contraction as compared to impaired contraction (Fig. 4H, p < 0.001, n = 3). On the opposite, the expression of VEGF121 and VEGF145 remained unchanged. This observation suggests that tissue deformation influences the expression of longer, heparin-binding VEGF isoforms. We speculate that the ECM and cellular-binding properties of the two isoforms reinforce the formation of the VEGF gradient by preventing protein diffusion.
Tissue Contractility Induces Local Proliferation of Endothelial Cells.
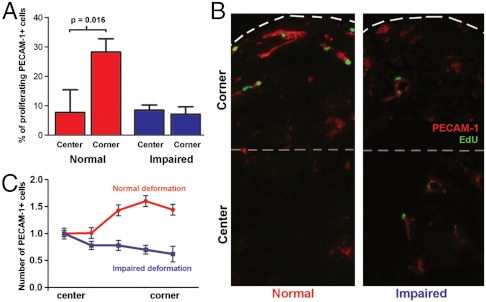
Because tissue deformation regulates the microenvironmental concentration of VEGF-A and VEGFR-2 and because VEGF-A is a potent mitogen acting via VEGFR-2, we quantified the local proliferation of EC. Using pulsed exposure to EdU (5-ethynyl-2’-deoxyuridine) (5-ethynyl-2’-deoxyuridine exposure over twelve hours, daily time point over five days), we observed that EdU+ cells were limited to PECAM-1+ cells. EdU+ cells represented 0.3 to 1% of the total cell number (Fig. S10). Upon tissue deformation, VS in regions of high deformation included more proliferating cells (EdU+) than VS in the tissue center (Fig. 5 A and B, 28% vs. 8%, p = 0.016). When deformation was impaired, the density of proliferating cells was lower (8%) and their distribution homogenous (Fig. 5 A and B). Finally, we quantified the number of PECAM-1+ cells per VS. Upon normal deformation, the number of PECAM-1+ cells in VS increased from tissue center to corner (Fig. 5C). When contraction was impaired, the number of PECAM-1+ cells in VS remained unchanged (Fig. 5C). VS in tissue corners included more PECAM-1+ cells upon normal contraction as compared to impaired contraction (Fig. 5C). We concluded that VS in regions of high deformation formed via a higher EC proliferation which resulted in a higher number of cells per VS.
Fig. 5.
Tissue deformation induces a local differential on endothelial cell proliferation. (A, B) EdU pulsed incorporation shows a higher percentage of proliferating EC in regions of high deformations. This local differential in EC proliferation is abolished when tissue deformation is impaired. (C) VS in regions of high deformation include a higher number of EC in deforming tissues as compared to tissues whose deformation is impaired.
We propose that tissue deformation can spatially modulate the expression of VEGF-A and VEGFR-2, and contribute to the formation of patterns of VS by inducing local differentials in EC proliferation.
Discussion
Vascular patterns can emerge from the spatial regulation of angiogenic signals including the concentration of and the EC sensitivity to mitogens (5, 28). Here, we suggest that tissue contraction and deformation can spatially modulate cellular density, the local expression of VEGF-A and VEGFR-2, and induce a local differential in EC proliferation.
Morphogen gradients form by regulated production, retention, controlled release, diffusion, and degradation (12, 29). Our data suggest that interstitial cells can form gradients of angiogenic microenvironments via local tissue compaction and a local regulation of VEGF signaling. The regulation of ECM-binding VEGF isoforms (VEGF165 and VEGF189) by tissue contractility could prevent VEGF protein diffusion and reinforce molecular gradients (30).
The agonist activity of VEGF on VEGFR-2 (i) guides the sprouting of the cell situated at the tip of the VS via local gradients (filopodia/lamellipodia extension along a differential of concentration) and (ii) induces the proliferation of the following, stalk EC based on local concentration (absolute concentration) (12). Our observations suggest that the higher local concentration of VEGF and of the local expression of the corresponding receptor VEGFR-2 induced a local differential in the proliferation of the stalk cells. Previous studies revealed that EC shape and cytoskeletal tension regulates a discrete cell cycle check-point at the G1/S border. The sensitivity to a mitogen is partly modulated by mechanical factors via the cdk inhibitor p27 and the activator cyclin D (31, 32). We speculate that the local proliferation of EC results from an appropriate combination of cytoskeletal tension and VEGF-A/VEGFR-2 density.
