Human Embryonic Stem Cells have Constitutively Active Bax at the Golgi and are Primed to Undergo Rapid Apoptosis (original) (raw)
. Author manuscript; available in PMC: 2013 Jun 8.
Abstract
Human embryonic stem (hES) cells activate a rapid apoptotic response after DNA damage but the underlying mechanisms are unknown. A critical mediator of apoptosis is Bax, which is reported to become active and translocate to the mitochondria only after apoptotic stimuli. Here we show that undifferentiated hES cells constitutively maintain Bax in its active conformation. Surprisingly, active Bax was maintained at the Golgi rather than at the mitochondria, thus allowing hES cells to effectively minimize the risks associated with having pre-activated Bax. After DNA damage, active Bax rapidly translocated to the mitochondria by a p53-dependent mechanism. Interestingly, upon differentiation, Bax was no longer active and cells were not acutely sensitive to DNA damage. Thus, maintenance of Bax in its active form is a unique mechanism that can prime hES cells for rapid death, likely to prevent the propagation of mutations during the early critical stages of embryonic development.
Keywords: Apoptosis, Bax, Human ES cells, Golgi, Ku70, DNA damage, p53, 6A7, mitochondria
Introduction
Human embryonic stem (hES) cells have the unique capability of differentiating into all cell types, leading to the development of an entire organism (Jaenisch and Young, 2008; Thomson et al., 1998). As the integrity of ES cells is critical for the developing embryo, these cells have likely evolved mechanisms that detect and respond rapidly to adverse stimuli. Indeed, hES cells have been shown to be highly sensitive to DNA damage, but the molecular mechanisms underlying this rapid death remain unclear (Filion et al., 2009; Grandela et al., 2008; Momčilović et al., 2009; Qin et al., 2007).
Caspases are critical mediators of apoptosis in mammalian cells and a key protein that controls their activation is Bax, a proapoptotic member of the Bcl-2 family (Tait and Green, 2010; Youle and Strasser, 2008). In healthy cells, Bax has been reported to be predominantly cytosolic, present in an inactive conformation. However, apoptotic stimuli such as DNA damage activate signaling pathways that result in the induction of BH3-only family proteins that promote a change in Bax conformation (Brunelle and Letai, 2009; Chipuk et al., 2010). Activated Bax then translocates to the mitochondria where it induces cytochrome c release and apoptosome-mediated caspase activation, resulting in apoptotic cell death (Dewson and Kluck, 2009; Taylor et al., 2008). Thus, Bax activation is a critical event in the commitment of cells to apoptosis.
Considerable details of the mechanism by which Bcl-2 family proteins regulate Bax activation have recently emerged. Bax can be directly activated via binding to a subset of BH3-only proteins known as “activators” (e.g. Bim, Bid, and Puma). However, the antiapoptotic members of the Bcl-2 family (e.g. Bcl-2, Bcl-XL) can also bind to Bax and prevent its function. A second subset of BH3-only proteins known as “sensitizers” (e.g. Bad, Noxa, Bmf) bind to and inactivate the antiapoptotic Bcl-2 family proteins and therefore, may also be needed to enable efficient Bax activation (Giam et al., 2008; Shamas-Din et al., 2011). Importantly, structural studies have shown that binding of the BH3-only activators to Bax induces a conformational change in Bax that exposes the N-terminus and mobilizes the C-terminus to insert into the mitochondrial outer membrane. These conformational changes enable Bax to oligomerize and form pores in the mitochondrial membrane that trigger the release of cytochrome c (Gavathiotis and Walensky, 2011). Adding to this complexity, Bax activation can also be regulated in cells by cytosolic factors such as Ku70, a protein also involved in non-homologous end joining DNA repair, that binds to inactive Bax and prevents its activation (Cohen et al., 2004; Gomez, 2007). Apoptotic stimuli result in the acetylation of Ku70 and the disruption of the Bax-Ku70 complex, facilitating Bax activation (Cohen et al., 2004; Li et al., 2007; Subramanian et al., 2005).
While the main components of the apoptotic pathway have been identified, exactly how this pathway is regulated in various primary cells remains unclear. Here, we examined the apoptotic pathway in hES cells and report a unique mechanism engaged by hES cells that can prime them to undergo rapid apoptosis in response to genotoxic damage.
Results
hES cells engage rapid apoptosis after DNA damage that is p53-dependent
To examine the apoptotic pathway in hES cells, we treated them with various stimuli that induce apoptosis. We found that DNA damage activates very rapid death in H9 hES cells, with all cells dying by 5 hours after etoposide treatment (Figures 1A, B). This death was apoptotic as it resulted in caspase-3 activation and was completely prevented by the addition of the pan-caspase inhibitor z-VAD-fmk (Figures 1A, B, C). hES cells were remarkably more sensitive to etoposide than fibroblasts in a time- and dose-dependent manner (Figure S1A). Furthermore, this sensitivity of hES cells was selective to DNA damage. While exposure to either etoposide or doxorubicin induced rapid apoptosis, tunicamycin and taxol treatments, which induce apoptosis by ER stress and microtubule stabilization respectively, did not induce hES cell death within this timeframe (Figure 1D).
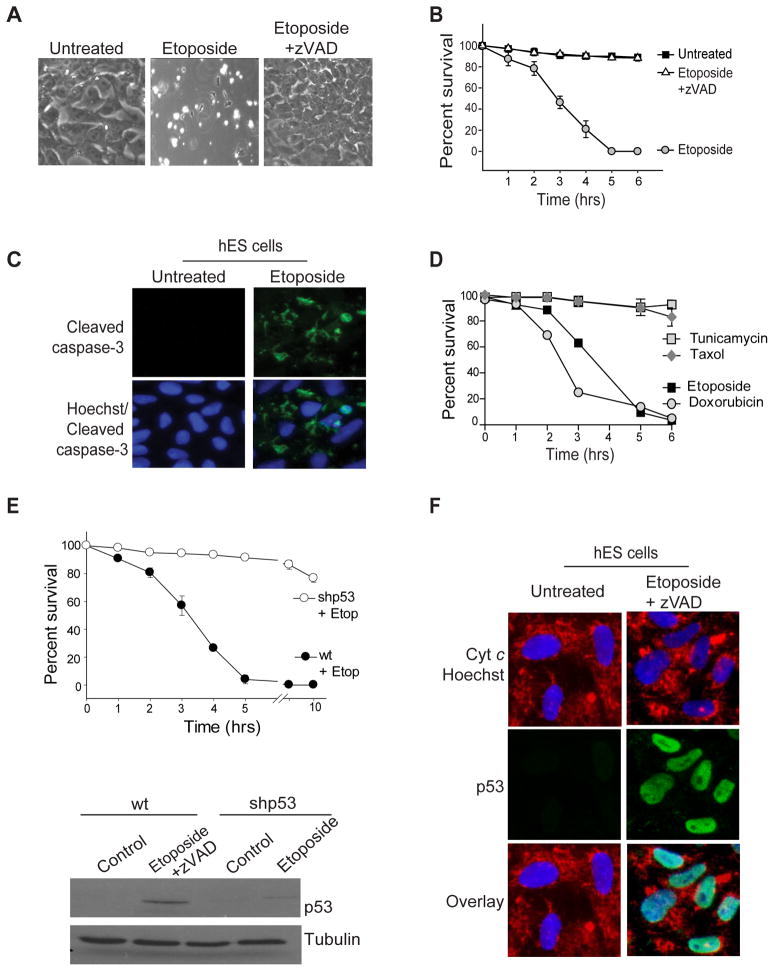
Figure 1.
