Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload (original) (raw)
Abstract
Thrombospondin-4 (TSP-4) expression increases dramatically in hypertrophic and failing hearts in rodent models and in humans. The aim of this study was to address the function of TSP-4 in the heart. TSP-4-knockout (_Thbs4_−/−) and wild-type (WT) mice were subjected to transverse aortic constriction (TAC) to increase left ventricle load. After 2 wk, _Thbs4_−/− mice had a significantly higher heart weight/body weight ratio than WT mice. The additional increase in the heart weight in TAC _Thbs4_−/− mice was due to increased deposition of extracellular matrix (ECM). The levels of interstitial collagens were higher in the knockout mice, but the size of cardiomyocytes and apoptosis in the myocardium was unaffected by TSP-4 deficiency, suggesting that increased reactive fibrosis was the primary cause of the higher heart weight. The increased ECM deposition in _Thbs4_−/− mice was accompanied by changes in functional parameters of the heart and decreased vessel density. The expression of inflammatory and fibrotic genes known to be influential in myocardial remodeling changed as a result of TSP-4 deficiency in vivo and as a result of incubation of cells with recombinant TSP-4 in vitro. Thus, TSP-4 is involved in regulating the adaptive responses of the heart to pressure overload, suggesting its important role in myocardial remodeling. Our study showed a direct influence of TSP-4 on heart function and to identify the mechanism of its effects on heart remodeling.—Frolova, E. G., Sopko, N., Blech, L., Popović, Z. B., Li, J., Vasanji, A., Drumm, C., Krukovets, I., Jain, M. K., Penn, M. S., Plow, E. F., Stenina, O. I. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload.
Keywords: heart hypertrophy, extracellular matrix, transverse aortic constriction
Thrombospondin-4 (TSP-4) is a secreted extracellular matrix protein, one of the 5 members of the thrombospondin protein family (TSP-1 through TSP-5; refs. 1, 2). The thrombospondin family members share homologous C-terminal regions but have unique N-terminal domains and are expressed with different spatial and temporal signatures (1–3), and hence exert distinct functions. Our observation of abundant expression of TSP-4 protein in the vascular wall (4) and now in the heart places it in locations to influence pathogenic processes in these tissues. Indeed, increased levels of TSP-4 have been reported in heart hypertrophy in humans and in animal models (5–8). Thrombospondins are regulators of matrix production and organization and influence tissue remodeling in different organs and pathologies (9–15). Furthermore, TSP-1 and TSP-2 have a well-established function in healing myocardial infarcts (16–19), limiting expansion of fibrosis into noninfarcted areas (19) and regulating integrity of the cardiac matrix (20). A recent study in mice identified TSP-4 as a myocyte-interstitial mechanosignaling molecule central to adaptive cardiac contractile responses to acute stress, which appears to play a crucial role in the transition to chronic cardiac dilatation and failure (21). However, despite the marked up-regulation of TSP-4 in heart failure, the function of the molecule in the heart and vasculature is essentially unknown, and molecular mechanisms underlying its potential effects on initiation and development of cardiovascular disease (CVD) are totally unknown.
To provide insight into the role of TSP-4 in the heart, we compared the response of _Thbs4_−/− and wild-type (WT; Thbs4+/+) mice to pressure overload induced heart hypertrophy. We find that TSP-4 deficiency markedly increases heart fibrosis and suggest that the increased levels of TSP-4 in failing human hearts may be of functional significance. On the basis of the alterations in matrix composition, vascularization of myocardium, and cellular functions that we observe in _Thbs4_−/− mice, we conclude that TSP-4 plays an important role in myocardial structure, function, and remodeling. Mechanistically, this role appears to arise from the effects of TSP-4 on matrix deposition and composition.
MATERIALS AND METHODS
_Thbs4_−/− mice
_Thbs4_−/− mice were described previously (22). They were crossed with C57BL/6 mice for 12 generations, and WT C57BL/6 mice, derived from breeding of Thbs4+/− mice, were used as controls. These mice had no overt phenotype. A decrease in body weight (BW) was detected in older _Thbs4_−/− mice with age but only became apparent in mice older than 80 wk of age [43.2±3.86 in WT mice vs. 38.3±4.54 in _Thbs4_−/− (_P_=0.2, _n_=8)]. The weight of the 58-wk-old mice at the end of the transverse aortic constriction (TAC) experiments reported in this study was similar for both genotypes: 38.3 ± 2.7 in WT sham-operated mice vs. 40.9 ± 2.7 in _Thbs4_−/− sham-operated mice (_P_=0.26, _n_=5 and 6, respectively) and 31.9 ± 1.1 in WT TAC-operated mice vs. 34 ± 2.6 in _Thbs4_−/− TAC-operated mice (_P_=0.25, _n_=6).
Blood pressure was measured with the computer-automated tail-cuff system (Hatteras MC-4000 Blood Pressure Analysis System; Hatteras Instruments, Cary, NC, USA) at the Physiology Core Facility of the Centre for Modeling Human Disease at Mount Sinai Hospital (Toronto, ON, Canada). All the animal experiments were approved by the Cleveland Clinic Institutional Animal Care and Use Committee and conformed to the current U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. The minimal number of animals resulting in statistically significant results was used in the experiments.
Antibodies
The polyclonal goat anti-TSP-4 antibody was from R&D Systems (Minneapolis, MN, USA). It was raised using purified recombinant human TSP-4 (Ala22-Asn961) and recognized both human and mouse TSP-4. The antibody did not react with tissues from _Thbs4_−/− mice in Western blots (not shown) or by immunohistochemistry (see Results) and, as previously reported by Frolova et al. (22), did not cross-react with other TSPs.
Anti-collagen I, anti-collagen III, anti-collagen IV, and anti-collagen V antibodies were from Abcam (Cambridge, MA, USA), and anti-collagen II was from R&D Systems. Full-length proteins were used as immunogens to produce all anti-collagen antibodies.
Western and dot blotting
Western blot analysis to analyze the levels of TSP-4 in tissue extracts was performed on 15 μg of extracted protein, as previously reported (22). To analyze collagens by dot blotting, 5 μg of cultured cell lysates was applied to the membrane and developed with antibodies specific for the different collagens, followed by secondary antibodies, as was done for the Western blots.
In situ messenger RNA (mRNA) hybridization
Hearts from TAC- and sham-operated WT mice were dissected, fixed in 10% neutral-buffered formalin, and embedded in paraffin. Sections (6 μm) were mounted on slides and analyzed at the Affymetrix ViewRNA assay service (Santa Clara, CA, USA). Slides were hybridized with the Thbs4 probe set specific for mouse Thbs4 and spanning the region 1470–2571 of mRNA. A mouse ubiquitin probe set was used as a positive control, and an Escherichia coli dihydrodipicolinate reductase probe set was used as a negative control. Images were taken with Leica DM5500B upright microscope (Leica Microsystems, Wetzlar, Germany).
Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed using reagents and commercially available specific primers from Applied Biosystems (Foster City, CA, USA), according to the manufacturer's instructions. Total RNA (5 μg) isolated using TRIzol reagent (Invitrogen, San Diego, CA, USA) was used to synthesize complementary DNA (cDNA) using SuperScript III First-Strand (Invitrogen). Levels of 18S mRNA were used to normalize the data. Quantitative real-time PCR was performed as described previously (23) using 2 μl of cDNA with TaqMan Gene Expression Master Mix and TaqMan probes (Applied Biosystems) for the genes of interest: Col1α2 (Mm01165187_m1), Col2α1 (Mm01309565_m1), Col3α1 (Mm01254476_m1), Col4α5 (Mm00801606_m1), Col5α (Mm00489289_m1), BNP (Mm01255770_g1), α1 skeletal actin (Mm00808218_g1), ANP (Mm01255747_g1), ADAMTS4 (Mm00556068_m1), MMP1 (Mm00439491_m1), MMP2 (Mm00439498_m1), MMP8 (Mm00439509_m1), MMP9 (Mm00442991_m1), MMP14 (Mm00485054_m1), MMP15 (Mm00485062_m1), MMP16 (Mm00490659_m1), MMP17 (Mm00449292_m1), and MMP24 (Mm00487721_m1).
To measure the expression of inflammation-related genes in heart tissue, we used a custom-prepared RT2 Profiler PCR Array from SABiosciences (Frederick, MD, USA). Real-time PCR to measure the expression of the inflammatory genes was performed on MyiQ2 detection system equipped with iQ5 optical system software, version 2.1, (Bio-Rad, Hercules, CA, USA) using RT2 SYBR Green Fluor qPCR Mastermix (Qiagen Sciences, Germantown, MD, USA). Relative changes in gene expression were calculated using the ΔΔ_C_t (threshold cycle) method (24). Data were normalized to the levels of β-actin and the sham values.
Immunohistochemistry
Hearts were harvested and placed in Tissue-Tek O.C.T. Compound (Sakura Finetek USA, Torrance, CA, USA), frozen in liquid nitrogen, and stored at −80°C until processing. Frozen sections (6–10 μm) were cut in a cryostat (Leica Microsystems) and placed on microscope slides (Superfrost; Fisher Scientific, Waltham, MA, USA). Sections were immediately blocked by incubation in PBS containing 5% bovine serum albumin (BSA; MB Biomedicals, Solon, OH, USA) and donkey IgG (1:50) for 30 min at 4°C. Following this treatment, the slides were incubated with primary antibodies (1:200) for 2 h at 4°C. After washing with PBS/BSA (3×10 min), primary antibodies were detected by incubating sections with Rhodamine Red-X-conjugated secondary antibodies for 45 min at 4°C. After washing with PBS/BSA (3×10 min), sections were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Secondary antibodies were tested without primary antibodies to assure that there was no staining background (Supplemental Fig. S1).
TAC
This procedure was performed as described previously (25, 26). Briefly, animals were anesthetized via intraperitoneal injection of pentobarbital sodium (50 mg/kg)/buprenorphine (2.5 mg/kg) and placed on a ventilator (Harvard Apparatus, Holliston, MA, USA) at a respiratory rate of 105 breaths/minute and a tidal volume of 0.25 ml and 60% oxygen. Under a dissecting microscope, the transverse aorta was isolated. A single 7-0 silk suture was placed around the transverse aorta and constricted against a 27-gauge needle placed externally on top of the aorta. After tightening, the needle was removed, leaving the aorta ∼75% occluded. Sham-operated animals underwent the same procedure without tightening of the suture around the aorta. Pulse-wave Doppler echocardiography performed 24 h after surgery showed a pressure gradient across the constriction in all TAC animals of ≥64 mmHg.
Echocardiography and Doppler imaging
To study the effect of TAC, operated and sham-operated WT and _Thbs4_−/− males (5 animals/group) were subjected to 2-dimensional Doppler echocardiography 1 and 2 wk after TAC using a 14-MHz linear array transducer interfaced with a Vivid 7 echocardiograph machine (GE Medical, Milwaukee, WI, USA). Data were digitally recorded at a rate of >160 frames/s (2-dimensional data) or at a sweep speed of 200 mm/s (Doppler data) and analyzed offline using Echopac PC (GE Medical) by an observer blinded to the treatment groups. From 2-dimensional data, left ventricle (LV) volume and ejection fraction (EF) were calculated by the Simpson's method, while LV mass was calculated by the bullet method (27). Each measurement in each animal was repeated 3 to 5 times, from 3 of 5 randomly chosen M-Mode clips. EF, end systolic volume (ESV), and end diastolic volume (EDV) were calculated from the M-Mode recordings. Pulsed-wave Doppler data obtained at the level of the mitral annulus in an apical 4-chamber view were used to obtain RR interval, filling time (FT), isovolumetric relaxation time (IVRT), and ejection time (ET). Both LV volume and mass were assessed by the bullet equation (28).
Staining of capillaries and image analysis
Fluorescein isothiocyanate (FITC)-conjugated lectin from Lycopersicon esculentum (Sigma, St. Louis, MO) was injected into the hearts of anesthetized animals (60 μg/25 g BW in 150–200 μl of PBS). Hearts were collected 10 min later and prepared for microscopy, as described above.
To detect changes in the vascularity of WT and _Thbs4_−/− mice, z series were collected from the same location in the myocardium using a Leica TCS-SP3-AOBS laser-scanning confocal microscope, as previously described (22). Image stacks from the z series were reconstructed using Volocity software (Improvision, Lexington, MA, USA). Matching areas from WT and _Thbs4_−/− 2-dimensional images of the reconstructions were selected for analysis using Image Pro Plus 6.1 (Media Cybernetics, Silver Spring, MD, USA). Grayscale images of the lectin labeling were extracted from each reconstructed image and analyzed as described below to determine the area of lectin labeling per total selected area.
Image acquisition and analysis of the vasculature in myocardium
Images of fluorescent histological cross sections of the hearts stained with lectin to visualize endothelial cells were acquired using a Leica DMR 4000B upright microscope fitted with a single-slide _x_-, _y_-, and _z_-axis motorized stage, a ×20 (dry) objective, a FITC fluorescence filter cube, and a Retiga 2000R CCD digital camera (Q-Imaging, Burnaby, BC, Canada). High-magnification image fields were raster-scanned across each cross section (Oasis 4i controller; Objective Imaging, Kansasville, MI, USA) and stitched together to form a single high-resolution, large field of view (FOV) image (∼289 tiles/mosaic). Each image field was background corrected prior to stitching to ensure continuity and minimize chromatic variability. For quantitative analysis of vascular content, large FOV images were batch-processed using customized macros and algorithms generated for Image-Pro Plus 6.1 (Media Cybernetics). For customized macros, in each large FOV image, a low-pass filter was applied to blur the entire cross section, followed by a user-defined intensity threshold to generate a filled tissue mask. Before calculating total tissue area, holes in each cross section resulting from the prior deletion of objects (e.g., lint and autofluorescence) were segmented and removed from the tissue mask. To segment the vasculature, the original large FOV image was first equalized using a local neighborhood examination of pixel intensity variations. This enhanced the low-intensity areas to produce a uniform intensity cross section. Finally, using a predefined threshold and size criteria for removal of objects not consistent with the size of vessels (i.e., noise and lint), a mask of segmented vessels was generated; the total vascular area and corresponding tissue area were then exported to Excel (Microsoft, Redmond, WA, USA) for each cross section.
