Mechanism-independent method for predicting response to multidrug combinations in bacteria (original) (raw)
Abstract
Drugs are commonly used in combinations larger than two for treating bacterial infection. However, it is generally impossible to infer directly from the effects of individual drugs the net effect of a multidrug combination. Here we develop a mechanism-independent method for predicting the microbial growth response to combinations of more than two drugs. Performing experiments in both Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) bacteria, we demonstrate that for a wide range of drugs, the bacterial responses to drug pairs are sufficient to infer the effects of larger drug combinations. To experimentally establish the broad applicability of the method, we use drug combinations comprising protein synthesis inhibitors (macrolides, aminoglycosides, tetracyclines, lincosamides, and chloramphenicol), DNA synthesis inhibitors (fluoroquinolones and quinolones), folic acid synthesis inhibitors (sulfonamides and diaminopyrimidines), cell wall synthesis inhibitors, polypeptide antibiotics, preservatives, and analgesics. Moreover, we show that the microbial responses to these drug combinations can be predicted using a simple formula that should be widely applicable in pharmacology. These findings offer a powerful, readily accessible method for the rational design of candidate therapies using combinations of more than two drugs. In addition, the accurate predictions of this framework raise the question of whether the multidrug response in bacteria obeys statistical, rather than chemical, laws for combinations larger than two.
Keywords: pairwise, entropy, prokaryotes, dose-response relationship
Combinations of three or more drugs have been studied in both clinical and laboratory settings as potential treatments for severe microbial infections (1–4). Drug interactions, including those that are clinically beneficial, have typically been studied using descriptive, rather than predictive, approaches that quantify the effects of a given drug pair on growth (5–7). For example, two drugs whose effects on microbial growth counteract one another, when used in combination, are known as antagonistic, whereas drugs whose potencies are significantly increased in combination are referred to as synergistic. As a result of these interactions, the effects of drug combinations cannot, in general, be predicted based on the effects of the drugs alone (7). Although combinations of two drugs have been studied extensively, little is known about the way in which more than two drugs combine to yield higher-order effects on bacterial growth, which is the relevant clinical outcome in treatments of bacterial infections. Here, we ask if it is possible to understand and to predict the effects of these larger drug combinations without relying on specific mechanistic details but rather on principles shared by a large number of biological systems.
For example, consider a classic three-drug combination of chloramphenicol (a protein synthesis inhibitor), ofloxacin (a fluoroquinolone DNA synthesis inhibitor), and trimethoprim (a folic acid synthesis inhibitor) at the following concentrations: [chloramphenicol]=1.5 μg/mL, [ofloxacin]=40 ng/mL, and [trimethoprim]=0.3 μg/mL. The growth rate of E. coli treated with each drug alone is about 0.58, 0.47, and 0.39 (normalized by the growth of untreated cells), respectively. Combining chloramphenicol and ofloxacin leads to a growth rate of 0.53, which is significantly higher than expected from a naive multiplication of the single drug rates (0.27) and consistent with previously observed antagonism between DNA synthesis inhibitors and protein synthesis inhibitors (8). On the other hand, combining ofloxacin with trimethoprim completely eradicates growth (growth < 0.01, compared with 0.18 expected from single drug growth rates), consistent with previously reported synergy between trimethoprim and fluoroquinolones (9). Finally, the combination of chloramphenicol and trimethoprim leads to a growth rate of 0.16, slightly smaller than the 0.23 predicted from single drug growth rates. The effects of all three pairs of drugs differ significantly from that predicted by multiplication of single drug effects. Therefore, there is seemingly little hope that such an assumption of independence will be useful when all three drugs are combined and the chemical complexity of the problem is increased. Surprisingly, the growth rate in the presence of all three drugs (0.11) is equal to the product of single drug growth rates, suggesting that the drugs act independently. Why have the previously strong interactions between drug pairs been eliminated when the three drugs are combined, leading to a mixture of effectively independent drugs? One hypothesis would be that the net effect of the drug combination arises from compensatory interactions that can only be measured when all three drugs are present. Alternatively, the net effect could follow directly from the accumulation of interactions between pairs of drugs. We wish to answer this question using a quantitative framework to provide insight into how the cell integrates signals from larger drug combinations.
