Pam17 and Tim44 act sequentially in protein import into the mitochondrial matrix (original) (raw)
. Author manuscript; available in PMC: 2012 May 16.
Published in final edited form as: Int J Biochem Cell Biol. 2009 Jul 3;41(11):2343–2349. doi: 10.1016/j.biocel.2009.06.011
Abstract
Import of proteins into the matrix is driven by the Tim23 presequence translocase-associated import motor PAM. The core component of PAM is the mitochondrial chaperone mtHsp70, which ensures efficient translocation of proteins across the inner membrane through interactions with the J-protein complex Pam16–18 (Tim16-Tim14) and its cochaperone Tim44. The recently identified non-essential Pam17 is a further member of PAM. Genetic and biochemical analyses reveal synthetic interactions between PAM17 and TIM44. Pam17 is involved in an early stage of protein translocation whereas Tim44 assists in a later step of transport, suggesting that both proteins can cooperate in a complementary manner in protein import.
1. Introduction
Since most of the mitochondrial proteins are nuclear-encoded and synthesized on cytosolic ribosomes, efficient protein import mechanisms into mitochondria are essential. Mitochondrial preproteins carry an N-terminal presequence that is recognized by receptors on the outer membrane. Translocation across the mitochondrial membranes is accomplished by the TOM (translocase of the outer mitochondrial membrane) complex and the TIM23 (translocase of the inner membrane) complex (Neupert and Herrmann, 2007). Import of matrix proteins requires both a membrane potential across the inner membrane and the ATP-consuming presequence translocase-associated import motor PAM in the matrix (Neupert and Brunner, 2002).
PAM couples the action of the mitochondrial Hsp70 chaperone Ssc1 to the translocation of a preprotein across the inner membrane. Ssc1 binds to preproteins in the ATP-bound state. ATP-hydrolysis, stimulated by the J-complex Pam16-Pam18 (also known as Tim16-Tim14) stabilizes this interaction (Bolender et al., 2008). The membrane-associated co-chaperone Tim44 can act as a membrane anchor for Ssc1. A purified Tim44-Ssc1(ATP) complex is destabilized upon binding of a peptide substrate to Ssc1, suggesting that in vivo another Ssc1(ATP) binds to freed Tim44 thus promoting further protein translocation (Liu et al., 2003). Recently two non-essential proteins, Tim21 and Pam17, which bind to the Tim23 channel, have been identified. Tim21 is involved in sorting of preproteins into the inner membrane, whereas Pam17 plays a role in import of preproteins into the matrix (Hutu et al., 2008; Mokranjac et al., 2005; Popov-Čeleketić et al., 2008; van der Laan et al., 2005, 2006).
The role of Pam17 in protein import is only poorly understood. _pam17_Δ mitochondria display an import defect with selected matrix-targeted precursors, but _pam17_Δ cells grow like wild type under most conditions. To understand this apparent discrepancy between the in vivo and the in vitro phenotype, I performed genetic and biochemical analyses of the PAM17 gene. Pam17 is required for efficient posttranslational protein import. The deletion of PAM17 in combination with mutants of essential genes of the import motor, SSC1 and TIM44, led to a synthetic enhancement of the single phenotypic effects. The data show that Pam17 is involved in an early step of import, thereby facilitating the interaction of Ssc1 with the incoming polypeptide, while Tim44 promotes further translocation of the protein into the matrix suggesting an overlapping functional cooperation of Pam17 and Tim44 in protein import.
2. Materials and methods
2.1. Strains used in this study
Yeast strains deleted for TIM44 and SSC1 have been constructed as described (Rassow et al., 1994; Voisine et al., 1999). Deletion of the PAM17 gene was carried out by replacing the open reading frame of PAM17 with the HPH gene from pAG32 (Goldstein and McCusker, 1999). Cell colonies resistant to hygromycin B were verified by PCR. Strains deleted for PAM17 and TIM44 or PAM17 and SSC1 were obtained by mating of the single deletion haploids. The resulting diploids were sporulated and the desired haploids were isolated by dissecting tetrads. The strain _pam17_Δ/_tim44_Δ carrying pRS314-tim44(R180A) was obtained by transforming _pam17_Δ/_tim44_Δ carrying pRS316-TIM44 with pRS314-tim44(R180A) (Schiller et al., 2008). Strain _pam17_Δ/_ssc1_Δ carrying pRS314-ssc1-2 was obtained by transforming _pam17_Δ/_ssc1_Δ cells carrying pRS316-SSC1 with pRS314-ssc1-2. In both cases transformants were selected on 5-fluoroorotic acid to select for candidates having lost the wild type copy of TIM44 and SSC1, respectively. Viable colonies were plated onto YPD plates and incubated at 23, 30, and 37°C.
2.2. Testing for in vivo phenotypes
To test for the accumulation of precursors in vivo, wild type and mutant strains were grown in YPD at 23°C to mid-logarithmic phase and then shifted to 30°C and 37°C for 6 h, respectively. Cell extracts (optical density at 600 nm (OD600), 0.2) were prepared and analyzed by SDS-PAGE and immunoblotting using antibodies specific for the mitochondrial matrix protein Hsp60.
