Ack1 Tyrosine Kinase Activation Correlates with Pancreatic Cancer Progression (original) (raw)
Abstract
Pancreatic cancer is a significant cause of cancer mortality worldwide as the disease has advanced significantly in patients before symptoms are evident. The signal transduction pathways that promote this rapid progression are not well understood. Ack1 or TNK2, an ubiquitously expressed oncogenic non–receptor tyrosine kinase, integrates signals from ligand-activated receptor tyrosine kinases to modulate intracellular signaling cascades. In the present study, we investigated the Ack1 activation profile in a pancreatic cancer tumor microarray, and observed that expression levels of activated Ack1 and pTyr284-Ack1 positively correlated with the severity of disease progression and inversely correlated with the survival of patients with pancreatic cancer. To explore the mechanisms by which Ack1 promotes tumor progression, we investigated the role of AKT/PKB, an oncogene and Ack1-interacting protein. Ack1 activates AKT directly in pancreatic and other cancer cell lines by phosphorylating AKT at Tyr176 to promote cell survival. In addition, the Ack1 inhibitor AIM-100 not only inhibited Ack1 activation but also suppressed AKT tyrosine phosphorylation, leading to cell cycle arrest in the G1 phase. This effect resulted in a significant decrease in the proliferation of pancreatic cancer cells and induction of apoptosis. Collectively, our data indicate that activated Ack1 could be a prognostic marker for ascertaining early or advanced pancreatic cancer. Thus, Ack1 inhibitors hold promise for therapeutic intervention to inhibit pancreatic tumor growth.
Pancreatic cancer is the fourth most common cause of cancer-related deaths in the United States.1 Median overall survival for patients with pancreatic cancer after resection of the tumor ranges from 15 to 20 months. Pancreatic carcinomas often exhibit resistance to conventional cytotoxic agents, and few effective chemotherapeutic agents are currently available for the treatment of advanced disease. New therapeutic strategies are urgently needed to improve survival outcomes. Protein kinases have emerged as a major therapeutic target for various cancers including pancreatic cancer.2 Most pancreatic cancers (∼90%) express epidermal growth factor receptor (EGFR).3 However, the phase III trial of the EGFR inhibitor erlotinib (Tarceva) with gemcitabine reported a marginal increase in the median survival of patients: 6.4 months compared with 5.9 months for patients treated with gemcitabine alone.4 Therefore, inhibition of EGFR alone may not be sufficient for improving survival outcomes. Inhibition of additional tyrosine kinases and their signaling networks is also needed to overcome the compensatory pathways that confer drug resistance in pancreatic cancer.
A key downstream effector of receptor tyrosine kinase (RTK) signaling is AKT/PKB kinase, which is required for the growth of normal cells and is frequently activated in many cancer types.5–7 Because of its ability to relay pro-survival signals, AKT has emerged as a major hallmark of tumor progression.5,7–9 AKT is frequently activated in pancreatic cancer, which is highly correlated with HER-2/neu overexpression.10 Although regulation of AKT activity by PI3K and negatively by PTEN (phosphatase and tensin homolog) is well studied, many of the pancreatic cell lines and tumors expressing activated AKT had retained wild-type PTEN.11,12 However, the precise mechanistic details of tyrosine kinase–mediated AKT activation in cancers with normal PTEN and PI3K activity is poorly understood.13,14 Recent studies have established alternative modes of AKT activation, and one such kinase is Ack1.15
Ack1, also known as TNK2, is an ubiquitously expressed tyrosine kinase that is rapidly activated by a number of activated RTKs including those mediated by epidermal growth factor (EGF), platelet-derived growth factor, and insulin signaling.16–19 Robust Ack1 activation in a variety of cancer cells by multiple RTKs has been reported; however, the role of Ack1 signaling in pancreatic cancer has not been explored. Ack1 is primarily phosphorylated at Tyr284, leading to its kinase activation.16,17 The Ack1 gene is also amplified in primary lung, ovarian, and prostate tumors, which correlates with poor prognosis.20,21 Auto-activating mutations in Ack1 have been reported in lung (W75R), ovarian (R99Q, E346K), and stomach (M409I) cancers.15,16 Our earlier studies have shown that Ack1 regulates prostate cancer progression to androgen independence by regulating the androgen receptor.17–19 We have also recently uncovered another major effector of Ack1, the oncogene AKT/PKB. Ack1-mediated phosphorylation of Tyr176 in the AKT kinase domain resulted in its activation, primarily assessed by Ser473 phosphorylation, in a PI3K/PTEN-independent manner promoting mitotic progression of the cells.15
In the present study, we demonstrated that activated Ack1 expression monitored by Tyr284 phosphorylation is significantly up-regulated in pancreatic intraepithelial neoplasia (PanIN) and in biopsy specimens of advanced metastatic pancreatic cancer. AKT Tyr176 phosphorylation gains prominence in late-stage pancreatic adenocarcinomas and is indicative of the severity of the disease. Moreover, higher levels of activated Ack1 correlates with poor survival outcomes, which suggests that Ack1/AKT signaling may have an important role in progression of pancreatic cancer. Toward the goal of understanding the role of Ack1/AKT signaling on the growth of pancreatic cells, we performed four sets of experiments. First, we developed pancreatic TMA and performed immunohistochemistry staining of pTyr284-Ack1 and pTyr176-AKT antibodies. Second, we developed pTyr176-AKT monoclonal antibodies and performed extensive characterization. Third, we undertook large-scale synthesis of Ack1 inhibitor AIM-100 to evaluate cytotoxicity. Fourth, we assessed suppression of pTyr284-Ack1, pTyr176-AKT, and pSer473-AKT levels in pancreatic and other tumor-derived cells treated with AIM-100.
