Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence (original) (raw)
Abstract
Invading an occupied niche is a formidable ecological challenge, and one of particular human importance in the context of food-borne microbial pathogens. We discuss distinct categories of invader-triggered environmental change that facilitate invasion by emptying their niche of competitors. Evidence is reviewed that gut bacteria use such strategies to manipulate their environment (via bacteriocins, temperate phage viruses or immuno-manipulation) at the expense of their competitors are reviewed. The possible virulence implications of microbial warfare among multiple co-infecting strains are diverse. Killing competitors can reduce virulence by reducing overall microbial densities, or increase virulence if for example the allelopathic mechanism involves immuno-manipulation. Finally, we place microbial anti-competitor strategies in a social evolution framework, highlighting how costly anti-competitor strategies can be understood as examples of microbial spite. We conclude by discussing other invasive species that have also developed such proactive strategies of invasion.
Keywords: disease biology, evolutionary theory, microbial biology, social evolution, virulence
Introduction
Given local adaptation between a resident population and its environment, how can a nonadapted competitor invade? Invasion can easily be conceived when the invaders are more adapted than the residents. However, if the invader is at a disadvantage in the context of the resident's environment, one solution could be to modify the environment into a configuration favouring the invaders. Many organisms are indeed known to modify the environment, such action is also called niche construction (Odling-Smee et al. 2003). When the environmental modification is triggered by the organisms that benefit from it, natural selection can retain it as an extended phenotype (Dawkins 1982). Building nests, spider webs or beaver dams are examples of extended phenotypes improving the actors’ niche, whereas the secretion of toxins is an example of niche-deterioration (although offering a relative inclusive fitness advantage to the actor), also referred to as interference competition, or allelopathy (Fitter 2003).
Here we review three broad classes of microbial allelopathy, each associated with the release of a distinct class of targeted weapon:
- The release of chemical toxins that directly kill competitors.
- The release of viral propagules that directly kill competitors and subsequently amplify to kill more competitors.
- The release of chemical toxins which provoke an inflammatory response within a vertebrate host, which preferentially kills competitors.
Chemical weapons
Bacteria can express allelopathy via the expression of bactericidal toxin genes. These ‘carrier’ lineages carry genes causing the explosive suicide of the host, with a low probability, the explosive suicide of the host, and the release of bactericidal toxins or bacteriocins (small peptides or heat stable proteins, Riley and Wertz 2002). Furthermore, nonlysed carriers are immune to the bacteriocin, thanks to the specific antidote coding genes carried along with the bacteriocin genes. Thus, the environmental change resulting from the release of bacteriocins can favour the carriers over any susceptible resident populations (Fig. 1a). This type of interference competition is found across a range of different bacterial species and analogous examples can even be found in eukaryotic yeasts (Wickner 1996; Riley and Wertz 2002).
Figure 1.
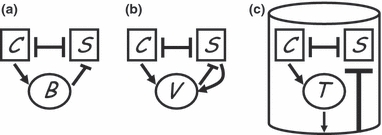
Schematic diagrams of (a) chemical, (b) viral and (c) immuno-manipulative weapons of mass microbial destruction. Squares represent microbial densities of competing weapons-carriers (C) and susceptibles (S). Circles represent the density of ‘weapon’, the agent of environmental change (B for bacteriocin, or other chemical toxin, V for temperate phage virus, and T for immuno-provocative toxin). The cylinder represents a multicellular host, the subject of immuno-manipulation by T. Pointed arrows represent a positive relationship (e.g. C increase the density of B), flat arrows represent a negative relationship (e.g. B decrease the density of S).
One well-documented example is that of Escherichia coli strains producing colicins, bacteriocins active against other E. coli. Previous experimental and theoretical studies of colicin-mediated bacterial competition have shown that colicin-producers can invade in a spatially structured environment and if unstructured, then only if sufficiently abundant (Chao and Levin 1981; Levin 1988; Frank 1994; Durrett and Levin 1997). In a spatially structured environment, the modification of the environment due to the secretion of colicins is a local phenomenon: with high local colicin concentrations killing neighbouring competitors, and thereby freeing nutrients for the invaders. Invaders can then spread as a consequence of sequential waves of colicins followed by colicin-mediated resident death leaving a competitive vacuum (Chao and Levin 1981; Levin 1988; Frank 1994; Durrett and Levin 1997).
