N-Glycosylation Determines Ionic Permeability and Desensitization of the TRPV1 Capsaicin Receptor (original) (raw)
Background: We studied effects of _N_-linked sugar residues on the sensory ion channel, TRPV1.
Results: Glycosylation of TRPV1 did not alter cell surface expression but was necessary for sustained cell calcium responses to allow uptake of YO-PRO-1 dye.
Conclusion: _N_-Glycosylation regulated inactivation and ion selectivity but not expression of TRPV1.
Significance: _N_-Glycosylation is a basic regulatory mechanism of TRPV1.
Keywords: Calcium Imaging, Glycosylation, Ion Channels, Receptor Desensitization, TRP Channels
Abstract
The balance of glycosylation and deglycosylation of ion channels can markedly influence their function and regulation. However, the functional importance of glycosylation of the TRPV1 receptor, a key sensor of pain-sensing nerves, is not well understood, and whether TRPV1 is glycosylated in neurons is unclear. We report that TRPV1 is _N_-glycosylated and that _N_-glycosylation is a major determinant of capsaicin-evoked desensitization and ionic permeability. Both _N_-glycosylated and unglycosylated TRPV1 was detected in extracts of peripheral sensory nerves by Western blotting. TRPV1 expressed in HEK-293 cells exhibited various degrees of glycosylation. A mutant of asparagine 604 (N604T) was not glycosylated but did not alter plasma membrane expression of TRPV1. Capsaicin-evoked increases in intracellular calcium ([Ca2+]i) were sustained in wild-type TRPV1 HEK-293 cells but were rapidly desensitized in N604T TRPV1 cells. There was marked cell-to-cell variability in capsaicin responses and desensitization between individual cells expressing wild-type TRPV1 but highly uniform responses in cells expressing N604T TRPV1, consistent with variable levels of glycosylation of the wild-type channel. These differences were also apparent when wild-type or N604T TRPV1-GFP fusion proteins were expressed in neurons from _trpv1_−/− mice. Capsaicin evoked a marked, concentration-dependent increase in uptake of the large cationic dye YO-PRO-1 in cells expressing wild-type TRPV1, indicative of loss of ion selectivity, that was completely absent in cells expressing N604T TRPV1. Thus, TRPV1 is variably _N_-glycosylated and glycosylation is a key determinant of capsaicin regulation of TRPV1 desensitization and permeability. Our findings suggest that physiological or pathological alterations in TRPV1 glycosylation would affect TRPV1 function and pain transmission.
Introduction
The regulation of glycosylation status and the ways it affects protein function are incompletely understood, and the lack of general principles means that they must be determined empirically for most proteins of interest. The balance of glycosylation states has functional consequences for many receptors and ion channels by several mechanisms. _N_-Glycosylation of newly synthesized proteins influences protein trafficking to the cell membrane, can alter functional characteristics and is likely to play a role in physiological regulation (1). The importance of glycosylation status in neurological states is demonstrated in congenital glycosylation disorders (2). In this study we evaluated N-glycosylation of the nonspecific cation channel, Transient Receptor Potential Vanilloid Type 1 (TRPV1)2 which is a key sensor of pain-sensing nerve fibers. We determined the glycosylation status of TRPV1 and investigated the functional consequences of glycosylation at asparagine 604 (N604) in primary neurons and transformed cells.
There are conflicting reports about the glycosylation state of TRPV1. Endogenous TRPV1 in dorsal root ganglia (DRG) was not found to be glycosylated (3). However, when heterologously expressed in HEK-293 cells and in DRG-derived F-11 cells, TRPV1 was reported to be _N_-glycosylated at asparagine 604, a position close to both the presumed pore-forming region (3, 4) and a glutamate important for activation by protons (5). _N_-Glycosylation can modulate the activities of TRPV1 and related channels (6–9). In these studies, mutation of the putative _N_-glycosylation site of TRPV1 to N604T reduced the maximum capsaicin current, the pH dependence of the capsaicin response and the antagonistic effects of capsazepine, but increased the potency of capsaicin (6). However, that study did not assess whether glycosylation influenced the expression or membrane-targeting of TRPV1, and electrophysiological experiments were at room temperature and used Ba2+ as the charge carrier in Ca2+-free solution to prevent normal TRPV1 desensitization (10). The influence of _N_-glycosylation of TRPV1 on desensitization and permeability are unknown. An understanding of the mechanisms of TRPV1 regulation is important because painful stimuli, such as capsaicin from chili peppers and protons that are generated during inflammation, directly activate TRPV1, and many receptors indirectly sensitize TRPV1 by post-translational mechanisms that include channel phosphorylation (11).
In addition to its permeability to small cations like Na+, K+, and Ca2+ and Ba2+, TRPV1 can also enter an open state that is permeable to larger cations, termed pore dilation (12, 13). Other TRP channels can also exhibit pore dilation, which can be assessed by measuring uptake of the blue fluorescent cationic dye YO-PRO-1 (14, 15). Pore dilation has physiological and pharmacological ramifications because it may enhance uptake of small cations such as Ca2+, which can influence TRPV1 desensitization and down-stream signals, including Ca2+-dependent neuropeptide release and neurotoxicity. Pore dilation may also facilitate the delivery of otherwise impermeant drugs into cells (16). The role of _N_-glycosylation in TRPV1 channel pore dilation is not known. Herein we report _N_-glycosylation of endogenous and heterologously expressed TRPV1 and describe a major effect of _N_-glycosylation on the TRPV1 desensitization and ion permeability.
EXPERIMENTAL PROCEDURES
Reagents
Cell culture reagents were from Invitrogen (Mulgrave, Victoria, Australia) and agonists were from Sigma unless otherwise stated.
Animals
Sensory neurons were cultured from 4–7-week-old male Sprague-Dawley rats or 4–6-week-old male _trpv1_−/− mice (B6.129X1-Trpv1tm1Jul/J) (Jackson Laboratories, Bar Harbor, ME). Animals had free access to food and water with a 12 h light/dark cycle. All procedures conformed to the National Health and Medical Research Council, Australia code of practice for the use of animals in research and were approved by the appropriate Animal Experimentation Ethics Committees at the University of Melbourne or the University of California, San Francisco.
Construction of TRPV1 Expression Vectors
Rat TRPV1 clone in pcDNA5/FRT/TO (17) was obtained from Novartis (Horsham, UK). An HA11 epitope tag was added to the carboxy-terminus to facilitate detection and site-directed mutagenesis was performed to generate untagged or tagged N604T TRPV1 (see supplemental Experimental Procedures). The N604T mutation was chosen for replication and extension of previous studies (4, 6).
