Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas (original) (raw)
Abstract
Basal-like breast carcinoma is characterized by the expression of basal/myoepithelial markers, undifferentiated phenotype, highly aggressive behaviour and frequent triple negative status (ESR−, PR−, Her2neu−). We have previously shown that epithelial–mesenchymal transition (EMT) occurs in basal-like breast tumours and identified Lysyl-oxidase-like 2 (LOXL2) as an EMT player and poor prognosis marker in squamous cell carcinomas. We now show that LOXL2 mRNA is overexpressed in basal-like human breast carcinomas. Breast carcinoma cell lines with basal-like phenotype show a specific cytoplasmic/perinuclear LOXL2 expression, and this subcellular distribution is significantly associated with distant metastatic incidence in basal-like breast carcinomas. LOXL2 silencing in basal-like carcinoma cells induces a mesenchymal-epithelial transition (MET) associated with a decrease of tumourigenicity and suppression of metastatic potential. Mechanistic studies indicate that LOXL2 maintains the mesenchymal phenotype of basal-like carcinoma cells by a novel mechanism involving transcriptional downregulation of Lgl2 and claudin1 and disorganization of cell polarity and tight junction complexes. Therefore, intracellular LOXL2 is a new candidate marker of basal-like carcinomas and a target to block metastatic dissemination of this aggressive breast tumour subtype.
Keywords: basal-like carcinomas, breast cancer, EMT, LOXL2, metastasis
INTRODUCTION
Breast carcinomas represent a highly heterogeneous tumour family in which different subtypes have been established. To date, histological grading, lymph node dissemination, hormone receptor status, expression of ErbB2neu and some additional markers are the most relevant criteria used to clinically classify breast carcinomas (Tavassoli & Deville, 2003). Nevertheless, the present classification does not allow discrimination of patients with increased metastatic and/or local recurrence risk or those that develop therapeutic resistance. Results of gene profiling studies performed during the last decade have allowed a new molecular classification of breast tumours with potential clinical impact for breast cancer diagnosis and personalized treatment. The molecular classification of breast tumours include: (i) luminal type (luminal A, luminal B subtypes) characterized by the expression of luminal markers and estrogen (ESR) and/or progesterone (PR) receptors; (ii) ErbB2neu type, exhibiting overexpression of the neu oncogene and other related genes; (iii) the normal-like group considered very similar to non-tumoural tissue, and (iv) basal-like type (Perou et al, 2000; Sorlie et al, 2001, 2003). Basal-like tumours are characterized by the expression of basal/myoepithelial markers (including cytokeratin 5/6, epidermal growth factor receptor and p63, among others) and they are frequently negative for ESR, PR and ErbB2neu expression (triple negative) (Nielsen et al, 2004; Rakha et al, 2007). Studies in a large collection of breast carcinoma cell lines have confirmed the molecular classification and extended it into two further sub-classes: the basal A subclass, with a mixed luminal/basal phenotype and the basal B subclass exhibiting mesenchymal-like characteristics (Blick et al, 2008; Charafe-Jauffret et al, 2006; Neve et al, 2006). Recently, a new tumour subtype, known as claudin-low tumours, has been described, which apparently discriminates metaplastic breast cancers from other breast tumour subtypes. These tumours are characterized by low expression of claudin 4 and 7 and high levels of stem cell markers (CD44high/CD24low) (Hennessy et al, 2009).
Epithelial–mesenchymal transition (EMT), an essential process in development, has been established as a key event for dissociation of carcinoma cells from the primary tumours concomitant to the acquisition of cell migration that provides tumour cells with the ability to invade into the adjacent tissues (Thiery et al, 2009). In addition, EMT is considered to be relevant for other stages of the metastatic cascade such as intravasation or extravasation (Polyak & Weinberg, 2009). EMT is currently considered as a focal and transient event occurring at specific tumour regions and being perhaps more relevant to specific tumour types (Peinado et al, 2007; Polyak & Weinberg, 2009; Thiery et al, 2009).
Several transcription factors have been described during the past decade as EMT inducers, including members of the Snail, bHLH and ZEB families (Peinado et al, 2007; Moreno-Bueno et al, 2008). A large amount of information has been accumulated on the plethora of signaling pathways that modulate their expression both in development and tumour progression (Peinado et al, 2007; Thiery et al, 2009). Significantly, post-transcriptional regulatory mechanisms of EMT inducers have also been uncovered, including miRNA and non-coding RNA regulation of ZEB factors (Brabletz & Brabletz, 2010; Cano & Nieto, 2008) as well as post-translational modifications of Snail1 influencing protein stability and repression function (MacPherson et al, 2010; Yook et al, 2005; Zhou et al, 2004). Indeed, we have previously described that Snail1 is regulated by lysyl oxidase-like 2 (LOXL2), which increases Snail1 protein levels contributing to E-cadherin repression and EMT induction (Peinado et al, 2005). LOXL2 is a member of the lysyl oxidase (LOX) family, constituted by five members, the prototypical LOX and four related members, lysyl oxidase-like (LOXL1-4). All LOX members share a highly conserved carboxy terminal catalytic domain required for the oxidative deamination of peptidyl-lysine residues in substrates to generate reactive aldehyde groups that initiate covalent inter and intramolecular crosslinks (Csiszar, 2001; Lucero & Kagan, 2006). LOX and LOXL1 are required for proper extracellular matrix assembly and homeostasis and cardiovascular system development (Maki et al, 2002; Liu et al, 2004). LOX and the LOX-like proteins have also been implicated in tumourigenesis (Lucero & Kagan, 2006; Maki, 2009; Payne et al, 2007) and a relevant role for LOX in establishment of the pre-metastatic niche has been recently described (Erler et al, 2009). LOXL2 has been previously recognized for its role in invasion of breast carcinoma cells (Akiri et al, 2003; Hollosi et al, 2009; Kirschmann et al, 2002) and its transcripts are associated with poor clinical outcome in lymph-node negative (N0) breast adenocarcinomas (Peinado et al, 2008) and with metastasis in estrogen receptor negative breast tumours (Barker et al, 2011). However, the role of LOXL2 in specific subtypes of breast carcinomas has not yet been established.
In this study, we show that LOXL2 is overexpressed in basal-like breast carcinomas. Analyses in a collection of human breast carcinoma cells demonstrated that LOXL2 is only expressed in cell lines with a basal-like phenotype in a cytoplasmic and perinuclear pattern. Interestingly, this subcellular distribution of LOXL2 is significantly associated with increased metastasis incidence in human basal-like carcinomas in a large series of grade 3 breast tumours. Indeed, the loss-of-function studies indicate that LOXL2 expression is required for cell invasion, tumour growth and lung metastasis of basal-like breast carcinoma cells. Interestingly, the action of LOXL2 in basal carcinoma cells is apparently independent of Snail1 and E-cadherin modulation, but affects the expression and organization of tight junction and cell polarity complexes by a novel mechanism involving transcriptional downregulation of cell polarity (Lgl2) and tight junction (claudin1) genes. These data strongly support the participation of intracellular LOXL2 in the maintenance of the mesenchymal phenotype and metastatic potential of basal-like carcinomas. Importantly, the results presented here indicate that LOXL2 is a new marker and a potential therapeutic target of metastatic basal-like breast tumours.
RESULTS
Gene expression analyses of grade 3 breast carcinomas identify LOXL2 as a marker of basal-like tumours
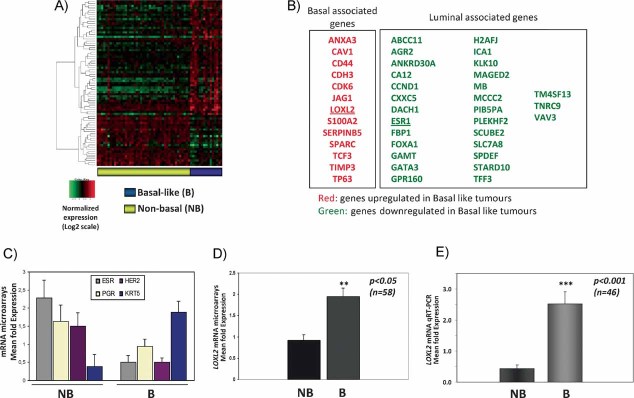
To identify the molecular signature associated to highly aggressive breast tumours an expression profile analysis was performed in a sample of 58 grade 3 infiltrative ductal carcinomas (IDC). After processing the arrays data, unsupervised hierarchical clustering sub-classified the samples into two clusters. The main cluster included 43 tumours that express ESR and PR receptors, some luminal markers and/or Her2neu indicating that this cluster included the luminal and Her2neu tumours and was defined as a non-basal breast carcinoma cluster (Supporting Information Fig S1A). The second cluster contained 15 tumours lacking expression of hormone receptors and the Her2neu oncogene, thus representing the basal-like tumours (Supporting Information Fig S1A). A supervised analysis identified a set of 311 genes able to classify the non-basal and basal-like tumours with a FDR <0.2 (Fig 1A). Around 23% of the genes represent genes previously characterized by their expression in luminal and basal/mesenchymal breast cancer cells (Charafe-Jauffret et al, 2006; Neve et al, 2006) (Fig 1B; Supporting Information Table S1). The basal-like tumours showed very low levels of ESR, PR and Her2neu transcripts as compared to the mean values obtained for the non-basal tumours (Fig 1C), indicating that the basal-like cluster indeed contains the triple negative tumours. In addition, the basal-like tumours showed upregulated expression of typical basal genes, like cytokeratins 5/6 (Fig 1C) as well as caveolin1 and p63 (Fig 1B; Supporting Information Table S1). To evaluate the biological relevance of the identified “basal-like breast signature” (BBS), we tested for its presence in several public breast cancer databases and found that it defines the basal-like tumours in the van 't Veer et al (2002) and Wang et al (2005) series, as well as basal breast cell lines in the Charafe-Jauffret et al (2006) series (Supporting Information Fig S1B).