The invasion of the bacteriocin producers favours the evolution of resistant bacteria. Resistance often comes at some cost to bacteria (either metabolically through the production of an immunity compound or through the loss of membrane receptors), and so allows for the coexistence, in a spatially structured environment (through negative frequency-dependent selection) of producer, sensitive, and resistant bacteria; producers out-compete sensitives, resistants beat producers, and sensitives beat resistants, creating a ‘rock-paper-scissors dynamic’ (Czárán et al. 2002; Kerr et al. 2002; Czárán and Hoekstra 2003). The pleiotropic fitness cost associated with resistance is also likely to explain the diversity in bacteriocin types seen within a microbial species. Being resistant to all bacteriocin types will often be too costly, so resistance to the most common bacteriocins will be frequent. This favours bacteria producing rare bacteriocins, which should then in turn become more widespread, thereby maintaining diversity (West et al. 2007).
More recently work on yeasts infected with double stranded RNA viruses has helped to further elucidate the ecological conditions that favour the production of anti-competitor toxins. These viruses can be considered similar to bacteriocins as there is no known horizontal transmission of the virus between yeast cells: daughter cells are instead infected via the cytoplasm of their parents (Magliani et al. 1997). In Saccharomyces cerevisiae, the K1 toxin system requires two viruses, one that encodes the toxin and immunity proteins and a second that is involved in encapsulating the virus. Unlike most bacteriocins, lysis is not required to release the toxin, but there is a pronounced fitness cost when carrying the virus (Wloch-Salamon et al. 2008).
Using this system, Grieg and Travisano (2008) were able to show the importance of density in determining the success of toxin-producing strains. Density dependence has previously been shown to be an important ecological determinant in allelopathic interactions (Brown et al. 2006), but experimental work has focused on the frequency of the producing and sensitive strains. Grieg and Travisano (2008) demonstrated that toxin producers, even when at intermediate frequencies, were unable to invade if the overall microbial density was low. This effect was also observed in structured environments, which tend to favour toxin production. When both producers and sensitive strains are rare there will only be limited interaction between them and there will be little benefit in producing toxins. Instead toxin producers will be at a disadvantage due to the metabolic and pleiotropic costs of producing toxins.
Nutrient availability, dispersal, and their effect on interference competition in yeast have also been considered experimentally. It has been suggested that toxin production might act as strategy of last resort among starving cells by killing competitors for nutrients (Ivanovska and Hardwick 2005). Wloch-Salamon et al. (2008) grew S. cerevisiae toxin-producing strains in competition with sensitives under low and high nutrient conditions. Nutrient availability was manipulated by varying the rate of nutrient replacement, following initial growth to carrying capacity (therefore controlling initial densities). Wloch-Salamon et al. (2008) found that toxin producers were able to out-compete sensitive cells only in high nutrient environments and were out-competed when grown under low nutrient conditions. They argued that the lack of toxin production seen in the low nutrient environment is unlikely to be solely due to a nutritional constraint, but rather due in addition to a physiological change in toxin production or resistance developing after the nutrients have been depleted (Wloch-Salamon et al. 2008). They conclude that this acts as support for Frank's (1994) theoretical prediction that toxin production has evolved to occur as a competitive strategy under conditions where resources are abundant and growth is allowed. This makes it unlikely that toxin production is used to acquire resources from sensitive cells, but is more important in the invasion of high nutrient patches. They also looked at the effect on dispersal, finding that limited dispersal favoured toxin producers. A benefit was still observed in treatments with high dispersal, but it was insufficient to compensate for the resource cost of carrying the toxin-producing virus. These results are consistent with Chao and Levin's (1981) results in spatially structured environments as dispersal will reduce the chances that related cells will profit from toxin production.
There is still much about the ecology and evolution of interference competition through toxin production that remains to be properly understood. A huge diversity exists in the types of toxins used from bacteriocins to antibiotics: they can differ in the specificity of killing action, in their ability to diffuse, and even in their respective half-lives in the environment (Ito et al. 1970; Michel-Briand and Baysse 2002; Riley and Wertz 2002; Cascales et al. 2007). All of these factors will greatly change how the environment is modified to allow toxin producers to invade. One constraint, however, that is faced by all toxin producers trying to invade a population is that for every halving of the invader population, the cost to individual invaders of successful environmental modification will double. Given limited resources, these increasing costs will ultimately present a rarity threshold to invasion (Chao and Levin 1981; Levin 1988; Frank 1994; Durrett and Levin 1997; Gordon and Riley 1999).
Escaping the rarity threshold: biological weapons
We have just seen that for chemical allelopathy in an unstructured environment, there exists a rarity threshold, below which allelopathic lineages cannot invade. Brown et al. (2006) demonstrated that this threshold can be reduced or eliminated for other forms of allelopathy. Specifically, weapons that are capable of self-amplification can allow vanishingly rare carrier lineages to invade and replace residents.