Transfections and Generation of Cell Lines
T-Rex-293 (HEK-293) FlpIn cells (Invitrogen) were used to generate stable cell lines expressing unmodified or HA-tagged wild type and N604T rat TRPV1, as previously described for CHO cells (17). Expression was induced with 1 μg/ml tetracycline 4 h prior to testing unless otherwise stated. Transient transfection of _trpv1_−/− mouse DRG cells with wild type- or N604T-rat TRPV1-eGFP fusion constructs was achieved using an Amaxa Nucleofector (Lonza, Walkersville, MD). The fusion construct, pZS5 was a kind gift from Dr Zoltán Sándor, University of Pécs, Hungary (18). Cells were imaged 24 to 48 h after transfection.
Western Blotting
Proteins were resolved in Criterion 4–15% Tris-glycine gels (Bio-Rad) and electroblotted onto nitrocellulose membrane (Protran, Whatman GmbH, Dassel, Germany). Blotted nitrocellulose filters were blocked with TBS-T (25 mm Tris-HCl (pH 7.2), 150 mm NaCl, 0.01% Tween 20) containing 5% dried nonfat milk overnight at 4 °C. Membranes were incubated with primary antibody (anti-TRPV1 Rb1–130-199-ws (from Flinders University, South Australia); monoclonal anti-HA 1:2000 (from Sigma-Aldrich); or anti-transferrin receptor 1:1000) from Invitrogen, Mulgrave, Victoria, Australia), in TBS-T and 5% dried nonfat milk for 2 h, washed three times in TBS-T and incubated with 1:5000 IRDye 680 goat anti-rabbit and/or IRDye 800 donkey anti-mouse IgG in TBS-T and 5% dried nonfat milk for 1 h. Membranes were imaged using an Odyssey Infrared imager (Li-Cor Biosciences, Nebraska). Signal density of anti-HA and TfR signal was quantified using ImageJ software (20).
Surface Biotinylation Assay and PNGase F Treatment
Cell-surface labeling was performed with EZ-Link Sulfo-NHS-LC-Biotin (Pierce) as described previously (19). Western blot pixel intensities were quantified relative to transferrin receptor protein (TfR). _N_-Glycosidase F (500 units) treatment of protein lysate was performed according to the manufacturer's instructions, without boiling (New England Biolabs, Ispwich, MA). To improve resolution rat tissue lysates were treated for 30 min at room temperature with 0.1 m DTT prior to SDS-PAGE.
Measurement of [Ca2+]i
Details of [Ca2+]i measurement are provided in detail in supplemental Experimental Procedures. For population studies, cells were seeded and grown to near-confluence over 48 h and [Ca2+]i was measured by Fura-2 fluorescence (340/380 nm excitation/510 nm emission; every 4 s) using a FlexStation 3 fluorimeter (Molecular Devices, Sunnyvale, CA) as described previously (19). Data were plotted as the means ± S.E. [Ca2+]i in individual cells was measured with a Leica AF-6000 LX fluorescent imaging system (Leica, Germany) every 5 s with 510 nm emission intensity at 340 nm/380 nm excitation. HEK-293 cell images were acquired with a 20× dry objective (310 ms exposure), neuron images acquired with a 10x dry objective (148 ms exposure) on a heated stage (37 °C) and HEPES-buffered solution pre-equilibrated to 37 °C. Image stacks were processed using ImageJ software (20). In neuronal cultures, capsaicin-responsive cell bodies in the microscopic field were selected as a region of interest (ROI). In HEK-293 cultures, each cell was automatically delineated into a ROI, and the time course of the median Fura-2 ratio for each ROI was combined and plotted as the mean ± standard deviation.
Data Analysis
Acute desensitization was measured as the difference between the maximum response (within the first 60 s) and the last measured response level (105 s after capsaicin addition). For cells (or wells) with well-defined early peaks followed by fading of the response, the desensitization index was a positive value. Cellular activation by capsaicin was determined by concentration-response curves using the maximum response (peak in first 60 s). The largest observed rate of change in the first 60 s (i.e. the steepest slope of the Fura-2 ratio response was also measured.
YO-PRO-1 Assay
YO-PRO-1 was injected into wells to a final concentration of 2 μm in HEPES buffered solution and monitored by fluorescence (485 nm excitation/516 emission) every 10 s using the FlexStation 3 fluorimeter. Capsaicin solutions were injected at 280 s.
RESULTS
TRPV1 is N-Glycosylated at the Plasma Membrane and in the Soma and Axons
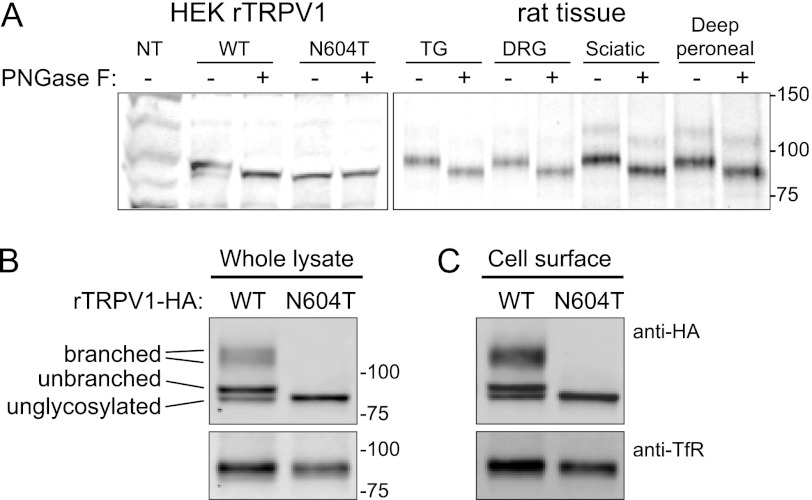
Because there are conflicting reports about TRPV1 glycosylation in neurons (3) and cell lines (6), we examined whether n-glycosidase F (PNGaseF) affected the apparent molecular weight of TRPV1 by Western blotting using a TRPV1 antibody. Analysis of HEK-293 cells expressing rat TRPV1, rat trigeminal and dorsal root ganglia, and peripheral sciatic and deep peroneal nerve segments, detected a predominant form of TRPV1 of 95 kDa (Fig. 1A). PNGase F-treatment reduced the molecular weight of TRPV1 to 86 kDa. Analysis of lysates of HEK-293 cells expressing HA11-tagged TRPV1 revealed bands that are consistent with previous studies (4) and have previously been predicted to correspond to simple and complex glycosylated forms of TRPV1 (Fig. 1B). In cells expressing a glycosylation-defective TRPV1 mutant (N604T), only unglycosylated TRPV1 (86 kDa) was detected. To assess whether TRPV1 is glycosylated at the plasma membrane, we biotinylated and enriched cell surface proteins by streptavidin pull-down, followed by Western analysis. Unglycosylated and glycosylated TRPV1 were detected at the plasma membrane of cells expressing wild-type but not N604T (Fig. 1C). By combining the signal densities of all glycosylated species, similar levels of expression were measured for wild-type and N604T TRPV1 in whole lysates. Despite the presence of multiple bands, signal density measurements of TRPV1 in the biotinylated cell surface fractions (relative to the TfR immuno-blot control) revealed no significant difference between wild-type and N604T TRPV1 protein (p = 0.08, paired t test, n = 6).
