The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 15.
Abstract
The CSF-1 receptor (CSF-1R) regulates CNS microglial development. However, the localization and developmental roles of this receptor and its ligands, IL-34 and CSF-1, in the brain are poorly understood. Here we show that compared to wild type mice, CSF-1R-deficient (Csf1r−/−) mice have smaller brains of greater mass. They further exhibit an expansion of lateral ventricle size, an atrophy of the olfactory bulb and a failure of midline crossing of callosal axons. In brain, IL-34 exhibited a broader regional expression than CSF-1, mostly without overlap. Expression of IL-34, CSF-1 and the CSF-1R were maximal during early postnatal development. However, in contrast to the expression of its ligands, CSF-1R expression was very low in adult brain. Postnatal neocortical expression showed that CSF-1 was expressed in layer VI, whereas IL-34 was expressed in the meninges and layers II–V. The broader expression of IL-34 is consistent with its previously implicated role in microglial development. The differential expression of CSF-1R ligands, with respect to CSF-1R expression, could reflect their CSF-1R-independent signaling. Csf1r−/− mice displayed increased proliferation and apoptosis of neocortical progenitors and reduced differentiation of specific excitatory neuronal subtypes. Indeed, addition of CSF-1 or IL-34 to microglia-free, CSF-1R-expressing dorsal forebrain clonal cultures, suppressed progenitor self-renewal and enhanced neuronal differentiation. Consistent with a neural developmental role for the CSF-1R, ablation of the Csf1r gene in Nestin-positive neural progenitors led to a smaller brain size, an expanded neural progenitor pool and elevated cellular apoptosis in cortical forebrain. Thus our results also indicate novel roles for the CSF-1R in the regulation of corticogenesis.
Keywords: CSF-1, IL-34, neural stem cells, neurogenesis, nestin, cerebral cortex
Introduction
Cytokines that regulate hematolymphopoiesis and bone morphogenesis also play significant roles in nervous system development beyond those ascribed to classical neurotrophins and CNS mitogens (Bernd, 2008; Mehler and Kessler, 1997). The intimate involvement of these cytokines in developing and adult brain reflect their increasingly well recognized, complex and context-dependent roles in orchestrating diverse cellular and molecular CNS processes, including regional trophic signaling, neuronal and glial cell specification, neuroblast migration, neurite outgrowth, and neuronal and neural network homeostasis, plasticity and connectivity (Bauer et al., 2007; Mehler and Gokhan, 1999; Mehler et al., 1993; Temple, 2001).
Colony-stimulating factor-1 (CSF-1), also known as macrophage-CSF (M-CSF) is the primary growth factor for tissue macrophages and osteoclasts (Byrne et al., 1981; Guilbert and Stanley, 1980; Stanley et al., 1983; Tanaka et al., 1993; Tushinski et al., 1982). The effects of CSF-1 are mediated by the CSF-1 receptor tyrosine kinase (CSF-1R) (Dai et al., 2002; Pixley and Stanley, 2004; Sherr et al., 1985). The receptor is expressed on macrophages (Byrne et al., 1981), osteoclasts (Kodama et al., 1991) and Langerhans cells (Ginhoux et al., 2006), as well as cells of non-hematopoietic origin (Arceci et al., 1989; Huynh et al., 2009) and CNS microglia (Sawada et al., 1990; Suzumura et al., 1990). Csf1op/Csf1op or Csf1op/op mice are homozygous for osteopetrotic mutation, a naturally occurring recessive mutation due to single nucleotide insertion in exon 4 of Csf1 gene leading to a frame-shift and a 63 amino acid long truncated, inactive protein (Marks and Lane, 1976; Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990). These mice exhibit a pleiotrophic phenotype including toothlessness, skeletal defects, reduced body weight, deficits in tissue macrophages and osteoclasts, as well as male and female reproductive defects (Cecchini et al., 1994; Cohen et al., 1996; Kodama et al., 1991; Marks and Lane, 1976; Pollard et al., 1991; Wiktor-Jedrzejczak et al., 1990; Wiktor-Jedrzejczak et al., 1982; Yoshida et al., 1990). In contrast to Csf1op/op mice that exhibit ~30% reduction in microglia, microglia are absent in CSF-1R-deficient, Csf1r −/Csf1r − (Csf1r−/−) mice (Dai et al., 2002; Erblich et al., 2011; Ginhoux et al., 2010), in agreement with the existence of a second CSF-1R ligand, interleukin-34 (IL-34) (Lin et al., 2008). Consistent with a role for IL-34 in brain, IL34 mRNA is expressed earlier and at 10-fold higher levels than Csf1 mRNA (Wei et al., 2010). Moreover, CSF-1 is required for maintenance of GABAergic cortical circuitry and promotion of neurite outgrowth (Michaelson et al., 1996), for control of neuroendocrine pathways in hypothalamus (Cohen et al., 2002) and for enhancing survival of a subset of adult neocortical neurons (Wang et al., 1999a), thereby suggesting that CSF-1R signaling has additional important trophic roles in neural cells.
In the present study, using CSF-1-reporter-LacZ mice coupled with immunochemistry, we demonstrate that CSF-1 and IL-34 exhibit a distinct regional and often complementary expression profiles, with a broader expression of IL-34. While CSF-1R expression decreases dramatically with development from neonatal to adult brain, IL-34 and to a lesser extent CSF-1 expression are maintained at higher levels. Focusing on the developing cortex, CSF-1 expression appears later than the CSF-1R and it is primarily localized to layer VI of neocortical neurons in the postnatal brain. In contrast, IL-34 is expressed by neocortical neurons in layers II–V, as well as by meningeal cells. We show that the CSF-1R is expressed by a small subset of Nestin-expressing dorsal forebrain progenitors and by microglia. Csf1r−/− mice exhibited forebrain developmental abnormalities, including increased proliferation of neural progenitor cells. In vitro clonal expansion and differentiation experiments, using _Nestin_-positive forebrain progenitors, indicate that both CSF-1 and IL-34 suppress progenitor self-renewal. The Csf1r−/− abnormalities of smaller brain size, increased dorsal forebrain progenitor numbers and elevated cellular apoptosis are partially recapitulated in Nestin-Cre/+; Csf1rflox/flox (Li et al., 2006; Tronche et al., 1999) mice. Together, these experiments demonstrate a direct role for the CSF-1R in progenitor cell-mediated neural development and further suggest that CSF-1 and IL-34 have broader biological functions than previously thought.
Materials and Methods
Mice
The generation, maintenance and genotyping of the TgN(Csf1-Z)7Ers/+ (TgZ7/+) (Ryan et al., 2001), Csf1op/op and Csf1r−/− (Dai et al., 2002; Dai et al., 2004b) mice on the FVB/NJ background and the Nestin-GFP transgenic reporter (Mignone et al., 2004), Nestin-Cre (Nes-Cre/+) transgenic (Tronche et al., 1999) and the Csf1rflox/flox (Csf1rfl/fl) (Li et al., 2006) mice on the C57BL/6 background were as described.
Brain weight measurement
After careful removal of the cranium, unperfused brains, including both olfactory bulbs (OB), were cut between pons and medulla. Brains were then removed from the skull sockets after severing the optic chiasma and weighed using a precision weighing balance.
Histology, Histochemistry and Immunohistochemistry (IHC)
Mice were perfused with 5 ml of cold PBS, followed by 15 ml of perfusate. For histological analysis, the perfused (4% paraformaldehyde (PFA) in PBS, pH 7.4) brains were sectioned (1–2 mm), fixed in perfusate (16 h, 4°C), embedded in paraffin and stained with hematoxylin and eosin. For whole mount X-gal staining, perfused (4% PFA) tissues were fixed in 4% PFA (30 min, 4°C), or in 2% PFA plus 0.01% glutaraldehyde, pH 7.4 (1h, 4°C), washed with PBS and incubated (16h, 32°C), on a rocker, in X-gal solution (Hennighausen et al., 1995). For X-gal staining of frozen sections, perfused (1.5% pre-chilled PFA, pH 7.4) brains were dissected, fixed in 1.5% PFA in 30% sucrose in PBS, pH 7.4 (1 h, 4°C), sliced into 1–2 mm thick sections, fixed (same fixative, 8–10 h), washed with PBS and embedded in OCT. Sections (10 μm) were stained with X-gal. For IHC, perfused (4% PFA) brains were sectioned (1–2 mm), fixed in perfusate (6 h, 4°C), incubated successively with 15%, then 30%, sucrose in PBS (6 h, 4°C, each), embedded in OCT and cut into 30μm sections. Images were captured by either an Olympus Bx51 upright fluorescent microscope with Olympus MicroSuite™- Five Biological Suite software, NY, USA, or by a Leica AOBS SP2 confocal microscope with Leica confocal software, Germany. Quantification of cell numbers following IHC was carried out either manually, or by using “Image J” software. Antibody dilution and isotype for IHC: Nestin, mouse IgG1 (1:250, BD Biosciences); β-tub III, mouse IgG2b (1:400, SIGMA); PDGFRα, polyclonal goat IgG (1:300, Chemicon); NG2, polyclonal rabbit IgG (1:300, Chemicon); O4, mouse IgM (1:300, Chemicon); S100 β, mouse IgG1 (1:100, SIGMA); NeuN mouse IgG1-Alexa fluor-488 (1:100, Chemicon); APC, mouse IgG2b (1:100, Calbiochem); GFAP, mouse IgG1 (1:400, SIGMA), or mouse IgG2b (1:200, BD Pharmingen); CSF-1R (IK), goat IgG (1.25 μg/ml,(Wang et al., 1999b)); β galactosidase, Goat IgG (1:100, AbD Serotec); IL-34, polyclonal rabbit IgG (1.25 μg/ml, a gift from Five Prime Therapeutics, CA, USA); BrdU, mouse IgG1 (1:100, Nova Castra); Iba1, polyclonal rabbit IgG (1:700, Wako Chemicals, USA); Pax6, polyclonal rabbit IgG (1:100, Milipore); Calbindin, polyclonal rabbit IgG (1:800, Milipore); Tbr1, polyclonal rabbit IgG (1:500, Abcam); CTIP2, rat IgG (1:500, Abcam); Satb2, mouse IgG1 (1:500, Abcam); Cux1, polyclonal rabbit IgG (1:100, Santa Cruz); Tbr2, polyclonal rabbit IgG (1:500, Abcam); MBP, mouse IgG2b (1:250, R&D). Secondary antibodies used were conjugated to Fluorescein Isothiocyanate (FITC, 1:200), Tetramethyl Rhodamine Isothiocyanate (TRITC, 1:200) (Southern Biotechnology Associates, Inc., Al, USA), Alexa fluor-594 or Alexa fluor-647 (1:1500) (Molecular Probes, Invitrogen, oregon, USA).