To tackle this question for a wide range of drug combinations, we develop a mechanism-independent model to quantify the relative contributions of combined chemical exposure—that is, one-drug effects, two-drug effects, and, in general, _N_-drug effects—to the multidrug growth response. We construct the model using a common statistical method, entropy maximization, which ensures that the model does not incorporate unwarranted statistical structure. We then test predictions of this framework using two species that represent Gram–negative (Escherichia coli) and Gram–positive (Staphylococcus aureus) bacteria. This predictive framework is a potentially powerful tool for studying multidrug effects, even without knowledge of the underlying network structure, molecular dynamics, or any other intracellular details.
Results
Response of E. coli to Single Drugs and Drug Pairs.
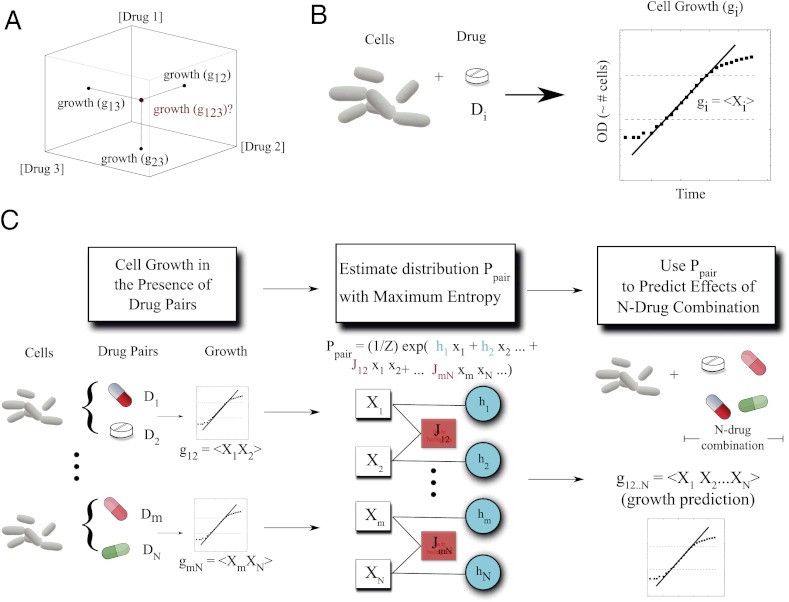
First, we measured the growth of E. coli in the presence of a single drug and then pairs of drugs by growing liquid cultures in Luria-Bertani media. We used a large variety of drugs, including several classes of protein synthesis inhibitors (with 30S and 50S ribosomal targets), DNA synthesis inhibitors (fluoroquinolones), folic acid synthesis inhibitors, and analgesics (Table S1). Using time series of optical density measured directly from a 96-well plate reader, we estimated growth with nonlinear least-squares fitting (Methods, Fig. S1). We define g1...N to be the measured growth rate of cells in our experiments exposed to a treatment with N drugs, D1, D2, ..., DN. All growth rates are normalized by growth rate in the absence of drugs. To understand the relationship between pairwise drug interactions and the net drug interaction between more than two drugs, we first asked whether one can estimate the growth response to three or four drugs using only our experimental measurements of single drug, gi, and two-drug, gij, growth rates (Fig. 1).
Fig. 1.
Growth in response to multiple drugs can be predicted from the growth in response to those drugs singly and in pairs using maximum entropy. (A) Schematic axes showing that the normalized growth responses of bacteria to pairs of drugs (g12, g23, g13) are used to predict the normalized growth response to all three drugs (g123). We use the three-drug case as an example; but growth in response to any number (N) of drugs can be predicted as long as we know all pairwise responses. (B) We estimate growth in the presence of drugs using nonlinear least-squares fitting to optical density time series. For each drug i, we define a random variable Xi whose expectation value is equal to the growth gi. (C) We made predictions by first estimating the maximum entropy distribution, P, using growth rate data from cells exposed to single drugs and drug pairs. The distribution takes an exponential form parameterized by resilience coefficients (hi, blue circles) and drug–drug coupling coefficients (Jij, pink boxes) that characterize the single drug response and the response to pairs of drugs, respectively. The resilience and coupling coefficients are chosen to ensure that the moments, <Xi> and <XiXj>, of Ppair match the two-drug growth rate data at each drug dosage. After determining the maximum entropy distribution, the _N_-drug growth response can be predicted by calculating the expectation values of the product X1X2...×N. We find that these expectation values are related to the moments <Xi> and <XiXj> by simple algebraic expressions (SI Appendix).