FIGURE 1.
TRPV1 expression and glycosylation. A, expression patterns of wild type and N604T TRPV1 in HEK-293 cells and from rat trigeminal ganglion (TG), dorsal root ganglion (DRG), peripheral sciatic and peroneal nerve segments were compared before and after digestion by PNGase F, as indicated. The Western blot in panel A was probed with anti-TRPV1 antibody. The expression levels of HA-tagged wild-type and N604T TRPV1 from (B) whole cell lysates and (C) biotinylated cell surface fractions were used to compare expression levels (normalized to transferrin receptor). No difference was observed between wild-type and N604T biotinylated protein (p = 0.08, paired t test, n = 6).
Thus, TRPV1 is N-glycosylated at asparagine 604, and glycosylated TRPV1 is present in the soma (ganglia) and in axons (nerve trunks). Glycosylated TRPV1 is present at the cell-surface, but glycosylation is not essential for the localization of TRPV1 at the plasma membrane in our expression system.
N-Glycosylation Impedes Acute TRPV1 Desensitization
To assess the functional importance of TRPV1 glycosylation, we compared capsaicin-induced increases in [Ca2+]i between populations of HEK-293 cells expressing wild-type or N604T TRPV1. The resting [Ca2+]i was slightly higher in cells expressing wild-type TRPV1 (Fura-2 ratios: wild-type 1.64 ± 0.04 versus N604T 1.36 ± 0.01, p < 0.001 _t_ test, Fig. 2, _A_ and _B_). Since wild-type and N604T TRPV1 were expressed at similar levels (Fig. 1, _B_ and _C_), this difference is a consequence of altered function of N604T TRPV1. However, there were marked differences in the duration of capsaicin signals between cells expressing wild-type and N604T TRPV1. In cells expressing wild-type TRPV1, capsaicin stimulated a concentration-dependent increase in [Ca2+]_i_ that was sustained for >105 s (Fig. 2A). In contrast, capsaicin responses in cells expressing N604T TRPV1 were sustained at low concentrations of capsaicin but transient at concentrations of capsaicin of ≥300 nm (Fig. 2B). This fading of responses likely represents acute TRPV1 desensitization. Altered desensitization was maintained in cells expressing HA-tagged wild-type or N604T TRPV1 (Fig. 2C).
FIGURE 2.
Time courses of TRPV1 HEK-293 cell responses to capsaicin. Capsaicin was applied at time 0 at concentrations from 1 nm to 10 μm to HEK-293 cells expressing TRPV1 (A) or N604T TRPV1 (B) for clarity, the responses to the 3, 30, 300, and 3000 nm have no marker points. C, responses to 10 μm capsaicin of cells expressing HA-tagged (squares) or untagged (circles) versions of the TRPV1 (open symbols) and N604T channels (filled symbols). Data are from n = 6 experiments conducted in triplicate.
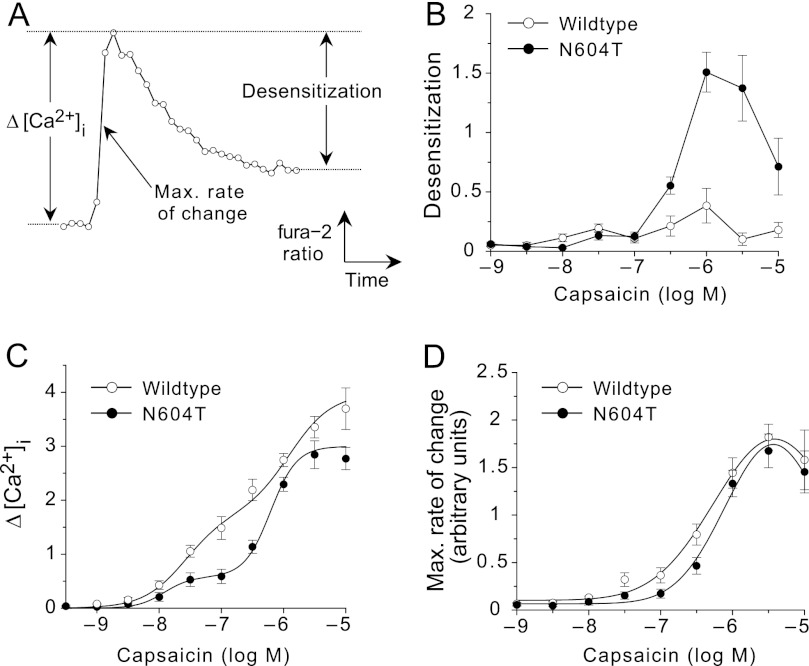
To evaluate this difference in capsaicin-evoked desensitization, we expressed the data as a capsaicin concentration-dependent desensitization curve. We defined desensitization as the maximal Fura-2 ratio detected within 60 s after capsaicin exposure minus the ratio at 105 s, and plotted this against the tested capsaicin concentrations (Fig. 3A). Whereas wild-type TRPV1 exhibited minimal desensitization to any concentration of capsaicin, N604T TRPV1 strongly desensitized to >300 nm capsaicin (Fig. 3B) and it had a bell-shaped concentration response, which is consistent with the ability of high concentrations of capsaicin to overcome desensitization induced by prior application of lower concentrations (21).
FIGURE 3.