hES cells engage rapid apoptosis after DNA damage. H9 hES cells were either left untreated or treated with 20 μM etoposide in the absence or presence of the caspase inhibitor z-VAD.fmk (50 μM). Shown are phase contrast images after 5 hrs of treatment (A) and quantification of cell survival. Error bars represent ±SD for triplicate experiments. (B). (C) Untreated and 3 hrs etoposide treated (20 μM) hES cells were fixed and stained with anti-cleaved caspase-3 antibody and Hoechst 33258 (nuclei). Immunofluorescence images show that the untreated hES cells do not have active caspase-3 but it becomes activated as early as 3 hrs after the initiation of the treatment. (D) Quantification of cell death (using nuclear fragmentation) of hES cells treated with Tunicamycin (5 μM), Taxol (1 μM), Etoposide (20 μM), or Doxorubicin (1 μM) as indicated in the figure. Error bars represent ±SD for triplicate experiments. (E) Upper panel: wildtype (wt) and p53 knock-down (shp53) hES cells were treated with 20 μM etoposide (Etop) and cell survival was quantified at various times after etoposide treatment. Lower panel: wildtype (wt) and p53-knock-down (shp53) hES cells were treated with 20 μM etoposide for 3 hrs and immunoblotted with an anti-p53 antibody. Wildtype hES cells were also treated with zVAD.fmk to prevent any cell death. Error bars represent ±SD for triplicate experiments. Western blot shows the efficient downregulation of p53. (F) Wildtype hES cells were treated with 20 μM etoposide for 2 hours and immunolabeled for p53, cyt c and the nuclear stain Hoechst 33258. The immunofluorescence images show the upregulation and nuclear localization of p53 after etoposide treatment.
hES cell death in response to DNA damage was also p53-dependent, as phosphorylated p53 (Ser15) accumulated after etoposide treatment (Figure S1B) and shRNA-mediated knock down of p53 completely blocked hES cell death (Figure 1E, Figure S1C). p53 can induce cell death either by localizing to the nucleus and activating proapoptotic genes such as Noxa or Puma (Nakano and Vousden, 2001; Oda et al., 2000; Yu et al., 2001) or by a faster, transcription independent mechanism whereby it translocates to the mitochondria and directly interacts with members of the Bcl-2 family to promote cytochrome c release (Chipuk et al., 2004; Mihara et al., 2003). Following DNA damage, p53 was detectable only in the nuclei of etoposide-treated hES cells (Figure 1F). Furthermore, addition of the protein synthesis inhibitor cycloheximide prevented the etoposide-induced death of hES cells (Figure S1D). Thus, the rapid apoptotic death of hES cells was likely mediated by a protein synthesis-dependent activity of p53, rather than by direct translocation to the mitochondria.
Bax is constitutively activated in hES cells
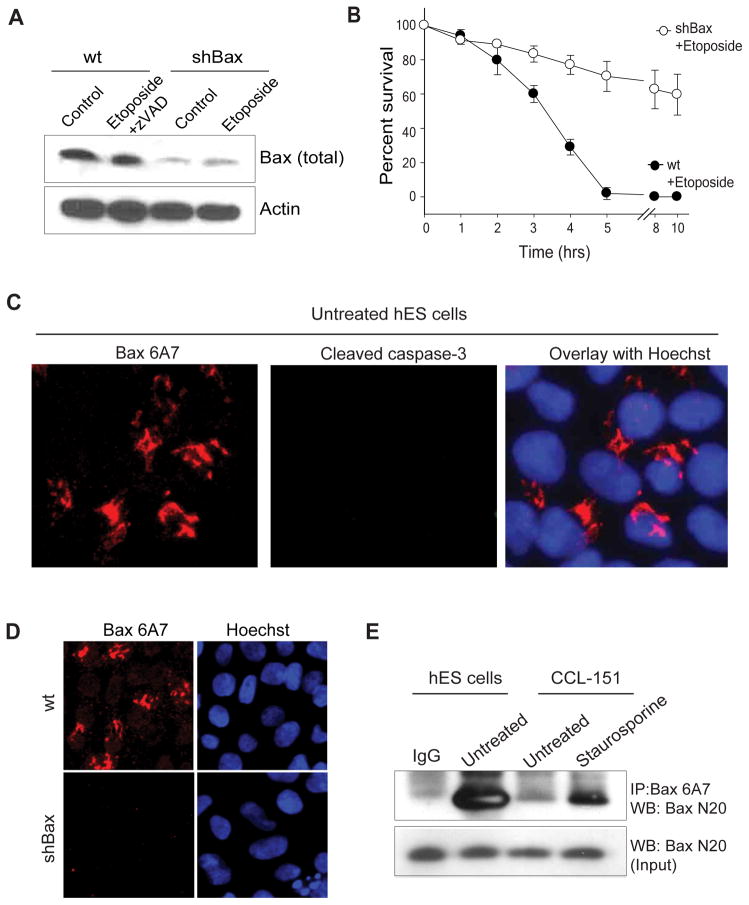
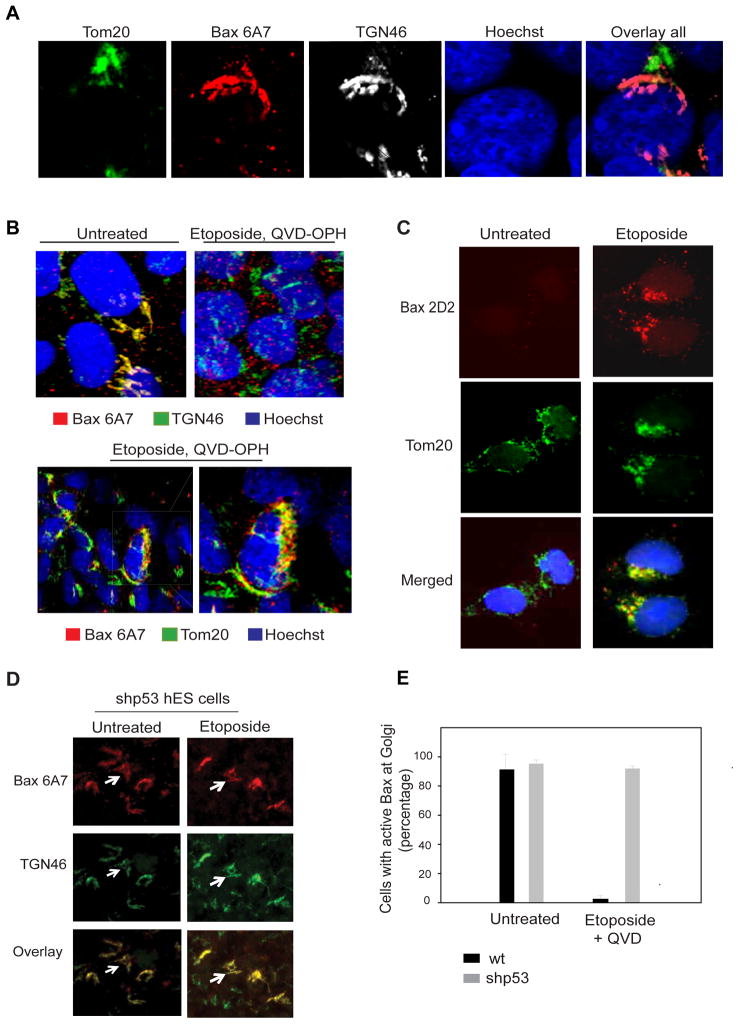
Bax and Bak are the two main proapoptotic members of the Bcl-2 family that directly mediate mitochondrial permeabilization during apoptosis. We found etoposide-induced hES cell death to be Bax-dependent but Bak-independent (Figures 2A, B, Figure S2A), indicating that Bax is the key inducer of apoptosis in these hES cells. Typically, in untreated cells, Bax is present in its inactive, monomeric conformation in the cytosol (Hsu, 1997). However, after the induction of apoptosis, Bax undergoes a conformational change, oligomerizes, and inserts into the outer mitochondrial membrane where it promotes the release of cytochrome c which results in caspase activation (Antonsson, 2001; Wolter et al., 1997; Youle and Strasser, 2008). To determine the kinetics of Bax activation in hES cells, we immunolabeled untreated and etoposide treated hES cells using the Bax 6A7 antibody, which detects Bax only in its active conformation (Hsu and Youle, 1997). Surprisingly, we found Bax to be in its active state in the untreated hES cells (Figures 2C). These results were unexpected because thus far Bax activation has been seen only in cells that are actively undergoing apoptosis (Youle and Strasser, 2008) (Figure S2B). However, we found no evidence of cell death in untreated hES cells that have activated Bax (Figure 2C and Figure S2C). We additionally