BrdU incorporation study
Postnatal 20-day old (P20) wild type (Wt), Csf1op/op and Csf1r−/− mice were injected intraperitoneally with 100μl of 20 mg/ml BrdU in 154 mM NaCl, 7 mM NaOH and sacrificed 2 h later. For IHC, brain sections were pretreated with 2N HCl (1 h, 37°C) neutralized with 0.1 M sodium borate solution, pH 8.5 for 10 min, quenched with 0.1% sodium borohydride for 10 min at room temperature and then incubated with anti-BrdU antibody.
Neurosphere culture and clonal analysis
Neurospheres (progenitor clones) were generated from cortical SVZ of P2 FVB Wt mice (Charles River), as described previously (Zhu et al., 1999). Cells were plated at a density of 30,000 cells per well (3 ml of culture media per well) and maintained in the presence of 10 ng/ml of EGF (R&D, MN, USA) for 7 DIV in the presence or absence of 60 ng/ml of recombinant human CSF-1 (rhCSF-1, a gift from Chiron Corporation, Emeryville, CA). CSF-1 was added on the first and fourth DIV. Secondary clones were obtained by dissociation of the primary clones at 7 DIV that were propagated under similar conditions. To study the role of CSF-1 on progenitor differentiation, primary and secondary clones, at 7 DIV, were plated onto poly-D-lysine coated-coverslips in the presence of 1 ng/ml of recombinant human bFGF (BD Biosciences, MA, USA) and 2 μg/ml of laminin (BD Biosciences, MA, USA). The adherent NPC clones were fixed at 4 days and 7 days with 4% PFA, pH 7.4 (20 min, 37°C) and studied for the expression of specific lineage markers by immunofluorescence. To study the effects of CSF-1 and IL-34 on purified progenitors, P2 cortical SVZ-derived NPC clonal cultures from pooled Wt, Nestin-GFP/+ and _Nestin-GFP/Nestin_-GFP brains were established and maintained in the presence of 10 ng/ml of EGF for 3 DIV, as described above. Cortical progenitor clones thus obtained were dissociated, sorted for GFP positivity using DakoMoflo XDP and 20,000 GFP+ cells were plated at 3 ml per well in 6–well tissue culture plates, in the presence of 80 ng/ml of either rhCSF-1 or mouse IL-34 (R&D, MN, USA), or both. CSF-1 and IL-34 were added on the first and fourth DIV. Secondary clones were generated as described above. To study the role of CSF-1 and IL-34 in progenitor differentiation, primary and secondary clones, at 7 DIV, were treated as described. Antibody dilution and isotype for immunocytochemistry: β tub-III, mouse IgG2b (1:700, SIGMA); O4, mouse IgM (1:700, SIGMA); GFAP, mouse IgG1 (1:700, SIGMA), CSF-1R (IK), goat IgG (1 μg/ml, (Wang et al., 1999b)), F4/80, rat IgG2b (1:200, (Hume et al., 1983)). Secondary antibodies used were conjugated to TRITC (1:200) (Southern Biotechnology Associates, Inc., Al, USA), Alexa fluor-350, Alexa fluor-594, or Alexa fluor-647 (1:1500) (Molecular Probes, Invitrogen, Oregon, USA). The number and the area of the progenitor clones were measured using the Olympus MicroSuite™- Five Biological Suite software, NY, USA.
RNA isolation and RT-PCR
RNA from FACS purified GFP+ fraction derived from cortical progenitor clones obtained from P2 Wt, Nestin-GFP/+ and Nestin-GFP/Nestin-GFP pups, following incubation with 10 ng/ml of EGF for 3 DIV and control RNA from L-cells (Stanley and Heard, 1977) and BAC1.2F5 macrophages (Morgan et al., 1987) was isolated using TRIzol reagent (Invitrogen, CA, USA) and RNeasy mini kit (QIAGEN, Maryland, USA) following manufacturer’s instructions. Reverse transcription (RT)-PCR reactions were carried out using SuperScript III One-step RT-PCR system with Platinum Taq DNA Polymerase (Invitrogen, CA, USA). Primers for RT-PCR:
- CSF-1R: (5′-TGCTGGCCACAGTTTGGCATG-3′ and
- 5′-CTTTGACATACAAGTGGATGGT-3′; 279bp),
- F4/80: (5′-ATGTGGGGCTTTTGGCTGCT-3′ and
- ′-TGAGTCACTTTGAAGACATT-3′; 270bp),
- β-actin: (5′-CGTGGGCCGCCCTAGGCACCA-3′ and
- 5′-TTGGCCTTAGGGTTCAGGGGGG-3′; 243 bp).
IL-34 staining of 293T cells and F4/80 staining of BAC1.2F5 cells
293T cells either untransfected or transfected with mouse IL-34 cDNA were cultured in DMEM (GIBCO, NY, USA) with 10% fetal calf serum (FCS) in the absence or presence of 5 μg/ml of puromycin respectively for 20 hours on the poly-D-lysine-coated coverslips. They were fixed with 4% PFA, pH 7.4 (20 min, 37°C) and stained with either purified rabbit IgG or affinity-purified rabbit anti-IL-34 antibody (1 μg/ml, a gift from Five Prime Therapeutics, CA, USA). BAC1.2F5 cells were similarly cultured in α MEM with 10% FCS in the presence of 36 ng/ml of CSF-1 for 16 hours, fixed and stained with either rat IgG or, anti-F4/80 monoclonal antibody (1:200, (Hume et al., 1983)).
Statistical Analysis
Data were expressed as means ± standard deviations (SD) or means ± standard error of means (SEM). Student’s t test (2 tailed) was used to test significance. Differences were considered statistically significant if comparison of data sets yielded P values of ≤0.05.
Results
Brain developmental abnormalities in Csf1op/op and Csf1r−/− mice
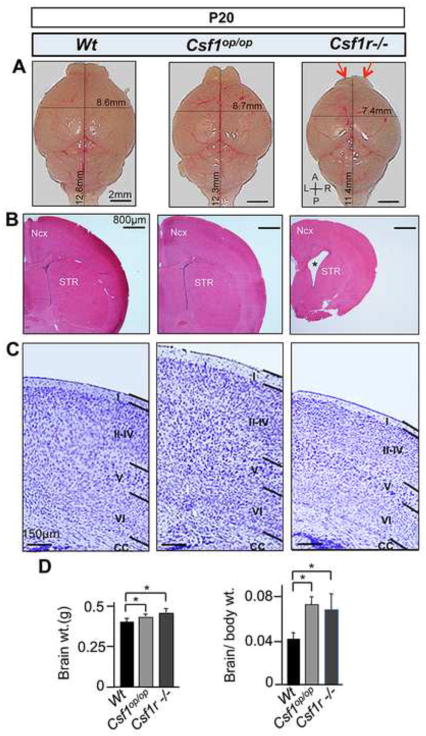
In contrast to the 40% survival rate of Csf1op/op FVB/NJ mice, Csf1r−/− FVB/NJ mice did not survive beyond one month of age (Dai et al., 2004a). Comparative analysis of P20 Csf1op/op and Csf1r−/− FVB/NJ mice revealed that, while both mutant brains are smaller in size (Fig. 1A), with a greater brain mass (Fig. 1D), than wild type brains, Csf1r−/− brains were smaller than Csf1op/op brains. The Csf1r−/− mice also displayed atrophy of the olfactory bulb (OB) (Fig. 1A, red arrows) and an expansion in the size of lateral ventricles (Fig. 1B, asterisk). Seventy-five percent of the Csf1r−/− mice had a thinner neocortex than wild type mice, with a general reduction in the thickness of all cortical layers (Fig. 1C). In contrast, Csf1op/op brains exhibited greater neocortical thickness than wild type brains (Fig. 1C). These observations suggest important roles for the CSF-1R in normal CNS homeostasis.
Fig. 1.
Gross anatomical and histological alterations in P20 Csf1op/op and Csf1r−/− brains. (A) A graded reduction in brain size along the A/P axis, from Csf1op/op to Csf1r−/− mice, with specific atrophy of the OB (red arrows) and a reduction in brain size along the mediolateral (L/R) axis in the Csf1r−/− mice (upper panels). (B) Coronal sections stained with hematoxylin and eosin (H&E), showing normal patterning but decrease in forebrain size of the Csf1r−/− mice. Asterisk indicates the increased size of the lateral ventricles in Csf1r−/− mice. (C) Nissl staining showing a normal laminar patterns, but an increase in thickness of neocortex (layers I–IV) in Csf1op/op mice and a reduction in thickness of the Csf1r−/− neocortex (all layers). (D) Whole brain weights (left panel) and brain to body weight ratios (right panel). *, p<0.01. n ≥ 5 per condition and mutant model. Ncx, neocortex; STR, striatum; A/P anterio-posterior; L/R, left-right; CC, corpus callosum; OB, olfactory bulb.