Capsaicin concentration-response curves for HEK-293 TRPV1 cells and N604T TRPV1 cells. Data are from the experiments shown in Fig. 2, expressed as mean ± S.E. A, schematic representing measurements for maximal rate of change, maximum [Ca2+]i and desensitization. Desensitization was calculated as the difference between the maximum [Ca2+]i during the first 60 s of agonist exposure and the Fura-2 ratio at 105 s post-application. TRPV1 cells (open symbols) and N604T TRPV1 cells (filled symbols). B, capsaicin concentration-dependent desensitization curves. C, Capsaicin concentration-dependent curves expressed as Δ[Ca2+]i represents that maximal change in [Ca2+]i in the first 60 s. D, responses expressed as the maximal rate of change to minimize effects of desensitization.
By altering the TRPV1 induction time with tetracycline, we were able to study the effect of channel expression on capsaicin-induced desensitization. Analysis of expression kinetics and degree of desensitization indicated that the differential desensitization of wild-type TRPV1 and N604T TRPV1 is a consequence of altered channel function rather than expression levels (supplemental Figs. S2 and S3).
Capsaicin concentration-response curves derived from the maximal Fura-2 ratios were biphasic in cells expressing wild-type and N604T TRPV1, but maximum responses were greater in cells expressing wild-type TRPV1 (Fig. 3C) (wild-type 3.93 ± 0.18 versus N604T 2.98 ± 0.21, p = 0.01 t test). Since the more rapid desensitization of N604T TRPV1 would be expected to depress the maximal responses to capsaicin, we also analyzed the maximal rate of change of [Ca2+]i, an early index of response that should be less affected by acute desensitization (Fig. 3A). The resulting concentration-response curves were almost identical, with similar maximum responses and potencies (Fig. 3D) (EC50, -log M, wild-type 6.36 ± 0.13 versus N604T 6.17 ± 0.08, p = 0.07 t test).
Thus, despite a similar potency and efficacy of the immediate response to capsaicin in cells expressing wild-type and N604T TRPV1, a sustained response to higher concentrations of capsaicin is only observed in cells expressing the wild-type channel. Our results suggest that _N_-glycosylation impedes TRPV1 desensitization.
Nonspecific removal of TRPV1 _N_-glycosylation using pharmacological and enzymatic approaches was also performed. Inhibiting glycoprotein synthesis of wild-type or N604T TRPV1 cells with tunicamycin (30 μg/ml for 4 h) causes comparable reductions in capsaicin responses. This is indicative of a role for glycoproteins in the synthesis and assembly of TRPV1 in both cell types (supplemental Fig. S4_A_). Treatment of living TRPV1 cells with the endoglycosidase PNGaseF (1000 units/ml for 4 h) also shows no effect on TRPV1 capsaicin responses (supplemental Fig. S4_B_). This supports a mutagenic approach for understanding the functional importance of TRPV1 _N_-linked glycosylation.
Cells Expressing Glycosylation-defective N604T TRPV1 Uniformly Desensitize to Capsaicin
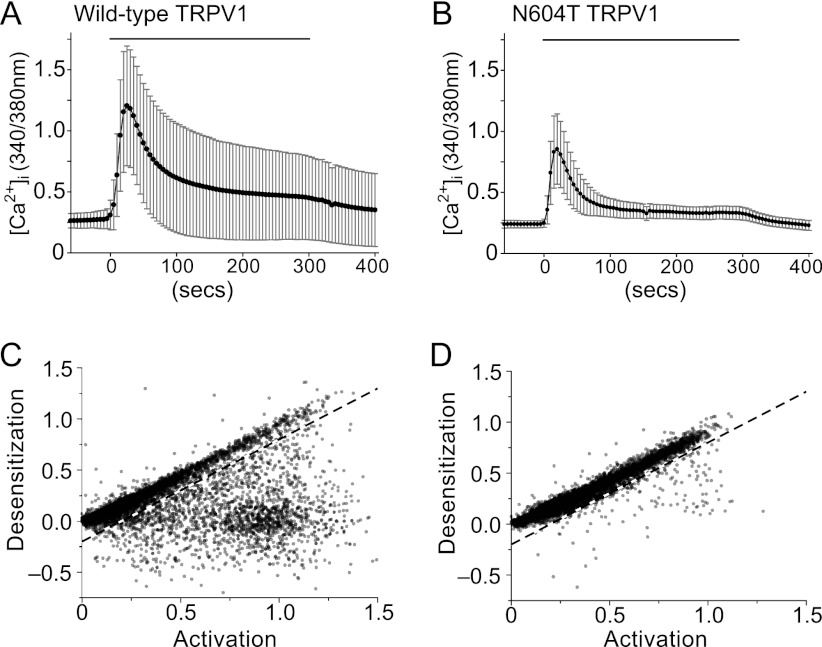
Western analysis of HEK cells expressing wild-type TRPV1 revealed various states of channel glycosylation, possibly between individual cells. If _N_-glycosylation impedes desensitization, then cell-to-cell variability in TRPV1 desensitization would be large in cells expressing wild-type but not N604T TRPV1. To examine this hypothesis, we studied the kinetics of capsaicin-evoked changes in [Ca2+]i in individual cells expressing wild-type or N604T TRPV1. Cells were challenged with a single concentration of capsaicin (wild-type, 0.3–3 μm, N604T 0.3–10 μm). Consistent with our observations in populations of cells, the average resting [Ca2+]i in cells expressing wild-type TRPV1 was slightly higher than in cells expressing N604T TRPV1 (95% confidence intervals, wild-type 0.216 to 0.219 versus N604T 0.185 to 0.187). However, the basal values were more variable between cells expressing wild-type than N604T TRPV1 (Fura-2 standard deviations, wild-type 0.140 versus N604T 0.093) (Fig. 4A). This heightened variability of [Ca2+]i in cells expressing TRPV1 remained in capsaicin-evoked responses, with some cells expressing a sustained response to capsaicin and others a transient response. In contrast, cells expressing N604T TRPV1 always displayed a transient response (Fig. 4B). A plot of desensitization versus the rate of activation of wild-type TRPV1 revealed two distinct populations of cells, one with direct proportionality between desensitization and activation, and another where there is little desensitization relative to the activation (Fig. 4C). An arbitrary linear boundary divides these populations, yielding 21.9 to 23.7% (95% confidence interval) of the cells expressing sustained responses. The responses of cells expressing N604T TRPV1 was much less variable and similar to the desensitizing response observed for this mutant in populations of cells (Fig. 4D). Almost all of the N604T cells gave transient responses, with only 2.4 to 3.1% (95% confidence interval) displaying sustained responses. It is likely that the subpopulation of wild-type TRPV1-expressing cells exhibiting sustained responses is also responsible for the difference in time course seen at the population level because while they are a relatively minor population (∼20%), their individual maximal Fura-2 response was more than twice that of the larger subpopulation (95% confidence intervals 1.09 to 1.12 versus 0.41 to 0.42). Thus, variability in desensitization rates between cells expressing wild-type TRPV1 is likely due to cell-to-cell differences in TRPV1 glycosylation.
