Effects of Leptin and Melanocortin Signaling Interactions on Pubertal Development and Reproduction (original) (raw)
Abstract
Leptin and melanocortin signaling control ingestive behavior, energy balance, and substrate utilization, but only leptin signaling defects cause hypothalamic hypogonadism and infertility. Although GnRH neurons do not express leptin receptors, leptin influences GnRH neuron activity via regulation of immediate downstream mediators including the neuropeptides neuropeptide Y and the melanocortin agonist and antagonist, α-MSH, agouti-related peptide, respectively. Here we show that modulation of melanocortin signaling in female db/db mice through ablation of agouti-related peptide, or heterozygosity of melanocortin 4 receptor, restores the timing of pubertal onset, fertility, and lactation. Additionally, melanocortin 4 receptor activation increases action potential firing and induces c-Fos expression in GnRH neurons, providing further evidence that melanocortin signaling influences GnRH neuron activity. These studies thus establish melanocortin signaling as an important component in the leptin-mediated regulation of GnRH neuron activity, initiation of puberty and fertility.
Obesity rates in humans are on the rise worldwide. Along with the increased risk for cardiovascular disease, diabetes, and cancer associated with obesity (1), fertility may also be impaired (2, 3). Loss of leptin signaling causes obesity, hyperphagia, hyperglycemia, hyperinsulinemia, and reduced body length as well as hypothalamic hypogonadism and infertility (4–10). Ablation of melanocortin 4 receptor (MC4R) signaling results in hyperphagia, with adult-onset obesity, hyperglycemia, and hyperleptinemia, whereas heterozygosity induces an intermediate phenotype (11–13).
Leptin regulates energy homeostasis and fertility by regulation of hypothalamic neurons releasing neuropeptide Y (NPY), agouti-related peptide (AgRP), proopiomelanocortin (POMC), and others (4). Although impairment of leptin signaling negatively impacts fertility, deleterious mutations of the preprohormone POMC, or the centrally expressed melanocortin receptors, MC3/4R, do not affect sexual development (14, 15). However, MC4R signaling regulates reproductive function and behavior both directly and indirectly (16–18), whereas transgenic overexpression of the MC3/4R endogenous antagonist, AgRP, leads to obesity and infertility (19).
AgRP and NPY overexpressions, as well as decreased melanocortin signaling, are cardinal features of leptin signaling deficiency. Both NPY and AgRP inhibit LH pulsatile release (20–23), and the overexpression of these neuropeptides may be one of multiple mechanisms responsible for infertility associated with leptin signaling deficiency. Data from multiple studies report that AGRP/NPY and POMC neurons in the arcuate nucleus project onto medial preoptic area GnRH neurons (24–26). Furthermore, fertility is restored in the ob/ob female mice with deletion of Npy or Npy4r (27, 28). The stimulatory effects of melanocortins on LH secretion and reproductive function are attributed to MC4R signaling (29, 30). Although in vivo evidence for MC4R expression on GnRH neurons is not reported, the GnRH secreting the GT-1 cell line endogenously expresses MC4R (31, 32).
Based on these data, we hypothesized that reducing inhibitory signals to GnRH neurons by either ablation of AgRP or deletion of MC4R could restore puberty in the leptin receptor knockout (db/db) mouse. Due to the opposite effects of AgRP and α-MSH on appetite and energy expenditure, we examined the effect of the loss of AGRP or MC4R in leptin receptor knockout mice (db/db) on metabolism, pubertal development, and fertility. We have developed a model based on our observations wherein Agrp and Mc4r are downstream of and exhibit antagonistic activities within the context of the reproductive axis. Accordingly, modulation of melanocortin signaling with AgRP antagonists or MC4R agonists may be useful for improving reproductive outcomes in women with obesity and leptin resistance.
Materials and Methods
Animals
Animals were housed in a barrier facility at 22 C on a 12-h light, 12-h dark cycle with ad libitum access to irradiated 5058 PicoLab Mouse Diet 20 chow (Québec, Canada) and sterilized water unless otherwise stated. Genotyping was performed using DNA from ear clips. Animals were euthanized by carbon dioxide asphyxiation; blood was collected at the time the animals were killed via cardiac puncture. The Albert Einstein College of Medicine Institutional Animal Care and Use Committee approved all protocols and procedures.
All animals were maintained on a mixed genetic background of C57BL/6J × FVB/NJ × 129. These animals include wild-type (homozygous wild type for leptin receptor, MC4R, and AgRP), leptin receptor knockout (db/db) (33), Agrp knockout (_Agrp_−/−) (34), MC4R knockout (_Mc4r_−/−) (12) and GnRH-green fluorescent protein (GFP) (35). db/+ mice were bred with _Agrp_−/− mice to generate db/+ Agrp+/−, which were then mated to generate db/+ _Agrp_−/− that were subsequently bred to create _Agrp_−/− and _db/db Agrp_−/− mice. _Mc4r_−/− mice were bred with db/+ mice to generate db/+ Mc4r+/−, which then were bred to generate wild-type, db/db, Mc4r+/−, and db/db Mc4r+/− mice.
Fasting glucose and insulin analysis
Animals were fasted 16 h before blood and plasma collection. Blood glucose levels were evaluated using a Bayer Ascensia Elite glucose meter (Tarrytown, NY). Plasma insulin was analyzed using an ultrasensitive mouse enzyme immunoassay from Alpco Diagnostics (Salem, NH). Body weights were measured on a scale with 0.01 g accuracy. Body composition was analyzed using the ECHO magnetic resonance spectroscopy instrument for the mouse (Echo Medical Systems, Houston, TX).
Relative quantification of neuropeptide expression by quantitative PCR
Total RNA was extracted from the hypothalami of 12- to 16-wk-old mice using an RNeasy lipid tissue minikit (QIAGEN, Venlo, The Netherlands). Reverse transcriptase reaction was conducted using 2 μg of total RNA using Invitrogen SuperScript III first-strand synthesis kit, (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed on the Roche LightCycler 480 real-time PCR system using the LightCycler 480 SYBR Green Master I kit (Roche Diagnostics, Mannheim, Germany). Primers (IDT-DNA, Coralville, IA) were designed to span at least one intron to distinguish between amplification products derived from genomic DNA and cDNA. Amplification efficiencies were similar for all primer pairs used. Primer sequences for each mRNA target can be found in the Supplemental Data, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. The PCR amplification protocol was as follows: 10 sec at 94 C, 20 sec at 65 C, and 30 sec at 72 C for 45 cycles. Results are displayed as fold change and calculated using the 2-ΔΔ cycle threshold method (36). Experiments were run in duplicate with n = 3–5 for each genotype.