Temporal expression of IL-34, CSF-1 and the CSF-1R in neocortex
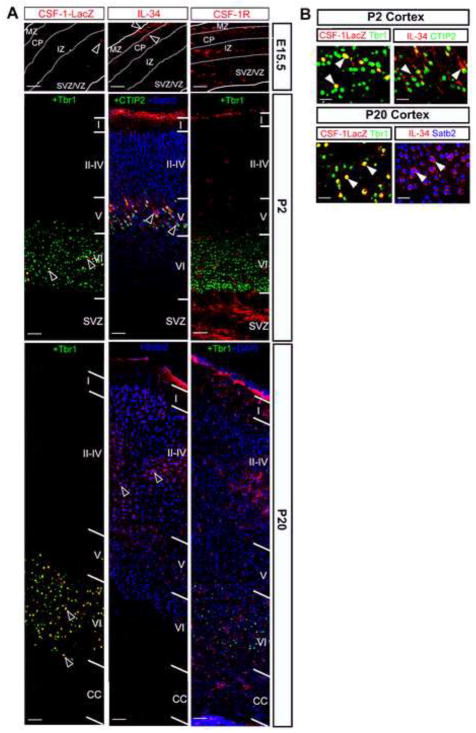
To visualize the expression profile of CSF-1 in developing brain, we employed a transgenic mouse _Csf1_-LacZ reporter line (TgZ7) that expresses a nuclear-localized β-galactosidase, under the control of the Csf1 promoter and first intron (Fig. S1A) in a cellular pattern recapitulating expression of the endogenous Csf1 gene (Ryan et al., 2001). β-galactosidase was detected by X-gal staining (Figs. S1B,C) and by immunochemistry employing an anti-β-galactosidase antibody. IL-34 and CSF-1R expression were detected using affinity-purified, specific polyclonal antibodies (Figs. S2, S3). Our initial analysis of CSF-1 reporter mice revealed that the Csf1 promoter is active in the developing telencephalon and cerebellum (Figs. S1 B,C). We further compared the temporal expression profiles of CSF-1R signaling molecules in the cortical forebrain by immunostaining of TgZ7 brain sections with anti-β-galactosidase, anti-IL-34 and anti-CSF-1R antibodies.
CSF-1R is expressed on microglia-like cells in E11.5 brain (Ginhoux et al., 2010). In addition, IL34 mRNA was present in the E11.5 telencephalon. However, we failed to detect any significant expression of either IL-34 (data not shown), or CSF-1 reporter protein at this stage (Fig. S1B). By E15.5, CSF-1 reporter expression appeared within the SVZ/VZ with IL-34 expression within the marginal zone and the cortical plate (Fig. 2A). At this stage, CSF-1R expression was observed throughout the dorsal telencephalon, including within the SVZ/VZ (Fig. 2A). Within the P2 neocortex, IL-34 was predominantly expressed in layer V and the meninges (Fig. 2A), consistent with the presence of microglia in the latter region (Boya et al., 1979; Perry et al., 1985). IL-34 expression was not detected on Cajal-Retzius cells (data not shown). In contrast, CSF-1 expression was detected solely in layer VI (Figs. S1B,2A). At P20, the expression of CSF-1 was similar, while IL-34 expression expanded into the upper layers (II–IV) (Figs. S1B, 2A). Interestingly, robust CSF-1R expression was observed in the P2 SVZ and in the meninges (Fig. 2A) and expression was reduced in the P20 generative zones with enhanced expression in the upper cortical layers (Fig. 2A).
Fig. 2.
Complementary expression of CSF-1 and IL-34 in the TgZ neocortex. (A) Temporal pattern of expression: E15.5: While the CSF-1 reporter is expressed in the SVZ/VZ, IL-34 is expressed in the MZ and in the CP. CSF-1R is expressed throughout the dorsal neocortex. P2: Complementary expression of the CSF-1 reporter (in layer VI) and IL-34 (in layer V and meninges) in distinct cortical laminar patterns. Strong CSF-1R staining is apparent in the SVZ and meninges, with lower expression levels in the cortical layers. P20: CSF-1 reporter and IL-34 expression patterns are similar to those observed at P2 but IL-34 expression expands to upper layers (in layers II–V). Note the decline of CSF-1R expression in the developing CC and its increased expression in the upper cortical layers that also strongly express IL-34. (B) Cellular expression profiles of IL-34 and CSF-1 in postnatal (P2 and P20) neocortex of the TgZ mouse. Blown-up images from (A) showing CSF-1 reporter-LacZ and IL-34 co-staining with markers: Tbr1 (layer VI-specific postmitotic neurons); CTIP2 (layer V-specific postmitotic neurons) and Satb2 (layers II–IV-specific postmitotic neurons). Arrowheads indicate overlap of staining profiles. CC, corpus callosum; VZ, ventricular zone; SVZ, sub-ventricular zone; CP, cortical plate; MZ, marginal zone; IZ, intermediate zone.
Our expression analysis revealed that CSF-1R and its signaling ligands are maximally expressed during postnatal development in the cerebral cortex. To further identify the neural cell subtypes expressing these signaling proteins, sections of postnatal TgZ7 cortices were stained for each of the CSF-1R signaling factors in combination with antibodies against different neural lineage markers. Within the P2 cortex, while CSF-1 reporter expression exhibited exclusive overlap with Tbr1 (Hevner et al., 2001), a marker present solely in mature neurons of layer VI, IL-34 was expressed on CTIP2 immunoreactive (Chen et al., 2008) neurons of layer V (Fig. 2B).
Interestingly, CSF-1R was expressed on a small subset of neural progenitors (Nestin+ cells) and some immature neurons (β-tubIII+ and NeuN-cells) (Fig. S4). Within the P20 cortex, while CSF-1 expression persisted on layer VI mature neurons, IL-34 expression was observed on Satb2 immunoreactive (Britanova et al., 2008) mature neurons of layers II–IV (Fig. 2B). At P20, CSF-1R expression was primarily restricted to microglia (Ginhoux et al., 2010).
Together, the co-localization studies and the temporal expression analysis indicate that IL-34 and CSF-1 exhibit complementary expression profiles in the developing cortex. While both ligands are expressed by a specific subset of mature cortical neurons, the CSF-1R, in addition to its expression on microglia, is also expressed on a small subset of dorsal forebrain progenitors.
CSF-1R suppresses the expansion of dorsal forebrain progenitors
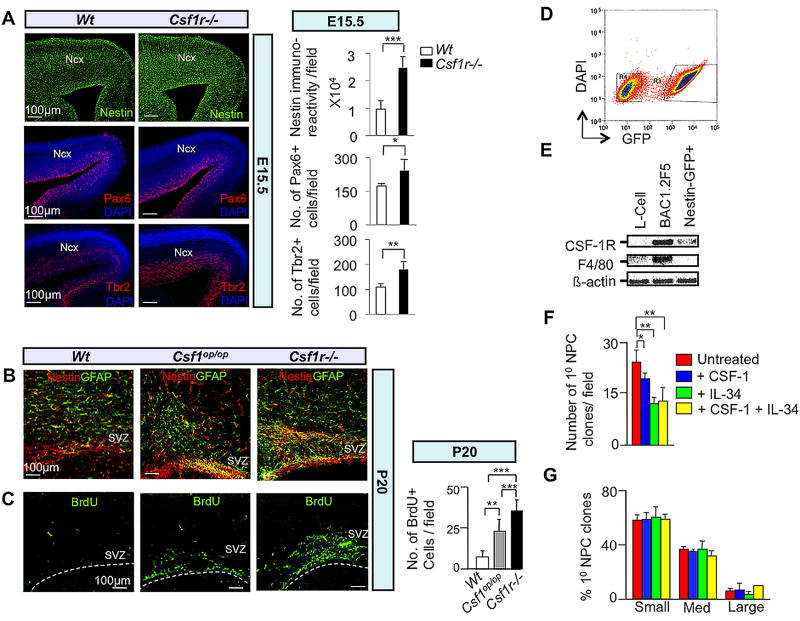
To examine the role of CSF-1R signaling in neural progenitor expansion in vivo, we first performed immunofluorescence microscopy of the embryonic and postnatal neocortex of Wt, Csf1op/op and Csf1r−/− mice using neural progenitor and cellular proliferation markers (Figs. 3A–3C). There was an increase in the number of radial glia (Nestin+ and Pax6+) and basal (Tbr2+) progenitors in the E15.5 Csf1r−/− cortex (Fig. 3A). Within the generative zone of the lateral ventricle, from Wt through Csf1op/op to Csf1r−/− P20 brains, there was a graded increase in the number of neural progenitors (Nestin+ GFAP+) (Fig. 3B). In addition, BrdU pulse-labeling indicated that there was a graded enhancement of cellular proliferation in this region of the mutant brains (Fig. 3C).
Fig. 3.