We model the effect of each drug, Di, using an associated stochastic variable, Xi. Specifically, we assume that the measured (normalized) growth rate is equal to the mean (i.e., expectation) value of that random variable, gi =< Xi>. Similarly, in the presence of two drugs, i and j, the normalized growth is taken to be gij =< Xi Xj> and, in general, the normalized growth in presence of a combination of N drugs, g1...N, equals the mean value <X1…XN> of the product of the Xis. The relevant experimental observable, growth, is associated with the moments (or joint moments) of the variables Xi, not to the stochastic variables themselves. By construction, then, drug interactions are represented as correlations between these abstract variables. In this framework, an absence of correlation between variables Xi and Xj indicates that the drugs do not interact, and therefore gij is equal to the product of the independent growth rate gi and gj. In the absence of interactions between the drugs, this statistical model is equivalent to the well-known Bliss independence model (5, 7) in pharmacology.
Drug Interactions Defined as a Mechanism-Independent Statistical Problem.
To characterize the apparent interactions between drugs (i.e., synergies and antagonisms), we introduce a probability density P(x) = P(x1, x2, …, xN) that describes the joint distribution of these random variables. Unfortunately, this probability distribution P(x) is not directly accessible, although as we will show, it can be estimated using experimental data. Specifically, we wish to estimate the probability density P(x) using only the growth rate data in response to single drugs and drug pairs. We call this estimate Ppair(x), because it depends only on the interactions between drug pairs and the effects of the drugs alone. Ppair(x) provides a picture of how the two-drug interactions would accumulate if there were no additional drug interactions, such as those requiring the presence of all three or four drugs. Of course, Ppair(x) will provide a good approximation to the true P(x) and, ultimately, to experiments only if the effects of higher-order interactions (three-drug, four-drug) are negligible.
To estimate Ppair(x) from experiments, we use entropy maximization (10, 11) (Fig. 1), a well-established statistical technique that guarantees that Ppair(x) contains only the information from our one-drug and two-drug data sets (SI Appendix). In this case, the form of the maximum entropy distribution is given by
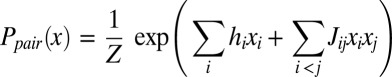
where subscripts label the components of x, and h and J represent the collection of free parameters determined by the data (SI Appendix, Figs. S2–S6), and Z is the normalization constant (i.e., partition function). It is straightforward to determine the parameters hi and Jij from our measurements of single and pairwise drug effects at each dosage (SI Appendix).
Three- and Four-Drug Interactions Arise from Accumulation of Pairwise Interactions.
Using the estimated distribution Ppair(x), one can easily calculate the expected growth response to a larger combination of drugs, g1,..N =< X1X2…XN>, where brackets represent an average using the distribution Ppair(x). This prediction would match experimental results only if the net effects of the drug combination were to arise entirely from the accumulation of pairwise interactions but not from higher drug interactions. To test this framework, we calculated expected growth response to various combinations of N drugs. We focus on the n = 3 and n = 4 cases, which are near the upper limit of current multidrug treatments in clinical settings. We then directly measured bacterial growth in the presence of these drug combinations and compared them to our expected results using the estimated distribution Ppair(x). Notably, the relationship between the _N_-drug response and the responses to single drugs and drug pairs—a relationship governed by the distribution Ppair(x) calculated from entropy maximization—is well described by simple algebraic expressions (12) (SI Appendix, Fig. S7). For example, the response to three drugs (gijk) is given by
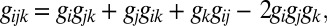
and the response to four drugs (gijkl) is given by
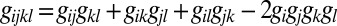
These well-known formulas are fully consistent with our numerical maximum entropy predictions and can be derived from the famous Isserlis theorem (12) in the specific case when Ppair(x) is a Gaussian distribution. The simple expressions provide a way to predict the effect of a drug combination on growth without using the sophisticated maximum entropy framework. However, the fact that these simple formulas yield predictions identical (Fig. S7) to those from maximum entropy calculations guarantees that they contain no hidden correlations, only correlations from measured pairwise and single drug effects.
