Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice (original) (raw)
Abstract
Tim-1, a type I transmembrane glycoprotein, consists of an IgV domain and a mucin domain. The IgV domain is essential for binding Tim-1 to its ligands, but little is known about the role of the mucin domain, even though genetic association of TIM-1 with atopy/asthma has been linked to the length of mucin domain. We generated a Tim-1–mutant mouse (Tim-1Δmucin) in which the mucin domain was deleted genetically. The mutant mice showed a profound defect in IL-10 production from regulatory B cells (Bregs). Associated with the loss of IL-10 production in B cells, older Tim-1Δmucin mice developed spontaneous autoimmunity associated with hyperactive T cells, with increased production of IFN-γ and elevated serum levels of Ig and autoantibodies. However, Tim-1Δmucin mice did not develop frank systemic autoimmune disease unless they were crossed onto the Fas-mutant lpr mice on a C57BL/6 background. Tim-1Δmucinlpr mice developed accelerated and fulminant systemic autoimmunity with accumulation of abnormal double-negative T cells and autoantibodies to a number of lupus-associated autoantigens. Thus, Tim-1 plays a critical role in maintaining suppressive Breg function, and our data also demonstrate an unexpected role of the Tim-1 mucin domain in regulating Breg function and maintaining self-tolerance.
Keywords: inflammation, hepatitis A virus cellular receptor 1, kidney injury molecule 1
The T-cell Ig mucin (Tim) family of genes consists of eight members in mice and three members in humans (1–4). The three human TIM genes, TIM-1, TIM-3, and TIM-4, are homologs of mouse Tim-1, Tim-3, and Tim-4, respectively. In addition, mouse Tim-2 is considered to be another ortholog of human TIM-1. The Tim proteins are type I cell-surface glycoproteins with common structural features including an N-terminal IgV domain, a mucin domain with numerous _O_-glycosylation sites, a stalk region with _N_-glycosylation site(s), a transmembrane domain, and a short cytoplasmic tail (1–5). The Tim gene family is located at chromosome 5q33.2 in humans and 11B1.1 in mice and frequently has been linked to asthma, allergy, and autoimmunity in both mice and humans (1–4, 6). Tim proteins have been reported to be expressed on various immune cells including T cells, macrophages, and dendritic cells (DCs) where they have been implicated in regulating broad immune responses, including asthma and allergy, autoimmunity, transplant tolerance, and responses to tumors and viral infection (1–4).
Tim-1 also has been identified as a cellular receptor for hepatitis A virus (HAVCR1); the IgV domain is crucial for binding to the virus, and the mucin domain is critical for uncoating the viral particles before entry and cellular infection (7–9). Interestingly, exposure/infection to hepatitis A virus (HAV) is associated with a reduced risk of developing asthma, atopy, and allergies in humans, and, similarly, Tim-1 has been linked genetically to Th2-driven murine airway hypersensitivity, leading to the identification of the Tapr locus and Tim-1 as an asthma susceptibility gene (6, 10–13). Although there are small allelic variations in the IgV domain, the genetic linkage to susceptibility to allergy following HAV infection was linked mainly to the length of the mucin domain of TIM-1 (14). An insertion of six amino acids forming a long TIM-1 mucin domain (157insMTTTVP) resulted in protection against asthma and allergy in subjects exposed to HAV (6, 11–13). Similarly, the mucin domain in Tim-1 is longer in BALB/c mice (6, 10, 11), which are susceptible to Th2-driven airway hypersensitivity, than in DBA/2 and C57BL/6 mice, which develop less airway reactivity following antigen challenge in murine airway hyperreactivity models. These data underscore the importance of the mucin domain of Tim-1 in regulating immune responses and in the development of atopic diseases. In addition, human NKT cells expressing the long form of TIM-1 showed greater cytolytic activity against HAV-infected liver cells (14). These data on genetic linkage to allergies, HAV infection, and immune responses demonstrate that the length of the mucin domain of TIM-1 has important functional consequences in human immune and infectious diseases, but the actual mechanism by which the TIM-1 mucin domain regulates immune responses has not been analyzed.
Surprisingly mice with either complete Tim-1 deficiency (Tim-1−/−) or with overexpression of the full-length Tim-1 molecule showed no defects in cellular phenotype, nor did they show any significant differences in Th2 responses and Th2-mediated airway inflammation (15, 16), again raising the question whether the mucin domain has critical biological functions in immune regulation.
