Major depressive disorder: new clinical, neurobiological, and treatment perspectives (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 16.
Abstract
In this Seminar we discuss developments from the past 5 years in the diagnosis, neurobiology, and treatment of major depressive disorder. For diagnosis, psychiatric and medical comorbidity have been emphasised as important factors in improving the appropriate assessment and management of depression. Advances in neurobiology have also increased, and we aim to indicate genetic, molecular, and neuroimaging studies that are relevant for assessment and treatment selection of this disorder. Further studies of depression-specific psychotherapies, the continued application of antidepressants, the development of new treatment compounds, and the status of new somatic treatments are also discussed. We address two treatment-related issues: suicide risk with selective serotonin reuptake inhibitors, and the safety of antidepressants in pregnancy. Although clear advances have been made, no fully satisfactory treatments for major depression are available.
Epidemiology, comorbidity, and diagnosis
Worldwide, depression is a seriously disabling public health problem of very high prevalence.1 Major depressive disorder has a 12-month prevalence of 6·6% and a lifetime prevalence of 16·2%, is twice as common in women as in men, and causes considerable impairment. Age-of-onset distributions suggest that depression is prevalent for the entire lifespan.2 The disorder not only produces decrements in health that are equivalent to those of other chronic diseases (eg, angina, arthritis, asthma, and diabetes), but also worsens mean health scores substantially more when comorbid with these diseases, than when the diseases occur alone.3 A crucial implication is that primary care providers should not ignore the presence of depression when patients have a chronic physical disorder.
Overdetection and underdetection are important factors that should be considered to ensure the appropriate diagnosis and management of clinical depression.4 Although a meta-analysis5 concluded that general practitioners correctly exclude depression in most individuals who are not depressed, overdetections (false positives) can outnumber missed cases. The presence of anxiety with depression can increase difficulties in diagnosis. Some researchers have argued that the establishment of anxious depression as a specific diagnosis would substantially improve identification of depression in primary care settings, and such a category has been proposed for the fifth edition of the diagnostic and statistical manual of mental disorders (DSM-5) and for the 11th revision of the international classification of diseases (ICD-11).6
Although in this Seminar we focus on major depressive disorder (bipolar disorder has been addressed in other Seminars in The Lancet7), studies that better elucidate the boundaries and phenotypical description of the disorder are highly relevant. In up to 40% of patients, major depression is associated with lifetime occurrences of isolated manic or hypomanic symptoms that do not cluster in a way that is consistent with a diagnosis of hypomania. Furthermore, such symptoms can be concurrent with syndromal-level major depressive disorder.8,9 Further investigation is needed to examine the treatment and prognosis of major depression that is associated either concurrently, or at other points in the patient’s history, with hypomanic symptoms.10 This investigation could be facilitated by proposed changes in DSM-5, which include the possibility of a mixed specifier indicating the presence of sub-threshold hypomanic symptoms in those with unipolar disorder.
Major depressive disorder was assumed to precede generalised anxiety disorder until a 32-year prospective follow-up study11 challenged this notion. Indeed, the reverse pattern seems to be frequently present, and the combination of generalised anxiety disorder and major depression might represent an additional burden. Social anxiety disorder (social phobia) is now also regarded as an important and consistent risk factor for the development of severe depression.12 Furthermore, comorbid personality disorder seems to confer a worse prognosis and poorer treatment response than does major depression alone.13 Some of the risk factors for the metabolic syndrome (eg, obesity), might also increase the risk of depression and, in turn, depression increases the risk for development of obesity.14 These two-way relations might be the reason for the increased association between depression and coronary artery disease.
Kendler and colleagues15 have shown a major relation between depression and coronary artery disease, mainly in acute states. A high severity of depression within several weeks of admission to hospital for an acute coronary syndrome, or an inadequate treatment response in depression, can double cardiac mortality in 6·7 years of follow-up.16 Studies examining depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease have shown a high likelihood of major adverse cardiac events in those with depression.17 These results have led to the recommendation that all patients with coronary artery disease be screened for depression;18 however, this recommendation is somewhat controversial.19 Studies of the relation between depression and diabetes have led to new conclusions—eg, that clinical depression is associated with a 65% increased risk of diabetes in elderly people.20 Major and minor depressions seem to be implicated in this relation.21 These studies22 emphasise the importance of identification and treatment of depression in the physically ill.
Advances in neurobiology
Genetic studies
Genetic, molecular, and neuroimaging studies continue to contribute to advances in our understanding of the neurobiological basis of major depressive disorder. However, the extent to which findings from neurobiological studies can help improve the clinical and functional outcome of individuals with the disorder is still uncertain. Thus, in the past 5 years, neurobiological research of depression has become two-tiered to: (1) understand the pathophysiology of the illness; and (2) identify the neurobiological measures for guiding treatment choice.
The identification of single candidate genes associated with major depression has been difficult because of the likelihood that complex psychiatric illnesses are under polygenic influence and are associated with interactions between genetic variants and environmental exposures.23 One approach has been to go beyond the conventional focus on monoamines in the search for a genetic association.24 For example, major depressive disorder has been associated with polymorphisms in the glucocorticoid receptor gene NR3C1,25 the monoamine oxidase A gene,26 the gene for glycogen synthase kinase-3β (which has a key role in the phosphorylation and regulation of metabolic enzymes and many transcription factors27), and a group-2 metabotropic glutamate receptor gene (GRM3).28 Success has been greater in the identification of candidate genes that are associated with known biological mechanisms and metabolic pathways for antidepressant drugs and, that in turn, can help predict the response from antidepressant treatment.