Extracellular matrix quantification, myocyte diameter, and apoptosis
Masson's trichrome was used to stain extracellular matrix in 5-μm-thick heart sections taken from the mitral level. Cardiomyocyte diameter was measured using the shortest transnuclear width of 60–100 cross-sectioned myocytes/section in ≥5 sections collected from 5 hearts (300–500 cells/group). Apoptotic cells were detected in myocardium by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining using an in situ cell death detection kit (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's protocol and were counterstained with DAPI. Sections of frozen hearts (6 μm) were selected from the same anatomical areas of WT and _Thbs4_−/− hearts (_n_≥4). The whole section was captured using a 20× objective to visualize and photograph fluorescein staining of apoptotic nuclei. The number of double-stained nuclei (fluorescein and DAPI) per heart section was counted using Image Pro 61.0.346 software (Media Cybernetics).
Statistical methods
Multivariate analysis of variance (MANOVA) was used to analyze the heart function data, treating the 2 weekly measurements as a repeated variable. Subsequent univariate analysis of TSP-4 effects was performed on each variable using a mixed models approach. Effects of TSP-4 deficiency on heart hypertrophy were evaluated from the mixed models using contrasts between the _Thbs4_−/− and WT groups under both TAC- and sham-surgery conditions. For each variable, the assumption of normality was examined, and appropriate transformations were done prior to analysis. Reported means ± sd were, however, based on the original scales. All analyses were done using the GLM procedure or the Mixed procedure in the statistical software package SAS 9.1 (SAS Institute, Cary, NC, USA). Results were considered significant at the level of α = 0.05.
RESULTS
_Thbs4_−/− mice
The TSP-4-deficient mice appeared normal at birth and displayed no obvious phenotype during early and adult development. There was no difference in the blood pressure of WT and _Thbs4_−/− mice (102.5±0.5 and 101.9±1.2, respectively; _P_=0.3).
Expression of TSP-4 in the mouse heart
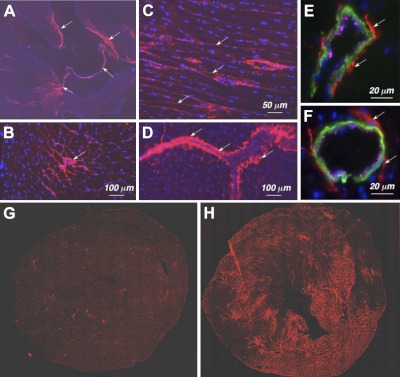
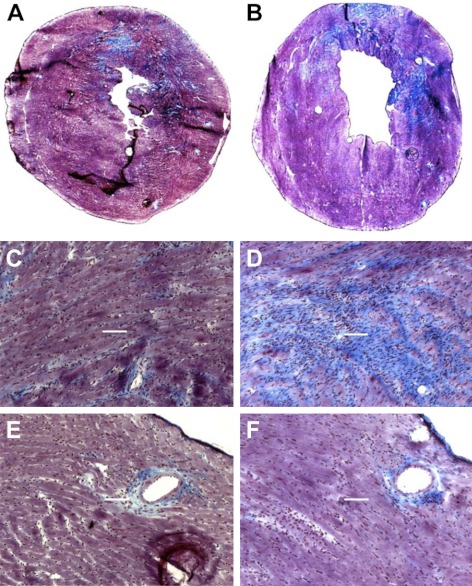
Studies of human and rodent heart tissue have shown that expression of TSP-4 mRNA increases in hypertrophic and failing hearts (5–8). However, the presence and localization of TSP-4 protein in the heart have not been investigated in detail with the exception of a single report (5) that localized TSP-4 protein to blood vessels in hearts of spontaneous hypertensive rats (SHRs). We did detect TSP-4 expression in the heart of WT C57BL/6 mice (Fig. 1). The highest expression was in the fibrous skeleton of the heart (Fig. 1A), which provides structure, tensile strength, and stiffness to the myocardium (29). Here, the protein was present in perimysium between bundles of cardiomyocytes (Fig. 1B) and the endomysium, the extracellular space between cardyomyocytes (Fig. 1C). Similar to our previous observations of TSP-4 protein in blood vessels in the brain (4), TSP-4 was consistently present in blood vessels of various sizes in the heart (Fig. 1D–F), in the adventitia of larger vessels and in subendothelial matrix of capillaries. When the hearts of TAC-operated mice were stained using anti-TSP-4 at 2 wk after surgery, TSP-4 expression increased dramatically and consistently (Fig. 1G, H).
Figure 1.
Expression of TSP-4 in the heart. A) TSP-4 (red; arrows indicate valves, membranous septum, interventricular septum) in the heart (blue denotes nuclei); composite figure from 4 images taken at ×20. B) TSP-4 in perimysium. C) TSP-4 in the matrix between cardiomyocytes. D) TSP-4 in the blood vessels. E, F) TSP-4 in two representative blood vessels: endothelial cells (anti-vWF, magenta); smooth muscle actin (anti-α-actin, green). Arrows (A–F) indicate TSP-4 staining. G, H) Expression of TSP-4 in the heart of sham-operated (G) and TAC-operated WT mouse (H), 2 wk after TAC.
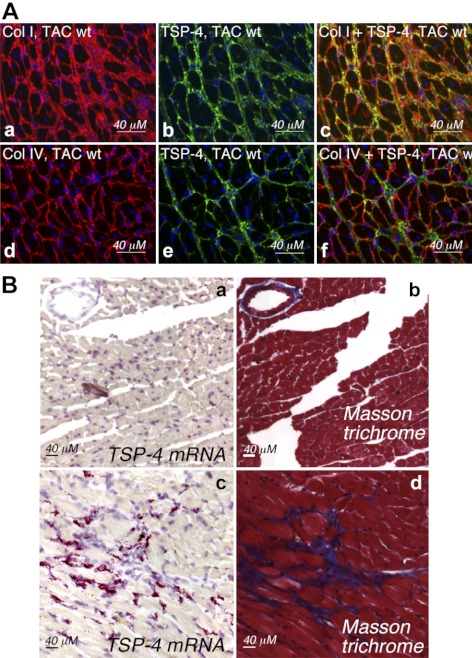
Costaining of collagens and endothelial cells (ECs) confirmed that TSP-4 resides in the extracellular matrix (ECM) between cardiomyocytes or ECM of blood vessels (Fig. 2A). To identify the cell type expressing TSP-4, in situ TSP-4 mRNA hybridization was performed (Fig. 2B). In sham-operated hearts, TSP-4 mRNA was detected at very low levels and only at the high magnification (not shown). As expression increased after TAC, TSP-4 mRNA was readily detected and was expressed by fibroblasts that resided in areas of increased ECM deposition (Fig. 2B). Blood vessels were visualized by the staining of serial sections with anti-vWF as an endothelial marker, but the expression of TSP-4 mRNA did not increase in blood vessels after TAC (not shown).