Figure 1. LOXL2 mRNA is upregulated in basal like carcinoma tumours.
- Supervised analysis of microarray expression profile of grade 3 breast carcinomas (n = 58) with a FDR ≤ 0.2. Tumours were classified in two groups: basal-like (B, n = 15, right column, purple) and non-basal (NB, n = 43, left column, yellow).
- Representative selection of genes differentially upregulated (red) and downregulated (green) in basal-like tumours. The previous identification of the genes expressed in basal and luminal breast carcinomas (Charafe-Jauffret et al, 2006) is indicated at the top.
- Mean expression values of ESR, PGR, ErbB2 (HER2), and cytokeratin 5 (KRT5) mRNAs from the microarray data in basal and non-basal groups.
- Mean expression value of LOXL2 mRNA detected in the microarray analysis in non-basal and basal-like tumours.
- Quantitative RT-PCR analyses of LOXL2 expression in breast carcinomas (n = 46) identified greater than fivefold increased expression in basal (n = 18) versus non-basal (n = 28) tumours.
We have previously reported that EMT occurs in basal-like breast tumours (Sarrio et al, 2008) and identified LOXL2 as an EMT regulator (Peinado et al, 2005). In agreement with this, we found that LOXL2 mRNA was upregulated in the basal-like cluster (Fig 1B) with a mean of around twofold increased expression over the non-basal tumours (Fig 1D). qRT-PCR analysis of LOXL2 performed in 46 tumours (28 with non-basal and 18 with basal-like phenotype) confirmed that LOXL2 expression was increased in basal-like tumours by a mean of 5.0-fold compared to non-basal tumours (Fig 1E). Interestingly, analyses of LOXL2 expression in public databases indicated that overexpression of LOXL2 mRNA associates with basal-like breast tumours and/or to distant metastasis in several independent series (Supporting Information Fig S2A and B). This finding is also in agreement with our previous observation that LOXL2 mRNA overexpression is associated with decreased overall survival in N0 breast tumours (Peinado et al, 2008) and with a recent report showing increased LOXL2 mRNA associated with metastasis in ER- breast tumours from the NKI database (Barker et al, 2011).
LOXL2 expression in breast carcinoma cell lines
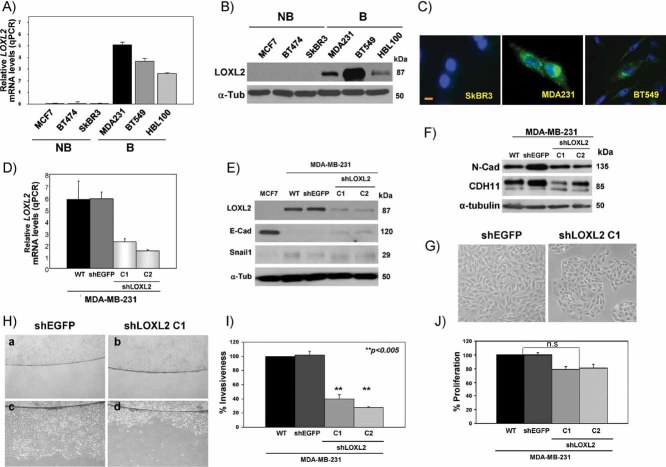
Once shown that LOXL2 was preferentially expressed in basal-like tumours, we analysed a series of breast carcinoma cell lines representing basal, luminal and ErbB2+ subtypes (Charafe-Jauffret et al, 2006; Neve et al, 2006) and found that LOXL2 mRNA expression was only detected in the basal-like carcinoma cell lines MDA-MB-231, BT549 and in the non-tumourigenic human mammary HBL100 (Fig 2A); the cell lines that lacked ESR/PR/Her2neu expression and exhibited a mesenchymal phenotype (Blick et al, 2008; Charafe-Jauffret et al, 2006; Neve et al, 2006). In contrast, no detectable levels of LOXL2 transcripts were present in the luminal (MCF7) and Her2neu+ (SkBR3, BT474) breast carcinoma cell lines (Fig 2A). Western blot analyses confirmed the expression of LOXL2 in the basal-like cell lines (Fig 2B) and immunofluorescence analyses showed the intracellular localization of LOXL2 with an intense punctuate cytoplasmic and/or perinuclear pattern in basal carcinoma cells (Fig 2C) as we have previously described in other cell systems (Peinado et al, 2005, 2008). As expected, no expression of LOXL2 was detected by immunofluorescence in non-basal carcinoma cells (Fig 2C; and data not shown).
Figure 2. LOXL2 is expressed in basal-like breast cell lines and its silencing induces MET.
- Quantitative RT-PCR analyses of LOXL2 expression in the indicated breast cell lines; high expression levels were detected in basal breast MDA-MB-231, BT549 and HBL100 cells; no LOXL2 transcripts were detected in luminal MCF7 and ErbB2+ BT474 and SkBR3 cells.
- Western blot of whole cell extracts for LOXL2 expression in the indicated cell lines. B, basal; NB, non-basal.
- Immunofluoresce staining for LOXL2 in the indicated cell lines; see the cytoplasmic and perinuclear stain in basal carcinoma cells and the absence of stain in non-basal SkBR3 cells. Bar, 50 µm.
- Quantitative RT-PCR analyses of LOXL2 expression in parental (wt), control (shEGFP) and two independent clones (C1, C2) generated after transfection of shLOXL2 in MDA-MB-231 cells.
- Western blots for LOXL2, E-cadherin (E-Cad) and Snail1 expression in parental MDA-MB-231 cells and in shEGFP- and shLOXL2-derived clones. MCF7 cells were included as a negative and positive control for LOXL2, Snail1 and E-cadherin expression, respectively.
- Western blot for N-cadherin and cadherin11 in the indicated cell lines. α-tubulin (α-Tub) was used as a loading control in (B), (E) and (F).
- Phase-contrast images of MDA-MB-231-shEGFP and -shLOXL2 (clone C1) cells: ×10 magnification.
- Cell motility of MDA-MB-231-shEGFP and -shLOXL2 (clone C1) cells analysed by a barrier assay. Images were taken at 0 h (a, b) and 48 h (c, d).
- Invasion assay on collagen type IV of parental MDA-MB-231 (WT) cells and shEGFP- and shLOXL2-derived clones; results represent the mean of two experiments performed in triplicate samples; bars, standard deviation (s.d.)
- Proliferation assay by incorporation of BrdU of parental MDA-MB-231 cells and shEGFP and shLOXL2-derived clones; results represent the mean of two experiments performed in quadruple samples; bars, s.d; n.s, not statistically significant.
LOXL2 silencing in basal carcinoma cell lines reduces invasiveness and cell motility
To obtain further insights into the role of LOXL2 in basal-like carcinomas, we generated stable basal breast carcinoma MDA-MB-231 cells in which LOXL2 was silenced. The MDA-MB-231-shLOXL2 clones showed significantly reduced LOXL2 mRNA (Fig 2D) and LOXL2 protein levels (Fig 2E), without significant changes in LOXL4 (Supporting Information Fig S3A), the other LOXL member expressed by MDA-MB-231 cells (Peinado et al, 2005). MDA-MB-231-shLOXL2 cells exhibited a more epithelial phenotype compared to control MDA-MB-231-shEGFP cells (Fig 2G). However, only a very modest re-expression of E-cadherin was detected by Western blot after LOXL2 silencing (Fig 2E). No expression of P-cadherin could be detected (data not shown) but MDA-MB-231-shLOXL2 cells maintained noticeable protein levels of N-cadherin and cadherin 11 (Fig 2F). On the other hand, Snail1 protein levels were not significantly modified in MDA-MB-231-shLOXL2 cells compared to controls (Fig 2E). Importantly, silencing of LOXL2 resulted in a marked decrease in migratory ability and motility of MDA-MB-231 cells as determined by barrier (Fig 2H) and wound healing assays (data not shown). Furthermore, LOXL2 silencing resulted in a highly significant decrease of the invasion capacity of MDA-MB-231 cells (Fig 2I) while their proliferation in bi-dimensional cultures was not significantly affected (Fig 2J), in agreement with a recent report (Barker et al, 2011).