All Tim-1 ligands identified thus far require the Tim-1 IgV domain for their ligand binding (3, 4, 17). For example, Tim-4 expressed on antigen-presenting cells (APCs) has been reported to costimulate T-cell responses by phosphorylating Tim-1 expressed on activated T cells (18, 19). The Tim-1 IgV domain also binds phosphatidylserine exposed on the surface of apoptotic cells and has been shown to clear apoptotic cells when expressed on kidney epithelial cells or when Tim-1 was overexpressed artificially on transfectants (20–23). The IgV domain therefore serves as the ligand-binding domain for Tim-1. Given that loss of full-length Tim-1 in the knockout mice did not show any phenotype and that genetic linkage to infection and allergies is associated with the length of the TIM-1 mucin domain, we generated a mutant mouse in which the Tim-1 was expressed at normal levels but did not contain the mucin domain (Tim-1Δmucin mice). Because the Tim-1–mutant mice expressed an intact ligand-binding IgV domain, we were able to analyze the role of Tim-1 in the immune system in the absence of the mucin domain.
For the most part, Tim-1Δmucin mice appeared normal at <6 mo of age, but as the mice aged (>10 mo), there was an impairment in IL-10 production by regulatory B cells (Bregs). Associated with the loss of Breg IL-10 production, Tim-1Δmucin mice developed features of systemic autoimmune disease including hyperactivated T cells with increased IFN-γ production and autoantibody formation. When introduced into Fas-mutant lpr mice on the C57BL/6 background, Tim-1Δmucin remarkably accelerated and worsened autoimmunity with increased accumulation of normal and abnormal double-negative T cells and an increase in autoantibodies to a number of lupus antigens including antibodies to dsDNA. These data suggest that the Tim-1 mucin domain is critical for IL-10 production by B cells and that in the absence of this domain mutant mice develop severe systemic autoimmunity when Tim-1Δmucin is expressed on a susceptible genetic background.
Results
Generation and Characterization of Tim-1Δmucin Mice.
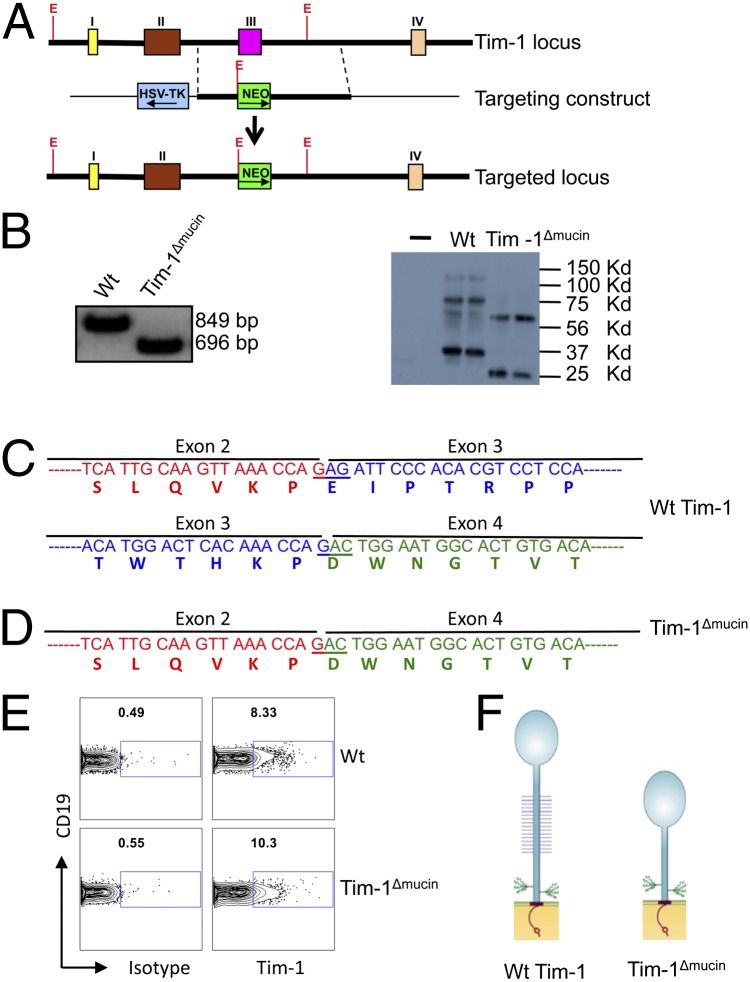
The mucin domain of Tim-1 is a highly _O_-glycosylated threonine-, serine-, and proline-rich region encoded by exon 3 of the Tim-1 gene in mice on the C57BL/6 background. To study the role of the mucin domain for Tim-1 function in vivo, we replaced the Tim-1 exon 3 with a PGK promoter-driven neomycin-resistance cassette (Fig. 1_A_) and generated a mutant mouse on the C57BL/6 background in which the Tim-1 proteins do not contain the mucin domain (Tim-1Δmucin mice). Tim-1Δmucin mice were