FIGURE 4.
TRPV1 desensitization in cell populations. A, typical Fura-2 ratiometric images and time-course of [Ca2+]i (340/380 nm; mean ± S.D.) from individual HEK-293 cells expressing TRPV1 in one microscopic field (200×, ∼500 cells), following application of 1 μm capsaicin. B, 200× microscopic field and time-course (mean ± S.D.) of N604T mutant TRPV1 cells following application of 3 μm capsaicin. 1 min vehicle only control and 5 min capsaicin treatments are indicated. C and D, relationship between maximal rate of change in [Ca2+]i (activation) within the first 60 s of capsaicin exposure and desensitization after 180 s are shown for individual HEK293 cells expressing TRPV1 (C) or N604T (D). Negative values of desensitization result from responses that increase between 60 s and 180 s. Each graph shows the results from multiple experiments in which responses of all cells in a microscopic field were assessed. Wild-type TRPV1 (C) data are from 8324 cells in 14 experiments with capsaicin concentrations of 0.3, 1, and 3 μm. N604T data (D) are from 10054 cells in 15 experiments with capsaicin concentrations of 0.3, 1, 3, and 10 μm. The dashed lines are an arbitrary boundary between cells expressing well-sustained responses and cells with desensitizing responses.
N-Glycosylation Determines TRPV1 Desensitization in Sensory Neurons
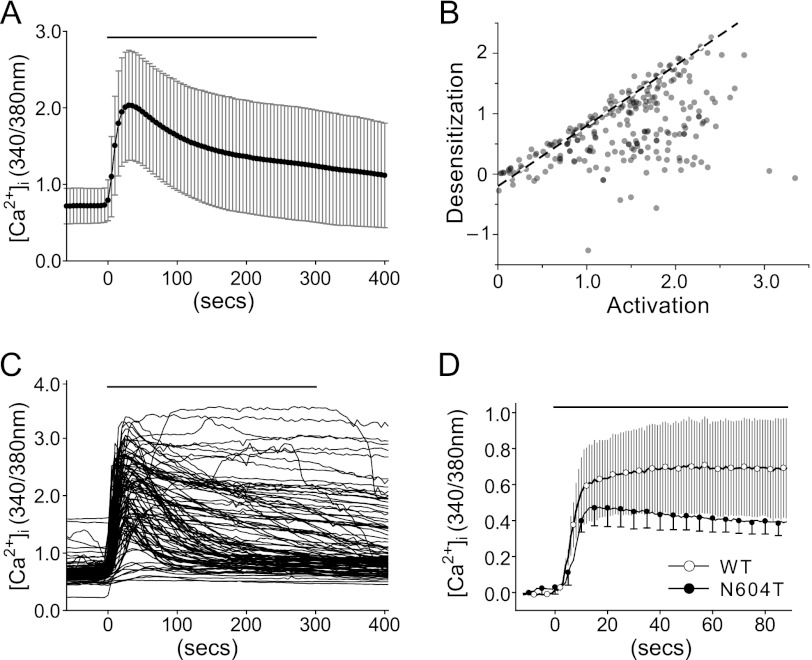
To determine if acute desensitization of native TRPV1 occurs in sensory neurons, we measured the capsaicin (1 μm) responses of neurons from trigeminal ganglia. A range of desensitization rates was detected (Fig. 5, A--C), consistent with a range of glycosylated forms of native TRPV1 to be present in sensory neurons. To determine if the differences in desensitization between wild-type and N604T TRPV1 are also retained in sensory neurons, we expressed wild-type and N604T rat TRPV1-GFP fusion proteins in DRG neurons from _trpv1_−/− mice. Consistent with responses measured in cell lines, 1 μm capsaicin evoked sustained increases in [Ca2+]i in neurons expressing wild-type TRPV1-GFP (over a 85 s period) and acute desensitization in neurons expressing N604T-GFP (Fig. 5D). Neurons expressing wild-type TRPV1 showed variable capsaicin desensitization rates, whereas variability was minimal in neurons expressing N604T TRPV1.
FIGURE 5.
Capsaicin-dependent responses in TRPV1-expressing sensory neurons. A, time course of native TRPV1 Fura-2 [Ca2+]i responses (mean ± S.D.) in cultured trigeminal neurons. Cells stimulated with 1 μm capsaicin for 5 min at time point 0 s (solid line). B, relationship between maximal rate of change in [Ca2+]i (activation) within the first 60 s of capsaicin exposure and desensitization after 180 s (described in Fig. 4). Data are from 253 neurons in 8 experiments from 3 rats. C, individual Fura-2 [Ca2+]i time course data from a random selection of 100 representative trigeminal neurons illustrates the range of possible capsaicin-dependent responses. D, Fura-2 [Ca2+]i responses (mean ± S.D.) for first 90s of 1 μm capsaicin response from cultured _trpv1_−/− mouse dorsal root ganglia transfected with wild-type- or N604T- TRPV1-GFP. For clarity some N604T error bars have been removed.