A microbial example is provided by bacteria and some of their phages (viruses that can infect bacteria), which can function as bacteriocins (Bossi et al. 2003) but with the additional capacity of autocatalytic amplification on victims (Brown et al. 2006). More specifically, we focus on temperate phages, which are viruses that are able to be transmitted either vertically or horizontally. Infection of susceptible bacteria by temperate phage can result in two possible outcomes. The most common is the lytic cycle (rapid host lysis and production of numerous horizontally transmissible viral particles). Very rarely, however, the phage can lysogenise the host, persisting in a dormant state while allowing the survival of the infected bacteria. This dormant phage is then replicated with the bacterial genome, and thus vertically transmitted upon bacterial division. Furthermore, this vertically transmitted carried-phage provides immunity to its carrier-bacteria against further horizontal infection by this phage (Adams 1959; Campbell 1996). Upon rare spontaneous induction of the carried-phage, viral progeny are released through host lysis.
Experimental competition of isogenic phage-carrying and susceptible bacterial lineages over a range of initial ratios illustrates that the ability of the released temperate phage ‘weapon’ to amplify itself on susceptible hosts (Fig. 1b) allows the phage-resistant carrier lineage to invade more rapidly when rare (Brown et al. 2006). Autocatalytic environmental modification, such as the one triggered by the release of phage, can be seen as a public good that is expensive to produce initially but which can then be amplified at a negligible cost, therefore allowing an escape from the rarity threshold (Brown et al. 2006).
The basic ‘indirect mutualist’ (the enemy of your enemy is your friend) interaction between bacteria and temperate phage can also be viewed in terms of parasite-mediated apparent competition, whereby the outcome of competition between two competitors is determined by their relative susceptibility to a shared parasite (Hudson and Greenman 1998; Tompkins et al. 2001; Joo et al. 2006). For instance, one of the leading hypotheses for the replacement of resident red squirrels by invasive grey squirrels in the UK is the differential susceptibility to a parapoxvirus brought by the invasive greys (Rushton et al. 2000; Tompkins et al. 2002). However, unlike the one-off invasion of the UK by grey squirrels plus a parapoxvirus, food-borne pathogens such as E. coli O157::H7 and its suite of phages continuously invade new digestive tracts. We will propose below that this distinction between once-off lucky and recurrent ‘proactive’ parasite-mediation can be explained in an evolutionary framework.
Both experimental and model results illustrate how the level of amplification is determined by the availability of susceptibles in the initial population (Brown et al. 2006). When susceptibles are sufficiently rare, the phage and colicin models converge as the phage ceases to gain any multiplicative advantage due to the rarity of susceptibles, acting purely through its direct killing effect. Whereas phage can act essentially as a colicin-style nonmultiplicative killer when their carriers are numerically dominant, they do so in a less efficient manner due to their relatively wasteful capacity for replication (e.g. effective colicin ‘burst-size’ is at least 10-fold greater, Gordon and Riley 1999). Therefore for a high carrier frequency, colicin production is more efficient. Given these differences between colicin and phage mediation, phage-carriers are more likely to be successful in invasive lineages, as they grow in the wake of the competitive vacuum left by their virulent indirect-mutualists. In contrast, a colicin-strategy specialising on killing without amplification by the susceptibles has an advantage over phage-carriers in a defensive context: their higher productivity ensures that they cannot be as easily invaded when residents (Brown et al. 2006).
Provoking the superpower: immuno-manipulation
To this point we have focused solely on a single invasion event into a resident population consisting entirely of susceptibles. However, given the diversity of normal microbial communities within the gut, only a minority of the resident bacteria will be susceptible to any specific bacteriocin or phage. Consider a distinct resistant lineage, and the problem is made plain. The carriers can only attack susceptibles, and resistants gain from the suppression of the susceptible population, along with the carriers. Following the eradication of susceptibles, resistants will outcompete carriers due to the latter's lytic behaviour, leaving an endpoint of pure resistants.
This might not be the end of the game; however, given that phage-resistant bacteria are likely to carry a cost relative to susceptibles (resistance is often generated via the loss of useful membrane proteins that are recognized by both phages and bacteriocins). Resistants could be outcompeted by susceptibles which are in turn outcompeted by carriers (at least, in a structured environment for bacteriocin producers) that are themselves outcompeted by these resistant bacteria, potentially promoting ‘rock/paper/scissors’ dynamics (Czárán et al. 2002; Kerr et al. 2002; Kirkup and Riley 2004; Brown et al. 2008).