confirmed that the Bax 6A7 staining was specific for Bax as shRNA-mediated knock down of Bax decreased active Bax staining in hES cells (Figure 2D). Consistent with the observation that Bax is maintained in an active state, active Bax was immunoprecipitated with the 6A7 antibody in untreated hES cells (Figure 2E). In contrast, in a human fibroblast line (CCL-151), the active form of Bax could only be detected upon the induction of apoptosis (Figure 2E). The observation that hES cells have Bax in its active conformation provides insight into how these cells are able to rapidly induce apoptosis after DNA damage. However, this raised the question of how hES cells are capable of maintaining Bax in its active state without spontaneously activating apoptosis.
Figure 2.
Bax is constitutively active in hES cells. (A) Wildtype (wt) and Bax knock-down (shBax) hES cells were treated with 20 μM etoposide for 5 hrs and immunoblotted for total Bax. Western blot shows efficient knock down in Bax levels in shBax expressing cells. (B) Quantification of cell survival in wt and Bax knock-down hES cells at various times after etoposide (20 μM) treatment. Error bars represent ±SD for triplicate experiments. (C) hES cells were immunostained with anti-Bax 6A7 and anti-cleaved caspase-3 antibodies. Images show active Bax but no caspase-3 activation in untreated hES cells. (D) Wildtype (wt) or Bax knockdown (shBax) hES cells were immunostained for active Bax with the 6A7 antibodies. Representative image shows no 6A7 positive staining in Bax-deficient hES cells demonstrating that the 6A7 staining is Bax-dependent. (E) Immunocomplexes were precipitated from hES and CCL-151 (human lung fibroblast cell line; untreated or treated with 200 nM staurosporine) extracts (prepared in CHAPS buffer) with the anti-Bax 6A7 antibody and immunoblotted with a total Bax antibody (N20). The input lane was loaded as 1/10 dilution of the pre-IP extract, and IgG served as a negative control.
Constitutively activated Bax is localized to the Golgi in hES cells
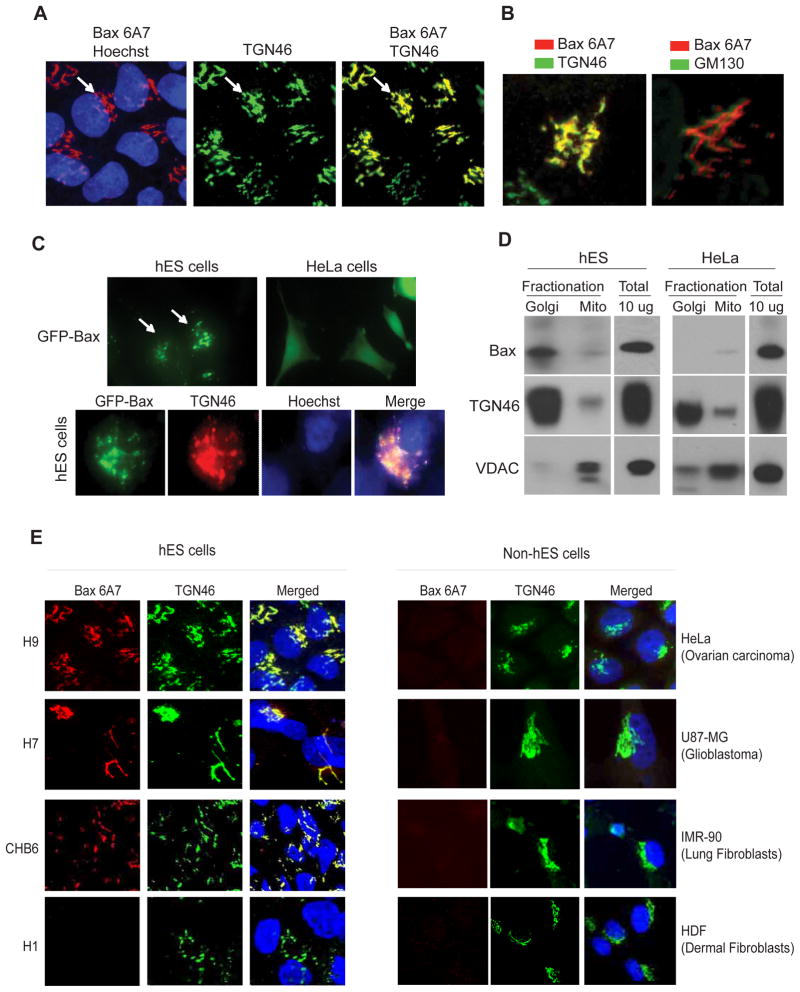
In cells undergoing apoptosis active Bax localizes to the mitochondria to promote caspase activation (Brunelle and Letai, 2009; Gross et al., 1999; Hsu, 1997; Wolter et al., 1997). Based on our observation that hES cells are Bax 6A7 positive, we asked whether active Bax was localized to the mitochondria of untreated hES cells. Unexpectedly, we found active Bax to be localized not at the mitochondria but at the Golgi in the untreated hES cells (Figure 3A and Figures S3A, B, C). Co-immunolabeling of hES cells with Bax 6A7 and TGN46, a protein maintained at the trans-Golgi network, showed that active Bax is specifically localized to the trans-Golgi compartment; no colocalization was seen with the cis-Golgi marker GM130 (Figures 3A, B). Consistent with the observation that active Bax was localized at the Golgi, treatment of hES cells with Brefeldin A, a known Golgi complex disassembly agent (Fujiwara et al., 1988), altered Bax localization in hES cells (Figure S3C). Importantly, in addition to our findings that the 6A7 antibody labeled Bax at the Golgi, we found ectopically expressed GFP-Bax to be localized to the trans-Golgi compartment in hES cells, in contrast to the cytosolic localization that is seen in other cell types (Figure 3C). To confirm Bax localization to the Golgi using biochemical techniques, we also conducted subcellular fractionation of hES cells by sucrose gradient ultracentrifugation. Our results show strong localization of Bax in the Golgi-enriched fractions of hES cells. In contrast, no Bax was detected in the Golgi fractions in HeLa cells (Figure 3D). To examine whether active Bax at the Golgi was also seen in other hES cell lines, we examined the status of Bax in a panel of four hES and four non-hES cell lines. Indeed, we found Bax to be in an active conformation and localized to the Golgi in three of the four hES cell lines we tested (H9, H7, CHB6; see Discussion section). Importantly, no active Bax was detected in any of the non-hES cells tested (Figure 3E). Thus, multiple experimental approaches show that activated Bax is found at the Golgi of healthy, untreated hES cells.