The CSF-1R directly mediates suppression of dorsal forebrain progenitor proliferation/self-renewal. (A–C) In vivo studies: Immunofluorescence microscopy of coronal sections of E15.5 (A) and P20 (B,C) Wt and mutant neocortex. (A) Nestin, Pax6 and Tbr2, (B) Nestin-GFAP double and (C) BrdU immunostaining. Quantification= cells/field. Mean ± SD of four representative fields per genotype. n=3: *, P<0.05; **, P<0.01 and ***, P< 0.001. Ncx, neocortex; SVZ, subventricular zone. Dotted lines in (C) delineate the ventricular lining. (D–G) In vitro studies: (D) FACS purification of cerebral cortical GFP+ progenitors from P2 Nestin-GFP transgenic pups. Cells were cultured in the absence of CSF-1 for 3 days and subjected to FACS. Cells gated in region R3 were used to set up neurosphere cultures. (E) Total RNA isolated from the GFP+ fraction was subjected to RT-PCR for assessment of Csf1r and F4/80 (Hume et al., 1983) mRNAs. RNA from L-cells and BAC1.2F5 macrophages, respectively, represent negative and positive controls for F4/80 and Csf1r mRNA expression. (F) FACS-purified GFP+ cells incubated in the presence of EGF and combinations of CSF-1 and IL-34 for 7 DIV. Reduced numbers of primary progenitor clones following incubation with CSF-1 and/or IL-34 for 7 DIV. (G) Failure of CSF-1 or IL-34 to affect the generation of small, medium or large-sized primary clones after 7 DIV. Clonal sizes: Small, 0.5–2.0 mm2; medium, 2.0–6.0 mm2; large, ≥ 6.0 mm2. Mean ± SEM of 16 different representative fields from three independent experiments between untreated and CSF-1, IL-34 and CSF-1+IL-34 treated conditions. *P<0.05, **P<0.01 and ***P<0.001.
To further investigate a possible inhibitory role of CSF-1R signaling in progenitor proliferation, we utilized in vitro clonal expansion assays. Initial neural differentiation paradigms that employ EGF-responsive neural progenitor clones derived from the P2 cortical SVZ of Wt mice revealed the presence of contaminating F4/80+ microglia (Hume et al., 1983) in these cultures (Fig. S10C). To generate microglia-free clones, we isolated EGF-responsive cortical SVZ neural progenitors from P2 Nestin-GFP mice (Mignone et al., 2004) (Fig. 3D). We expanded these cells in the presence of EGF for 3 days in vitro (DIV) and dissociated the resulting small EGF-responsive clones for FACS-purification of GFP+ cells. We then verified that the pooled GFP+ cells express mRNA for the CSF-1R, but not for F4/80 (Fig. 3E). In vitro clonal expansion analysis of these purified Nestin-GFP P2 cortical SVZ-derived neural progenitor cells (Fig. S5A) demonstrated that either CSF-1 or IL-34 was sufficient to inhibit the self-renewal (Fig. 3F), but not to promote the proliferation (Fig. 3G) of these neural progenitors. Together, our in vivo and in vitro results strongly suggest a direct role for CSF-1R-signaling in the suppression of dorsal forebrain progenitor expansion.
CSF-1R enhances neuronal differentiation of dorsal forebrain progenitors
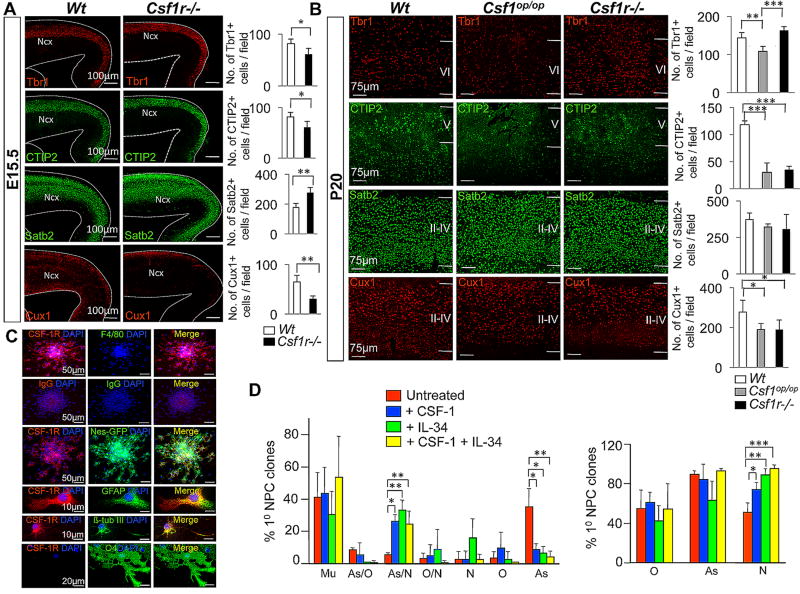
To examine the role of CSF-1R signaling in neuronal differentiation in vivo, we performed immunofluorescence microscopy of the embryonic and postnatal neocortex of Wt, Csf1op/op and Csf1r−/− mice using markers that permitted analysis of the laminar distribution of mature excitatory neuronal subtypes (Figs. 4A,B). At E15.5, the Csf1r−/− cortex displayed a reduction in the number of Tbr1+ and CTIP2+ lower layer and Cux1+ upper layer neurons, while Satb2+ neurons in layers II–IV were increased (Fig. 4A). In P20, we observed a consistent reduction in CTIP2+ as well as Cux1+ neuronal subtypes in both Csf1op/op and Csf1r−/− mice and a reduction of Tbr1+ neurons only in Csf1op/op mice (Fig. 4B). Although Nissl staining of neocortex showed an apparently normal distribution of neurons in both mutants (Fig. 1C), excitatory neuronal subtype analyses revealed loss of layer-specific neuronal subtypes in both Csf1op/op and _Csf1r−/−_mice.
Fig. 4.
The CSF-1R directly enhances neuronal differentiation of dorsal forebrain progenitors. (A,B) In vivo studies: Immunofluorescence microscopy of coronal sections of E15.5 (A) and P20 (B) Wt and mutant neocortex. Tbr1, CTIP2, Satb2 and Cux1 immunostaining. Quantification, cells/field. Mean ± SD of four representative fields per genotype. n=3: Ncx, neocortex. *P<0.05, **P<0.01 and ***P<0.001. (C,D) In vitro studies: Adherent GFP+ primary cortical progenitor clones (generated under proliferation conditions, with EGF only, for 7 DIV, as described in Fig. 3D) incubated under differentiation conditions for 4 DIV. (C) Expression of the CSF-1R and the absence of F4/80 immunoreactivity (upper panels). Control IgGs for the CSF-1R and F4/80 antibodies indicate specificity (second row of panels). Three lower rows: Overlap of CSF-1R staining with GFP, GFAP and β tub-III. (D) Adherent GFP+ clones generated in the presence of EGF and combinations of CSF-1 and IL-34 for 7 DIV, then subsequently incubated under differentiation conditions for 4 DIV. Left panel: Percentage of primary progenitor clones that were multipotent (Mu) (β tub-III+/GFAP+/O4+), bipotent (GFAP+/O4+ (As/O), β tub-III+/GFAP+ (As/N) and β tub-III+/O4+ (O/N)) and unipotent (O4+ (O), β tub-III+ (N), or GFAP+ (As)). CSF-1 or IL-34 facilitated the generation of astrocyte/neuron (As/N) bipotent clones at the expense of unipotent astrocyte clones (As). Right panel: Percentage of primary progenitor clones containing β tub-III+ neurons (N), GFAP+ astrocytes (As) and O4+ oligodendrocytes (O). Mean ± SEM of 16 different representative fields from three independent experiments between untreated and CSF-1, IL-34 and CSF-1+IL-34 treated conditions. *P<0.05, **P<0.01 and ***P<0.001.
To further assess the role of CSF-1R signaling in neuronal differentiation, we utilized in vitro clonal differentiation assays using Nestin-GFP mice. Indeed, adherent GFP+ neural progenitor clones continued to express the CSF-1R in the absence of significant microglial contamination (Fig. 4C). The CSF-1R was expressed by protoplasmic astrocytes (Doetsch et al., 1999; Miller and Raff, 1984) and by neurons, but not by oligodendrocytes (Fig. 4C). In vitro clonal differentiation analysis further demonstrated that either CSF-1 or IL-34 facilitated the generation of bipotent neuronal/astroglial clones at the expense of unipotent astroglial clones (Figs. 4D, S5B). Co-application of CSF-1 and IL-34 resulted in a further increase in the generation of neuron-containing clones without corresponding changes in the generation of astrocyte-containing clones, independent of overall clonal lineage composition (Fig. 4D). Together, these in vivo and in vitro results indicate a direct role for CSF-1R-signaling in the enhancement of neuronal differentiation of dorsal forebrain progenitors.
