Evolution of the metabolic and regulatory networks associated with oxygen availability in two phytopathogenic enterobacteria (original) (raw)
Abstract
Background
Dickeya dadantii and Pectobacterium atrosepticum are phytopathogenic enterobacteria capable of facultative anaerobic growth in a wide range of O2 concentrations found in plant and natural environments. The transcriptional response to O2 remains under-explored for these and other phytopathogenic enterobacteria although it has been well characterized for animal-associated genera including Escherichia coli and Salmonella enterica. Knowledge of the extent of conservation of the transcriptional response across orthologous genes in more distantly related species is useful to identify rates and patterns of regulon evolution. Evolutionary events such as loss and acquisition of genes by lateral transfer events along each evolutionary branch results in lineage-specific genes, some of which may have been subsequently incorporated into the O2-responsive stimulon. Here we present a comparison of transcriptional profiles measured using densely tiled oligonucleotide arrays for two phytopathogens, Dickeya dadantii 3937 and Pectobacterium atrosepticum SCRI1043, grown to mid-log phase in MOPS minimal medium (0.1% glucose) with and without O2.
Results
More than 7% of the genes of each phytopathogen are differentially expressed with greater than 3-fold changes under anaerobic conditions. In addition to anaerobic metabolism genes, the O2 responsive stimulon includes a variety of virulence and pathogenicity-genes. Few of these genes overlap with orthologous genes in the anaerobic stimulon of E. coli. We define these as the conserved core, in which the transcriptional pattern as well as genetic architecture are well preserved. This conserved core includes previously described anaerobic metabolic pathways such as fermentation. Other components of the anaerobic stimulon show variation in genetic content, genome architecture and regulation. Notably formate metabolism, nitrate/nitrite metabolism, and fermentative butanediol production, differ between E. coli and the phytopathogens. Surprisingly, the overlap of the anaerobic stimulon between the phytopathogens is also relatively small considering that they are closely related, occupy similar niches and employ similar strategies to cause disease. There are cases of interesting divergences in the pattern of transcription of genes between Dickeya and Pectobacterium for virulence-associated subsystems including the type VI secretion system (T6SS), suggesting that fine-tuning of the stimulon impacts interaction with plants or competing microbes.
Conclusions
The small number of genes (an even smaller number if we consider operons) comprising the conserved core transcriptional response to O2 limitation demonstrates the extent of regulatory divergence prevalent in the Enterobacteriaceae. Our orthology-driven comparative transcriptomics approach indicates that the adaptive response in the eneterobacteria is a result of interaction of core (regulators) and lineage-specific (structural and regulatory) genes. Our subsystems based approach reveals that similar phenotypic outcomes are sometimes achieved by each organism using different genes and regulatory strategies.
Background
Dickeya dadantii and Pectobacterium atrosepticum cause soft-rot diseases characterized by maceration of plant tissues through the action of multiple secreted plant cell wall degrading enzymes [1]. D. dadantii strain 3937 (D. dadantii) was originally isolated from African violet and is better known by its former name Erwinia chrysanthemi 3937, and P. atrosepticum strain SCRI1043 was isolated from potato [2,3], but individual strains and these genera as a whole have broad host range, affecting over 50% of angiosperm plant orders [4]. They are a world-wide problem for economically important crops and ornamental plants [5]. Both D. dadantii and P. atrosepticum are relatively well-studied model organisms for understanding the molecular biology of soft-rot pathogenesis [6,7]. Like most enterobacteria, Dickeya and Pectobacterium are facultative anaerobes that are able to grow with or without O2 by shifting metabolic strategies from aerobic respiration to anaerobic respiration or fermentation [8]. They experience a wide range of O2 concentrations in different plant tissues and natural reservoirs like soil and water [9]. Lack of O2 is thought to be one of the factors that can trigger rapid expansion of latent infections leading to devastating post-harvest destruction of entire crops in storage [5].
Apart from a small number of important virulence factors, such as pectinases PelA, D and E [10], little is known about which genes are regulated by O2 availability in these two soft-rot pathogens. In contrast, O2 -regulated genes have been extensively studied in the model animal-associated enterobacteria Escherichia coli and Salmonella enterica, where available data includes genome-scale expression profiling of the anaerobic stimulon of wild-type strains as well as mutants of key regulators FNR, ArcA, NarPQ and NarXL [11-16]. Most of these regulators, and many of the known target genes associated with anaerobic metabolism are conserved across the enterobacteria and among more distantly related gamma-proteobacteria [17]. Thus, we expect a conserved core transcriptional response to O2 limitation that includes the basic cellular machinery required to generate energy in an anaerobic environment. Yet, some of the O2-regulated genes found in E. coli are simply not present in other genera, and other genes that may be O2 - responsive in the phytopathogens are not shared with animal-associated organisms. A more complete picture of the anaerobic stimulon of plant-pathogenic enterobacteria requires direct experimentation in these organisms.
Here, we characterize the transcript profiles of P. atrosepticum and D. dadantii grown with and without O2 under controlled laboratory conditions. We performed our experiments in defined media to illuminate solely the O2-responsive regulatory network. These conditions are not expected to mirror the complex, dynamic, and largely undefined environment of a plant host. Rather, we seek to identify components of the anaerobic stimulon for follow-up experimentation, provide a framework for identification of characteristics of the response to O2 in more complex datasets, and investigate the conservation and divergence in O2-mediated regulation among the enterobacteria.
Results and discussion
A large number of genes are in the O2-response stimulon
Using a conditional false-discovery rate (cFDR) of 0.01 (permissive criterion for differential expression), EBarrays [18] detects over 2204 differentially expressed genes in D. dadantii (48.5%), and 599 in P. atrosepticum (13.4%). Particularly in D. dadantii where the extremely good agreement between replicates improves the sensitivity, many of these are genes that show small changes between the aerobic and anaerobic conditions. Requiring the changes (anaerobic/aerobic) be at least 3-fold reduces the numbers to 443 differentially expressed genes in D. dadantii (9.8%) and 320 genes in P. atrosepticum (7.3%). Thus, a substantial fraction of each genome is involved in the anaerobic stimulon even using our stringent criteria (cFDR = 0.01 and fold change > 3), consistent with published reports for E. coli K-12 [12] despite differences in array platforms and analysis methods. The most extreme and conserved transcriptional responses are associated with anaerobic metabolism indicating that these organisms are responding to O2 availability.
Differences in the genetic architecture of the O2-responsive stimulon are illuminated by a biological subsystems approach
Pectobacterium and Dickeya (with Brenneria) form a monophyletic clade of phytopathogens distinct from other genera of enterobacteria, like Escherichia and Salmonella [4], where the transcriptional response to O2 has been extensively studied. Nevertheless, all free-living enterobacteria with sequenced genomes share a substantial fraction of ancestral genes that are thought to reflect clonal or vertical descent. Gene losses, duplications, and lateral gene transfers lead to content differences among genomes. Little is known about the extent to which these types of events factor into variation in the response of different enterobacteria to O2 availability.
We used OrthoMCL [19] to cluster protein-coding genes from D. dadantii, P. atrosepticum, and E. coli, and used these ortholog groups to compare transcript profiles across organisms. Genes for some of the well-characterized components of the E. coli anaerobic energy metabolism architecture are entirely missing from one or both of the phytopathogens. Some functional equivalents within and between organisms are carried out by genes that are not orthologous. For these reasons, we find it useful to approach the comparison from a biological subsystem-oriented perspective that accommodates the complexity of the evolutionary history and functional redundancy, and considers related gene products, such as genes associated with a single molecular complex or biological process [20]. In the following sections, we report our findings from a largely statistical perspective. We discuss the possible biological significance in later sections following a subsystems oriented approach that groups orthologous, paralogous and even analogous genes with functionally related products.
Transcriptional response to O2 limitation for genes orthologous in the two phytopathogens
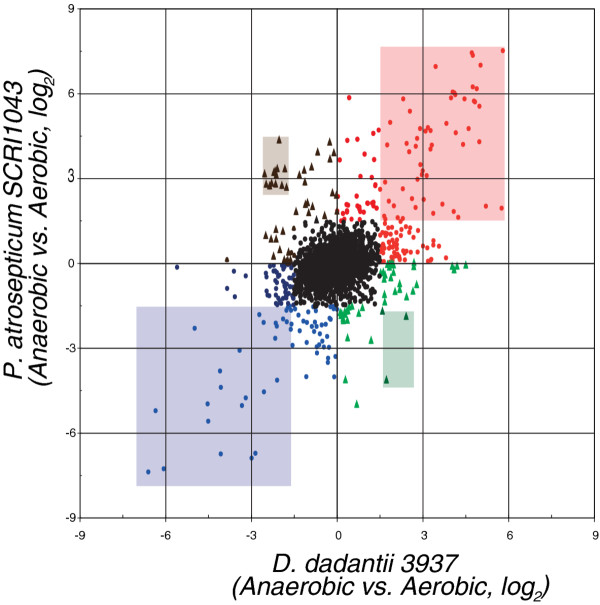
In total, our OrthoMCL analysis clustered 3110 D. dadantii genes and 3094 P. atrosepticum genes into groups that contained at least one gene from both phytopathogens. Of these, 2889 groups are simple 1-1 cases and their expression patterns are summarized in Figure 1. Overall, far more of the 1-1 orthologs differentially expressed under aerobic and anaerobic conditions exhibit congruent rather than divergent expression patterns. This observation is not surprising since the two pathogens supposedly share a common ancestor and occupy similar ecological niches. Among genes that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3), 247 D. dadantii genes and 196 P. atrosepticum genes have predicted orthologs in the other genome (Additional File 1). Of these, 96 show fold changes > 3 for both orthologs. Eighty-one ortholog sets show congruent changes in expression in both organisms; transcripts for 51 are "up-regulated" (transcript abundance is higher under anaerobic conditions) and 30 are "down-regulated" (transcript abundance is lower under anaerobic conditions). Together these represent a minimal conserved transcriptional response shared by both plant pathogens (Table 1). Not surprisingly, the majority of the up-regulated genes encode essential cellular functions under anaerobic conditions. Beside these, 15 ortholog sets change expression in opposite directions in D. dadantii and P. atrosepticum (Table 2). Interestingly, 12 of the 15 ortholog sets belong to a single biological system, namely type VI secretion system.
Figure 1.
Scatterplot of fold changes for the 2889 orthologous genes in D. dadantii vs P. atrosepticum. Fold change values are represented as log ratio of expression in anaerobic vs. aerobic condition. The categories represented are: Statistically significant up-regulation with fold changes greater than 3 (log2 > 1.5) in both organisms (orange box), Statistically significant down-regulation with fold changes greater than 3 (log2 > 1.5) in both organisms (purple box), Statistically significant expression in both organisms but in opposite directions (up-regulated in D. dadantii: green box, down-regulated in D. dadantii: brown box) and Equivalently expressed in both organisms (black). At least 81 genes are part of a highly differentially regulated core of genes conserved across D. dadantii and P. atrosepticum (orange and purple boxes) and this minimum core grows to 222 genes if we allow smaller, but statistically significant differences between aerobic and anaerobic samples (red and blue filled circles). We observe divergent expression patterns in which orthologs are up-regulated > 3- fold in one organism and down-regulated > 3- fold (log2 > 1.5) in the other organism for 15 ortholog sets (brown and green boxes), and at least 35 additional ortholog sets show a less extreme, but nevertheless divergent pattern (brown and green filled triangles). Genes that are differentially expressed in one organism only, have not been distinguished in the figure. Given the overall larger number of genes called differentially expressed for D. dadantii relative to P. atrosepticum, there are many cases where the ortholog in one organism is called differentially expressed (and with fold change > 3), while the ortholog in the other genome is not (966 ortholog groups in D. dadantii and 77 different groups in P. atrosepticum). In most cases both members of the ortholog group trend in the same direction (766 orthologs) rather than exhibiting divergent expression (277 orthologs).
Table 1.
Minimal conserved anaerobic transcriptional response shared by D. dadantii 3937 and P. atrosepticum SCRI1043 and its comparison to E. coli.