To ascertain whether the marked effect of LOXL2 silencing on the phenotype and migratory capacity was restricted to MDA-MB-231 cells or rather represents a general effect on basal cells, stable knockdown of LOXL2 was also performed in BT549 and HBL100 basal cell lines (Supporting Information Fig S3B). Again, LOXL2 silencing in both of these cell lines resulted in a marked reversion towards the epithelial phenotype that was particularly evident in high density cultures of BT549-shLOXL2 cells (Supporting Information Fig S3C). LOXL2 silencing also led to a dramatic inhibition of cell motility in BT549 and HBL100 cells (Supporting Information Fig S3D and E) and suppression of invasiveness in BT549 cells (Supporting Information Fig S4C). Noteworthy, no re-expression of E-cadherin was detected in BT549 or HBL100 cells after LOXL2 silencing (Supporting Information Fig S3B), but BT549-shLOXL2 cells maintained noticeable N-cadherin and cadherin11 levels (Supporting Information Fig S4A). Moreover, BT549 cells do not express endogenous Snail1 and remained unchanged after LOXL2 silencing (Supporting Information Fig S4A). In addition, reorganization of F-actin and downregulation of vimentin is observed after LOXL2 silencing in BT549 cells (Supporting Information Fig S4B). These results indicate that LOXL2 silencing induces a MET-like process in basal cell lines associated with decreased invasive and migratory properties. They are also in concordance with our previous observation and those by other groups showing that ectopic expression of LOXL2 in luminal MCF7 cells induces a mesenchymal-like phenotype and migratory and invasive potential (Fig 3B; Akiri et al, 2003; Hollosi et al, 2009). The present results support that LOXL2 is involved in the maintenance of the mesenchymal phenotype in basal carcinoma cells.
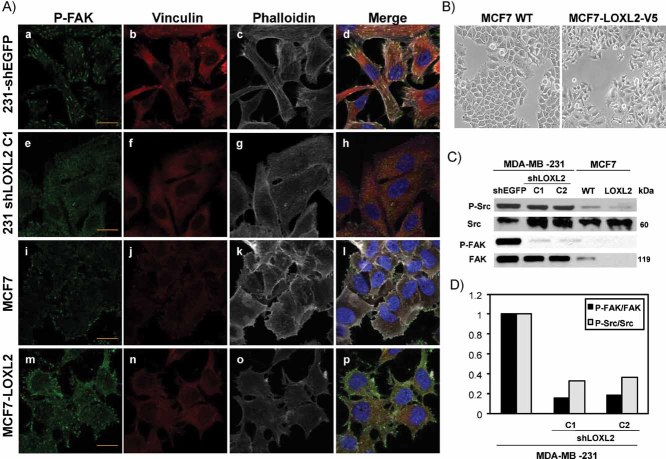
Figure 3. LOXL2 induces FAK activation and organization of focal contacts.
- Confocal immunofluoresce images for P-FAKY397 (green), vinculin (red) and F-actin (white) organization in control MDA-MB-231-shEGFP (a–d), -shLOXL2 (clone C1) (e–h), parental MCF7 (i–l) and MCF7-LOXL2 (m–p) cells. Merged images are shown at the right panels. Bars, 50 µm.
- Phase contrast images of parental MCF7 (left) and MCF-LOXL2-V5 (right) cells.
- Western blot analyses of Src and FAK activation and total expression levels in the indicated cell lines. α-tubulin (α-tub) was used as a loading control.
- Ratio of P-Src/Src and P-FAK/FAK levels from densitometry analysis of the blot shown for MDA-MB-231 cells in (C). Similar results were obtained in two independent experiments.
LOXL2 modulates focal adhesion, tight junctions and cell polarity complexes in a dual fashion in basal carcinoma cells
Previous studies have reported the positive modulation of FAK and Src pathways by LOXL2 in gastric carcinoma cells (Peng et al, 2009), while a negative modulation of the FAK pathway has been reported for the LOX pro-peptide in breast carcinoma cells (Zhao et al, 2009). We analysed the influence of LOXL2 on FAK and Src pathways and focal adhesion organization after LOXL2 silencing in MDA-MB-231 cells, in parallel with analyses in the gain of function system, MCF7-LOXL2-V5 cells (Fig 3B), we recently described (Hollosi et al, 2009). Control MDA-MB-231 cells presented high levels of activated P-FAK and P-Src that were reduced in MDA-MB-231-shLOXL2 cells (Fig 3C and D); on the contrary, control MCF7 cells showed undetectable P-FAK and very low levels of P-Src that were not apparently changed in MCF7-LOXL2 cells (Fig 3C). The influence of LOXL2 on focal contact organization was then analysed by confocal immunofluorescence of P-FAK, vinculin and F-actin (Fig 3A). Control MDA-MBA-231 cells showed prominent focal contacts on membrane extensions resembling filopodia as well as on the lower cell surface area with clear co-localization of P-FAK, vinculin and F-actin bundles (Fig 3A, a–d). In striking contrast, MDA-MBA-231-shLOXL2 cells exhibited low and diffuse staining of P-FAK and vinculin, and showed very few and small focal contacts organized apparently only at the cell periphery (Fig 3A, e–h). In agreement with their epithelial phenotype, MDA-MBA-231-shLOXL2 cells also showed strongly decreased F-actin stress fibres and apparent reorganization to cortical F-actin as compared to control cells (Fig 3A, compare panels c and g). Interestingly, focal adhesion and F-actin organization of MCF7 cells was alike to that of MDA-MBA-231-shLOXL2 cells (Fig 3A, i–l), while MCF7-LOXL2 cells showed increased focal contact organization at cell membrane extensions resembling filopodia (Fig 3A, m–p), thus being more similar to the pattern observed in control MDA-MBA-231 cells. Additional analyses of P-Src and focal contact organization corroborated these findings (Supporting Information Fig S5). These results indicate that LOXL2 positively modulates the FAK and Src signaling pathways and focal contact organization of basal-like carcinoma cells that can contribute to the cell migratory behaviour.
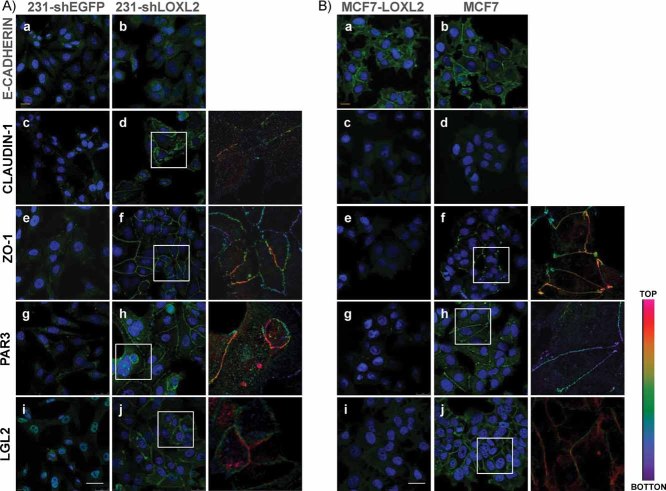
The striking induction of MET by LOXL2 silencing in basal-like carcinoma cells led us to analyse in further detail the expression/organization of cell junctions. As demonstrated above, the cell phenotype induced by LOXL2 silencing in basal-like carcinoma cells was apparently independent of E-cadherin (Fig 2E, Supporting Information Fig S3B). Indeed, confocal analyses indicated that the low E-cadherin protein levels detected in MDA-MBA-231-shLOXL2 cells were not properly organized at cell–cell contacts in most adherent cells (Fig 4A, a,b). In contrast, MCF7-LOXL2 cells, although showing reduced E-cadherin levels (Supporting Information Fig S6C), organized E-cadherin at cell–cell contacts in most adherent cells in a pattern similar to that present in parental MCF7 cells (Fig 4B, a,b). The expression and organization of other adhesion complexes, in particular tight junctions, was then investigated in both cell systems. Western blot analyses indicated increased expression of claudin1 in MDA-MBA-231-shLOXL2 cells while the soluble ZO1 protein levels decreased slightly compared to parental cells (Supporting Information Fig S6A). On the other hand, strongly decreased expression of ZO1 was detected in MCF7-LOXL2 cells compared to parental MCF7 cells, while no expression of claudin1 was detected in parental or MCF7-LOXL2 cells (Supporting Information Fig S6A). Importantly, confocal immunofluorescence analyses showed that ZO1 and claudin1 were properly organized at cell–cell contacts at the upper lateral membrane in MDA-MBA-231-shLOXL2 cells (Fig 4A, d,f and insets) in a pattern strikingly similar to that present for ZO1 in MCF7 cells (Fig 4B, f and inset). In agreement with these observations, ZO1 and claudin1 were fully disorganized or not detected in MDA-MB-231-shEGFP and MCF7-LOXL2 cells (Fig 4A/B, c,e). Together, these data indicate a negative role for LOXL2 in the expression and organization of tight junction complexes of basal-like carcinoma cells.
Figure 4. LOXL2 silencing in basal carcinoma cells induces expression and reorganization of tight junctions and cell polarity complexes.
- A, B. Confocal immunofluorescence analyses in MDA-MB-231-shEGFP and -shLOXL2 (Clone 1) (A) and MCF7 and MCF-LOXL2 (B) cells for E-cadherin (a, b); claudin1 (c, d), ZO1, (e, f), Par3 (g, h) and Lgl2 (i, j). x_–_y maximal projections are shown for E-cadherin (A, B) and claudin1 (B) stain; z_–_x planes are shown in the rest of panels. Insets in panels d, f, h, j, indicate enlarged areas (right) showing positive immunostaining along the lateral membrane. Colour scale indicates the intensity of staining from basal (bottom, purple) to apical (top, red) localization. Bars, 50 µm.