AgRP neuron number assessments in wild-type and _Mc4r_−/− females
Female w_ild-type_ and _Mc4r_−/− mice (n = 3) were fasted overnight, anesthetized, and then perfused via the left ventricle with 0.1 m PBS followed by 10% formalin the following morning. Brains were removed, placed in cryoprotectant (30% sucrose in PBS) for 48 h, and sectioned (40 μm) using a Leica VT1000S vibratome (Buffalo Grove, IL). Sections were stained for AgRP immunoreactivity (37). Staining of Agrp-null mice showed a lack of immunoreactivity similar to that observed with omission of the anti-AGRP primary. Images were acquired with a Zeiss LSM 510 NLO multiphoton confocal microscope (Jena, Germany). Images were processed with ImageJ (National Institutes of Health, Bethesda, MD) (38).
Fertility assessment
Assessment for pubertal onset was performed daily from weaning (18–21 d) by visual inspection for vaginal opening. Estrous cycle monitoring was conducted in 8- to 12-wk-old female mice by daily vaginal lavage (21 consecutive days) and microscopic analysis of vaginal epithelial cell type (39). To assess fertility, 8- to 16-wk-old females were individually mated to a wild-type stud male for 6 wk. Females were weighed weekly. After wk 3, cages were checked daily for the presence of pups. Litters were monitored for survival until weaning age.
Mammary and ovarian tissue processing and histological staining
Whole-mount staining of the left inguinal mammary glands excised from virgin females was done using alum carmine (http://mammary.nih.gov/). Digital images of the whole mounts were taken using a Nikon D70 camera with a 60 mm 1:2.8 macro lens (Shinjuku, Tokyo, Japan). The right inguinal mammary gland and ovaries were fixed in Z-FIX (Anatech, Battle Creek, MI) for up to 2 wk and overnight, respectively, and then paraffin embedded, sectioned (5 μm), and stained with hematoxylin and eosin (H&E).
Ovaries used for ovarian follicle count studies were serially sectioned (5 μm) and stained with H&E. Follicles were counted from every fifth section using light microscopy. Morphological follicle classifications were based on the system proposed by Pedersen and Peters (40). To avoid bias, researchers were blinded to the genotype of the ovary from which follicles were counted. We uniformly multiplied our raw follicle number counts by a correction factor of 5, based on the sampling at every fifth ovarian section, to account for the remainder of follicles throughout the whole ovary not counted.
Estradiol and progesterone hormone assays
The Einstein Hormone Assay Core using ultra-sensitive 17β-estradiol RIA (DSL-4800) and progesterone RIA (DSL-3900) from Diagnostic Systems Laboratories (Webster, TX) measured serum 17β-estradiol and progesterone concentrations. Animals were in proestrus as determined by vaginal cytology at the time of collection, except for db/db, which were in metestrus. Reported sensitivities for the 17β-estradiol and progesterone assays were 2 pg/ml and 5 ng/ml, respectively. Intraassay coefficients of variation were 7.2 and 7.5%, respectively.
Single-cell RT-PCR
Medial preoptic area GFP-positive cell samples from 4- to 6-wk-old male (n = 4) and female (n = 6) GnRH-GFP mice were collected from brain slice preparations via aspiration into the patch pipette. The initial reverse transcriptase reaction was conducted after pressure ejection of single-cell samples as described (41) The sequences of the primers are located in the Supplemental Data. After a second round of amplification with nested primers, PCR products were analyzed on 1.5% agarose gels. Primers were purchased from IDT-DNA and amplified at similar efficiencies.
Electrophysiology slice preparation
Coronal brain slices were prepared from GnRH-GFP mice at postnatal age 28–35 d (n = 16). Animals were anesthetized with a mixture of ketamine and xylazine. After decapitation, the brain was transferred into a sucrose-based solution bubbled with 95% O2-5% CO2 and maintained at approximately 3 C. This solution contained (millimoles): sucrose, 248; KCl, 2; MgCl2, 1; KH2PO4, 1.25; NaHCO3, 26; and glucose, 10. Transverse coronal brain slices (200 mm) were prepared using a Leica VT1000S. Slices were equilibrated with an oxygenated artificial cerebrospinal fluid (aCSF) for more than 1 h before transfer to the recording chamber. The slices were continuously superfused with aCSF at a rate of 2 ml/min containing (in millimoles); NaCl, 113; KCl 3; NaH2PO4, 1; NaHCO3, 26; CaCl2, 2.5; MgCl2, 1; and glucose, 5 in 95% O2-5% CO2 at room temperature.
Electrophysiological recordings
Brain slices were placed on the stage of an upright, infrared-differential interference contrast microscope (Olympus BX50WI; Shinjuku, Tokyo, Japan) mounted on a Gibraltar X-Y table (Burleigh, Thorlabs, Inc., Newton, NJ) and visualized with a ×40 water immersion objective by infrared microscopy (DAGE-MTI camera; Michigan City, IN). GnRH neurons were identified by the presence of enhanced GFP. Membrane potentials were recorded at 28 C with a Multiclamp 700B amplifier in the whole-cell configuration. The aCSF contained (in millimoles): NaCl, 113; KCl, 3; NaH2PO4, 1; NaHCO3, 26; CaCl2, 2.5; MgCl2, 1; and glucose, 5 in 95% O2-5% CO2. 6-cyano-7-nitroquinoxaline-2,3-dione (10 μm), DL-amino-phosphonovaleric acid (DL-AP-5, 50 μm), picrotoxin (100 mm), and strychnine (1 mm) were continuously present in aCSF except as noted to block excitatory and inhibitory synaptic transmission. The internal solution contained (millimoles): K acetate, 115; KCl, 10; MgCl2, 2; EGTA, 10; HEPES, 10; Na2ATP, 2; Na2GTP, 0.5; and Na2phosphocreatine, 10. Pipette resistance ranged from 2.5 to 4MΩ. The MC3/4R agonist, melanotan (MTII) (Tocris, Ellisville, MO), and AgRP (Phoenix Pharmaceuticals, Burlingame, CA) were prepared as 1 mm stock solutions and dissolved at a final concentration in aCSF just before electrophysiological recording.
MTII dosing and c-Fos immunohistochemistry
Female GnRH-GFP mice (8–12 wk old) were acclimatized to handling and ip injections by weighing and giving a daily injection of sterile PBS (n = 3) similar in volume to that given for MTII (n = 3) treatment. Diestrous females were treated with either 10 mg/kg body weight MTII or saline for 1.5 h. Coronal vibratome sections were blocked with 2% normal donkey serum (Vector Laboratories, Burlingame, CA) for 2 h at room temperature and then incubated with rabbit anti-c-Fos antibody (1:500; EMD Biosciences, San Diego, CA) diluted in 0.4% Triton X-100 and 0.5% BSA in PBS for 48 h at 4 C. After incubation in primary antiserum, sections were washed three times in PBS and incubated in Alexa Fluor 568 donkey antirabbit IgG (1:750; Molecular Probes, Invitrogen, Carlsbad, CA) diluted in 0.4% Triton X-100 and 1% donkey serum in PBS for 3 h at room temperature. Tissues were washed three times in PBS, dried, and mounted with Vectashield HardSet mounting media (Vector Laboratories) with 4′,6′-diamino-2-phenylindole. Images were acquired using a Leica SP2 AOBS scanning confocal microscope (Heidelberg, Germany) and processed with ImageJ software (38).