| ASAP Feature ID | Gene Name | Product | Fold change | ||||
|---|---|---|---|---|---|---|---|
| D. dadantii | P. atrosepticum | E. coli | D. dadantii | P. atrosepticum | E. coli | ||
| A. Orthologs down-regulated ≥3-fold in both D. dadantii and P. atrosepticum | |||||||
| ABF-0016541 | ABL-0060576 | ABE-0009869 | exbB | membrane spanning protein in TonB-ExbB-ExbD complex | -6.6 | -3.5 | -3.3 |
| ABF-0020799 | ABL-0061445 | ABE-0007383 | nrdA | ribonucleotide reductase of class Ia (aerobic), alpha subunit | -4.3 | -4.7 | -2.2* |
| ABF-0020798 | ABL-0061444 | ABE-0007386 | nrdB | ribonucleotide reductase of class Ia (aerobic), beta subunit | -3.7 | -3.9 | -2.0* |
| ABF-0017172 | ABL-0063446 | ABE-0009169 | sdaC | serine transporter | -3.1 | -3.2 | 1.3* |
| ABF-0020233 | ABL-0063426 | sfuA | iron-binding periplasmic protein | -9.1 | -26.9 | ||
| ABF-0019943 | ABL-0062041 | ABE-0003583 | yceI | secreted protein | -3.1 | -5.0 | -6.0 |
| ABF-0019942 | ABL-0062042 | ABE-0003585 | yceJ | predicted cytochrome b561 | -4.4 | -4.3 | -8.6 |
| ABF-0020068 | ABL-0062100 | ABE-0005694 | ydiU | hypothetical protein | -3.4 | -4.2 | -2.2* |
| ABF-0015019 | ABL-0064500 | ABE-0012474 | yigI | conserved protein | -2.9 | -7.4 | -3.1 |
| ABF-0019535 | ABL-0061414 | ABE-0001021 | ykgM | predicted ribosomal protein | -97.0 | -165.4 | -1.4* |
| ABF-0019536 | ABL-0061413 | ABE-0285027 | ykgO | predicted ribosomal protein | -66.7 | -153.3 | |
| ABF-0017084 | ABL-0062748 | ABE-0006191 | znuA | zinc ABC transporter, periplasmic-binding protein ZnuA | -4.3 | -17.5 | -1.2* |
| ABF-0017082 | ABL-0062750 | ABE-0006201 | znuB | high-affinity zinc transport system membrane protein | -3.3 | -2.9 | 1.4* |
| ABF-0017083 | ABL-0062749 | ABE-0006198 | znuC | high-affinity zinc transport system ATP-binding protein | -3.1 | -2.8 | -1.1* |
| ABF-0018178 | ABL-0063535 | Iron dicitrate-binding protein | -10.6 | -8.4 | |||
| ABF-0018571 | ABL-0064593 | putative iron ABC transporter permease protein | -5.9 | -23.3 | |||
| ABF-0018572 | ABL-0064592 | putative iron ABC transporter, periplasmic-binding protein | -7.9 | -117.8 | |||
| ABF-0018573 | ABL-0064591 | putative iron ABC transporter ATP-binding protein | -7.3 | -104.7 | |||
| ABF-0018864 | ABL-0063083 | TonB-dependent ferric achromobactin receptor | -31.6 | -4.9 | |||
| ABF-0019222 | ABL-0064073 | putative ABC transporter substrate-binding protein | -16.8 | -106.2 | |||
| ABF-0019223 | ABL-0064074 | putative ABC transporter substrate-binding protein | -22.8 | -47.8 | |||
| ABF-0019568 | ABL-0061801 | putative transport system permease protein | -10.1 | -32.4 | |||
| ABF-0019569 | ABL-0061802 | putative ABC transporter substrate-binding protein | -23.1 | -31.3 | |||
| ABF-0019570 | ABL-0061804 | putative ABC transporter substrate-binding protein | -16.7 | -20.8 | |||
| ABF-0019572 | ABL-0061805 | putative ABC transporter substrate-binding protein | -17.0 | -13.9 | |||
| ABF-0020094 | ABL-0060663 | ABC-type transporter, periplasmic component | -4.5 | -6.3 | |||
| ABF-0020095 | ABL-0060662 | ABC transporter, permease protein | -5.9 | -4.3 | |||
| ABF-0020096 | ABL-0060661 | ABC transporter, permease protein | -3.0 | -4.4 | |||
| ABF-0020097 | ABL-0060660 | ABC transporter ATP-binding protein | -3.4 | -3.2 | |||
| ABF-0046525 | ABL-0064075 | ABC transporter substrate-binding protein | -81.6 | -37.0 | |||
| B. Orthologs up-regulated ≥3-fold in both D. dadantii and P. atrosepticum | |||||||
| ABF-0020642 | ABL-0062590 | ABE-0004164 | adhE | iron-dependent alcohol dehydrogenase | 16.8 | 3.6 | 3.9 |
| ABF-0018570 | ABL-0064258 | ABE-0002090 | ahpC | alkyl hydroperoxide reductase, C22 subunit | 2.8 | 6.1 | -2.2* |
| ABF-0019339 | ABL-0060528 | budC | 2,3-butanediol dehydrogenase | 36.8 | 4.1 | ||
| ABF-0018628 | ABL-0061786 | ABE-0013503 | dcuB | C4-dicarboxylate transporter DcuB | 16.4 | 66.3 | 8.5 |
| ABF-0018914 | ABL-0063040 | ABE-0002776 | dps | Fe-binding and storage protein | 3.2 | 2.9 | -2.5* |
| ABF-0019603 | ABL-0062860 | ABE-0003073 | focA | formate transporter | 8.1 | 4.8 | 3.1 |
| ABF-0017842 | ABL-0064288 | ABE-0013604 | frdA | fumarate reductase (anaerobic) NAD/flavoprotein subunit | 7.5 | 11.2 | 3.5 |
| ABF-0017841 | ABL-0064289 | ABE-0013602 | frdB | fumarate reductase (anaerobic), Fe-S subunit | 8.8 | 8.6 | 5.3 |
| ABF-0017839 | ABL-0064290 | ABE-0013598 | fr d C | fumarate reductase (anaerobic), membrane anchor subunit | 7.9 | 9.6 | 3.7 |
| ABF-0017837 | ABL-0064291 | ABE-0013595 | frdD | fumarate reductase (anaerobic), membrane anchor subunit | 7.0 | 7.8 | 4.5 |
| ABF-0019825 | ABL-0062949 | ABE-0002893 | grxA | glutaredoxin 1,coenzyme for ribonucleotide reductase | 4.3 | 3.1 | 1.0* |
| ABF-0017078 | ABL-0061498 | hoxN | high-affinity nickel transport protein | 6.7 | 17.6 | ||
| ABF-0017349 | ABL-0061473 | ABE-0009830 | hybB | predicted hydrogenase 2 cytochrome b type component | 5.4 | 18.8 | 2.6* |
| ABF-0017353 | ABL-0061476 | ABE-0009824 | hybE | hydrogenase 2-specific chaperone | 5.7 | 15.3 | 2.1* |
| ABF-0017346 | ABL-0061471 | ABE-0009834 | hybO | hydrogenase 2, small subunit | 3.4 | 18.1 | 14.6 |
| ABF-0015747 | ABL-0061483 | ABE-0008931 | hycI | protease involved in processing C-terminal end of HycE | 9.6 | 25.8 | 1.4* |
| ABF-0015752 | ABL-0061495 | ABE-0008919 | hydN | formate dehydrogenase-H, ferredoxin subunit | 55.3 | 183.5 | 1.7* |
| ABF-0015735 | ABL-0061493 | 2 orthologs | hyfA | hydrogenase 4, 4Fe-4S subunit | 32.2 | 128.0 | MO |
| ABF-0015736 | ABL-0061492 | 2 orthologs | hyfB | hydrogenase 4, membrane subunit | 26.7 | 163.1 | MO |
| ABF-0015737 | ABL-0061491 | 2 orthologs | hyfC | hydrogenase 4, membrane subunit | 10.9 | 123.6 | MO |
| ABF-0015738 | ABL-0061490 | ABE-0008185 | hyfD | hydrogenase 4, membrane subunit | 26.2 | 173.6 | -1.7* |
| ABF-0015739 | ABL-0061489 | ABE-0008188 | hyfE | hydrogenase 4, membrane subunit | 29.4 | 72.0 | 1.5* |
| ABF-0015740 | ABL-0061488 | ABE-0008191 | hyfF | hydrogenase 4, membrane subunit | 15.8 | 57.3 | 2.7* |
| ABF-0015741 | ABL-0061487 | 2 orthologs | hyfG | hydrogenase 4, subunit | 26.7 | 75.1 | MO |
| ABF-0015742 | ABL-0061486 | ABE-0008942 | hyfH | hydrogenase 4, Fe-S subunit | 17.0 | 64.9 | 1.9* |
| ABF-0015744 | ABL-0061485 | 2 orthologs | hyfI | hydrogenase 4, Fe-S subunit | 17.5 | 62.2 | MO |
| ABF-0015745 | ABL-0061484 | 2 orthologs | hyfJ | predicted processing element hydrogenase 4 | 31.3 | 46.9 | MO |
| ABF-0017358 | ABL-0061480 | ABE-0008960 | hypB | GTP hydrolase involved in nickel liganding into hydrogenases | 5.8 | 41.4 | 2.3* |
| ABF-0020729 | ABL-0061479 | ABE-0008962 | hypC | [NiFe] hydrogenase metallocenter assembly protein HybG | 21.1 | 18.4 | 3.8 |
| ABF-0017360 | ABL-0061478 | ABE-0008965 | hypD | protein required for maturation of hydrogenases | 3.6 | 31.6 | 2.4* |
| ABF-0047122 | ABL-0062652 | ABE-0006058 | manZ | mannose-specific enzyme IID component of PTS | 3.9 | 4.1 | -2.1* |
| ABF-0016556 | ABL-0060593 | ABE-0013865 | nrdD | anaerobic ribonucleoside-triphosphate reductase | 17.8 | 24.4 | 4.4 |
| ABF-0016554 | ABL-0060592 | ABE-0013860 | nrdG | anaerobic ribonucleotide reductase activating protein | 4.9 | 6.2 | 4.5 |
| ABF-0017768 | ABL-0062712 | ABE-0003800 | pepT | peptidase T | 31.3 | 19.7 | 3.6 |
| ABF-0019604 | ABL-0062861 | 2 orthologs | pflB | pyruvate formate lyase I | 10.3 | 3.4 | MO |
| ABF-0174126 | ABL-0064936 | ABE-0003227 | rmf | ribosome modulation factor | 8.3 | 3.2 | 1.9* |
| ABF-0015967 | ABL-0063825 | ABE-0008501 | trxC | thioredoxin 2 | 7.0 | 21.3 | -3.8 |
| ABF-0016966 | ABL-0061596 | ABE-0002386 | ybfA | predicted protein | 6.7 | 3.9 | 2.2* |
| ABF-0019390 | ABL-0062816 | ABE-0003125 | ycbJ | conserved protein | 6.1 | 4.9 | 3.3 |
| ABF-0018000 | ABL-0062073 | ABE-0003740 | ycfP | conserved protein | 3.1 | 3.2 | 1.7* |
| ABF-0020593 | ABL-0063322 | ABE-0007565 | yfbS | predicted transporter | 3.5 | 4.6 | 1.0* |
| ABF-0020590 | ABL-0063324 | ABE-0007571 | yfbU | conserved protein | 3.2 | 7.3 | 1.4* |
| ABF-0020347 | ABL-0063577 | ABE-0008489 | yfiD | pyruvate formate lyase subunit | 18.8 | 3.1 | 5.9 |
| ABF-0018102 | ABL-0060954 | ABE-0010380 | yhbU | predicted peptidase (collagenase-like) | 8.5 | 25.5 | 5.9 |
| ABF-0018103 | ABL-0060953 | ABE-0010382 | yhbV | predicted protease | 9.9 | 18.0 | 3.7 |
| ABF-0015647 | ABL-0060483 | ABE-0010667 | yhdH | predicted oxidoreductase, Zn-dependent and NAD(P)-binding | 4.9 | 3.0 | 1.8* |
| ABF-0020757 | ABL-0062521 | ABE-0005319 | ynfK | predicted dethiobiotin synthetase | 9.6 | 16.3 | 5.3 |
| ABF-0017163 | ABL-0063441 | putative membrane protein | 7.8 | 8.8 | |||
| ABF-0018208 | ABL-0060635 | hypothetical protein | 14.1 | 30.7 | |||
| ABF-0018787 | ABL-0063809 | lactoylglutathione lyase-like lyase | 53.8 | 3.8 | |||
| ABF-0019032 | ABL-0061661 | ABE-000492_1_ | formate dehydrogenase, cytochrome B556 subunit | 24.3 | 27.1 | 1.5* |
Table 2.
Comparison of divergent and differentially expressed genes in the phytopathogens to E. coli.
| ASAP Feature ID | GeneName | Product | Fold change | ||||
|---|---|---|---|---|---|---|---|
| D. dadantii | P. atrosepticum | E. coli | D. dadantii | P. atrosepticum | E. coli | ||
| ABF-0014955 | ABL-0064548 | ABE-0003424 | putA | Proline dehydrogenase | 5.3 | -3.7 | 1* |
| ABF-0015852 | ABL-0063738 | ABE-0002201 | putative lipoprotein | -3.4 | 6.3 | 1.2* | |
| ABF-0015853 | ABL-0063739 | putative membrane protein | -5.1 | 6.5 | |||
| ABF-0015854 | ABL-0063740 | IcmF-related protein | -4.9 | 7 | |||
| ABF-0015858 | ABL-0063744 | putative chaperone | -4.3 | 10 | |||
| ABF-0015859 | ABL-0063745 | putative membrane protein | -5.8 | 8.8 | |||
| ABF-0015860 | ABL-0063746 | hypothetical protein | -3.6 | 9.9 | |||
| ABF-0015861 | ABL-0063747 | putative lipoprotein | -4.4 | 8.6 | |||
| ABF-0015862 | ABL-0063748 | hypothetical protein | -4.5 | 9.4 | |||
| ABF-0015864 | ABL-0063749 | hypothetical protein | -4.4 | 6.8 | |||
| ABF-0015865 | ABL-0063750 | hypothetical protein | -5.6 | 6.8 | |||
| ABF-0015866 | ABL-0063751 | hypothetical protein | -4.6 | 9.1 | |||
| ABF-0015868 | ABL-0063752 | hypothetical protein | -3.8 | 6.6 | |||
| ABF-0018340 | ABL-0061543 | ABE-0002155 | lipA | lipoate synthase | 3 | -3.3 | -1.4* |
| ABF-0018771 | ABL-0062484 | cybC | soluble cytochrome b562 | 3.3 | -17.6 |
If we include simple ortholog groups where both orthologs are detected as differentially expressed (cFDR = 0.01), but one or the other, or even both have fold changes < 3, then we identify 222 ortholog groups in total that are differentially expressed in a congruent direction, and 51 that are differentially expressed in divergent directions across the two phytopathogens (Additional File 2). This provides a more generous estimate of the conserved core and divergently expressed members of the stimulon. This more permissive congruent set includes operons associated with anaerobiosis in other organisms and closer inspection shows that in these cases, some genes do meet our stringent criteria. This observation provides evidence that our more permissive congruent set includes real members of the anaerobic stimulon. Below, where we detail the genes and biological processes that are implicated in the transcriptional response to O2, we guide inclusion by the stringent set (96 genes), but do not limit discussion to genes meeting the stringent significance criteria.
Comparison of the expression patterns for orthologs shared by D. dadantii, P. atrosepticum and E. coli
D. dadantii and P. atrosepticum are more closely related to each other than to E. coli. Our OrthoMCL analysis identified 2231 groups that include at least one gene from each of the three organisms (totaling 2309 E. coli genes, 2283 D. dadantii genes, and 2263 P. atrosepticum genes). Of these, 2124 ortholog groups include a single gene from each organism.1124 of the 2124 groups have a differentially expressed gene (permissive criteria) in at least one of the three organisms, and 261 groups have at least one gene that shows fold change > 3 (114 genes in P. atrosepticum, 111 in E. coli, and 153 in D. dadantii, see Additional File 3). Only 20 ortholog groups contain genes that show a congruent expression pattern with fold change greater than 3 for all three orthologs (Table 1, bold gene names), suggesting that the conserved response to O2 is small in terms of the number of genes involved, or that the conserved response lies in orthologs with smaller magnitude changes. This set is mainly comprised of genes known to function in cellular metabolism under anaerobic conditions namely, frdABCD, dcuB, adhE, hypC, focA, hybO, yfiD, nrdG, nrdD, beside others such as the collagenase encoding genes yhbUV, a peptidase coding gene pepT, and two other genes ynfK and ycbJ which are all up-regulated. The stringent congruent set also includes four down-regulated genes. These are exbB that encodes a component of the TonB-exbBD complex, yceI that encodes a cytochrome b561 and two other genes (yceJ, yigI), which encode uncharacterized proteins.
Relaxing the analysis stringency to consider all 1-1-1 orthologs that are differentially expressed (no fold change threshold) in all three organisms results in only a small increase to 39 ortholog groups with congruent expression pattern (see Additional File 2, bold gene names). This suggests that the magnitude of the response is not the primary reason the conserved stimulon is so small. Even our most permissive analysis suggests that there are more genes in 1-1 ortholog groups that are differentially expressed in a subset of the organisms than congruent across all three.
Transcriptional response to O2 limitation for genes orthologous in the two phytopathogens and not shared with E. Coli
D. dadantii and P. atrosepticum share lifestyle characteristics, including a plant-host environment, not common to E. coli. They are also more closely related to each other and have acquired genes by lateral transfer events along the shared branch since their divergence from E. coli. We examined the set of orthologs shared by the two phytopathogens, but absent from E. coli to examine which if any of these lineage-specific genes are O2-responsive. A total of 780 OrthoMCL groups include genes from both phytopathogens and none from E. coli, and 716 of these are simple 1-1 ortholog groups. Only 22 genes without E. coli orthologs are differentially expressed with fold changes greater than 3 in both D. dadantii and P. atrosepticum (see Table 1). Of these 5 are up-regulated and encode butanediol dehydrogenase, lactoylglutathione lyase, a nickel transporter (different from the E. coli nikABCDE nickel transport system), a putative membrane protein and a hypothetical protein. All of the 17 down-regulated genes encode proteins that constitute transport systems, and many of them likely transport iron. These are discussed in greater detail in later sections.
Transcriptional response of genes shared with E. Coli and only one of the phytopathogens
Forty P. atrosepticum genes shared with E. coli but not with D. dadantii are O2 responsive in P. atrosepticum in our experiments, and for 26 of them transcript levels change more than 3-fold between the conditions. Similarly, 70 D. dadantii genes that have orthologs in E. coli but not in P. atrosepticum are differentially expressed in D. dadantii, of which 18 show fold changes > 3 (see Additional File 4).
Each phytopathogen shows a distinct response to O2 limitation - differential expression of genes without orthologs in the other organism
D. dadantii and P. atrosepticum each encode a substantial number of genes that are not predicted to have orthologs in the other phytopathogen or in E. coli. There are 1267 D. dadantii protein-coding genes in the ASAP database that were not found in OrthoMCL groups. Of these, 501 are differentially expressed and 142 show fold changes greater than 3. These 142 genes include several which were previously implicated in virulence or growth and survival in plant hosts (Additional File 5), and other recognizable biological processes, but 57 genes encode proteins of unknown function underscoring our incomplete understanding of the response to O2 limitation. In P. atrosepticum, there are 1130 ungrouped protein-coding genes, of which 144 are differentially expressed and 73 show fold changes greater than 3. The 73 genes include the coronofacic acid synthesis genes all of which are > 3- fold up-regulated, three genes that encode putative oxidoreductases, at least ten genes that encode putative exported proteins, and 13 genes of unknown function (Additional File 6).
We attribute the larger number of unique O2-regulated genes from D. dadantii to the smaller variance among replicates. But even using the numbers from P. atrosepticum, the number of genes (144 and 73) in the organism-specific transcriptional response is comparable to the number of genes (81) in the conserved transcriptional response, or greatly exceeds it, if we limit the core to the 20 differentially expressed genes with fold changes greater than 3 that are shared across all three organisms.