produced in normal Mendelian ratios and showed no obvious abnormalities before the age of 6 mo. As expected, RT-PCR combined with sequencing assays showed that B cells from WT mice expressed the full-length 849-bp Tim-1 mRNA, whereas B cells from Tim-1Δmucin mice expressed a 696-bp Tim-1 mRNA, which lacks the 159-bp Tim-1 exon 3 (Fig. 1_B_). Consistent with the loss of 53 amino acids in Tim-1Δmucin proteins as predicted based on the sequencing data (Fig. 1 C and D), Western blot data showed that Tim-1Δmucin+ cells expressed shortened Tim-1 proteins (mainly an ∼25-KDa unglycosylated band and an ∼60-KDa glycosylated band), whereas WT Tim-1+ cells expressed primarily ∼35-kDa unglycosylated and ∼75-kDa glycosylated Tim-1 proteins (Fig. 1_B_). Furthermore, flow cytometric data showed that Tim-1Δmucin proteins, like the WT Tim-1 proteins, were expressed normally on the surface of B cells (Fig. 1_E_). These data confirmed that we generated mice that expressed a mutant mucinless Tim-1 protein. The extracellular portion of this Tim-1 mutant was composed of the IgV domain and the stalk domain containing two _N_-glycosylation sites but completely lacked _O_-glycosylated mucin domain (Fig. 1_F_).
Fig. 1.
Generation and characterization of Tim-1Δmucin mice. (A) The gene structures of the WT Tim-1 allele, the Tim-1Δmucin targeting construct, and the targeted Tim-1 allele. Colored boxes represent coding sequences; Roman numerals represent exons. E, EcoR I sites. (B) (Left) CD19+ B cells isolated from WT and Tim-1Δmucin mice were used to determine Tim-1 mRNA expression by RT-PCR. (Right) 293T cells were overexpressed with WT Tim-1 and Tim-1Δmucin and then were used to determine Tim-1 protein expression by Western blot. (C and D) RT-PCR products were sequenced, and parts of cDNA and predicted encoding amino acid sequences of WT Tim-1 (C) and Tim-1Δmucin (D) are shown. (E) Isolated WT and Tim-1Δmucin B cells were examined for Tim-1 expression by flow cytometry. (F) Schematic representation of Tim-1 and Tim-1Δmucin protein structures.
Tim-1Δmucin Mice Develop an Activated Immune Phenotype with Age.
We first evaluated whether the mucin deletion affected the development and phenotype of immune cells. Up to 6 mo of age, Tim-1Δmucin mice were apparently healthy and did not show any significant abnormality in cellularity of immune compartments or any defect in kidneys or other organs. For example, Tim-1Δmucin mice showed normal T-cell development in thymi, normal distribution of T and B cells, DCs, and macrophages, and normal activation status of T cells and APCs in peripheral immune compartments (Fig. 2_A_).