CSF-1R promotes survival of neural progenitors and committed precursors
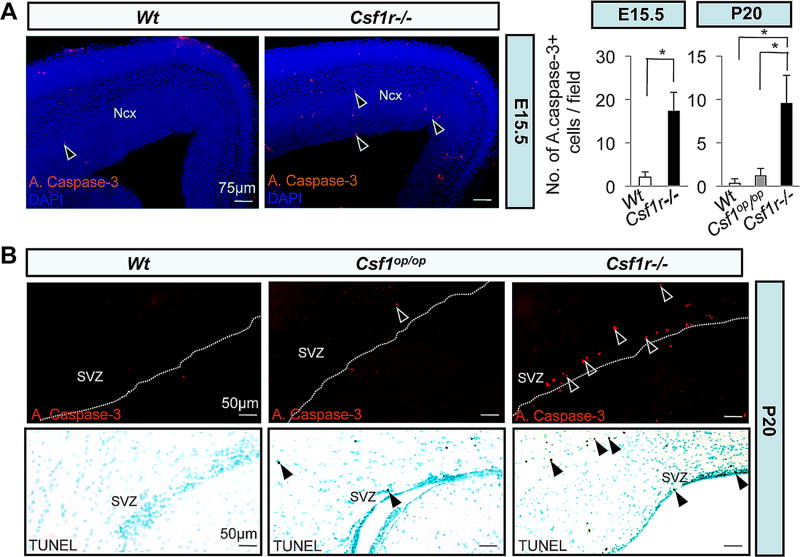
We next studied whether CSF-1R-signaling was associated with regulation of survival of distinct cortical cells. We examined the Wt and the mutant brains for active caspase-3 expression and TUNEL activity. Our results revealed an increase in the number of apoptotic cells in the E15.5 (Fig. 5A) and P20 (Fig. 5B) Csf1r−/− cortex and that the rate of apoptosis in Csf1r−/− cortex was comparable between these two developmental stages (Figs. 5A,B). Suggestive of a significant pro-survival role for IL-34, Csf1op/op cortex compared to Csf1r−/− cortex had significantly fewer apoptotic cells (Fig. 5B). Interestingly, the majority of the apoptotic cells in Csf1r−/− cortex resided in the SVZ/VZ at E15.5 and in the anterior SVZ at P20. To further determine the identities of apoptotic cells in the Csf1r−/− cortex, we employed immunofluorescence microscopy of P20 brain sections using active caspase-3 and various neural developmental markers. Within the anterior SVZ, there was preferential co-localization of active capase-3 staining with markers of neural progenitors (Nestin, GFAP), significant association with neuronal and glial cell precursors (β-tub III, S100β, PDGFRα, O4), but absence of co-localization with markers of post-mitotic neurons (NeuN) and mature oligodendrocytes (APC) (Table 1). These findings suggest that CSF-1R signaling either directly promotes the survival of forebrain neural progenitors, or limits the differentiation potential of specific neuronal and glial lineages, the failure of which in the Csf1r−/− brains, would lead to their surplus production and subsequent pruning by a CSF-1R-independent mechanism. In both Csf1op/op and Csf1r−/− mice, we also observed the presence of apoptosis in the granule cell layers of the OB and the DG of the hippocampus, two areas associated with adult neurogenesis (Fig. S6). In both mouse mutants, the numbers of apoptotic cells observed in the OB were similar and significantly higher than in the SVZ and DG regions, reflecting a predominant pro-survival role for CSF-1 acting through the CSF-1R in OB.
Fig. 5.
Cellular apoptosis in the developing Csf1r−/− neocortex. (A, B) Photomicrographs of active caspase-3+ (red dots) (A) and (B, upper panels) as well as TUNEL+ (brown dots) (B, lower panels) apoptotic cells in the SVZ/VZ region of E15.5 (A) and P20 (B) Wt and mutant mice. Arrowheads in (A, B) indicate apoptotic cells. Counterstained with DAPI (A) and hematoxylin (B). Dotted lines in (B) delineate the ventricular lining. Quantitation of the number of active caspase-3+ apoptotic cells/field. Means ± SD of ten different representative low-power (20X) fields per region per genotype from three different mice per genotype; *, P<0.001.
Table 1.
Distribution of neural cell types among active caspase-3+ cells in the anterior SVZ of P20 Csf1r−/− mice
| Neural cell-specific marker | % double + cells |
|---|---|
| Nestin | 69.1 (29/42)* |
| GFAP | 40.0 (22/55) |
| β-tub III | 45.8 (22/48) |
| S100β | 19.5 (16/82) |
| PDGFRα | 33.3 (27/81) |
| O4 | 27.8 (27/97) |
| NeuN | 0.0 (0/50) |
| APC | 0.0 (0/50) |
The CSF-1R mediates crossing of axons through the midline in the corpus callosum
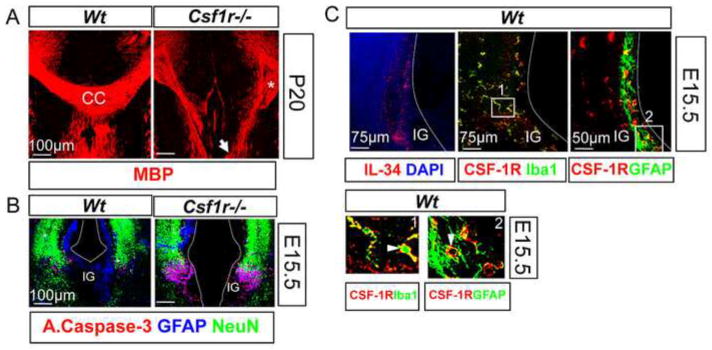
An important aspect of cortical development is the midline crossing of the corpus callosum. This requires an orchestrated development of radial glia that subsequently express GFAP and populate the cortical midline and indusium griseum (IG) by E14.5, prior to axonal routing (Kriegstein and Gotz, 2003; Shu et al., 2003; Shu and Richards, 2001; Silver et al., 1993). Our initial observations showed that the forebrain callosal commissures failed to cross and instead formed Probst bundles near the midline of 80% (n=9) of Csf1r−/− P20 forebrain cortex (Fig. 6A). In the E15.5 Csf1r−/− forebrain, there was increased apoptosis of GFAP+ cells with normal survival of neurons (Fig. 6B), suggesting that CSF-1R-mediated survival and development of GFAP+ cells could ensure a normal midline crossing of the axons. In addition to microglia (Fig. 6C, upper middle panel and box 1), a subset of these GFAP+ cells (Fig. 6C, upper right panel and box 2), also express the CSF-1R. Interestingly, commissural development occurs normally in 78.8% (n=7) of age-matched Csf1op/op brains (data not shown). This suggests a primary role of IL-34 in regulating this process. Consistent with this, IL-34 expression was observed in the IG, as well as along the dorsal midline of the E15.5 cortex (Fig. 6C, upper left panel). These observations demonstrate that CSF-1R-signaling facilitates midline crossing of the corpus callosum, in a non-cell autonomous manner, that is likely to be predominantly regulated by IL-34-dependent survival and development of GFAP+ cell populations at the dorsal midline of the embryonic brain.
Fig. 6.
Abnormalities of midline crossing of the corpus callosum in the Csf1r−/− mice. (A) P20 forebrain sections immunostained for MBP, showing the failure of the callosal axons to cross the midline in the Csf1r−/− brains (white arrow). Asterisk indicates the formation of the Probst bundles (P). (B) Apoptosis of GFAP+ cells in the IG and along the midline of E15.5 Csf1r−/− dorsal forebrains. Overlap of active caspase-3 (red) with GFAP (blue) staining, but not with NeuN (green) staining. (C) Expression of IL-34 and the CSF-1R at the midline and the IG of E15.5 dorsal forebrains. Upper panels: Immunostaining reveals the expression of IL-34 (red, left) and the CSF-1R (red, middle and right). Note the presence of CSF-1R+(red) Iba1+(green) microglia (middle). A subset of GFAP+ (green) population also expresses the CSF-1R (red, right). Lower panels: Insets 1 and 2 from upper panels. Arrows indicate overlap of CSF-1R staining with Iba1 and GFAP staining. Dotted line indicates the contour of each hemisphere. CC, corpus callosum; IG, indusium griseum.
The neonatal lethality and the forebrain phenotypes of Csf1op/op and Csf1r−/− mice are partially recapitulated in Nes-Cre/+; Csf1rflox/flox mice
To further assess its neural role, we selectively ablated the CSF-1R in Nestin-positive neural progenitors by mating Csf1rflox/flox (Csf1rfl/fl) mice (Li et al., 2006), with mice expressing the Cre transgene driven by the rat Nestin promoter and 2nd intron (Nes-Cre/+) (Tronche et al., 1999). These crosses were on the C57BL/6 background. On this background, the Csf1op/op and Csf1r−/− phenotypes are more severe than on the FVB/NJ background. Both mutant progeny die between E18.5 and P2 and heterozygous Csf1r+/− progeny also have reduced survival (S.N., X-M.D. and E.R.S., unpublished observations). Progeny of matings of Nes-Cre/+; Csf1rfl/+ with Csf1rfl/fl mice yielded normal Mendelian genetic ratios at E18.5, but significantly lower than expected numbers of Nes-Cre/+; Csf1rfl/fl and Nes-Cre/+; Csf1rfl/+ progeny at P20 (Table 2).
Table 2.