Figure 3.
Constitutively active Bax is localized to the Golgi in hES cells. (A) Untreated hES cells were immunostained with anti-Bax 6A7 and anti-TGN46 antibodies. Hoechst 33258 was used to label the nuclei. Immunofluorescence images show the localization of active Bax at the Golgi (arrows). (B) Active Bax is localized at the trans-Golgi (anti-TGN46 antibody) and not at the cis-Golgi network (anti-GM130 antibody). (C) Upper panels: Ectopically expressed GFP-Bax shows a distinct subcellular localization in hES cells in contrast to the cytoplasmic localization seen in HeLa cells. Lower panels: GFP-Bax immunofluorescence (GFP antibody) shows the localization of Bax to the Golgi (TGN46) in hES cells. (D) Subcellular fractionation of Golgi and mitochondria (Mito) in hES and HeLa cells. Representative Western blots demonstrate that Bax (N20 antibody) can be detected in the Golgi of hES but not of HeLa cells. TGN46 and VDAC were used as markers of Golgi and mitochondrial, respectively. (E) A panel of hES cells (H9, H7, CHB6, H1) and human non-ES cells (HeLa, U87-MG, IMR-90, HDF) were probed for the status of active Bax by immunostaining with 6A7 and TGN46 (for Golgi) antibodies. Images show that active Bax localized to the Golgi was seen in the H9, H7 and CHB6 hES cells.
Early hES cell differentiation changes Bax status and sensitivity to DNA damage
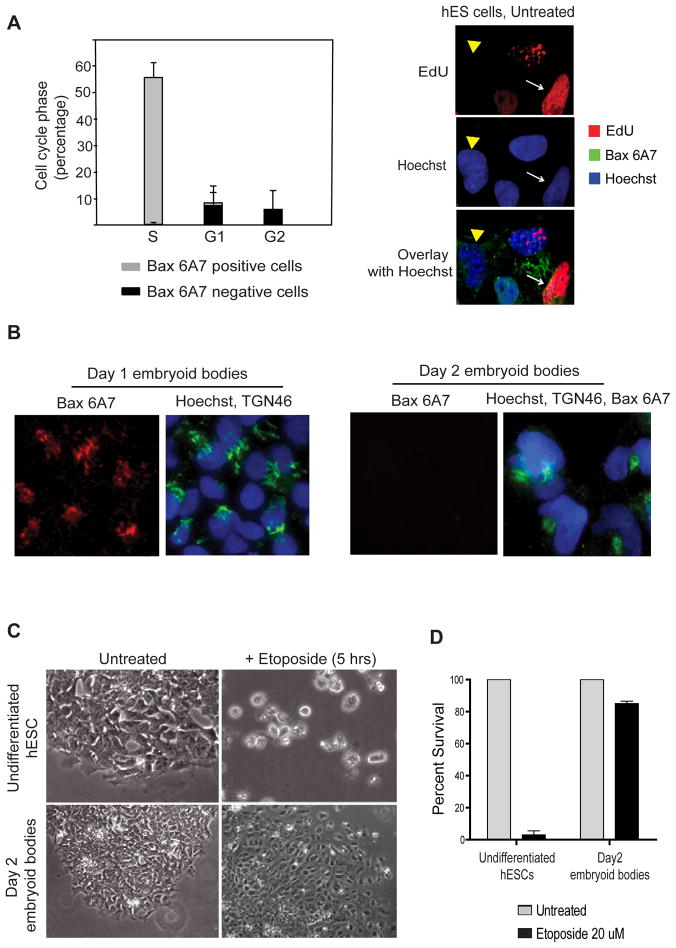
We noticed that a subset (approximately 30–35%) of untreated hES cells did not have Bax in its active conformation. Since the cell cycle distribution in a population of hES cells is reported to be mostly in S phase (65%), followed by G0-G1 (20%) and G2-M (15%) (Becker et al., 2006), we investigated whether the presence of active Bax in hES cells was correlated with the cell cycle state. We labeled untreated hES cells with Bax 6A7, EdU (S phase marker), and DAPI (for DNA) and conducted FACS analysis to identify the population of cells with active Bax. Our results showed that Bax 6A7 positive cells were almost exclusively (greater than 95%) in the S phase of the cell cycle (Figure 4A).
Figure 4.
Active Bax is present predominantly in hES cells in the S phase ; Bax is no longer active in hES cells after differentiation. (A) Left panel: Untreated hES cells were stained with EdU, anti-Bax 6A7 antibody and DAPI and subjected to FACS analysis as described in the Materials and Methods. Analysis showed that Bax 6A7 positivity was seen almost exclusively (greater than 95%) in cells going through the S phase of the cell cycle (~60% of the total cells are in S phase). Right panel: Representative fluorescent images show that cells that are actively replicating (EdU positive; white arrows) have Bax in its active state (6A7 positive), whereas cells that are not in the S phase (EdU negative; yellow arrowhead) do not have active Bax (6A7 negative). Error bars represent ±SD for triplicate experiments. (B) hES cells after one and two days of differentiation were immunostained with anti-Bax 6A7 and anti-TGN46 antibodies. Immunofluorescence images show that 2-day differentiated hES cells are no longer positive for Bax 6A7. (C) Phase contrast images show that embryoid bodies (2 days of differentiation) are no longer sensitive to rapid apoptosis induced by etoposide. (D) Quantification of cell death by nuclear morphology after treatment with 20 μM etoposide. Error bars represent ±SD for triplicate experiments.