Apico-basal polarity is an essential property of epithelial cells that is lost during EMT (Dow & Humbert, 2007; Moreno-Bueno et al, 2008). Several recent reports have shown the influence of EMT factors, like ZEB1 or Snail, in the transcriptional repression of cell polarity components (Aigner et al, 2007; Spaderna et al, 2008; Whiteman et al, 2008). To obtain further insights into the regulation of EMT mediated by LOXL2 in basal-like carcinoma cells, we next studied the expression and organization of cell polarity components. We choose to analyse Par3 and Lgl2 molecules as representative of the Par and Scribble cell polarity complexes, respectively (Dow & Humbert, 2007; Moreno-Bueno et al, 2008). The protein expression levels of Par3 and Lgl2 strongly increased in MDA-MBA-231-shLOXL2 compared to control cells, while they decreased significantly in MCF7-LOXL2 cells compared to parental cells (Supporting Information Fig S6B). More importantly, confocal immunofluorescence analyses showed weak cytoplasmic and/or nuclear stain of Par 3 and Lgl2, respectively, in control MDA-MBA-231-shEGFP cells (Fig 4A, g,i). Strikingly, both Lgl2 and Par3 stained strongly and organized at the upper lateral region in MDA-MBA-231-shLOXL2 cells (Fig 4A, h,j and insets). Interestingly, the reverse situation was observed in MCF7 cell system, in which the organized expression of Par3 and Lgl2 containing cell polarity complexes of parental cells (Fig 4B, h,j and insets) was fully lost in MCF7-LOXL2 cells (Fig 4B, g,i). These results strongly support that LOXL2 negatively modulates the expression and organization of cell polarity complexes in basal-like carcinoma cells.
The punctuate cytoplasmic and perinuclear stain of LOXL2 detected in basal breast cells (Fig 2C) suggested its association with the Golgi network and vesicle trafficking that could affect the transport and/or organization of cell–cell adhesion and polarity complexes. Confocal immunofluorescence analyses showed that intracellular LOXL2 associates to the trans-Golgi network as demonstrated by its colocalization with the GM130 Golgi marker in both MDA-MB-231 control and MCF7-LOXL2 cells (Supporting Information Fig S7). This latter analysis also indicated that the overall organization of the trans-Golgi network is not disturbed by the expression of LOXL2 or its silencing (Supporting Information Fig S7), thus discarding an overall effect of LOXL2 on intracellular Golgi trafficking.
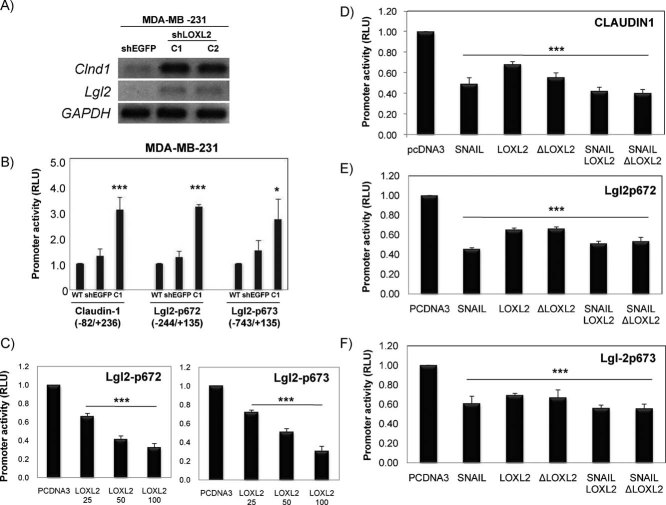
LOXL2 represses claudin1 and Lgl2 promoters independently of Snail1 and of its enzymatic activity
The above results led us to further investigate the mechanisms underlying LOXL2 mediated downregulation of tight junctions and cell polarity complexes. To this end, we chose claudin1 and Lgl2 as representative components of tight junctions and cell polarity complexes, respectively, whose expression has been previously shown to be downregulated by EMT factors, like Snail1/Snail2 and/or ZEB1 (Aigner et al, 2007; Martinez-Estrada et al, 2006; Ohkubo and Ozawa, 2004; Spaderna et al, 2008). RT-PCR analyses of claudin1 and Lgl2 transcripts showed an inverse association with LOXL2 expression in MDA-MB-231 cells (Fig 5A). We then speculated that LOXL2 could downregulate claudin1 and Lgl2 gene transcription. Promoter assays indicated that the activity of claudin1 and two independent Lgl2 promoters were upregulated in MDA-MBA-231-shLOXL2 cells compared to parental and control shEGFP cells (Fig 5B), supporting a negative influence of LOXL2. Indeed, transient promoter assays indicated that LOXL2 represses the activity of both Lgl2 promoter constructs in a dose-dependent fashion up to 60% (Fig 5C). The activity of the claudin1 promoter was also repressed by LOXL2 to about 40% (Fig 5D), a lower potency than that previously shown by Snail1 (up to 60%) (Fig 5D; Martinez-Estrada et al, 2006). Interestingly, the repressive action of LOXL2 on the claudin1 and Lgl2 promoters was independent of Snail1 and, surprisingly, also of the LOXL2 catalytic activity since a LOXL2 deletion mutant lacking the catalytic domain (ΔLOXL2; aa 548–688 deleted) showed the same repressor potency as the wild type LOXL2 on either promoter (Fig 5D–F). Taken together, these data indicate that LOXL2 downregulates tight junction and cell polarity complexes in basal-like carcinoma cells at the transcriptional level by a mechanism independent of Snail1 and LOXL2 enzymatic activity.
Figure 5. Claudin1 and Lgl2 are transcriptionally repressed by LOXL2.
- A. RT-PCR of claudin1 and Lgl2 transcripts in the indicated cell lines. GAPDH is used as a loading control.
- B. Activity of claudin1 and Lgl2 promoters in parental MDA-MB-231 cells (WT) and in control shEGFP and shLOXL2 (clone C1) cells. The activity was determined as relative luciferase units (RLU) and normalized to the activity detected in WT cells for each promoter.
- C. Activity of the two Lgl2 promoters in HEK293T cells in the presence of the indicated amounts (ng) of LOXL2. The activity, as RLU, was normalized to that obtained in the presence of control pcDNA3 vector (100 ng).
- D.–F. Activity of claudin1 (D) and the two Lgl2 promoters (E, F) in the presence of the indicated expression vectors (50 ng). The activity, as RLU, was normalized to that obtained in the presence of control pcDNA3 vector (100 ng). Results in (B) to (F) represent the mean +/− s.d of three (B–D) and two (E, F) independent experiments performed in quadruple samples. (*p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001)
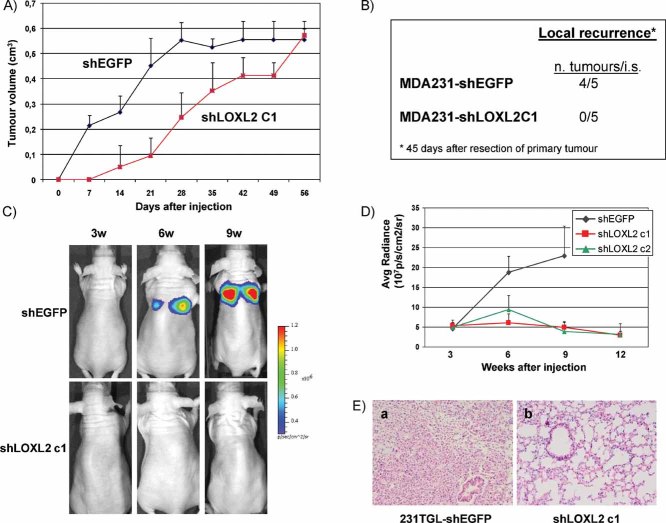
Silencing of LOXL2 reduces tumour growth and inhibits local recurrence and metastatic capacity of basal breast carcinoma cells
The former results demonstrated the participation of LOXL2 in basal-like carcinoma phenotype. To obtain further information about the biological function of LOXL2 in basal breast carcinomas, we orthotopically injected control MDA-MB-231-shEGFP and MDA-MB-231-shLOXL2 cells into the mammary fat pad of nude mice and analysed their tumourigenic potential. Control MDA-MB-231-shEGFP cells gave rise to tumours at all injection sites (4/4) reaching a volume of 0.4 cm3 20 days after injection (Fig 6A). In contrast, silencing of LOXL2 strongly reduced the tumour growth potential of MDA-MB-231 cells requiring 42 days to reach a similar tumour volume while the tumour incidence remained the same (4/4) (Fig 6A). No histological differences were detected between control and shLOXL2 xenografts (data not shown). The influence of LOXL2 silencing on the metastatic burden of MDA-MB231 cells was also analysed in spontaneous metastasis assays, by surgical removal of the primary tumours (1 × 106 cells/mammary fat pad) after they reached 0.4 cm3 volume. No lung metastasis could be detected in nude mice injected with either MDA-MB-231 control or -shLOXL2 after 4 months, in agreement with the low spontaneous metastatic capacity reported for the parental cell line (Minn et al, 2005; Olmeda et al, 2007; Price et al, 1990). Interestingly, however, local tumour recurrence was detected in mice injected with control MDA-MB231-shEGFP cells after 45 days of tumour resection but not in mice injected with MDA-MB231-shLOXL2 cells (Fig 6B), suggesting a role for LOXL2 in local tumour recurrence.
Figure 6. LOXL2 knockdown induces decreased tumourigenicity and inhibition of lung metastatic capacity of basal MDA-MB-231 cells.