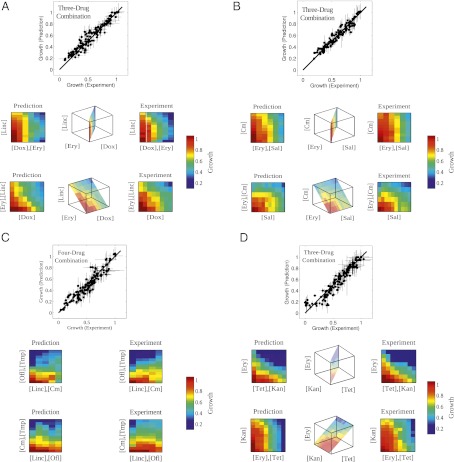
Fig. 2_A_ shows representative data collected from bacteria exposed to various concentrations of the combination of three antibiotics, erythromycin, doxycycline, and lincomycin. All three drugs inhibit protein synthesis, erythromycin by inhibiting translocation of peptidyl tRNA, doxycycline by disrupting aminoacyl-t-RNA binding to the ribosome, and lincomycin by inhibiting enzymatic activity of peptidyl transferase. We previously found that lincomycin is antagonistic with both doxycycline and erythromycin, whereas the latter two drugs are synergistic (Fig. S2). However, because the mode of action is similar for the three drugs, it is possible that these mechanisms might interact in a unique way when all three drugs are present (see also SI Appendix, Fig. S8 for drugs with identical modes of action). Therefore, it is not clear whether the overall effect could be predicted solely from the accumulation of the measured pairwise interactions. Interestingly, Fig. 2_A_ demonstrates that the pairwise interactions are indeed sufficient to accurately predict the growth response to the combination of these three protein synthesis inhibitors.
Fig. 2.
Three- and four-drug interactions arise from the accumulation of pairwise interactions. Maximum entropy predictions of growth, using only data from pairwise drug interactions, match experimental growth responses in E. coli (A–C) and S. aureus (D) in the presence of three-drug (A, B, and D) and four-drug (C) combinations. In each panel, Lower Insets are heat maps showing the model’s predictions (left) and experimental data (right) for various planes through the three- or four-dimensional spaces of drug concentrations. White squares indicate drug dosages for which the maximum entropy algorithm did not converge. Experimental error bars and 95% confidence intervals from nonlinear fitting are shown; error bars on predictions are shown, ±2 SDs of an ensemble of predictions from maximum entropy distributions calculated with random initial conditions (SI Appendix). Cm, chloramphenicol; Dox, doxycycline; Ery, erythromycin; Kan, kanamycin; Linc, lincomycin; Ofl, ofloxacin; Sal, salicylate; Tet, tetracycline; Tmp, trimethoprim.
Next, we tested this approach using chloramphenicol, erythromycin, and salicylate. The former two drugs are protein synthesis inhibitors. The binding of chloramphenicol to its ribosomal target has been shown to enhance the ribosomal binding of erythromycin (13), and it is therefore not surprising that we found chloramphenicol and erythromycin to be synergistic when used together. Salicylate, the active component of the analgesic aspirin, is known to be a potent inducer of a multidrug efflux pump that contributes to _E. coli_’s resistance to chloramphenicol (14). Consequently, it is also not surprising that chloramphenicol and salicylate are strongly antagonistic. Although interactions between salicylate and erythromycin have not been studied, we found them to be weakly antagonistic. What happens when the three drugs are combined together? A priori, one might expect a novel effect when all three drugs are present. The presence of salicylate decreases the intracellular concentration chloramphenicol, which might then decrease the binding affinity of erythromycin in a manner that depends on the dosages of salicylate and chloramphenicol. However, we find that pairwise interactions again yield accurate predictions of multidrug effects (Fig. 2_B_).
We found similar results for three additional three-drug combinations and also for two four-drug combinations. In all experiments, the predictions from the pairwise experiments provide accurate descriptions of the data (Fig. 2_C_, Figs. S9–S15, and Table S2). Interestingly, although most pairs of drugs interact either synergistically or antagonistically, we found that some three-drug combinations, such as doxycycline-erythromycin-lincomycin, act almost independently in larger combinations, whereas others, such as chloramphenicol-salicylate-ofloxacin, display extremely strong interactions and deviate significantly from Bliss independence (Fig. S9). Using standard model selection techniques (SI Appendix, Fig. S10), we verified that the Bliss independence model may be applicable for select drug combinations, but as a whole, the pairwise model (_R_2 = 0.90) performs significantly better than the independent model (_R_2 = 0.33) for describing the effects of three or four drugs in combination (Figs. S11–S15). In addition to the previous results, which include drug combinations over a large range of drug dosages, we also surveyed various multidrug interactions by performing five combinatorial experiments yielding 93 unique three-drug combinations and a total of 120 unique dosage combinations (SI Appendix, Figs. S16 and S17 and Table S3). We included a large range of drug types, including pain relievers, food preservatives, and inhibitors of DNA synthesis, folic acid synthesis, cell wall synthesis, and protein synthesis. Again, the pairwise model (_R_2 = 0.95) significantly outperforms the independent model (_R_2 = 0.29) and provides an excellent description of the data. Overall, these results suggest that for a wide range of antimicrobial drugs, the net effect of a drug combination is dominated by the accumulation of pairwise drug interactions, independent of the modes of action of the specific drugs involved.