Figure 2.
TSP-4 is localized in the extracellular matrix of myocardium. A) Costaining of myocardium tissue from TAC-operated WT mice (2 wk after TAC) with anti-collagen I (red; a), anti-collagen IV (red; d), and anti-TSP-4 (green; b, e) antibodies, and overlay images (c, f). B) In situ TSP-4 mRNA hybridization. a, b) Heart of a sham-operated WT mouse. c, d) Heart of a TAC-operated WT mouse. a, c) TSP-4 mRNA (red). b, d) Masson trichrome staining (blue, ECM; red, cardiomyocytes and smooth muscle cells).
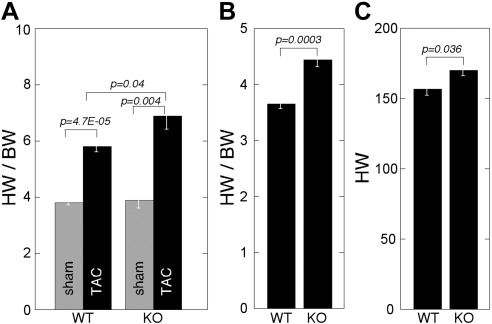
Increased heart weight (HW)/BW ratio in _Thbs4_−/− mice in response to pressure overload
HW was unchanged in sham-operated _Thbs4_−/− as compared to WT animals: 2 wk after sham surgery, HW/BW ratio was 3.8 ± 0.3 in WT mice and 3.9 ± 0.6 in _Thbs4_−/− mice (_P_=0.3; Fig. 3A), and the HW was 143.9 ± 19 and 159.1 ± 14 (_P_=0.1), respectively. There was no difference in the average tibia length (TL, a measure of a mouse size) or average weight between the two genotypes, and the ratios of HW/BW and HW/TL were similar in the two genotypes in sham-operated mice (not shown). However, TAC accelerated development of hypertrophy and higher HW/BW (Fig. 3A) in _Thbs4_−/− mice. In WT mice, TAC induced a 28.6% increase in HW; in _Thbs4_−/− mice, a 37% increase was detected. This difference was statistically significant (P<0.05). The HW increased from 143 ± 8.5 mg to 185 ± 7.2 mg in WT mice and from 159.1 ± 6.3 to 218 ± 9.8 mg in the _Thbs4_−/− mice. The increase in HW/BW was 52.6% in WT and 76.9% in _Thbs4_−/− mice (Fig. 3_A_). In older _Thbs4_−/− mice (>80 wk old), we did observe an increase in HW/BW and HW in nonoperated mice as compared to WT mice (Fig. 3B, C).
Figure 3.
Increased HW in _Thbs4_−/− mice. A) HW/BW (mg/g) in sham- and TAC-operated mice of 58 wk of age (2 wk after TAC); n = 5. B) HW/BW (mg/g) in nonoperated mice of 80 wk of age; WT: n = 7; _Thbs4_−/−: n = 8. C) HW (mg) in nonoperated mice of 80 wk of age; WT: n = 7, _Thbs4_−/−: n = 8. Values are means ± se.
Functional changes in the hearts of _Thbs4_−/− mice in response to pressure overload
Heart function in of _Thbs4_−/− mice changed significantly in response to pressure overload as compared to WT animals (see Table 1 for data summary). LV mass was significantly increased and EF was depressed in TAC animals (_P_=5E-5, _n_=5), but the drop in EF to (45%) in TAC-operated _Thbs4_−/− mice was significantly greater than in WT TAC-operated animals (_P_=0.003, _n_=5). Thus, the systolic dysfunction in _Thbs4_−/− mice was more pronounced than in WT mice (Table 1). LV ESV was depressed in TAC-operated animals. ESV increased in WT animals after TAC (P<0.0001, _n_=5), but it was increased even more in _Thbs4_−/− mice (_P_=0.002, _n_=5), suggesting that TSP-4 is necessary to control heart contractility. Both genotypes increased their ESV after TAC; however, the effect of TSP-4 deficiency was significant in both sham- and TAC-operated animals, indicating that _Thbs4_−/− mice have depressed systolic function with or even without elevated afterload.
Table 1.
Heart function in TAC- and sham-operated _Thbs4_−/− and WT mice 2 wk after surgery
| Genotype | Function | ||||||
|---|---|---|---|---|---|---|---|
| EDV (μl) | ESV (μl) | EF (%) | IVRT (ms) | ET (ms) | FT (ms) | LV mass (mg) | |
| Sham WT | 30.4 ± 7.3 | 6.9 ± 3.0 | 77.6 ± 5.5 | 13.6 ± 1.2 | 34.2 ± 2.4 | 37.1 ± 3.4 | 156.8 ± 31.9 |
| Sham _Thbs4_−/− | 33.8 ± 11.5* | 10.5 ± 4.5* | 69.2 ± 6.1* | 14.0 ± 2* | 38.5 ± 4.0* | 39 ± 3.4 | 158.9 ± 35.1 |
| TAC WT | 42.0 ± 12.7 | 18.3 ± 10.9 | 58.2 ± 4.0 | 13.3 ± 0.8 | 38.7 ± 2.4 | 32.7 ± 1.6 | 182.7 ± 45.4 |
| TAC _Thbs4_−/− | 62.6 ± 20.6* | 36.5 ± 19.7* | 44.4 ± 13.8* | 16.9 ± 1.4* | 45.4 ± 5.2* | 33.2 ± 2.5 | 207.4 ± 41.4 |
As parameters of diastolic function, we assessed LV FT and LV IVRT, both uncorrected and corrected by RR interval duration. FT is an overall measure of LV filling, while IVRT strongly correlates with time constant of isovolumetric pressure decay, a gold standard measure of ventricular relaxation. IVRT was increased in _Thbs4_−/− mice, both sham and TAC operated. FT decreased in banded animals (_P_=0.0002, _n_=5); however, the genotype of the mice did not have any effect on the decrease in FT function. Because there was a significant difference between groups in RR-interval duration, we reanalyzed the data after correcting for RR-interval duration by expressing IVRT and FT as a percentage of RR duration. The corrected FT was also significantly shorter in banded animals (_P_=6E-6, _n_=5), as compared to sham-operated mice and in _Thbs4_−/− mice as compared to WT mice. However, there was no effect of genotype on the corrected FT decrease after TAC. _Thbs4_−/− mice increased corrected IVRT significantly after banding, as compared to sham-operated mice (_P_=0.009, _n_=5). These data suggest that TSP-4 deficiency has broad and marked effects on the functional response of the heart to chronic afterload.
Cardiomyocyte size and apoptosis
The diameter of cardiomyocytes increased after TAC in both genotypes (Supplemental Fig. S2_A_). The increase of diameter in response to banding was statistically significant within each genotype, but the size was not different between the 2 genotypes. Nuclei of apoptotic cells were visualized and counted (Supplemental Fig. S2_B_, C). In TAC-operated mice, as expected, the number of apoptotic nuclei increased dramatically (30). However, no difference was detected in the number of apoptotic nuclei between the two genotypes.