Statistics
Data were analyzed and statistical calculations performed using GraphPad Prism version 5.0 for Mac OS X (GraphPad Software, La Jolla, CA). One-way ANOVA followed by Bonferroni multiple comparison tests was used to determine statistical significance among multiple groups unless otherwise stated.
Statistical analyses for both electrophysiology and AGRP neuron number studies were performed on all GnRH neurons or wild-type and _Mc4r_−/− mice, respectively, examined using an unpaired t test. The mean values were reported from the entire population tested (Origin 7.0; OriginLab, Northampton, MA). Data were considered significantly different when the P value was less than 0.05. Results are given as mean ± sem.
Results
Body weight, fat distribution, and fasting serum glucose and insulin concentrations in leptin receptor-deficient female mice
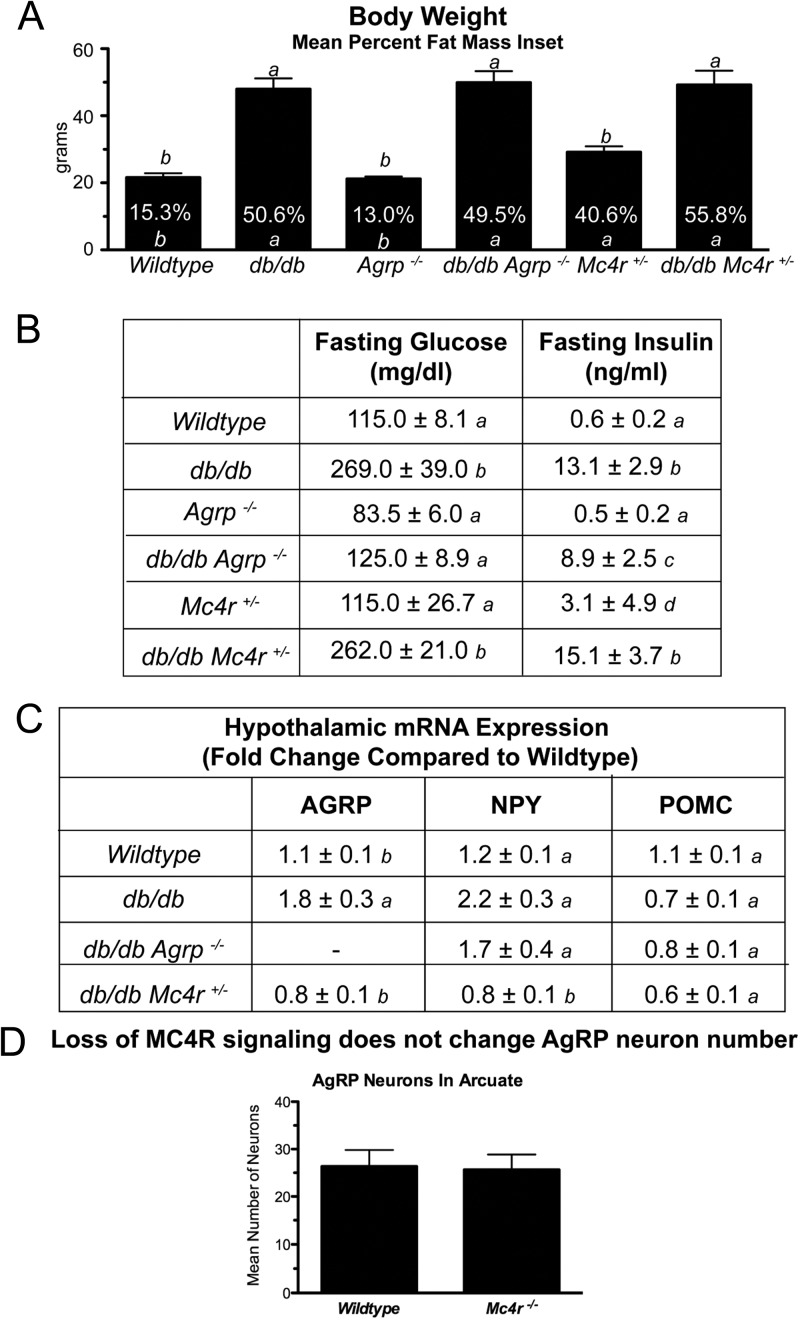
We examined the effects of AgRP ablation (_Agrp_−/−) and MC4R heterozygosity (Mc4r+/−/−) on body weight and fat distribution, as well as serum glucose and insulin concentrations, in leptin receptor-deficient animals. Mean body weights of 8- to 12-wk-old db/db, _db/db Agrp_−/−, db/db Mc4r+/−, and Mc4r+/−/− females were significantly greater (P < 0.05) than those of wild-type littermates (Fig. 1A). There were no significant differences in body weight between db/db, _db/db Agrp_−/−, and db/db Mc4r+/− females. The fractional fat mass was significantly greater (P < 0.05) in all leptin receptor-deficient and Mc4r+/− females than in wild-type and _Agrp_−/− females (Fig. 1A). However, there was no significant difference in fractional fat mass among the various subgroups of leptin receptor-deficient females, suggesting melanocortin-signaling modulation in these models did not alter fat deposition. We further examined whether ablation of AgRP or Mc4r heterozygosity altered the type 2 diabetes phenotype that develops in leptin signaling-deficient animals. We found that although fasting glucose levels were significantly elevated in db/db and db/db Mc4r+/− females, glucose values were normalized in _db/db Agrp_−/− females to values similar to those of wild-type controls (Fig. 1B). Fasting insulin levels were significantly elevated (P < 0.05) in all leptin receptor knockout females relative to wild-type females. However, there were no significant differences in fasting insulin levels among the leptin receptor-deficient females (Fig. 1B).
Fig. 1.
Impact of alterations of melanocortinergic tone on metabolic characteristics of db/db mice.
POMC, AgRP, and NPY mRNA expression
Leptin regulates the expression of the anorexigenic peptide POMC and the orexigenic peptides AgRP and NPY. In the leptin signaling-deficient state, hypothalamic POMC mRNA expression is reduced (42), whereas AgRP and NPY mRNA are elevated (43, 44). We compared AgRP, NPY, and POMC mRNA expression from the hypothalami of 8- to 12-wk-old _ad libitum_-fed females. Actin (ACTB) was used as an internal control for each sample. As expected, AgRP mRNA levels of db/db females were significantly greater (P < 0.05) than those of wild-type females (Fig. 1C). Interestingly, AgRP mRNA levels in hypothalami of db/db Mc4r+/− females resembled those of wild type (Fig. 1C), suggesting intact melanocortin signaling may be required for leptin signaling deficiency induced AgRP production. AgRP mRNA levels were not detectable in either the _Agrp_−/− or the _db/db Agrp_−/− females. NPY mRNA expression was higher in db/db (P < 0.05) than in wild-type females (Fig. 1C). Similar to the AgRP results, NPY mRNA expression was lower in db/db Mc4r+/− compared with db/db females (P < 0.05). POMC expression was lower in all leptin receptor-deficient animals than in wild types. There were no differences in ACTB mRNA levels among groups similar to a prior report (45).