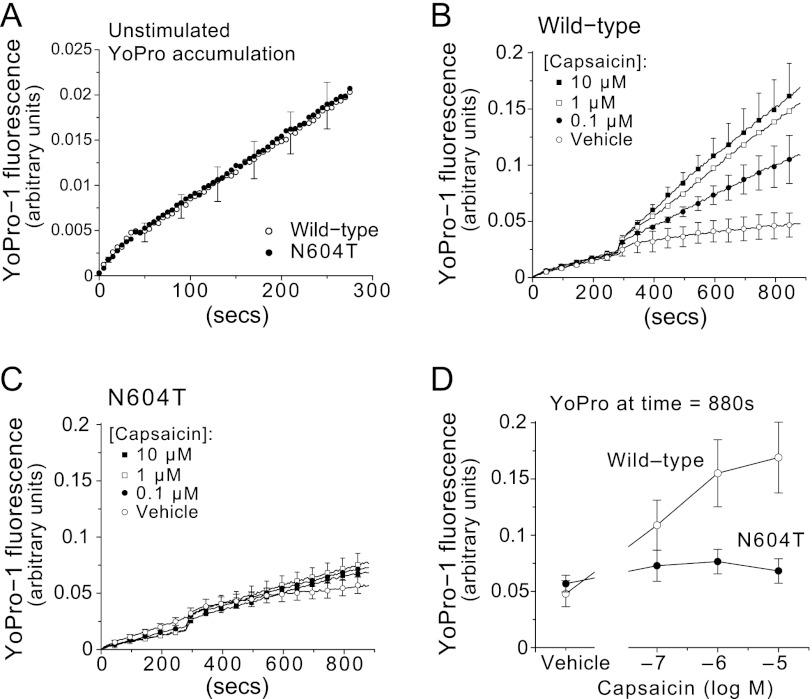
N-Glycosylation Is Required for Capsaicin-evoked Pore Dilation of TRPV1
Since _N_-glycosylation of residues adjacent to the channel pore may influence ion selectivity of TRPV1, we investigated capsaicin-dependent uptake of the large fluorescent cationic dye, YO-PRO-1, into HEK-293 cells expressing wild-type or N604T TRPV1 using fluorimetry (15). A low resting uptake of YO-PRO-1 was identical for cells expressing wild-type or N604T TRPV1 (Fig. 6A). Consistent with the previous uptake studies (15), capsaicin evoked a large, concentration-dependent uptake of YO-PRO-1 into cells expressing wild-type TRPV1 (Fig. 6B) but not into cells expressing N604T TRPV1 (Fig. 6C). Analysis of YO-PRO-1 uptake demonstrated a large and significant difference in permeability between wild-type and N604T TRPV1 (Fig. 6D). Thus, _N_-glycosylation is required for capsaicin-evoked permeation of large ions by TRPV1.
FIGURE 6.
TRPV1 glycosylation is required for capsaicin concentration-dependent accumulation of YO-PRO-1. A, following YO-PRO-1 addition at time 0, the accumulation of YO-PRO-1 was measured (excitation 485 nm/emission 516 nm) in wild type and N604T TRPV1 HEK-293 cells prior to capsaicin application (mean ± S.E.). Some error bars and symbols have been omitted for clarity. YO-PRO-1 accumulation was then measured over time following capsaicin addition (at time 280 s; symbols for capsaicin concentrations as indicated) to cells expressing wild-type TRPV1 (B) or N604T mutant (C). D, concentration-effect curves from the data in B and C using the last measurement of YO-PRO-1 fluorescence (10 min after capsaicin addition) as the response index (wild-type, open circles; N604T, closed circles). All data average of n = 5 experiments, each with three replicates.
DISCUSSION
This study investigates the effect of _N_-glycosylation on the function of TRPV1. Our results show a clear difference between the wild-type and N604T mutant TRPV1 channels with respect to their propensity to acute desensitization and permeability to the cationic dye, YO-PRO-1. These differences are a result of the absence of glycosylation in the N604T mutant and thus show an important role of _N_-glycosylation of the TRPV1 channel.
The rat TRPV1 channel protein has a single site for _N_-glycosylation in the pore loop (Asn-604) and mutagenesis and PNGase treatment results confirm that both simple (most likely high-mannose) and complex glycosylation at that site occurs in HEK-293 cells. The cell surface biotinylation experiments show that multiple glycosylated forms occur on the cell surface, and that there is no significant difference in the total amounts of wild-type and N604T TRPV1 receptor protein at the cell surface.
Since the _N_-glycans attached to TRPV1 are estimated by gel migration to contribute ∼10% to the mass of the protein, it is probable that glycosylation is important and can influence channel function as for other TRP channels (7, 22). TRPV4 has a glycosylation site at Asn-651 which, like N604 of TRPV1, is in the pore-forming region of the channel. A glycosylation-null mutant of TRPV4 exhibits increased [Ca2+]i in response to hypotonic stress (9), an effect ascribed to increased plasma membrane targeting of the mutant relative to wild-type TRPV4. We saw no significant difference in the cell surface expression between the wild-type and N604T mutant TRPV1 channels and so, in contrast, our findings indicate changes in channel function rather than trafficking or expression. Functional regulation of TRP channel activity by glycosylation has been described for TRPC3 and TRPC6 channels, which are glycosylated at sites distant from the pore. TRPC6 mutation to express the TRPC3 pattern of glycosylation confers a TRPC3 level of constitutive activity (23). Our observation of higher basal intracellular [Ca2+]i in cells expressing wild-type TRPV1 compared with the N604T-expressing cells is consistent with such an effect of glycosylation on unstimulated channel activity. Although basal [Ca2+]i in both cells types were dependent on the time of channel induction, the N604T-expressing cells had lower [Ca2+]i than wild-type TRPV1-expressing cells at any time point. Thus, it is possible that the absence of glycosylation reduces constitutive TRPV1 channel activity. Channels that do not desensitize might more strongly affect basal [Ca2+]i than channels that rapidly desensitize so that the difference between TRPV1 and N604T basal activity might be due to the different desensitization properties. We note that for channels like TRPV1, that respond to temperature and pH, it is difficult to draw a distinction between constitutive activity and alterations in the set-point for those constitutively present stimuli.
The glycosylation of TRP channels may be physiologically regulated. Activity of TRPV5 in renal tubules is increased by the endogenous enzyme klotho via hydrolysis of terminal sialic acid residues from the _N_-glycan, which causes retention of the constitutively active channel in the plasma membrane (7, 8). The existence of the TRPV1 channels on the cell surface with differing degrees of glycosylation indicates that such a mechanism is also possible with TRPV1 channels, but there is no evidence regarding differential behavior of the TRPV1 channels with simple and complex glycosylation.
The presence of glycosylation on TRPV1 was first demonstrated in 2001 (3, 4), but the first functional study of the role of glycosylation was by Wirkner et al. in 2005 (6). That study showed that the N604T mutation altered the influence of pH on the responses to capsaicin, and, in contrast to our results, increased sensitivity to capsaicin while reducing the maximal response. However, that study could not investigate the influence of the N604T mutation on channel desensitization because it involved the use of Ba2+ ions in place of Ca2+ ions thereby preventing TRPV1 channel desensitization, which is largely dependent on calcium-dependent enzymes (10, 13, 24–29). There are several other important methodological differences between that study and ours. Whereas we studied TRPV1 stably expressed in isogenic, inducible HEK-293 cells and we measured [Ca2+]i at 37 °C, the other study involved expression of the channels in transiently transfected cells and made electrophysiological measurements at room temperature. We reason that a stable isogenic cell line should yield more consistent responsiveness than transiently transfected cells, and previous electrophysiological studies have shown that there are differences between the behavior of TRPV1 channels at 25 °C and 37 °C (21). Our results show that the average response to TRPV1 activation is sustained over the period of the assay whereas the N604T-expressing cells exhibit appreciable acute desensitization. Measurements at the individual cell level showed that those average responses of wild type TRPV1 cells consist of a mixture of desensitizing and non-desensitizing responses.