A possible mechanism allowing a broader niche emptying involves the production of toxins active against the animal host. If the toxin carrier had superior resistance to the resulting immunological response, manipulating the host immune state would then competitively benefit a suitably protected pathogen (Fig. 1c, Brown et al. 2008). Consistently with this hypothesis, phages from E. coli O157::H7 carry genes coding for both immune-provoking toxins expressed just before lysis and for protection against the immune response (Plunkett et al. 1999; Hendrix et al. 2000;Ohnishi et al. 2001) expressed in the lysogenic association. Residents that are susceptible to the invaders’ phage can act as a phage-multiplying factory, and consequently multiply the phage-coded toxin as well (Gamage et al. 2003). Upon infection by the virus, susceptibles are turned into factories producing more viruses. If the virus also coded for niche-modifying proteins (proteins impacting the extracellular environment), the susceptibles would then also become factories for such proteins, directly or indirectly triggering a change in the environment benefiting the phage carrier. The resultant immune response can then favour the protected invaders, at the expense of a broad spectrum of residents including those that resist the phage but are susceptible to the immune response. As genes allowing even further protection from the inflammatory response are also carried in the E. coli O157::H7 genome outside of the released phage (Hayashi et al. 2001; Ohnishi et al. 2001; Perna et al. 2001), even if rare residents are able to become lysogenised, they will not carry all the genes required for long-term persistence. We propose below that E. coli O157::H7 would thus preserve a competitive advantage during invasions. Given the long co-existence of multiple phages within the E. coli O157::H7 lineage (Feng et al. 1998; Ohnishi et al. 2001) these bacteria may have been proactive invaders for a long time.
Several more microbial examples of an immuno-manipulative anti-competitor strategy have been recently proposed (Lysenko et al. 2005, Stecher et al. 2007,Brown et al. 2008). For example, Lysenko et al. 2005 demonstrated in vivo that Haemophilus influenze outcompetes Streptococcus pneumoniae in the upper respiratory tract by recruiting and then by activating neutrophils and complement, to which Streptococcus is more sensitive than Haemophilus. Similarly, Raberg et al. (2006) demonstrated that more virulent strains of the rodent malaria parasite Plasmodium chabaudi experience a stronger within-host competitive advantage in immuno-competent hosts, relative to competition in immunodeficient hosts. Stecher et al. (2007) demonstrated that host inflammatory responses triggered by Salmonella enterica serovar Typhimurium aid its ability to invade resident gut microbiota.
Discussion
Social evolution
A broad strand of experimental and theoretical work on the ecology of bacteriocin-mediated competition highlights the importance of spatial structure in mediating the outcome of competition, with a broad consensus recognising that structured environments promote the invasion of rare killers by increasing the local density of chemical weapons to an effective dose (Chao and Levin 1981; Levin 1988; Frank 1994; Durrett and Levin 1997; Gordon and Riley 1999).
The impact of spatial structure on the relative costs and benefits of investment in allelopathy also have strong implications on an evolutionary time scale. Modelling work by Gardner et al. (2004) showed that the evolution of bacteriocin production should be most favoured when producers and sensitives interact locally at intermediate frequencies. When toxin producers are locally scarce, they are unable to generate sufficient chemical weapons (and therefore sufficient competitor deaths) to compensate for the cost of production. In contrast when toxin producers are locally dominant, producing toxins has little benefit as there are few competitors to kill, and hence less available resources to gain from their death. Only when producers are at intermediate local frequencies will bacteriocin production confer the greatest fitness advantage, by maximising gains from competitive escape for a given investment in killing.
Gardner et al. (2004) go on to illustrate that the production of anti-competitor chemical weapons can be understood as an example of microbial spite. A spiteful trait imposes costs on both actor and recipient (Hamilton 1964, 1–16; Hamilton 1970; Gardner and West 2006; Gardner et al. 2007), and so allelopathy can be considered a spiteful trait as it has a negative fitness impact on the actor cell producing the toxin (due to metabolic costs of toxin production, and for many bacteriocins, cell lysis so as to release the toxins) and imposes a clear cost on recipient cells that are sensitive to the action of the toxin. For spite to evolve, Hamilton's rule rb > c must be satisfied. For a spiteful trait, c refers to the cost to the actor (c > 0) and b refers to the cost (or ‘negative benefit’, b < 0) to the recipients. Given _c_ > 0 > b, Hamilton's rule can be satisfied, only if relatedness r is negative. Negative relatedness occurs between individuals that are less genetically similar (at the social trait of interest) than individuals chosen at random from the competitive arena (Hamilton 1970; Grafen 1985; Queller 1994). Thus, if a spiteful bacterium killed a random selection of neighbours (who by definition have relatedness of zero, Queller 1994), then this trait would not be favoured. However, the specificity of bacteriocin action allows for the molecular discrimination between kin (who carry appropriate immunity genes) and non-kin (who do not), and therefore the generation of negative relatedness between discriminately spiteful actor and unfortunate recipient. On the level of a clonal bacteriocin-producing lineage, bacteriocins are selfish (i.e. returning direct benefits to the lineage through release from competition); however bacteriocin production is certainly spiteful (c > 0 > b) at the level of a self-destructing bacterium producing the toxins (Gardner et al. 2004).