Chi square analysis of progeny genotypes from the cross between Nes-Cre/+; Csf1rfl/+ and Csf1rfl/fl mice
| Cross | Progeny | |||||||
|---|---|---|---|---|---|---|---|---|
| Nes-Cre/+; Csf1rfl/+ X Csf1rfl/fl | Nes-Cre/+ | +/+ | Number of progeny | Number of litters | *χ2 | P | ||
| Csf1r fl/+ | Csf1r fl/fl | Csf1r fl/+ | Csf1r fl/fl | |||||
| Age | O/E | O/E | O/E | O/E | ||||
| P20 | 18/22 | 12/22 | 24/22 | 20/22 | 74 | 12 | 5.26 | 0.02 |
| E18.5 | 16/11 | 13/11 | 8/11 | 12/11 | 49 | 6 | 1.38 | 0.10 |
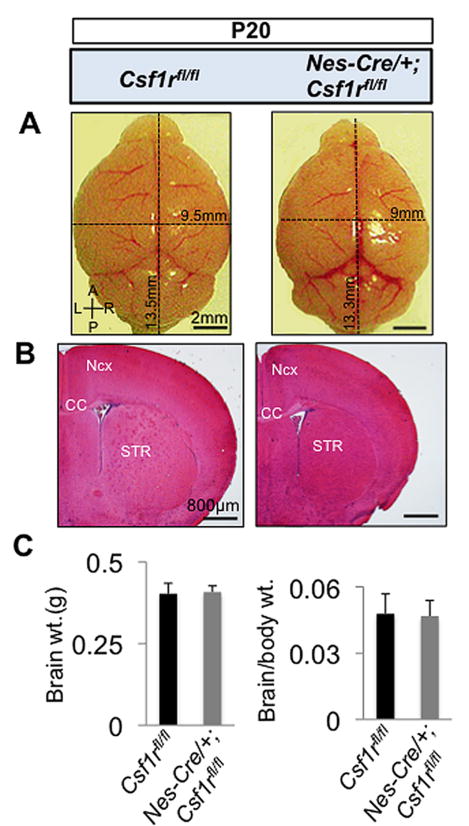
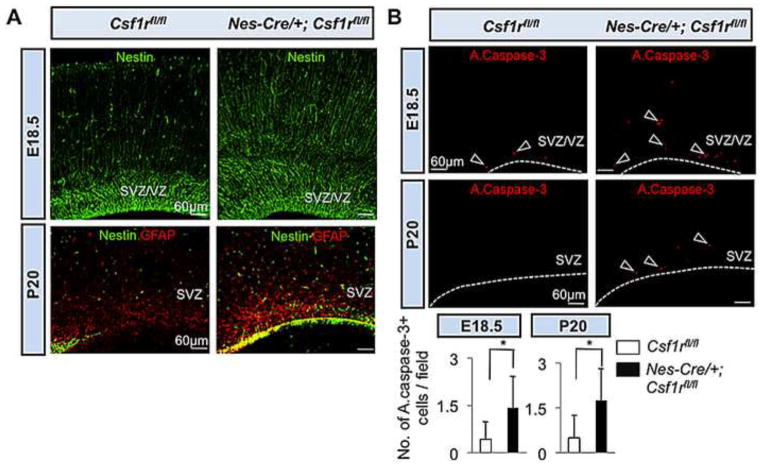
Compared with Csf1rfl/fl mice, the majority of the Nes-Cre/+; Csf1rfl/fl mice that survived to P20 had a smaller brain size, but a similar brain mass (Fig. 7). They also possessed smaller forebrains (Fig. 7B), but exhibited variable neocortical thickness (data not shown). No abnormalities were observed in the midline crossing of the corpus callosum, in the size of the ventricles (Fig. 7B) or the OB (Fig. 7A) (n=5). However, Nes-Cre/+; Csf1rfl/fl mice displayed an increase in the size of the forebrain progenitor pool (Fig. 8A) and elevated levels of forebrain cellular apoptosis (Fig. 8B). Thus the neonatal lethality, smaller brain size, enhanced forebrain progenitor proliferation and forebrain cellular apoptosis phenotypes of the Nes-Cre/+; Csf1rfl/fl mice partially recapitulated those of the Csf1r−/− and Csf1op/op mice (Table 3). However, in Nes-Cre/+; Csf1rfl/fl mice, some specific Csf1r−/− abnormalities (atrophy of the OB, enlarged lateral ventricle size and the failure of midline crossing of callosal axons) were not observed, while others (altered cortical thickness and impaired neuronal differentiation) were only partially recapitulated (Table 3).
Fig. 7.
Gross anatomical and histological abnormalities in P20 Nes-Cre/+; Csf1rfl/fl brains. (A) Reduction in brain size along the A/P and the L/R axes, but normal development of the OB in Nes-Cre/+; Csf1rfl/fl mice. (B) Coronal sections stained with hematoxylin and eosin (H&E), showing normal patterning, but a decrease in the forebrain size in Nes-Cre/+; Csf1rfl/fl mice. Note a normal ventricular size and midline crossing of the CC in Nes-Cre/+; Csf1rfl/fl mice. (C) Whole brain weight (left panel) and brain to body weight ratios (right panel). n = 5 mice per group. Ncx, neocortex; STR, striatum; A/P anterio-posterior; L/R, left-right; CC, corpus callosum.
Fig. 8.
Expansion of forebrain progenitor pools and enhanced cellular apoptosis in Nes-Cre/+; Csf1rrfl/fl mice. (A) Immunofluorescence microscopy of coronal sections of E18.5 (upper panels) and P20 (lower panels) Wt and mutant neocortex. Upper panels, Nestin. Lower panels, Nestin-GFAP double immunostaining. (B) Photomicrographs of active caspase-3+ (red) apoptotic cells in the SVZ/VZ region of E18.5 (upper panels) and SVZ region of P20 (middle panels) Wt and mutant mice. Arrowheads indicate apoptotic cells. Dotted lines in (B) delineate the ventricular border. Lower panels: Quantitation of the number of active caspase-3+ apoptotic cells/field. Means ± SD of eight representative low-power (20X) fields per region per genotype from two different mice per genotype; *, P<0.01.
Table 3.
Brain phenotypes of Csf1op/op, Csf1r−/− and Nes-Cre/+; Csf1rfl/fl mice compared to those of wild type mice.
| Mouse Mutant | |||
|---|---|---|---|
| Phenotype | Csf1op/op | Csf1r−/− | Nes-Cre/+; Csf1rfl/fl |
| Brain size | Reduced | Reduced | Reduced |
| Brain mass | Increased | Increased | Unchanged |
| OB atrophy | Absent | Present | Absent |
| Ventricular size | Unchanged | Increased | Unchanged |
| CC midline crossing defect | Present (22%*) | Present (80%*) | Absent |
| Cortical thickness | Increased | Reduced | Variable |
| Cortical NPC proliferation | Increased | Increased | Increased |
| Excitatory neuronal differentiation | Reduced | Reduced | Variable |
| Sub-cortical OL differentiation | Reduced | Reduced | Unchanged |
| Cortical cellular apoptosis | Unchanged | Increased | Increased |
| Cortical microglia | Reduced | Absent | Unchanged |
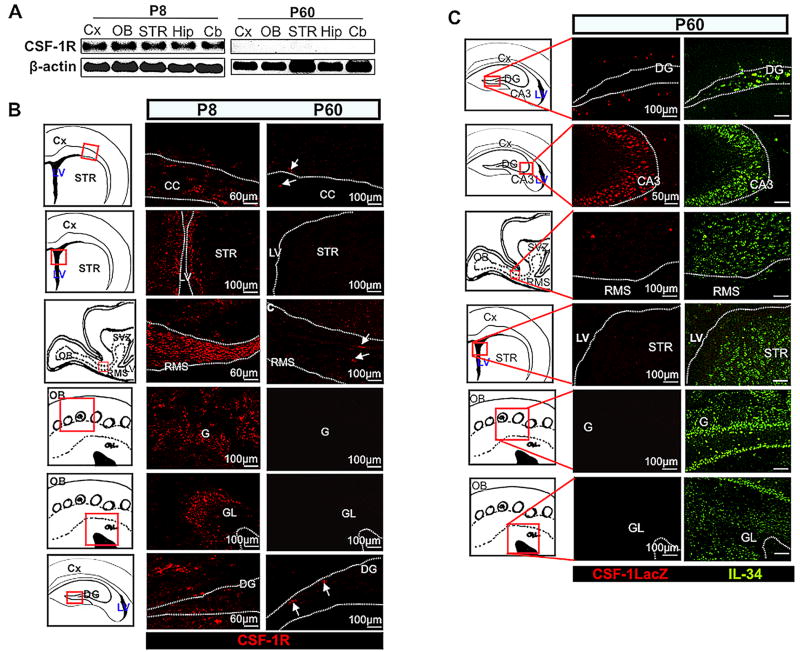
Predominant expression of IL-34, reduced expression of CSF-1 and minimal expression of the CSF-1R in adult brain
We have previously shown that mRNAs for both IL34 and Csf1 are expressed in adult brain and that the expression of IL34 is approximately 10 times higher than CSF-1 expression (Wei et al., 2010). Interestingly, expression of Csf1r mRNA (Fig. 9A) and protein (Fig. 9B) were minimal in various adult (P60) brain regions, although transcript and protein expression were at significant levels during early postnatal (P8) development, in agreement with the previous observations (Michaelson et al., 1996). In the absence of CSF-1 reporter expression, IL-34 was expressed and occasionally co-expressed with the CSF-1R in various early postnatal (P8) forebrain regions (Fig. S7). In contrast, IL-34 protein was expressed in various adult brain regions (Fig. 9C), where we failed to detect significant expression of the CSF-1R (Fig. 9B). In addition, the CSF-1 reporter protein was not detected in most adult brain regions where IL-34 was expressed, except in the RMS and hippocampus (Fig. 9C). The persistently high levels of IL-34 in different areas of adult brain, in the absence of CSF-1R expression, suggest the existence of additional IL-34 receptors.
Fig. 9.
Expression of IL-34, CSF-1 and the CSF-1R in the adult brain. (A,B) Decline in the level of CSF-1R expression in adult brain. (A) Semi-quantitative RT-PCR showing relative abundance of Csf1r mRNA from various P8 and P60 brain regions. (B) CSF-1R (red) immunostaining of various P8 and P60 Wt brain regions. (C) Exclusive expression of IL-34 as well as regional co-expression with CSF-1 in adult brains. Immunostaining of P60 TgZ brain sections showing expression of IL-34 (green) and CSF-1 reporter (red). Dotted lines delineate the contour of the structures. Arrows in (B) indicate a few CSF-1R+ cells in P60 brains. OB, olfactory bulb; G, glomerulus; GL, granule cell layer; CC, corpus callosum; Cx, cerebral cortex; LV, lateral ventricles; SVZ, subventricular zone; DG, dentate gyrus; RMS, rostral migratory stream; STR, striatum; CA3, CA3 region of hippocampus; hip, hippocampus; Cb, cerebellum.