- Comparative tumourigenic analysis of control MDA-MB-231-shEGFP and shLOXL2 (clone C1) cells. The indicated cell lines were injected into the mammary fat pad of female nude mice; tumour volume was measured every 4 days after injection. Data show the mean of four independent mice for each injected cell line; bars, s.d.
- Local recurrence determined by the appearance of tumours at the mammary gland region after 45 days of resection of primary mammary tumours.
- Representative bioluminescence images of mice intravenous (i.v.) injected with control MDA-MB-231TGL-shEGP (upper) and -shLOXL2 (clone C1) cells (bottom) obtained at 3, 6 and 9 weeks after tail vein injection. The colour scale represents the photon flux (photons per second) emitted from the lung region of xenografted mice.
- Bioluminescence (BLI) plots showing the quantification of the luminescence signal, represented as average radiance, emitted as a function of time from mice i.v. injected with control MDA-MB-231TGL-shEGP (black) and two independent shLOXL2 clones (c1, red; c2, green). Results represent the mean +/− s.d. obtained from six injected mice with each cell line.
- Representative images of hematoxilin and eosin staining of lung sections obtained from mice i.v. injected with MDA-MB-231TGL-shEGFP (a) and -shLOXL2 (b) cells; ×10 magnification.
Experimental metastasis assays were then performed by tail vein injections of control and MDA-MB231-shLOXL2 cells. To this end, LOXL2 was silenced in MDA-MB-231-TGL cells expressing luciferase and GFP (Minn et al, 2005) to allow for non-invasive bioluminescence analysis. MDA-MB231-TGL cells were characterized for efficient LOXL2 silencing (Supporting Information Fig S8A). MDA-MB231-TGL-shLOXL2 cells exhibited a marked epithelial phenotype albeit they lacked E-cadherin re-expression (Supporting Information Fig S8B and C) as observed for MDA-MB231-shLOXL2 cells (Fig 2E and G).
Silencing of LOXL2 resulted in a dramatic decrease of lung colonization of MDA-MB231-TGL cells (Fig 6C and D). Mice injected with control MDA-MB231-TGL-shEGFP cells started to show detectable luciferase activity in the lung region 3 weeks after intravenous injection that steadily increased for up to 9 weeks (Fig 6C and D) when the mice had to be sacrificed. In contrast, mice injected with MDA-MB231-TGL-shLOXL2 cells showed a dramatic suppression of lung colonization, as indicated by the lack of bioluminescent emission in the lung region up to 12 weeks after injection (Fig 6C and D). Mice injected with MDA-MB231-TGL-shLOXL2 cells survived in healthy conditions for up to 3 months after injection at which time they were sacrificed. Lung inspection at necropsy revealed macrometastasis in all samples derived from mice injected with control shEGFP cells, while mice injected with shLOXL2 cells were devoid of metastatic lung lesions (Fig 6E). These observations indicate that LOXL2 silencing in MDA-MB231 cells strongly impairs lung tumour homing and/or the efficient growth of the metastatic lung foci.
LOXL2 expression in breast cancer and its correlation with clinical and pathological data
To address the biological significance of LOXL2 in human breast cancer, we analysed a cohort of 195 formalin-embedded grade 3 infiltrative ductal breast carcinomas for LOXL2 expression (see Supporting Information Table S2, for clinical and molecular description of the tumour series). We used our home-made, previously characterized, polyclonal antiserum (herein called KC) (Fong et al, 2007; Hollosi et al, 2009). This study revealed intracellular LOXL2 expression in tumour cells with two different staining patterns, similar to those previously identified in human squamous cell carcinomas (Peinado et al, 2008): a diffuse and homogeneous cytoplasmic localization was found in 79.5% (Fig 7A, b; Supporting Information Table S2), and increased staining of LOXL2 in the cytoplasm and perinuclear region was detected in 20.5% of tumours (Fig 7A, c; Supporting Information Table S2). The normal mammary gland adjacent to the tumour area showed a faint cytoplasmic LOXL2 staining, which was absent in myoepithelial cells (Fig 7A, a). Interestingly, LOXL2 was not detected in normal and tumoural stroma in the present breast tumour series, in agreement with our own previous observations in other tumour types stained with the same antisera (Fong et al, 2007; Hollosi et al, 2009; Peinado et al, 2008). Other recent studies have reported the expression of extracellular LOXL2 in a reduced series of breast adenocarcinoma using a different, non-commercial, anti-LOXL2 antiserum (herein called Arresto) (Barry-Hamilton et al, 2010). We therefore also stained part of our breast tumour series (n = 84) with the latter antiserum detecting 47 cases (55.9%) with positive expression of LOXL2 (Supporting Information Table S2); specifically, intracellular LOLX2 localization was found in 12 cases (14.3% of total), and intracellular plus stromal distribution of LOXL2 observed in 35 biopsies (41.7%) (Supporting Information Fig S9A b,d; Supporting Information Table S2). Interestingly, almost 65% of the cases with intracellular LOXL2 localization correspond to basal carcinomas, a similar percentage as the cytoplasmic/perinuclear stain detected with our home-made antiserum (Supporting Information Fig S9B). These results corroborate the intracellular LOXL2 distribution in basal-like breast cancer.
Figure 7. LOXL2 expression is increased in basal-like tumours with a specific cytoplasmic/perinuclear staining pattern and associated to distant metastasis.
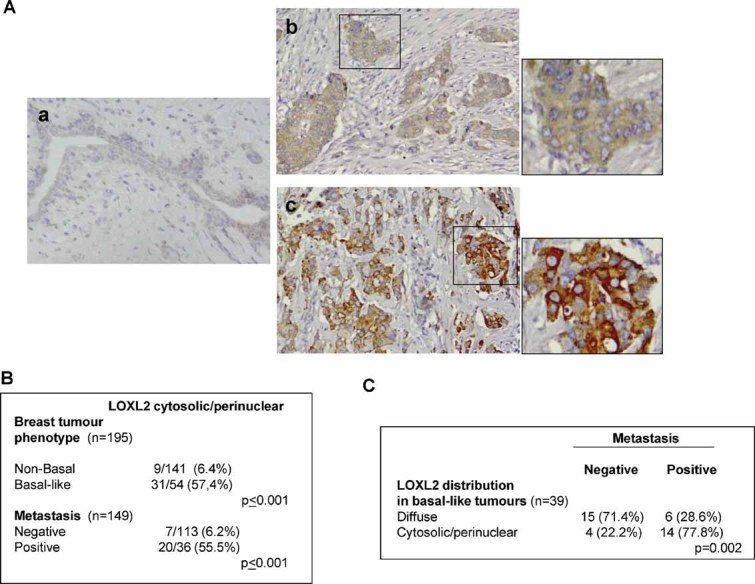
- Immunohistochemical analysis of LOXL2 in paraffin-embedded grade 3 IDC (n = 195). (a) Weak diffuse stain in normal mammary gland. (b) Diffuse cytosolic expression pattern in tumour cells; (c) intense cytosolic and perinuclear expression pattern in tumour cells; ×40 magnification. Insets in (b) and (c) show magnification (×160) areas (right). See the absence of expression in the normal and tumoural stroma.
- Correlation between LOXL2 cytoplasmic/perinuclear pattern and the basal-like phenotype and metastasis incidence of grade 3 IDC tumours.
- Correlation between LOXL2 stain pattern and metastastic incidence in basal-like breast carcinomas.
Basal-like breast tumours within the present tumour series were subclassified according to the established immunohistochemical (IHC) criteria for this tumour subtype (Nielsen et al, 2004; Rakha et al, 2007) (Supporting Information Fig S10 and Supporting Information Tables S2 and S3). Fifty-four cases (28%) were classified as basal-like breast tumours and the rest (n = 139, 72%) were considered non-basal tumours (Supporting Information Table S2). A highly significant statistical correlation was found between the intense cytoplasmic/perinuclear LOXL2 expression pattern detected with our antiserum and basal-like tumours (p ≤ 0.001; Fig 7B and Supporting Information Table S3). Comparison of LOXL2 expression with clinico-pathological parameters showed a significant correlation between the heterogeneous LOXL2 pattern and negative ESR and/or PR expression (p ≤ 0.001 and p = 0.003, respectively; Supporting Information Table S3). Furthermore, a statistical tendency was observed between the heterogeneous pattern of LOXL2 expression and high levels of the proliferation marker Ki67 (p = 0.073), while no correlation with p53 status was found (Supporting Information Table S3). The expression of E-cadherin and other established EMT markers in basal-like breast carcinomas (Sarrio et al, 2008) was also analysed. E-cadherin expression was detected in a high percentage of the analysed tumours (55 out of 92) and no association with LOXL2 expression was found (Supporting Information Fig S10 and Table S3). Perinuclear LOXL2 expression was associated with CK5, EFGR and cadherin-11 staining in 82.6% (p ≤ 0.001), 64% (p ≤ 0.001) and 62% (p = 0.045) of the cases, respectively (Supporting Information Fig S10 and Table S3). In addition, a statistical tendency was found between heterogeneous LOXL2 and SPARC expression (p = 0.068), further supporting the association of those markers with basal-like tumours. Importantly, LOXL2 cytoplasmic/perinuclear expression strongly correlated with distant metastasis incidence (p < 0.001; Fig 7B, Supporting Information Table S3). From the 54 basal-like tumours, data for metastasis and LOXL2 pattern were available for 39 samples. Noteworthy, 18 cases of the basal tumours showed intracellular/perinuclear LOXL2 localization and, among them, 14 (77.8%) were metastatic. On the other hand, only a small fraction of basal tumours that showed a diffuse LOXL2 pattern (6 out of 21, 28.6%) displayed distant metastasis. Considering that a similar number of basal lesions was analysed for the two LOXL2 staining pattern (18 cytoplasmic/perinuclear and 21 diffuse), we conclude that there is a clear connection between cytoplasmic/perinuclear LOXL2 staining, basal phenotype and metastasis risk.