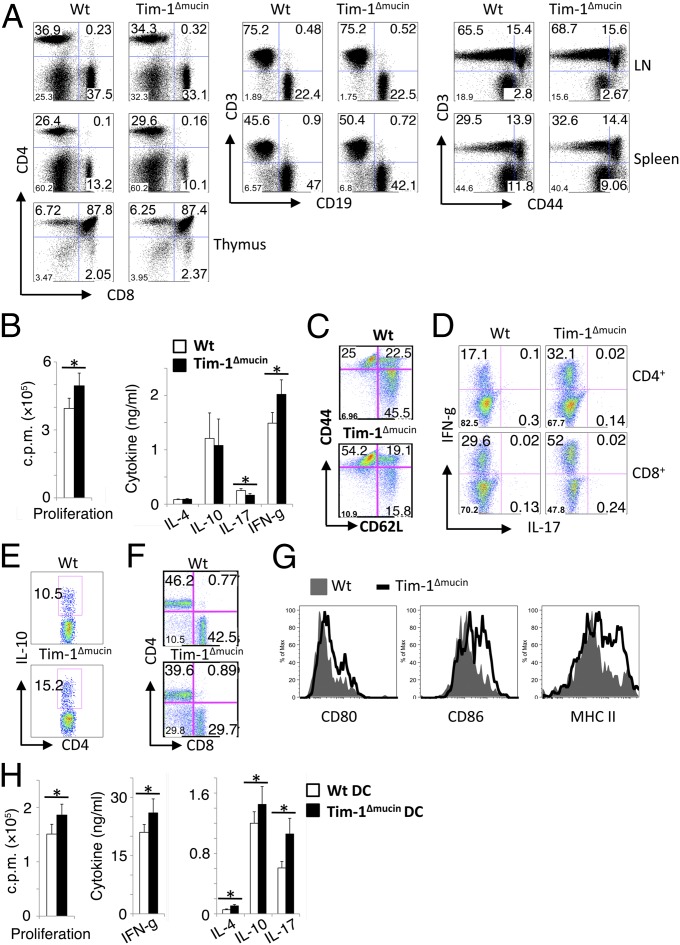
Fig. 2.
Immune phenotypes in Tim-1Δmucin mice. (A) Spleen, lymph nodes, and thymi were analyzed for the expression of indicated markers by flow cytometry. Data shown are representative of n = 8–10 per group of 3- to 6-mo-old mice. (B) CD4+ T cells isolated from 3- to 6-mo-old mice were treated with anti-CD3 and anti-CD28. Cytokines in 48-h culture supernatants were measured by cytometric bead array. Proliferation was measured by [3H]-thymidine incorporation. (C) Splenocytes from 10- to 12-mo-old mice were examined for CD62L and CD44 expression on CD3+ cells by flow cytometry. (D and E) Splenocytes from 10- to 12-mo-old mice also were stained for IFN-γ and IL-17 (D) and IL-10 (E) expression after stimulation with phosphomolybdic acid (PMA)/ionomycin. (F) Splenocytes from 20-mo-old WT and Tim-1Δmucin mice were analyzed for CD3, CD4, and CD8 expression by flow cytometry (gated on CD3+ cells). (G) Splenocytes from 10- to 12-mo-old mice were examined for CD80, CD86, and MHC class II expression on CD11c+ cells by flow cytometry. (H) DCs isolated from WT and Tim-1Δmucin mice were cocultured with WT CD4+ T cells in the presence of anti-CD3. Cytokines in 48-h culture supernatants were measured by cytometric bead array. Proliferation was measured by [3H]-thymidine incorporation. *P < 0.05.
Because Tim-1 has been reported to be expressed on T cells upon activation and to regulate T-cell responses (24, 25), we assessed the responses of T cells from Tim-1Δmucin mice. After activation, Tim-1Δmucin CD4+ cells showed slightly increased proliferation and IFN-γ production but slightly decreased levels of IL-17 compared with WT CD4+ cells (Fig. 2_B_). No obvious differences in IL-4 or IL-10 production were observed between WT and Tim-1Δmucin CD4+ cells (Fig. 2_B_). These data suggest that the net effect of Tim-1Δmucin in CD4+ cells is mainly an enhanced Th1 response, as indicated by IFN-γ production.
Similar to the younger mice, WT and Tim-1Δmucin mice at 10–12 mo of age did not exhibit any dramatic differences in the percentage or number of CD4+ or CD8+ T cells or B cells in spleen or lymph nodes. However, the percentage and number of activated/memory (CD44hi) T cells in Tim-1Δmucin mice were much higher than in WT mice (Fig. 2_C_). Also, the frequency and number of both IFN-γ+CD4 and IFN-γ+CD8 T cells were much higher in the older Tim-1Δmucin mice than in WT mice, although the number of IL-17+ T cells remained similar between the two groups of mice, and IL-4+ T cells were not detectable (Fig. 2_D_). CD4+ T cells from the older Tim-1Δmucin mice also showed slightly higher IL-10 production, probably as a result of ongoing activation and inflammation (Fig. 2_E_). More interestingly, when Tim-1Δmucin mice were more than 18 mo old, many developed enlarged spleens, and almost all their T cells were CD44hi. Of interest, there was an increase in CD3+CD4−CD8− double-negative (normal DN) T cells in Tim-1Δmucin mice that were more than 18 mo old (Fig. 2_F_). This subset is reminiscent of a T-cell subset expanded in systemic autoimmunity, such as lupus, in both human and mouse (26, 27).