Many studies have focused on the functional insertion-deletion promoter variant (serotonin transporter-linked polymorphic region [5HTTLPR]) in the serotonin transporter gene (SLC6A4). A meta-analysis29 showed two associations, first between the 5HTTLPR long allele and increased response to selective serotonin reuptake inhibitor (SSRI) antidepressant drugs (although not to nortriptyline30), and reduced side-effects to SSRIs;31 and second between the 5HTTLPR short allele and increased paroxetine-induced, but decreased mirtazapine-induced adverse effects.32 Several single-nucleotide polymorphisms (SNPs) in the gene for the serotonin type-2a receptor are associated with outcomes of SSRI treatment.31,33–35
Studies36–38 of candidate genes associated with other biological mechanisms have emphasised associations between glutamatergic genes (eg, GRIK4) and citalopram response and adverse effects; between the Met allele of the functional Val/Met polymorphism (rs6265) in brain-derived neurotrophic factor (BDNF) and SSRI response;39 and between several other BDNF SNPs and desipramine response.31 Genetic variation in FKBP5—a protein that helps to regulate cortisol binding to the glucocorticoid receptor40—is associated with antidepressant response;41,42 whereas genetic variants in _TREK1_—a potassium channel mediating SSRI mechanism of action—are associated with non-response to several antidepressants.43 Genetic variation in the catechol-O-methyltransferase (COMT) gene, which alters COMT activity, is associated with response to treatment with several antidepressants.44,45
Genome-wide association studies further suggest that effectiveness of antidepressants can be predicted by genetic markers other than traditional candidate genes. These genes include those for the corticotrophin-releasing hormone (CRH) receptor-1 (CRHR1) and CRH binding protein (CRHBP), which predict SSRI response in anxious depression,46,47 and genes for uronyl-2 sulphotransferase and interleukin-11, which predict response to nortriptyline and escitalopram oxalate, respectively.48
Molecular studies
At least three main categories of peripheral hormone-type factors, for which genetic variants are associated with major depressive disorder, are implicated in the pathophysiology of the illness: (1) neurotrophic factors and other growth factors, including BDNF, vascular endothelial growth factor, and insulin-like growth factor-1; (2) proinflammatory cytokines, including interleukin-1β, interleukin-6, and tumour necrosis factor-α; and (3) impaired regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis. For example, serum BDNF is decreased in individuals with major depression, and antidepressant treatment reverses this decrease.49,50
The secretion and production of proinflammatory cytokines are increased in individuals who are stressed and depressed,51,52 and, in major depression, anti depressant drugs can return concentrations of these cytokines to normal or suppress their synthesis.53 Impaired regulation of the HPA axis in an acute episode of depression has long been documented,54 and published work has indicated attenuation of neuroendocrine response to the combined dexamethasone-corticotrophin-releasing hormone test—a sensitive measure of altered regulation of the HPA system—during antidepressant treatment.55
Neuroimaging studies
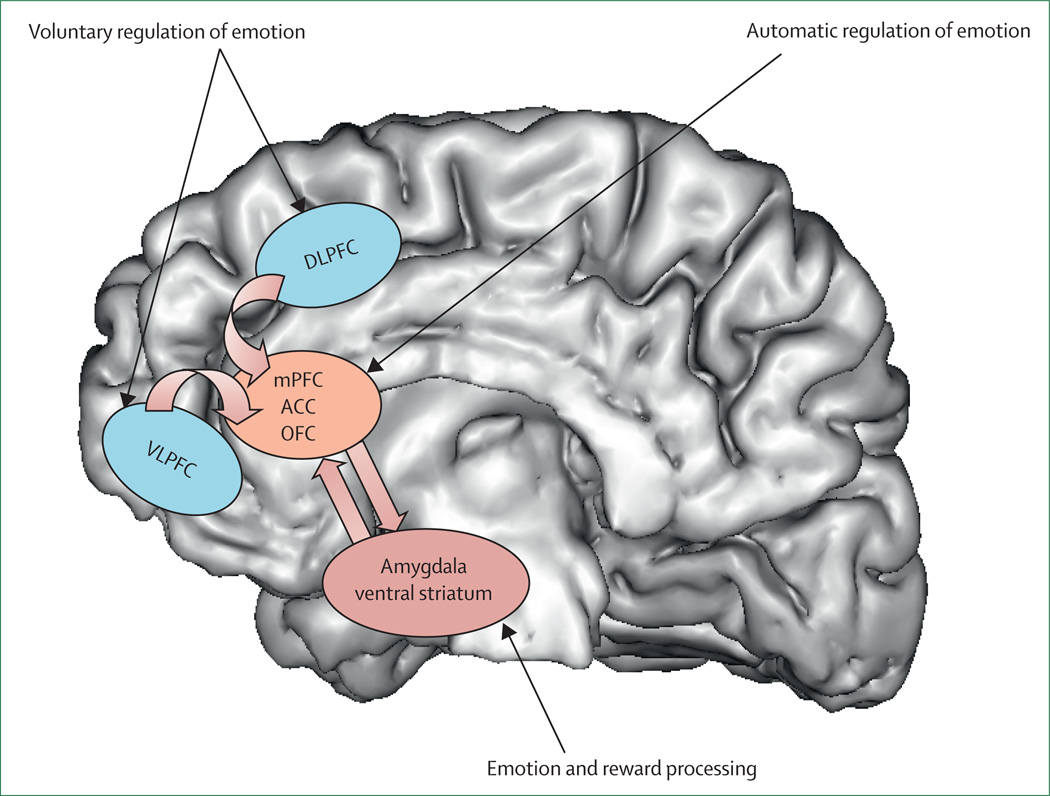
Neural systems that are important to understand major depressive disorder include those that support emotion processing, reward seeking, and regulate emotion, all of which are dysfunctional in the disorder. These systems include subcortical systems involved in emotion and reward processing (eg, amygdala, ventral striatum); medial prefrontal and anterior cingulate cortical regions involved in processing emotion and automatic or implicit regulation of emotion; and lateral prefrontal cortical systems (eg, ventrolateral prefrontal cortex and dorsolateral prefrontal cortex) involved in cognitive control and voluntary or effortful regulation of emotion.56 These systems can be conceptualised as a medial prefrontal-limbic network, including amygdala, anterior cingulate cortex, and medial prefrontal cortex that is modulated by serotonin neurotransmission,57–59 and a reward network centred on ventral striatum and interconnected orbitofrontal and medial prefrontal cortices that is modulated by dopamine (figure 1).60
Figure 1. Neural systems of relevance to major depressive disorder.
Key neural regions implicated in emotion and reward processing, and voluntary and automatic regulation of emotion are shown superimposed on a greyscale depiction of the human brain. DLPFC=dorsolateral prefrontal cortex. mPFC=medial prefrontal cortex. ACC=anterior cingulate cortex. OFC=orbitofrontal cortex. VLPFC=ventrolateral prefrontal cortex. Adapted with permission from reference 56.