DISCUSSION
Basal-like carcinomas are highly aggressive tumours with visceral metastasis, poor prognosis and a high tendency to develop chemoresistance (Foulkes et al, 2010; Nielsen et al, 2004; Reis-Filho et al, 2006; Rodriguez-Pinilla et al, 2006). Basal-like breast carcinomas are presently considered a heterogenous group that may include the recently identified claudin-low (Hennessy et al, 2009; Taube et al, 2010), metaplastic tumours (Reis-Filho et al, 2006), BRCA1-associated tumours and, at least part of the triple negative tumours (ER−/PR−/ErbB2neu−) (Foulkes et al, 2010). It is still presently undefined whether basal-like breast tumours originate from precursor cells distinct from those of other breast tumour types (Blick et al, 2010; Foulkes et al, 2010; Hennessy et al, 2009; Korsching et al, 2005; Shipitsin et al, 2007) or if different entities of basal-like tumours derive from different precursors, as recently proposed for the luminal origin of BRCA1-associated basal-like cancers (Lim et al, 2009; Molyneux et al, 2010). Unfortunately, the therapeutic targets for basal-like tumours have not been defined yet hindering the treatment of this specific breast tumour type. Interestingly, basal-like, as well as the claudin-low subtype and metaplastic breast tumours have been recently identified by us and others as likely candidates prone to undergo EMT processes (Hennessy et al, 2009; Sarrio et al, 2008; Taube et al, 2010).
In previous studies, we identified LOXL2 as an EMT regulator (Peinado et al, 2005) and defined LOXL2 as a prognostic marker in larynx squamous cell carcinomas (Peinado et al, 2008). In this work, we identify LOXL2 as a marker of basal-like breast tumours and provide functional evidence for its involvement in the invasion and metastatic dissemination of basal carcinoma cells. Gene profiling and qRT-PCR analysis of grade 3 IDC identified a basal-like gene signature that defines the basal-like tumours in independent public databases from N0 breast series and in a dataset from breast carcinoma cell lines (Supporting Information Fig S1B). Notably, LOXL2 mRNA was highly upregulated in the basal-like cluster identified in the present study (Fig 1) that we validated in several public databases (Supporting Information Fig S2A). Furthermore, the association of LOXL2 expression with basal-like breast tumours was confirmed in studies using breast cell lines showing that LOXL2 expression was only observed in those previously sub-classified as basal B/mesenchymal subtype with predominant mesenchymal gene expression and enhanced invasiveness (Blick et al, 2008; Charafe-Jauffret et al, 2006; Neve et al, 2006). Interestingly, LOXL2 was localized in a cytoplasmic and perinuclear pattern in basal cells, as we found in previous studies in LOXL2 overexpressing cells (Peinado et al, 2005).
Silencing of LOXL2 greatly decreases the migratory and invasive capacities of basal cell lines (MDA-MB-231 and BT549) as well as migration of HBL100 cells. These results confirm and extend the previously proposed pro-invasive role of LOXL2 derived from LOXL2 gain-of-function studies in luminal breast MCF7 cells (Akiri et al, 2003; Hollosi et al, 2009) and are also in agreement with recent reports of LOXL2 silencing in MDA-MB-435 and MDA-MB-231 carcinoma cells (Barker et al, 2011; Barry-Hamilton et al, 2010). Remarkably, in addition to inhibition of invasion, LOXL2 knockdown has a significant inhibitory effect on the tumour growth potential, local recurrence and, importantly, on the lung metastatic dissemination of MDA-MB-231 cells. The tumourigenic data obtained in the present study differ from those recently reported by Barker and colleagues in which no differences in tumour growth capacity were detected in MDA-MB-231 cells and even increased tumour growth was detected in mouse 4T1 breast carcinoma cells after LOXL2 silencing (Barker et al, 2011). The differences in MDA-MB-231 cells could be explained by the higher cell dose injected in the mammary fat pad (1 × 107) in the latter study that may erase or attenuate the differences in the tumourigenic behaviour we observed here between control and shLOXL2 cells after injecting a lower cell dose (1 × 106). On the other hand, the reported data on 4T1 cells could unfortunately not be corroborated, since in our hands endogenous LOXL2 transcripts were not detected in 4T1 cells obtained from the ATCC collection (data not shown), thus precluding further studies on the influence of LOXL2 silencing in these cells. In this regard, it is also worth mentioning that significant differences in tumour growth after inhibition or silencing of LOXL2 in other breast or gastric carcinoma cells have been recently reported (Barry-Hamilton et al, 2010; Peng et al, 2009). Despite the tumourigenic differences, our present data support the positive influence of LOXL2 in tumour recurrence and experimental lung metastasis of MDA-MB-231 cells (Fig 6B–D).
Importantly, the IHC analyses performed with our home-made antiserum (Fong et al, 2007) in a series of grade 3 human breast IDC (n = 195) showed that increased LOXL2 expression with a specific cytoplasmic/perinuclear pattern is significantly associated with the basal-like subtype (Fig 7B; 57.4% tumours). Noteworthy, the heterogeneous cytoplasmic/perinuclear staining of LOXL2 observed in basal-like tumours was similar to the one we previously reported in larynx squamous cell carcinomas, which proved to be of prognostic value (Peinado et al, 2008), and in a small series of breast adenocarcinomas (Hollosi et al, 2009). Moreover, the cytoplasmic/perinuclear LOXL2 pattern in breast carcinomas was significantly associated with distant metastasis incidence (Fig 7B and C), further supporting a role of LOXL2 in metastatic dissemination of basal-like carcinomas. These results are also in agreement with our previous observation that LOXL2 overexpression is associated with poor clinical outcome of N0 breast adenocarcinomas (Peinado et al, 2008) and with the association of increased LOXL2 transcripts with metastasis incidence in the van de Vijver et al (2002) and Wang et al (2005) series (Supporting Information Fig S2B), as well as with ER- tumours from the NKI series recently reported (Barker et al, 2011). No extensive data of LOXL2 protein expression on breast tumours were so far available, since previous IHC studies of LOXL2 were restricted to a few samples of breast adenocarcinomas of non-specific histological subtypes and lacking information of other clinico-pathological features (Barry-Hamilton et al, 2010; Hollosi et al, 2009). Our present IHC analyses in a large series of grade 3 breast tumours identify for the first time the basal-like carcinomas as the breast tumour subtype with highly significant expression of intracellular LOXL2 protein associated with distant metastasis. Interestingly, the LOXL2 expression detected in breast tumours with our polyclonal antiserum is exclusively present in the tumour cells but not in the stromal component (Fig 7A), supporting a likely intracellular action of LOXL2 in metastasis. The staining of 32 basal-like tumours included in the present series with a distinctly different antiserum (described in Barry-Hamilton et al, 2010) corroborates the LOXL2 intracellular stain in about 22% of the cases. In addition, intracellular plus stromal stain was detected in 44% of the cases (Supporting Information Fig S9B), suggesting an additional extracellular action of LOXL2 in a subset of basal-like tumours, as supported from recent studies in other tumour types (Barker et al, 2011; Barry-Hamilton et al, 2010). Taken together, these results might suggest a dual role and/or substrates for LOXL2 in regulating intracellular and extracellular components that may prevail in distinct pathological contexts or tumour subtypes. Indeed, previous studies have indicated an important role for LOX in hypoxia-induced metastasis (Erler et al, 2006) and the specific participation of LOX in the establishment of the pre-metastatic niche through extracellular matrix remodelling (Erler et al, 2009). A role for extracellular LOXL2 in the development of pathological microenvironment and the effectiveness of anti-LOXL2 antibody or copper chelator inhibitors in inhibition of tumour or metastatic burden of carcinoma MDA-MB-435 cells or in PyMT induced tumours has been recently reported (Barker et al, 2011; Barry-Hamilton et al, 2010). Other recent studies have also reported the participation of secreted LOXL2 in gastric carcinoma metastasis (Peng et al, 2009). Further studies will clarify the contribution of extracellular LOXL2 to the metastasis potential of basal-like breast tumours.