2.3. Mitochondrial assays
Mitochondria were isolated from strains grown in liquid YPG medium (1% yeast extract, 2% Bacto peptone, 3% glycerol) at 25°C as described (Liu et al, 2001). For analysis of the steady state protein levels, 5 μg of mitochondrial protein was analyzed by SDS-PAGE and immunoblotting using antibodies specific for components of the TIM23 translocase and the PAM import motor. Recombinant precursor proteins were purified from Escherichia coli cells as described (Lim et al., 2001) and added to isolated mitochondria at a final concentration of 1 nmol precursor per mg mitochondrial protein. For import assays the purified precursor was added to energized mitochondria in import buffer (10 mM morpholinepropanesulfonic acid [MOPS]-KOH pH 7.2, 80 mM KCl, 5 mM KPi pH 7.2, 5 mM MgCl2, 250 mM sucrose, 1% (w/v) BSA fatty acid-free) including an ATP-regenerating system (4 mM ATP, 2 mM NADH, 5 mM creatine phosphate, and 0.1 mg/ml creatine kinase). At times indicated the import reaction was stopped by addition of 1 mM valinomycin and cooling on ice. Following protease treatment with proteinase K (ratio of mitochondrial protein to protease mass was 10:1) for 20 min on ice to remove unimported precursor, protease resistant recombinant Cytochrome b2-DHFR was detected by SDS-PAGE and immunoblotting using affinity-purified antibodies specific for DHFR. For arresting a precursor in a membrane-spanning fashion, the precursor was incubated with 10 μM methotrexate (MTX) and import was carried out in the presence of 10 μM MTX in the import buffer. For import into mitoplasts, the outer membrane was disrupted by hypoosmotic swelling in ice-cold buffer (10 mM MOPS-KOH pH 7.2, 1mM EDTA) prior to import. Mitoplasts were sedimented and washed once in ice-cold swelling buffer. The mitoplasts were resuspended in import buffer and energized for 10 min before adding precursor. The efficiency of disrupting the outer membrane was tested by monitoring the presence of the inter membrane space protein Cytochrome b2 using Cyt b2-specific antibodies. Import of denatured precursor was carried out by incubating the precursor in 8 mM Urea, 50 mM DTT, 10 mM Tris/HCl pH 7.2 for 15 min at 23°C prior to adding to energized mitochondria.
2.4. Coimmunoprecipitation in mitochondrial lysates
To analyze the interaction of mtHsp70 with the presequence of an arrested precursor, Cytochrome b2(47)-DHFR was imported into mitoplasts in the presence of MTX under high (4 mM ATP, 2 mM NADH) and low (10 U apyrase, 20 μM oligomycin) mitochondrial ATP levels. Import was stopped by cooling the reaction on ice. Mitochondria were sedimented and resuspended in lysis buffer (20 mM Tris/HCl pH 7.2, 80 mM KCl, 1 mM PMSF) and incubated at 4°C for 20 min. Insoluble material was sedimented and the supernatant was incubated with anti Ssc1 antibodies cross-linked to proteinA beads as described (Schiller et al., 2008). Immunoprecipitated precursor was detected by SDS-PAGE and immunoblotting using affinity-purified antibodies specific for DHFR.
2.5. Precursor accumulation and processing in vivo
The accumulation and processing of mitochondrial precursors in vivo was tested by growing cells in liquid YPD to early-logarithmic phase. After addition of the protonophore carbonylcyanide _m_-chlorophenylhydrazone [CCCP] to a final concentration of 20 μM, cells were further incubated. Samples (OD600 = 0.1) were withdrawn and resuspended in double-concentrated SDS-PAGE sample buffer containing 1 mM PMSF. The samples were boiled for 3 min, centrifuged to sediment insoluble material and the supernatant was subjected to SDS-PAGE and western-blot analysis using antibody against the mitochondrial matrix protein Mdj1. To compare the processing efficiency cells were treated like described and further incubated for 30 min in the presence of CCCP. 10 mM β-mercaptoethanol was added to reverse the effect of the uncoupler (Reid and Schatz, 1982) and samples were analyzed as described.
2.6. Miscellaneous
Affinity purification of antibody against Ssc1 and cross-linking to protein A beads by the use of dimethylpimelimidate dihydrochloride was carried out as described previously (Liu et al., 2003). Immunoblot analysis was carried out using an ECL system (Amersham Pharmacia Biotech) according to the manufacturer’s instructions, using polyclonal antibodies specific for Tim17, Tim23, Tim50, Tim44, Pam16, and Pam18. For quantifying the amount of imported precursor proteins into mitochondria calibration curves with defined amounts of purified precursor proteins were determined by densitometry using antibodies specific for DHFR and compared to the signal intensity of detected imported protein. The relative membrane potential of mutant mitochondria was assessed by quenching the fluorescence of the membrane potential-sensitive dye DiSC3(5) (Geissler et al., 2000). All results presented were obtained from a minimum of two completely independent experiments.