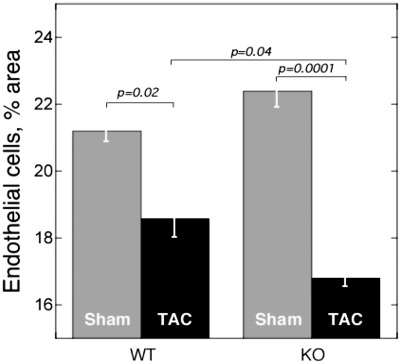
Decreased number of microvessels in _Thbs4_−/− mice in response to pressure overload
ECs were visualized using lectin staining, and the area occupied by ECs was quantified. In both WT and _Thbs4_−/− mice, the percentage of area occupied by microvessels dramatically decreased after TAC (Fig. 4). However, the decrease was significantly greater in _Thbs4_−/− mice as compared to WT mice.
Figure 4.
Microvessels in hearts of _Thbs4_−/− mice subjected to TAC. Endothelial cells were stained by injection of fluorescein-labeled lectin; percentage of stained area (_n_=5, 2 sections/mouse), 2 wk after TAC.
Increased deposition of extracellular matrix in _Thbs4_−/− mice
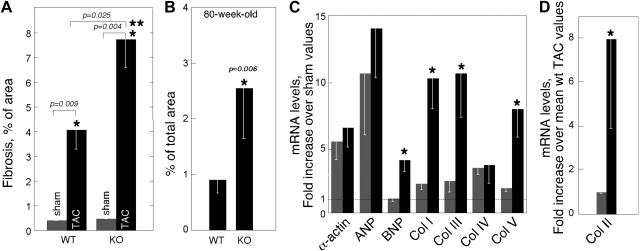
Initial responses of hearts to pressure overload may include increased cardiomyocyte volume and/or interstitial/perivascular fibrosis. As noted above, the mean diameter of cardiomyocytes was not significantly different between _Thbs4_−/− and WT mice in TAC-operated or sham-operated mice. However, the increased hypertrophy in TAC _Thbs4_−/− mice was associated with higher deposition of ECM, as was visualized by Masson's trichrome staining (ref. 31 and Fig. 5). Striking differences were detected in interstitial ECM (Fig. 5A–D), while perivascular matrix appeared unaffected by TSP-4 deficiency (Fig. 5E, F). A difference of almost 2-fold was detected in the ECM staining between the two genotypes after TAC, as was quantified using ImagePro 6.1 (Fig. 6A). The ECM staining was similar in sham-operated animals of two genotypes: 0.4 ± 0.015% in WT and 0.47 ± 0.021% in _Thbs4_−/− mice (_n_=5). However, the increase in fibrosis after TAC was not proportional in two genotypes: 9-fold in WT and 15.4-fold increase in _Thbs4_−/− mice. With age, _Thbs4_−/− mice developed increased heart fibrosis without pressure overload (Fig. 6B): the area of fibrosis was >2.5 fold larger in these mice, as compared to WT mice of the same age.
Figure 5.
Extracellular matrix deposition in _Thbs4_−/− mice subjected to TAC. Masson trichrome-stained heart sections of age-matched TAC-operated WT (A, C, E) and _Thbs4_−/− (B, D, F) mice, 2 wk after banding (scale bar=400 m). Extracellular matrix is stained blue.
Figure 6.
Interstitial fibrosis and the expression of collagens and hypertrophy markers in _Thbs4_−/− mice 2 wk after TAC. A) Fibrosis in sham-and TAC-operated mice, percentage fibrosis of the tissue section (total area=100%); n = 5. B) Fibrosis in nonoperated 80-wk-old mice, percentage fibrosis of the tissue section (total area=100%); n = 5. C) Expression of mRNA of hypertrophy markers and collagens in hearts of TAC-operated mice. D) Expression of collagen II mRNA. Shaded bars, WT mice; solid bars, _Thbs4_−/− mice. Values are means ± se.
Expression of collagen and other hypertrophy markers in _Thbs4_−/− mice
As was evident in Masson trichrome-stained sections (Fig. 6), interstitial rather than perivascular fibrosis was responsible for ECM differences between the two genotypes. Masson's trichrome staining visualizes multiple ECM proteins, including collagens (31), and we sought to evaluate collagen deposition specifically. By RT-PCR, the mRNA levels of collagen IV, the major perivascular collagen type, were similar in both genotypes (Fig. 6C). Remarkably, mRNA for collagen II was highly expressed in hearts of TAC-operated mice (Fig. 6D) but was not detected in sham-operated animals. Levels of collagens I, II, III, and V were significantly higher in _Thbs4_−/− mice after TAC (Fig. 6C, D). For comparison, the levels of skeletal muscle α1-actin and ANP mRNA, markers of cardiomyocyte hypertrophy, were not statistically different (Fig. 6C), which is consistent with the lack of an increase in cardiomyocyte size in _Thbs4_−/− mice compared to TAC-operated WT mice.
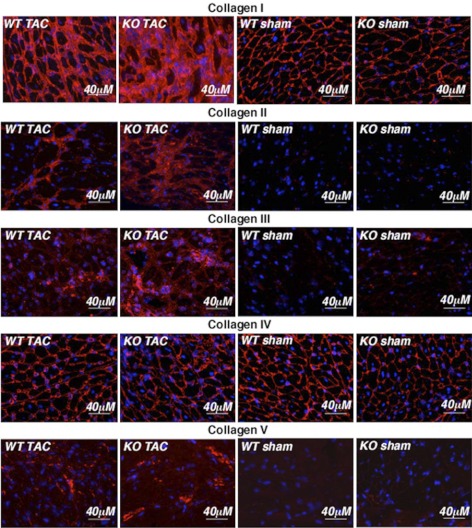
Collagen I–V protein levels were examined by immunohistochemistry. Collagen I and IV were the main isoforms detected in hearts of sham-operated mice and were at similar levels in both genotypes (Fig. 7, demonstrating the localization of staining in the ECM, and Supplemental Fig. S3, demonstrating the overall levels of collagens at a lower magnification). After TAC, no difference between genotypes was detected in levels of collagen IV, consistent with the RT-PCR data (Fig. 6B, C). However, the level of collagen I was higher in _Thbs4_−/− mice compared to WT TAC-operated mice. Collagens II, III, and V were barely detectable in sham-operated mice, but all three increased substantially after TAC with the levels being higher in _Thbs4_−/− mice than WT mice.
Figure 7.
Collagens in hearts of _Thbs4_−/− mice subjected to TAC. Collagens I–V visualized in the ECM of myocardium tissue sections of sham- and TAC-operated WT and _Thbs4_−/− mice (2 wk after TAC). Red, collagen; blue, nuclei. Scale bars = 40 μm.