The anaerobic stimulon
The enterobacteria have considerable flexibility, both regulatory and enzymatically, in adapting their metabolism to changing environments. Orthologous genes that demonstrate a response to O2 limitation, many of which have 3-fold or more differences in gene expression, are presented according to biological subsystems. The anaerobic growth conditions used in our experiments have limited amounts of alternative electron acceptors (i.e.-nitrate) and are expected to favor fermentation as opposed to anaerobic respiration. Data in the following sections detail the similarities and differences in gene expression patterns of metabolic subsystems conserved across all three organisms (Figure 2) as well as those within and between phytopathogen species(Additional File 7). Additionally, in examining the genomes of these species, we have found many genes associated with the anaerobic stimulon have also undergone considerable changes in genomic architecture. These changes may have an influence on transcriptional differences across each species. These data also demonstrate how the anaerobic stimulon has evolved to include interactions not specifically associated with anaerobic metabolism.
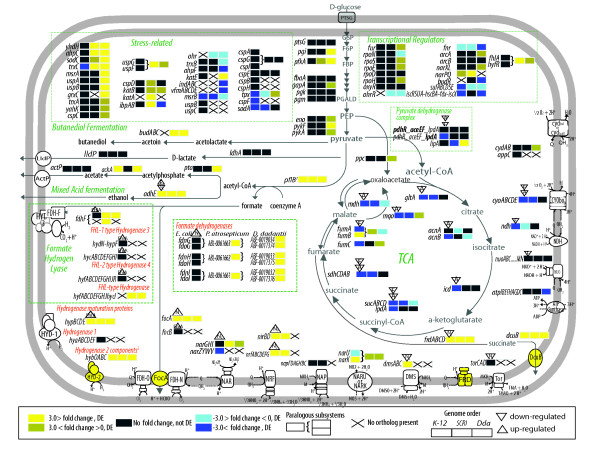
Figure 2.
Metabolic overview of conserved pathways in E. coli, P. atrosepticum and D. dadantii. Changes in gene expression under anaerobic conditions are shown for all three organisms and are represented by different colors. Fold change patterns and genome order are as follows: E. coli, P. atrosepticum and D. dadantii (see key within the figure). Each orthologous group of genes is represented by three blocks colored by fold change (dark blue: down-regulated, fold change > 3; light blue: down-regulated, fold change < 3; bright yellow: up-regulated, fold change > 3; dirty yellow: up-regulated, fold change < 3; black: no change in expression, X: ortholog absent in that organism). Fold change values for poly-cistronic operons are averaged across genes. The transcriptional regulators FNR, ArcA, NarP, NarL and FhlA, for which there are known targets, are denoted in the figure based on their mode of regulation: (▲) up regulated (▼) down-regulated. Several components in this figure, such as fermentation and respiratory chains are adapted from Unden and Dunnwald and Sawers et al. 2004 [34,46]. A more detailed diagram of the genomic structure for formate hydrogen lyase complex and accompanying hydrogenases (HYD 1-4) is shown in Figure 3.
Global transcriptional regulators associated with anaerobiosis
In E. coli, the key O2 responsive transcriptional regulators include FNR, ArcAB, NarXL, and NarPQ. FNR is an iron-sulfur cluster-containing protein that dimerizes in the absence of O2, and can act as either a transcriptional activator or repressor [21]. The amino acid sequence of FNR from the two phytopathogens is 97.6% identical to the E. coli protein with no differences in important functional domains. Surprisingly, fnr is more down regulated in D. dadantii under anaerobic conditions than either P. atroscepticum or E. coli. If this decreased expression is mediated by FNR as it is in E. coli, it suggests that FNR represses its own synthesis to a much greater extent in D. dadantii than in E. coli.
ArcB is the sensor kinase of the two-component regulatory system, ArcAB, which detects signals emanating from the aerobic respiratory chain, such as the oxidation state of ubiquinones [22]. Under anaerobic conditions ArcB phosphorylates ArcA, activating its site-specific DNA binding activity, which primarily represses genes required for aerobic metabolism [23]. D. dadantii arcB contains a nonsense mutation at codon 383, a change that is predicted to interfere with the multi-step phosphorelay transfer to ArcA [24]. It is possible that ArcA is partnered with a different sensor kinase in D. dadantii, or that this organism does not have a functional ArcA-mediated regulatory system. Curiously, arcA is up-regulated in D. dadantii, while arcA and arcB transcript levels are unaffected in P. atrosepticum and E. coli.
NarPQ and NarXL are paralogous two-component regulatory systems associated with nitrate/nitrite regulation under anaerobic conditions in E. coli. P. atrosepticum encodes NarXL and NarPQ of which the latter is missing in D. dadantii. The sensor kinase NarX responds to higher concentrations of nitrate than its paralog NarQ, which also responds to nitrite and aeration [25,26]. While each sensor kinase can phosphorylate both response regulators, dephosphorylation to the inactive state for NarP is restricted to the cognate partner [27,28]. Both response regulators activate genes associated with nitrate and nitrite catabolism and repress genes involved in other anaerobic respiratory and fermentative pathways. In P. atrosepticum, narPQ is up-regulated, while its ortholog in E. coli, although not detected as differentially expressed in Kang et al., is up-regulated less than 3- fold.
TCA cycle and glycolysis
Many genes associated with the central metabolic enzymes of glycolysis and the aerobic TCA cycle, have simple 1-1-1 orthologous relationships. While the transcription patterns are largely conserved, however, most are congruent between E. coli and P. atrosepticum but different in D. dadantii (Figure 2). For example, all four genes of the TCA cycle enzyme succinate dehydrogenase (sdhCDAB) are > 3-fold down-regulated in E. coli and P. atrosepticum but in D. dadantii only sdhD is down-regulated. These differences may be related to the arcB mutation found in D. dadantii, which may prevent the anaerobic repression of ArcA regulated genes. In genes that encode isozymes, such as aconitase, all three organisms have at least one isozyme with a conserved response. The fumarase paralogs (fumA, fumB) are the only TCA cycle genes that do not share simple orthology relationships according to OrthoMCL, although the fumC isozymes do. Nevertheless, there is some level of anaerobically induced expression in one of the isozymes across all three organisms (Figure 2).
Fermentation
Conserved genes that participate in fermentation include those associated with the reduction of pyruvate to lactate (ldhA) or the non-oxidative conversion to acetyl-coenzyme-A and formate by pyruvate formate lyase (PFL, pflB), and the subsequent conversion to acetate and ethanol (pta, ackA and adhE). In E. coli, fermentative lactate dehydrogenase (ldhA) is induced under low pH [29]; however under our growth conditions and in the Kang et al. data, no significant changes in gene expression were detected in any organism. The remaining genes pflB and adhE are up-regulated in all three, however, only pta is upregulated in E. coli and D. dadantii and ackA in P. atrosepticum (see Figure 2).
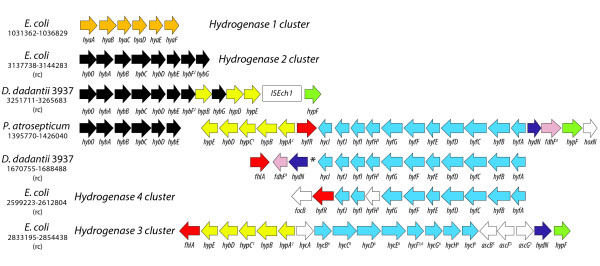
There is remarkable genomic and transcriptional variation across all three organisms in the genes associated with formate metabolism. In E. coli, formate, in the absence of a terminal electron acceptor, is further dismutated to CO2 and H2 via the formate hydrogen lyase complex (FHL), a multienzyme complex that includes a cytoplasmic formate dehydrogenase (FDH-H, fdhF), a hydrogenase (Hyd-3, encoded by the hyc operon) and other components. In E. coli, the FHL type hydrogenase groups with genes of the hydrogenase 3 and hydrogenase 4 (hyf) systems. Differences in the structure and content of the hydrogenases in the phytopathogens are shown in Figure 3. D. dadantii encodes a similar complement of hydrogenases to P. atrosepticum, but they are divided into two distinct loci. Expression of the genes encoding hydrogenase 3 in E. coli, and the single FHL type hydrogenases in both D. dadantii and P. atrosepticum are up-regulated.
Figure 3.
Genomic architecture of hydrogenase gene clusters from E. coli, D. dadantii and P. atrosepticum. Gene order and orientation of hydrogenase gene clusters from all three organisms are illustrated, including 4 clusters from E. coli, 2 from D. dadantii 3937 and 1 from P. atrosepticum SCRI1043. Direction (forward or reverse complement indicated by (rc)) was selected to maximize collinearity with the single hydrogenase cluster from P. atrosepticum. Colors are indicative of OrthoMCL grouping unless otherwise indicated by footnotes such that each color marks the genes associated with E. coli clusters and members of orthologous groups from D. dadantii 3937 and P. atrosepticum SCRI1043 labeled with the same name. White genes are singletons with no orthologs in the other two organisms. 1. hypC has an ortholog in D. dadantii 3937 that is located elsewhere, 2. hybF and hypA are grouped by OrthoMCL, 3. hycF (E. coli) and hyfH (D. dadantii 3937 and P. atrosepticum SCRI1043) are grouped by OrthoMCL, but E. coli hyfH is not part of the cluster (singleton), 4. fdhF has an ortholog in E. coli that is located elsewhere, 5. ascBF and ascG have orthologs in P. atrosepticum SCRI1043 that are located elsewhere, 6. OrthoMCL groups most members of the E. coli hydrogenase 3 and 4 systems.
Expression of _fdhF (_including a paralog of fdhF (ABL-0061761) in P. atrosepticum) is up-regulated in both phytopathogens, but remains unaffected in E. coli where FHL expression is known to be dependent on formate and an acidic pH under fermentative conditions (Figure 2). FHL is regulated by the formate-dependent regulator (fhlA) and sigma-54 [30,31], but are not differentially expressed in E. coli. The E. coli FhlA regulon includes many components (for example, hydrogenases 3, 4) missing in the phytopathogens, and is an obvious example of regulatory divergence where the network is not only smaller in the phytopathogen lineage, but is transcriptionally divergent (Figure 2). Additionally, OrthoMCL groups flhA with the E. coli regulator of hydrogenase 4 (hyfR), together with single genes, which are differentially expressed, in D. dadantii and P. atrosepticum.
Two additional E. coli formate dehydrogenase and hydrogenase isozymes linked to respiration are discussed in the following section. Unlike the phytopathogens, E. coli formate dehydrogenases are the only proteins in E. coli that require selenocysteine for assembly and maturation [32-34]. The specific requirement for selenocysteine in the oxidation of formate has been shown for formate dehydrogenase-H (fdhF) where replacement with a cysteine residue resulted in a 20-fold less active protein than the wild-type [32,33]. The phytopathogens are missing genes for tRNASec (selC), selenocysteine synthase, the specialized translation elongation factor and the selenophosphate synthase (selD). The phytopathogens encode cysteine residues into each of the formate dehydrogenases raising the question of whether they have reduced specific activity.
Both D. dadantii and P. atrosepticum have a budAB operon (encoding alpha-acetolactate decarboxylase and acetolactate synthase) adjacent to a divergently transcribed _budR_-like gene encoding a LysR family transcriptional regulator and budC (encoding 2, 3-butanediol dehydrogenase). In our experiments budC is up-regulated in both phytopathogens. The bud genes enable fermentative butanediol production, a feature that limits the channeling of pyruvate to acid producing pathways and thus counteracts the lethal effects of acidification [35]. It has recently been shown that during soft-rot infection the bud genes play the essential role of increasing the pH of the plant apoplast to facilitate activity of pectate lyases [36]. The pathway is not present in Escherichia or Salmonella but is present in other members of the enterobacteria such as Serratia, Enterobacter, Erwinia, and Klebsiella [37]. In these organisms, butanediol is involved in interactions among plant, animal and insect hosts by acting as a signaling molecule. The mechanism of insect-attraction has been described [38]. It has been shown to produce an anti-inflammatory response in endotoxin-induced lung injury in rats [39,40].
Aerobic and anaerobic respiration
In the absence of O2, E. coli is able to reduce a variety of alternate electron acceptors, including fumarate, dimethyl sulfoxide (DMSO), trimethylamine N-oxide (TMAO), nitrate and nitrite [41], to conserve energy through anaerobic respiration using electrons from a variety of donors. The ability to respire fumarate, nitrate and nitrite anaerobically has been reported for D. dadantii and P. atrosepticum [42] and a subset of the pathways are conserved with E. coli, for example, fumarate reductase (frdABCD) and nitrate reductase (narGHI). Other nitrate/nitrite reductases, which have a complex evolutionary history, are discussed in more detail below. There are no phytopathogen orthologs of the E. coli DMSO reductase genes (dmsABC), the two TMAO reductases (torCAD and torYZ) and their associated regulators (torR, torS and torT).
E. coli has three nitrate reductases and two nitrite reductases, which are part of the NarL and NarP regulons (see Figure 2). These include two membrane-bound proton-translocating nitrate reductases, (narGHJI and narZYWV operons), the periplasmic nitrate reductase (napFDAGHBC operon), a formate-dependent respiratory nitrite reductase (nrfABCDEFG) and the NADH-dependent nitrite reductase (nirBDC). The E. coli narGHJI operon has orthologs in both phytopathogens, but narZYWV does not. The narGHJI operon is up-regulated in both D. dadantii and P. atrosepticum, but is not differentially expressed in E. coli. However, narGHI in E. coli is known to be induced by FNR during anaerobic growth and further induced by NarL [43,44]. Although there are predicted binding sites for NarL and FNR in the regulatory region of narG in the phytopathogens, the sequence upstream of the conserved FNR binding site, 53 bp from the transcriptional start, has diverged.
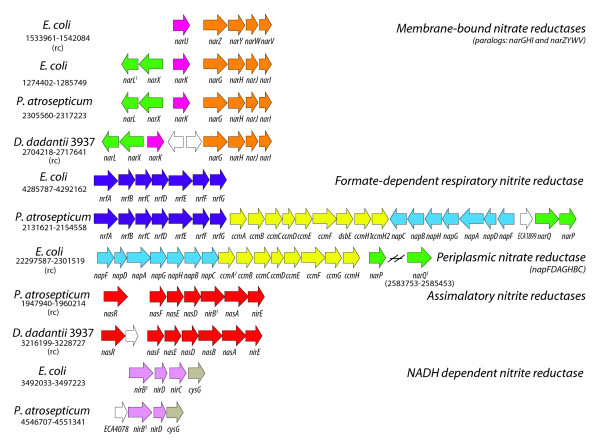
Comparative analysis reveals differences in genomic content of genes involved in nitrate/nitrite metabolism (Figure 4). The locus for the periplasmic nitrate reductase (nap), including part of the ccm operon and narP, is conserved between E. coli and P. atrosepticum, but is missing in D. dadantii. In E. coli, the genes for NarQ, and the respiratory nitrite reductase Nrf, which are also missing in D. dadantii, are not grouped in the same locus as found in P. atrosepticum (Figure 4). The napABCDFGH operon is not differentially expressed in P. atrosepticum or E. coli under the growth conditions used in our experiments. The P. atrosepticum nrf genes are > 3-fold up-regulated, while in E. coli, only nrfB shows a greater than 3-fold change. The E. coli NADH dependent nitrite reductase (nirBDC-cysG operon) is a cytoplasmic enzyme that does not produce a proton gradient, and is thought to be involved with detoxification of nitrite (Figure 4).
Figure 4.
Genomic architecture of genes involved with nitrate/nitrite metabolism in E. coli, D. dadantii and P. atrosepticum. Direction (forward or reverse complement indicated by (rc)) was selected to maximize collinearity. Colors are indicative of OrthoMCL grouping unless otherwise indicated by footnotes such that each color marks the genes associated with E. coli clusters and members of orthologous groups from D. dadantii 3937 and P. atrosepticum SCRI1043 labeled with the same name. White genes are singletons with no orthologs in the other two organisms. 1. narPQ and narXL are paralogous components in E. coli and P. atrosepticum SCRI1043. The narQ locus is located elsewhere in the chromosome, 2. ccmABCDEFGH, type 1 cytochrome C biogenesis system contains a duplication of ccmH in P. atrosepticum, 3. OrthoMCL cluster that includes the nirB encoded large subunit from E. coli, a single protein from D. dadantii 3937 annotated as nirB, and two paralogs from P. atrosepticum SCRI1043, annotated as nirB and nasB.