3. Results
3.1. Pam17 is required for posttranslational protein import
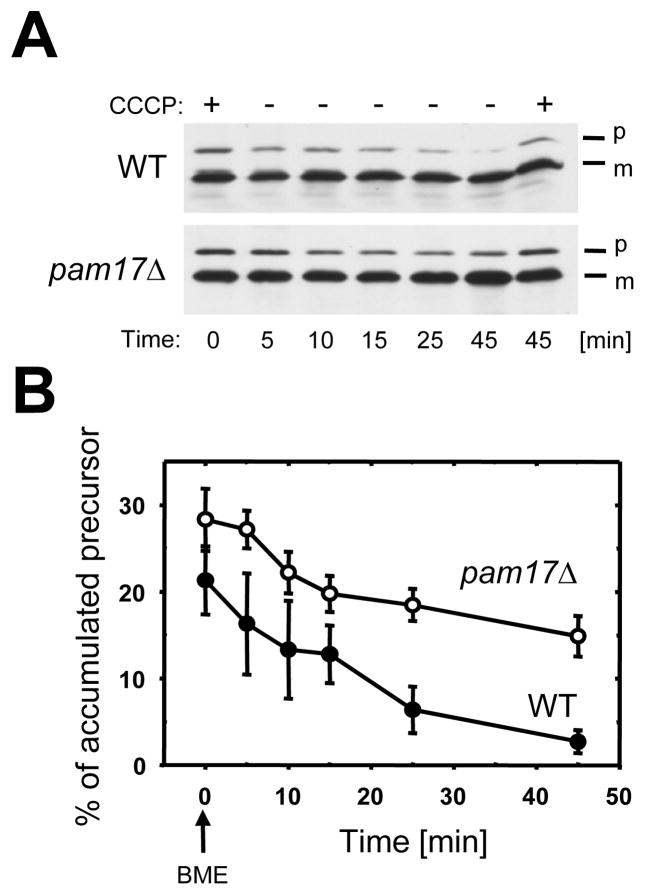
To test the role of Pam17 in protein import I accumulated preproteins in the cytosol of pam17Δ cells by reducing the mitochondrial membrane potential with the protonophore CCCP and monitored subsequent import of precursors after restoration of the membrane potential ΔΨ Reid and Schatz, 1982). Cell lysates were analyzed by western-blot using antibodies against Mdj1, a mitochondrial J-protein involved in folding, but not in import of matrix proteins (Rowley et al., 1994). In wild type cells the amount of accumulated precursor decreased from 21.3% to 2.7% within 45 minutes, compared to a decrease from 28.3% to 14.9% of preMdj1 in pam17Δ cells in this period (Fig. 1). I concluded that PAM17 is involved in posttranslational protein import into mitochondria.
Fig. 1.
Pam17 facilitates posttranslational protein import defect in vivo. (A) Cells were grown to mid-logarithmic phase. After addition of the uncoupler CCCP incubation was continued for 30 minutes. β-mercaptoethanol was added to reverse the effect of CCCP and to restore the membrane potential across the mitochondrial inner membrane. At the times indicated, cell extracts were analyzed by immunoblotting using antibodies against the mitochondrial matrix protein Mdj1. (B) The signal intensities from four independent experiments were quantified by densitometry and the amount of preMdj1 was plotted against the incubation time using the formula: preMdj1/(preMdj1 + mMdj1). p, premature; m, mature Mdj1. BME, β-mercaptoethanol.
3.2. PAM17 genetically interacts with SSC1 and TIM44
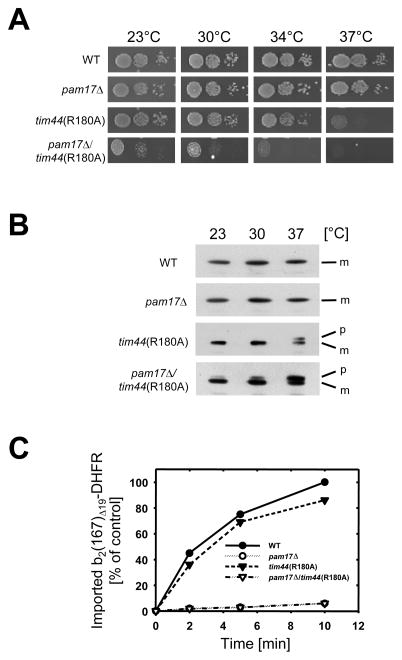
I combined the genomic deletion of PAM17 with mutant alleles of SSC1 and TIM44, encoding for two essential central components of the import motor involved in binding and translocation of mitochondrial matrix proteins. The single mutants ssc1-2 and tim44(R180A) display temperature-sensitive phenotypes and are defective in protein import, due to a deregulated Tim44-Ssc1 interaction in the presence of polypeptide substrate, as described (D’Silva et al., 2004; Schiller et al., 2008). The double mutant pam17_Δ/ssc1-2_ was inviable (Supplemental Fig. S1). _pam17_Δ _tim44_Δ cells, however, having Tim44(R180A) as the only functional Tim44 were compromised in growth at all temperatures (Fig. 2A). In contrast, tim44(R180A) cells alone showed a growth defect at 34°C and 37°C, while cells lacking PAM17 were not compromised in growth. I concluded that the tim44 and ssc1 mutations in the _pam17_Δ background cause a synthetic enhancement of the single phenotypic effects.