As hES cells have the potential to be differentiated into all cell lineages, we examined whether the constitutively active status of Bax seen in undifferentiated hES cells changes with differentiation. Differentiation of hES cells can be achieved by removing cells from feeder layers or matrixes and allowing them to grow in suspension on a non-adherent surface (Odorico et al., 2001). hES cells begin to differentiate under these conditions and form aggregates called embryoid bodies which can be dissociated for analysis. Interestingly, Bax was found in its active conformation only in undifferentiated hES cells; Bax was no longer present in its active state after just two days of differentiation (Figure 4B and Figure S4). Consistent with the idea that the presence of active Bax in undifferentiated hES cells primes them for rapid apoptosis, the two-day differentiated hES cells that do not have active Bax were also no longer acutely sensitive to DNA damage (Figures 4C, D). These results illustrate how the apoptotic machinery undergoes dynamic changes to set apoptotic thresholds even at the earliest stages of hES cell differentiation.
p53 is required for translocation of active Bax to mitochondria after DNA damage
Confocal analysis using triple labeling with Bax 6A7, TGN46 (Golgi), and Tom20 (mitochondria) antibodies confirmed active Bax to be localized at the Golgi in untreated hES cells (Figure 5A). Next, we examined whether active Bax changes localization after DNA damage. As early as 3 hours after etoposide treatment Bax 6A7 co-localized with the mitochondrial marker Tom20, indicating that active Bax translocates from the Golgi to the mitochondria in hES cells undergoing apoptosis (Figure 5B and Figure S5). Bax was localized to distinct focal points at the mitochondria during apoptosis, as previously described (Karbowski et al., 2002; Nechushtan et al., 2001). Bax translocation to the mitochondria was also confirmed with another Bax antibody (2D2) that showed a similar pattern of Bax localization to mitochondrial foci upon etoposide treatment in hES cells (Figure 5C). As our results show that apoptosis after DNA damage in hES cells was p53-dependent, we investigated whether p53 was necessary for the translocation of active Bax from the Golgi to mitochondria. Indeed, we found active Bax to be maintained at the Golgi after etoposide treatment in the p53-knock down hES cells, indicating that p53 was required for the Golgi-to-mitochondria translocation of active Bax after DNA damage (Figures 5D, E).
Figure 5.
p53 is required for Bax translocation to mitochondria after DNA damage. (A) Triple labeled confocal images of untreated hES cells demonstrate active Bax (6A7) colocalizing to the Golgi (TGN46) but not the mitochondria (Tom20). (B) hES cells were either left untreated or treated with etoposide for 5 hours (in the presence of caspase inhibitor 25 μM QVD-OPH). Immunostaining with anti-Bax 6A7 and anti-TGN46 antibodies (and Hoechst 33258 to label nuclei) shows no colocalization of active Bax with Golgi after etoposide treatment. Bottom panels show distinct sites of Bax 6A7 co-localization with mitochondria (anti-Tom20 antibody) after etoposide treatment. (C) hES cells were left untreated or treated with etoposide (20 μM) for 5 hours (in the presence of caspase inhibitor 25 μM QVD-OPH). Immunostaining with anti-Bax 2D2 and anti-Tom20 antibodies shows translocation of Bax to the mitochondria after etoposide treatment. (D) p53 knock-down (shp53) hES cells were either left untreated or were treated with etoposide for 5 hours and immunostained with anti- Bax 6A7 and anti-TGN46 antibodies. Immunofluorescence images show that Bax 6A7 remains colocalized with TGN46 at the Golgi, even after DNA damage. (E) Quantification of results in (D), indicating the number of wild-type (wt) and p53 knock-down (shp53) hES cells with active Bax at Golgi before and after etoposide treatment. Error bars represent ±SD for triplicate experiments.
Inactivation of Bcl-2 and Bcl-XL does not alter Golgi localization of Bax in hES cells
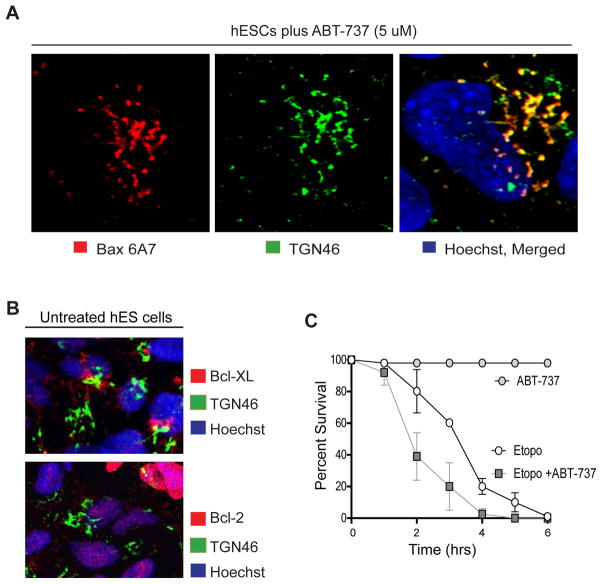
The antiapoptotic members of the Bcl-2 family such as Bcl-2 and Bcl-XL have been shown to inhibit Bax-induced mitochondrial permeabilization (Youle and Strasser, 2008). We examined whether these proteins were important for maintaining Bax at the Golgi and restricting its translocation to the mitochondria in untreated hES cells. Addition of ABT-737, an inhibitor of Bcl-2 and Bcl-XL (Oltersdorf et al., 2005), did not change localization of active Bax at the Golgi (Figure 6A). Consistent with this observation, we did not detect either Bcl-2 or Bcl-XL at the Golgi in hES cells (Figure 6B). Treatment with ABT-737 alone also did not induce hES cells to undergo apoptosis. However, ABT-737 addition increased the rate of etoposide-induced hES cell death, with nearly 60% of cells dying by 2 hours after etoposide treatment (Figure 6C). These results suggest that inactivation of Bcl-2 and Bcl-XL alone is not sufficient to trigger Bax translocation to the mitochondria in the absence of an apoptotic stimuli in hES cells.
Figure 6.
Inactivation of Bcl-2 and Bcl-XL does not change Bax status at the Golgi in hES cells (A) hES cells were treated with 5 μM ABT-737 for 18 hrs and immunostained with anti- Bax 6A7 and anti-TGN46 antibodies. Immunofluorescence images show that ABT-737 treatment did not affect localization of active Bax to the Golgi. (B) hES cells were immunostained with anti-Bcl-XL or anti-Bcl-2 and anti-TGN46 antibodies. Immunostaining shows no localization of Bcl-2 and Bcl-XL to Golgi. (C) Quantification of survival (with nuclear morphology) in hES cells treated with ABT-737 (5 μM) or etoposide alone (20 μM) or a combination of ABT-737 and etoposide. hES cells treated with ABT737 alone did not die but cells treated with etoposide in combination with ABT-737 died faster than cells treated with etoposide alone. Error bars represent ±SD for triplicate experiments.