We provide here new mechanistic insights into the action of intracellular LOXL2 in the regulation of the mesenchymal and migratory phenotype of basal-like carcinoma cells that may likely underlie its metastatic potential. In agreement with the reported induction of EMT by LOXL2 (Peinado et al, 2005), we now report that LOXL2 silencing induces a MET process in the human basal-like breast cell lines analysed. Interestingly, the phenotypic change induced by LOXL2 silencing is associated with downregulation of some mesenchymal markers and increased expression of tight junction and cell polarity components but without significant changes in E-cadherin expression (Fig 2E, Supporting Information Fig S3B). Importantly, LOXL2 silencing in basal MDA-MB-231 cells induces the expression and organization of tight junctions and Lgl2- and Par3-containing cell polarity complexes at the upper lateral membrane, indicating their functional organization as in ‘bona-fide’ epithelial cells. The reverse situation occurs in MCF7-LOXL2 cells, thus further supporting the implication of LOXL2 in the absence/disorganization of tight junctions and cell polarity complexes in breast carcinoma cells. Surprisingly, this situation appears to occur independently of E-cadherin expression or organization at cell–cell contacts. Since MDA-MB-231- and BT549-shLOXL2 cells maintain elevated levels of N-cadherin and cadherin11 (Fig 2G and Supporting Information Fig S4A), we speculate that either of those cadherins may substitute for E-cadherin in the maintenance of cell–cell contacts and collaborate in the functional organization of tight junctions and cell polarity complexes in the absence of LOXL2. In this regard, some situations in which N-cadherin can replace E-cadherin, like in the functional organization of embryonic intestinal epithelium, have been recently reported (Libusova et al, 2010). Further studies will contribute to clarify this interesting possibility. Independent of this aspect, we present here evidence for a novel mechanism of LOXL2 mediated downregulation of tight junction and cell polarity complexes at transcriptional level. LOXL2 significantly represses the activity of claudin1 and Lgl2 promoters and both the promoter activity and mRNA levels of these genes are upregulated after LOXL2 silencing in basal MDA-MB-231 cells (Fig 5A–C). The LOXL2 repressive action on both promoters is independent of Snail1 and, interestingly, of the LOXL2 catalytic domain (Fig 5D–F), suggesting a new role of intracellular LOXL2 apart from its enzymatic activity. Our previous studies also provided evidence for Snail1-dependent and -independent pathways in LOXL2 function on EMT and epidermal terminal differentiation of squamous carcinoma cells (Peinado et al, 2008). The present data provide strong support for a novel intracellular action of LOXL2, independent of Snail1, in the downregulation of tight junction and cell polarity genes, thus actively contributing to the maintenance of the mesenchymal phenotype of basal-like carcinoma cells. A role for intracellular LOXL2 mediated by RAMP3 in tumourigenicity of MDA-MB-231 cells has been recently reported (Breckhman et al, 2011). On the other hand, LOXL2 contributes positively to activation of the FAK signaling pathway and participates in the organization of focal adhesion complexes in basal carcinoma cells in a pattern fully compatible with a motile phenotype (Fig 4 and Supporting Information Fig S5). Whether the influence of LOXL2 on focal contacts is mediated by the extracellular catalytically active form, as reported for FAK/Src signaling in gastric carcinoma cells (Peng et al, 2009), or can be also mediated by intracellular LOXL2 remains to be established in future studies.
In summary, our present results define intracellular LOXL2 as a new marker of basal-like breast tumours associated with metastasis incidence and demonstrate its active participation in lung metastatic dissemination of basal breast carcinoma cells. The downregulation by LOXL2 of tight junction and cell polarity components at transcriptional level unveil a new and unexpected molecular action for LOXL2 that can provide new targets for anti-metastatic therapies of basal-like breast carcinomas.
MATERIALS AND METHODS
Tumour samples
The present study was performed using a total of 202 infiltrating ductal breast carcinoma (IDC) tumours acquired from the archives of the Pathology Department of La Paz Hospital, Madrid, Spain and from the MD Anderson Cancer Center Madrid, Madrid, Spain. All of the tumours were grade 3. Patients underwent surgery between 2003 and 2004. The mean patient age at surgery was 56.3 years (range, 30–81 years). In the microarrays analysis and qRT-PCR study we used a total of 58 frozen specimens. The IHC analyses included all of the 202 IDC. According to the TNM Classification staging, 82 of the tumours were stage I, 66 were stage II and 54 were stage III–IV. Histological and IHC studies were all carried out on formalin-fixed, paraffin-embedded tissue samples. Clinical data of the tumour sample are provided in Supporting Information Table S2. This study was performed following standard ethical procedures of the Spanish regulation (Ley de Investigación Orgánica Biomédica, 14 July 2007) and was approved by the ethic committees of La Paz Hospital, and MD Anderson Cancer Center Madrid, Madrid, Spain.
Gene profiling of breast tumours
Microarray analyses on 58 frozen specimens were performed using Human 1A Microarray (V2) G4110B (Agilent technologies). Details for RNA extraction, probe synthesis, hybridization on oligonucleotide arrays and statistical analyses are provided as Supporting Information.
Quantitative RT-PCR
cDNA from the different cell lines and tumour samples was obtained from 1 µg of total RNA using random primers and Superscript II system (Life Technologies Inc.) as previously described (Moreno-Bueno et al, 2009). Quantitative real-time PCR (TaqMan) was performed with Iq5 BIORAD (BioRad Laboratories SA), using the manufacturer's recommended conditions. The comparative threshold cycle (_C_t) method was used to calculate the amplification factor as specified by the manufacturer, and the amount of target and endogenous reference was determined from a standard curve for each experimental sample. The sequence of oligonucleotides and TaqMan probes used for the analysis of LOXL2 were obtained using the Assays-by-Design (SM) File Builder program (Applied Biosystems); a B2M probe (Applied Biosystems) was used as endogenous gene control.
The paper explained
PROBLEM
Basal-like carcinomas are highly aggressive tumours with visceral metastasis, poor prognosis and a high tendency to develop chemoresistance. Therapeutic targets for basal-like carcinomas have not been yet defined hindering the treatment of this specific breast tumour type. Basal-like tumours have been identified as expressing a distinct set of EMT markers, being likely candidates to suffer EMT. LOXL2, a member of the LOX family, has been identified as an EMT regulator and as a prognostic marker in SCC. We have investigated the role of LOXL2 in breast carcinomas.
RESULTS
We define a basal-like signature in grade 3 breast IDC tumours and detect LOXL2 overexpression in basal-like carcinomas. LOXL2 expression defines the basal-like tumours and is associated to distant metastasis of breast tumours in several public datasets. LOXL2 expression is restricted to breast carcinoma cell lines with a basal-like phenotype showing a specific cytoplasmic/perinuclear LOXL2 pattern. Knockdown of LOXL2 induces a MET phenotype and suppresses lung metastasis dissemination of basal carcinoma cells. We demonstrate that LOXL2 negatively modulates the expression and organization of tight junctions and cell polarity complexes, by transcriptional repression of tight junction (claudin1) and cell polarity (Lgl2) genes independent of Snail1. Analyses of LOXL2 in a large series of grade 3 breast IDC show that intracellular LOXL2 stain is significantly associated with basal-like tumours, and importantly, with distant metastatic incidence of basal-like breast carcinomas.
IMPACT
The data bring to light the importance of LOXL2 in basal-like breast tumours and we propose intracellular LOXL2 as a new marker of this aggressive breast carcinoma subtype. The LOXL2 implication in the downregulation of tight junction and cell polarity components unveils a new and unexpected molecular action for LOXL2 that can provide new targets for antimetastatic therapies of basal-like breast carcinomas.
Immunohistochemical analysis
LOXL2 IHC staining was performed by the LSAB (Dako) method with a heat-induced antigen retrieval step, as previously described (Moreno-Bueno et al, 2009; Peinado et al, 2008). Briefly, sections were immersed in boiling 10 mM sodium citrate at pH 6.0 for 3 min in a pressure cooker. The rabbit polyclonal antisera against human LOXL2 were previously described and they are refered here as KC (Fong et al, 2007) and Arresto (Barry-Hamilton et al, 2010) antibodies. The antibodies and conditions used for staining of basal and EMT markers are described in Supporting Information Table S4. The primary antibodies were omitted in the negative controls. LOXL2 staining with our home-made antiserum was defined as intense cytoplasmic/perinuclear when it was detected in at least 20% of tumour cells when compared with the diffuse staining pattern detected in the cytoplasm in most tumour cells (Peinado et al, 2008). LOXL2 staining with Arresto antiserum was categorized as: negative, intracellular and intracellular plus stromal. IHC analyses for ESR, PR, ErbB2 and EMT markers were performed as previously described (Sarrio et al, 2008).
Cell cultures and transfection assays
Human breast carcinoma cell lines MCF7, MDA-MB-231, SkBR3, BT474, BT549 and non-tumourigenic HBL100 cells were obtained from the ATCC-LGC Promochem Partnership and were cultured according to the indicated supplier conditions. The generation and culture conditions of MCF7-LOXL2-V5 cells were previously described (Hollosi et al, 2009). MDA-MB-231-TGL cells were kindly provided by Dr. J. Massagué (Memorial Sloan-Kettering Cancer Canter, New York) and cultured as previously described (Minn et al, 2005). Cells were maintained as monolayer cultures at 37°C in an atmosphere with 5% CO2. For generation of shLOXL2 and shEGFP cells, the indicated cell lines were transfected with pSuper-shLOXL2 and pSuper-shEGFP vectors, respectively, and independent clones isolated by selection in the presence of puromycin (1 µg/ml) for 2–3 weeks. Two independent shLOXL2 sequences (Supporting Information Table S5a), obtained from SuperArray (Qiagen), were tested in transient transfection assays and shLOXL2-3, giving the most effective silencing (60% reduction of LOXL2 protein level) was used for stable transfections. pSuper-shEGFP has been previously described (Olmeda et al, 2007).
Proliferation, migration and invasion assays
Details for proliferation, barrier migration and invasion in collagen IV transwells assays are provided as Supporting Information.