We believe nirB gene was erroneously assigned in D. dadantii, because this gene is part of a larger cluster conserved among the two phytopathogens that include a transcriptional regulator (nasR), an ABC transporter (nasF, nasE and nasD), two subunits of an assimilatory nitrate reductase (nasB and nasA), and an uroporphyrin-III C-methyltransferase (nirE). We hesitate to assign orthology of the nas systems between the phytopathogens because the genome context is not conserved beyond the nas subsystem itself, but it is clear that the phytopathogen loci are more structurally conserved (order and content) than either is with the nir system of E. coli (Figure 4).
E. coli has two characterized aerobic terminal reductases that are conserved in the phytopathogens. These are: the cytochrome bo3 type quinol oxidase (encoded by cyoABCD), which has a low affinity for O2, and the high-affinity bd-type cytochrome oxidase (encoded by cydABCD). The cyoABCD operon is down-regulated in E. coli and P. atrosepticum, but shows very little change in D. dadantii. The cydABCD operon is only up-regulated in D. dadantii. The cytochrome oxidase loci are regulated in part by ArcAB in E. coli, which may explain why D. dadantii shows the most divergent expression patterns. Additional Cyt bd type oxidases are encoded in E. coli (appCB) and P. atrosepticum, which may be strain-specific, as suggested by their genomic context and OrthoMCL clustering
E. coli encodes additional cytochromes with predicted roles in the electron transport chain. One of which, yceJ, is > 3-fold down-regulated in all three organisms under anaerobic conditions. The others show divergent patterns of gene expression. The gene cybBD (cytochrome b561) groups with yodB in E. coli, and single orthologs from both phytopathogens, of which only the D. dadantii ortholog is differentially expressed. The E. coli cybC, a pseudogene in strain MG1655, is a soluble cytochrome b562 of unknown function [45] but has orthologs that are divergently expressed in the phytopathogens (Additional File 8).
In E. coli there are 15 known dehydrogenases that donate electrons to the respiratory chain [46]. Some of these have already been mentioned, but most are conserved in the phytopathogen lineage (sdhCDAB, mqo, glpD, ndh, nuo). Only the operon encoding NADH dehydrogenase I, nuo, is differentially expressed in the phytopathogens while ndh remains unaffected by O2.
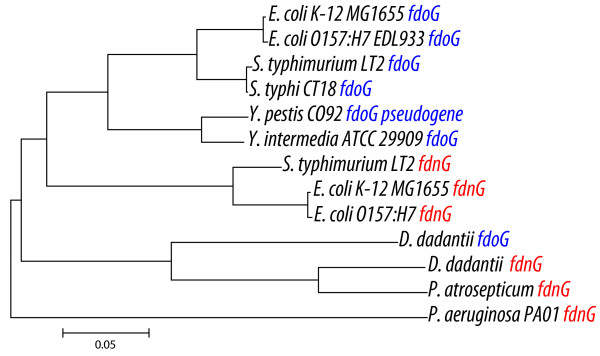
The dehydrogenases involved in formate metabolism and reuse of dihydrogen have diverged in the phytopathogen lineage. These include the formate dehydrogenase N (Fdh-N, fdnGHI), which functions in the formate-nitrate respiratory chain and the structurally related formate dehydrogenase O (Fdh-O, fdoGHI). Fdh-O is expressed at relatively low levels independent of either O2 or nitrate availability and is thought to provide this critical activity during the transition to anaerobic growth [47]. D. dadantii encodes two formate dehydrogenases homologous to Fdh-O and Fdh-N, while P. atrosepticum has only one formate dehydrogenase. There is insufficient conservation of genome context to confidently assign orthology of these genes to either Fdn-N or Fdn-O. Phylogenetic analysis (Figure 5) of the major subunit genes of the two formate dehydrogenases showed that all three phytopathogen loci are more similar to each other than either of those found in E. coli, suggesting that none of them are orthologous to the E. coli loci. Furthermore, all the formate dehydrogenases in the phytopathogens are up-regulated even though nitrate is not present in the medium. In E. coli, expression of these genes is below detectable levels. An alignment of phytopathogen and E. coli sequences upstream of fdnG revealed a conserved promoter and an FNR binding site relative to the -42.5 position, however there are no NarL heptamer sites relative to the -77,-100,-109, or -124 positions in the phytopathogens.
Figure 5.
Phylogenetic analysis of the major subunit of formate dehydrogenases from select enterobacteria. Sequences of the major subunit of formate dehydrogenase from Fdh-O and Fdh-N were aligned using CLUSTALW. The tree was constructed using NJ with default parameters of MEGA 4.0.
There are two systems for the oxidation of hydrogen under anaerobic conditions in E. coli: the Tat dependent periplasmic uptake [48] hydrogenases hydrogenase 1 and hydrogenase 2 that are involved in hydrogen oxidation coupled to quinone reduction under anaerobic conditions. Like the FHL-type hydrogenases there are differences in the structure and content of these loci between E. coli and the phytopathogens (Figure 3). There are no phytopathogen orthologs for any genes of the E. coli hydrogenase 1 operon, hyaABCDEF. In P. atrosepticum, all hydrogenase related genes are clustered in a single chromosomal locus that OrthoMCL groups with E. coli hydrogenase 2. In E. coli, several genes of the hydrogenase 1 and 2 are up-regulated with others changing in a congruent though not statistically significant way. In D. dadantii and P. atrosepticum, all genes associated with the hydrogenase 2-like system are up-regulated.
It appears that much of the transcriptional variation between the phytopathogens and E. coli with respect to metabolic responses to O2, is mainly the result of changes in gene content, genome rearrangements of both important regulatory and metabolic components of the anaerobic stimulon, specifically in relation to the loss of the NarP regulon and a mutation in ArcB in D. dadantii and also of genes involved in nitrate/nitrite metabolism. Variation in the components of the respiratory formate dehydrogenases and hydrogenases in both phytopathogens, exhibit more complex evolutionary histories with equivalent functions carried out by paralogous or analogous systems, and many of these exhibit differential responses to O2 across these organisms. The only obvious energy metabolism subsystem present in the phytopathogens that is missing from E. coli involves butanediol fermentation.
Sequences of the arcB locus from D. dadantii strains in our lab confirm the arcB nonsense mutation (data not shown), but we have yet to confirm whether this mutation is found in D. dadantii 3937 strains from other labs, although it is not present in any of the recently sequenced Dickeya species. Whether this mutation is laboratory derived or is a lineage specific event remains to be determined. Regardless, this strain has been successfully used to identify and test various pathogenicity related phenotypes in planta suggesting that it may not affect its ability to macerate host tissues.
Stress responses
Analysis of the expression pattern of genes associated with various types of stress responses reveals interesting similarities and differences among the three organisms. Overall, the patterns are suggestive of a phytopathogen-specific oxidative stress response. For example, ahpC, trxC, sodC and dps encode bona fide virulence factors in bacterial pathogens [49-53] and they counteract damage due to reactive O2 species. Counterintuitively, these genes are up-regulated in the phytopathogens under anaerobic conditions and as expected, remain unaffected in E. coli. It is possible that prolonged growth in an O2 limited environment simulates a situation that soft-rotting bacteria experience prior to encountering the host oxidative burst. Our data suggest the possibility that both P. atrosepticum and D. dadantii may be able to anticipate and respond to a host induced oxidative environment before its onset, leading to the speculation that in these two phytopathogens the regulatory networks that govern responses to two opposite stressors (anaerobic stress and oxidative stress) may be linked to increase chances of survival in the plant environment. Such anticipatory responses are proposed to occur in organisms living in environments that change in predictable ways [54]. The phytopathogen-lineage specific up-regulation of both the narGHJI genes and the nitrate-dependent formate dehydrogenase genes (fdnGHI) involved in respiratory nitrate reduction, despite the absence of nitrate in our growth media, may also be regarded as an anticipatory response in view of the fact that nitrate is an abundant anion in plant. Similar to E. coli, it is possible that a complex regulatory network that includes PecS [55], OxyR [56], FNR, (represses sodC in E. coli), RpoS (induces sodC) as well as regulatory elements of nitrate metabolism may be involved.
Some oxidative stress responsive genes that are down-regulated in the phytopathogens, as expected in an anaerobic environment, are either lineage-specific (ohrR and ohr) or strain-specific (indABC, vfmABCDE) and are not present in E. coli. The expression pattern of several other genes associated with oxidative stress is similar in E. coli and P. atrosepticum, but differs in D. dadantii and for two of these genes, sodA and tpx, ArcA-mediated regulation has been demonstrated for the E. coli ortholog [57]. Orthologs of universal stress proteins and cold shock proteins, which are involved in the response to a variety of environmental stresses in enterobacteria [58-62], are up-regulated in one or both phytopathogens but remain unaffected in E. coli (see Figure 2)
Metal transport systems
A variety of transition metals including iron, manganese, nickel, zinc and copper are required by bacteria for the activity and stabilty of proteins, including FNR and most of the respiratory enzymes. A large number of genes are devoted to their acquisition, uptake, storage and efflux in order to maintain homeostasis. Since several of the anaerobic respiratory enzymes require a different complement of metals (e.g. nickel) than the aerobic respiratory enzymes, and because unbound iron oxidation states is readily influenced by the O2 status of the growth media, we are not surprised to observe transcriptional changes for associated genes, and some of them are described below.
Each of the three organisms up-regulates at least one nickel uptake system (nikABCDE in E. coli, hoxN in the phytopathogens) indicating an increased requirement for this metal during anaerobiosis. In E. coli the NikABCDE system transports nickel for the NiFe hydrogenases. Genes encoding transport systems for copper and zinc largely show phytopathogen-specific responses. For example, the Cus metal efflux system (cusCFBA operon) is up-regulated in both phytopathogens and is down-regulated in E. coli whereas, the copA gene is down-regulated in both phytopathogens, but not in E. coli. The Cus system and CopA are associated with copper homeostasis under anaerobic and aerobic conditions respectively, in E. coli [63]. Similarly, zinc uptake systems (znuABC) are down-regulated only in the phytopathogens. Additionally, both phytopathogens down-regulate transcripts (> 3-fold) for an ABC transport system predicted to transport zinc (according to the D. dadantii annotations). Genes for this system are not present in E. coli. All three organisms encode a zinc uptake regulator (Zur), the gene for which is down-regulated only in D. dadantii.
All three organisms have a substantial number of genes involved in synthesis and transport of iron chelating siderophores and other iron-containing compounds. Overall, these subsystems are down-regulated in the phytopathogens, and to a lesser extent in E. coli suggesting that the phytopathogens may have a reduced demand for iron during anaerobiosis or may reduce iron levels to avoid damage in the anticipated oxidative environment of the host. Many of these genes are likely regulated by Fur in all three organisms.
Genes that belong to the same ortholog group do not necessarily synthesize the same siderophore although they might be able to transport some of them. For example, OrthoMCL clusters genes for synthesis of the D. dadantii siderophore, chrysobactin, with enterobactin synthesis genes of E. coli, but the siderophores are distinct [64]. D. dadantii does not synthesize enterobactin, although it is capable of uptake and utilization of exogenous enterobactin [65]. The OrthoMCL clusters also include single members from P. atrosepticum, but none of the genes share extended conserved genomic context across organisms, and there is no data on whether P. atrosepticum produces enterobactin, chrysobactin or another siderophore using these genes. It is also established that some bacteria can "steal" siderophores from their neighbors as seen in P. atrosepticum which is unlikely to produce achromobactin, but may be able to uptake and transport it via genes orthologous to the D. dadantii cbrABCD [66]. A detailed comparison of the D. dadantii and P. atrosepticum iron homeostasis systems is found in Franza and Expert [64].
Genes for several iron transporting ABC transport systems shared only between the phytopathogens are all largely down-regulated in both organisms (for example OrthoMCL groups 3161-3163, yfeABCD, sfuABC [67]). In E. coli, under anaerobic conditions uptake of ferrous iron is expected to increase relative to oxidized ferric iron. In line with this expectation, the feoAB genes that are involved in transport of ferrous iron are up-regulated in D. dadantii. However, in the Kang et al. experiments, neither the efeBOU genes nor the feoAB genes, which encode ferrous iron transporters were up-regulated. Several genes encoding iron storage proteins are up-regulated in the phytopathogens and remain unaffected or are down-regulated in E. coli (see Additional File 7). These include the bfr gene, encoding a bacterioferritin which contributes differentially to the virulence of D. dadantii depending on the host [68] and dps which encodes a ferritin-like protein, Dps, with pleiotropic functions. D. dadantii also up-regulates transcripts for a second strain-specific Dps-like protein (ABF-0015905).
Other transporters
Most of the transporters for amino acids are down-regulated in the phytopathogens, and unaffected in E. coli. Only one gene (sulfate transporter; yfbS) shows > 3-fold congruent up-regulation between the phytopathogens and a sodium-serine transporter gene (sst) is up-regulated both in E. coli and P. atrosepticum. Even though OusA has been implicated in anaerobiosis in D. dadantii [69], we did not detect changes in expression for its gene in our experiments.
Cell-wall degradation
In P. atrosepticum and D. dadantii, pathogenicity is largely due to their capacity to depolymerize plant cell wall polymers including cellulose, hemicellulose and pectic substances, as well as other components such as lignin and proteins, through the coordinate production of multiple cell wall degrading enzymes (CWDE). Because of their indispensable role in pathogenicity, expression of CWDE is strictly regulated at the transcriptional level by multiple regulators and further fine tuned by environmental factors including pH, osmolarity and O2 concentrations all of which influence the successful onset of disease symptoms [70]. Anaerobic regulation in the presence of an inducer has been demonstrated for pelA, pelD, pelE and pelL [71] in D. dadantii. Except for pelD none of these four genes has an ortholog in P. atrosepticum (see Additional File 7). As expected for cells grown in non-inducing conditions, all the reported pectate lyase genes (pelA to E, L, Z, I) are repressed in D. dadantii. This trend is not seen in P. atrosepticum where in fact, one gene, pelB, is up-regulated. The only CWDE-encoding gene that is down-regulated in P. atrosepticum is a pectin lyase, pnl, which is a member of a complex ortholog group. It is interesting to note that a D. dadantii -specific gene, xynA (ABF-0019026), encoding a putative endoxylanase, characterized in a related corn pathogen [72], is > 3-fold up-regulated.
Phytopathogen secretion systems
Both D. dadantii and P. atrosepticum encode a diverse collection of secretion systems, several of which are known to play key roles in interaction with plant hosts. The T6SS shows one of the most dramatic divergent expression patterns observed in our experiments. The T6SS mediates secretion of proteins encoded within repetitive clusters of genes found distributed throughout the genome, often, though not reliably, annotated as hcp and vgrG. OrthoMCL clusters paralogs of each type into two groups. The hcp cluster includes three members from P. atrosepticum and two from D. dadantii. The vgrG cluster includes three members from D. dadantii and five from P. atrosepticum. The D. dadantii T6SS is down-regulated in the absence of O2 and the P. atrosepticum T6SS is up-regulated. A previous report demonstrated that the P. atrosepticum T6SS cluster and proteins secreted via the T6SS (four hcp genes and three vgrG genes) are induced by plant host extracts [73,74]. Mutants of two genes, believed to correspond to a structural component of the secretion apparatus (vasK) and a sigma-54 dependent regulator (vasH), showed increased virulence relative to wild-type, a phenotype attributed to increased growth (higher density) and associated increases in pectic enzyme production in the mutants. In our analyses, vasK and vasH are up-regulated, and the corresponding orthologs in D. dadantii are both down-regulated, typical of the T6SS clusters as a whole. All members of both hcp and vgrG groups are up-regulated and exhibit an expression pattern congruent with the T6SS in each organism.