Fig. 2.
Synthetic phenotype of tim44(R180A) in the _pam17_Δ strain background. (A) Growth phenotypes of mutant strains _pam17_Δ, tim44(R180A), or _pam17_Δ/tim44(R180A). Tenfold serial dilutions were plated onto rich medium and incubated for 3 days (23°C) or 2 days (30°C, 34°C, and 37°C). (B) Accumulation of a preprotein in vivo. Cells were grown at 23°C to mid-logarithmic phase and subsequently shifted to 30°C or 37°C. Cell extracts were analyzed by immunoblotting using Hsp60-specific antibodies. p, premature Hsp60; m, mature Hsp60. (C) Import of the recombinant precursor cytochrome b2(167)Δ19-DHFR into isolated mitochondria. Kinetically saturating amounts (1 nmol/mg of mitochondrial protein) of purified preprotein was incubated with energized mitochondria for the indicated times. Following proteinase K treatment to remove unimported precursor, the amount of imported processed protein was determined by immunoblotting using antibodies against DHFR. The amount of precursor processed in wild type mitochondria after 10 minutes was set to 100% (control). The data points represent the average value of at least two independent experiments.
I asked whether the observed phenotype was due to a defective import of mitochondrial preproteins. Lysates from cells grown at different temperatures were analyzed by immunoblotting using antibody directed against the matrix chaperonin Hsp60 (Fig. 2B). Consistent with the observed growth phenotype, the precursor form of Hsp60 was present in lysates of _pam17_Δ tim44(R180A) cells under all temperatures tested, while tim44(R180A) cells accumulated the precursor only at 37°C, as described (Schiller et al., 2008). No precursor could be detected in lysates of _pam17_Δ cells. The steady state levels of mitochondrial proteins were not significantly reduced in the mutant mitochondria (Supplemental Fig. S2).
I compared the import efficiencies of a recombinant fusion protein consisting of the first 167 amino acids of cytochrome b2 fused to mouse dihydrofolate reductase (DHFR) into the matrix of mutant mitochondria (cf. Supplemental Fig. S3). Deletion of 19 amino acids in the membrane sorting signal directs this precursor into the matrix. Import of b2(167)Δ 9-DHFR into both _pam17_Δ (van der Laan et al., 2005), and _pam17_Δ/tim44(R180A) mitochondria was strongly impaired, even though a membrane potential like wild type was generated (Fig. 2C and Fig. S4). In contrast, tim44(R180A) mitochondria imported the precursor similar to wild type, suggesting that the absence of PAM17 in _pam17_Δ/tim44(R180A) mitochondria causes a dominant negative effect in the double mutant. Interestingly, facilitating the import by providing an extended substrate upon Urea-induced unfolding of the entire precursor prior to import did not alter the import efficiencies in the single mutant mitochondria (Supplemental Fig. S5 G). Since import was not compromised in tim44(R180A), but defective in _pam17_Δ mitochondria, both proteins assist in import of the precursor b2(167)Δ19-DHFR in a different manner.