The relative weighting of the indirect and direct effects of microbial spite change markedly when we consider the more complex dynamics created by temperate phage weapons amplifying on susceptibles (Dionisio 2007), or immunological responses being ramped up by a sensitive multicellular host (Fig. 1). In particular, the autocatalytic dynamic most evident in the amplification of phage on susceptibles will allow a greater destruction of competitors when the focal spiteful lineage is rare and sensitives are common, broadening the ecological conditions favouring microbial spite. Of course, the temperate phage symbiont cannot be considered a mere adaptation of its carrier. Rather, one should consider the strategic interests of both players in such dangerous liaisons (van Baalen and Jansen 2001). The functional similarities from the carrier perspective between bacteriocins and temperate phage propagules (Bossi et al. 2003; Brown et al. 2006; Joo 2006) present an intrguing bridge between public goods (weapons are a public good of the producer lineage, liberating them from competitors) and symbionts (Brown and Taddei 2007).
Virulence
The impact of within-host competition on the evolution of virulence has been the subject of a diverse range of models, offering contrasting explanations for either an increase or a decrease in virulence as within-host diversity increases (Van Baalen and Sabelis 1995; Frank 1996; Brown et al. 2002; West and Buckling 2003). In contrast, in the case of chemical warfare overall virulence is predicted to be maximised at intermediate within-host diversities, at the point where spiteful killing is maximised and therefore overall microbial growth is most disrupted (Gardner et al. 2004). A partial test of this theory using Photorhabdus spp. in caterpillars showed that single strain infections were more virulent than mixes of bacteriocin producer and a sensitive species of Photorhabdus (Massey et al. 2004). Further work looking at the effect of migration on virulence in a bacteria/nematode system found that virulence is reduced at high migration rates, leading to more local diversity and therefore more bacterial interference competition is likely to occur (Vigneux et al. 2008).
The relationship between virulence and within-host diversity changes yet again when alternative mechanisms of microbial interference competition are considered. Temperate phage mediated killing will create the greatest demographic disruption to overall microbial growth when phage carriers are scarce and susceptibles are common (Brown et al. 2006). In the case of immuno-manipulative strategies, when pro-inflammatory toxins are involved, virulence is likely to become decoupled from overall bacterial density, and be largely driven by the provocative actions of the toxin-producing lineage (Brown et al. 2008). In contrast, immuno-manipulation may reduce virulence in other systems, if the manipulation is carried out by resident chronic infections to skew or neutralise an inflammatory response (Riffkin et al. 1996; Graham 2002; Graham et al. 2005; Sansonetti and Di Santo 2007; Alizon and van Baalen 2008) or to increase resistance to superinfection (Brown and Grenfell 2001). More generally, the complex links between microbes and immunopathology are likely to limit the applicability of general rules for the evolution of virulence (Graham et al. 2005; Day et al. 2007).
Proactive invaders
In this review we have briefly sketched three distinct mechanisms of microbial allelopathy, each with a distinct mediator of competition: chemicals, viruses or immunity. The study of chemically mediated microbial competition is by far the most advanced experimentally and theoretically, and can therefore serve as a template for future studies on virus- and immune-mediated allelopathy (e.g. understanding and characterising density and frequency dependence, specificity and diversity). In return, the study of virus- and immune-mediated allelopathy bring to the fore issues of co-evolutionary ‘dangerous liaisons’ between microbial provocator and their ‘weapon’ (virus or multicellular host) that are also present, yet less evident, in chemically mediated competition. For instance, numerous bacteriocinogenic bacteria owe their allelopathy to mobile genetic elements, mostly plasmids but also transposons and even to extinct phages (Riley and Wertz 2002).