Materials and Methods
Cell Lines and Materials
Panc-1, CD18, HEK293, MCF-7, H292, A2780-CP, OV90, and MDA-MB-468 cells were obtained from the American Type Tissue Culture Collection (Manassas, VA). Cells were grown in DMEM (Panc-1, CD18, HEK293 and MDA-MB-468) supplemented with 10% fetal bovine serum and penicillin or streptomycin. H292, A2780-CP, and OV90 cells were grown in RPMI 1640, and MCF-7 cells were grown in minimal essential medium supplemented with 10% fetal bovine serum and penicillin or streptomycin. HPNE cells were grown in DMEM (Dulbecco's modified Eagle's medium; Invitrogen Corp., Carlsbad, CA) supplemented with 25% M3F base medium, 10% fetal bovine serum, and penicillin or streptomycin. α-Tubulin (TU-O2) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-phospho-Ack1 (Tyr284 [Upstate]; Millipore Corp., Billerica, MA); AKT (Cell Signaling Technology, Inc., Beverly, MA), and actin monoclonal antibody (Abcam, Inc., Cambridge, MA) were purchased commercially. AIM-100 was synthesized as described earlier.19 Recombinant insulin and EGF were purchased from Invitrogen Corp. Control and Ack1 small-interfering RNAs (siRNAs) were custom synthesized from Qiagen GmbH (Hilden, Germany); the siRNA sequence information has been described previously.15
Generation and Purification of pTyr176-AKT Monoclonal Antibody
Two AKT peptides coupled to immunogenic carrier proteins were synthesized: the phosphopeptide Ac-ATGRY[pY]AMKIL-Ahx-C-amide and the non-phosphopeptide Ac-ATGRYYAMKIL-Ahx-C-amide. Two rabbits were immunized twice with phosphopeptide, several weeks apart, and an enzyme-linked immunosorbent assay was performed to determine the relative titer of serum samples against phosphorylated and non-phosphorylated peptides. For generation of monoclonal antibodies, rabbits were sacrificed, and splenocytes were isolated. Rabbit monoclonal antibodies expressing clones were custom generated by Epitomics, Inc. (Burlingame, CA). Several clones were assessed for specificity, and one clone, 96.3, was used for large-scale production. The cell supernatant was used for antibody purification using a protein-A sepharose column (Montage Kit; Millipore Corp.).
TMA Analysis
A pancreatic TMA was used in the present study, which was exempt from institutional review board approval because no personal information about patients was sought. Four-micrometer sections were transferred to adhesive-coated slides. The tissue array slides (four slides including two test duplicate slides, and positive and negative controls) were stained for pTyr284-Ack1 and pTyr176-AKT using respective rabbit polyclonal antibodies. The specificity of pTyr284-Ack1 and pTyr176-AKT antibodies for detection of activated Ack1 and Tyr-phosphorylated AKT, respectively, by immunohistochemistry (TMA) staining have been previously validated in biopsy specimens of breast and prostate tumors.15,19 The slides were dewaxed, tissues were rehydrated, and antigen retrieval was performed. After blocking, the samples were incubated with rabbit polyclonal pTyr284-Ack1 antibody (1:300 dilution; Millipore Corp.) and rabbit polyclonal pTyr176-AKT antibody (1:25 dilution) at 4°C overnight. The sections were incubated with biotin-labeled secondary and streptavidin-peroxidase for 30 minutes each (Dako A/S, Glostrop, Denmark). The samples were developed using 3,3′-diaminobenzidine substrate (Vector Laboratories, Inc., Burlingame, CA), and counterstained with hematoxylin. Negative controls were included by omitting pTyr284-Ack1/pTyr176-AKT antibody during primary antibody incubation. The stainings were examined in a blinded fashion by two independent pathologists (D.C. and A.L). Positive reactions were scored into four grades according to the intensity of staining: 0, 1+, 2+, and 3+. The percentages of positive cells were also scored into four categories: 0 (0%), 1+ (1% to 33%), 2+ (34% to 66%), and 3+ (more than 66%). The product of the intensity and percentage scores was used as a final staining score.
Statistical Analysis
Spearman's correlation coefficient was estimated to examine whether there was an increasing trend for pTyr284-Ack1 and pTyr176-AKT insofar as various stages of pancreatic cancer progression. Analysis of variance was performed to examine whether the expression levels differed among various progression stages. Boxplots were used to summarize the intensity distribution at each stage. When differences were detected, the Tukey-Kramer method was performed to examine in which pairs of stages the expression levels were different. This post hoc procedure adjusts for all pairwise comparisons and simultaneous inference. Correlation between pTyr284-Ack1 and pTyr176-AKT was examined using Spearman's ranked correlation analysis. The association of the expression levels of pTyr284-Ack1 and pTyr176-AKT and the overall survival of patients were assessed using the Kaplan-Meier method. When more than one sample was obtained from a patient, the intensity of the most advanced stage was used for the survival analysis. For pancreatic cancer data, there were 85 individuals with available pTyr284-Ack1 staining and survival information, and 78 individuals with available pTyr176-AKT staining and survival information. Survival differences between the groups were determined using the log-rank test, with P = 0.05 considered statistically significant.