Neuroimaging studies of major depressive disorder61–65 have provided evidence for specific functional abnormalities in these neural systems in adults. Converging findings from these studies suggest abnormally increased amygdala, ventral striatal, and medial prefrontal cortex activity, mostly to negative emotional stimuli, such as fearful faces. Abnormally reduced ventral striatal activity to positive emotional stimuli,61,66 and during receipt and anticipation of reward in adults67 and adolescents68 with depression have also been reported. These findings support a bias of attention towards negative emotional stimuli, and away from positive emotional and reward-related stimuli in individuals with major depression. Further studies are needed to examine neural activity in the prefrontal cortical regions that are implicated in voluntary and implicit regulation of emotion. These functional neuroimaging studies are complemented by increased findings suggesting reductions in grey matter in key regions of these neural systems (including amygdala69), age-related decreases in volume of the anterior cingulate cortex,70 and post-mortem findings indicating neural-cell and glial-cell pathology in prefrontal cortical regions.71
Additional evidence for abnormalities in neural systems for emotion and reward processing and regulation of emotion in major depressive disorder has come from neuroimaging studies of emotion processing, which used measures of functional connectivity, including effective connectivity—ie, the effect that activity in one region exerts over that in another. For example, one study72 reported abnormal-inverse effective connectivity in medial prefrontal cortical-amygdala during emotion labelling of happy faces in individuals with major depression. These findings suggest abnormally increased top-down regulation of the amygdala by medial prefrontal cortex in these individuals, which again suggests a bias away from attendance to positive emotional stimuli. Other studies73 have applied diffusion tensor imaging, which uses measures of the diffusivity of water in different directions—either parallel or perpendicular to the direction of white-matter fibres—to construct whole-brain measures of patterns of white-matter connectivity between different neural regions. Findings from these studies, have shown abnormal prefrontal cortico-subcortical white-matter connectivity between regions supporting emotion regulation in adults with depression.73
Examination of brain activity during rest can provide valuable insights into how the brain functions during self-reflection, which could be abnormal in individuals with major depression. Studies have provided evidence for a malfunction of the default mode network.74 This network includes several midline regions, including the inferior (ventral) part of the medial prefrontal cortex (vmPFC). Anterior parts of this network (eg, vmPFC) are involved in self-referential processing74 and tend to deactivate during cognitive tasks as cognitive resources are redirected. The normal decrease in vmPFC activity during difficult tasks might indicate intact emotional gating and, conversely, absence of this deactivation might indicate impaired gating. Findings from analysis of task-independent deactivations suggest an abnormality of the default mode network in vmPFC in major depressive disorder. A method to examine resting-state-related neural activity is measurement of the correlation of low-frequency blood oxygen level-dependent temporal fluctuations (LFBF) in steady-state functional MRI data between different neural regions. One study75 showed that individuals with depression had increased resting-state connectivity in medial prefrontal cortex regions in the default mode network, including the anterior cingulate cortex and the vmPFC.
A meta-analysis76 of several neuroimaging studies of major depressive disorder identified two neural systems of importance to the illness. One network that centred on the dorsolateral prefrontal cortex and more dorsal regions of the anterior cingulate cortex, but that also included other regions implicated in cognitive control, was characterised by reduced activity in the resting state, which returned to normal with treatment. A second network, centred on medial prefrontal cortex and subcortical regions, was hyperactive to emotional stimuli in the depressed state, but returned to normal after antidepressant treatment. This meta-analysis76 provides further evidence of increased activity in neural systems supporting emotion processing (amygdala and medial prefrontal cortex), and reduced activity in neural systems supporting regulation of emotion (eg, dorsolateral prefrontal cortex).
Neuroimaging studies have also examined neural predictors of outcome and neuroimaging measures that change with response to different antidepressant treatments. These studies have focused mainly on the role of the serotonergically-mediated medial prefrontal-limbic network. In major depression, SSRI drugs modulate emotion-induced activity in this network, and response to SSRIs is predicted by activity in this region.77 LFBF correlations between subcortical regions and the anterior cingulate cortex can also increase after SSRI treatment in individuals with depression.78,79 However, less is known about the effects of non-serotonergic drugs on the medial prefrontal-limbic network, and the extent to which pretreatment activity in this region predicts subsequent response to non-serotonergic antidepressants needs to be examined. Desynchronisation of the anterior cingulate cortex, and its functional connectivity with the amygdala predicts rapid antidepressant response to ketamine,80 but response to cognitive behavioural therapy is predicted by reduced medial prefrontal activity, and increased amygdala activation.81 Response to deep-brain stimulation correlates with reduced activity in ventral (subgenual) regions of the anterior cingulate cortex,82,83 and increased metabolism in ventral striatum.83
Integration of measures
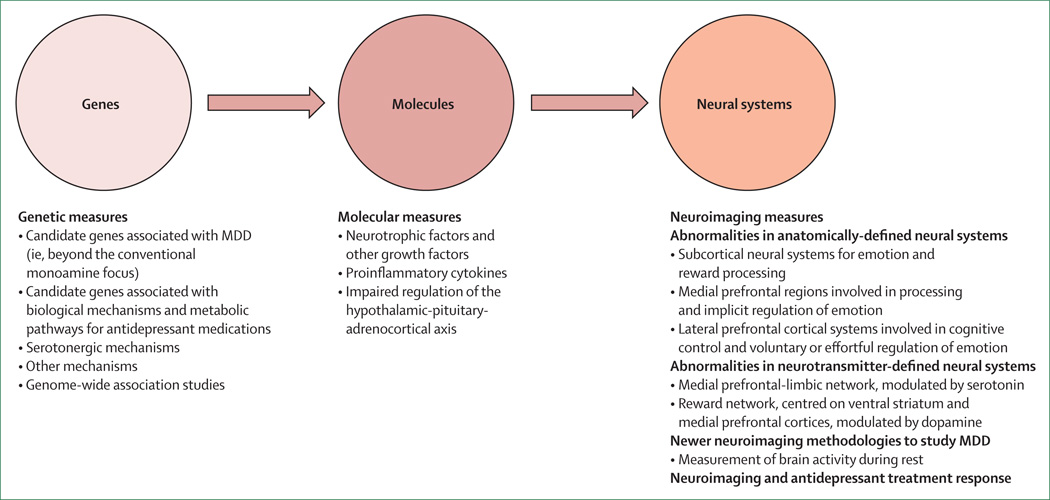
Studies examining a combination of genetic, molecular, and neuroimaging measures to identify relations among genes, molecules, neural systems, and behaviour in major depressive disorder could increase our understanding of the underlying pathophysiological processes and prediction of treatment response (figure 2). Relations between genetic variation in neutrophic factors, and variation in concentrations of these factors, such as BDNF and vascular endothelial growth factor, and structural and functional variation in neural regions supporting mood regulation and associated behaviours and neurocognitive function, are well documented.84–88 This variation might in turn reduce the ability to respond to treatments dependent on intact neurocognition, such as cognitive behavioural therapy.
Figure 2. Advances in neurobiology of MDD.
MDD=major depressive disorder.