Effects of Three-Drug Combinations in Staphylococcus aureus.
Because this approach does not rely on assumptions about molecular mechanisms, it should then be applicable to other bacterial species. As a model system, we used the bacterium Staphylococcus aureus, a common source of clinical infections. S. aureus are Gram-positive bacteria whose response to antibiotics differ substantially from that of E. coli (15). As for E. coli, we first measured the growth of S. aureus in response to three drugs: tetracycline, kanamycin, and erythromycin. All three drugs inhibit protein synthesis via different mechanisms. We performed the measurements for all drugs alone, and then repeated the measurement for all pairs of drugs.
Using the single drug and pairwise measurements, we then estimated the distribution Ppair(x), which allowed us to calculate the expectation of the growth response to the three-drug combination. We tested these predictions by comparing them with direct measurements of S. aureus growth in the presence of all three drugs. Remarkably, Fig. 2_D_ demonstrates that the mechanism-independent framework correctly predicts the experimentally measured growth response to multidrug exposure in S. aureus based solely on the responses to single and drug pairs.
Quantifying the Contribution of Pairwise Interactions to the Multidrug Response.
Overall, these results suggest that the integrated growth response of bacteria to three-drug and four-drug combinations can be directly inferred from the measured interactions between drug pairs. The data and the predictions are in excellent agreement, and the pairwise model performs significantly better than the Bliss independence model according to model selection techniques. However, the maximum entropy framework (16, 17) provides an additional metric that allows us to further quantify exactly how well the pairwise model captures deviations from independence. To do so, we used the maximum entropy distributions Pi (i = 1,2,3), which are consistent with the measured effects of all combinations composed of up to i drugs, to calculate the fraction of total correlations, fc, captured by the pairwise hypothesis (Table S4). Strikingly, this analysis demonstrates that there is very little additional information (∼3%) encapsulated by pure three-drug interactions. The answer to our original question is therefore surprising: the combined effects of these three-drug combinations follow almost entirely from the effects of the drugs alone and in pairs.
How Exactly Do Pairwise Interactions Accumulate?
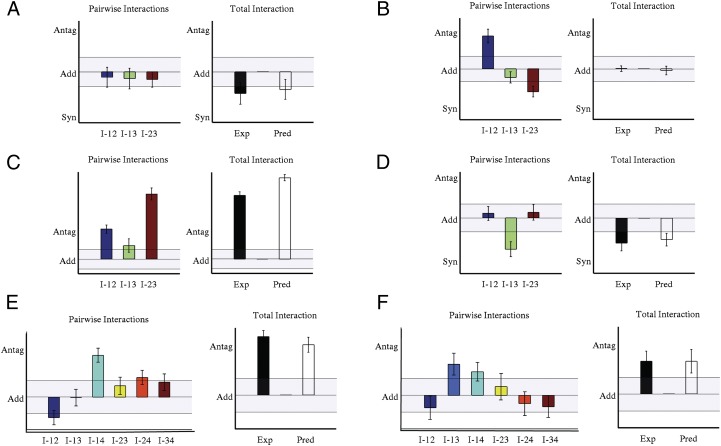
Our results demonstrate that for a large variety of antimicrobial drug combinations, no new apparent chemical interactions arise when three or four drugs are combined together. Instead, the net effect of the drug combination arises from the cumulative effect of the pairwise interactions. Given this drastic simplification, what outcomes are possible when drugs are combined at specific dosages? Surprisingly, there are still numerous ways that pairwise interactions can be combined to yield higher-order drug combinations (Fig. 3), even without requiring novel three-drug or four-drug effects. For example, weak synergistic interactions between drug pairs, such as those between chloramphenicol and erythromycin or erythromycin and trimethoprim, can combine to yield a cumulative effect that is strongly synergistic at particular doses (Fig. 3_A_). Conversely, as we saw, with the initial example of chloramphenicol, ofloxacin, and trimethoprim (Fig. 3_B_), that strong pairwise drug–drug interactions can combine to yield a cumulative drug effect weaker than or similar to the strongest pairwise interaction (Fig. 3_D_). In the case of salicylate, chloramphenicol, and ofloxacin, which interact antagonistically when used in pairs, the net result is an antagonistic three-drug effect whose magnitude is similar to that of the pure salicylate–ofloxacin interaction (Fig. 3_C_). In all cases, the net effect can be predicted using only the response to drug pairs (Fig. 3 A–F), illustrating that a wide range of cumulative effects are possible, depending on the dosages of each drug, even in the absence of pure three-drug or four-drug interactions. Overall, these results offer a mechanism-independent framework for predicting the cooperative effect of drug combinations on bacterial growth using only the information from the response to isolated drugs and drug pairs.