Tumourigenesis and spontaneous metastasis assays
Control MDA-MB-231-shEGPP and MDA-MB-231-shLOXL2 cells from subconfluent cultures were orthotopically injected (1 × 106 in 0.05 ml serum free growth medium) into the left fifth mammary fat pad of female Balb/c immunocompromised mice, aged 8 weeks (Charles River) as described (Olmeda et al, 2007). Tumour growth was measured twice a week by determination of the two orthogonal external diameters using a caliper. When tumours reached a size of 0.7 cm3 they were surgically excised and processed for histology. A minimum of four tumours from each cell line were generated. For spontaneous metastasis assay, primary tumours were excised when reached 0.4 cm3 volume. Afterwards, mice were left alive for additional 4 months and then sacrificed. Mice were housed and maintained under specific pathogen-free conditions and used in accordance with institutional guidelines and approved by the Use Committee for Animal Care from the Universidad Autónoma de Madrid (UAM).
Experimental metastasis assays and bioluminescence imaging
Control MDA-MB-231-TGL-shEGFP and MDA-MB-231-TGL-shLOXL2 cells from subconfluent cultures were injected (1 × 106 in 0.05 ml serum free growth medium) into the tail vein of female Balb/c immunocompromised mice, aged 8 weeks (Charles River). For in vivo bioluminescence imaging, mice were anesthetized and injected retro-orbitally with 1.5 mg of d-luciferin (15 mg/ml in PBS). Imaging was completed between 2 and 5 min after injection with a Xenogen IVIS (IVISR Lumina II) system coupled to Living Image acquisition and analysis software (Xenogen Corporation). For bioluminescence intensity (BLI) plots, photon flux was calculated as described (Minn et al, 2005). Measurements were performed once a week starting 3 weeks after tail vein injection and up to 9 (control) and 12 (experimental) weeks. Mice were housed and maintained under specific pathogen-free conditions and used in accordance with institutional guidelines and approved by the Use Committee for Animal Care from the Universidad Autónoma de Madrid (UAM).
Immunofluorescence and western blots
Western blots and immunofluoresce analyses were performed basically as described (Moreno-Bueno et al, 2009) on cell extracts and cells grown on cover slips and fixed on methanol or formaldehyde, respectively. Details for antibodies and confocal imaging are provided as Supporting Information and in Supporting Information Table S4.
Promoter assays
Promoter assays were performed basically as described (Peinado et al, 2005), using the human claudin1 promoter (−82/+236) (Martinez-Estrada et al, 2006) and two human Lgl2 promoters (p672:−244/+135; p673:−743/+135) (Spaderna et al, 2008) (kindly provided by T. Brabletz, Freiburg University), in the indicated cell lines in the presence or absence of the indicated amounts of wild type LOXL2, Snail1 and/or ΔLOXL2. Generation of ΔLOXL2 mutant (carrying an internal deletion from 558 to 688 aa) and details for transfection efficiency and quantification are provided as Supporting Information.
Statistical analysis
The Chi-square contingency test Yates correction, or Fisher's exact test, was used to determine the statistical significance of the relationships between IHC and clinico-pathological features. Values of p < 0.05 were considered statistically significant. These analyses were carried out using the SPSS 17.0 for statistical program (SPSS Inc.).
Acknowledgments
The authors thank Ángela Nieto (Alicante-Spain) for insightful discussions and suggestions and reading of the manuscript, Joan Massagué for TGL cells, Thomas Brabletz for Lgl2 promoters, Denis Strand (Mainz-Germany) for Lgl2 antisera, Victoria Smith and Vivian Barry-Hamilton (Gilead Sciences Inc) for IHC with Arresto's antisera, David Olmeda and Diego Megías (Madrid-Spain) for help in the bioluminescence and confocal analyses, respectively. This work was supported by grants from the Spanish Ministry of Science and Innovation, MICINN, (SAF2007-53061; SAF2010-21143; Consolider Ingenio CSD2007/00017, to AC; SAF2007-63075; SAF2010-20175 to GM-B); Fundación Mutua Madrileña (2007, 2009 to AC and GM-B); Instituto de Salud Carlos III (ISCIII) (PI 080971 to JP), and Junta de Andalucía (PI-0384/2007; PI 080971, P07-CVI-03100 to JP). FS and A Martín are recipients of JAE-pre and JAE-postdoc contracts from the Spanish Research Council (CSIC), respectively; MAC is founded by the RETICS (ISCIII).
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
GMB, FP and AC designed the experiments; GMB performed the gene profiling and IHC studies; FS, AM, AF and HP performed LOXL2 silencing and cellular and molecular characterization; EPC performed the promoter assays; A Montes performed cell proliferation, wound healing and western blot assays; VS and SM performed tumourigenic and metastasis assays and together with GMB the bioluminescence analyses. A Martinez, ARS, and DH provided the tumour samples and clinical data; JP and MAC did the pathological characterization and qRT-PCR of tumours; KC provided LOXL2 antibody and MCF-LOXL2-V5 model; AC and GMB wrote the paper with input of FP, KC and HP.
Supplementary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Aigner K, Dampier B, Descovich LM, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist D, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–6988. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- Barker HE, Chang J, Cox TR, Lang G, Bird D, Nicolau M, Evans HR, Gartland A, Erler JT. LOXL2-mediated matrix remodelling in metastasis and mammary gland involution. Cancer Res. 2011;71:1561–1572. doi: 10.1158/0008-5472.CAN-10-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transitions traits in human breast cancer cell lines parallel the CD44hi/CD24lo− stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop_motor of cellular plasticity in development and cancer. EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckhman V, Lugassie J, Zaffryar-Eilot S, Sabo E, Kessler O, Smith V, Golding H, Neufeld G. Receptor activity modifying protein-3 mediate the protumorigenic activity of lysyl oxidase-like protein-2. FASEB J. 2011;25:55–65. doi: 10.1096/fj.10-162677. [DOI] [PubMed] [Google Scholar]
- Cano A, Nieto MA. Non-coding RNA take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. doi: 10.1016/j.tcb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adélaïde J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–2284. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, Parker MI, Csiszar K. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer. 2007;46:644–655. doi: 10.1002/gcc.20444. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollosi P, Yakushiji JK, Fong KS, Csiszar K, Fong SF. Lysyl oxidase like-2 promotes migration in non-invasive breast cancer cells but not in normal breast epithelial cells. Int J Cancer. 2009;125:318–327. doi: 10.1002/ijc.24308. [DOI] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- Korsching E, Packeisen J, Liedtke C, Hungermann D, Wülfing P, van Diest PJ, Brandt B, Boecker W, Buerger H. The origin of vimentin expression in invasive breast cancer: epithelial-mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential. J Pathol. 2005;206:451–457. doi: 10.1002/path.1797. [DOI] [PubMed] [Google Scholar]
- Libusova L, Stemmler MP, Hierholzer A, Schwarz H, Kemler R. N-cadherin can structurally substitute for E-cadherin during instestinal development but leads to polyp formation. Development. 2010;137:2297–2305. doi: 10.1242/dev.048488. [DOI] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson MR, Molina P, Souchelnytskyi S, Wernstedt C, Martin-Pérez J, Portillo F, Cano A. Phosphorylation of Serine 11 and Serine 92 as new positive regulators of human Snail1 function. Potential involvement of casein kinase-2 (CK2) and cAMP activated kinase (PKA) Mol Biol Cell. 2010;21:244–253. doi: 10.1091/mbc.E09-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, Reina M, Cano A, Fabre M, Vilaro S. The transcription factors Slug and Snail act as repressors of claudin1 expression. J Biochem. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;3:271–272. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6058–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Peinado H, Molina P, Olmeda D, Cubillo E, Santos V, Palacios J, Portillo F, Cano A. The morphological and molecular features of the epithelial to mesenchymal transition. Nat Protocols. 2009;4:1591–1613. doi: 10.1038/nprot.2009.152. [DOI] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independent of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231cells. Cancer Res. 2007;67:11721–11731. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- Payne SL, Hendrix MJ, Kirschman DA. Paradoxical roles for lysyl oxidases in cancer-a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- Peinado H, Iglesias-de la Cruz MC, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype. Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, et al. Lysyl oxidase-like2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- Peng L, Ran YL, Hu H, Yu L, Liu Q, Zhou Z, Sun YM, Sun LC, Pan J, Sun LX, et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis. 2009;30:1660–1669. doi: 10.1093/carcin/bgp178. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- Rakha EA, El-Sayed ME, Green AR, Paish EC, Lee AH, Ellis IO. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology. 2007;50:434–438. doi: 10.1111/j.1365-2559.2007.02638.x. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland JM, Pereira EM, Lakhani SR, Schmitt FC. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pinilla SM, Sarrió D, Honrado E, Hardisson D, Calero F, Benitez J, Palacios J. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12:1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli F, Deville P, editors. 2003. World Health Organization: Tumours of the Breast and Female Genital Organs (Who/IARC Classification of Tumours), IARC Lyon.
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, et al. Gene-expresssion profiles to predict distant metastasis of lymph-node-negative breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Liu C-J, Fearon ER, Margolis B. The transcription factor Snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene. 2008;27:3875–3879. doi: 10.1038/onc.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Min C, Vora SR, Trackman PC, Soneshein GE, Kirsch KH. The lysyl oxidase pro-peptide attenuates fibronectin-mediated activation of focal adhesion kinase and p130Cas in breast cancer cells. J Biol Chem. 2009;284:1385–1393. doi: 10.1074/jbc.M802612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial–mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.