Cell Proliferation, Drug Sensitivity, and Apoptosis Assays
CD18, Panc-1, HPNE, OV90, MCF-7, MDA-MB466, and MEF (mouse embryonic fibroblast) cells were untreated or treated with 2 to 10 μmol/L AIM-100 for 48 hours, and an MTT assay was performed as previously described.19 A WST-1 cell proliferation assay was performed per the manufacturer's protocol (Clontech Laboratories, Inc., Mountain View, CA). The cell death assay was performed using CellEvent Caspase-3/7 Green Detection Reagent (Invitrogen Corp.).
Results
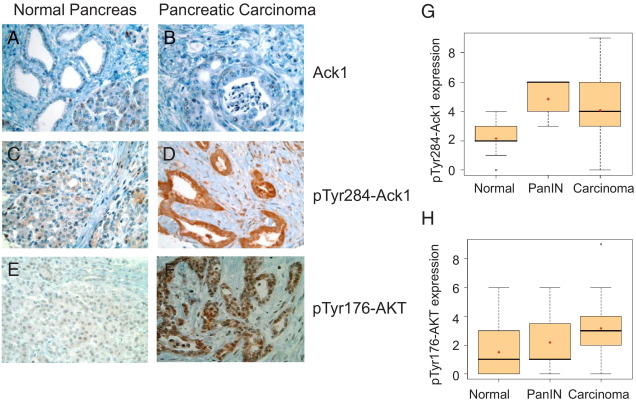
To examine the role of activated Ack1 in pancreatic tumor progression, we performed TMA analysis of clinically annotated pancreatic tumor samples (n = 281 for pTyr284-Ack1; n = 293 for pTyr176-AKT). Tyr284 is the primary autophosphorylation site in Ack1; hence, pTyr284-Ack1 antibodies were used to assess Ack1 activation. Earlier, we had characterized the specificity of pTyr284-Ack1 and pTyr176-AKT polyclonal antibodies for immunohistochemical detection.15 Immunohistochemical staining of pancreatic TMA was performed using total Ack1, pTyr284-Ack1, and pTyr176-AKT polyclonal antibodies. The total Ack1 levels remained unchanged between normal and pancreatic carcinoma samples (Figure 1, A and B). In contrast to normal tissue, a significant increase in pTyr284-Ack1 expression was evident in the samples of pancreatic carcinoma (compare Figures 1C and D). Tyr176-phosphorylated AKT expression was also up-regulated, specifically in pancreatic carcinoma compared with normal cells, and seemed to accumulate in the nuclear compartment of the cancer cell. It is consistent with the nuclear translocation of AKT and its role in export of the pro-apoptotic FoxO proteins for cell survival8,9 (Figure 1, E and F).
Figure 1.
pTyr284-Ack1 and pTyr176-AKT expressions correlate with disease progression in pancreatic cancer. Shown are TMA sections representing normal and pancreatic cancer stages stained with Ack1 (A and B), pTyr284-Ack1 (C and D) and pTyr176-AKT antibodies (E and F). G and H: Significant increase in pTyr284-Ack1 (G) and pTyr176-AKT (H) expression was seen in PanIN and pancreatic carcinoma as compared with normal pancreatic tissue samples. Spearman's correlation analysis showed that expression levels of both pTyr284-Ack1 and pTyr176-AKT increased significantly from normal to carcinoma (P < 0.0001 for each protein). Boxplots were used to summarize the intensity distribution at each stage. The boxplots have boxes with lines at the lower quartile (25%), median (50%), and upper quartile (75%) values, whereas the red cross within the circle marks the mean value. Whiskers extend from each end of the box to the most extreme values within 1.5 times the interquartile range from the ends of the box. Data with values beyond the ends of the whiskers, indicated with black open circles, are potential outliers.
Of the 281 samples with pTyr284-Ack1 expression, there were 114 normal, 13 PanIN, and 154 carcinomas samples. Further, of the 293 samples with pTyr176-AKT expression, there were 137 normal, 11 PanIN, and 145 carcinomas samples. The expression levels of these samples were used to generate boxplots (Figure 1, G and H). A statistically significant increase in expression of activated Ack1 (pTyr284-Ack1) was observed, from normal to PanIN to carcinoma (ρ = 0.51, P < 0.0001; Figure 1G). Similarly, AKT Tyr176 phosphorylation showed an increasing trend among stages of progression (ρ = 0.41, P < 0.0001; Figure 1H). Analysis of variance indicated that expression levels of both pTyr284-Ack1 and pTyr176-AKT differed significantly between stages of progression (P < 0.0001 for each protein) (see Supplemental Figure S1 at http://ajp.amjpathol.org). The Tukey-Kramer method was used to examine pairwise differences between stages, and the expression levels of pTyr284-Ack1 were significantly lower in the normal samples compared with the PanIN (P < 0.0001) and carcinoma (P < 0.0001) samples. Expression levels of pTyr176-AKT were also significantly lower in the normal samples when compared with the carcinoma samples (P < 0.0001) (see Supplemental Table S1 at http://ajp.amjpathol.org).