Fig. 3.
Predictions highlight ways that pairwise interactions accumulate to yield higher-order interactions. Total drug interactions and pairwise drug interactions for three-drug (A–D) and four-drug (E and F) combinations in E. coli (A–C, E, and F) and S. aureus (D). Each panel shows pairwise (all panels, left, I_-ij = g_ ij -g i g j and three-drug (A–D, right, g 123 -g 1 g 2 g 3) or four-drug (E and F, right, g 1234 -g 1 g 2 g 3 g 4) interactions at a given drug dosage. Light bars indicate maximum entropy prediction; dark bars indicate experimental result. Shaded portions of each plot indicate regions of approximately additive behavior (add, |interaction| < 0.1). In all panels, antagonism (antag) and synergy (syn) labels correspond to interactions of +0.3 and −0.3, respectively. Error bars are shown, ±SE (SI Appendix). Interactions that cannot be statistically explained from the pairwise predictions are less than 0.05 (units of relative growth rate) in all cases (Fig. S17). Drug combinations: A, chloramphenicol-erythromycin-trimethoprim; B, chloramphenicol-ofloxacin-trimethoprim; C, chloramphenicol-ofloxacin-salicylate; D, kanamycin-erythromycin-tetracycline; E, doxycycline-erythromycin-lincomycin-salicylate; F, chloramphenicol-ofloxacin-trimethoprim-lincomycin.
Discussion
Our experiments reveal that, for many antimicrobial drug combinations, interactions involving exactly three or more drugs do not appreciably contribute to the overall effect of the combination. The results are complementary to detailed mechanistic models because they impose an upper limit on how much mechanistic information is required to predict bacterial growth. Mechanistic and empirical approaches remain essential to characterize the effects of specific drug pairs (18–23). Remarkably, however, our results reveal that additional information is often not required to predict the effects of larger combinations of drugs. Consequently, these findings may provide a powerful strategy for the rational design of candidate therapies using combinations of three or more drugs, even when full mechanistic descriptions are not available.
Nevertheless, the approach does have practical limitations. First, the distribution Ppair(x) (or equivalently, the single drug and two-drug effects, gi, and gij) measured for a particular bacterial strain cannot, in general, be used to predict the multidrug response in a different strain. Using this approach to screen for multidrug combinations to combat drug-resistant mutants, for example, would require measurements of the relevant two-drug effects in each specific strain. Second, it is important to note that we chose maximum entropy as a systematic way to incorporate deviations from Bliss independence without adding spurious statistical structure. However, there may exist other pairwise models that could also be used to estimate the effects of larger drug combinations. Our primary finding is that at least one such pairwise model exists that provides excellent predictive power. Finally, one can design ad hoc examples in which any pairwise model is likely to fail. For example, if one drug were an enzyme that required two substrates, then the combination of the enzyme with both substrates might yield a completely novel three-body interaction that could not be predicted from the pairwise effects. Interestingly, however, we do not find evidence for such strong three-body interactions in any of our experiments.
Previous studies have also used pairwise approximations in other contexts, but the underlying variables represented the dynamics of specific cellular components or other physical entities such as proteins or neurons (24–30). Most notably, a recent study in cancer cells demonstrated that the expression of some proteins in response to combinations of drugs can be predicted from their responses to smaller drug combinations (24). Elucidating the biological connection between these results, at the level of individual proteins, and the integrated responses of entire cells, such as growth, remains an intriguing issue for future work. Unfortunately, fully mechanistic models of the transcriptional, metabolic, and posttranslational networks governing the multidrug response may be intractable, highlighting the need for phenomenological or statistical models to bridge this gap. To circumvent the difficulties associated with building a mechanistic model, we have formulated the problem using a mechanism-independent statistical approach. By using coarse-grained stochastic variables, Xi, whose moments <X1..Xn> reflect the effects of a combination of N drugs, we have replaced large, intractable mechanistic models with a remarkably small statistical model of interacting drugs. Although the variables do not have a direct microscopic interpretation, they do offer a very powerful tool for inferring the relationship between the _N_-drug response and the response to drug pairs. Moreover, we find that simple formulas can yield accurate predictions as well, making the approach widely applicable and easy to implement. From a basic science perspective, the picture emerging from our analysis is surprising because it suggests that the chemical complexity underlying the cellular response to drug combinations often does not exceed that of drug pairs. These findings therefore raise the possibility that the multidrug response in bacteria obeys statistical, rather than chemical, laws for combinations larger than two. Finally, because our findings do not depend on details of any specific cellular system, they offer a powerful predictive framework that may be applicable to other bacteria and even to eukaryotes.