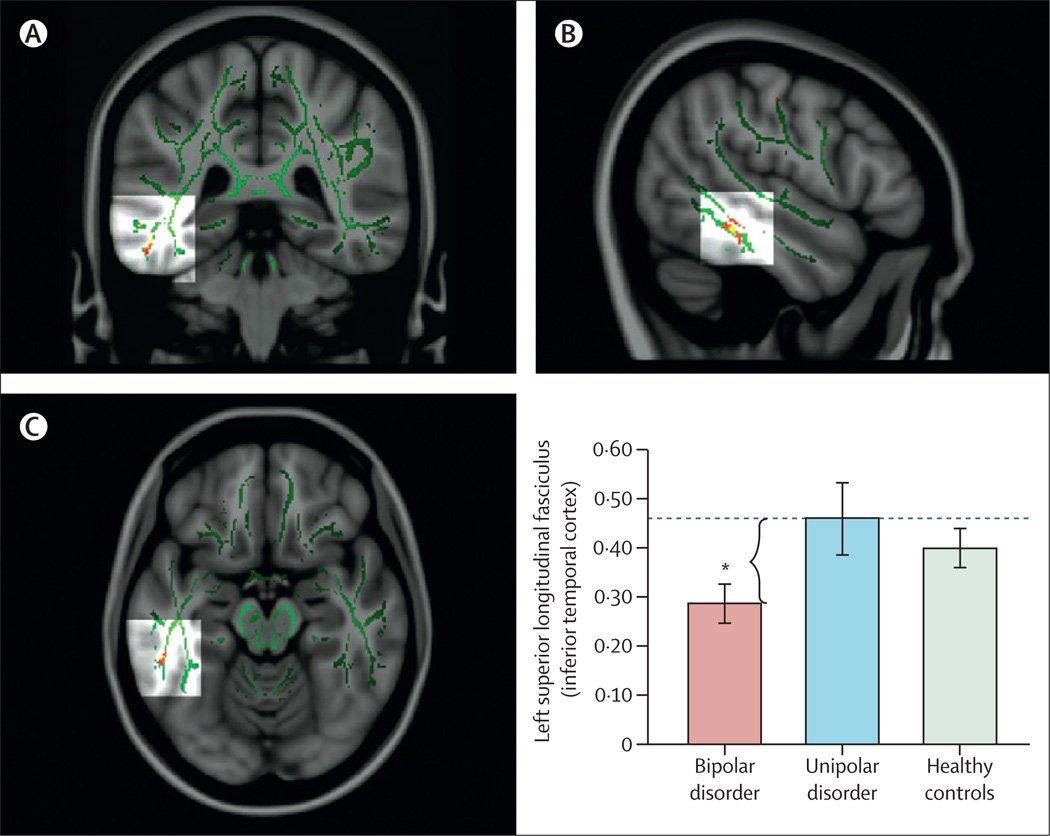
Reports89–93 have emphasised associations between genetic variation in function of the HPA axis, genetic variation between—and variation in concentrations of—inflammatory cytokines, and functional and structural variation in key neural regions supporting mood regulation. Studies have also indicated how neuroimaging techniques can be applied to diagnosis and how specific neuroimaging measures might help to distinguish major depressive disorder from bipolar depression.72,94,95 In turn, these measures can facilitate early diagnosis and can inform treatment choice (figure 3). Computerised machine learning and techniques for pattern recognition in combination with neuroimaging techniques can be used to classify individuals, case-by-case, into diagnostic groups on the basis of neuroimaging measures.64,96
Figure 3. Distinguishing bipolar and unipolar depression with measures of white-matter connectivity.
Fractional anisotropy (FA) maps showing three orthogonal ([A] coronal, [B] sagittal, and [C] axial) views of the main effect of group on FA in the region of the left superior longitudinal fasciculus for adults with bipolar depression, unipolar depression, and for healthy controls. MNI152 brain and white-matter skeleton (green) used for randomised analysis. The images represent findings (in red-yellow) projected onto the white-matter skeleton (green). The red-yellow spectrum represents a significance range—ie, 2<f<20. Monte Carlo simulation with the alphasim approach was done on uncorrected F-statistical and t-statistical maps (p<0·001), obtaining a dual thresholding of both type I error (alpha; p<0·05) and cluster-size thresholding. Bar graphs show mean FA values and 95% CIs for every group in the region of the left superior longitudinal fasciculus (MNI x, y, z = −51, −38, −17; in the inferior temporal cortex). *Significant pairwise comparison between adults with bipolar depression (red), and adults with unipolar depression (blue) that resulted in the main effect of group. Adapted with permission from reference 94.
Advances in treatment
Depression-specific psychotherapies
Pharmacotherapy and manual-driven depression-specific psychotherapy are both effective treatments for unipolar depression, either as monotherapies, or in combination.97,98 Although similar results are reported for depression-specific psychotherapy in primary-care samples, fewer published works are available in this area than in psychiatric samples.99,100 These studies suggest that inter personal psychotherapy alone, or in combination with pharmacotherapy, is effective for the acute treatment of depression.
In a clinical trial of inpatients with depression,101 response rates of patients receiving a combination of interpersonal psychotherapy (modified for inpatients) and pharmacotherapy were higher than those of patients receiving pharmacotherapy alone. The effects of acute interpersonal psychotherapy can be sustained even after remission.101,102 Similarly, several trials in the USA and Europe continue to support the acute efficacy of cognitive therapy and its usefulness as an intervention to achieve full remission and reduce the risk of recurrence.103,104 Research has also indicated that cognitive behavioural therapy can be effectively implemented in non-traditional ways—eg, via telephone and the internet—that accommodate the unique needs of primary care practice.105,106 A randomised clinical trial comparing cognitive behavioural therapy and interpersonal psychotherapy in participants with major depression found that, on average, the treatments were equally effective. However, for a subset of patients with severe depression (Montgomery-Åsberg Depression Rating Scale [MADRS] score >30), those allocated to cognitive behaviour therapy had a greater percentage improvement in MADRS score and also had a greater likelihood of response than did those receiving interpersonal psychotherapy. No difference between the two treatments was noted for individuals with melancholic depression.107 For patients with three or more previous depressive episodes, mindfulness-based cognitive therapy has an additive benefit to usual care. Problem-solving therapy might be as effective as alternative psychotherapies and pharmacotherapies for the treatment of depression, and more effective than control treatments, and might be particularly well-suited for use in primary care.108,109
Antidepressant pharmacotherapy
Although expansion of antidepressant treatment in the USA has been substantial, low rates persist in racial and ethnic minorities. Antidepressants were the most commonly prescribed class of drugs in office-based and hospital outpatient-based medical practice in 2005.110 The table lists the antidepressants that have been approved by the US Food and Drug Administration (FDA), and the proposed mechanisms of action for the various classes of antidepressants. A complete assessment of their use can be found in the American Psychiatric Association guidelines for depression.111
Since the latest update on depression in The Lancet,112 the results of the sequenced treatment alternatives to relieve depression (STAR*D) study113—the largest depression study ever done outside the pharmaceutical industry—have been reported. This study was a practical clinical trial with broad inclusion criteria, resulting in a highly representative sample of the US population. Undertaken in both psychiatric and primary care settings, STAR*D used up to four successive treatment steps, including a switch to and augmentation with additional drug or cognitive therapy in an equipoise randomisation design. The goal was for full remission, rather than just response. Remission rates in steps one to four were disappointing at 36·8%, 30·6%, 13·7%, and 13·0%, respectively, with a cumulative remission rate of 67% after all four steps.114 These rates were low compared with efficacy trials of antidepressants, which suggests that, in actual practice, most patients need several sequential treatment steps to achieve remission. The STAR*D trial showed no clear advantage of one strategy of drug over another for patients who did not achieve remission after one or more acute treatments. Furthermore, because there was no placebo control in this hybrid (efficacy-effectiveness) trial, there is no way to know whether any of the strategies were better than maintenance of the original treatment for an additional period. Too few patients received psychotherapy, either as an augmentation or a switch strategy, to make firm conclusions about its role.115 Neither sociodemographic nor clinical (anxious, atypical, and melancholic) features moderated the effect of various switching options after the first non-successful attempt at acute treatment. No differences in outcomes were found between primary care and psychiatric settings in the first two stages of acute treatment, suggesting that primary care physicians can be reasonable providers of care for patients with less complex depression.116
Although switching of antidepressants is a common strategy for management of depression, whether effectiveness can be improved remains controversial.117 Many combination treatment trials of antidepressant drugs or antidepressant and antipsychotic combinations have been done, but caution is warranted for the recommendation of such combinations as first-step treatments.118 One review119 also emphasises the discrepancy between practice patterns and evidence for combination therapy. Lithium carbonate continues to be used as an augmentation strategy, and second-generation anti-psychotics have been intensively studied.120,121 Drugs recommended for treatment-resistant depression are aripiprazole, quetiapine fumurate, and the combination of olanzapine with fluoxetine.