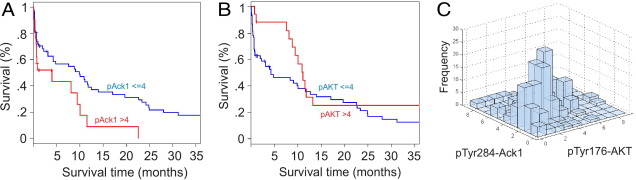
Statistical analysis showed that patients with pancreatic cancer with higher pTyr284-Ack1 expression levels (with staining intensity >4) had significantly worse overall survival outcome than did those with lower expression levels, with staining intensity ≤4 (P = 0.02 for log-rank test; Figure 2, A and B;see also Supplemental Table S2 at http://ajp.amjpathol.org). We also performed survival analysis excluding the panIN samples; the conclusion for the log-rank test results remained the same with minor P difference: P = 0.02 for pTyr284-Ack1 and P = 0.15 for pTyr176-AKT (see Supplemental Figure S2 at http://ajp.amjpathol.org). No significant association was observed between pTyr176-AKT expression levels and overall survival (P = 0.16; Figure 2B). However, expression of pTyr284-Ack1 significantly correlated with pTyr176-AKT in situ (Spearman rank correlation coefficient ρ = 0.40, P < 0.0001; Figure 2C), suggesting that in addition to AKT, Ack1 may require additional proteins to promote survival.
Figure 2.
pTyr284-Ack1 expression correlated negatively with survival in patients with pancreatic cancer. A: Kaplan-Meier analysis shows that patients with pancreatic cancer have higher pTyr284-Ack1 expression levels (with staining intensity >4) and significantly worse overall survival outcome when compared with those with lower expression levels (with staining intensity ≤4) (log-rank test, P = 0.02). B: Kaplan-Meier analysis in patients with pancreatic cancer shows that no significant association was observed between pTyr176-AKT levels and overall survival (log-rank test, P = 0.16). C: Expression of pTyr284-Ack1 was also significantly correlated with that of pTyr176-AKT in pancreatic cancer (Spearman's rank correlation coefficient, ρ = 0.40, P < 0.0001).
Immunohistochemistry analysis of pancreatic TMA revealed a role of Ack1/AKT signaling nexus in pancreatic cancer for the first time (Figures 1 and 2). This presented the possibility that AIM-100, a small molecule inhibitor of Ack1, could potentially inhibit pancreatic cancer cell growth. AIM-100, a 4-amino-5,6-biaryl-furo[2,3-d]pyrimidine derivatives,22 was identified as a potent Ack1 inhibitor, and has been shown to inhibit prostate cancer cell proliferation.19 We established the specificity of AIM-100 by performing kinase assays in the presence of increasing concentrations of AIM-100. This revealed that AIM-100 specifically inhibits Ack1 with an IC50 (half maximal inhibitory concentration) of 22 nmol/L, but not the other 30 kinases including PI 3-kinase subfamily members and the three known AKT isoforms (submitted for publication).
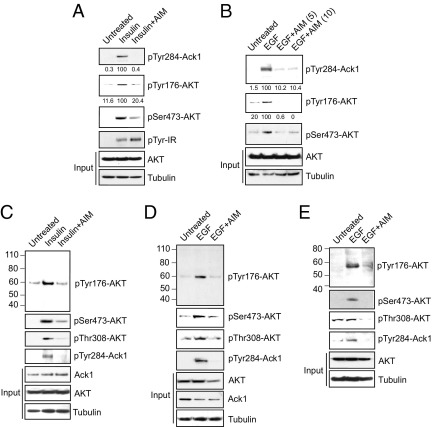
Because of the limited availability of polyclonal antibodies, we generated and characterized pTyr176-AKT monoclonal antibodies (detailed in Materials and Methods). To test the specificity of the antibodies, HEK293 cells were transfected with constitutively active Ack1 (caAck or L487F mutant) or kinase dead Ack1 (kdAck or K158R mutant).18 Cells were harvested, and immunoblot analysis was performed. caAck phosphorylated AKT at Tyr176; however, kdAck failed to phosphorylate AKT (see Supplemental Figure S3A at http://ajp.amjpathol.org). Further, we have reported characterization of four somatic mutations in Ack1: R34L, R99Q, E346K, and M409I.15 We observed that E346K mutant undergoes robust auto-activation and causes AKT Tyr176-phosphorylation; in contrast, phosphorylation of Tyr176 to Phe mutant of AKT (or Y176F) was not detected (see Supplemental Figure S3B at http://ajp.amjpathol.org). These data indicate that the AKT-specific rabbit monoclonal antibodies can be used to specifically detect Tyr176-phosphorylated AKT.
Ack1 integrates signals from a variety of RTKs including EGFR and insulin receptor.15,16 Insulin treatment of human pancreatic cells CD18 led to Tyr-phosphorylation of insulin receptor and Tyr284-phosphorylation of Ack1 (Figure 3A). Similarly, EGF treatment of Panc-1 cells showed Ack1 activation (Figure 3B). These data are consistent with our earlier observation that Ack1 can be activated on stimulation of more than one RTK.16 EGF- and insulin-stimulated pancreatic cells also exhibited Tyr176-phosphorylation of AKT (Figure 3A and B, panel 2). pTyr284-Ack1 and pTyr176-AKT expression was also significantly elevated in insulin-treated breast (MCF-7) and EGF-stimulated lung (H292) and ovarian (A2780-CP) cancer cells (Figure 3, C–E, top two panels). On treatment with the Ack1 inhibitor AIM-100, Ack1 Tyr284-phosphorylation was abrogated, which led to a concomitant decrease in AKT Tyr176-phosphorylation (Figure 3, A–E; compare lanes 2 and 3). Previously, we have demonstrated that Ack1-mediated AKT Tyr176-phosphorylation resulted in AKT activation, as noted by AKT Ser473- and Thr308-phosphorylations.15 We observed that inhibition of Ack1 activation by AIM-100 not only suppressed AKT Tyr176-phosphorylation but also inhibited AKT activation, as noted by a significant decrease in AKT Ser473- and Thr308-phosphorylations (Figure 3, C–E). Collectively, these data suggest that AIM-100 not only inhibits phosphorylation of Ack1 Tyr-284 but also suppresses activation of AKT.