Expression of inflammatory and fibrotic genes in _Thbs4_−/− mice after TAC
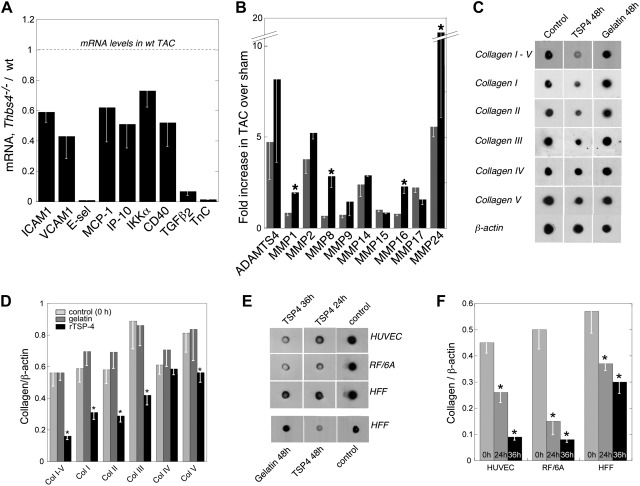
We reported that TSP4 deficiency prevented the development of local inflammation in atherosclerotic lesions by decreasing the expression of endothelial surface adhesion proteins and chemokines (22). The levels of mRNA of 7 representative inflammation-associated proteins were measured in WT and _Thbs4_−/− hearts. The markers were endothelial surface adhesion proteins that facilitate leukocyte extravasation, intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and E-selectin; chemokines, monocyte chemotactic protein-1 (MCP-1) and IP-10, interferon γ-induced protein 10 (IP-10); activator of proinflammatory transcription factor nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), inhibitor of κB (IκB) kinase α; and CD40. No difference in expression of these inflammation markers in sham-operated mice was detected. However, after TAC, the levels of all these proteins were lower in _Thbs4_−/− mice than WT mice (Fig. 8A), consistent with our previous data on atherosclerotic lesions (22). The expression of transforming growth factor (TGF)-β2 and tenascin C, two proteins implicated in fibrosis in hypertrophied hearts (32, 33), was also decreased in _Thbs4_−/− mice. Matrix metalloproteinases (MMPs) are key regulators of collagen turnover, and we considered whether the increased deposition of collagen might reflect altered MMP levels. The levels of mRNA of 10 MMPs were measured in heart tissue, but no differences between two genotypes in sham-operated animals were noted (not shown). The levels either remained similar between two genotypes after TAC or increased (MMP1, MMP8, MMP16, and MMP24; Fig. 7B), but did not decrease, i.e., did not account for increased collagen deposition in the _Thbs4_−/− mice.
Figure 8.
Regulation of inflammatory and fibrosis-related genes in vivo and of collagen production in vitro by TSP-4. A) Decreased levels of inflammatory markers in hearts of _Thbs4_−/− mice subjected to TAC. Real-time quantitative RT-PCR (qRT-PCR); levels of mRNA in TAC-operated WT mice = 100%; mean ± se, n = 5, 2 wk after TAC. Levels are normalized to corresponding values in sham-operated mice. B) Levels of MMPs in hearts of _Thbs4_−/− mice subjected to TAC. For real-time qRT-PCR, levels are normalized to corresponding values in sham-operated mice; means ± se, n = 5, 2 wk after TAC. C) Human foreskin fibroblasts (HFFs) were incubated with 50 μg/ml recombinant TSP-4 or gelatin for 24–48 h. Solubilized cells and their matrices were tested in dot-blot assays (_n_=3) using anti-collagen I-V, anti-collagen I, anti-collagen II, anti-collagen III, anti-collagen IV, anti-collagen V, or anti-β-actin (loading control) antibodies. Representative blot. D) Quantification of the results of experiment in C, n = 3. E) Cells [human umbilical vein endothelial cells (HUVECs), RF/6A microvascular endothelial cells, and HFFs] were treated as in C. Representative blot. F) Quantification of the results of experiment in E; n = 3.
Effect of recombinant TSP-4 on collagen production by cultured cells
In view of the increased collagen levels in the _Thbs4_−/− mice, the effect of TSP-4 on collagen accumulation was assessed in cultured human fibroblasts and ECs. Cells were incubated with purified rTSP-4 (4, 34, 35). After 24–48 h, the cells were lysed in sodium dodecyl sulfate to solubilize both cellular and ECM proteins. These samples were then used to detect total collagen using a mixture of anti-collagens I–V and individual anti-collagens I–V (Fig. 8C–F). Collagen production was suppressed by TSP-4 in both cell types. Gelatin used as a control protein at the same concentration as TSP-4 did not affect the collagen levels. Consistent with the data on collagen protein and mRNA levels in mouse hearts, rTSP-4 affected the level of collagen I, the major collagen of interstitial ECM, and of collagens II, III, and V, while the level of collagen IV, the major type of collagen in perivascular matrix, was unaffected. Thus, these data recapitulated our in vivo observations.
DISCUSSION
Although TSP-4 mRNA expression increases in hypertrophied and failing hearts (5–8), very limited information is available as to its presence, localization, and function of the protein in the myocardium. We did report that TSP-4 protein can be detected in the ECM of blood vessels of different sizes (4, 22), and Mustonen et al. (5) observed TSP-4 in the blood vessels in the hearts of SHRs. A recent study has identified TSP-4 as a myocyte-interstitial mechanosignaling molecule (21). We now show abundant TSP-4 expression in fibrous tissue throughout the myocardium, suggesting that TSP-4 may play an important role in maintenance of heart structure and function. The fibrous matrix of the heart not only provides structure by determining tensile strength and stiffness (29) but also contributes to ventricular function through transmission of myocyte-generated force to the atrial and ventricular chambers and to the relengthening of myocytes in diastole (36).
The increase in expression of TSP-4 in hypertrophied and failing hearts may reflect either a pathogenic or a protective response. Our data favor a role for TSP-4 in protection of the heart from pathological remodeling and functional change by decreasing fibrosis. This interpretation is in agreement with the recently observed association between decreased TSP-4 levels, improved heart function, and attenuated remodeling after metoprolol treatment (37). In our study, TAC resulted in an accelerated increase in HW in _Thbs4_−/− mice, as compared to WT mice of the same age; the difference in HW/BW between _Thbs4_−/− mice and WT mice was ∼2-fold. Cardiomyocyte size and the number of apoptotic cells in the myocardium increased after TAC, but the mean diameter of myocytes was not larger in _Thbs4_−/− compared to WT mice with or without surgery. Instead, we found that the primary mechanism for and the accelerated increase in HW in _Thbs4_−/− mice was an increased reactive fibrosis. Both at the mRNA level and the protein level, the expression of collagens I, II, III, and V was higher in _Thbs4_−/− mice, suggesting that these collagens, most probably in combination with other ECM proteins and other collagens, are responsible for the increased pressure overload-induced fibrosis in the absence of TSP-4. Our in vitro experiments demonstrating that rTSP-4 directly lowers levels of collagens I, II, III, and V in fibroblasts and endothelial cells provides an explanation for the increased ECM deposition observed in vivo in _Thbs4_−/− mice. TGF-β is a major initiator of fibrosis (38), and TSP-1 is a primary activator of TGF-β (39). However, TSP-4 does not contain the domain or sequence implicated in the binding and activation of TGF-β by TSP-1 (2, 40). Our observations suggest that TSP-4 regulates mRNA levels of collagens. These observations emphasize the need for further investigation of molecular and cellular mechanisms by which TSP-4 directly controls collagen synthesis. Although increased inflammation is often associated with fibrosis, inflammation, and fibrosis may represent two distinct stages of development of heart hypertrophy (32). In _Thbs4_−/− mice, the two processes are clearly uncoupled, consistent with our previous report on decreased local inflammation in _Thbs4_−/− mice (22); the expression of inflammatory markers was lower in _Thbs4_−/− hearts, while the expression of collagens and MMPs was increased or unchanged. The observation of increased fibrosis in _Thbs4_−/− hearts accompanied by the decreased local inflammation suggests that TSP4 may have a direct role on regulating fibrosis independent of inflammation. TSP-4 appears to regulate specific fibrotic events: only selected collagens and MMPs were up-regulated, while others are not affected, and the level of the key regulator of the fibrotic response, TGF-β, was decreased.