The notion of sequential combinations of pharmacotherapy or psychotherapy represents a shift in the treatment of mood disorders. Such two-stage approaches are based on awareness that one treatment strategy alone is unlikely to treat the varied symptoms of depression. Because the switch to a different treatment is dependent on the patient’s response to the first treatment, reassessment of design and methodological approaches is needed.122
Treatment of psychotic depression
Patients with psychotic depression (presence of delusions or hallucinations) are often difficult to treat and need several interventions. Although new pharmacological strategies are being tested, electroconvulsive therapy is important as a frequently used and effective treatment. Antipsychotics and antidepressants are being used more often to treat psychotic depression. In two studies,123,124 the combination of an antidepressant with an antipsychotic showed greater efficacy than did the antidepressant alone.
Drug development
In the past 5 years, several strategies have been developed to improve depression outcomes, including the use of new compounds and several older drugs. An example is a report showing the benefit of ademetionine (S-adenosyl methionine [SAMe]) augmentation in major depressive disorder.125,126 One strategy focuses on the use of N-methyl-D-aspartate glutamate-receptor antagonists providing the promise of rapid antidepressant action,127 including clinically significant effects from one dose that were sustained for up to 1 week.128 Another study129 has suggested the use of repeated dose intravenous ketamine for the acute treatment of treatment-resistant depression. Although the effect of glutamate on depression is promising, this has not necessarily been the case for other novel pharmacological mechanisms. The failures of CRF1 antagonist compounds and substance-P antagonists have continually been noted. Another advance is the introduction of agomelatine—a melatonin (MT1 and MT2) agonist and a 5-HT2C-receptor antagonist. Agomelatine has shown a generally favourable tolerability and efficacy, therefore providing a promising alternative for patients who do not respond to existing pharmacotherapies, or who cannot tolerate their side-effects.130 Placebo-controlled research provides evidence for the effectiveness of agomelatine as both an acute and a continuation treatment for major depression.131–133
Suicide risk with SSRIs
The safety of SSRIs has been debated because of their potential association with suicidal ideation and behaviour as noted from studies of adolescents with depression.134 Some work suggests that adults treated with antidepressant drugs, including SSRIs, are no more likely to attempt or complete suicide than those not treated with an antidepressant.134,135 However, other research suggests a reduced risk of suicide attempt in adults after start of SSRI treatment, particularly with sertraline,135 and in men.134 In 226 866 male veterans with depression, rates of suicide attempt were lower after the start of SSRI treatment than before, and compared with treatment with other antidepressants or no antidepressant.135 Similarly, a study of county-level data in the USA showed that a decrease in suicide rate was associated with SSRIs when prescribed in combination with non-SSRIs or non-tricyclic antidepressants.136 Despite these findings, caution is needed against strong conclusions being made on the basis of limited ecological analyses.137
Safety in pregnancy
Data before 2000 provided weak evidence for an association between SSRIs and major fetal malformation. Since then, data have shown that paroxetine might be associated with major malformations, especially cardiac defects.138 Some evidence is available of an association between a neonatal behavioural syndrome and exposure to SSRIs in utero during the last trimester. This syndrome can be managed with supportive treatment in a special-care nursery.139 Presence of persistent pulmonary hyper tension in the neonate can also be associated with SSRIs taken late in pregnancy.138 Infants with continuous exposure to mother’s depression and continuous exposure to SSRIs throughout gestation were more likely to be born preterm than were infants with partial or no exposure.140 Guidelines suggest that SSRIs should be used with caution during pregnancy, and that paroxetine be avoided.141,142
Status of new somatic treatments
Although deep brain stimulation is still in the early investigational stages and not approved by the FDA or the European Medicines Agency, it is promising as a treatment for treatment-resistant depression.143,144 In deep brain stimulation, electrodes are implanted bilaterally in the brain and are connected to an internal pulse generator.145 This procedure is fully reversible and the stimulation it provides can be adjusted on the basis of the patient’s needs.145,146 The treatment might exert its effect by modulating neurotransmission in the cortico-striatal-thalamic-cortical circuit.143 Work has shown the antidepressant effect of deep brain stimulation when used within the subgenual cingulate white matter (Cg25WM), the nucleus accumbens,83,147 the subcollosal cingulated gyrus,148 and the ventral capsule or ventral stiatum.149 This treatment has also been discussed for use in the anterior limb of the internal capsule, globus pallidus internus, inferior thalamic peduncle, rostral cingulated cortex, and lateral haenula.144,145 Although deep brain stimulation seems to be well tolerated, the possibility of suicide should be monitored.147–149 One study149 notes that although stable remission can be achieved with deep brain stimulation, the affective instability induced by the treatment merits specific attention.
Transcranial magnetic stimulation (TMS) has been approved by the FDA for the treatment of major depressive disorder, especially for those who have not responded to a course of one antidepressant drug.150 Although this treatment is regarded as safe and well-tolerated, seizures can be a rare side-effect.150,151 TMS produces a magnetic field around the brain; the left and right dorsolateral prefrontal cortex (DLPFC) are common target areas for TMS in depression.150 Several meta-analyses150,152 support the use of DLPFC TMS or repetitive TMS to treat depression, because they were more effective than placebo or sham. Some studies150 suggest that the sizes of these effects are comparable to treatment with antidepressants; however, the sizes are moderate150,152 and repetitive TMS seems to be less effective than electroconvulsive therapy.151 A multisite sham-controlled study153 of repetitive TMS, which included an open-label extension for patients who did not improve, provided the opportunity to examine predictors of acute outcome. In this sample, low degree of previous drug resistance in the current episode, short duration of current episode, absence of anxiety disorder, high severity of baseline depression, and female sex, were associated with improved outcome in one or both parts of this trial.153
Conclusions
Increased data for imaging and genetics in major depressive disorder, and other neurobiological data, provide potential biomarkers for the assessment of treatment outcomes. If a description of precise subgroups based on such data were to emerge, short-term and long-term benefits of treatment might be improved. Although new reports about treatment response in multisite studies have emerged in the past 5 years, treatment advances are somewhat lagging because of an inability to undertake adequate studies with the appropriate predictors of response.
Table.