3.3.Pam17 and Tim44 are involved at different stages of preprotein translocation
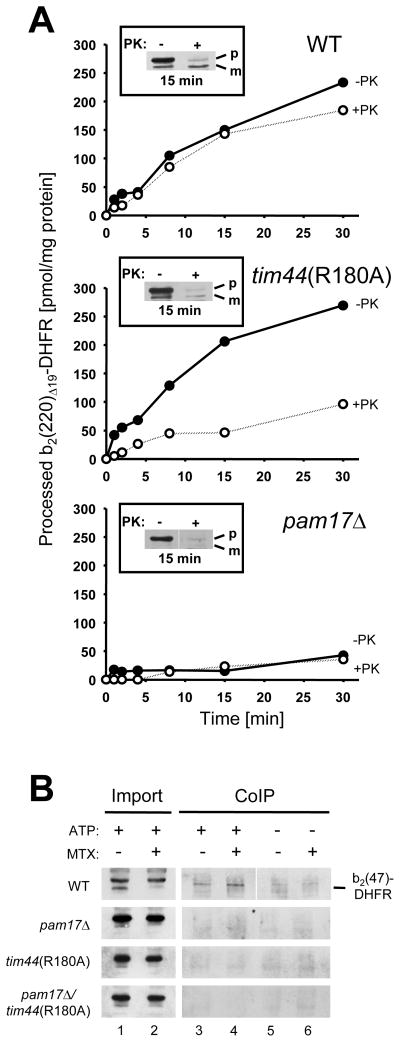
To analyze at what stage of protein translocation the mutants are defective, I tested a precursor whose presequence is already cleaved by the matrix processing peptidase (MPP) while the remainder of the protein is still being imported. In the import assay the ratio of processed precursor before and after treatment with proteinase K indicates whether an early, i.e. import of the presequence, or later step (i.e. translocation of the folded carrier protein) is compromised. I used purified b2(220)Δ19-DHFR consisting of the first 220 amino acids of cytochrome b2 with the folding-competent full-length heme binding domain preceding DHFR (cf. Supplemental Fig. S3). In control mitochondria, the amount of processed precursor after 30 minutes was comparable before and after protease treatment, indicating that all mitochondria-associated precursor was imported into the protease-protected matrix (Fig. 3A upper panel). In contrast, in tim44(R180A) mitochondria, only a fraction of processed protein was detectable after proteinase K treatment, whereas the amount of processed precursor before protease treatment was comparable to wild type, indicating an inefficient import of this precursor into the matrix (Fig. 3A, middle panel). In _pam17_Δ mitochondria, the amount of processed protein was low (Fig. 3A, lower panel). Thus, Pam17 is involved in an early stage of import i.e. before processing of the presequence by the matrix-localized processing peptidase occurs, while Tim44 acts in a later step of preprotein import, when import motor-catalyzed unfolding of extra mitochondrial protein domains is required.
Fig. 3.
PAM17 is involved in an early stage of preprotein translocation. (A) The recombinant precursor cytochrome b2(220)Δ19-DHFR was imported into isolated mitochondria for the times indicated. Samples were split in half and treated with proteinase K (+PK) or left untreated (-PK). The amount of processed precursor in each assay was determined and plotted against the incubation time. Inset: western blot of mitochondria-associated precursor after 15 minutes of import (−PK and +PK, respectively). (B) Coimmunoprecipitation of b2(47)-DHFR as arrested precursor in mitoplasts. b2(47)-DHFR was incubated in the presence or absence of 10 μM MTX with energized mitoplasts for 10 minutes in the presence of an ATP-regenerating system (+ATP) or in the absence of ATP (-ATP). After lysis the supernatant was incubated with antibodies against Ssc1 cross linked to protein A beads. The immunoprecipitate was subjected to immunoblotting using antibodies against DHFR. p, premature b2(47)DHFR; m, mature b2(47)DHFR. The data points represent the average value of at least two independent experiments.
Ssc1(ATP) can bind to a translocated presequence in the mitochondrial matrix even before its processing occurs (Ungermann et al., 1994). To test whether such an early Ssc1-presequence interaction appears in mutant tim44 and pam17 mitochondria, b2(47)-DHFR was imported into mitoplasts and immunoprecipitated with antibodies against Ssc1 (Fig. 3B). This precursor was chosen because of its short N-terminal sequence followed by the folded DHFR domain. Since about 20 amino acids of a helix are required to span the inner membrane, in this assay only about 25 amino acids of the presequence would emerge into the matrix, while the folded DHFR domain remained outside, since a methotrexate(MTX)-stabilized DHFR domain cannot be unfolded by the mitochondrial protein import motor under these conditions (Eilers and Schatz, 1986; Rassow et al., 1990). To arrest the precursor in transit, the folded state of DHFR was stabilized by the addition of the ligand methotrexate (MTX). When importing b(47)-DHFR(MTX) into energized mitoplasts, only wild type mitochondria were capable of a stable Ssc1-b2(47)-DHFR(MTX) interaction due to a fully functional import motor at the exit of the Tim23 channel (Fig. 3B). In tim44(R180A) mitochondria, however, a functional Ssc1-precursor interaction fails most likely due to the stable complex that is formed between Ssc1 and Tim44(R180A) even in the presence of peptide substrate preventing the binding of Ssc1 to the precursor, as previously reported (Schiller et al., 2008). Interestingly also in _pam17_Δ mitochondria no stable Ssc1-precursor interaction could be detected, even though the peptide substrate regulated dissociation of a Ssc1-Tim44 complex was similar to wild type when immunoprecipitating the protein complex in mitochondrial lysates, suggesting that the interaction of Ssc1 with Tim44 per se is not affected by the absence of Pam17 (Supplemental Fig. S6). Thus I concluded that the impaired Ssc1-presequence interactions close to the exit of the Tim23 channel in both _pam17_Δ and tim44 mitochondria have different causes, since efficient import of preproteins into tim44(R180A) mitochondria but not in the pam17 mutant occurs (cf. Fig. 2C). To further test this hypothesis I measured the ATP-driven inward-driving force on an arrested precursor in transit in isolated mitochondria. In these experiments the precursor spans both mitochondrial membranes while the N-terminal sequence interacts with the import motor such that the extra mitochondrial folded domain is pulled against the outer membrane preventing its proteolytic degradation by externally added proteinase K (Krayl et al., 2007. When testing the arrested precursor b2(167)Δ19-DHFR(MTX), a functional interaction of its N-terminal portion with the import motor could be detected as judged by the resistance of the stabilized extra mitochondrial DHFR domain to proteolytic degradation. In contrast, no stable interaction of Ssc1 with the precursor could be detected in _pam17_Δ mitochondria, whereas tim44(R180A) mitochondria still exerted a moderate import-driving force (Supplemental Fig. S7). Thus, consistent with the observed import defect, no detectable efficient interaction of Ssc1 with the preprotein in transit occurs in _pam17_Δ mitochondria.