Figure 3.
AIM-100 inhibited pTyr284-Ack1 and pTyr176-AKT expression in insulin-treated cells. A: Serum-depleted CD18 cells were untreated or were treated with 0.8 μg/mL insulin for 30 minutes and with 10 μmol/L AIM-100 overnight, and lysates were immunoblotted using pTyr284-Ack1, pSer473-AKT, pTyr-IR, panAKT, and tubulin antibodies. The lysates were also immunoprecipitated using pTyr176-AKT antibodies, followed by immunoblotting using AKT antibodies. B: Serum-depleted Panc-1 cells were untreated or were treated with 10 ng/mL EGF for 10 minutes and with 5 and 10 μmol/L AIM-100 overnight, and lysates were immunoblotted using pTyr284-Ack1, pSer473-AKT, panAKT, and tubulin antibodies. The lysates were also immunoprecipitated using pTyr176-AKT antibodies, followed by immunoblotting using AKT antibodies. C–E: Serum-depleted MCF-7 (C), H292 (D), and A2780-CP (E) cells were untreated or were treated with 0.8 μg/mL insulin for 30 minutes or 10 ng/mL EGF for 10 minutes and with 0.8 μmol/L AIM-100 overnight, and lysates were immunoblotted using pTyr284-Ack1, pSer473-AKT, pThr308-AKT, PanAKT, Ack1, and tubulin antibodies. The lysates were also immunoprecipitated using pTyr176-AKT antibodies, followed by immunoblotting using AKT antibodies (top panels).
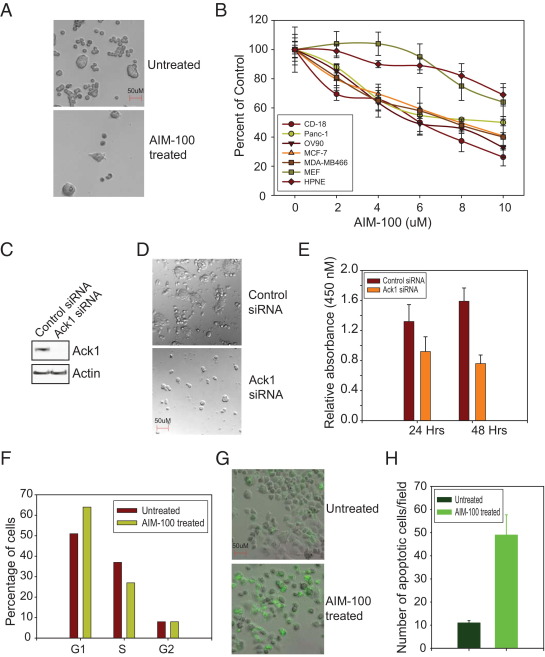
AKT Tyr176-phosphorylation is important for mitotic progression,15 which suggests that the loss of Ack1/AKT Tyr-phosphorylation may lead to decreased cell proliferation. To determine whether Ack1 inhibition affects pancreatic cell proliferation, CD18 cells were treated with 10 μmol/L AIM-100 for 48 hours. Phase contrast imaging revealed that AIM-100 treatment significantly inhibited the growth of pancreatic cells (Figure 4A). To obtain quantitative measurement of cell growth, an MTT assay was performed. Pancreatic (CD18 and Panc-1), ovarian (OV90), and breast (MCF-7 and MDA-MB-468) cancer cells were treated with increasing concentrations of AIM-100 (2 to 10 μmol/L) for 48 hours. Normal human pancreatic ductal epithelial (HPNE) cells were also included as normal control, and MEF cells as “nonmalignant” control. A significant decrease in pancreatic, ovarian, and breast cancer cell growth is evident on AIM-100 treatment with GI50 (absolute IC50) of approximately 7 to 8 μmol/L (Figure 4B). A similar decrease in cell proliferation was observed in two lung cancer cell lines, H292 and H1975, on AIM-100 treatment (see Supplemental Figure S4, A and B, at http://ajp.amjpathol.org). MEF and HPNE cells were significantly less sensitive to AIM-100 treatment with GI50 of approximately 14 to 15 μmol/L.
Figure 4.
AIM-100 inhibited growth of pancreatic cancer cells. A: CD18 cells were untreated or were treated with 10 μmol/L AIM-100 for 48 hours, and cells were photographed at 10× magnification using differential interference contrast imaging. AIM-100 treatment significantly inhibited the growth of cells. B: CD18, Panc-1, HPNE, OV90, MCF-7, MDA-MB-468, and MEF cells were untreated or were treated with 2 to 10 μmol/L AIM-100 for 48 hours, and an MTT assay was performed. The experiment was performed twice with eight replicates; a representative data set is shown. C: CD18 cells were electroporated (Lonza) with control and Ack1 siRNA, and cell lysates were immunoblotted using Ack1 and actin antibodies. D: CD18 cells were transfected with Ack1 or control siRNA, and cells were photographed at 10× magnification using differential interference contrast imaging. E: CD18 cells were transfected with Ack1 or control siRNA, and a WST-1 cell proliferation assay was performed. F: Cell cycle analysis was performed using flow cytometry. Panc-1 cells were untreated or were treated with 6 μmol/L AIM-100 for 48 hours. Cells were stained with propidium iodide, and DNA content was measured. The experiment was performed three times, and a representative data set is shown. G: Panc-1 cells were untreated or were treated with 10 μmol/L AIM-100 for 48 hours. The cell death/caspase reagent (Invitrogen Corp.) was added, and caspase activity was assessed by monitoring green fluorescence using a microscope. H: The number of cells positive for green fluorescence was counted in multiple fields, and the mean number per field is plotted.