The study of microbial allelopathy may have broader lessons for the study of competition. A population that increases on arriving in a new habitat can do so in one of three ways. First, because of some chance preadaptation, mutation or environmental change, organisms find themselves favoured in their new environment (Sakai et al. 2001). Parasites can play a determining role in these chance events, both through preadapted resistance (Rushton et al. 2000; Tompkins et al. 2002) or through chance losses in parasitic environment (competitive release hypothesis, Torchin et al. 2003; Mitchell and Power 2003). Second, organisms which exploit, through repeated generations, regularly appearing environmental disturbances (for instance new forest patches following the death of a mature tree) can evolve adaptations favouring their growth in these disturbed environments (Crawley 1997). Third, given certain genotypes become better adapted than their competitors to disturbed environments, they can then evolve adaptations to increase the environmental disturbance from which they benefit, i.e. become proactive invaders (Brown et al. 2008). Cheatgrass, Bromus tectorum, is one candidate example, a plant that not only grows well on niches emptied by fire, but also favours fire itself (Melgoza et al. 1990; Billings 1993; Kerr et al. 1999). Similarly, bacteria inhabiting hosts where inflammation is recurrent will be selected to resist the associated immune response. Given better adaptation to inflammation, selection could then favour the provocation of inflammation itself, through the acquisition of virulence factors. This principle of relative advantage suggests that invaders can gain by actively deteriorating their own environment. When the invader is a pathogen and the environment is a host, this strategy can have important implications for virulence.
Acknowledgments
We thank Angus Buckling and Troy Day for helpful comments on an earlier draft, and the Wellcome Trust (SPB), NERC (RFI) and EURYI (FT) for funding.
Literature cited
- Adams MH. Bacteriophages. London: Interscience Publishers Ltd; 1959. [Google Scholar]
- Alizon S, Van Baalen M. Multiple infections, immune dynamics and the evolution of virulence. American Naturalist. 2008;172:E150–E168. doi: 10.1086/590958. [DOI] [PubMed] [Google Scholar]
- Van Baalen M, Jansen VAA. Dangerous liaisons: the ecology of private interest and common good. Oikos. 2001;95:211–224. [Google Scholar]
- Billings WD. Ecology, Management and Restoration of Inter-Mountain Annual Rangelands. Ogdon Utah: US Forest Service; 1993. [Google Scholar]
- Bossi L, Fuentes JA, Mora G, Figueroa-Bossi N. Prophage contribution to bacterial population dynamics. Journal of Bacteriology. 2003;185:6467–6471. doi: 10.1128/JB.185.21.6467-6471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Grenfell BT. An unlikely partnership: parasites, concomitant immunity and host defence. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268:2543–2549. doi: 10.1098/rspb.2001.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Taddei F. The durability of public goods changes the dynamics and nature of social dilemmas. PLoS ONE. 2007;2:e593. doi: 10.1371/journal.pone.0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Hochberg ME, Grenfell BT. Does multiple infection select for raised virulence? Trends in Microbiology. 2002;10:401–405. doi: 10.1016/s0966-842x(02)02413-7. [DOI] [PubMed] [Google Scholar]
- Brown SP, Le Chat L, De Paepe M, Taddei F. Ecology of microbial invasions: Amplification allows virus carriers to invade more rapidly when rare. Current Biology. 2006;16:2048–2052. doi: 10.1016/j.cub.2006.08.089. [DOI] [PubMed] [Google Scholar]
- Brown SP, Le Chat L, Taddei F. Evolution of virulence: triggering host inflammation allows invading pathogens to exclude competitors. Ecology Letters. 2008;11:44–51. doi: 10.1111/j.1461-0248.2007.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AM. Cryptic prophages. In: Neidhardt FC, editor. Escherichia coli and Salmonella. Washington, D.C: ASM Press; 1996. pp. 2325–2338. [Google Scholar]
- Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, Postle K, Riley M, et al. Colicin biology. Microbiology and Molecular Biology Reviews. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Levin BR. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ. Plant Ecology. Oxford: Blackwell Science; 1997. [Google Scholar]
- Czárán TL, Hoekstra RF. Killer-sensitive coexistence in metapopulations of micro-organisms. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2003;270:1373–1378. doi: 10.1098/rspb.2003.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czárán TL, Hoekstra RF, Pagie L. Chemical warfare between microbes promotes biodiversity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:786–790. doi: 10.1073/pnas.012399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. The Extended Phenotype. San Francisco: Freeman; 1982. [Google Scholar]