Under the anaerobic conditions used in our experiments, genes for type I secreted proteases (PrtA, PrtB, PrtC, PrtG) and for their accessory proteins (PrtD, PrtE and PrtF) show a similar trend as the CWDE; they are down regulated in D. dadantii, and remain unaffected in P. atrosepticum. The Type II secretion system (T2SS) is responsible for secretion of CWDE as well as several other targets [75]. In addition, it has been linked to iron homeostasis in D. dadantii with interactions between inner membrane components of the T2SS and the machinery for achromobactin synthesis [76]. E. coli has an orthologous secretion system that is not expressed in wild-type E. coli strains but is functional in hns mutant strains [77], and the corresponding genes are unaffected in E. coli. Genes associated with the T2SS are nearly all down-regulated in D. dadantii and unaffected in P. atrosepticum, where absolute expression levels remain high regardless of O2 availability. D. dadantii also encodes a second locus similar to genes of a T2SS, which is associated with targeting proteins to the outer membrane [78]. Several genes from this system in D. dadantii are also down-regulated. Both phytopathogens encode a Type III secretion system (T3SS), a syringe-like apparatus employed by numerous Gram-negative pathogens to inject bacterial proteins into host cells. Although the T3SS is required for full virulence in D. dadantii [79] and P. atrosepticum [80], far fewer secreted effector proteins have been identified in soft-rot associated pathogens than many other bacteria, and some pathogenic Pectobacterium lack a T3SS altogether [81]. The D. dadantii T3SS has also been implicated in multicellular behavior and biofilm formation [82]. Genes associated with the T3SS are largely unaffected in both D. dadantii and P. atrosepticum, with low absolute expression levels with and without O2. However, in D. dadantii several related genes are down-regulated including, hrpS, which encodes a σ54-enhancer binding regulatory protein, two secreted harpin genes, hrpN and hrpW and dspE encoding a T3 secreted effector. None of these genes show a similar response in P. atrosepticum. Genes that encode a putative two partner secreted adhesin and its associated activator/transporter constitute a complex OrthoMCL group that has two D. dadantii genes and three P. atrosepticum genes (includes hecA/B). Only the P. atrosepticum orthologs are up-regulated under anaerobiosis. In E. chrysanthemi strain EC16 a role for HecA in early pathogenesis has been suggested [83].
Chemotaxis
Methyl-accepting chemotaxis proteins (MCPs) transduce environmental and cellular signals to the flagella [84]. The D. dadantii genome has 45 genes that encode proteins whose products are annotated as MCPs, while there are 36 such genes in P. atrosepticum. The C-terminal signal transduction domain of MCPs is highly conserved across all members of the family, while the N-terminal sensory domain varies extensively. This complicates reliable prediction of orthology. In our OrthoMCL analysis, 12 out of 15 _D. dadantii_-specific MCPs and one of 5 _P. atrosepticum_-specific MCPs are differentially expressed. Of the MCPs shared between the phytopathogens 17 are differentially expressed in D. dadantii and 9 in P. atrosepticum. Setting aside the potential errors with prediction of orthology, there is clearly an O2-availability regulated motility response in both D. dadantii and P. atrosepticum.
Though not an MCP, the E. coli Aer has been associated with aerotaxis via sensing of cellular redox potential using an FAD cofactor [85]. The phytopathogens each have three homologs that show similarity to Aer throughout the entire length of the alignment (aer1, aer2 and ABL-0063893 in P. atrosepticum and aer1, ABF-0014726 and ABF-0014843 in D. dadantii). Both phytopathogens include at least one putative aerotaxis receptor up-regulated and one down-regulated under anaerobic conditions. Transcripts of the E. coli aer gene decrease, though not statistically significantly, during anaerobiosis.
Flagella, motility and attachment
In the soft-rot pathogens, the contribution of flagella to motility is important for virulence [86-88]. Genes encoding regulatory elements of flagellar assembly as well as some of the genes of the flagellar apparatus are affected under anaerobic conditions in one or both phytopathogens. None of the differentially expressed genes show a similar trend in both phytopathogens (see Additional File 7) suggesting that these genes may be regulated differently during anaerobiosis in these two phytopathogens.
Polysaccharides
Genes encoding enzymes that produce a range of polysaccharides such as lipopolysaccharides (rfa and waa genes), exopolysaccharides/O-antigens (wza and rfb genes), enterobacterial common antigen (wec and rff genes) and membrane derived oligosaccharides such as periplasmic glucans (opg genes) play important roles in virulence, adhesion, resistance to host-derived compounds and are considered virulence factors in P. atrosepticum and/or D. dadantii [89-93]. In our experiments, the expression of these genes is mostly unaffected in P. atrosepticum and some of them are down-regulated in D. dadantii.
Toxins
The P. atrosepticum genome contains a cluster of 9 cfa and cfl genes that are > 3- fold up-regulated in the absence of O2. They encode a putative polyketide biosynthesis system predicted to synthesize a compound similar to coronafacic acid, a component of the coronatine phytotoxin produced by P. syringae [94], and mutations in the P. atrosepticum genes dramatically reduces virulence on potato. A similar gene cluster was recently characterized from a phytopathogenic Streptomyces, with mutants in the polyketide synthesis system showing reduced virulence on tobacco [95].
D. dadantii is pathogenic to pea aphids under laboratory conditions and this trait appears relatively widely distributed among species of enterobacteria [96]. Deleting a cluster of four genes encoding proteins similar to cytolytic delta-endotoxins from the gram-positive entomopathogen Bacillus thuringiensis significantly reduced virulence of D. dadantii on aphids. These four genes (typically expressed as a single transcriptional unit) are up-regulated in our experiments. Interestingly these genes were regulated by many of the same regulators that control expression of virulence factors for the plant host but in opposite directions [97].
Pathogenicity-associated transcriptional regulators (TR)
In P. atrosepticum and D. dadantii, several regulators coordinate expression of virulence factors in response to environmental or physiological conditions [98-103]. O2 dependent modulation has been demonstrated for very few virulence factors or associated regulators.
In our experiments, D. dadantii and P. atrosepticum each appear to have at least one O2-responsive strain-specific global regulatory gene whose expression is influenced by anaerobiosis. They are a gene for PecM [103-107] in D. dadantii and that for RdgB in P. atrosepticum [108,109]. Beside these, the PecS repressed expI gene [110] coding for the LuxI homolog in D. dadantii for the production of AHL, the expR gene that activates PecS and which encodes the AHL receptor are down-regulated only in D. dadantii, and their orthologs are unaffected in P. atrosepticum. Interestingly, many regulators shared between the phytopathogens are differentially expressed in a lineage-specific pattern that may indicate regulatory divergence for these loci. It is possible that at least some of these regulators respond to host-derived signals under the O2-limiting and inducing conditions encountered within the plant.
Most of the other global regulators of virulence including KdgR [111,112], Crp [99,101] and Fur (fur shows statistically significant up-regulation but only minimal (~1.7) fold-change), that are highly conserved between the phytopathogens are unaffected in either organism. They all show moderate levels of expression regardless of O2 availability. Although transcription of several KdgR target genes is affected in our experiments, the involvement of KdgR is unlikely to account for the change in expression for these targets since the inducer 2-keto-3-deoxygluconate (KDG), a pectin degradation compound was not present in our medium. Furthermore other than CWDE genes mentioned above, which are members of complex overlapping regulons, none of the co-regulated transporter genes such as KdgMN and togMNAB are affected in our experiments.
Non-protein coding genes in the anaerobic stimulon
Small RNAs
Small RNA genes are increasingly recognized as important global regulators of diverse biological processes, but relatively few small RNAs have been directly linked to the response to O2, even in E. coli where small RNAs have been most extensively investigated. In D. dadantii, a total of 12 small RNA genes were differentially expressed with a 3-fold or greater magnitude, and in P. atrosepticum, 6 small RNA genes met these criteria (Table 3). Small RNA genes that show a consistent anaerobic response in both phytopathogens include fnrS, a gene known to be O2-responsive in E. coli, as well as ffs (4.5S) and ssrS (6S), both of which are conserved in E. coli, though previously unlinked to the anaerobic stimulon and unaffected in the Kang et al. experiments. FnrS and ArcZ have been implicated in anaerobic regulation of a variety of targets in E. coli. In E. coli transcriptional activation of fnrS by FNR during anaerobiosis leads to translational repression of cydDC, metE, sodA, sodB. FnrS also activates at least one target gene (yhaO); [113,114]. In the phytopathogens, fnrS is > 3- fold up-regulated in both D. dadantii and in P. atrosepticum. In E. coli, a second small RNA, ArcZ, is important under anaerobic conditions and is regulated by ArcAB [115]. In our experiments, arcZ is detected as differentially expressed in D. dadantii, but not in P. atrosepticum.
Table 3.
List of small RNAs that are O2-responsive in at least one of the two phytopathogens D. dadantii and P. atrosepticum
| ASAP Feature ID | Gene Name | Fold Change | References | ||||
|---|---|---|---|---|---|---|---|
| D. dadantii | P. atrosepticum | E. coli | D. dadantii | P. atrosepticum | E. coli | ||
| ABF-0061315 | ABL-0064934 | arcZ | 1.9 | -1.2* | [115] | ||
| ABF-0061324 | ABL-0061410 | ABE-0001579 | ffs | 12.8 | 4.3 | 1.4* | [116-118] |
| ABF-0174125 | ABL-0064933 | fnrS | 28.3 | 52.4 | [113,114] | ||
| ABF-0061309 | ABL-0064917 | glmY | 3 | 1.4* | [119-123] | ||
| ABF-0061313 | ABL-0064935 | glmZ | -0.8* | 1.9* | [119-123] | ||
| ABF-0061316 | ABL-0060542 | ABE-0010269 | rnpB | 2.7 | 1.2* | 1.6* | [12] |
| ABF-0061322 | ABL-0061263 | rsmB | 3.7 | 1.2* | [124-126] | ||
| ABF-0061325 | ABL-0062736 | ryeA | 2.3 | -2.1 | [127,128] | ||
| ABF-0061326 | ABL-0062735 | ryeB | 5.4 | -2.3 | [127,128] | ||
| ABF-0061311 | ABL-0063956 | rygA | 6.3 | -2.0* | [129,130] | ||
| ABF-0061314 | ABL-0060225 | ABE-0012621 | spf | 16.3 | -1.1* | 2.08* | [131] |
| ABF-0061318 | ABL-0060877 | sraF | 0.6* | -3 | [132] | ||
| ABF-0061317 | ABL-0060677 | ABE-0009556 | ssrS | 5.2 | 2 | 1.75* | [133] |
| ABF-0061323 | ABL-0061273 | tff | -1.8 | -1.1* | [134] |
Several other small RNA genes show fairly compelling evidence of differential transcriptional regulation between the two phytopathogens including spf (Spot 42), rygA/omrA and ryeA, and possibly glmY, glmZ, and rsmB. In D. dadantii, one up-regulated small RNA corresponds to the spf gene, an ortholog of the E. coli Spot 42 small RNA, which plays a role in anti-sense mediated down-regulation of the third gene (galK) of the galactose operon [116,135], and whose expression is known to be affected by carbon source available in the media and is cAMP-CRP responsive [136]. The corresponding gene in P. atrosepticum does not change expression, rather levels are intermediate in both conditions, similar to the E. coli ortholog in the Kang experiments. The rygA gene is > 3-fold up-regulated in D. dadantii. The P. atrosepticum ortholog is not differentially expressed, and trends in the opposite direction. Targets of the two E. coli orthologs, neither of which have previously been implicated in anaerobiosis, include both transcriptionally up and down regulated genes many of which are involved with cell surface structures or functions. They also negatively regulate fepA and fecA, two genes associated with iron homeostasis, and fimbrial genes associated with adhesion and biofilm formation [130]. The ryeB RNA is up-regulated in D. dadantii and down-regulated in P. atrosepticum. In E. coli, RyeB interacts with RyeA, encoded on the opposite strand, to mediate RNAse III-dependent cleavage [127] and is known to be pH-responsive in O2-limited conditions [128]. We detect ryeA as differentially expressed in D. dadantii, although not in P. atrosepticum, where it trends in the same direction as ryeB. GlmY, a small RNA implicated in amino-sugar metabolism, is detected as up-regulated in D. dadantii and unaffected in P. atrosepticum. Amino sugars are important precursors of the peptidoglycan and lipopolysaccharide components of the cell wall. The rsmB gene is highly expressed under both aerobic and anaerobic conditions in D. dadantii and P. atrosepticum. It is up-regulated in the absence of O2 only in D. dadantii. This gene, like several others, was also not present on the Kang et al. arrays. RsmB has been linked to production of extracellular enzymes, quorum sensing and T3SS in Dickeya as well as in a related species [124-126]. This collection of lineage-specific expression patterns suggests that altering regulation of small RNAs may be a particularly labile mechanism of regulatory diversification.
Conclusions
We investigated the transcriptional response to O2 under simple controlled laboratory conditions for two soft rot-associated phytopathogenic enterobacteria to begin to enumerate the regulatory and metabolic networks associated with a key environmental parameter that impacts the interaction of these organisms with plant hosts, and to explore the extent of regulatory divergence that occurs among enterobacteria. We analyzed data from D. dadantii and P. atrosepticum individually, and compared them to each other, as well as the model organism E. coli K12, using predicted gene-by-gene orthology and by grouping related genes into subsystems. The latter approach provides insights into larger scale patterns of conserved and lineage-specific biological processes regulated by O2 availability.
The O2-responsive stimulon for each organism is large, and includes genes conserved across the family enterobacteria, as well as lineage-specific and organism-specific genes that were likely acquired through lateral gene transfer events. Some conserved genes show a conserved response to O2, but others vary across organisms in the magnitude or even direction of response. D. dadantii and P. atrosepticum are more closely related to each other than to E. coli K12, and overall, their gene expression profiles are more conserved in terms of total number of orthologous genes (including those not shared with E. coli) responding in a congruent way, and in the proportion of genes shared across all three organisms responding in a congruent way.
A subset of genes show a different trend, with the expression profile more similar between E. coli and P. atrosepticum, with D. dadantii behaving differently. We attribute many of these to the likely deficiency of ArcAB regulation in D. dadantii where the sensor kinase of this two-component regulatory system is a pseudogene; however, it is not possible from these experiments to rule out that ArcA may be partnered with a different signal transducer, opening up the possibility that existing binding sites for ArcA could be coopted to respond to an entirely different signal.
A relatively small number of conserved genes are > 3-fold up-regulated or down-regulated in all three organisms. While these include some known components of the well characterized E. coli anaerobic stimulon, other important components are missing from this conserved core. Detailed investigation of the orthology relationships and the subsystem-based approach reveal a broader group of processes implicated in a net conserved response to O2, but with variation across the organisms in the number of functionally redundant paralogs and/or non-paralogous isofunctional subsystems, which we refer to as changes in subsystem architecture. For example, hydrogenases are highly up-regulated under anaerobic conditions in all three organisms, but the genes involved show complex homology relationships, involving duplications, and or deletions, as well as genome rearrangements, that obscure the common response. We detailed these types of relationships and the associated expression patterns for subsystems involved with regulation, metabolism and a variety of processes associated with interactions of the phytopathogens with plant hosts.
Our results indicate that the O2-responsive gene network includes a variety of virulence and pathogenicity-relevant processes including secretion, response to environmental stress, metal homeostasis, and taxis. Further experiments aimed at investigating the specific role of O2 regulation of these biological processes may be fruitful. Several virulence-associated subsystems exhibit strikingly divergent behavior in the two phytopathogens, most notably the T6SS. More subtle differences, like down-regulation of the complete complement of pectate lyases in D. dadantii, which are largely unaffected in P. atrosepticum, may lead to insights into the differences in virulence of these two phytopathogens under O2-limited conditions, but this requires exploration under a broader number of experimental conditions. The experimental conditions explored here are very limited, and the number of genes in the plant-pathogen anaerobic stimulons will only increase as additional variables (carbon sources, nitrate availability, intermediate O2 levels, time series of shifting O2 availability, etc.) are investigated.
The genes differentially expressed in one or both phytopathogens include known targets of regulators associated with quorum sensing, oxidative stress, iron homeostasis, nitrate/nitrite, and carbohydrate availability, as well as the established key regulators of anaerobiosis under the experimental conditions we chose, FNR and ArcAB. The underlying regulatory network behind the O2-responsive stimulon we have described is complex, involving a larger number of regulators, and it clearly differs between the two phytopathogens. Further dissection of this network bioinformatically will certainly require simultaneous consideration of a large number of regulators. We do not attempt a comprehensive dissection in this paper. We observe that at least one known key regulator of virulence genes, PecM, is among the genes differentially affected between D. dadantii and P. atrosepticum.