Ku70 is constitutively acetylated and does not interact with Bax in untreated hES cells
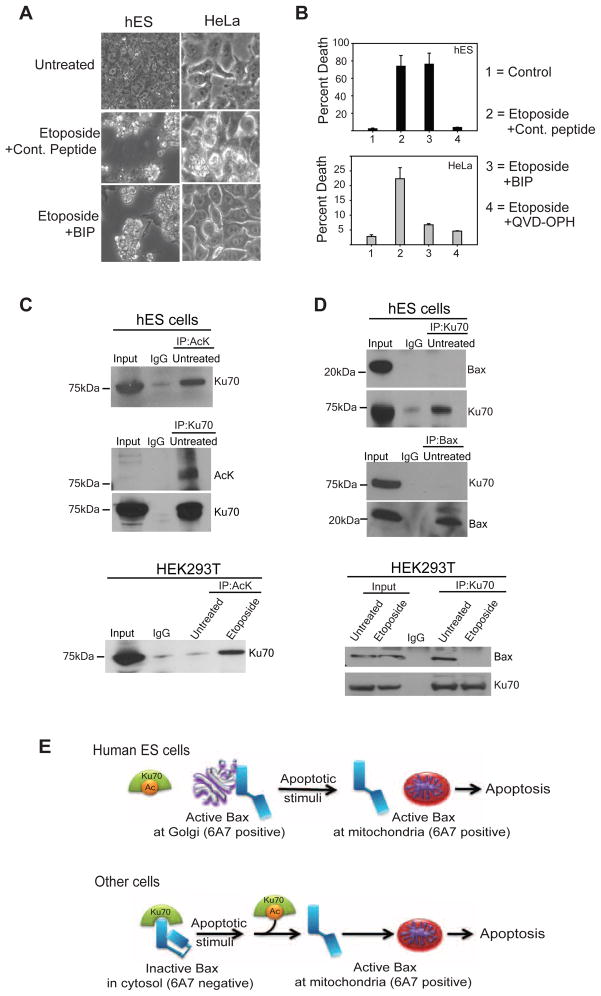
Previous studies have found Bax to be maintained in an inactive form in a complex with Ku70 (Cohen et al., 2004; Subramanian et al., 2005). A peptide containing the Ku70 binding domain (Bax inhibitory peptide, BIP) potently binds inactive Bax and prevents its activation (Yoshida et al., 2004). Because Ku70 cannot bind active Bax, BIP is unable to block apoptosis once Bax is activated. We investigated the ability of BIP to block apoptosis in hES cells following DNA damage as an alternative method of confirming that Bax is pre-activated in hES cells. As anticipated, BIP could effectively inhibit apoptosis in HeLa cells but not in hES cells where Bax is already in its active conformation (Figures 7A, B).
Figure 7.
Ku70 is constitutively acetylated and does not interact with Bax in untreated hES cells. (A)hES and HeLa cells were either left untreated or treated with etoposide either in the presence of Bax Inhibiting Peptide (200 μM BIP) or a negative control peptide at the same concentration. Results show that while BIP inhibited cell death in HeLa cells, it failed to do so in hES cells. (B) Quantification of cell death (propidium iodide staining) after 5 hrs (hES cells) or 16 hours (HeLa cells) of etoposide treatment (20 μM). Error bars represent ±SD for triplicate experiments. (C) Immunocomplexes were precipitated from untreated hES and untreated or etoposide treated HEK293T cells with the anti-pan acetylated lysine (AcK) antibody and immunoblotted with an anti-Ku70 antibody. For untreated hES cells, a reverse IP with anti-Ku70 antibodies and immunoblot for acetylated lysine are also shown. The input lane was loaded as 1/10 dilution of the pre-IP extract, and IgG served as a negative control. (D) Immunocomplexes were precipitated from untreated hES and untreated or etoposide treated HEK293T cells with the anti-Ku70 antibody and immunoblotted with an anti-Bax (N20) antibody. For untreated hES cells, a reverse IP with anti-Bax antibodies and immunoblot for Ku70 are also shown. The input lane was loaded as 1/10 dilution of the pre-IP extract, and IgG served as a negative control. (E) Model showing the unique mechanisms engaged by hES cells where Bax is present in its constitutively active form at the Golgi, thus priming these cells for rapid apoptosis after DNA damage.
In response to DNA damage, Ku70 becomes acetylated by histone acetyl-transferases, resulting in the release of Bax and the subsequent translocation of Bax to mitochondria (Cohen et al., 2004; Subramanian et al., 2005). Because we found that Bax was in its active conformation in untreated hES cells, we examined the acetylation status of Ku70 in these cells. Our immunoprecipitation results show that Ku70 is constitutively acetylated in the untreated hES cells (Figure 7C). In contrast, Ku70 was acetylated only after DNA damage in HEK293 and HeLa cells (Figure 7C, data not shown). Consistent with the model that acetylated Ku70 can no longer bind Bax, we found no interaction between Ku70 and Bax in untreated hES cells (Figure 7D). These results in hES cells are distinct from those in HEK293 and HeLa cells, where Bax co-immunoprecipitated with Ku70. The interaction between Bax and Ku70 was disrupted only after the acetylation of Ku70 as a consequence of DNA damage in these cells (Figures 7C, D, data not shown). These results identify an interesting feature of hES cells in which Ku70 is constitutively acetylated in the absence of DNA damage, and therefore incapable of sequestering Bax.
Discussion
Here we describe the striking observation that healthy undifferentiated hES cells maintain Bax in its pre-activated state at the Golgi. This is in contrast to other cell types in which Bax is typically present in an inactive form in the cytosol. Importantly, as early as 3 hours after DNA damage, active Bax translocates from the Golgi to the mitochondria in a p53-dependent manner. We propose that by sequestering active Bax at the Golgi, hES cells are able to effectively minimize the risks associated with having pre-activated Bax yet maintain their ability to respond rapidly to genotoxic insults.
Detailed biochemical and structural studies on the mechanism of how Bax is activated have emerged in recent years. However, exactly how Bax activation is regulated in various primary cells remains largely unexplored. This is particularly important as different cell types may modify their apoptotic threshold in order to function in, and adapt to, different physiological conditions. Typically, Bax is activated by the BH3-only proteins in a rapid sequence of events. The direct binding of the activator BH3-only proteins to Bax induces a conformation change in the N-terminus (recognized by the 6A7 antibody) which is followed immediately by the mobilization of the C-terminal transmembrane domain and the insertion of Bax on the mitochondrial outer membrane (Walensky and Gavathiotis, 2011). Our results show that these steps of Bax activation are uncoupled in hES cells with the 6A7 positive form of Bax stabilized without mitochondrial translocation. Whether the maintenance of active Bax is dependent on some BH3-only proteins in hES cells is not known. The full repertoire of BH3-only proteins expressed in hES cells is unknown and the simultaneous knock down of multiple members may be needed to discern their importance in inducing Bax activation. Recently, a constitutively active isoform of Bax (Baxβ) was detected in various cell lines. However, in contrast to our results in hES cells, the Baxβ isoform was found at the mitochondria and only detected in the presence of proteasome inhibitors in the cell lines analyzed (Fu et al., 2009).