3.4. Different import modes reveal complementary functions of PAM17 and TIM44
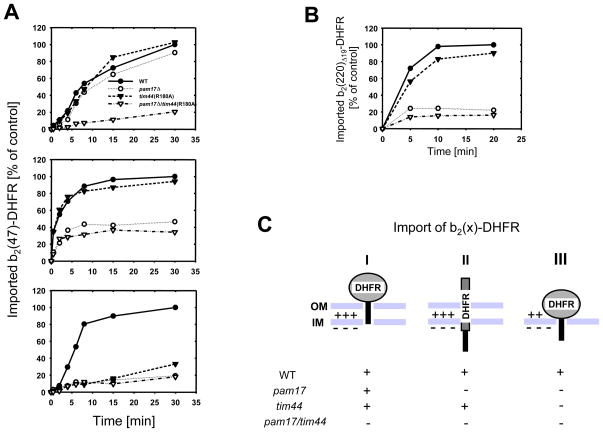
tim44(R180A) mitochondria are defective in import of b2(220)Δ19-DHFR, whereas the shorter version b2(167)Δ19-DHFR is imported with wild type efficiency, suggesting a selective defect depending on the required import mode for a given preprotein (cf. Fig. 2C). I asked whether such variability in import occurs in _pam17_Δ mitochondria and tested different precursors varying in both the length of the N-terminal sequence preceding the carrier protein and the nature of the carrier protein. Interestingly I found precursors which were efficiently imported into _pam17_Δ mitochondria (Supplemental Fig. S8). To further analyze this apparent precursor-dependent import efficiency I imported the same precursor in kinetically saturating amounts but under 3 different conditions (Fig. 4A). First I imported native b2(47)-DHFR into mitochondria. Since 50 amino acids are required to span both mitochondrial membranes, interaction of the N-terminal segment of b2(47)-DHFR with matrix Ssc1 and subsequent import can only occur upon spontaneous unfolding of the native DHFR domain prior to import (Krayl et al., 2007; Rassow et al., 1990). Under this condition pam17_Δ and tim44(R180A) mitochondria imported native b2(47)-DHFR with an efficiency of about 90% compared to the control reaction (Fig. 4A, upper panel). Import into pam17_Δ/tim44(R180A) mitochondria, however, was strongly impaired. Next, I unfolded the native precursor by urea treatment prior to import to provide a substrate, whose presequence easily reaches the matrix since the otherwise folded DHFR-domain is absent (Fig. 4A, middle panel). Only tim44(R180A) mitochondria imported the unfolded precursor similar to wild type, whereas import into both _pam17_Δ and _pam17_Δ/tim44(R180A) mitochondria was compromised. Finally, I combined the characteristics of the two preceding assays, by providing a binding site for Ssc1 to the presequence in the matrix while the folded DHFR domain remained outside (cf. Fig. 3B). Therefore, I imported native b2(47)-DHFR into mitoplasts (Fig. 4A, lower panel). As expected, no efficient import into either mutant compared to wild type was observed. This is consistent with the observed import defects of tim44(R180A) and _pam17_Δ mitochondria when using b2(220)Δ19-DHFR as a substrate (cf. Fig. 3A), where the import motor needs to unfold first the cytochrome b2 heme binding domain (HBD) before the subsequent DHFR domain can be translocated (cf. supplemental Fig. S3). I hypothesized that if import motor-catalyzed unfolding of HBD is the rate-limiting step in import of native b2(220)Δ19-DHFR, then transport of the unfolded precursor should only be impaired in the _pam17_Δ mutant. Indeed, only _pam17_Δ and _pam17_Δ/tim44(R180A), but not tim44(R180A) mitochondria were defective in import of denatured b2(220)Δ19-DHFR (Fig. 4B).
Fig. 4.