- Day T, Graham A, Read A. Evolution of parasite virulence when host responses cause disease. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2007;B 274:2685–2692.554. doi: 10.1098/rspb.2007.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio F. Selfish and spiteful behaviour through parasites and pathogens. Evolutionary Ecology Research. 2007;9:1199–1210. [Google Scholar]
- Durrett R, Levin S. Allelopathy in spatially distributed populations. Journal of Theoretical Biology. 1997;185:165–171. doi: 10.1006/jtbi.1996.0292. [DOI] [PubMed] [Google Scholar]
- Feng P, Lampel KA, Karch H, Whittam TS. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. Journal of Infectious Diseases. 1998;177:1750–1753. doi: 10.1086/517438. [DOI] [PubMed] [Google Scholar]
- Fitter A. Ecology. Making allelopathy respectable. Science. 2003;301:1337–1338. doi: 10.1126/science.1089291. [DOI] [PubMed] [Google Scholar]
- Frank SA. Spatial polymorphism of bacteriocins and other allelopathic traits. Evolutionary Ecology. 1994;8:369–386. [Google Scholar]
- Frank SA. Models of parasite virulence. Quarterly Review of Biology. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Gamage SD, Strasser JE, Chalk CL, Weiss AA. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infection and Immunity. 2003;71:3107–3115. doi: 10.1128/IAI.71.6.3107-3115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- Gardner A, West SA. Spite. Current Biology. 2006;16:R662–R664. doi: 10.1016/j.cub.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Gardner A, West SA, Buckling A. Bacteriocins, spite and virulence. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271:1529–1535. doi: 10.1098/rspb.2004.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Hardy ICW, Taylor PD, West SA. Spiteful soldiers and sex ratio conflict in polyembryonic parasitoid wasps. American Naturalist. 2007;169:519–533. doi: 10.1086/512107. [DOI] [PubMed] [Google Scholar]
- Gordon DM, Riley MA. A theoretical and empirical investigation of the invasion dynamics of colicinogeny. Microbiology. 1999;145:655–661. doi: 10.1099/13500872-145-3-655. [DOI] [PubMed] [Google Scholar]
- Grafen A. A geometric view of relatedness. Oxford Surveys in Evolutionary Biology. 1985;2:28–89. [Google Scholar]
- Graham AL. When T-helper cells do not help: immuno-pathology during concomitant infection. Quarterly Review of Biology. 2002;77:409–434. doi: 10.1086/344414. [DOI] [PubMed] [Google Scholar]
- Graham AL, Allen JE, Read AF. Evolutionary causes and consequences of immunopathology. Annual Review of Ecology, Evolution and Systematics. 2005;36:373–397. [Google Scholar]
- Greig D, Travisano M. Density-dependent effects of allelopathic interactions in yeast. Evolution. 2008;62:521–527. doi: 10.1111/j.1558-5646.2007.00292.x. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. The evolution of altruistic behaviour. American Naturalist. 1963;97:354–356. [Google Scholar]
- Hamilton WD. The genetical evolution of social behaviour. I. Journal of Theoretical Biology. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Selfish and spiteful behaviour in an evolutionary model. Nature. 1970;228:1218–1220. doi: 10.1038/2281218a0. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Altruism and related phenomena, mainly in social insects. Annual Review of Ecology and Systematics. 1972;3:193–232. [Google Scholar]
- Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Research. 2001;8:11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- Hendrix RW, Lawrence JG, Hatfull GF, Casjens S. The origins and ongoing evolution of viruses. Trends in Microbiology. 2000;8:504–508. doi: 10.1016/s0966-842x(00)01863-1. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Greenman JV. Competition mediated by parasites: biological and theoretical progress. Trends in Ecology & Evolution. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. [DOI] [PubMed] [Google Scholar]
- Ito S, Kageyama M, Egami F. Isolation and characterization of pyocins from several strains of pseudomonas aeruginosa. Journal of General and Applied Microbiology. 1970;16:205–214. [Google Scholar]
- Ivanovska I, Hardwick JM. Viruses activate a genetically conserved cell death pathway in a unicellular organism. Journal of Cell Biology. 2005;170:391–399. doi: 10.1083/jcb.200503069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo J, Gunny M, Cases M, Hudson P, Albert R, Harvill E. Bacteriophage-mediated competition in Bordetella bacteria. Proceedings Royal Society B. 2006;273:1843–1848. doi: 10.1098/rspb.2006.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Schwilk DW, Bergman A, Feldman MW. Rekindling an old flam: a haploid model for the evolution and impact of flammability in resprouting plants. Evolutionary Ecology Research. 1999;1:807–833. [Google Scholar]