Discussion
Requirement of CSF-1R signaling in neural development
Comparative histological examination of brain regions in Csf1r−/− mice at P20, just prior to their demise, revealed relatively normal gross developmental anatomy. However, the reduced size and the greater mass of Csf1r−/− brains, the reduced thickness of the neocortex, the midline crossing defect in the corpus callosum, the atrophy of the OB and the expansion of the area of the lateral ventricles, suggest that CSF-1R signaling is essential for the developmental specification and early maturation of these brain regions. Consistent with our observations, olfactory deficits and hydrocephaly have recently been reported in Csf1op/op and Csf1r−/− mice respectively (Erblich et al., 2011). The early perinatal lethality and some of the Csf1r−/− abnormalities were observed in Nes-Cre/+; Csf1rfl/fl mice (Table 3), demonstrating that CSF-1R-signaling in neural progenitors partially contributes to CSF-1R-mediated neural developmental effects. Consistent with a direct action of CSF-1R ligands on neural progenitors, in vitro clonal expansion and differentiation assays, performed using early postnatal dorsal forebrain progenitors derived from Nestin-GFP mice, revealed suppressed progenitor self-renewal and enhanced neurogenesis mediated by each ligand, in the absence of the ancillary effects of microglia. Supporting these in vitro results, our in vivo observations of Csf1op/op and Csf1r−/− mice suggest that the size of postnatal cortical progenitor pools is regulated, in part, through the developmental effects of CSF-1R signaling on inhibition of neural progenitor cell proliferation and promotion of regional neurogenesis (Fig. S8B). Consistent with our results, expression of the CSF-1R has been previously reported on a subset of cortical, hippocampal, cerebellar and brain stem neurons (Wang et al., 1999a), including cerebellar Purkinje cells (Murase and Hayashi, 1998). In contrast, Erblich et al (2011) concluded, on the basis of studies using the transgenic CSF-1R reporter (CSF-1R-EGFP MacGreen (Sasmono et al., 2003)) mice, that the CSF-1R is solely expressed by microglia. However, our anti-CSF-1R antibody that was formally demonstrated to be sensitive and highly specific, revealed CSF-1R expression in both microglial and neural cell types.
Our failure to completely recapitulate the Csf1r−/− mouse phenotype in the Nes-Cre/+; Csf1rfl/fl mice (Table 3), that exhibit normal microglial development (Fig. S9), together with the evidence for a microglia-dependent role of the CSF-1R in oligodendroglial differentiation (Fig. S10), indicates that CSF-1R-signaling in microglia is required for normal CNS development. Indeed, it has been previously suggested that paracrine signaling originating from CSF-1R-expressing microglia is involved in the mediation of normal neural developmental processes (Papavasiliou et al., 1997).
Both Csf1op/op and Csf1r−/− mice exhibit a failure of microglial homeostasis to different degrees (a 20% decrease in Csf1op/op and a 100% decrease in Csf1r−/−), as well as subcortical oligodendrocyte and neocortical neuronal differentiation failures in both mutants. These cellular deficits could explain the smaller brains of these mutants. Although it is not clear why the brain mass of both mutants is increased, it may in part be explained by the reduced clearance of cell corpses due to the microglial deficiency.
Our observation of the increased thickness of the neocortex of Csf1op/op mice and its decreased thickness in Csf1r−/− mice is surprising in view of the significantly stronger effect of CSF-1R deficiency in increasing the number of neural progenitors. It is possible that this difference is explained by the effects of microglia in the Csf1op/op mice (absent in Csf1r−/− mice), despite our failure to observe greater excitatory neuronal differentiation in these mice compared with Csf1r−/− mice. Alternatively, CSF-1 could also act through another receptor in neural cells with actions opposing CSF-1R-mediated effects.
Distinct regional and cellular expression profiles of CSF-1 and IL-34
Analysis of the CSF-1 reporter mice showed that CSF-1 was predominantly restricted to mature neurons of the dorsal forebrain (Fig. 2B) and cerebellum (Fig. S11C) and that the CSF-1R is expressed by a small subset of forebrain progenitors (Fig. S4) and neurons of the cerebellar molecular layer and Purkinje cells, as well as microglia (Fig. S11C). Compared with CSF-1, IL-34 was similarly expressed by mature neurons, but located within distinct cortical laminae, as well as in meningeal cells. While IL-34 expression was limited to specific forebrain structures (neocortex, OB and striatum) (Figs. 2, 9C), CSF-1 expression was also observed in hindbrain regions (cerebellum) (Fig. S1B,C) and in the spinal cord (Wei et al., 2010). During embryogenesis, cerebellar CSF-1 expression was localized to the rhombic lip (Fig. S11B), the region that gives rise to excitatory glutamatergic and granule neurons (Carletti and Rossi, 2008; Hatten et al., 1997). During postnatal development, CSF-1 was expressed in layer VI of the neocortex, where excitatory neurons reside, whereas IL-34 was expressed in layers II–V of the neocortex, where both excitatory as well as inhibitory neurons and mature glial cell subtypes are found (Nadarajah et al., 2003), as well as the meninges. Together, these data suggest that CSF-1 and IL-34 have complementary and non-redundant functions in developing and adult CNS.
The complementary expression profiles of IL-34 and CSF-1 are consistent with the requirement of CSF-1R signaling for the development and maintenance of microglia
Microglia are macrophages that are broadly distributed throughout the central nervous system, constitute a first line of immunological defense and are activated in response to inflammation (Altman, 1994; Dickson et al., 1993). Recently, we showed that adult microglia derive predominantly from primitive extra-embryonic progenitors that are recruited to the brain primordia after development of the embryonic circulation at E9.5 (Ginhoux et al., 2010). In that study, we confirmed the partial reduction in the number of microglia (~20%) in Csf1op/op brains (Kondo et al., 2007; Wegiel et al., 1998) and showed that Csf1r−/− brains are completely devoid of microglia. These findings, coupled with our current and earlier (Wei et al., 2010) observations of the complementary and broad expression of IL-34 and CSF-1, are consistent with regulation of microglia by both factors, as their expression profiles are maintained in the adult brain. Interestingly, there was a higher frequency of amoeboid microglia in Wt compared with Csf1op/op brains (Fig. S12), suggesting that CSF-1 may be important for microglial activation. Microglial activation has been implicated in neurodegenerative diseases (Paresce et al., 1996). In addition, the phagocytic activity of microglia has been implicated in the removal of apoptotic cells during development as well as in disease states (Magnus et al., 2002; Takahashi et al., 2005) and the absence of microglia may, in part, contribute to the increase in apoptotic cells we observed in Csf1r−/− brains in the present study. How such CSF-1R signaling in microglia might contribute to paracrine regulation of normal brain development is not presently clear. However, microglia-derived soluble factors, including IL-1, IL-6 and insulin-like growth factor-1, have been reported to modulate the survival and behavior of diverse neural cell types (Choi et al., 2008; Lalancette-Hebert et al., 2007; Nakanishi et al., 2007).
CSF-1R-independent roles of IL-34 and CSF-1
Consistent with the previous demonstration that IL34 mRNA is expressed at higher levels during brain development than either Csf1 or Csf1r mRNA (Wei et al., 2010), we have shown that IL-34 protein is persistently and broadly expressed during development and in the adult brain. IL-34 and CSF-1 display infrequent co-expression patterns in adult brain, whereas CSF-1R expression is markedly reduced (Fig.S8A). Also, as mentioned above, our analyses of the mutant brains showed that the absence of CSF-1 results in larger forebrain size with increased cortical thickness, abnormalities distinct from CSF-1R-deficiencies. Thus the differential pattern of expression of the CSF-1R and its ligands, together with the unique Csf1op/op phenotype, could reflect the use of additional receptors by IL-34 and CSF-1.
CSF-1R signaling in neural progenitors
In hematopoiesis, CSF-1R signaling instructs macrophage lineage choice (Rieger et al., 2009) and regulates mononuclear phagocyte survival, proliferation and differentiation (reviewed in (Pixley and Stanley, 2004) and may stimulate myeloid lineage commitment (Sarrazin et al., 2009; Stanley, 2009). CSF-1R-mediated processes affecting developing and possibly mature forebrain neural progenitor pools involve inhibition of cellular expansion, while ensuring proper selective neuronal and glial cell differentiation. Thus CSF-1R-mediated differentiation of neural progenitor cells appears to be somewhat analogous to CSF-1R action on granulocyte/macrophage progenitors (Rieger et al., 2009), where CSF-1 commits cells to a macrophage fate. Moreover, while this lineage commitment results in less proliferation during myeloid expansion, the inhibition of more differentiated myeloid cell proliferation by CSF-1 has not been reported. However, CSF-1R signaling has been shown to induce G1 cell cycle arrest by the coordinate up-regulation of the cyclin-dependent kinase inhibitor, p21cip1/waf1 and cyclin D1 in MCF-7 human breast cancer cells (Lee et al., 1999). Furthermore, c-myb, a Csf1r transcriptional repressor (Reddy et al., 1994), facilitates neural progenitor proliferation by up regulation of the transcription factors, Pax-6 and Sox-2 (Malaterre et al., 2008). As Pax-6 and Sox-2 assist in maintaining neural progenitor cell pools by inhibiting progenitor differentiation (Estivill-Torrus et al., 2002; Graham et al., 2003), it is possible that these transcription factors are targets of CSF-1R signaling.
Conclusion
Previous studies have implicated CSF-1 in the regulation and maintenance of microglia (Sawada et al., 1990; Suzumura et al., 1990) and suggested that CSF-1 might directly regulate neural cells (Michaelson et al., 1996; Wang et al., 1999a). We have recently shown that microglia are established in early embryogenesis and that their lineage elaboration and maintenance are absolutely dependent on CSF-1R signaling (Ginhoux et al., 2010). Together with our present finding of the broad and complementary regional and local expression profiles of IL-34 and CSF-1 and our earlier demonstration of high levels of expression of IL34 mRNA in developing and adult brain (Wei et al., 2010), these studies implicate IL-34, as well as CSF-1 in CNS microglia regulation. In contrast, the focus of the present study has been on the nature and significance of expression of the CSF-1R on neural progenitors. We have shown that the CSF-1R, via the action of its two known ligands, plays a very significant and direct role in the regulation of progenitor cell proliferation and differentiation. That the direct regulation of neural progenitors through the CSF-1R is biologically significant has been demonstrated by the perinatal lethality of the Nes-Cre/+; Csf1rfl/fl mice, which phenocopies the perinatal death of Csf1r−/− mice. Similar to its different roles in the commitment and differentiation to the myeloid lineage and macrophages, CSF-1R signaling is also involved in regulation of the proliferation, differentiation and survival of neural progenitor cells. Relevant to our study, dominant point mutations in the Csf1r gene have recently been shown to cause hereditary diffuse leukoencephalopathy, a disease primarily affecting the subcortical white matter tracts (Rademakers et al., 2011).