Ablation of MC4R does not change AgRP neuron numbers
To determine whether reductions in hypothalamic AgRP and NPY mRNA expression in the db/db Mc4r+/− females were due to functional or structural changes, we next investigated whether loss of MC4R signaling could affect AgRP/NPY neuron number. We found no observable differences in the number of AgRP-immunoreactive neurons in arcuate nuclei of wild-type (26.3 ± 3.5) and _Mc4r_−/− (25.7 ± 3.2) females (Fig. 1D).
Ablation of Agrp and heterozygosity of Mc4r restores puberty and improves reproductive function in female db/db mice
Animals with impaired leptin signaling are infertile as a result of hypothalamic hypogonadism characterized by delayed pubertal development and reduced serum gonadal steroids (7, 46). The most commonly used marker for the onset of puberty in rodents is age at vaginal opening. In db/db females, pubertal onset was significantly delayed when compared with wild-type females (44.3 ± 2.7 vs. 33.7 ± 1.6 d; P < 0.05). Ablation of Agrp or Mc4r heterozygosity in db/db females reduced the age at vaginal opening to 35.0 ± 2.1 and 41.3 ± 0.9 d, respectively. This reduction was significant (P < 0.05) in _db/db Agrp_−/− when compared with db/db females. Pubertal onset was similar between _Agrp_−/−, Mc4r +/−, and wild-type females (Table 1).
Table 1.
Effects of melanocortin signaling modulation on puberty and fertility in the db/db female
| Age at vaginal opening (d) (n = 6–12) | Uterine weight (mg) (n = 6–11) | Mean estrous cycle length (days) (n = 10–14) | Mean number of pups surviving to weaning (n = 9–13) | |
|---|---|---|---|---|
| Wild type | 33.7 ± 1.6_a_ | 131.6 ± 10.0_a_ | 5.1 ± 0.4_a_ | 9.0 ± 0.8_a_ |
| db/db | 44.3 ± 2.7_b_ | 66.2 ± 6.4_b_ | 0.0 cycles_b_ | 0.0 ± 0.0_b_ |
| _Agrp_−/− | 28.7 ± 0.7_a_ | 108.6 ± 12.1_a_ | 4.6 ± 0.2_a_ | 9.1 ± 0.7_a_ |
| _db/db Agrp_−/− | 35.0 ± 2.1_a_ | 108.2 ± 29.2_a_ | 6.3 ± 0.4_a_ | 5.1 ± 0.8_c_ |
| Mc4r+/− | 33.5 ± 1.0_a_ | 95.2 ± 10.3_a_ | 5.2 ± 0.8_a_ | 8.7 ± 0.6_a_ |
| db/db Mc4r+/− | 41.3 ± 0.9_b_ | 83.7 ± 16.0_a_ | 8.3 ± 1.6_c_ | 4.5 ± 0.7_c_ |
We next examined vaginal cytology/estrous cyclicity in these mice (Table 1) after vaginal opening. The wild-type mice cycled on average every 5.1 ± 0.4 d, whereas db/db mice were in a state of persistent metestrus. Average estrous cycle lengths of _Agrp_−/− and Mc4r+/− were similar to wild type. Ablation of Agrp or heterozygosity of Mc4r in db/db females restored estrous cyclicity to a mean cycle length of 6.3 ± 0.4 and 8.3 ± 1.6 d, respectively.
Similar to previous studies (8, 47), serum 17β-estradiol and progesterone levels were reduced in db/db females when compared with proestrous wild-type females (Table 2). Ablation of Agrp in db/db animals restored proestrous estradiol and progesterone values to levels detected in wild-type animals. Proestrous estradiol and progesterone values in female db/db Mc4r+/− mice were not significantly greater than those of db/db females (Table 2).
Table 2.
Effects of melanocortin signaling modulation on gonadal steroids
| 17β-Estradiol (pg/ml) | Progesterone (ng/ml) | |
|---|---|---|
| Wild type | 29.0 ± 4.8_a_ | 23.8 ± 7.7_a_ |
| db/db | 6.9 ± 0.5_b_ | 3.4 ± 0.7_b_ |
| Agrp −/− | 25.1 ± 4.2_a_ | 20.62 ± 5.8_a_ |
| db/db Agrp −/− | 20.8 ± 4.2_a_ | 19.3 ± 4.9_a_ |
| Mc4r+/− | 20.8 ± 4.3_a_ | 10.0 ± 2.6_a_ |
| db/db Mc4r+/− | 8.1 ± 0.6_b_ | 7.2 ± 1.0_b_ |
Reproductive function was examined by mating 8- to 12-wk-old females to an experienced and proven fertile 10- to 12-wk-old wild-type male for a period of up to 6 wk (Table 1). All wild-type mating pairs examined (n = 12) achieved parturition in an average of 21.4 ± 0.5 d and successfully suckled and weaned an average of 9.0 pups per litter. Matings between db/db females (n = 11) and wild-type males resulted in only one pregnancy 38 d after initial introduction of males. The single pup born to the db/db female did not survive beyond 48 h after birth due to the inability of the dam to lactate. Ablation of Agrp and Mc4r heterozygosity restores fertility and lactational competence in db/db females. Pairings between _db/db Agrp_−/− females and wild-type males produced viable offspring in 11 of 12 pairs, with an average of 5.1 ± 0.8 pups weaned per litter at 21 d postnatally. Five of nine matings between db/db Mc4r+/− females and wild-type males produced viable offspring, with an average of 4.5 ± 0.7 pups per litter weaned. The mean time from mating to parturition was 22.3 ± 0.9 d for the _db/db Agrp_−/− females and 23.8 ± 1.0 d for the db/db Mc4r+/− females. We found no significant differences in length of gestation, litter size, and survival rates among wild-type, _Agrp_−/−, and Mc4r+/− females. Surprisingly, neither Agrp ablation nor Mc4r heterozygosity restored fertility in db/db male mice (n = 10 each). We are continuing to characterize the reproductive deficit in these males.
We also evaluated uterine weights (Table 1) among the groups. Previous studies reported a reduction in uterine weights with defective leptin signaling due to reduced estradiol levels (5, 7, 9, 46, 48). Accordingly, we found uterine weights were significantly reduced in db/db females compared with estrus wild-type females (P < 0.05). There were no significant differences in uterine weights among wild-type, _Agrp_−/−, Mc4r+/−, db/db Mc4r+/−, or _db/db Agrp_−/− females during estrus (Table 1).