Antidepressants approved by the FDA
| Drugs | Proposed mechanism of action | |
|---|---|---|
| Selective serotonin reuptake inhibitors | Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline | Selectively inhibits the reuptake of serotonin |
| Tricyclic antidepressants | Amitriptyline, desipramine, doxepin, imipramine, maprotiline, nortriptyline, protriptyline, trimipramine | Nonselectively inhibits the reuptake of monoamines, including serotonin, dopamine, and norepinephrine |
| Norepinephrine-dopamine reuptake inhibitor | Bupropion | Inhibits the reuptake of norepinephrine and dopamine |
| Serotonin modulator | Nefazodone, trazodone | Primarily antagonises 5-HT2 receptors |
| Serotonin-norepinephrine reuptake inhibitors | Desvenlafaxine, duloxetine, venlafaxine | Inhibits the reuptake of serotonin and norepinephrine |
| Noradrenergic and specific serotonergic modulator | Mirtazapine | Primarily antagonises α-2 and 5-HT2C receptors |
| Serotonin reuptake inhibitor and 5-HT1A-receptor partial agonist | Vilazodone | Potently and selectively inhibits serotonin reuptake and acts as a partial agonist at the 5-HT1A receptor |
| MAO inhibitors | Isocarboxazid, phenylzine, tranylcypromine; Selegiline | Nonselectively inhibits enzymes (MAO-A and MAO-B) involved in the breakdown of monoamines, including serotonin, dopamine, and norepinephrine MAO-B selective inhibitor |
Acknowledgments
DK and EF were supported by a grant from the National Institute of Mental Health (MH081003). EF was supported by a grant from the National Institute of Mental Health (MH065376). MP was supported by a grant from the National Institute of Mental Health (MH076971). We thank Tanya Fabian, Jessica Levenson, and Meredith Lotz for their assistance with the literature search for the section about advances in treatment.
Footnotes
Contributors
EF, DK, and MP wrote and revised the first and final versions of the Seminar, did the literature searches, and interpreted the data.
Conflicts of interest
EF serves on an advisory board for Servier. DK and MP declare that they have no conflicts of interest.
References
- 1.Kessler RC, Berglund P, Demler O, et al. for the National Comobordity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- 3.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 4.Tyrer P. Are general practitioners really unable to diagnose depression? Lancet. 2009;374:589–590. doi: 10.1016/S0140-6736(09)61156-9. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009;374:609–619. doi: 10.1016/S0140-6736(09)60879-5. [DOI] [PubMed] [Google Scholar]
- 6.Das-Munshi J, Goldberg D, Bebbington PE, et al. Public health significance of mixed anxiety and depression: beyond current classification. Br J Psychiatry. 2008;192:171–177. doi: 10.1192/bjp.bp.107.036707. [DOI] [PubMed] [Google Scholar]
- 7.Benazzi F. Bipolar disorder—focus on bipolar II disorder and mixed depression. Lancet. 2007;369:935–945. doi: 10.1016/S0140-6736(07)60453-X. [DOI] [PubMed] [Google Scholar]
- 8.Akiskal HS, Pinto O. The soft bipolar spectrum: footnotes to Kraepelin on the interface of hypomania, temperament and depression. In: Marneros A, Angst J, editors. Bipolar disorders: 100 years after manic-depressive insanity. Dorderecht, Netherlands: Kluwer Academic; 2000. pp. 37–62. [Google Scholar]
- 9.Cassano GB, Rucci P, Frank E, et al. The mood spectrum in unipolar and bipolar disorder: arguments for a unitary approach. Am J Psychiatry. 2004;161:1264–1269. doi: 10.1176/appi.ajp.161.7.1264. [DOI] [PubMed] [Google Scholar]
- 10.Schneck CD. Mixed depression: the importance of rediscovering subtypes of mixed mood states. Am J Psychiatry. 2009;166:127–130. doi: 10.1176/appi.ajp.2008.08111669. [DOI] [PubMed] [Google Scholar]
- 11.Moffitt TE, Harrington H, Caspi A, et al. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Arch Gen Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 12.Beesdo K, Bittner A, Pine DS, et al. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch Gen Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- 13.Michels R. Personality disorders in the depressed: seeing clearly through blue lenses. Am J Psychiatry. 2010;167:487–488. doi: 10.1176/appi.ajp.2010.10020172. [DOI] [PubMed] [Google Scholar]
- 14.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Gardner CO, Fiske A, Gatz M. Major depression and coronary artery disease in the Swedish twin registry: phenotypic, genetic, and environmental sources of comorbidity. Arch Gen Psychiatry. 2009;66:857–863. doi: 10.1001/archgenpsychiatry.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glassman AH, Bigger JT, Jr, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry. 2009;66:1022–1029. doi: 10.1001/archgenpsychiatry.2009.121. [DOI] [PubMed] [Google Scholar]
- 17.Frasure-Smith N, Lespéance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2009;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 18.Thombs BD, de Jonge P, Coyne JC, et al. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 19.Mitka M. Routine depression screening advised for patients with coronary heart disease. JAMA. 2008;300:2356–2357. doi: 10.1001/jama.2008.622. [DOI] [PubMed] [Google Scholar]
- 20.Campayo A, de Jonge P, Roy JF, Saz P, et al. for the ZARADEMP Project. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- 21.Lyketsos CG. Depression and diabetes: more on what the relationship might be. Am J Psychiatry. 2010;167:496–497. doi: 10.1176/appi.ajp.2010.10020243. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg D. The detection and treatment of depression in the physically ill. World Psychiatry. 2010;9:16–20. doi: 10.1002/j.2051-5545.2010.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uher R. The role of genetic variation in the causation of mental illness: an evolution-informed framework. Mol Psychiatry. 2009;14:1072–1082. doi: 10.1038/mp.2009.85. [DOI] [PubMed] [Google Scholar]
- 24.Shyn SI, Hamilton SP. The genetics of major depression: moving beyond the monoamine hypothesis. Psychiatr Clin North Am. 2010;33:125–140. doi: 10.1016/j.psc.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Rossum EF, Binder EB, Majer M, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Fan M, Liu B, Jiang T, Jiang X, Zhao H, Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- 27.Yoon HK, Kim YK. Association between glycogen synthase kinase-3β gene polymorphisms and major depression and suicidal behavior in a Korean population. Prog NeuroPscyhopharmacol Biol Psychiatry. 2010;34:331–334. doi: 10.1016/j.pnpbp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Tsunoka T, Kishi T, Ikeda M, et al. Association analysis of group II metabotropic glutamate receptor genes (GRM2 and GRM3) with mood disorders and fluvoxamine response in a Japanese population. Prog NeuroPsychopharmacol Biol Psychiatry. 2009;33:875–879. doi: 10.1016/j.pnpbp.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Serretti A, Kato M, De Ronchi D, Kinoshita T. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12:247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 30.Huezo-Diaz P, Uher R, Smith R, et al. Moderation of antidepressant response by the serotonin transporter gene. Br J Psychiatry. 2009;195:30–38. doi: 10.1192/bjp.bp.108.062521. [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- 32.Murphy GM, Jr, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61:1163–1169. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 33.Horstmann S, Lucae S, Menke A, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35:727–740. doi: 10.1038/npp.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkie MJ, Smith G, Day RK, et al. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics J. 2009;9:61–70. doi: 10.1038/sj.tpj.6500491. [DOI] [PubMed] [Google Scholar]