Further, to complement inhibitor studies, CD18 cells were transfected with Ack1 or control siRNA followed by cell proliferation assay. Ack1 siRNA-transfected cells exhibited a significant decrease in cell number as compared with control siRNA-transfected cells (Figure 4, C and D). Further, a WST-1 cell proliferation assay was performed. In contrast to control siRNA-transfected CD18 cells, Ack1 siRNA-transfected cells exhibited a significant decrease in cell proliferation (Figure 4E). To determine whether this decrease in cell proliferation was due to cell cycle arrest, we performed cell cycle analysis. Cells were untreated or treated with 5 μmol/L AIM-100 and stained with propidium iodide. DNA content was measured using flow cytometry. In contrast to untreated cells, AIM-100 treatment increased the percentage of cells in the G1 phase of the cell cycle by 13% and concurrently decreased cells in the S phase by 10% (Figure 4F), which suggests that AIM-100 treatment results in cell cycle arrest in the G1 phase.
To determine whether AIM-100 treatment induces apoptosis, a cell death assay was performed using CellEvent Caspase-3/7 Green Detection Reagent (Invitrogen Corp.), which consists of the DEVD peptide sequence conjugated to a nucleic acid–binding dye. On initiation of apoptosis and caspase-3/7 activation, the dye is cleaved from the DEVD peptide and binds DNA, producing fluorescence emission that can be detected at 520 nm. In contrast to untreated cells, Panc-1 cells treated with 10 μmol/L AIM-100 for 48 hours exhibited significant increase in green fluorescence–producing cells (Figure 4, G and H). Collectively, these data suggest that AIM-100 treatment arrests cells in the G1 phase, causes apoptosis, and, thus, inhibits pancreatic cancer cell growth.
Discussion
Recent studies underscore the oncogenic role of Ack1/TNK2 in breast and prostate cancer.16 Of importance in biopsy samples of human primary breast and prostate cancer, Ack1 activation positively correlates with breast cancer progression, and negatively with patient survival.15,19 Consistent with these data, Ewing's sarcomas cells seem to require Tnk2/Ack1 for growth and survival.23 In the present study, we demonstrated for the first time that PanIN and pancreatic adenocarcinomas also exhibit significantly higher levels of pTyr284-Ack1 or activated Ack1, which correlate with poor prognosis. The substantial activation of Ack1 in these tumors is likely the consequence of more than one growth factor–activated RTK signaling pathway feeding into Ack1, as is evident in that Ack1 can be activated by more than one ligand, for example, EGF and insulin. In addition to these, other mechanisms of Ack1 activation could also be operational in pancreatic cancer. Ack1 gene amplification, another mechanism of Ack1 activation, has been reported in lung and prostate cancers.20 Also, Ack1 may be activated by somatic auto-activating mutations. Second-generation sequencing from our laboratory and those of others has identified more than a dozen auto-activating mutations in lung, ovarian, and stomach cancers (four of eight mutations have been published15). Whether somatic auto-activating mutations occur in Ack1/Tnk2 in pancreatic cancer is not known. We are currently sequencing pancreatic primary tumor biopsy samples for Ack1 auto-activating mutations and gene amplifications.
We observed high levels of pTyr284-Ack1 but significantly lower levels of pTyr176-AKT in PanIN. PanIN is an early precancerous lesion. Inasmuch as Ack1 kinase is a “driver,” high pTyr284-Ack1 levels can be detected in PanIN because of its early activation. However, optimal Tyr176-phosphorylation of AKT has not yet been achieved in PanIN, which could be why significantly lower levels of pTyr176-AKT were observed.
While AKT is active in many pancreatic tumors, direct nucleotide sequencing of AKT2, PIK3CA, RPS6K1, STK11, PDPK1, and FRAP1-mTOR, which are six key genes of the AKT/mTOR (mammalian target of rapamycin) pathway, revealed no mutations in 36 primary pancreatic endocrine tumors.24 Whether these tumor biopsy samples harbor Ack1-activating mutations is not known. We found it striking that 96% of the pancreatic endocrine tumors (135 of 140) were immunopositive for PDGFR-α, another RTK that activates Ack1.16 Our immunohistochemistry analysis has revealed significant expression of Tyr176-phosphorylated AKT in carcinomas, which suggests the possibility that the Ack1/AKT signaling nexus may be operational in pancreatic cancers. Because activated Ack1 maintains active AKT pools even in the presence of PI3K inhibitors,15 the present study highlights the importance of PI3K-independent pathways in AKT activation in pancreatic tumors and may explain the resistance of pancreatic cancers to PI3K inhibitors.
Our data suggest that in addition to AKT, Ack1 may require additional proteins to promote survival. One of the major downstream effecters of Ack1 is tumor-suppressor Wwox (WW domain-containing oxidoreductase).17,25,26 We have demonstrated that Ack1 phosphorylates Wwox at Tyr 287, leading to its polyubiquitination and degradation.17 Thus, Ack1 could stimulate pancreatic cancer progression by positively regulating oncogenic kinase AKT and negatively regulating the pro-apoptotic tumor suppressor Wwox. Further studies will be needed to assess the precise contributions of these two signaling events.