Our data favor a model in which TSP-4 primarily regulates matrix production by fibroblasts rather than directly affecting the hypertrophy, proliferation, or apoptosis of cardiomyocytes. Similar mechanism of regulation of heart remodeling was recently reported for microRNA-21 (41): miR-21 was expressed in cardiac fibroblasts and contributed to myocardial disease by directly affecting matrix secretion by these cells. In combination with our study, these data suggest that dysfunction of cardiac fibroblasts and ECM production may play an important and causative role in heart hypertrophy and the associated functional changes. Increased levels of TSP-4 mRNA were associated with fibrotic areas and fibroblasts but were never detected in cardiomyocytes or vascular cells. Interestingly, increased heart size and fibrosis developed in nonchallenged _Thbs4_−/− mice with age: HW/BW and fibrotic areas were significantly increased in _Thbs4_−/− mice at 80 wk of age, suggesting that TSP-4 has an important role in maintaining myocardium structure and homeostasis in physiological conditions.
Isumiya et al. (42) reported that the coronary vascular bed was decreased on remodeling in response to TAC and was inversely proportional to the extent of interstitial fibrosis. TSP-4 deficiency appears to potentiate the effects of TAC on the number of capillaries. The effect of TSP-4 deficiency on vascularity may be a consequence of increased fibrosis or may be a direct regulation of vascular remodeling and angiogenesis by TSP-4. TSP-4 has receptors and initiates signaling in ECs (4). Thus, TSP-4 may influence cardiac remodeling in response to pressure overload by affecting several complementary mechanisms—directly suppressing the ECM production and decreasing ischemia through up-regulation of angiogenesis.
In the pressure overload model, the structural changes in the heart were accompanied by the functional changes, indicating altered systolic and diastolic function of cardiomyocytes. Echocardiograms and Doppler imaging confirmed that heart function was altered in TAC-operated _Thbs4_−/− mice compared to WT animals, resulting in higher volumes and decreased EFs in the _Thbs4_−/− mice. The systolic function of _Thbs4_−/− mice is depressed under normal hemodynamic conditions and was even more suppressed by chronic afterload. In addition to an altered hypertrophic response, _Thbs4_−/− mice have depressed contractility and a relaxation abnormality that become even more pronounced under chronic pressure, as indicated by higher increases of both IVRT and corrected IVRT in _Thbs4_−/− mice after TAC. The increase in IVRT of the _Thbs4_−/− mice suggests pathological changes in cellular function and primary diastolic dysfunction.
In summary, our data suggest an important role of TSP-4 in myocardial function and cardiac tissue remodeling and demonstrate that TSP-4 is involved in regulation of adaptive responses of the heart to pressure overload. A mechanism for this effect appears to reside in the capacity of TSP-4 to regulate the composition and production of the myocardial matrix and most likely angiogenesis.
Supplementary Material
Supplemental Data
Acknowledgments
The authors thank Drs. Judy Drazba and Satyamangla Prasad (Cleveland Clinic) for helpful discussions.
This work was supported by U.S. National Institutes of Health grants (P50 HL077107 to E.F.P. and R01 DK067532 to O.I.S.).
Abbreviations:
BSA
bovine serum albumin
BW
body weight
cDNA
complementary DNA
CVD
cardiovascular disease
EC
endothelial cell
ECM
extracellular matrix
EDV
end diastolic volume
EF
ejection fraction
ESV
end systolic volume
ET
ejection time
FITC
fluorescein isothiocyanate
FT
filling time
HW
heart weight
ICAM1
intercellular adhesion molecule 1
IκB
inhibitor of κB
IP-10
interferon γ-induced protein 10
IVRT
isovolumetric relaxation time
LV
left ventricle
MANOVA
multivariate analysis of variance
MCP-1
monocyte chemotactic protein-1
MMP
matrix metalloproteinase
mRNA
messenger RNA
NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
PCR
polymerase chain reaction
RT
reverse transcription
SHR
spontaneous hypertensive rat
TAC
transverse aortic constriction
TGF
transforming growth factor
TL
tibia length
TSP-4
thrombospondin-4
TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
VCAM1
vascular cell adhesion molecule 1
WT
wild type
REFERENCES
- 1.Bornstein P. (2001) Thrombospondins as matricellular modulators of cell function. J. Clin. Invest. 107, 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams J. C., Lawler J. (2004) The thrombospondins. Int. J. Biochem. Cell Biol. 36, 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams J. C. (2001) Thrombospondins: multifunctional regulators of cell interactions. Annu. Rev. Cell Dev. Biol. 17, 25–51 [DOI] [PubMed] [Google Scholar]
- 4.Stenina O. I., Desai S. Y., Krukovets I., Kight K., Janigro D., Topol E. J., Plow E. F. (2003) Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation 108, 1514–1519 [DOI] [PubMed] [Google Scholar]
- 5.Mustonen E., Aro J., Puhakka J., Ilves M., Soini Y., Leskinen H., Ruskoaho H., Rysa J. (2008) Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem. Biophys. Res. Commun. 373, 186–191 [DOI] [PubMed] [Google Scholar]
- 6.Rysa J., Leskinen H., Ilves M., Ruskoaho H. (2005) Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 45, 927–933 [DOI] [PubMed] [Google Scholar]
- 7.Tan F. L., Moravec C. S., Li J., Apperson-Hansen C., McCarthy P. M., Young J. B., Bond M. (2002) The gene expression fingerprint of human heart failure. Proc. Natl. Acad. Sci. U. S. A. 99, 11387–11392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrielsen A., Lawler P. R., Yongzhong W., Steinbruchel D., Blagoja D., Paulsson-Berne G., Kastrup J., Hansson G. K. (2007) Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J. Mol. Cell. Cardiol. 42, 870–883 [DOI] [PubMed] [Google Scholar]
- 9.Krady M. M., Zeng J., Yu J., MacLauchlan S., Skokos E. A., Tian W., Bornstein P., Sessa W. C., Kyriakides T. R. (2008) Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am. J. Pathol. 173, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alford A. I., Hankenson K. D. (2006) Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone 38, 749–757 [DOI] [PubMed] [Google Scholar]
- 11.Hankenson K. D., James I. E., Apone S., Stroup G. B., Blake S. M., Liang X., Lark M. W., Bornstein P. (2005) Increased osteoblastogenesis and decreased bone resorption protect against ovariectomy-induced bone loss in thrombospondin-2-null mice. Matrix Biol. 24, 362–370 [DOI] [PubMed] [Google Scholar]