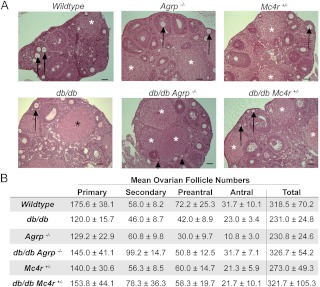
Moreover, previous studies reported reduced primary and secondary ovarian follicle numbers with defective leptin signaling (5, 7, 9, 46, 48). We examined ovarian morphology and histology and found no significant differences in the number of primary, secondary, preantral, or antral follicles among the genotypes examined, although there was a trend toward reduced primary and preantral follicles in all genotypes relative to wild-type females (Fig. 2, A and B). In the db/db females, there are structures that bear the morphological hallmarks of corpora lutea. However, functionality of these structures is likely to be compromised due to low progesterone concentrations (Table 2). We are unable to determine whether they are sufficient to sustain gestation as pregnancy in db/db females is nearly impossible to ascertain and the rarity of the event.
Fig. 2.
Mutations of Lepr, Agrp and Mc4r do not alter follicular development.
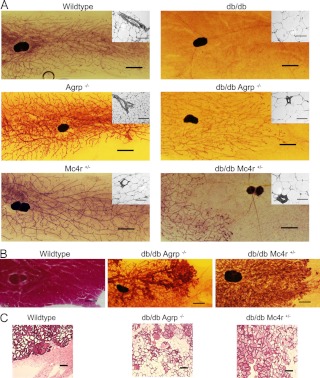
A significant feature of the improved fertility in _db/db Agrp_−/− and db/db Mc4r+/− females was the restoration of lactational competence as demonstrated by survival of pups to weaning. Whole mounts of fourth inguinal mammary glands from wild-type, _Agrp_−/−, and Mc4r+/− mice showed extensive ductal tree development extending throughout the fat pad, whereas the mammary glands from db/db females had negligible to no ductal tree development or lateral branching (Fig. 3A). Agrp ablation or Mc4r heterozygosity restored mammary ductal tree development and lateral branching in db/db females to near normal levels (Fig. 3A). Paraffin-embedded, H&E-stained mammary gland sections from wild-type, _Agrp_−/−, and Mc4r+/− virgin females showed the presence of multiple lactiferous ducts embedded in adipose tissue, whereas db/db fat pads had very few or none (Fig. 3A, inset). This is improved in the mammary glands of _db/db Agrp_−/− and db/db Mc4r+/− female mice (Fig. 3A, inset).
Fig. 3.
Restoration of mammary gland development and milk production in db/db females by mutations of Agrp and Mc4r.
Histological examination of mammary glands from lactating animals provided further evidence of the restoration of mammary gland function. Lateral ductal branching into the mammary fat pads of _db/db Agrp_−/− and db/db Mc4r+/− lactating mammary glands was comparable with those from nulliparous females, suggesting there was no further ductal tree development during pregnancy (Fig. 3B). Mammary glands removed 2 d postpartum from wild-type, _db/db Agrp_−/−, and db/db Mc4r+/− demonstrated densely stained, milk-filled alveolar structures (Fig. 3C). The single db/db female that achieved pregnancy and delivery of a single pup had mammary gland development comparable with nulliparous db/db females (data not shown).
GnRH neurons express MC4R mRNA
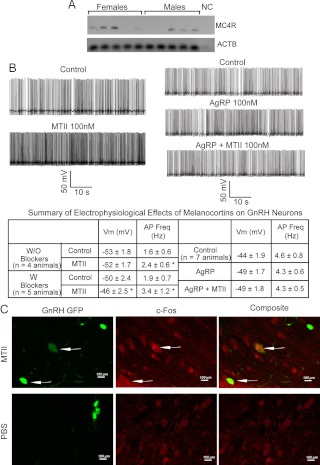
We used single-cell RT-PCR (scRT-PCR) to determine whether GnRH neurons express MC4R. We examined five GnRH neurons in the medial preoptic area in brain slices from each of 10 GnRH-GFP mice (n = 4 males, n = 6 females). We found that more than half of the GnRH neurons expressed MC4R mRNA (Fig. 4A). This finding is consistent with several other (scRT-PCR) studies, which indicate that not all cells tested provide positive signals for receptors that are expressed at relatively low levels (49, 50). We performed scRT-PCR for GnRH on some of the GFP-positive neurons to confirm that we were selecting GnRH neurons. All neurons examined (two neurons from four mice (n = 2 males, n = 2 females) were positive for GnRH (data not shown).
Fig. 4.
Melanocortins regulate GnRH neurons via MC4R.
Activation of melanocortin receptors increases GnRH neuron activity
To examine the potential electrophysiological impact of melanocortin signaling on GnRH neurons, whole-cell, patch-clamp recordings were made from GnRH neurons in the medial preoptic area of brain slices of GnRH-GFP mice (Fig. 4B). We first examined the action of MTII, an MC3/4 receptor agonist, on GnRH neurons by measuring membrane potential and firing rate in current-clamp mode. After at least 10 min of stable recording, treatment with MTII (100 nm) for 5 min increased the firing rate of GnRH neurons (firing rate: control, 1.6 ± 0.6 Hz; plus MTII, 2.4 ± 0.6 Hz; P < 0.05; n = 4 animals). The enhanced electrical activity was not reversible after washout (>30 min). We next eliminated presynaptic influences by adding a cocktail of glutamate, γ-aminobutyric acidA, and glycine receptor blockers and determined whether MTII directly modulated GnRH neurons. Under these conditions, MTII significantly depolarized as well as increased the firing rate of GnRH neurons (Fig. 4B; Vm: control, −50 ± 2.4 mV; plus MTII, −46 ± 2.5 mV; P < 0.05; firing rate: control, 1.9 ± 0.7 Hz; plus MTII, 3.4 ± 1.2 Hz; P < 0.05; n = 5 animals). Most GnRH neurons responded to MTII in the two conditions (n = 7 of eight neurons without blockers and five of six neurons with blockers). This depolarization was completely blocked by AgRP. Although treatment with AgRP (100 nm for 5 min) alone did not modulate GnRH neuron electrical activity, MTII (100 nm) had no effect on GnRH neurons in the presence of AgRP (Fig. 4B). This implies that MTII can directly increase the activity of GnRH neurons through melanocortin receptors expressed in GnRH neurons and that AgRP can prevent such increases.
Induction of c-Fos expression in the brains of GnRH-GFP mice after MTII treatment was used to further test whether melanocortin signaling modulates GnRH neuron activity. We found that in MTII-treated animals, 42 ± 13% (n = 3) of the GnRH neurons in the medial preoptic area were FOS positive, significantly more (Mann-Whitney U test, P < 0.05) than in PBS-treated animals with only 21 ± 5% (n = 3) of the GnRH neurons coexpressing FOS (Fig. 4C).