We assessed TRPV1 desensitization by measuring [Ca2+]i, whereas electrophysiological approaches have been used previously to examine TRPV1 desensitization. The methods have their own strengths and weaknesses. The [Ca2+]i assay we used measures influx and accumulation of just one ion through the channel rather than current carried by all permeant ions and it has low temporal resolution. However, physiologically relevant changes in [Ca2+]i that are subject to intracellular homeostatic control are measured at 37 °C in hundreds of cells per experiment. Despite these useful characteristics, future studies employing electrophysiological measurement of ion fluxes under highly controlled conditions will be needed to precisely study the biophysical basis of the regulation. Comparisons between these approaches are not straightforward for the reasons noted above but it is worth noting that the maximal rate of increase of Fura-2 ratio that we used as an index of activation (to avoid the confounding influence of desensitization on the maximal response) is, at least mathematically, equivalent to a calcium flux and so might be comparable to a calcium current. Using a microscope-based fluorescence assay, multiple types of TRPV1 responses were identified in HEK-293 cells: many of the TRPV1-expressing cells desensitize rapidly, similar to the N604T TRPV1, some have stable responses and other responses increase in amplitude during the period of exposure to capsaicin. These findings are in accord with reports of substantial variability in the desensitization of capsaicin-induced responses, both in electrophysiological and [Ca2+]i assays (27, 30–33). The differences between wild-type and N604T TRPV1 cells indicate that glycosylation is necessary, but may not be sufficient for sustained responses in HEK-293 cells.
Our results show that TRPV1 glycosylation is required for capsaicin-induced pore dilation as indicated by the permeation of YO-PRO-1 in a concentration and time-dependent manner (15). Dilated TRPV1 channels are resistant to desensitization by several possible mechanisms. The dilated state may preclude conformational transition to a desensitized state, phosphorylation events necessary for dilation may preclude interactions with accessory proteins responsible for desensitization, the dilated channels may be in specialized membrane microdomains, or due to a combination of these mechanisms. We cannot be sure that pore dilation is correlated with sustained responses because our YO-PRO-1 uptake experiments measure dye uptake by the whole cell population, although it seems likely. However, because the YO-PRO-1 uptake experiments were conducted in the absence of extracellular Ca2+ ions, the absence of YO-PRO-1 accumulation in the N604T cells cannot be ascribed to the normal Ca2+-dependent desensitization.
The detection of glycosylated TRPV1 in neuronal cell lysates, the detection of variable capsaicin-induced TRPV1 responses in primary sensory neurons, and the presence of a sub-population of neurons exhibiting a lack of desensitization with a remarkable similarity to capsaicin responses from wild-type TRPV1 in HEK cells are consistent with physiologically-relevant regulation of TRPV1 by glycosylation. The terminals of pain-sensing primary afferent neurons are very sensitive to the pungent TRPV1 agonists, capsaicin and resiniferatoxin (34–36). Treatment of sensory neurons with capsaicin or resiniferatoxin leads to Wallerian-like degeneration and retraction of nerve processes, which then slowly re-grow over a period of several weeks (37) and this approach has been used to treat intractable pain. The observation that the glycosylation state of TRPV1 affects the extent of desensitization provides a mechanism whereby neurons could show differential sensitivity to TRPV1 agonists. We speculate that differences in the pattern of glycosylation could also occur within individual neurons and that this could provide a plausible explanation of why neurites die and cell bodies are preserved from potentially lethal ion influx (38).
In conclusion, we found that a sustained response to capsaicin requires TRPV1 glycosylation and the propensity to acutely desensitize is altered by TRPV1 glycosylation. The uptake of large cations, as measured by YO-PRO-1, requires TRPV1 glycosylation, indicating that channel permeability is modulated by this post-translational modification. These observations may have clinical relevance since TRPV1 activation and loss of ion selectivity can facilitate the delivery of local anesthetics to nociceptive neurons to inhibit pain generation and transmission (16).
Acknowledgments
We thank Alastair Brown, Stuart Bevan, and Linda McClatchie for initial studies into the effects of glycosylation on TRPV1 function.
*
This work was supported in whole or in part, by NHMRC Grant 566834 (to P. M. and N. W. B.), 633033 (to N. W. B.) and National Institutes of Health Grants DK57840, DK43207, DK39957 (to N. W. B.).
2
The abbreviations used are:
TRPV1
transient receptor potential vanilloid 1
DRG
dorsal root ganglia
TG
trigeminal ganglia
TfR
transferrin receptor
PNGase F
_N_-glycosidase F
[Ca2+]i
intracellular calcium concentration.