- 12.Hankenson K. D., Hormuzdi S. G., Meganck J. A., Bornstein P. (2005) Mice with a disruption of the thrombospondin 3 gene differ in geometric and biomechanical properties of bone and have accelerated development of the femoral head. Mol. Cell. Biol. 25, 5599–5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinale F. G. (2004) Cell-matrix signaling and thrombospondin: another link to myocardial matrix remodeling. Circ. Res. 95, 446–448 [DOI] [PubMed] [Google Scholar]
- 14.Qian X., Wang T. N., Rothman V. L., Nicosia R. F., Tuszynski G. P. (1997) Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp. Cell Res. 235, 403–412 [DOI] [PubMed] [Google Scholar]
- 15.Kyriakides T. R., Zhu Y. H., Yang Z., Huynh G., Bornstein P. (2001) Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am. J. Pathol. 159, 1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatila K., Ren G., Xia Y., Huebener P., Bujak M., Frangogiannis N. G. (2007) The role of the thrombospondins in healing myocardial infarcts. Cardiovasc. Hematol. Agents Med. Chem. 5, 21–27 [DOI] [PubMed] [Google Scholar]
- 17.Bonnefoy A., Moura R., Hoylaerts M. F. (2008) The evolving role of thrombospondin-1 in hemostasis and vascular biology. Cell. Mol. Life Sci. 65, 713–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sezaki S., Hirohata S., Iwabu A., Nakamura K., Toeda K., Miyoshi T., Yamawaki H., Demircan K., Kusachi S., Shiratori Y., Ninomiya Y. (2005) Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp. Biol. Med. (Maywood) 230, 621–630 [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis N. G., Ren G., Dewald O., Zymek P., Haudek S., Koerting A., Winkelmann K., Michael L. H., Lawler J., Entman M. L. (2005) Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 111, 2935–2942 [DOI] [PubMed] [Google Scholar]
- 20.Schroen B., Heymans S., Sharma U., Blankesteijn W. M., Pokharel S., Cleutjens J. P., Porter J. G., Evelo C. T., Duisters R., van Leeuwen R. E., Janssen B. J., Debets J. J., Smits J. F., Daemen M. J., Crijns H. J., Bornstein P., Pinto Y. M. (2004) Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ. Res. 95, 515–522 [DOI] [PubMed] [Google Scholar]
- 21.Cingolani O. H., Kirk J. A., Seo K., Koitabashi N., Lee D. I., Ramirez-Correa G., Bedja D., Barth A. S., Moens A. L., Kass D. A. (2011) Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ. Res. 109, 1410–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frolova E. G., Pluskota E., Krukovets I., Burke T., Drumm C., Smith J. D., Blech L., Febbraio M., Bornstein P., Plow E. F., Stenina O. I. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ. Res. 107, 1313–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salcedo L., Sopko N., Jiang H. H., Damaser M., Penn M., Zutshi M. (2011) Chemokine upregulation in response to anal sphincter and pudendal nerve injury: potential signals for stem cell homing. Int. J. Colorectal. Dis. 26, 1577–1581 [DOI] [PubMed] [Google Scholar]
- 24.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 25.Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J., Jr., Chien K. R. (1991) Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. U. S. A. 88, 8277–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockman H. A., Wachhorst S. P., Mao L., Ross J., Jr. (1994) ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am. J. Physiol. Heart Circ. Physiol. 266, H2468–H2475 [DOI] [PubMed] [Google Scholar]
- 27.Popovic Z. B., Sun J. P., Yamada H., Drinko J., Mauer K., Greenberg N. L., Cheng Y., Moravec C. S., Penn M. S., Mazgalev T. N., Thomas J. D. (2005) Differences in left ventricular long-axis function from mice to humans follow allometric scaling to ventricular size. J. Physiol. 568, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins K. A., Korcarz C. E., Shroff S. G., Bednarz J. E., Fentzke R. C., Lin H., Leiden J. M., Lang R. M. (2001) Accuracy of echocardiographic estimates of left ventricular mass in mice. Am. J. Physiol. Heart Circ. Physiol. 280, H1954–H1962 [DOI] [PubMed] [Google Scholar]
- 29.Weber K. T., Brilla C. G., Janicki J. S. (1993) Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc. Res. 27, 341–348 [DOI] [PubMed] [Google Scholar]
- 30.Dorn G. W., 2nd, Diwan A. (2008) The rationale for cardiomyocyte resuscitation in myocardial salvage. J. Mol. Med. (Berl.) 86, 1085–1095 [DOI] [PubMed] [Google Scholar]
- 31.Bradshaw A. D., Baicu C. F., Rentz T. J., Van Laer A. O., Boggs J., Lacy J. M., Zile M. R. (2009) Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Y., Lee K., Li N., Corbett D., Mendoza L., Frangogiannis N. G. (2009) Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 131, 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A. K., Bradham W. S., Gleaves L. A., De Taeye B., Murphy S. B., Covington J. W., Vaughan D. E. (2010) Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation 122, 1200–1209 [DOI] [PubMed] [Google Scholar]
- 34.Stenina O. I., Ustinov V., Krukovets I., Marinic T., Topol E. J., Plow E. F. (2005) Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. FASEB J. 19, 1893–1895 [DOI] [PubMed] [Google Scholar]
- 35.Pluskota E., Stenina O. I., Krukovets I., Szpak D., Topol E. J., Plow E. F. (2005) Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood 106, 3970–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson T. F., Factor S. M., Sonnenblick E. H. (1986) The heart as a suction pump. Sci. Am. 254, 84–91 [DOI] [PubMed] [Google Scholar]
- 37.Mirochnik Y., Kwiatek A., Volpert O. V. (2008) Thrombospondin and apoptosis: molecular mechanisms and use for design of complementation treatments. Curr. Drug Targets 9, 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Creemers E. E., Pinto Y. M. (2011) Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc. Res. 89, 265–272 [DOI] [PubMed] [Google Scholar]
- 39.Murphy-Ullrich J. E., Poczatek M. (2000) Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 11, 59–69 [DOI] [PubMed] [Google Scholar]
- 40.Schultz-Cherry S., Lawler J., Murphy-Ullrich J. E. (1994) The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J. Biol. Chem. 269, 26783–26788 [PubMed] [Google Scholar]
- 41.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., Castoldi M., Soutschek J., Koteliansky V., Rosenwald A., Basson M. A., Licht J. D., Pena J. T., Rouhanifard S. H., Muckenthaler M. U., Tuschl T., Martin G. R., Bauersachs J., Engelhardt S. (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 [DOI] [PubMed] [Google Scholar]
- 42.Izumiya Y., Shiojima I., Sato K., Sawyer D. B., Colucci W. S., Walsh K. (2006) Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 47, 887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data