APCs, such as DCs, from 10- to 12-mo-old Tim-1Δmucin mice showed increased expression of CD80, CD86, and MHC class II molecules, compared with WT mice, that was not seen in younger Tim-1Δmucin mice (Fig. 2_G_). We then determined the effects of Tim-1Δmucin DCs on T-cell responses. WT and Tim-1Δmucin splenic DCs were cocultured with WT CD4+ T cells in the presence of anti-CD3. Tim-1Δmucin DCs slightly increased T-cell proliferation and variably increased the production of cytokines including IFN-γ, IL-17, IL-10, and IL-4 (Fig. 2_H_). These data suggest that Tim-1Δmucin DCs can increase effector T-cell responses modestly. Taken together, the data showed that Tim-1Δmucin mice did not have any obvious defects when young (<6 mo old); however, when these mice become older (typically >10 mo old), they begin to show signs of autoimmunity, mainly characterized by hyperactivated effector T-cell responses.
Tim-1Δmucin Mice Have Normal Foxp3+ Regulatory T Cells but Impaired Breg IL-10 Production.
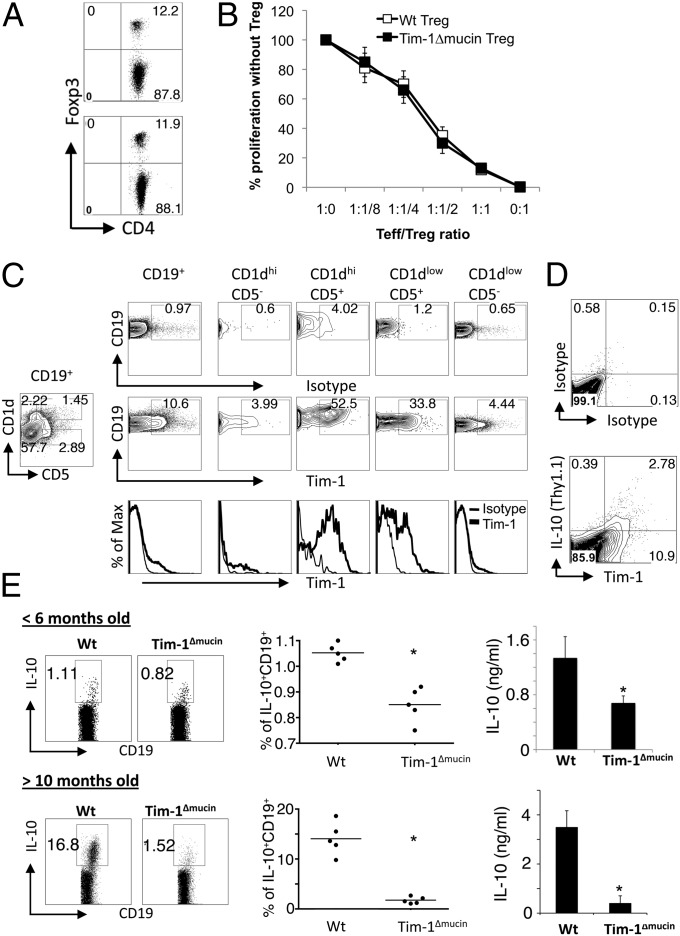
Regulatory T cells (Tregs) have been shown to play a critical and essential role in the maintenance of immune tolerance (28). Therefore, we next asked whether the T-cell hyperactivity and DC activation developed in Tim-1Δmucin mice was caused by a Treg defect. To study the frequency and function of Foxp3+ Treg cells, we generated Tim-1ΔmucinFoxp3/GFP-knockin (KI) mice by breeding Tim-1Δmucin mice with Foxp3/GFP-KI mice (29). We did not observe any obvious differences between WT and Tim-1Δmucin mice in the number or frequency of Foxp3+ Tregs or in their suppressive capacity (Fig. 3 A and B).