Discussion
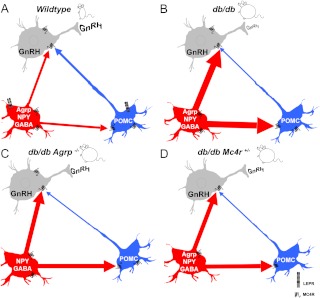
Here we describe the complete restoration of pubertal development, fertility, and lactation to leptin receptor-deficient females via modulations in melanocortin signaling. (Figure 5 briefly illustrates the potential mechanisms by which melanocortin signaling improves fertility in the db/db female.) Previous studies have shown restoration of puberty, and, to varying degrees, reproductive and lactational competence, in leptin signaling-deficient animals. These methods include treatment with exogenous leptin (51, 52), neuron-specific leptin receptor transgene expression in db/db mice (53, 54), unilateral reexpression of leptin receptor in the ventral premammillary nuclei of db/db female mice (55), and ablation of NPY or its receptor Y4R in ob/ob animals (27, 28). One key difference is that our study demonstrates restoration of normal pubertal onset and fertility to db/db females is independent of improvements in their obesity and diabetes phenotypes. Obesity and hyperinsulinemia persist in both the db/db Mc4r+/− and _db/db Agrp_−/− females, although the latter have normalized fasting glucose, which is likely be due to melanocortin signaling-mediated enhancements to insulin signaling (56).
Fig. 5.
Modeling of a network whereby leptin sensitive melanocortinergic neurons (both AGRP/NPY neurons and POMC neurons) directly regulate GnRH neuron activity via MC4R.
Female mice with deficient leptin signaling have delayed puberty, reduced uterine and ovarian weight as well as a decreased number of primary and secondary follicles in the ovary, abnormal estrous cyclicity, reduced sex steroid hormone concentrations, and impaired mammary gland morphology and function (5, 7, 46, 57). Consistent with previous reports, pubertal onset in db/db females was significantly delayed relative to wild-type animals (5). The age at pubertal onset, although still delayed in db/db Mc4r+/−, was restored to normal in _db/db Agrp_−/− females. Estrous cycling was restored in both db/db Mc4r +/− and _db/db Agrp_−/− females, indicating a correction of the acyclicity observed in db/db females. Serum 17β-estradiol and progesterone were restored to levels found in wild-type in _db/db Agrp_−/− females, but there were no significant improvements in these steroid levels in the db/db Mc4r+/− females despite evidence of estrous cycling. Interestingly, we failed to find significant differences in ovarian follicle numbers, regardless of follicular stage in any of our animals, suggesting that defects in follicular development are unlikely to be a major cause of infertility in female db/db mice. The presence of corpora lutea in db/db females is evidence of ovulatory events, although we have not assessed the competency of the db/db uterus and hormonal milieu for gestation. Although others have reported reduced ovarian follicle numbers with leptin signaling deficiency (58), several studies indicate that the ovaries of leptin signaling deficient animals are functional: ovaries from leptin signaling-deficient females respond to gonadotropin stimulation (5) and produce litters when transplanted into normal females (10, 46). Additionally, obese, leptin receptor-deficient (fa/fa) Zucker female rat ovaries have elevated total follicle numbers and ovarian dysfunction due to increased follicular atresia (59), which has also been previously reported in leptin-deficient mice (58). We believe that the differences in follicle numbers observed in our studies may be due to several factors including the younger ages at which animals were examined (in these studies 8–16 wk old) and the mixed strain used for these experiments.
Leptin signaling is important for normal mammary gland development (60). Animals with leptin signaling deficiencies have reduced secretion of the hormones estradiol, progesterone, prolactin, and GH (61), all of which are necessary for proper mammary gland development and function (62). Restoration of mammary gland development and function in the _db/db Agrp_−/− and db/db Mc4r +/− females is likely due to improvements in estradiol and progesterone production but possibly also increased prolactin and GH secretion from the pituitary as a result of modulations of melanocortin signaling (20, 30, 63). Although mammary ductal growth and side branching in _db/db Agrp_−/− and db/db Mc4r+/− females is incomplete, the successful rearing of litters to weaning confirms that ductal tissue improvements are sufficient for lactation.
It is increasingly apparent that melanocortin signaling plays an important role in reproductive function and behavior (18, 21, 63–66). POMC neurons in the arcuate nucleus project onto GnRH-immunoreactive cells in the medial preoptic area (26) in which activation of MC4R stimulates LH secretion (30). Using scRT-PCR and immunohistochemistry, we show MC4R expression in preoptic area GnRH neurons. Moreover, MTII treatment increased action potential firing and induced FOS expression in medial preoptic area GnRH neurons, suggesting that melanocortin signaling can regulate GnRH neuron activity. The improved fertility in _db/db Agrp_−/− and db/db Mc4r+/− female mice may also involve improvements in arousal and mating behavior, which we did not test. NPY is known to inhibit sexual behavior (67), and NPY overexpression along with AgRP in leptin signaling deficient rodents is likely to contribute to the diminished reproductive behaviors found in these animals. MC4R activation elicits lordosis behavior in female progesterone-primed rats (68), and its expression in hypothalamic regions is important for the control of female sexual function (64). Moreover, recent studies indicate that melanocortin receptor agonists can stimulate arcuate nucleus kisspeptin expression in ewes, thus providing an additional mechanism by which melanocortin signaling can impact reproductive outcome (18).
Conditions such as food restriction and obesity that induce overexpression of AgRP and NPY, or administration of exogenous AgRP or NPY, inhibit pulsatile LH release and reproductive function (21). Both POMC and AgRP/NPY neurons originating in the arcuate nucleus project onto GnRH neurons in the medial preoptic area (26, 69), likely regulate GnRH neuron function. The reduced AgRP and NPY mRNA levels detected in db/db Mc4r+/− females indicate that melanocortin signaling may regulate expression of these neuropeptides. Reductions in hypothalamic AgRP and NPY mRNA expression were also described in studies of MC4R knockout mice (70). We found that loss of MC4R signaling did not reduce AgRP/NPY neuron numbers in arcuate nuclei of MC4R knockout females when compared with wild types, suggesting that the reduced expression of AgRP and NPY in the db/db Mc4r+/− females is due to functional rather than structural changes. Despite normalization of AgRP and NPY mRNA levels in the db/db Mc4r+/− females improving fertility, there was no improvement in the obesity and diabetes phenotype. This may be in part due to reduced melanocortin signaling caused by leptin deficiency- mediated reductions of POMC, reduced MC4R expression, and estradiol deficiency (71, 72). Overall, the reproductive phenotype of the db/db Mc4r+/− females showed less improvement than the _db/db Agrp_−/− females. This discrepancy may be due to several factors, including reduced gonadal steroid levels, severe hyperglycemia, and greater insulin resistance in the db/db Mc4r+/− females.
It is well documented that leptin signaling deficiency has disparate effects, depending on the mouse strain examined (73–75). We believe that some of the results from these studies may be due to unique contributions from the mixed background of our breeding colonies. Specifically, we believe that the 129-strain background, on which most knockout alleles are generated, is a major contributor to the restoration of fertility to db/db females. However, infertility is retained in 129/J db3J/db3J mice, suggesting that a mixed strain background is also an important feature (76). We were able to identify MC4R expression and show its activity in GFP-expressing GnRH neurons from a mouse strain (C57BL/6) that was not representative of the mixed strain used in our studies. Although strain-specific modifier genes may be responsible for the unveiling of the fertility-promoting effects observed in these studies, it is apparent that melanocortin signaling is important for leptin dependent and independent regulation of reproductive function.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Drs. Anne Etgen, Genevieve Neal-Perry, and Genevieve Marcelin for advice, insight, and technical assistance as well as Robin Squeglia (Albert Einstein College of Medicine Hormone Assay Core Facility).