Methods
Bacterial Strains.
We used the WT BW25113 strain for all experiments on E. coli (Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514) (31). We used the clinically isolated strain Newman for all experiments on S. aureus (32).
Drugs.
We prepared all drug solutions from solid stocks (Table S1 lists drugs, their classes, and their mode of action). All antibiotic stock solutions were stored in the dark at −20 °C in single-use daily aliquots. All drugs were thawed and diluted in sterilized broth for experimental use.
Media.
We used Lennox LB broth (Fisher) for experiments on E. coli and Tryptic Soy Broth (BD) for experiments on S. aureus.
Growth Conditions and Drug Treatments.
For both E. coli and S. aureus experiments, we inoculated 3 mL fresh medium with a single colony and grew the cells overnight (12 h) in 14-mL culture tubes at 30 °C, with shaking at 200 rpm. Following overnight growth, stationary phase cells were diluted (5,000-fold for E. coli, 20,000 fold for S. aureus) in medium and grown for an additional 2 h at 30 °C, with shaking at 200 rpm. We then transferred 195 μL cells plus medium to 96-well plates (round-bottomed, polystyrene; Corning), and to each well we added a given combination of one, two, three, or four drugs. Specifically, we set up a 2D matrix of one-, two-, three-, or four-drug combinations, with the concentration of one or more drugs increasing along each direction of the plate. In the presence of the drugs, we grew the cells for 10–18 h at 30 °C, with shaking at 1,000 rpm on four identical vibrating plate shakers. We measured the absorbance at 600 nm (A600) at time intervals dt (dt = 20 min for E. coli, 30 min for S. aureus) using a Wallac Victor-2 1420 Multilabel Counter (Perkin-Elmer) combined with an automated robotic system (Twister II, Caliper Life Sciences) to transfer plates between shakers and the reader.
Growth Rate Calculation.
From the time series of A600, we determined growth rates by fitting the early exponential phase portion of curves (0.01 < A600 <0.1) to an exponential function (MATLAB 7.6.0 curve fitting toolbox, Mathworks). We normalized growth rates in the presence of single drugs (gi) or multiple drugs (gij, gijk, gijkl) by the growth rate of cells in the absence of drugs. An example growth curve is shown in Fig. S1. SEs of the fitted growth parameter are used to estimate uncertainty in growth rates.
To minimize the small effects of day-to-day fluctuations in drug efficacy (typically <5%), we generated a standard dose–response curve (and IC50 value) for each drug by combining all data involving only exposure to that drug. In all subsequent three and four-drug experiments, we remeasured the IC50 value for each drug and scaled all concentrations to ensure that it agreed with the IC50 from the standard curve. Single drug (gi) and pairwise (gij) growth rates at a given set of concentrations were then estimated by interpolating, if necessary, between data points measured at nearby concentrations.