A recent study found Bax to shuttle between the cytosol and mitochondria in healthy cells and that Bcl-XL is important for the retrotranslocation of Bax from the mitochondria to cytosol (Edlich et al., 2011). We specifically examined whether Bcl-2 and Bcl-XL were important for maintaining active Bax at the Golgi and preventing its spontaneous translocation to mitochondria. While both Bcl-2 and Bcl-XL are known to promote hES cell survival (Ardehali et al., 2011; Bai et al., 2012), our results show that inhibition of these proteins alone did not change the status of active Bax or trigger apoptosis in hES cells. The association of Bax with Ku70 has been reported as one mechanism by which Bax is maintained in an inactive conformation (Cohen et al., 2004; Subramanian et al., 2005). Importantly, we found that Ku70 is constitutively acetylated and therefore unable to bind Bax in hES cells. These results contrast the findings in other cell types where the Ku70-Bax complex is disrupted only when Ku70 is acetylated by histone acetyltransferases (e.g. p300/CBP, PCAF) in response to apoptotic stimuli (Cohen et al., 2004; Subramanian et al., 2005). The observation that Ku70 is constitutively acetylated and unable to bind to Bax provides insight into how Bax is available for activation in hES cells.
Our results also highlight the fact that the apoptotic machinery undergoes dynamic changes even at early stages of differentiation. While undifferentiated hES cells have constitutively active Bax and undergo rapid apoptosis in response to DNA damage, just 2 days of differentiation induced significant changes such that Bax was no longer active, and the cells were no longer highly sensitive to DNA damage. This could be manifested with even greater complexity in vivo as cells during early embryogenesis undergo rapid proliferation and differentiation. While most of our studies were done in the H9 hES cell line, we also examined the status of Bax in a panel of other hES cells. Like the H9 hES cells, the H7 and CHB6 hES cells also exhibited 6A7 positive Bax staining at the Golgi (Figure 3E). However, the staining intensity was weaker in the H7 and CHB6 hES cells (requiring confocal imaging) as compared to the H9 hES cells, and negative in the H1 hES cells. Thus, we anticipate that there may be differences in the status of the apoptosis machinery between various hES cell lines potentially as a consequence of their derivation from the inner cell mass at different time points during blastocyst outgrowth.
Recent evidence demonstrates that sustained treatment of ES cells with DNA damaging agents can predispose ES cells to karyotypic abnormalities (Bueno et al., 2009; Stambrook and Tichy, 2010). Therefore, the ability to maintain genomic integrity in ES cells is of critical importance to prevent propagation of mutations to the developing embryo and reduce the risk of carcinogenesis. Because hES cells are critical for embryogenesis, yet need to be sensitive to genomic perturbations, their ability to engage a rapid death after DNA damage is likely a key evolutionary adaptation for optimal organismal development.
Materials and Methods
Cell Culture
Human embryonic stem cell lines H9 (WA09) and H7 (WA07), H1 (WA01) were obtained from WiCell Research Institute (Wisconsin). CHB6 hES cells were obtained from Dr. George Daley (Children’s Hospital, Boston). Cells were seeded as undifferentiated colonies on plates coated with fibronectin (Sigma), maintained at 37°C and 5% CO2, and fed mouse embryo fibroblasts (MEF) conditioned human ES culture medium plus bFGF daily. For CHB6 hES cells and certain experiments that required larger number of cells, hES cells were seeded on matrigel and fed daily with mTeSR media (Stem Cell Technologies). Differentiation of hES cells was induced by plating 4-day-old colonies onto non-adherent plates without fibronectin and fed non-conditioned hES cell media daily. All experiments with hES cells were conducted with the H9 cell line. As indicated, the experiment in Figure S3C was conducted in the H7 cell line.
The human lung fibroblast cells (CCL-151), HEK293T cells, and HeLa cells were received from ATCC and maintained and cultured as recommended by the supplier in modified Ham’s F12 (F-12K) medium supplemented with 15% FBS (CCL-151) and Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 1% L-glutamine (HEK293 T cells). Human dermal fibroblasts (HDF UNCH1) were received from the Tissue Culture Facility at UNC and maintained in DMEM/F12 + 5% FBS.
Cell Treatments
All treatments were performed on day 4–5 for hES cells or at 80% confluency for fibroblasts. Etoposide (Sigma) was added at a final concentration of 20 μM to hES cells and fibroblasts unless otherwise stated. Staurosporine (Sigma) was added to a final concentration of 200 nM. The pan-caspase inhibitors Z-VAD-FMK (MP Biomedicals) and Q-VD-OPH (SM Biochemicals) were added to the cells at a concentration of 50 μM (Z-VAD) and 25 μM (QVD-OPH). Cycloheximide (Sigma) was added at a final concentration of 1 μg/ml for 5 hrs. The cell impermeable dyes propidium iodide and sytox green (Molecular Probes) were added to hES cells at a final concentration of 1 μg/ml (propidium iodide) and 10 nM (sytox green) and incubated at 37ºC in the dark for 20 min. Membrane permeable peptides, negative control and Bax inhibiting peptide (BIP) were purchased from Calbiochem. The stock solutions were prepared in DMSO and the peptides were added directly into medium (final concentration: 200 μM) at 37 °C 1hr before apoptosis treatments. ABT-737 was purchased from Selleck Chemicals LLC (Houston TX, USA) and stock solutions were prepared in DMSO. hES cells were treated with etoposide (20 μM) for 5 hours and HeLa cells for 16–24 hours. Cell survival was assessed at multiple time points by morphology (by counting the total number of cells attached with intact morphology in a defined area at each timepoint). Wherever indicated, cell death was also quantitated by counting apoptotic nuclei (condensed or fragmented) stained with Hoechst Staining or with propidium iodide dye exclusion. Golgi was disrupted using Brefeldin A (5 μg/ml; Sigma) for 15 minutes at 37ºC. All experiments were repeated at least 3 times and error bars indicate standard deviation.
Lentiviral Transduction
To generate lentiviral-mediated knock down lines of hES cells, day 3 hES cell colonies were transduced with p53, Bax or Bak lenti-shRNAs (Open Biosystems) for 10 hours. After 10 hours, the cells were allowed to recover for 12–14 hours in fresh conditioned medium. Selection with puromycin (10 μg/ml; Sigma) was started 24 hours after the initiation of the transduction and was continued for 4 days, with replacement of the conditioned medium and puromycin every day. Transduction efficiency was validated by Western blotting on healthy, recovered hES cell colonies.