- Kerr B, Riley MA, Feldman MW, Bohannan BJM. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- Kirkup BC, Riley MA. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature. 2004;428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- Levin BR. Frequency-dependent selection in bacterial populations. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1988;319:459–472. doi: 10.1098/rstb.1988.0059. [DOI] [PubMed] [Google Scholar]
- Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of inter- species competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliani W, Conti S, Gerloni M, Bertolotti D, Polonelli L. Yeast killer systems. Clinical Microbiology Reviews. 1997;10:369–400. doi: 10.1128/cmr.10.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey RC, Buckling A, Ffrench-Constant R. Interference competition and parasite virulence. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004;271:785–788. doi: 10.1098/rspb.2004.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgoza G, Nowak RS, Tausch RJ. Soil-water exploitation after fire – competition between Bromus tectorum (cheatgrass) and 2 native species. Oecologia. 1990;83:7–13. doi: 10.1007/BF00324626. [DOI] [PubMed] [Google Scholar]
- Michel-Briand Y, Baysse C. The pyocins of Pseudomonas aeruginosa. Biochimie. 2002;84:499–510. doi: 10.1016/s0300-9084(02)01422-0. [DOI] [PubMed] [Google Scholar]
- Mitchell CE, Power AG. Release of invasive plants from fungal and viral pathogens. Nature. 2003;421:625–627. doi: 10.1038/nature01317. [DOI] [PubMed] [Google Scholar]
- Odling-Smee FJ, Laland KN, Feldman MW. Niche Construction The neglected process in evolution. Princeton and Oxford: Princeton University Press; 2003. [Google Scholar]
- Ohnishi M, Kurokawa K, Hayashi T. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends in Microbiology. 2001;9:481–485. doi: 10.1016/s0966-842x(01)02173-4. [DOI] [PubMed] [Google Scholar]
- Perna NT, Plunkett G, III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, et al. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- Plunkett G, III, Rose DJ, Durfee TJ, Blattner FR. Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. Journal of Bacteriology. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller DC. Genetic relatedness in viscous populations. Evolutionary Ecology. 1994;8:70–73. [Google Scholar]
- Raberg L, De Roode JC, Bell AS, Stamou P, Gray D, Read AF. The role of immune-mediated apparent competition in genetically diverse malaria infections. Am. Nat. 2006;168:41–53. doi: 10.1086/505160. [DOI] [PubMed] [Google Scholar]
- Riffkin M, Seow HF, Jackson D, Brown L, Wood P. Defence against the immune barrage: helminth survival strategies. Immunology and Cell Biology. 1996;74:564–574. doi: 10.1038/icb.1996.90. [DOI] [PubMed] [Google Scholar]
- Riley MA, Wertz JE. Bacteriocins: Evolution, ecology, and application. Annual Review of Microbiology. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- Rushton SP, Lurz PWW, Gurnell J, Fuller R. Modelling the spatial dynamics of parapoxvirus disease in red and grey squirrels: a possible cause of the decline in the red squirrel in the UK? J Appl Ecol. 2000;37:997–1012. [Google Scholar]
- Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. 2007;26:149–161. doi: 10.1016/j.immuni.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PloS Biology. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins DM, Greenman JV, Hudson PJ. Differential impact of a shared nematode parasite on two gamebird hosts: implications for apparent competition. Parasitology. 2001;122:187–193. doi: 10.1017/s0031182001007247. [DOI] [PubMed] [Google Scholar]
- Tompkins DM, Sainsbury AW, Nettleton P, Buxton D, Gurnell J. Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2002;269:529–533. doi: 10.1098/rspb.2001.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. Introduced species and their missing parasites. Nature. 2003;421:628–630. doi: 10.1038/nature01346. [DOI] [PubMed] [Google Scholar]
- Van Baalen M, Sabelis MW. The dynamics of multiple infection and the evolution of virulence. American Naturalist. 1995;146:881–910. [Google Scholar]
- Vigneux F, Bashey F, Sicard M, Lively CM. Low migration decreases interference competition among parasites and increases virulence. Journal of Evolutionary Biology. 2008;21:1245–1251. doi: 10.1111/j.1420-9101.2008.01576.x. [DOI] [PubMed] [Google Scholar]
- West SA, Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc. Biol. Sci. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA, Diggle SP, Buckling A, Gardner A, Griffin AS. The social lives of microbes. Annual Review of Ecology, Evolution and Systematics. 2007;38:53–77. [Google Scholar]
- Wickner RB. Double-stranded RNA viruses of saccharomyces cerevisiae. Microbiological Reviews. 1996;60:250–265. doi: 10.1128/mr.60.1.250-265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloch-Salamon DM, Gerla D, Hoekstra RF, De Visser JAGM. Effect of dispersal and nutrient availability on the competitive ability of toxin-producing yeast. Proceedings of the Royal Society B: Biological Sciences. 2008;275:535–541. doi: 10.1098/rspb.2007.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]