Supplementary Material
Supporting Information
Acknowledgments
We thank C. Guet, L. Bruneaux, E. Balleza, and A. Subramaniam for technical guidance and Ilya Nemenman, Jonathon D. Shlens, Remi Monasson, Simona Cocco, Kris Wood, and all members of the Cluzel laboratory for many helpful discussions. We also thank K. Dave for editorial advice and assistance. This work was supported in part by the National Institutes of Health (NIH) Award P50GM081892-02 to the University of Chicago, AFOSR FA9550-11-1-0247 and NIH 1R01GM086881 through Rutgers University (E.D.S.), and by a National Science Foundation Postdoctoral Fellowship 0805462 (K.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Baddour LM, et al. Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease. Council on Cardiovascular Disease in the Young. Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia. American Heart Association. Infectious Diseases Society of America Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–e434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 2.Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2009;49:1072–1079. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- 3.Herbert D, et al. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2296–2299. doi: 10.1128/aac.40.10.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mory F, Fougnot S, Rabaud C, Schuhmacher H, Lozniewski A. In vitro activities of cefotaxime, vancomycin, quinupristin/dalfopristin, linezolid and other antibiotics alone and in combination against Propionibacterium acnes isolates from central nervous system infections. J Antimicrob Chemother. 2005;55:265–268. doi: 10.1093/jac/dkh521. [DOI] [PubMed] [Google Scholar]
- 5.Bliss CI. The calculation of microbial assays. Bacteriol Rev. 1956;20:243–258. doi: 10.1128/br.20.4.243-258.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 7.Greco WR, Bravo G, Parsons JC. The search for synergy: A critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 8.Bollenbach T, Quan S, Chait R, Kishony R. Nonoptimal microbial response to antibiotics underlies suppressive drug interactions. Cell. 2009;139:707–718. doi: 10.1016/j.cell.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huovinen P, Wolfson JS, Hooper DC. Synergism of trimethoprim and ciprofloxacin in vitro against clinical bacterial isolates. Eur J Clin Microbiol Infect Dis. 1992;11:255–257. doi: 10.1007/BF02098092. [DOI] [PubMed] [Google Scholar]
- 10.Cover TM, Thomas JA. Elements of Information Theory, XXIII. Hoboken, NJ: Wiley-Interscience; 2006. [Google Scholar]
- 11.Jaynes ET. Information theory and statistical mechanics. Phys Rev. 1957;106:620–630. [Google Scholar]
- 12.Isserlis L. On a formula for the product-moment coefficient of any order of a normal frequency distribution in any number of variables. Biometrika. 1918;12:134–139. [Google Scholar]
- 13.Langlois R, Cantor CR, Vince R, Pestka S. Interaction between the erythromycin and chloramphenicol binding sites on the Escherichica coli ribosome. Biochemistry. 1977;16:2349–2356. doi: 10.1021/bi00630a007. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SP, Levy SB, Foulds J, Rosner JL. Salicylate induction of antibiotic resistance in Escherichia coli: Activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haight TH, Finland M. Laboratory and clinical studies on erythromycin. N Engl J Med. 1952;247:227–232. doi: 10.1056/NEJM195208142470701. [DOI] [PubMed] [Google Scholar]
- 16.Amari S. Information geometry on hierarchy of probability distributions. IEEE Trans Inf Theory. 2001;47:1701–1711. [Google Scholar]
- 17.Schneidman E, Still S, Berry MJ, 2nd, Bialek W. Network information and connected correlations. Phys Rev Lett. 2003;91:238701. doi: 10.1103/PhysRevLett.91.238701. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK. Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol. 2006;2:458–466. doi: 10.1038/nchembio817. [DOI] [PubMed] [Google Scholar]
- 19.Jonker DM, Visser SAG, van der Graaf PH, Voskuyl RA, Danhof M. Towards a mechanism-based analysis of pharmacodynamic drug-drug interactions in vivo. Pharmacol Ther. 2005;106:1–18. doi: 10.1016/j.pharmthera.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 21.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: From targets to networks. Nat Rev Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehár J, Stockwell BR, Giaever G, Nislow C. Combination chemical genetics. Nat Chem Biol. 2008;4:674–681. doi: 10.1038/nchembio.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehár J, et al. Chemical combination effects predict connectivity in biological systems. Mol Syst Biol. 2007;3:80. doi: 10.1038/msb4100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geva-Zatorsky N, et al. Protein dynamics in drug combinations: A linear superposition of individual-drug responses. Cell. 2010;140:643–651. doi: 10.1016/j.cell.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Margolin AA, et al. ARACNE: An algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics. 2006;7(Suppl 1):S7. doi: 10.1186/1471-2105-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora T, Walczak AM, Bialek W, Callan CG., Jr Maximum entropy models for antibody diversity. Proc Natl Acad Sci USA. 2010;107:5405–5410. doi: 10.1073/pnas.1001705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlens J, et al. The structure of large-scale synchronized firing in primate retina. J Neurosci. 2009;29:5022–5031. doi: 10.1523/JNEUROSCI.5187-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shlens J, et al. The structure of multi-neuron firing patterns in primate retina. J Neurosci. 2006;26:8254–8266. doi: 10.1523/JNEUROSCI.1282-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneidman E, Berry MJ, 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440:1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee MS, Purvis JE, Brass LF, Diamond SL. Pairwise agonist scanning predicts cellular signaling responses to combinatorial stimuli. Nat Biotechnol. 2010;28:727–732. doi: 10.1038/nbt.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006–2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008;190:300–310. doi: 10.1128/JB.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information