Several small regulatory RNAs are also differentially expressed under aerobic and anaerobic conditions, including ones that are found in E. coli, but have not previously been characterized as O2-responsive. These expression patterns should be experimentally validated using additional techniques, both because of their novelty, and because small genes may be particularly subject to measurement errors using arrays.
Many of the organism-specific and phytopathogen-specific genes in the anaerobic stimulon were likely acquired through lateral gene transfer events. These genes may have become a part of the stimulon in a variety of ways. A lateral transfer can include regulatory elements from the donor that also function in the recipient or a laterally acquired gene could be integrated into the recipient genome in a way that makes use of native O2-responsive regulatory elements. Alternately, evolutionary events (point mutations, rearrangements, further lateral transfers in the same region) subsequent to acquisition of a gene could render it O2-responsive. We expect there to be examples of all of these, but further examination awaits comparison with additional representatives of each genus from ongoing genome projects that will permit more precise definition of the boundaries of laterally acquired elements and reconstruction of the ancestral regulatory states. Finally, these experiments addressed only a single representative of each species, and further investigation will be required to determine whether the expression patterns we observed here are typical of each species, and which aspects of the anaerobic stimulon vary within each species.
Methods
Bacterial growth and RNA extraction
We grew three replicates each of Dickeya dadantii 3937 and Pectobacterium atrosepticum SCRI1043 in MOPS minimal medium (purchased from Teknova, Inc.) supplemented with 0.1% glucose at 30°C and 23°C respectively. Overnight cultures of each bacterium were diluted to an O.D.600 of 0.05 in fresh medium and cultured in a gas sparging system [137] apparatus described in Kang et al.) that permits precise control over the mixtures of O2, N2 and CO2. Cultures were grown to early log phase under aerobic (70% N2, 25% O2 and 5% CO2) and anaerobic (95% N2 and 5% CO2) conditions. 20 ml samples were collected in tubes containing 2 ml phenol-ethanol. RNA was extracted using the hot-phenol method [138]. Quality of RNA samples was assessed using the Agilent Bioanalyzer 2100 nanochip system (Agilent Technologies).
Microarray design and hybridization
334647 genome-specific probes for the D. dadantii and 344859 probes for the P. atrosepticum genomes were selected using chipD (target melting temperature 78°C, target probe length 40 to 70 -mers, interval size 12) [139] and oligonucleotide arrays were synthesized by Nimblegen Inc. Procedures described in the Nimblegen Arrays User's guide (http://www.nimblegen.com/products/lit/05434505001_NG_Expression_UGuide_v6p0.pdf) were followed for cDNA synthesis, labeling and hybridization. Arrays were scanned at 532 nm and signals were extracted using NimbleScan software (NimbleGen, Inc.).
Analysis of gene expression data
Signals from the 3 replicates of the hybridization experiments from each organism were normalized using RMA [129] implemented in the NimbleScan software and imported into a custom MicroSoft Access database. Normalized signal intensities for each set of three replicates are in good agreement (R2 ranging from 0.92-0.99). The median of signals from multiple probes (from coding and non-coding strand) for each individual gene was calculated using R, from which log2 expression values were derived. To determine directional changes in gene expression, log2 ratios were determined by calculating the difference between the log2 median anaerobic signal and log2 median aerobic signal.
To identify differentially expressed genes between aerobic and anaerobic growth, an empirical Bayesian analysis, EBArrays [18] was executed within the free statistical analysis software package R [140] and Bioconductor v2.1 [141]. The posterior probability for each pattern was calculated using a hierarchical log-normal normal expression model with the conditional false discovery rate (cFDR) at 0.01 to determine the appropriate threshold (cFD(τ)). The E. coli WT data set for aerobic and anaerobic conditions derived from Kang et al. was reanalyzed to identify differentially expressed genes as described above. The critical thresholds for the three data sets are as follows:
D. dadantii : 0. 8734342, P. atrosepticum: 0.9067187, E. coli: 0.9289846. Throughout the manuscript, we have used the term "differentially expressed" to denote genes that qualify our permissive criterion namely the probability for observed differential expression is higher than the critical threshold, for the dataset, when the conditional false discovery rate is set at 0.01 and the prefix "highly" is used to denote differentially expressed genes whose transcripts show more than a 3-fold change between the conditions (stringent criteria). Genes whose transcript abundance is higher under anaerobic conditions are referrred to as "up-regulated" and those with lower transcript abundance under anaerobic conditions are referred to as "down-regulated". In some instances, fold change values for poly-cistronic operons that are conserved across all three organisms are averaged across genes to simplify our analyses. Additional File 9, Additional File 10, and Additional File 11 contain the complete datasets for D. dadantii, P. atrosepticum and E. coli, respectively.
Analysis of expression data for non protein coding RNA (small RNA)
Our oligonucleotide arrays included probes tiled across the entire genomes for both D. dadantii and P. atrosepticum. Thus, we are also able to analyze the O2-response for genes typically not included in gene expression arrays, like those that encode small RNAs (sRNAs), even if they were not present in the original genome annotations. OrthoMCL considers only protein-coding genes, and there are not comprehensive predictions of orthology for non-coding RNAs (ncRNAs) or tRNAs in the ASAP database (the sRNAbase [142] has predictions for 34 small RNAs in P. atrosepticum) so we do not include them in the cross-species analyses above. Instead, we used BLASTn to find orthologs in both phytopathogens on a case-by-case basis. Most small RNA genes discussed in the results and discussion are missing or incorrectly annotated in at least one of the two phytopathogen GenBank genome sequences. We have corrected them in the ASAP database. These RNAs are short, and consequently, inferences about expression patterns are based on a relatively small number (typically around 10) of probes. We manually investigated probe behavior consistency in most cases, and found the expression patterns persuasive. All small RNA genes detected as differentially expressed in either organism are shown in Table 3.
Comparative analysis
Sequences and annotations for predicted protein-coding genes for D. dadantii 3937, P. atrosepticum SCRI1043 and E. coli were obtained from the ASAP database [143] and clustered using OrthoMCL [19] using default parameters. OrthoMCL clustered genes from each of the three organisms into simple or complex ortholog groups depending on whether one or more than one ortholog is present for the gene in the organsims being compared as described using the following example. E. coli encodes paralogous genes for fumarase, fumA and fumB that are homologous to the gene identified as fumA in the phytopathogens. All of these four genes are clustered together in a single orthologous group (complex group) by OrthoMCL. The third E. coli fumarase isozyme, encoded by fumC has a simple 1-1-1 relationship with fumC of D. dadantii and P. atrosepticum and these three genes are clustered together as a different group (simple group). Strain-specific genes do not belong to any OrthoMCL group. Additional File 8 lists all protein-coding genes from all three organisms, OrthoMCL group identifiers, and counts of the number of members of the group from each organism along with a short form of the experimental data results. Operon structures and known and predicted regulator binding sites for E. coli were obtained from EcoCyc [144] unless otherwise noted.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors contributed to the overall concept and in the writing of the manuscript. LB and VB performed the experiments designed by JA, LB, JDG and NP. LB and JA participated in statistical analysis. PL performed the OrthoMCL analysis. All authors read, contributed to revisions of and approved the final manuscript.
Supplementary Material
Additional file 1
List of orthologous genes in D. dadantii and P. atrosepticum that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 2
List of simple orthologs in the phytopathogens (P. atrosepticum and D. dadantii) that are detected as differentially expressed (cFDR = 0.01) in both phytopathogens, but one or the other, or even both have fold changes < 3. Orthologs that show congruent expression patterns as well as those that show divergent expression patterns are listed.
Additional file 3
List of simple orthologs in all three organisms (E. coli, P. atrosepticum and D. dadantii) that are detected as differentially expressed in at least one using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 4
List of genes shared with E. coli and only one of the phytopathogens that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 5
List of strain-specific genes in D. dadantii that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 6
List of strain-specific genes in P. atrosepticum that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 7
Fold change patterns for P. atrosepticum and D. dadantii genes implicated in virulence and for their orthologs in E. coli predicted by OrthoMCL. Anaerobic growth in the presence of glucose alters expression of virulence-associated genes in both P. atrosepticum and D. dadantii. This includes genes associated with cell wall degradation and the Out system (down-regulated in D. dadantii only), T6SS (down-regulated in D. dadantii and up-regulated in P. atrosepticum), iron uptake (down-regulated in both), methyl-accepting chemotaxis and regulators associated with virulence. Fold change patterns and genome order are as follows: E. coli, P. atrosepticum and D. dadantii (see key within the figure). Each orthologous group of genes is represented by three blocks colored by fold change (dark blue: down-regulated, fold change > 3; light blue: down-regulated, fold change < 3; bright yellow: up-regulated, fold change > 3; dirty yellow: up-regulated, fold change < 3; black: no change in expression, X: ortholog absent in that organism). Genes are represented by their symbols, or by ASAP feature IDs (especially for strain-unique genes) or by OrthoMCL group designations ("G" followed by number).
Additional file 8
Complete OrthoMCL analysis.
Additional file 9
Normalized signals, fold changes and significance for features in D. dadantii 3937.
Additional file 10
Normalized signals, fold changes and significance for features in P. atrosepticum SCRI1043.
Additional file 11
Normalized signals, fold changes and significance for features in E. coli K12-MG1655.
Contributor Information
Lavanya Babujee, Email: babujee@wisc.edu.
Jennifer Apodaca, Email: japodaca@wisc.edu.
Venkatesh Balakrishnan, Email: vbalakrishn2@wisc.edu.
Paul Liss, Email: paul@genome.wisc.edu.
Patricia J Kiley, Email: pjkiley@wisc.edu.
Amy O Charkowski, Email: acharkowski@wisc.edu.
Jeremy D Glasner, Email: jglasner@wisc.edu.
Nicole T Perna, Email: ntperna@wisc.edu.
Acknowledgements
JA is supported by the NHGRI training grant to the “Genomic Sciences Training Program” (5T32HG002760) and the NLM training grant to the “Computation and Informatics in Biology and Medicine Training Program” (NLM 5T15LM007359). Both LB and JA are also supported by the “Molecular Evolution of Microbial Pathogen Genomes”, NIH R01-GM62994, as were PL and JDG (P.I. NTP; Co-P.I.s JDG, PJK). LB was also supported in part by a Vilas Life Cycle Award to NTP. We would like to thank the Gene Expression Center at the University of Wisconsin-Madison for their support in providing technical support, facilities and equipment for use in our research.
References
- Perombelon MCM. Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 2002;51(1):1–12. 10.1046/j.0032-0862.2001.Short title.doc.x. [Google Scholar]
- Lemattre P. Chimie horticole, Nouvelle éd. edn. Paris,: J.-B. Baillière; 1972. [Google Scholar]
- Harrison MD, Nielsen LW. In: Compendium of Potato Diseases. Hooker WJ, St. Paul MN, editor. American Phytopathological Society; 1981. Blackleg and bacterial soft rot; pp. 27–29. [Google Scholar]
- Ma B, Hibbing ME, Kim HS, Reedy RM, Yedidia I, Breuer J, Glasner JD, Perna NT, Kelman A, Charkowski AO. Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology. 2007;97(9):1150–1163. doi: 10.1094/PHYTO-97-9-1150. [DOI] [PubMed] [Google Scholar]
- Perombelon MCM, Kelman A. Ecology of the Soft Rot Erwinias. Annu Rev Phytopathol. 1980;18(1):361–387. doi: 10.1146/annurev.py.18.090180.002045. [DOI] [Google Scholar]
- Kotoujansky A. Molecular Genetics of Pathogenesis by Soft-Rot Erwinias. Annu Rev Phytopathol. 1987;25(1):405–430. doi: 10.1146/annurev.py.25.090187.002201. [DOI] [Google Scholar]
- Robert-Baudouy J. Molecular biology of Erwinia: from soft-rot to antileukaemics. Trends Biotechnol. 1991;9(1):325–329. doi: 10.1016/0167-7799(91)90103-O. [DOI] [Google Scholar]
- Smid EJ, Jansen AH, Tuijn CJ. Anaerobic Nitrate Respiration by Erwinia carotovora subsp. atroseptica during Potato Tuber Invasion. Appl Environ Microbiol. 1993;59(11):3648–3653. doi: 10.1128/aem.59.11.3648-3653.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CE, Bardin M, Kinkel LL, Moury B, Nicot PC, Sands DC. Expanding the Paradigms of Plant Pathogen Life History and Evolution of Parasitic Fitness beyond Agricultural Boundaries. PLoS Pathog. 2009;5(12):e1000693. doi: 10.1371/journal.ppat.1000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N, Dominguez H, Robert-Baudouy J. Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi3937. J Bacteriol. 1992;174(23):7807–7818. doi: 10.1128/jb.174.23.7807-7818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon KA, Hung SP, Steffen NR, Krupp R, Baldi P, Hatfield GW, Gunsalus RP. Global gene expression profiling in Escherichia coli K12: effects of oxygen availability and ArcA. J Biol Chem. 2005;280(15):15084–15096. doi: 10.1074/jbc.M414030200. [DOI] [PubMed] [Google Scholar]
- Kang Y, Weber KD, Qiu Y, Kiley PJ, Blattner FR. Genome-Wide Expression Analysis Indicates that FNR of Escherichia coli K-12 Regulates a Large Number of Genes of Unknown Function. J Bacteriol. 2005;187(3):1135–1160. doi: 10.1128/JB.187.3.1135-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon K, Hung SP, Mekjian K, Baldi P, Hatfield GW, Gunsalus RP. Global gene expression profiling in Escherichia coli K12. The effects of oxygen availability and FNR. J Biol Chem. 2003;278(32):29837–29855. doi: 10.1074/jbc.M213060200. [DOI] [PubMed] [Google Scholar]
- Cho BK, Knight EM, Palsson BO. Transcriptional regulation of the fad regulon genes of Escherichia coli by ArcA. Microbiology. 2006;152(Pt 8):2207–2219. doi: 10.1099/mic.0.28912-0. [DOI] [PubMed] [Google Scholar]
- Constantinidou C, Hobman JL, Griffiths L, Patel MD, Penn CW, Cole JA, Overton TW. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. J Biol Chem. 2006;281(8):4802–4815. doi: 10.1074/jbc.M512312200. [DOI] [PubMed] [Google Scholar]
- Overton TW, Griffiths L, Patel MD, Hobman JL, Penn CW, Cole JA, Constantinidou C. Microarray analysis of gene regulation by oxygen, nitrate, nitrite, FNR, NarL and NarP during anaerobic growth of Escherichia coli : new insights into microbial physiology. Biochem Soc Trans. 2006;34(Pt 1):104–107. doi: 10.1042/BST0340104. [DOI] [PubMed] [Google Scholar]