Transfection, Immunofluorescence, and Immunoprecipitation
To visualize GFP-tagged Bax, 3-day colonies of hES cells were transfected with 2 μg of hBaxC3-EGFP (Addgene) with FuGENE HD transfection reagent (Roche). EdU labeling and detection was performed using a flow cytometry kit according to manufacturer’s instructions (Click-iT EdU Alexa Fluor 594, Invitrogen, Carlsbad, CA). DAPI (2 μg/ml; Sigma, St. Louis, MO) was used to measure the DNA content. Samples were subjected to FACS analysis as measured on a Dako CyAN ADP instrument. For immunofluorescence, cells were fixed with 4% paraformaldehyde for 30 min at 4ºC and permeabilized in 0.3% Triton X-100 (or 0.04% Saponin in Supplementary Fig. S3B) for 1 hr at room temperature. After blocking, cells were then treated with primary and secondary antibodies using standard methods. Immunoprecipitation experiments were performed in order to detect the active form of Bax (using CHAPS buffer), the acetylation status of Ku70 (using Triton X-100 buffer), and the interaction status between Bax and Ku70 (using CHAPS buffer).
Subcellular Fractionation
To separate the Golgi from the mitochondrial fraction we performed subcellular fractionation as previously described (Jesch and Linstedt, 1998). Briefly, hES cells were collected by centrifugation (2,000 rpm) for 5 min, washed once in PBS (containing 1 mM EDTA), washed once in sucrose buffer (250 mM sucrose, 10 mM triethylamine pH 7.4, 1 mM EDTA, with protease inhibitors), homogenized by 20 passages through a 25-gauge needle in 0.5 ml homogenization buffer (50 mM NaCl, 10 mM triethylamine, pH 7.4, 1 mM EDTA, with protease inhibitors), and centrifuged at 1,000 × g for 2 min. The postnuclear supernatant was adjusted to 1.6 M sucrose (1.1 ml), layered on 2 M sucrose (0.5 ml), covered with three sucrose layers (1.1 ml each: 1.4 M, 1.2 M, 0.8 M), and centrifuged at 105,000 × g for 105 min in a MLS-50 rotor (Beckman), and 0.4-ml fractions were collected from the top. All steps were carried out at 0–4°C. Immunoblotting was used to assay each fraction for the presence of Bax (N20), Golgi (TGN46) and mitochondria (VDAC).
Image acquisition
Images were acquired using a Hamamatsu ORCA-ER digital B/W CCD camera mounted on a Leica inverted fluorescence microscope (DMIRE 2). The software used for image acquisition was Metamorph version 5.0 (Universal Imaging Corporation). Confocal images were taken on an Olympus FV1000 confocal microscope.
Additional detailed description of the methods is included in the Supplementary Information.
Supplementary Material
01
Highlights.
- hES cells have constitutively active Bax and are highly sensitive to DNA damage
- Constitutively active Bax is localized to the Golgi in hES cells
- DNA damage triggers a p53 dependent translocation of Bax from Golgi to mitochondria
- hES cell differentiation changes status of active Bax and sensitivity to DNA damage
Acknowledgments
We thank members of the Deshmukh Lab for helpful discussions and critical review of this manuscript. We also thank Dr. Adam Listedt, Dr. Terry Magnuson, Dr. Vladmir Ghukasyan and Dr. Lucy Williams for technical advice. This work was supported by NIH grants GM078366 (to MD) and UNC’s University Cancer Research Fund (to MD). RD was supported by the Helen Lyng White Fellowship. VG was supported by the American Heart Association and American Brain Tumor Association postdoctoral fellowships. hES cells were maintained in the UNC Human Embryonic Stem Cell Core Facility. Confocal imaging was supported by Core 5 of NINDS Center Grant P30 NS04892.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- Ardehali R, Inlay MA, Ali SR, Tang C, Drukker M, Weissman IL. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc Natl Acad Sci USA. 2011;108:3282–3287. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Chen K, Gao YX, Arzigian M, Xie YL, Malcosky C, Yang YG, Wu WS, Wang ZZ. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res. 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno C, Catalina P, Melen GJ, Montes R, Sanchez L, Ligero G, Garcia-Perez JL, Menendez P. Etoposide induces MLL rearrangements and other chromosomal abnormalities in human embryonic stem cells. Carcinogenesis. 2009;30:1628–1637. doi: 10.1093/carcin/bgp169. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 Family Reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, KJB, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C-terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- Dewson G, Kluck RM. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci. 2009;122:2801–2808. doi: 10.1242/jcs.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland Megan M, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle Richard J. Bcl-xL Retrotranslocates Bax from the Mitochondria into the Cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:586–592. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxbeta: a constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Gavathiotis E, Walensky LD. Tracking BAX once its trigger is pulled. Cell cycle. 2011:10. doi: 10.4161/cc.10.6.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27(Suppl 1):S128–136. doi: 10.1038/onc.2009.50. [DOI] [PubMed] [Google Scholar]
- Gomez JA, Gama V, Yoshida T, Sun W, Hayes P, Leskov K, Boothman D, Matsuyama S. Bax-inhibiting peptides derived from Ku70 and cell-penetrating pentapeptides. Biochem Soc Trans. 2007;35:797–801. doi: 10.1042/BST0350797. [DOI] [PubMed] [Google Scholar]
- Grandela C, Pera MF, Wolvetang EJ. p53 is required for etoposide-induced apoptosis of human embryonic stem cells. Stem Cell Res. 2008;1:116–128. doi: 10.1016/j.scr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol- to- membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem Cells, the Molecular Circuitry of Pluripotency and Nuclear Reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch SA, Linstedt AD. The Golgi and Endoplasmic Reticulum Remain Independent during Mitosis in HeLa Cells. Mol Bio Cell. 1998;9:623–635. doi: 10.1091/mbc.9.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yokota T, Gama V, Yoshida T, Gomez JA, Ishikawa K, Sasaguri H, Cohen HY, Sinclair DA, Mizusawa H, et al. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ. 2007;14:2058–2067. doi: 10.1038/sj.cdd.4402219. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Momčilović O, Choi S, Varum S, Bakkenist C, Schatten G, Navara C. Ionizing Radiation Induces Ataxia Telangiectasia Mutated-Dependent Checkpoint Signaling and G2 But Not G1 Cell Cycle Arrest in Pluripotent Human Embryonic Stem Cells. Stem Cells. 2009;27:1822–1835. doi: 10.1002/stem.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Odorico JS, Kaufman DS, Thomson JA. Multilineage Differentiation from Human Embryonic Stem Cell Lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, et al. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–5852. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Stambrook PJ, Tichy ED. Preservation of Genomic Integrity in Mouse Embryonic Stem Cells. In: Meshorer E, Plath K, editors. The Cell Biology of Stem Cells. Springer; US: 2010. pp. 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Gavathiotis E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem Sci. 2011;36:642–652. doi: 10.1016/j.tibs.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, Gama V, Okuno M, Chen Y, Abe Y, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun. 2004;321:961–966. doi: 10.1016/j.bbrc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01