This work was supported by Grants U54HD058155, 5T32DK007513-24, R01DK057621, and P30DK02668 (to S.C.C.); Grant R01HD053529 (to D.J.S.), and American Diabetes Association A.D.A. Junior Faculty Award (to Y.-H.J.).
Disclosure Summary: The authors have no conflict to disclose.
Footnotes
Abbreviations:
aCSF
Artificial cerebrospinal fluid
AgRP
agouti-related peptide
GFP
green fluorescent protein
H&E
hematoxylin and eosin
MC4R
melanocortin 4 receptor
MTII
melanotan
NPY
neuropeptide Y
POMC
proopiomelanocortin
scRT-PCR
single-cell RT-PCR.
References
- 1.World Health Organization 2006. Obesity and overweight. In: Fact sheet no. 311. Geneva: World Health Organization Media Centre [Google Scholar]
- 2.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. 2003. Obesity and reproductive disorders in women. Hum Reprod Update 9:359–372 [DOI] [PubMed] [Google Scholar]
- 3.Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. 2004. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82:863–870 [DOI] [PubMed] [Google Scholar]
- 4.Israel D, Chua S., Jr 2010. Leptin receptor modulation of adiposity and fertility. Trends Endocrinol Metab 21:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batt RA, Everard DM, Gillies G, Wilkinson M, Wilson CA, Yeo TA. 1982. Investigation into the hypogonadism of the obese mouse (genotype ob/ob). J Reprod Fertil 64:363–371 [DOI] [PubMed] [Google Scholar]
- 6.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147 [DOI] [PubMed] [Google Scholar]
- 7.Ingalls AM, Dickie MM, Snell GD. 1950. Obese, a new mutation in the house mouse. J Hered 41:317–318 [DOI] [PubMed] [Google Scholar]
- 8.Saiduddin S, Bray GA, York DA, Swerdloff RS. 1973. Reproductive function in the genetically obese “fatty” rat. Endocrinology 93:1251–1256 [DOI] [PubMed] [Google Scholar]
- 9.Swerdloff RS, Batt RA, Bray GA. 1976. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology 98:1359–1364 [DOI] [PubMed] [Google Scholar]
- 10.Johnson LM, Sidman RL. 1979. A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod 20:552–559 [DOI] [PubMed] [Google Scholar]
- 11.Trevaskis JL, Butler AA. 2005. Double leptin and melanocortin-4 receptor gene mutations have an additive effect on fat mass and are associated with reduced effects of leptin on weight loss and food intake. Endocrinology 146:4257–4265 [DOI] [PubMed] [Google Scholar]
- 12.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 13.Sina M, Hinney A, Ziegler A, Neupert T, Mayer H, Siegfried W, Blum WF, Remschmidt H, Hebebrand J. 1999. Phenotypes in three pedigrees with autosomal dominant obesity caused by haploinsufficiency mutations in the melanocortin-4 receptor gene. Am J Hum Genet 65:1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. 1998. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- 15.Butler AA, Cone RD. 2002. The melanocortin receptors: lessons from knockout models. Neuropeptides 36:77–84 [DOI] [PubMed] [Google Scholar]
- 16.Schiöth HB, Watanobe H. 2002. Melanocortins and reproduction. Brain Res Brain Res Rev 38:340–350 [DOI] [PubMed] [Google Scholar]
- 17.Backholer K, Bowden M, Gamber K, Bjørbaek C, Iqbal J, Clarke IJ. 2010. Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology 91:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Backholer K, Smith J, Clarke IJ. 2009. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 150:5488–5497 [DOI] [PubMed] [Google Scholar]
- 19.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138 [DOI] [PubMed] [Google Scholar]
- 20.Schioth HB, Kakizaki Y, Kohsaka A, Suda T, Watanobe H. 2001. Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport 12:687–690 [DOI] [PubMed] [Google Scholar]
- 21.Vulliémoz NR, Xiao E, Xia-Zhang L, Wardlaw SL, Ferin M. 2005. Central infusion of agouti-related peptide suppresses pulsatile luteinizing hormone release in the ovariectomized rhesus monkey. Endocrinology 146:784–789 [DOI] [PubMed] [Google Scholar]
- 22.Catzeflis C, Pierroz DD, Rohner-Jeanrenaud F, Rivier JE, Sizonenko PC, Aubert ML. 1993. Neuropeptide Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology 132:224–234 [DOI] [PubMed] [Google Scholar]
- 23.Pierroz DD, Catzeflis C, Aebi AC, Rivier JE, Aubert ML. 1996. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology 137:3–12 [DOI] [PubMed] [Google Scholar]
- 24.Nilsson I, Johansen JE, Schalling M, Hökfelt T, Fetissov SO. 2005. Maturation of the hypothalamic arcuate agouti-related protein system during postnatal development in the mouse. Brain Res Dev Brain Res 155:147–154 [DOI] [PubMed] [Google Scholar]
- 25.Cheung S, Hammer RP., Jr 1995. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in arcuate neurons that innervate the medial preoptic area of the rat. Neuroendocrinology 62:283–292 [DOI] [PubMed] [Google Scholar]
- 26.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. 1988. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res 449:167–176 [DOI] [PubMed] [Google Scholar]
- 27.Erickson JC, Hollopeter G, Palmiter RD. 1996. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274:1704–1707 [DOI] [PubMed] [Google Scholar]
- 28.Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, Herzog H. 2002. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev 16:1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. 2011. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanobe H, Schiöth HB, Wikberg JE, Suda T. 1999. The melanocortin 4 receptor mediates leptin stimulation of luteinizing hormone and prolactin surges in steroid-primed ovariectomized rats. Biochem Biophys Res Commun 257:860–864 [DOI] [PubMed] [Google Scholar]
- 31.Chai B, Li JY, Zhang W, Newman E, Ammori J, Mulholland MW. 2006. Melanocortin-4 receptor-mediated inhibition of apoptosis in immortalized hypothalamic neurons via mitogen-activated protein kinase. Peptides 27:2846–2857 [DOI] [PubMed] [Google Scholar]
- 32.Khong K, Kurtz SE, Sykes RL, Cone RD. 2001. Expression of functional melanocortin-4 receptor in the hypothalamic GT1–1 cell line. Neuroendocrinology 74:193–201 [DOI] [PubMed] [Google Scholar]
- 33.Kowalski TJ, Liu SM, Leibel RL, Chua SC., Jr 2001. Transgenic complementation of leptin-receptor deficiency. I. Rescue of the obesity/diabetes phenotype of LEPR-null mice expressing a LEPR-B transgene. Diabetes 50:425–435 [DOI] [PubMed] [Google Scholar]
- 34.Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ. 2002. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 22:5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. 1999. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−ΔΔC(T)] method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 37.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr 2008. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149:1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magelhaes PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int 11:36–42 [Google Scholar]