In conclusion, we have demonstrated for the first time a functional role for the Ack1/AKT signaling nexus in progression of pancreatic cancer. Further, we have generated rabbit monoclonal antibodies that specifically recognize pTyr176-AKT. Availability of this important research resource enabled us to assess loss of AKT Tyr176-phosphorylation on treatment with the Ack1 inhibitor AIM-100. AIM-100 not only inhibited Ack1/AKT Tyr-phosphorylation but also suppressed growth of cell lines derived from pancreatic, breast, and lung tumors. Considered together, these data highlight the clinical relevance of targeted inhibition of Ack1 signaling for suppression of pancreatic cancer. Results of this study have clinical implications because they offer a new drug target for therapeutic intervention in pancreatic cancer.
Acknowledgments
We thank Dr. Alexis Lopez for pathologic scoring of the TMA immunostaining, Dr. Mokenge Malafa for providing HPNE cells, and Sridevi Challa, Jeremy McGuire, and Noopur Ghade for technical assistance.
Footnotes
Supported by the Chemical Biology, Tissue and Analytical Microscopy Cores at the Moffitt Cancer Center. N.P.M. is a recipient of NIH grant 1R01CA135328, a Donald A. Adam Comprehensive Melanoma Research Center Award, a Miles for Moffitt award, and Career Development Awards in Lung Cancer.
Disclosure: K.M. and N.P.M. are named inventors on patent application No. 13/205,171, titled “AKT Tyrosine 176 Phosphorylation as Cancer Biomarker.”
Supplementary data
Supplemental Figure S1
pTyr284-Ack1 and pTyr176-AKT expression in pancreatic cancer. Top panel: pTyr284 expression in pancreatic cancer TMA sections, representing 0 to 3 staining scores. Bottom panel: pTyr176-AKT expression in pancreatic cancer TMA sections, representing 0 to 3 staining scores.
Supplemental Figure S2
Survival analysis of patients with pancreatic cancer, excluding PanIN.A: Kaplan-Meier analysis comparing higher pTyr284-Ack1 expression levels (staining intensity >4) with lower expression levels (staining intensity ≤4) (log-rank test, P = 0.02). B: Kaplan-Meier analysis comparing pTyr176-AKT levels and overall survival (log-rank test, P = 0.15).
Supplemental Figure S3
Activation of Ack1 leads to pTyr176-AKT expression. A: HEK293 cells were transfected with caAck1 or kdAck, and AKT constructs and cell lysates were immunoblotted using pTyr176-AKT (top panel), pTyr284-Ack1, AKT, and Ack1 antibodies. B: HEK293 cells were transfected with Ack1 mutant E346K and FLAG-tagged AKT or Y176F mutant constructs. Whole cell lysates were immunoprecipitated using FLAG antibodies, followed by immunoblotting with pTyr176-AKT antibodies (top panel). The lysates were also immunoblotted using FLAG antibodies (middle panel) and Ack1 antibodies (bottom panel).
Supplemental Figure S4
>and B: Lung cancer–derived H292 (A) and H1975 (B) cells were untreated or were treated with 1 to 5 μmol/L AIM-100 for 24 to 72 hours, and an MTT assay was performed. Experiments were performed three times with eight replicates; a representative data set is shown.
Supplemental Table S1
Supplemental Table S2
References
- 1.Greenlee R.T., Murray T., Bolden S., Wingo P.A. Cancer Statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Burris H., 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist. 2008;13:289–298. doi: 10.1634/theoncologist.2007-0134. [DOI] [PubMed] [Google Scholar]
- 3.Xiong H.Q., Abbruzzese J.L. Epidermal growth factor receptor–targeted therapy for pancreatic cancer. Semin Oncol. 2002;29:31–37. doi: 10.1053/sonc.2002.35645. [DOI] [PubMed] [Google Scholar]
- 4.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., Au H.J., Murawa P., Walde D., Wolff R.A., Campos D., Lim R., Ding K., Clark G., Voskoglou-Nomikos T., Ptasynski M., Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Manning B.D., Cantley L.C. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellacosa A., Kumar C.C., Di Cristofano A., Testa J.R. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 7.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Rev. 2002;4:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 9.Huang H., Tindall D.J. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 10.Schlieman M.G., Fahy B.N., Ramsamooj R., Beckett L., Bold R.J. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto J., Kaneda M., Tada M., Hamada J., Okushiba S., Kondo S., Katoh H., Moriuchi T. Differential mechanisms of constitutive Akt/PKB activation and its influence on gene expression in pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1317–1326. doi: 10.1111/j.1349-7006.2002.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurada A., Suzuki A., Sato M., Yamakawa H., Orikasa K., Uyeno S., Ono T., Ohuchi N., Fujimura S., Horii A. Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary. Jpn J Cancer Res. 1997;88:1025–1028. doi: 10.1111/j.1349-7006.1997.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibes R., Kornblau S.M., Qiu Y., Mousses S.M., Robbins C., Moses T., Carpten J.D. PI3K/AKT pathway activation in acute myeloid leukaemias is not associated with AKT1 pleckstrin homology domain mutation. Br J Haematol. 2008;140:344–347. doi: 10.1111/j.1365-2141.2007.06920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X., Tan M., Stone Hawthorne V., Klos K.S., Lan K.H., Yang Y., Yang W., Smith T.L., Shi D., Yu D. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779–6788. doi: 10.1158/1078-0432.CCR-04-0112. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan K., Coppola D., Challa S., Fang B., Chen Y.A., Zhu W., Lopez A.S., Koomen J., Engelman R.W., Rivera C., Muraoka-Cook R.S., Cheng J.Q., Schonbrunn E., Sebti S.M., Earp H.S., Mahajan N.P. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS One. 2010;5:e9646. doi: 10.1371/journal.pone.0009646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan K., Mahajan N.P. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol. 2010;224:327–333. doi: 10.1002/jcp.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahajan N.P., Whang Y.E., Mohler J.L., Earp H.S. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005;65:10514–10523. doi: 10.1158/0008-5472.CAN-05-1127. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan N.P., Liu Y., Majumder S., Warren M.R., Parker C.E., Mohler J.L., Earp H.S., Whang Y.E. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci USA. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahajan K., Challa S., Coppola D., Lawrence H., Luo Y., Gevariya H., Zhu W., Chen Y.A., Lawrence N.J., Mahajan N.P. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate. 2010;70:1274–1285. doi: 10.1002/pros.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Horst E.H., Degenhardt Y.Y., Strelow A., Slavin A., Chinn L., Orf J., Rong M., Li S., See L.H., Nguyen K.Q., Hoey T., Wesche H., Powers S. Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proc Natl Acad Sci USA. 2005;102:15901–15906. doi: 10.1073/pnas.0508014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor B.S., Schultz N., Hieronymus H., Gopalan A., Xiao Y., Carver B.S., Arora V.K., Kaushik P., Cerami E., Reva B., Antipin Y., Mitsiades N., Landers T., Dolgalev I., Major J.E., Wilson M., Socci N.D., Lash A.E., Heguy A., Eastham J.A., Scher H.I., Reuter V.E., Scardino P.T., Sander C., Sawyers C.L., Gerald W.L. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMauro E.F., Newcomb J., Nunes J.J., Bemis J.E., Boucher C., Buchanan J.L., Buckner W.H., Cheng A., Faust T., Hsieh F., Huang X., Lee J.H., Marshall T.L., Martin M.W., McGowan D.C., Schneider S., Turci S.M., White R.D., Zhu X. Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: development of an expedient and divergent synthetic route and preliminary SAR. Bioorganic Med Chem Lett. 2007;17:2305–2309. doi: 10.1016/j.bmcl.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 23.Arora S., Bisanz K.M., Peralta L.A., Basu G.D., Choudhary A., Tibes R., Azorsa D.O. RNAi screening of the kinome identifies modulators of cisplatin response in ovarian cancer cells. Gynecol Oncol. 2010;118:220–227. doi: 10.1016/j.ygyno.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Corbo V., Beghelli S., Bersani S., Antonello D., Talamini G., Brunelli M., Capelli P., Falconi M., Scarpa A. Pancreatic endocrine tumours: mutational and immunohistochemical survey of protein kinases reveals alterations in targetable kinases in cancer cell lines and rare primaries. Ann Oncol. 2011;23:127–134. doi: 10.1093/annonc/mdr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudol M., Recinos C.C., Abraczinskas J., Humbert J., Farooq A. WW or WoW: the WW domains in a union of bliss. IUBMB Life. 2005;57:773–778. doi: 10.1080/15216540500389039. [DOI] [PubMed] [Google Scholar]
- 26.Aqeilan R.I., Donati V., Palamarchuk A., Trapasso F., Kaou M., Pekarsky Y., Sudol M., Croce C.M. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res. 2005;65:6764–6772. doi: 10.1158/0008-5472.CAN-05-1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1
pTyr284-Ack1 and pTyr176-AKT expression in pancreatic cancer. Top panel: pTyr284 expression in pancreatic cancer TMA sections, representing 0 to 3 staining scores. Bottom panel: pTyr176-AKT expression in pancreatic cancer TMA sections, representing 0 to 3 staining scores.
Supplemental Figure S2
Survival analysis of patients with pancreatic cancer, excluding PanIN.A: Kaplan-Meier analysis comparing higher pTyr284-Ack1 expression levels (staining intensity >4) with lower expression levels (staining intensity ≤4) (log-rank test, P = 0.02). B: Kaplan-Meier analysis comparing pTyr176-AKT levels and overall survival (log-rank test, P = 0.15).
Supplemental Figure S3
Activation of Ack1 leads to pTyr176-AKT expression. A: HEK293 cells were transfected with caAck1 or kdAck, and AKT constructs and cell lysates were immunoblotted using pTyr176-AKT (top panel), pTyr284-Ack1, AKT, and Ack1 antibodies. B: HEK293 cells were transfected with Ack1 mutant E346K and FLAG-tagged AKT or Y176F mutant constructs. Whole cell lysates were immunoprecipitated using FLAG antibodies, followed by immunoblotting with pTyr176-AKT antibodies (top panel). The lysates were also immunoblotted using FLAG antibodies (middle panel) and Ack1 antibodies (bottom panel).
Supplemental Figure S4
>and B: Lung cancer–derived H292 (A) and H1975 (B) cells were untreated or were treated with 1 to 5 μmol/L AIM-100 for 24 to 72 hours, and an MTT assay was performed. Experiments were performed three times with eight replicates; a representative data set is shown.
Supplemental Table S1
Supplemental Table S2