- Ravcheev DA, Gerasimova AV, Mironov AA, Gelfand MS. Comparative genomic analysis of regulation of anaerobic respiration in ten genomes from three families of gamma-proteobacteria (Enterobacteriaceae, Pasteurellaceae, Vibrionaceae) BMC Genomics. 2007;8:54. doi: 10.1186/1471-2164-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendziorski CM, Newton MA, Lan H, Gould MN. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat Med. 2003;22(24):3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M, de Crecy-Lagard V, Diaz N, Disz T, Edwards R. et al. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33(17):5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol Rev. 1998;22(5):341–352. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Georgellis D, Kwon O, Lin EC. Quinones as the redox signal for the arc two-component system of bacteria. Science. 2001;292(5525):2314–2316. doi: 10.1126/science.1059361. [DOI] [PubMed] [Google Scholar]
- Lynch AS, Lin EC. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J Bacteriol. 1996;178(21):6238–6249. doi: 10.1128/jb.178.21.6238-6249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O, Georgellis D, Lin EC. Phosphorelay as the sole physiological route of signal transmission by the arc two-component system of Escherichia coli. J Bacteriol. 2000;182(13):3858–3862. doi: 10.1128/JB.182.13.3858-3862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V, Chen LL, Wu HC. Response to culture aeration mediated by the nitrate and nitrite sensor NarQ of Escherichia coli K-12. Mol Microbiol. 2003;50(4):1391–1399. doi: 10.1046/j.1365-2958.2003.03776.x. [DOI] [PubMed] [Google Scholar]
- Noriega CE, Schmidt R, Gray MJ, Chen LL, Stewart V. Autophosphorylation and dephosphorylation by soluble forms of the nitrate-responsive sensors NarX and NarQ from Escherichia coli K-12. J Bacteriol. 2008;190(11):3869–3876. doi: 10.1128/JB.00092-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol. 2010;75(2):394–412. doi: 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G, Bongaerts J. Alternative respiratory pathways of Escherichia coli:energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta. 1997;1320(3):217–234. doi: 10.1016/S0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- Jiang GR, Nikolova S, Clark DP. Regulation of the ldhA gene, encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology. 2001;147(Pt 9):2437–2446. doi: 10.1099/00221287-147-9-2437. [DOI] [PubMed] [Google Scholar]
- Leonhartsberger S, Korsa I, Bock A. The molecular biology of formate metabolism in enterobacteria. J Mol Microbiol Biotechnol. 2002;4(3):269–276. [PubMed] [Google Scholar]
- Rossmann R, Sawers G, Bock A. Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol. 1991;5(11):2807–2814. doi: 10.1111/j.1365-2958.1991.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Axley MJ, Bock A, Stadtman TC. Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc Natl Acad Sci USA. 1991;88(19):8450–8454. doi: 10.1073/pnas.88.19.8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axley MJ, Grahame DA. Kinetics for formate dehydrogenase of Escherichia coli formate-hydrogenlyase. J Biol Chem. 1991;266(21):13731–13736. [PubMed] [Google Scholar]
- Sawers RG, Blokesch M, Böck A. In: EcoSal-Escherichia coli and Salmonella: Cellular and Molecular Biology. Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery DA, editor. Washington: ASM Press; 2004. Anaerobic Formate and Hydrogen Metabolism. [Google Scholar]
- Mayer D, Schlensog V, Bock A. Identification of the transcriptional activator controlling the butanediol fermentation pathway in Klebsiella terrigena. J Bacteriol. 1995;177(18):5261–5269. doi: 10.1128/jb.177.18.5261-5269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Villavicencio MdP, Weber B, Witherell RA, Willis DK, Charkowski AO. The 3-Hydroxy-2-Butanone Pathway Is Required for Pectobacterium carotovorum Pathogenesis. PLoS One. 2011;6(8):e22974. doi: 10.1371/journal.pone.0022974. doi:doi:10.1371/journal.pone.0022974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist K, Nikkola M, Lehtovaara P, Suihko ML, Airaksinen U, Straby KB, Knowles JK, Penttila ME. Characterization of the genes of the 2,3-butanediol operons from Klebsiella terrigena and Enterobacter aerogenes. J Bacteriol. 1993;175(5):1392–1404. doi: 10.1128/jb.175.5.1392-1404.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Rochat D, Morin JP, Kakul T, Beaudoin-Ollivier L, Prior R, Renou M, Malosse I, Stathers T, Embupa S, Laup S. Activity of male pheromone of Melanesian rhinoceros beetle Scapanes australis. J Chem Ecol. 2002;28(3):479–500. doi: 10.1023/A:1014531810037. [DOI] [PubMed] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134(3):1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SC, Lu CC, Horng YT, Soo PC, Chang YL, Tsai YH, Lin CS, Lai HC. The bacterial metabolite 2,3-butanediol ameliorates endotoxin-induced acute lung injury in rats. Microbes Infect. 2007;9(12-13):1402–1409. doi: 10.1016/j.micinf.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Dickey RS. Erwinia chrysanthemi: A Comparative Study of Phenotypic Properties of Strains from Several Hosts and Other Erwinia Species. Phytopathology. 1979;69:324–329. doi: 10.1094/Phyto-69-324. [DOI] [Google Scholar]
- Darwin AJ, Li J, Stewart V. Analysis of nitrate regulatory protein NarL-binding sites in the fdnG and narG operon control regions of Escherichia coli K-12. Mol Microbiol. 1996;20(3):621–632. doi: 10.1046/j.1365-2958.1996.5491074.x. [DOI] [PubMed] [Google Scholar]
- Melville SB, Gunsalus RP. Isolation of an oxygen-sensitive FNR protein of Escherichia coli: interaction at activator and repressor sites of FNR-controlled genes. Proc Natl Acad Sci USA. 1996;93(3):1226–1231. doi: 10.1073/pnas.93.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkila H, Gennis RB, Sligar SG. Cloning and expression of the gene encoding the soluble cytochrome b562 of Escherichia coli. Eur J Biochem. 1991;202(2):309–13. doi: 10.1111/j.1432-1033.1991.tb16377.x. [DOI] [PubMed] [Google Scholar]
- Unden G, Dünnwald P. In: EcoSal-Escherichia coli and Salmonella: Cellular and Molecular Biology. Böck A, III RC, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery DA, editor. Washington: ASM Press; 2004. The Aerobic and Anaerobic Respiratory Chain of Escherichia coli and Salmonella enterica: Enzymes and Energetics. [Google Scholar]
- Abaibou H, Pommier J, Benoit S, Giordano G, Mandrand-Berthelot MA. Expression and characterization of the Escherichia coli fdo locus and a possible physiological role for aerobic formate dehydrogenase. J Bacteriol. 1995;177(24):7141–7149. doi: 10.1128/jb.177.24.7141-7149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews BJ, Lehman JA, Turchi JJ. Kinetic analysis of the Ku-DNA binding activity reveals a redox-dependent alteration in protein structure that stimulates dissociation of the Ku-DNA complex. J Biol Chem. 2006;281(19):13596–13603. doi: 10.1074/jbc.M512787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gort AS, Ferber DM, Imlay JA. The regulation and role of the periplasmic copper, zinc superoxide dismutase of Escherichia coli. Mol Microbiol. 1999;32(1):179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- Battistoni A, Pacello F, Folcarelli S, Ajello M, Donnarumma G, Greco R, Ammendolia MG, Touati D, Rotilio G, Valenti P. Increased expression of periplasmic Cu, Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells. Infect Immun. 2000;68(1):30–37. doi: 10.1128/IAI.68.1.30-37.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzau S, Bossi L, Figueroa-Bossi N. Differential accumulation of Salmonella[Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: correlation with their relative contribution to pathogenicity. Mol Microbiol. 2002;46(1):147–156. doi: 10.1046/j.1365-2958.2002.03145.x. [DOI] [PubMed] [Google Scholar]
- Abremski K, Gottesman S. The form of the DNA substrate required for excisive recombination of bacteriophage lambda. J Mol Biol. 1979;131(3):637–649. doi: 10.1016/0022-2836(79)90012-3. [DOI] [PubMed] [Google Scholar]
- Piddington DL, Fang FC, Laessig T, Cooper AM, Orme IM, Buchmeier NA. Cu, Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst. Infect Immun. 2001;69(8):4980–4987. doi: 10.1128/IAI.69.8.4980-4987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, Romano GH, Groisman B, Yona A, Dekel E, Kupiec M, Dahan O, Pilpel Y. Adaptive prediction of environmental changes by microorganisms. Nature. 2009;460(7252):220–224. doi: 10.1038/nature08112. [DOI] [PubMed] [Google Scholar]
- Hommais F, Oger-Desfeux C, Van Gijsegem F, Castang S, Ligori S, Expert D, Nasser W, Reverchon S. PecS is a global regulator of the symptomatic phase in the phytopathogenic bacterium Erwinia chrysanthemi3937. J Bacteriol. 2008;190(22):7508–7522. doi: 10.1128/JB.00553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz D, Patel H, Doan B, Zheng M, Aslund F, Storz G, Beckwith J. Thioredoxin 2 is involved in the oxidative stress response in Escherichia coli. J Biol Chem. 2000;275(4):2505–2512. doi: 10.1074/jbc.275.4.2505. [DOI] [PubMed] [Google Scholar]
- Tang Y, Quail MA, Artymiuk PJ, Guest JR, Green J. Escherichia coli aconitases and oxidative stress: post-transcriptional regulation of sodA expression. Microbiology. 2002;148(4):1027–1037. doi: 10.1099/00221287-148-4-1027. [DOI] [PubMed] [Google Scholar]
- Nachin L, Nannmark U, Nystrom T. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J Bacteriol. 2005;187(18):6265–6272. doi: 10.1128/JB.187.18.6265-6272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WT, Karavolos MH, Bulmer DM, Allaoui A, Hormaeche RD, Lee JJ, Khan CM. Role of the universal stress protein UspA of Salmonella in growth arrest, stress and virulence. Microb Pathog. 2007;42(1):2–10. doi: 10.1016/j.micpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Farewell A, Kvint K, Nystrom T. uspB, a new σS-regulated gene in Escherichia coli which is required for stationary-phase resistance to ethanol. J Bacteriol. 1998;180(23):6140–6147. doi: 10.1128/jb.180.23.6140-6147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadtare S, Inouye M. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J Bacteriol. 2001;183(4):1205–1214. doi: 10.1128/JB.183.4.1205-1214.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Zheng W, Crooke E, Wang YH, Inouye M. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol Microbiol. 2001;39(6):1572–1584. doi: 10.1046/j.1365-2958.2001.02345.x. [DOI] [PubMed] [Google Scholar]
- Outten FW, Huffman DL, Hale JA, O'Halloran TV. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem. 2001;276(33):30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- Franza T, Expert D. In: Iron Uptake and Homeostasis in Microorganisms. Cornelis P, Andrews SC, editor. Norfolk, UK: Caister Academic Press; 2010. Iron Uptake in Soft Rot Erwinia; p. 292. [Google Scholar]
- Rauscher L, Expert D, Matzanke BF, Trautwein AX. Chrysobactin-dependent iron acquisition in Erwinia chrysanthemi. Functional study of a homolog of the Escherichia coli ferric enterobactin esterase. J Biol Chem. 2002;277(4):2385–2395. doi: 10.1074/jbc.M107530200. [DOI] [PubMed] [Google Scholar]
- Mahe B, Masclaux C, Rauscher L, Enard C, Expert D. Differential expression of two siderophore-dependent iron-acquisition pathways in Erwinia chrysanthemi 3937: characterization of a novel ferrisiderophore permease of the ABC transporter family. Mol Microbiol. 1995;18(1):33–43. doi: 10.1111/j.1365-2958.1995.mmi_18010033.x. [DOI] [PubMed] [Google Scholar]
- Braun V, Killmann H. Bacterial solutions to the iron-supply problem. Trends Biochem Sci. 1999;24(3):104–109. doi: 10.1016/S0968-0004(99)01359-6. [DOI] [PubMed] [Google Scholar]
- Boughammoura A, Matzanke BF, Bottger L, Reverchon S, Lesuisse E, Expert D, Franza T. Differential role of ferritins in iron metabolism and virulence of the plant-pathogenic bacterium Erwinia chrysanthemi3937. J Bacteriol. 2008;190(5):1518–1530. doi: 10.1128/JB.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloux K, Touze T, Pagot Y, Jouan B, Blanco C. Mutations of ousA alter the virulence of Erwinia chrysanthemi. Mol Plant Microbe Interact. 2005;18:150–157. doi: 10.1094/MPMI-18-0150. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N, Condemine G, Nasser W, Reverchon S. Regulation of pectinolysis in Erwinia chrysanthemi. Annu Rev Microbiol. 1996;50(1):213–257. doi: 10.1146/annurev.micro.50.1.213. [DOI] [PubMed] [Google Scholar]
- Shevchik VE, Kester HCM, Benen JAE, Visser J, Robert-Baudouy J, Hugouvieux-Cotte-Pattat N. Characterization of the exopolygalacturonate lyase PelX of Erwinia chrysanthemi3937. J Bacteriol. 1999;181(5):1652–1663. doi: 10.1128/jb.181.5.1652-1663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen NT, Boyd C, Henrissat B. Cloning and characterization of a xylanase gene from corn strains of Erwinia chrysanthemi. Mol Plant Microbe Interact. 1996;9(7):651–657. doi: 10.1094/MPMI-9-0651. [DOI] [PubMed] [Google Scholar]
- Mattinen L, Somervuo P, Nykyri J, Nissinen R, Kouvonen P, Corthals G, Auvinen P, Aittamaa M, Valkonen JP, Pirhonen M. Microarray profiling of host-extract-induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum. Microbiology. 2008;154(Pt 8):2387–2396. doi: 10.1099/mic.0.2008/017582-0. [DOI] [PubMed] [Google Scholar]
- Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics. 2007;7(19):3527–3537. doi: 10.1002/pmic.200600759. [DOI] [PubMed] [Google Scholar]
- Kazemi-Pour N, Condemine G, Hugouvieux-Cotte-Pattat N. The secretome of the plant pathogenic bacterium Erwinia chrysanthemi. Proteomics. 2004;4(10):3177–3186. doi: 10.1002/pmic.200300814. [DOI] [PubMed] [Google Scholar]
- Douet V, Expert D, Barras F, Py B. Erwinia chrysanthemi iron metabolism: the unexpected implication of the inner membrane platform within the type II secretion system. J Bacteriol. 2009;191(3):795–804. doi: 10.1128/JB.00845-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francetic O, Belin D, Badaut C, Pugsley AP. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 2000;19(24):6697–6703. doi: 10.1093/emboj/19.24.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, Humphris S, Burr T, Takle G, Brurberg MB, Birch PR. et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008;4(6):e1000093. doi: 10.1371/journal.ppat.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Gavilanes-Ruiz M, Okinaka Y, Vedel R, Berthuy I, Boccara M, Chen JW, Perna NT, Keen NT. hrp genes of Erwinia chrysanthemi 3937 are important virulence factors. Mol Plant Microbe Interact. 2002;15(5):472–480. doi: 10.1094/MPMI.2002.15.5.472. [DOI] [PubMed] [Google Scholar]
- Bell KS, Sebaihia M, Pritchard L, Holden MT, Hyman LJ, Holeva MC, Thomson NR, Bentley SD, Churcher LJ, Mungall K. et al. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc Natl Acad Sci USA. 2004;101(30):11105–11110. doi: 10.1073/pnas.0402424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Ma B, Perna NT, Charkowski AO. Phylogeny and virulence of naturally occurring type III secretion system-deficient Pectobacterium strains. Appl Environ Microbiol. 2009;75(13):4539–4549. doi: 10.1128/AEM.01336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MN, Yang CH, Barak JD, Jahn CE, Charkowski AO. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J Bacteriol. 2005;187(2):639–648. doi: 10.1128/JB.187.2.639-648.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas CM, Ham JH, Deng WL, Doyle JJ, Collmer A. HecA, a member of a class of adhesins produced by diverse pathogenic bacteria, contributes to the attachment, aggregation, epidermal cell killing, and virulence phenotypes of Erwinia chrysanthemi EC16 on Nicotiana clevelandii seedlings. Proc Natl Acad Sci USA. 2002;99(20):13142–13147. doi: 10.1073/pnas.202358699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuichet K, Alexander RP, Zhulin IB. Comparative genomic and protein sequence analyses of a complex system controlling bacterial chemotaxis. Methods Enzymol. 2007;422:1–31. doi: 10.1016/S0076-6879(06)22001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179(12):4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AA, Lauga E. Propulsion by passive filaments and active flagella near boundaries. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82(4 Pt 1):041915. doi: 10.1103/PhysRevE.82.041915. [DOI] [PubMed] [Google Scholar]
- Mulholland V, Hinton JC, Sidebotham J, Toth IK, Hyman LJ, Perombelon MC, Reeves PJ, Salmond GP. A pleiotropic reduced virulence (Rvi-) mutant of Erwinia carotovora subspecies atroseptica is defective in flagella assembly proteins that are conserved in plant and animal bacterial pathogens. Mol Microbiol. 1993;10(5):1154. doi: 10.1111/j.1365-2958.1993.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Jahn CE, Willis DK, Charkowski AO. The flagellar sigma factor fliA is required for Dickeya dadantii virulence. Mol Plant Microbe Interact. 2008;21(11):1431–1442. doi: 10.1094/MPMI-21-11-1431. [DOI] [PubMed] [Google Scholar]