- 35.McMahon FJ, Buervenich S, Charney D, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perlis RH, Laje G, Smoller JW, Fava M, Rush AJ, McMahon FJ. Genetic and clinical predictors of sexual dysfunction in citalopram-treated depressed patients. Neuropsychopharmacology. 2009;34:1819–1828. doi: 10.1038/npp.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paddock S, Laje G, Charney D, et al. Association of GRIK4 with outcome of antidepressant treatment in the STAR*D cohort. Am J Psychiatry. 2007;164:1181–1188. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 38.Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 39.Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009;66:488–497. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(suppl):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- 42.Lekman M, Laje G, Charney D, et al. The FKBP5-gene in depression and treatment response—an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perlis RH, Moorjani P, Fagerness J, et al. Pharmacogenetic analysis of genes implicated in rodent models of antidepressant response: association of TREK1 and treatment resistance in the STAR(*)D study. Neuropsychopharmacology. 2008;33:2810–2819. doi: 10.1038/npp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baune BT, Hohoff C, Berger K, et al. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33:924–932. doi: 10.1038/sj.npp.1301462. [DOI] [PubMed] [Google Scholar]
- 45.Perlis RH, Fijal B, Adams DH, et al. Variation in catechol-O-methyltransferase is associated with duloxetine response in a clinical trial for major depressive disorder. Biol Psychiatry. 2009;65:785–791. doi: 10.1016/j.biopsych.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Binder EB, Owens MJ, Liu W, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Zhu F, Wang G, et al. Association study of corticotropin-releasing hormone receptor 1 gene polymorphisms and antidepressant response in major depressive disorders. Neuroscience Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Uher R, Perroud N, Ng MY, et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am J Psychiatry. 2010;167:555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- 49.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 51.Raison CL, Borisov AS, Majer M, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–907. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 55.Ising M, Horstmann S, Kloiber S, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression—a potential biomarker? Biol Psychiatry. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 56.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertolino A, Arciero G, Rubino V, et al. Variation of human amygdala response during threatening stimuli as a function of 5’HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 58.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 59.Heinz A, Smolka MN, Braus DF, et al. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61:1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Surguladze S, Brammer MJ, Keedwell P, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 62.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 63.Dannlowski U, Ohrmann P, Bauer J, et al. Amygdala reactivity to masked negative faces is associated with automatic judgmental bias in major depression: a 3 T fMRI study. J Psychiatry Neurosci. 2007;32:423–429. [PMC free article] [PubMed] [Google Scholar]
- 64.Fu CH, Mourao-Miranda J, Costafreda SG, et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry. 2008;63:656–662. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 67.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kronenberg G, Tebartz van Elst L, Regen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res. 2009;43:1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- 71.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–128. doi: 10.1016/j.brainres.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 74.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anand A, Li Y, Wang Y, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30:1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- 79.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salvadore G, Cornwell BR, Sambataro F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 82.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 84.Blumberg HP, Wang F, Chepenik LG, et al. Influence of vascular endothelial growth factor variation on human hippocampus morphology. Biol Psychiatry. 2008;64:901–903. doi: 10.1016/j.biopsych.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pezawas L, Meyer-Lindenberg A, Goldman AL, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- 88.Joffe RT, Gatt JM, Kemp AH, et al. Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: implications for depressive illness. Hum Brain Mapp. 2009;30:1246–1256. doi: 10.1002/hbm.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zobel A, Jessen F, von Widdern O, et al. Unipolar depression and hippocampal volume: impact of DNA sequence variants of the glucocorticoid receptor gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:836–843. doi: 10.1002/ajmg.b.30709. [DOI] [PubMed] [Google Scholar]
- 90.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baune BT, Dannlowski U, Domschke K, et al. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry. 2010;67:543–549. doi: 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Gaiteri C, Guilloux JP, Lewis DA, Sibille E. Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS One. 2010;5:e9970. doi: 10.1371/journal.pone.0009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dillon DG, Bogdan R, Fagerness J, Holmes AJ, Perlis RH, Pizzagalli DA. Variation in TREK1 gene linked to depression-resistant phenotype is associated with potentiated neural responses to rewards in humans. Hum Brain Mapp. 2010;31:210–221. doi: 10.1002/hbm.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Versace A, Almeida JR, Quevedo K, et al. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry. 2010;68:560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Craddock RC, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. Disease state prediction from resting state functional connectivity. Magn Reson Med. 2009;62:1619–1628. doi: 10.1002/mrm.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuijpers P, Dekker J, Hollon SD, Andersson G. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219–1229. doi: 10.4088/JCP.09r05021. [DOI] [PubMed] [Google Scholar]
- 98.Cuijpers P, van Straten A, van Oppen P, Andersson G. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. J Clin Psychiatry. 2008;69:1675–1685. doi: 10.4088/jcp.v69n1102. [DOI] [PubMed] [Google Scholar]
- 99.Bortolotti B, Menchetti M, Bellini F, Montaguti MB, Berardi D. Psychological interventions for major depression in primary care: a meta-analytic review of randomized controlled trials. Gen Hosp Psychiatry. 2008;30:293–302. doi: 10.1016/j.genhosppsych.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 100.Wolf NJ, Hopko DR. Psychosocial and pharmacological interventions for depressed adults in primary care: a critical review. Clin Psychol Rev. 2008;28:131–161. doi: 10.1016/j.cpr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 101.Schramm E, van Calker D, Dykierek P, et al. An intensive treatment program of interpersonal psychotherapy plus pharmacotherapy for depressed inpatients: acute and long-term results. Am J Psychiatry. 2007;164:768–777. doi: 10.1176/ajp.2007.164.5.768. [DOI] [PubMed] [Google Scholar]
- 102.Frank E, Kupfer DJ, Buysse DJ, et al. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164:761–767. doi: 10.1176/appi.ajp.164.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vittengl JR, Clark LA, Jarrett RB. Continuation-phase cognitive therapy’s effects on remission and recovery from depression. J Consult Clin Psychol. 2009;77:367–371. doi: 10.1037/a0015238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–422. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 105.Simon GE, Ludman EJ, Rutter CM. Incremental benefit and cost of telephone care management and telephone psychotherapy for depression in primary care. Arch Gen Psychiatry. 2009;66:1081–1089. doi: 10.1001/archgenpsychiatry.2009.123. [DOI] [PubMed] [Google Scholar]