REFERENCES
- 1.Tyrrell L., Renganathan M., Dib-Hajj S. D., Waxman S. G. (2001) Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J. Neurosci. 21, 9629–9637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeze H. H. (2006) Genetic defects in the human glycome. Nat. Rev. Genet. 7, 537–551 [DOI] [PubMed] [Google Scholar]
- 3.Kedei N., Szabo T., Lile J. D., Treanor J. J., Olah Z., Iadarola M. J., Blumberg P. M. (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J. Biol. Chem. 276, 28613–28619 [DOI] [PubMed] [Google Scholar]
- 4.Jahnel R., Dreger M., Gillen C., Bender O., Kurreck J., Hucho F. (2001) Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem. 268, 5489–5496 [DOI] [PubMed] [Google Scholar]
- 5.Welch J. M., Simon S. A., Reinhart P. H. (2000) The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc. Natl. Acad. Sci. U.S.A. 97, 13889–13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirkner K., Hognestad H., Jahnel R., Hucho F., Illes P. (2005) Characterization of rat transient receptor potential vanilloid 1 receptors lacking the _N_-glycosylation site N604. Neuroreport 16, 997–1001 [DOI] [PubMed] [Google Scholar]
- 7.Lu P., Boros S., Chang Q., Bindels R. J., Hoenderop J. G. (2008) The β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol. Dial Transplant 23, 3397–3402 [DOI] [PubMed] [Google Scholar]
- 8.Chang Q., Hoefs S., van der Kemp A. W., Topala C. N., Bindels R. J., Hoenderop J. G. (2005) The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310, 490–493 [DOI] [PubMed] [Google Scholar]
- 9.Xu H., Fu Y., Tian W., Cohen D. M. (2006) Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am. J. Physiol. Renal Physiol 290, F1103–F1109 [DOI] [PubMed] [Google Scholar]
- 10.Koplas P. A., Rosenberg R. L., Oxford G. S. (1997) The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J. Neurosci. 17, 3525–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studer M., McNaughton P. A. (2010) Modulation of single-channel properties of TRPV1 by phosphorylation. J. Physiol. 588, 3743–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binshtok A. M., Bean B. P., Woolf C. J. (2007) Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449, 607–610 [DOI] [PubMed] [Google Scholar]
- 13.Chung M. K., Güler A. D., Caterina M. J. (2008) TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11, 555–564 [DOI] [PubMed] [Google Scholar]
- 14.Chen J., Kim D., Bianchi B. R., Cavanaugh E. J., Faltynek C. R., Kym P. R., Reilly R. M. (2009) Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol. Pain 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banke T. G., Chaplan S. R., Wickenden A. D. (2010) Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am. J. Physiol. Cell Physiol. 298, C1457–C1468 [DOI] [PubMed] [Google Scholar]
- 16.Binshtok A. M., Gerner P., Oh S. B., Puopolo M., Suzuki S., Roberson D. P., Herbert T., Wang C. F., Kim D., Chung G., Mitani A. A., Wang G. K., Bean B. P., Woolf C. J. (2009) Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology 111, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntyre P., McLatchie L. M., Chambers A., Phillips E., Clarke M., Savidge J., Toms C., Peacock M., Shah K., Winter J., Weerasakera N., Webb M., Rang H. P., Bevan S., James I. F. (2001) Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br J. Pharmacol. 132, 1084–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sándor Z., Varga A., Horváth P., Nagy B., Szolcsányi J. (2005) Construction of a stable cell line uniformly expressing the rat TRPV1 receptor. Cell Mol. Biol. Lett. 10, 499–514 [PubMed] [Google Scholar]
- 19.Dragoni I., Guida E., McIntyre P. (2006) The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J. Biol. Chem. 281, 37353–37360 [DOI] [PubMed] [Google Scholar]
- 20.Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Image Processing with ImageJ. Biophotonics International 11, 36–42 [Google Scholar]
- 21.Novakova-Tousova K., Vyklicky L., Susankova K., Benedikt J., Samad A., Teisinger J., Vlachova V. (2007) Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience 149, 144–154 [DOI] [PubMed] [Google Scholar]
- 22.Cohen D. M. (2006) Regulation of TRP channels by _N_-linked glycosylation. Semin Cell Dev. Biol. 17, 630–637 [DOI] [PubMed] [Google Scholar]
- 23.Dietrich A., Mederos y Schnitzler M., Emmel J., Kalwa H., Hofmann T., Gudermann T. (2003) _N_-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J. Biol. Chem. 278, 47842–47852 [DOI] [PubMed] [Google Scholar]
- 24.Mandadi S., Tominaga T., Numazaki M., Murayama N., Saito N., Armati P. J., Roufogalis B. D., Tominaga M. (2006) Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCϵ-mediated phosphorylation at S800. Pain 123, 106–116 [DOI] [PubMed] [Google Scholar]
- 25.Cholewinski A., Burgess G. M., Bevan S. (1993) The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience 55, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 26.Vyklický L., Nováková-Tousova K., Benedikt J., Samad A., Touska F., Vlachová V. (2008) Calcium-dependent desensitization of vanilloid receptor TRPV1: a mechanism possibly involved in analgesia induced by topical application of capsaicin. Physiol. Res. 57, S59–S68 [DOI] [PubMed] [Google Scholar]
- 27.Mohapatra D. P., Nau C. (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 280, 13424–13432 [DOI] [PubMed] [Google Scholar]
- 28.Numazaki M., Tominaga T., Toyooka H., Tominaga M. (2002) Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 277, 13375–13378 [DOI] [PubMed] [Google Scholar]
- 29.Jung J., Shin J. S., Lee S. Y., Hwang S. W., Koo J., Cho H., Oh U. (2004) Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 279, 7048–7054 [DOI] [PubMed] [Google Scholar]
- 30.Tóth A., Wang Y., Kedei N., Tran R., Pearce L. V., Kang S. U., Jin M. K., Choi H. K., Lee J., Blumberg P. M. (2005) Different vanilloid agonists cause different patterns of calcium response in CHO cells heterologously expressing rat TRPV1. Life Sci 76, 2921–2932 [DOI] [PubMed] [Google Scholar]
- 31.Lam P. M., McDonald J., Lambert D. G. (2005) Characterization and comparison of recombinant human and rat TRPV1 receptors: effects of exo- and endocannabinoids. Br J Anaesth 94, 649–656 [DOI] [PubMed] [Google Scholar]
- 32.Ursu D., Knopp K., Beattie R. E., Liu B., Sher E. (2010) Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur J Pharmacol 641, 114–122 [DOI] [PubMed] [Google Scholar]
- 33.Laínez S., Valente P., Ontoria-Oviedo I., Estévez-Herrera J., Camprubí-Robles M., Ferrer-Montiel A., Planells-Cases R. (2010) GABAA receptor-associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 24, 1958–1970 [DOI] [PubMed] [Google Scholar]
- 34.Jancso-Gabor A., Szolcsanyi J., Jansco N. (1967) A simple method for measuring the amount of azovan blue exuded into the skin in response to an inflammatory stimulus. J Pharm. Pharmacol. 19, 486–487 [DOI] [PubMed] [Google Scholar]
- 35.Jancsó N., Jancsó-Gábor A., Szolcsányi J. (1967) Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother. 31, 138–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kárai L. J., Russell J. T., Iadarola M. J., Oláh Z. (2004) Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 279, 16377–16387 [DOI] [PubMed] [Google Scholar]
- 37.Olah Z., Szabo T., Karai L., Hough C., Fields R. D., Caudle R. M., Blumberg P. M., Iadarola M. J. (2001) Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J. Biol. Chem. 276, 11021–11030 [DOI] [PubMed] [Google Scholar]
- 38.Grant E. R., Dubin A. E., Zhang S. P., Zivin R. A., Zhong Z. (2002) Simultaneous intracellular calcium and sodium flux imaging in human vanilloid receptor 1 (VR1)-transfected human embryonic kidney cells: a method to resolve ionic dependence of VR1-mediated cell death. J. Pharmacol. Exp. Ther. 300, 9–17 [DOI] [PubMed] [Google Scholar]