- 39.Goldman JM, Murr AS, Cooper RL. 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- 40.Pedersen T, Peters H. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- 41.Chun SK, Jo YH. 2010. Loss of leptin receptors on hypothalamic POMC neurons alters synaptic inhibition. J Neurophysiol 104:2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton JE, Cheung CC, Clifton DK, Steiner RA. 1997. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066 [DOI] [PubMed] [Google Scholar]
- 43.Mizuno TM, Mobbs CV. 1999. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140:814–817 [DOI] [PubMed] [Google Scholar]
- 44.Wilding JP, Gilbey SG, Bailey CJ, Batt RA, Williams G, Ghatei MA, Bloom SR. 1993. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology 132:1939–1944 [DOI] [PubMed] [Google Scholar]
- 45.Chua SC, Jr, Leibel RL, Hirsch J. 1991. Food deprivation and age modulate neuropeptide gene expression in the murine hypothalamus and adrenal gland. Brain Res Mol Brain Res 9:95–101 [DOI] [PubMed] [Google Scholar]
- 46.Hummel KP, Dickie MM, Coleman DL. 1966. Diabetes, a new mutation in the mouse. Science 153:1127–1128 [DOI] [PubMed] [Google Scholar]
- 47.Garris DR, Garris BL. 2003. Diabetes (db/db) mutation-induced ovarian involution: progressive hypercytolipidemia. Exp Biol Med (Maywood, NJ) 228:1040–1050 [DOI] [PubMed] [Google Scholar]
- 48.Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. 1998. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18:213–215 [DOI] [PubMed] [Google Scholar]
- 49.Skynner MJ, Sim JA, Herbison AE. 1999. Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140:5195–5201 [DOI] [PubMed] [Google Scholar]
- 50.Pape JR, Skynner MJ, Sim JA, Herbison AE. 2001. Profiling γ-aminobutyric acid [GABA(A)] receptor subunit mRNA expression in postnatal gonadotropin-releasing hormone (GnRH) neurons of the male mouse with single cell RT-PCR. Neuroendocrinology 74:300–308 [DOI] [PubMed] [Google Scholar]
- 51.Chehab FF, Lim ME, Lu R. 1996. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320 [DOI] [PubMed] [Google Scholar]
- 52.Farooqi IS, O'Rahilly S. 2005. Monogenic obesity in humans. Annu Rev Med 56:443–458 [DOI] [PubMed] [Google Scholar]
- 53.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr 2005. Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest 115:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chua SC, Jr, Liu SM, Li Q, Sun A, DeNino WF, Heymsfield SB, Guo XE. 2004. Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am J Physiol Endocrinol Metab 286:E384–E392 [DOI] [PubMed] [Google Scholar]
- 55.Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. 2011. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121:355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chai B, Li JY, Zhang W, Wu X, Zhang C, Mulholland MW. 2010. Melanocortin-4 receptor activation promotes insulin-stimulated mTOR signaling. Peptides 31:1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane PW. 1959. The pituitary-gonad response of genetically obese mice in parabiosis with thin and obese siblings. Endocrinology 65:863–868 [DOI] [PubMed] [Google Scholar]
- 58.Hamm ML, Bhat GK, Thompson WE, Mann DR. 2004. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod 71:66–72 [DOI] [PubMed] [Google Scholar]
- 59.Kajihara T, Uchino S, Suzuki M, Itakura A, Brosens JJ, Ishihara O. 2009. Increased ovarian follicle atresia in obese Zucker rats is associated with enhanced expression of the forkhead transcription factor FOXO1. Med Mol Morphol 42:216–221 [DOI] [PubMed] [Google Scholar]
- 60.Hu X, Juneja SC, Maihle NJ, Cleary MP. 2002. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94:1704–1711 [DOI] [PubMed] [Google Scholar]
- 61.Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263–307 [DOI] [PubMed] [Google Scholar]
- 62.Watson CJ, Khaled WT. 2008. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135:995–1003 [DOI] [PubMed] [Google Scholar]
- 63.Watanobe H, Yoneda M. 2003. Evaluation of the role of melanocortin 3 and 4 receptors in leptin-stimulated and spontaneous growth hormone secretion in rats. Neuroendocrinology 78:331–338 [DOI] [PubMed] [Google Scholar]
- 64.Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Alexandre L, Giuliano F. 2010. Neuroanatomical evidence for a role of central melanocortin-4 receptors and oxytocin in the efferent control of the rodent clitoris and vagina. J Sex Med 7:2056–2067 [DOI] [PubMed] [Google Scholar]
- 65.Shadiack AM, Sharma SD, Earle DC, Spana C, Hallam TJ. 2007. Melanocortins in the treatment of male and female sexual dysfunction. Curr Top Med Chem 7:1137–1144 [DOI] [PubMed] [Google Scholar]
- 66.Wessells H, Levine N, Hadley ME, Dorr R, Hruby V. 2000. Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II. Int J Impot Res 12(Suppl 4):S74–S79 [DOI] [PubMed] [Google Scholar]
- 67.Marin-Bivens CL, Kalra SP, Olster DH. 1998. Intraventricular injection of neuropeptide Y antisera curbs weight gain and feeding, and increases the display of sexual behaviors in obese Zucker female rats. Regul Pept 75–76:327–334 [DOI] [PubMed] [Google Scholar]
- 68.Rössler AS, Pfaus JG, Kia HK, Bernabé J, Alexandre L, Giuliano F. 2006. The melanocortin agonist, melanotan II, enhances proceptive sexual behaviors in the female rat. Pharmacol Biochem Behav 85:514–521 [DOI] [PubMed] [Google Scholar]
- 69.Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. 2003. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology 144:4967–4974 [DOI] [PubMed] [Google Scholar]
- 70.Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. 2005. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun 326:638–644 [DOI] [PubMed] [Google Scholar]
- 71.Mystkowski P, Schwartz MW. 2000. Gonadal steroids and energy homeostasis in the leptin era. Nutrition 16:937–946 [DOI] [PubMed] [Google Scholar]
- 72.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK. 2010. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hummel KP, Coleman DL, Lane PW. 1972. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet 7:1–13 [DOI] [PubMed] [Google Scholar]
- 74.Ewart-Toland A, Mounzih K, Qiu J, Chehab FF. 1999. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology 140:732–738 [DOI] [PubMed] [Google Scholar]
- 75.Qiu J, Ogus S, Mounzih K, Ewart-Toland A, Chehab FF. 2001. Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology 142:3421–3425 [DOI] [PubMed] [Google Scholar]
- 76.Leiter EH, Coleman DL, Eisenstein AB, Strack I. 1980. A new mutation (db3J) at the diabetes locus in strain 129/J mice. I. Physiological and histological characterization. Diabetologia 19:58–65 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data