- Bouchart F, Boussemart G, Prouvost AF, Cogez V, Madec E, Vidal O, Delrue B, Bohin JP, Lacroix JM. The virulence of a Dickeya dadantii3937 mutant devoid of osmoregulated periplasmic glucans is restored by inactivation of the RcsCD-RcsB phosphorelay. J Bacteriol. 2010;192(13):3484–3490. doi: 10.1128/JB.00143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TJ, Ind A, Komitopoulou E, Salmond GP. Phage-selected lipopolysaccharide mutants of Pectobacterium atrosepticum exhibit different impacts on virulence. J Appl Microbiol. 2010;109(2):505–514. doi: 10.1111/j.1365-2672.2010.04669.x. [DOI] [PubMed] [Google Scholar]
- Toth IK, Thorpe CJ, Bentley SD, Mulholland V, Hyman LJ, Perombelon MC, Salmond GP. Mutation in a gene required for lipopolysaccharide and enterobacterial common antigen biosynthesis affects virulence in the plant pathogen Erwinia carotovora subsp. atroseptica. Mol Plant Microbe Interact. 1999;12(6):499–507. doi: 10.1094/MPMI.1999.12.6.499. [DOI] [PubMed] [Google Scholar]
- Kotoujansky A, Diolez A, Boccara M, Bertheau Y, Andro T, Coleno A. Molecular cloning of Erwinia chrysanthemi pectinase and cellulase structural genes. EMBO J. 1985;4(3):781–785. doi: 10.1002/j.1460-2075.1985.tb03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page F, Altabe S, Hugouvieux-Cotte-Pattat N, Lacroix JM, Robert-Baudouy J, Bohin JP. Osmoregulated periplasmic glucan synthesis is required for Erwinia chrysanthemi pathogenicity. J Bacteriol. 2001;183(10):3134–3141. doi: 10.1128/JB.183.10.3134-3141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CL, Alarcon-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 1999;63(2):266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell DR, Seipke RF, Huguet-Tapia JC, Chambers AH, Parry RJ, Loria R. Streptomyces scabies 87-22 contains a coronafacic acid-like biosynthetic cluster that contributes to plant-microbe interactions. Mol Plant Microbe Interact. 2010;23(2):161–175. doi: 10.1094/MPMI-23-2-0161. [DOI] [PubMed] [Google Scholar]
- Grenier AM, Duport G, Pages S, Condemine G, Rahbe Y. The phytopathogen Dickeya dadantii(Erwinia chrysanthemi3937) is a pathogen of the pea aphid. Appl Environ Microbiol. 2006;72(3):1956–1965. doi: 10.1128/AEM.72.3.1956-1965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costechareyre D, Dridi B, Rahbe Y, Condemine G. Cyt toxin expression reveals an inverse regulation of insect and plant virulence factors of Dickeya dadantii. Environ Microbiol. 2010;12(12):3290–3301. doi: 10.1111/j.1462-2920.2010.02305.x. [DOI] [PubMed] [Google Scholar]
- Nasser W, Faelen M, Hugouvieux-Cotte-Pattat N, Reverchon S. Role of the nucleoid-associated protein H-NS in the synthesis of virulence factors in the phytopathogenic bacterium Erwinia chrysanthemi. Mol Plant Microbe Interact. 2001;14(1):10–20. doi: 10.1094/MPMI.2001.14.1.10. [DOI] [PubMed] [Google Scholar]
- Nasser W, Robert-Baudouy J, Reverchon S. Antagonistic effect of CRP and KdgR in the transcription control of the Erwinia chrysanthemi pectinolysis genes. Mol Microbiol. 1997;26(5):1071–1082. doi: 10.1046/j.1365-2958.1997.6472020.x. [DOI] [PubMed] [Google Scholar]
- Nasser W, Reverchon S. H-NS-dependent activation of pectate lyases synthesis in the phytopathogenic bacterium Erwinia chrysanthemi is mediated by the PecT repressor. Mol Microbiol. 2002;43(3):733–748. doi: 10.1046/j.1365-2958.2002.02782.x. [DOI] [PubMed] [Google Scholar]
- Reverchon S, Expert D, Robert-Baudouy J, Nasser W. The cyclic AMP receptor protein is the main activator of pectinolysis genes in Erwinia chrysanthemi. J Bacteriol. 1997;179(11):3500–3508. doi: 10.1128/jb.179.11.3500-3508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulchre JA, Reverchon S, Nasser W. Modeling the onset of virulence in a pectinolytic bacterium. J Theor Biol. 2007;244(2):239–257. doi: 10.1016/j.jtbi.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Lebeau A, Reverchon S, Gaubert S, Kraepiel Y, Simond-Cote E, Nasser W, Van Gijsegem F. The GacA global regulator is required for the appropriate expression of Erwinia chrysanthemi3937 pathogenicity genes during plant infection. Environ Microbiol. 2008;10(3):545–559. doi: 10.1111/j.1462-2920.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- Praillet T, Reverchon S, Robert-Baudouy J, Nasser W. The PecM protein is necessary for the DNA-binding capacity of the PecS repressor, one of the regulators of virulence-factor synthesis in Erwinia chrysanthemi. FEMS Microbiol Lett. 1997;154(2):265–270. doi: 10.1111/j.1574-6968.1997.tb12654.x. [DOI] [PubMed] [Google Scholar]
- Perera IC, Grove A. Urate is a ligand for the transcriptional regulator PecS. J Mol Biol. 2010;402(3):539–551. doi: 10.1016/j.jmb.2010.07.053. [DOI] [PubMed] [Google Scholar]
- Praillet T, Reverchon S, Nasser W. Mutual control of the PecS/PecM couple, two proteins regulating virulence-factor synthesis in Erwinia chrysanthemi. Mol Microbiol. 1997;24(4):803–814. doi: 10.1046/j.1365-2958.1997.3901755.x. [DOI] [PubMed] [Google Scholar]
- Glasner JD, Marquez-Villavicencio M, Kim HS, Jahn CE, Ma B, Biehl BS, Rissman AI, Mole B, Yi X, Yang CH. et al. Niche-specificity and the variable fraction of the Pectobacterium pan-genome. Mol Plant Microbe Interact. 2008;21(12):1549–1560. doi: 10.1094/MPMI-21-12-1549. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chatterjee A, Chatterjee AK. Nucleotide sequence, organization and expression of rdgA and rdgB genes that regulate pectin lyase production in the plant pathogenic bacterium Erwinia carotovora subsp. carotovora in response to DNA-damaging agents. Mol Microbiol. 1994;14(5):999–1010. doi: 10.1111/j.1365-2958.1994.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Nguyen HA, Kaneko J, Kamio Y. Temperature-dependent production of carotovoricin Er and pectin lyase in phytopathogenic Erwinia carotovora subsp. carotovora Er. Biosci Biotechnol Biochem. 2002;66(2):444–447. doi: 10.1271/bbb.66.444. [DOI] [PubMed] [Google Scholar]
- Reverchon S, Chantegrel B, Deshayes C, Doutheau A, Cotte-Pattat N. New synthetic analogues of N-acyl homoserine lactones as agonists or antagonists of transcriptional regulators involved in bacterial quorum sensing. Bioorg Med Chem Lett. 2002;12(8):1153–1157. doi: 10.1016/S0960-894X(02)00124-5. [DOI] [PubMed] [Google Scholar]
- Rodionov DA, Gelfand MS, Hugouvieux-Cotte-Pattat N. Comparative genomics of the KdgR regulon in Erwinia chrysanthemi3937 and other gamma-proteobacteria. Microbiology. 2004;150(Pt 11):3571–3590. doi: 10.1099/mic.0.27041-0. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N, Robert-Baudouy J. Isolation of Erwinia chrysanthemi mutants altered in pectinolytic enzyme production. Mol Microbiol. 1989;3(11):1587–1597. doi: 10.1111/j.1365-2958.1989.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Boysen A, Moller-Jensen J, Kallipolitis B, Valentin-Hansen P, Overgaard M. Translational regulation of gene expression by an anaerobically induced small non-coding RNA in Escherichia coli. J Biol Chem. 2010;285(14):10690–10702. doi: 10.1074/jbc.M109.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Storz G. Reprogramming of anaerobic metabolism by the FnrS small RNA. Mol Microbiol. 2010;75(5):1215–1231. doi: 10.1111/j.1365-2958.2010.07044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandin P, Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29(18):3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Small RNAs shed some light. Cell. 2004;118(1):1–2. doi: 10.1016/j.cell.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Brown S, Fournier MJ. The 4.5 S RNA gene of Escherichia coli is essential for cell growth. J Mol Biol. 1984;178(3):533–550. doi: 10.1016/0022-2836(84)90237-7. [DOI] [PubMed] [Google Scholar]
- Wickstrom D, Wagner S, Baars L, Ytterberg AJ, Klepsch M, van Wijk KJ, Luirink J, de Gier JW. Consequences of depletion of the signal recognition particle in Escherichia coli. J Biol Chem. 2011;286(6):4598–4609. doi: 10.1074/jbc.M109.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel Y, Luttmann D, Heroven AK, Reichenbach B, Dersch P, Gorke B. Common and divergent features in transcriptional control of the homologous small RNAs GlmY and GlmZ in Enterobacteriaceae. Nucleic Acids Res. 2011;39(4):1294–1309. doi: 10.1093/nar/gkq986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JH, Vogel J. Two seemingly homologous noncoding RNAs act hierarchically to activate glmS mRNA translation. PLoS Biol. 2008;6(3):e64. doi: 10.1371/journal.pbio.0060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JH, Vogel J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007;35(3):1018–1037. doi: 10.1093/nar/gkl1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach B, Maes A, Kalamorz F, Hajnsdorf E, Gorke B. The small RNA GlmY acts upstream of the sRNA GlmZ in the activation of glmS expression and is subject to regulation by polyadenylation in Escherichia coli. Nucleic Acids Res. 2008;36(8):2570–2580. doi: 10.1093/nar/gkn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorke B, Vogel J. Noncoding RNA control of the making and breaking of sugars. Genes Dev. 2008;22(21):2914–2925. doi: 10.1101/gad.1717808. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cui Y, Mukherjee A, Chatterjee AK. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol Microbiol. 1998;29(1):219–234. doi: 10.1046/j.1365-2958.1998.00924.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Cui Y, Ma W, Liu Y, Chatterjee AK. hexA of Erwinia carotovora ssp. carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA, a global regulator of extracellular proteins, plant virulence and the quorum-sensing signal, N-(3-oxohexanoyl)-L-homoserine lactone. 215. 2000;2(2):203. doi: 10.1046/j.1462-2920.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- Yang S, Peng Q, Zhang Q, Yi X, Choi CJ, Reedy RM, Charkowski AO, Yang CH. Dynamic regulation of GacA in type III secretion, pectinase gene expression, pellicle formation, and pathogenicity of Dickeya dadantii(Erwinia chrysanthemi3937) Mol Plant Microbe Interact. 2008;21(1):133–142. doi: 10.1094/MPMI-21-1-0133. [DOI] [PubMed] [Google Scholar]
- Vogel J, Bartels V, Tang TH, Churakov G, Slagter Jager JG, Huttenhofer A, Wagner EGH. RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res. 2003;31(22):6435–6443. doi: 10.1093/nar/gkg867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes ET, Wilks JC, Sanfilippo P, Yohannes E, Tate DP, Jones BD, Radmacher MD, BonDurant SS, Slonczewski JL. Oxygen limitation modulates pH regulation of catabolism and hydrogenases, multidrug transporters, and envelope composition in Escherichia coli K-12. BMC Microbiol. 2006;6:89. doi: 10.1186/1471-2180-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier M, Gottesman S. The 5' end of two redundant sRNAs is involved in the regulation of multiple targets, including their own regulator. Nucleic Acids Res. 2008;36(21):6781–6794. doi: 10.1093/nar/gkn742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier M, Gottesman S. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol Microbiol. 2006;59(1):231–247. doi: 10.1111/j.1365-2958.2005.04929.x. [DOI] [PubMed] [Google Scholar]
- Adhya S. Suboperonic regulatory signals. Sci STKE 2003. 2003. p. pe22. [DOI] [PubMed]
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11(12):941–950. doi: 10.1016/S0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Geissen R, Steuten B, Polen T, Wagner R. E. coli 6S RNA: A universal transcriptional regulator within the centre of growth adaptation. RNA Biol. 2010;7(5):564–568. doi: 10.4161/rna.7.5.12969. [DOI] [PubMed] [Google Scholar]
- Rivas E, Klein RJ, Jones TA, Eddy SR. Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr Biol. 2001;11(17):1369–1373. doi: 10.1016/S0960-9822(01)00401-8. [DOI] [PubMed] [Google Scholar]
- Moller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002;16(13):1696–1706. doi: 10.1101/gad.231702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polayes DA, Rice PW, Dahlberg JE. DNA polymerase I activity in Escherichia coli is influenced by spot 42 RNA. J Bacteriol. 1988;170(5):2083–2088. doi: 10.1128/jb.170.5.2083-2088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton VR, Kiley PJ. Techniques for studying the oxygen-sensitive transcription factor FNR from Escherichia coli. Methods Enzymol. 2003;370:300–312. doi: 10.1016/S0076-6879(03)70027-5. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Lin P-H, Cohen SN, Lin-Chao S. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. PNAS. 2004;101(9):2758–2763. doi: 10.1073/pnas.0308747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour YS, Wesenberg GE, Tritt AJ, Glasner JD, Perna NT, Mitchell JC, Donohue TJ. chipD: a web tool to design oligonucleotide probes for high-density tiling arrays. Nucleic Acids Res. 2010;38(Suppl):W321–325. doi: 10.1093/nar/gkq517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2011.
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathesh-Kumar S, Sridhar J, Rafi ZA. sRNAbase: A web resource for small RNAs in Enterobacteriaceae. Silico Biol. 2009. p. 35. [DOI] [PubMed]
- Glasner JD, Liss P, Plunkett G, Darling A, Prasad T, Rusch M, Byrnes A, Gilson M, Biehl B, Blattner FR. et al. ASAP, a systematic annotation package for community analysis of genomes. Nucleic Acids Res. 2003;31(1):147–151. doi: 10.1093/nar/gkg125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keseler IM, Collado-Vides J, Gama-Castro S, Ingraham J, Paley S, Paulsen IT, Peralta-Gil M, Karp PD. EcoCyc: a comprehensive database resource for Escherichia coli. Nucleic Acids Res. 2005. pp. D334–D337. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1
List of orthologous genes in D. dadantii and P. atrosepticum that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 2
List of simple orthologs in the phytopathogens (P. atrosepticum and D. dadantii) that are detected as differentially expressed (cFDR = 0.01) in both phytopathogens, but one or the other, or even both have fold changes < 3. Orthologs that show congruent expression patterns as well as those that show divergent expression patterns are listed.
Additional file 3
List of simple orthologs in all three organisms (E. coli, P. atrosepticum and D. dadantii) that are detected as differentially expressed in at least one using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 4
List of genes shared with E. coli and only one of the phytopathogens that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 5
List of strain-specific genes in D. dadantii that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 6
List of strain-specific genes in P. atrosepticum that are detected as differentially expressed using stringent criteria (cFDR = 0.01 and fold change > 3).
Additional file 7
Fold change patterns for P. atrosepticum and D. dadantii genes implicated in virulence and for their orthologs in E. coli predicted by OrthoMCL. Anaerobic growth in the presence of glucose alters expression of virulence-associated genes in both P. atrosepticum and D. dadantii. This includes genes associated with cell wall degradation and the Out system (down-regulated in D. dadantii only), T6SS (down-regulated in D. dadantii and up-regulated in P. atrosepticum), iron uptake (down-regulated in both), methyl-accepting chemotaxis and regulators associated with virulence. Fold change patterns and genome order are as follows: E. coli, P. atrosepticum and D. dadantii (see key within the figure). Each orthologous group of genes is represented by three blocks colored by fold change (dark blue: down-regulated, fold change > 3; light blue: down-regulated, fold change < 3; bright yellow: up-regulated, fold change > 3; dirty yellow: up-regulated, fold change < 3; black: no change in expression, X: ortholog absent in that organism). Genes are represented by their symbols, or by ASAP feature IDs (especially for strain-unique genes) or by OrthoMCL group designations ("G" followed by number).
Additional file 8
Complete OrthoMCL analysis.
Additional file 9
Normalized signals, fold changes and significance for features in D. dadantii 3937.
Additional file 10
Normalized signals, fold changes and significance for features in P. atrosepticum SCRI1043.
Additional file 11
Normalized signals, fold changes and significance for features in E. coli K12-MG1655.