Among many remaining questions to be addressed are: How do the non-overlapping regional expression profiles of IL-34 and CSF-1 contribute differentially to neural development? Do IL-34 and CSF-1 instruct lineage commitment, as observed for CSF-1 action on bipotential granulocyte/macrophage progenitors? Is IL-34 required for the generation and maintenance of a majority of the CNS microglia? What is the nature of CSF-1 action on microglia in brain? How do these cytokines contribute to regulation of migration of microglial progenitors from the yolk sac to the primordial brain and of neural progenitors and neurons in the developing cerebral cortex and RMS? Irrespective of the answers to these questions, the present study demonstrates that CSF-1 and IL-34 play important roles in the regulation of diverse neural cell types in brain.
Supplementary Material
Supplementary Data
Highlights.
- Detailed CNS expression pattern of CSF-1, IL-34 and the CSF-1R
- Expression of the CSF-1R on neural progenitor cells (NPC)
- Altered proliferation, survival and differentiation of NPC in Csf1r−/− mice
- Direct regulation of neurogenesis by CSF-1 or IL-34 in vitro
- Increased lethality & brain abnormalities in Nestin-Cre/+; Csf1rflox/flox mice
Acknowledgments
We thank Halley Ketchum and Xiao-Hua Zong for technical assistance and Dr. J.W. Pollard for the Csf1rfl/fl mice. We also thank the Einstein histopathology, FACS and analytical imaging facilities. This work was supported by: NIH grants CA32551 and CA26504 (to ERS) and NS071571 and HD071593 (to MFM), NIMH and NYSTEM (to GE) and the Albert Einstein College of Medicine Cancer Center grant 5P30-CA13330.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J. Microglia emerge from the fog. Trends Neurosci. 1994;17:47–49. doi: 10.1016/0166-2236(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Arceci RJ, Shanahan F, Stanley ER, Pollard JW. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989;86:8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Bernd P. The role of neurotrophins during early development. Gene Expr. 2008;14:241–250. doi: 10.3727/105221608786883799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya J, Calvo J, Prado A. The origin of microglial cells. J Anat. 1979;129:177–186. [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnar Z, Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Byrne PV, Guilbert LJ, Stanley ER. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981;91:848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PE, Chisholm O, Arceci RJ, Stanley ER, Pollard JW. Absence of colony-stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice results in male fertility defects. Biol Reprod. 1996;55:310–317. doi: 10.1095/biolreprod55.2.310. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Nishimura K, Pollard JW. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology. 2002;143:1413–1422. doi: 10.1210/endo.143.4.8754. [DOI] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Dai XM, Zong XH, Akhter MP, Stanley ER. Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res. 2004a;19:1441–1451. doi: 10.1359/JBMR.040514. [DOI] [PubMed] [Google Scholar]
- Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004b;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer’s disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Guilbert LJ, Stanley ER. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980;85:153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Wall RJ, Tillmann U, Li M, Furth PA. Conditional gene expression in secretory tissues and skin of transgenic mice using the MMTV-LTR and the tetracycline responsive system. J Cell Biochem. 1995;59:463–472. doi: 10.1002/jcb.240590407. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hume DA, Robinson AP, MacPherson GG, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983;158:1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh D, Dai XM, Nandi S, Lightowler S, Trivett M, Chan CK, Bertoncello I, Ramsay RG, Stanley ER. Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine. Gastroenterology. 2009;137:136–144. 144 e131–133. doi: 10.1053/j.gastro.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama H, Nose M, Niida S, Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991;173:1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Lemere CA, Seabrook TJ. Osteopetrotic (op/op) mice have reduced microglia, no Abeta deposition, and no changes in dopaminergic neurons. J Neuroinflammation. 2007;4:31. doi: 10.1186/1742-2094-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Gotz M. Radial glia diversity: a matter of cell fate. Glia. 2003;43:37–43. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Nambirajan S, Moffat JG. CSF-1 activates MAPK-dependent and p53-independent pathways to induce growth arrest of hormone-dependent human breast cancer cells. Oncogene. 1999;18:7477–7494. doi: 10.1038/sj.onc.1203123. [DOI] [PubMed] [Google Scholar]
- Li J, Chen K, Zhu L, Pollard JW. Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto-oncogene) in mice. Genesis. 2006;44:328–335. doi: 10.1002/dvg.20219. [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- Magnus T, Chan A, Savill J, Toyka KV, Gold R. Phagocytotic removal of apoptotic, inflammatory lymphocytes in the central nervous system by microglia and its functional implications. J Neuroimmunol. 2002;130:1–9. doi: 10.1016/s0165-5728(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Malaterre J, Mantamadiotis T, Dworkin S, Lightowler S, Yang Q, Ransome MI, Turnley AM, Nichols NR, Emambokus NR, Frampton J, Ramsay RG. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells. 2008;26:173–181. doi: 10.1634/stemcells.2007-0293. [DOI] [PubMed] [Google Scholar]
- Marks SC, Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. J Hered. 1976;67:11–18. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Gokhan S. Postnatal cerebral cortical multipotent progenitors: regulatory mechanisms and potential role in the development of novel neural regenerative strategies. Brain Pathol. 1999;9:515–526. doi: 10.1111/j.1750-3639.1999.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993;362:62–65. doi: 10.1038/362062a0. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, Pollard JW, Kessler JA. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Pollard JW, Stanley ER. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987;130:420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Murase S, Hayashi Y. Expression pattern and neurotrophic role of the c-fms proto-oncogene M-CSF receptor in rodent Purkinje cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:10481–10492. doi: 10.1523/JNEUROSCI.18-24-10481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Neuronal migration in the developing cerebral cortex: observations based on real-time imaging. Cereb Cortex. 2003;13:607–611. doi: 10.1093/cercor/13.6.607. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Papavasiliou AK, Mehler MF, Mabie PC, Marmur R, Qingbin S, Keating RF, Kessler JA. Paracrine regulation of colony-stimulating factor-1 in medulloblastoma: implications for pathogenesis and therapeutic interventions. Neurosurgery. 1997;41:916–923. doi: 10.1097/00006123-199710000-00028. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Pollard JW, Hunt JS, Wiktor-Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991;148:273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, Dejesus-Hernandez M, Adamson J, Kouri N, Sundal C, Shuster EA, Aasly J, Mackenzie J, Roeber S, Kretzschmar HA, Boeve BF, Knopman DS, Petersen RC, Cairns NJ, Ghetti B, Spina S, Garbern J, Tselis AC, Uitti R, Das P, Van Gerpen JA, Meschia JF, Levy S, Broderick DF, Graff-Radford N, Ross OA, Miller BB, Swerdlow RH, Dickson DW, Wszolek ZK. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet. 2011 doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Yang BS, Yue X, Barnett CJ, Ross IL, Sweet MJ, Hume DA, Ostrowski MC. Opposing actions of c-ets/PU.1 and c-myb protooncogene products in regulating the macrophage-specific promoters of the human and mouse colony-stimulating factor-1 receptor (c-fms) genes. J Exp Med. 1994;180:2309–2319. doi: 10.1084/jem.180.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, Otto C, Sieweke MH. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, Yamamoto H, Marunouchi T. Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Res. 1990;509:119–124. doi: 10.1016/0006-8993(90)90317-5. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shu T, Puche AC, Richards LJ. Development of midline glial populations at the corticoseptal boundary. Journal of neurobiology. 2003;57:81–94. doi: 10.1002/neu.10252. [DOI] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial wedge during the development of the corpus callosum. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Edwards MA, Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. The Journal of comparative neurology. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- Stanley ER. Lineage commitment: cytokines instruct, at last! Cell Stem. Cell. 2009;5:234–236. doi: 10.1016/j.stem.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Guilbert LJ, Tushinski RJ, Bartelmez SH. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stanley ER, Heard PM. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252:4305–4312. [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Effects of colony stimulating factors on isolated microglia in vitro. J Neuroimmunol. 1990;30:111–120. doi: 10.1016/0165-5728(90)90094-4. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kishi K, McCarron RM, Miyatake T. The generation of macrophages from precursor cells incubated with brain endothelial cells--a release of CSF-1 like factor from endothelial cells. Tohoku J Exp Med. 1993;171:211–220. doi: 10.1620/tjem.171.211. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tushinski RJ, Oliver IT, Guilbert LJ, Tynan PW, Warner JR, Stanley ER. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982;28:71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Berezovska O, Fedoroff S. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J Neurosci Res. 1999a;57:616–632. [PubMed] [Google Scholar]
- Wang Y, Yeung YG, Stanley ER. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J Cell Biochem. 1999b;72:119–134. [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM, Dziewiatkowski J, Tarnawski M, Kozielski R, Trenkner E, Wiktor-Jedrzejczak W. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 1998;804:135–139. doi: 10.1016/s0006-8993(98)00618-0. [DOI] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Zhu G, Mehler MF, Mabie PC, Kessler JA. Developmental changes in progenitor cell responsiveness to cytokines. J Neurosci Res. 1999;56:131–145. doi: 10.1002/(sici)1097-4547(19990415)56:2<131::aid-jnr3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data