Import of b2(47)DHFR under different conditions. (A) b2(47)-DHFR was imported into energized mitochondria: wild type (closed circles), _pam17_Δ (open circles), tim44(R180A) (closed triangles), and _pam17_Δ/tim44(R180A) (open triangles). Import of the native precursor into intact mitochondria (upper panel). The precursor was denatured by Urea-treatment prior to import (middle panel). The native precursor was imported into mitoplasts, whose outer membrane has been disrupted by hypo-osmotic swelling prior to import (lower panel). (B) Import of denatured radiolabeled b2(220)Δ19-DHFR into mitochondria. The precursor was unfolded by Urea-treatment prior to import. (C) Evaluation of the different import modes of b2(47)-DHFR into mutant mitochondria: The native (I and III) or unfolded (II) domain of DHFR (gray) and the N-terminal presequence (black rectangle) are shown. The relative import of b2(47)-DHFR as native (I and III) or denatured (II) precursor is shown. +, efficient import; -, inefficient import. OM, outer membrane, IM, inner membrane. The data points represent the average value of at least two independent experiments.
4. Discussion
The data presented here show that Pam17 and Tim44 act in a complementary fashion with Pam17 being involved in an early stage of protein import, i.e. the translocation of the presequence across the mitochondrial inner membrane and Tim44 in a later stage of protein import, catalyzing the complete translocation of the mitochondrial matrix protein. The import efficiencies of recombinant cytochrome b2-DHFR preproteins into tim44 and pam17 mitochondria depend on the required translocation mode dictated by the nature of the respective precursor (Fig. 4C): Three import modes were tested: (i) If the presequence provides binding site(s) for mtHsp70 in the matrix, while the folded carrier protein is still outside, then import motor-initiated unfolding prevails and the extra-mitochondrial domain of DHFR is subsequently translocated (Fig. 4C, right panel). Since both proteins are required for an efficient early Ssc1-presequence interaction (cf. Fig. 3B), Tim44 and Pam17 are required for this import mode independently of each other. (ii) If translocation of an unfolded precursor is required, only pam17 mutants are defective, suggesting an exclusive role for Pam17 in this process (Fig. 4C, middle panel). (iii) If the initiation of the precursor import is dependent on spontaneous unfolding of the extra-mitochondrial DHFR domain first before further import can be catalyzed, both proteins are required, suggesting that Tim44 and Pam17 cooperate in a complementary manner (Fig. 4C, left panel). This cooperation does not require a direct physical interaction of the two proteins. As a matter of fact a more dynamic sequential assembly and functional rearrangement of the import motor with the Tim23 translocase has been proposed, recently (Hutu et al., 2008; Popov-Čeleketić et al., 2008; Wiedemann et al., 2007). In this regard the observations made herein are conclusive with a working model in which Pam17 associates transiently with the import-motor-free Tim23 translocase first, while the Pam16-Pam18 complex assembles with the import channel before Tim44 with Pam17 presumably having already left the complex. This view is supported by a recent observation in which the association of Pam17 and Pam16-Pam18 with the TIM23 complex shows an opposite dependence on the functionality of Tim44, with inactivated Tim44 leading to an increased binding of Pam17 while the Pam16-Pam18 association with the import channel is reduced (Hutu et al., 2008). This model is also in line with the observed lethality of the double mutant _pam17_Δ/ssc1-2. In the ssc1-2 mutant the interaction of Ssc1 with Tim44 is defective in a similar way as has been observed for the tim44(R180) mutant, namely a failure of the Tim44-Ssc1 complex to dissociate in the presence of peptide substrate (D’Silva et al., 2004; Schiller et al., 2008). It is tempting to speculate that Pam17 might act as a membrane adaptor for matrix Ssc1 at the Tim23 channel, initiating a Tim44-independent early interaction of Ssc1 with incoming polypeptide and thus promoting the functional assembly of the Pam16-Pam18 complex and Tim44 to complete the translocation of the matrix protein. The existence of such a potential additional membrane adaptor for Ssc1 has been proposed recently (Bömer et al., 1997). However a direct interaction of Pam17 with Ssc1 has not been reported so far and might not be required under most growth conditions, since -dependent on the precursor- efficient protein import into pam17D mitochondria can occur (cf. Supplemental Fig. S8).
In conclusion Pam17 contributes to the translocation of the presequence across the inner membrane, whereas Tim44 is required for completing the initiated import of the processed protein into the matrix. Each protein assists at a different import stage so that the single tim44 and pam17 mutations are complemented by the respective wild type allele, but only their combination is deleterious in vivo. A complementary functional cooperation of Pam17 and Tim44 would ensure efficient import of hundreds of mitochondrial proteins under any given growth condition, regardless of whether a post- or cotranslational import mode prevails.
Supplementary Material
01
02
Acknowledgments
This work was done in the laboratory of Dr. E. A. Craig, UW-Madison, WI, U.S.A. I thank B. Guiard for plasmids encoding for cytochrome b2-DHFR fusion proteins and R. Abele, S. Butcher, D. Nikles, A. Prunuske, E. Schleiff, and R. Tampé for helpful comments on the manuscript. Special thanks to Willy Walter for experimental assistance and Brenda Schilke for creating the _pam17_Δ mutant. This work was supported by a National Institutes of Health grant (to E.A.Craig) and by a research fellowship of the Deutsche Forschungsgemeinschaft (to D.S.).
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version, at .....