- 106.Kessler D, Lewis G, Kaur S, et al. Therapist-delivered Internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet. 2009;374:628–634. doi: 10.1016/S0140-6736(09)61257-5. [DOI] [PubMed] [Google Scholar]
- 107.Luty SE, Carter JD, McKenzie JM, et al. Randomised controlled trial of interpersonal psychotherapy and cognitive-behavioural therapy for depression. Br J Psychiatry. 2007;190:496–502. doi: 10.1192/bjp.bp.106.024729. [DOI] [PubMed] [Google Scholar]
- 108.Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Rev. 2009;29:348–353. doi: 10.1016/j.cpr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Schreuders B, van Marwijk H, Smit J, Rijmen F, Stalman W, van Oppen P. Primary care patients with mental health problems: outcome of a randomised clinical trial. Br J Gen Pract. 2009;57:886–891. doi: 10.3399/096016407782317829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 111.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. [accessed Feb 16, 2011];2010 Nov; http://www.psychiatryonline.com/pracGuide/pracGuideTopic_7.aspx. [Google Scholar]
- 112.Ebmeier KP, Donaghey C, Steele JD. Recent developments and current controversies in depression. Lancet. 2006;367:153–167. doi: 10.1016/S0140-6736(06)67964-6. [DOI] [PubMed] [Google Scholar]
- 113.Rush AJ, Fava M, Wisniewski SR, et al. for the STAR*D Investigators Group. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 114.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 115.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 116.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 117.Bschor T, Baethge C. No evidence for switching the antidepressant: systematic review and meta-analysis of RCTs of a common therapeutic strategy. Acta Psychiatr Scand. 2010;121:174–179. doi: 10.1111/j.1600-0447.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 118.Rush AJ. Combining antidepressant medications: a good idea? Am J Psychiatry. 2010;167:241–243. doi: 10.1176/appi.ajp.2009.09121768. [DOI] [PubMed] [Google Scholar]
- 119.Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. doi: 10.2165/11587620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 120.Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62:36–42. doi: 10.1159/000314308. [DOI] [PubMed] [Google Scholar]
- 121.Papakostas GI, Shelton RC, Smith J, Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry. 2007;68:826–831. doi: 10.4088/jcp.v68n0602. [DOI] [PubMed] [Google Scholar]
- 122.Tomba E, Fava GA. The sequential combination of pharmacotherapy and psychotherapy in mood disorders. J Contemp Psycho. 39:101–109. [Google Scholar]
- 123.Meyers BS, Flint AJ, Rothschild AJ, et al. for the STOP-PD Group. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) Arch Gen Psychiatry. 2009;66:838–847. doi: 10.1001/archgenpsychiatry.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wijkstra J, Burger H, van den Broek WW, et al. Treatment of unipolar psychotic depression: a randomized, double-blind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;121:190–200. doi: 10.1111/j.1600-0447.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 125.Nelson JC. S-adenosyl methionine (SAMe) augmentation in major depressive disorder. Am J Psychiatry. 2010;167:889–891. doi: 10.1176/appi.ajp.2010.10040627. [DOI] [PubMed] [Google Scholar]
- 126.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 127.Krystal JH. N-methyl-D-aspartate glutamate receptor antagonists and the promise of rapid-acting antidepressants. Arch Gen Psychiatry. 2010;67:1110–1111. doi: 10.1001/archgenpsychiatry.2010.138. [DOI] [PubMed] [Google Scholar]
- 128.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 129.aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 130.Ghosh A, Hellewell JS. A review of the efficacy and tolerability of agomelatine in the treatment of major depression. Expert Opin Investig Drugs. 2007;16:1999–2004. doi: 10.1517/13543784.16.12.1999. [DOI] [PubMed] [Google Scholar]
- 131.Olié JP, Kasper S. Efficacy of agomelatine, a MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007;10:661–673. doi: 10.1017/S1461145707007766. [DOI] [PubMed] [Google Scholar]
- 132.Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A. Agomelatine in the treatment of major depressive disorder: an 8-week, multicenter, randomized, placebo-controlled trial. J Clin Psychiatry. 2010;71:616–626. doi: 10.4088/JCP.09m05471blu. [DOI] [PubMed] [Google Scholar]
- 133.Goodwin GM, Emsley R, Rembry S, Rouillon F for the Agomelatine Study Group. Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:1128–1137. doi: 10.4088/JCP.08m04548. [DOI] [PubMed] [Google Scholar]
- 134.Olfson M, Marcus SC. A case-control study of antidepressants and attempted suicide during early phase treatment of major depressive episodes. J Clin Psychiatry. 69:425–432. doi: 10.4088/jcp.v69n0313. [DOI] [PubMed] [Google Scholar]
- 135.Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case-control study. Arch Gen Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- 136.Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Mann JJ. Relationship between antidepressants and suicide attempts: an analysis of the Veterans Health Administration data sets. Am J Psychiatry. 2007;164:1044–1049. doi: 10.1176/ajp.2007.164.7.1044. [DOI] [PubMed] [Google Scholar]
- 137.Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry. 2005;62:165–172. doi: 10.1001/archpsyc.62.2.165. [DOI] [PubMed] [Google Scholar]
- 138.Tuccori M, Testi A, Antonioli L, et al. Safety concerns associated with the use of serotonin reuptake inhibitors and other serotonergic/noradrenergic antidepressants during pregnancy: a review. Clin Ther. 2009;31:1426–1453. doi: 10.1016/j.clinthera.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 139.Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293:2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 140.Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166:557–566. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.US Food and Drug Administration. Public health advisory: paroxetine. [accessed Dec 8, 2010]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051731.htm.
- 142.Medicines and Healthcare Products Regulatory Agency. MHRA update on the risks of birth defects in babies born to mothers taking paroxetine—q&a. [accessed Dec 8, 2010]; http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/con2022700.pdf.
- 143.Ward HE, Hwynn N, Okun MS. Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol Dis. 2010;38:346–353. doi: 10.1016/j.nbd.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 144.Kuhn J, Grüdler TO, Lenartz D, Sturm V, Klosterköter J, Huff W. Deep brain stimulation for psychiatric disorders. Dtsch Arztebl Int. 2010;107:105–113. doi: 10.3238/arztebl.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giacobbe P, Kennedy SH. Deep brain stimulation for treatment-resistant depression: a psychiatric perspective. Curr Psychiatry Rep. 2006;8:437–444. doi: 10.1007/s11920-006-0048-5. [DOI] [PubMed] [Google Scholar]
- 146.Kopell BH, Greenberg B, Rezai AR. Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 2004;21:51–67. doi: 10.1097/00004691-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 147.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 148.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 149.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim DR, Pesiridou A, O’Reardon JP. Transcranial magnetic stimulation in the treatment of psychiatric disorders. Curr Psychiatry Rep. 2009;11:447–452. doi: 10.1007/s11920-009-0068-z. [DOI] [PubMed] [Google Scholar]
- 151.Dell’osso B, Altamura AC. Augmentative transcranial magnetic stimulation (TMS) combined with brain navigation in drug-resistant rapid cycling bipolar depression: a case report of acute and maintenance efficacy. World J Biol Psychiatry. 2009;10:673–676. doi: 10.1080/15622970701806192. [DOI] [PubMed] [Google Scholar]
- 152.Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 153.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]