This experiment links tissue contraction and deformation to the formation of a gradient of angiogenic microenvironments. This correlation might explain, in further investigations, how contractile forces generated within a tissue or between adjacent tissues might have a long-range control over capillary formation. These findings may be relevant in wound healing -vascular morphogenesis was reported to be mechanically driven by the contracting wound (2)-, or in cancer biology -tumors are dependent on angiogenesis and stiffer than normal tissues due to an elevated endogeneous tension (33).
Self-organization can emerge from tissue contractility and geometry. Such properties induce an heterogeneous tissue deformation and pattern the behavior of cells. We suggest that tissue contractility and deformation can modulate the formation of gradients of angiogenic microenvironments and angiogenesis. We speculate that such a mechanism might contribute to the long-range patterning of the vascular system.
Methods
Agarose Chips.
Micropatterned agarose chips for nonadherent cell culture were formed by replica molding. Patterned elastomeric stamps of poly(dimethylsiloxane) (PDMS; Sylgard 184, Dow Corning) were replicated from either etched silicon wafers or SU-8/silicon wafers. PDMS stamps were used to routinely replicate the microstructures in 4% agarose.
Coculture.
A coculture of 92% hMSC and 8% huvEC was used for all experiments. The culture medium for the coculture (Exp. Medium) was serum-free and composed of Dulbecco’s Modified Eagle’s Medium supplemented with 10-7 M dexamethasone, 50 mg/mL ascorbate 2-phosphate, 40 mg/mL proline, 100 mg/mL pyruvate, and 50 mg/mL ITS 1 Premix (Becton-Dickinson, MA: 6.25 mg/mL insulin, 6.25 mg/mL transferrin, 6.25 ng/mL selenious acid, 1.25 mg/mL bovine serum albumin, 5.35 mg/mL linoleic acid).
Supplementary Material
Supporting Information
Acknowledgments.
We thank M. Berger, K. Ma for their technical suggestions and assistance; H.Tank and J. de Boer for comments on the manuscript. This work was supported by funds from the STW (Stichting Technologie en Wetenschappen), The Netherlands, project TKG 6716.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Perryn ED, Czirok A, Little CD. Vascular sprout formation entails tissue deformations and VE-cadherin-dependent cell-autonomous motility. Dev Biol. 2008;313:545–555. doi: 10.1016/j.ydbio.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15:657–664. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 3.Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 4.Nelson CM, Chen CS. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J Cell Sci. 2003;116:3571–3581. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 5.Mammoto A, et al. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–887. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 7.Herbert SP, Stainier DY. Molecular control of endothelial cell behavior during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 9.Nelson CM, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shay-Salit A, et al. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 12.Gerhardt H, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivron NC, et al. Tissue assembly and organization: developmental mechanisms in microfabricated tissues. Biomaterials. 2009;30:4851–4858. doi: 10.1016/j.biomaterials.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Laib AM, et al. Spheroid-based human endothelial cell microvessel formation in vivo. Nat Protoc. 2009;4:1202–1215. doi: 10.1038/nprot.2009.96. [DOI] [PubMed] [Google Scholar]
- 15.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun. 2009;390:834–838. doi: 10.1016/j.bbrc.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furumatsu T, et al. Vascular endothelial growth factor principally acts as the main angiogenic factor in the early stage of human osteoblastogenesis. J Biochem. 2003;133:633–639. doi: 10.1093/jb/mvg081. [DOI] [PubMed] [Google Scholar]
- 18.Rouwkema J, de Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–2693. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 19.Knapp DM, Barocas VH. Estimation of cell traction and migration in an isometric cell traction assay. AIChE J. 2004;45:2628–2640. [Google Scholar]
- 20.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 22.Rivron NC, Liu JJ, Rouwkema J, de Boer J, van Blitterswijk CA. Engineering vascularised tissues in vitro. Eur Cell Mater. 2008;15:27–40. doi: 10.22203/ecm.v015a03. [DOI] [PubMed] [Google Scholar]
- 23.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 24.Yung YC, Chae J, Buehler MJ, Hunter CP, Mooney DJ. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci USA. 2009;106:15279–15284. doi: 10.1073/pnas.0905891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beerepoot LV, Shima DT, Kuroki M, Yeo KT, Voest EE. Up-regulation of vascular endothelial growth factor production by iron chelators. Cancer Res. 1996;56:3747–3751. [PubMed] [Google Scholar]
- 26.Giacca M. Non-redundant functions of the protein isoforms arising from alternative splicing of the VEGF-A pre-mRNA. Transcr. 2010;1:149–153. doi: 10.4161/trns.1.3.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa CR, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Ingber DE. A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp Cell Res. 2002;275:255–264. doi: 10.1006/excr.2002.5504. [DOI] [PubMed] [Google Scholar]
- 33.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information