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bömer U, Meijer M, Maarse AC, Hönlinger A, Dekker PJ, Pfanner N, Rassow J. Multiple interactions of components mediating preprotein translocation across the inner mitochondrial membrane. EMBO J. 1997;16:2205–16. doi: 10.1093/emboj/16.9.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolender N, Sickmann A, Wagner R, Meisinger C, Pfanner N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 2008;9:42–9. doi: 10.1038/sj.embor.7401126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva P, Liu Q, Walter W, Craig EA. Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat Struct Mol Biol. 2004;11:1084–91. doi: 10.1038/nsmb846. [DOI] [PubMed] [Google Scholar]
- Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–32. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Geissler A, Krimmer T, Bömer U, Guiard B, Rassow J, Pfanner N. Membrane potential-driven protein import into mitochondria. The sorting sequence of cytochrome b(2) modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Mol Biol Cell. 2000;11:3977–91. doi: 10.1091/mbc.11.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–53. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hutu DP, Guiard B, Chacinska A, Becker D, Pfanner N, Rehling P, van der Laan M. Mitochondrial protein import motor: differential role of tim44 in the recruitment of pam17 and j-complex to the presequence translocase. Mol Biol Cell. 2008;19:2642–9. doi: 10.1091/mbc.E07-12-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayl M, Lim JH, Martin F, Guiard B, Voos W. A cooperative action of the ATP-dependent import motor complex and the inner membrane potential drives mitochondrial preprotein import. Mol Cell Biol. 2007;27:411–25. doi: 10.1128/MCB.01391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Martin F, Guiard B, Pfanner N, Voos W. The mitochondrial Hsp70-dependent import system actively unfolds preproteins and shortens the lag phase of translocation. EMBO J. 2001;20:941–50. doi: 10.1093/emboj/20.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Krzewska J, Liberek K, Craig EA. Mitochondrial Hsp70 Ssc1: role in protein folding. J Biol Chem. 2001;276:6112–8. doi: 10.1074/jbc.M009519200. [DOI] [PubMed] [Google Scholar]
- Liu Q, D’Silva P, Walter W, Marszalek J, Craig EA. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science. 2003;300:139–41. doi: 10.1126/science.1083379. [DOI] [PubMed] [Google Scholar]
- Mokranjac D, Popov-Čeleketić D, Hell K, Neupert W. Role of Tim21 in mitochondrial translocation contact sites. J Biol Chem. 2005;280:23437–40. doi: 10.1074/jbc.C500135200. [DOI] [PubMed] [Google Scholar]
- Neupert W, Brunner M. The protein import motor of mitochondria. Nat Rev Mol Cell Biol. 2002;3:555–65. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–49. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- Popov-Čeleketić D, Mapa K, Neupert W, Mokranjac D. Active remodelling of the TIM23 complex during translocation of preproteins into mitochondria. EMBO J. 2008;27:1469–80. doi: 10.1038/emboj.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J, Hartl FU, Guiard B, Pfanner N, Neupert W. Polypeptides traverse the mitochondrial envelope in an extended state. FEBS Lett. 1990;275:190–4. doi: 10.1016/0014-5793(90)81469-5. [DOI] [PubMed] [Google Scholar]
- Rassow J, Maarse AC, Krainer E, Kubrich M, Muller H, Meijer M, Craig EA, Pfanner N. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix hsp70 and the inner membrane protein MIM44. J Cell Biol. 1994;127:1547–56. doi: 10.1083/jcb.127.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid GA, Schatz G. Import of proteins into mitochondria. Extramitochondrial pools and post-translational import of mitochondrial protein precursors in vivo. J Biol Chem. 1982;257:13062–7. [PubMed] [Google Scholar]
- Rowley N, Prip-Buus C, Westermann B, Brown C, Schwarz E, Barrell B, Neupert W. Mdj1p, a novel chaperone of the DnaJ family, is involved in mitochondrial biogenesis and protein folding. Cell. 1994;77:249–59. doi: 10.1016/0092-8674(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Schiller D, Cheng YC, Liu Q, Walter W, Craig EA. Residues of Tim44 involved in both association with the translocon of the inner mitochondrial membrane and regulation of mitochondrial Hsp70 tethering. Mol Cell Biol. 2008;28:4424–33. doi: 10.1128/MCB.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–3. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- van der Laan M, Chacinska A, Lind M, Perschil I, Sickmann A, Meyer HE, Guiard B, Meisinger C, Pfanner N, Rehling P. Pam17 is required for architecture and translocation activity of the mitochondrial protein import motor. Mol Cell Biol. 2005;25:7449–58. doi: 10.1128/MCB.25.17.7449-7458.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan M, Wiedemann N, Mick DU, Guiard B, Rehling P, Pfanner N. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr Biol. 2006;16:2271–6. doi: 10.1016/j.cub.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Voisine C, Craig EA, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–74. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- Wiedemann N, van der Laan M, Hutu DP, Rehling P, Pfanner N. Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J Cell Biol. 2007;179:1115–22. doi: 10.1083/jcb.200709087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02