Fig. 3.
Foxp3+ Tregs are normal, but IL-10–producing Bregs are impaired in Tim-1Δmucin mice. (A) Frequency of Foxp3/GFP+ Tregs in spleens of 4- to 6-mo-old WT Foxp3/GFP-KI and Tim-1ΔmucinFoxp3/GFP-KI mice was analyzed by flow cytometry. (B) Effector T cells (Teff) and DCs from WT Foxp3/GFP-KI mice were cocultured with different ratios of Tregs in the presence of anti-CD3, and cell proliferation was measured by 3[H]-thymidine incorporation. (C) Splenocytes from 3-mo-old WT mice were stained with CD19, CD5, CD1d, and Tim-1 mAb, and Tim-1 expression on different CD19+ B-cell subsets was analyzed by flow cytometry. (D) Splenocytes from IL-10/Thy1.1 reporter mice were activated with LPS/PMA/ionomycin for 5 h, and Tim-1 expression and IL-10 production (Thy1.1+) in CD19+ B cells were analyzed by flow cytometry. (E) Splenocytes from WT and Tim-1Δmucin mice were activated with LPS/PMA/ionomycin for 5 h, and IL-10 production in CD19+ B cells was analyzed by intracellular cytokine staining (Left and Middle). Isolated splenic CD19+ cells were activated with LPS for 24 h, and IL-10 production in culture supernatants was measured by cytokine bead array (Right). *P < 0.05.
Recent studies also have suggested that IL-10–producing Bregs are essential in immune tolerance and that loss of IL-10–producing Bregs leads to the development of autoimmune disease (30). We asked whether the autoimmunity developed in Tim-1Δmucin mice with age was caused by a defect in Bregs. Although there is not a clear marker for Bregs, at this stage they are defined best by a combination of cell-surface markers that include CD19+CD5+CD1dhi cells. Because a portion of B cells express Tim-1 (16), and because CD19+CD5+CD1dhi B cells are the most competent B-cell subset for IL-10 production (30), we first determined whether the CD19+CD5+CD1dhi B-cell subset expressed Tim-1. We found that ∼10% of splenic CD19+ B cells from naive mice expressed Tim-1, and Tim-1+ cells were enriched mainly in CD5+ B cells: ∼50% of CD5+CD1dhi and ∼30% of CD5+CD1dlow B cells expressed Tim-1, whereas <5% of CD5− B cells were Tim-1+ regardless their CD1d levels (Fig. 3_C_). We then examined whether Tim-1+ B cells produced IL-10 using the IL-10/Thy1.1 reporter mice (31). Interestingly, we found that IL-10 production in B cells was almost exclusively from Tim-1+ cells (Fig. 3_D_), consistent with a recent report suggesting that Tim-1 identifies IL-10–producing B cells regardless of other markers (32). In fact, it was shown that Tim-1 ligation could induce Tim-1+ Bregs, which could transfer long-term acceptance of islet allografts and inhibit allergic airway disease (32).
We then compared IL-10 production from WT and Tim-1Δmucin B cells. When mice were <6 mo old, Tim-1Δmucin B cells clearly produced less IL-10 than WT B cells, as determined by both intracellular cytokine staining and ELISA (Fig. 3_E_). When mice were >10 mo old, the difference became even more dramatic. Although the frequency of IL-10+ B cells increased in aged WT mice, as reported previously (33), the frequency of IL10+ B cells decreased markedly in Tim-1Δmucin mice (Fig. 3_E_).
Taken together, these data suggest that Tim-1+ B cells are the major B cells for IL-10 production. Lack of the mucin domain in Tim-1 protein impairs IL-10–producing Bregs, and the difference in IL-10 production becomes even more dramatic with age. Also, the defect in IL-10 production is CD19+ B-cell specific in Tim-1Δmucin mice, because there was no obvious difference in IL-10 production between WT and Tim-1Δmucin CD19- cells from young mice. Furthermore, Tim-1Δmucin CD19− cells from old mice produced slightly higher IL-10 levels than did WT cells (Fig. S1). In fact, Tim-1Δmucin CD4+ cells from old mice produced higher IL-10 than did WT T cells (Fig. 2_E_).
Immune Phenotypes in Tim-1Δmucinlpr Mice.
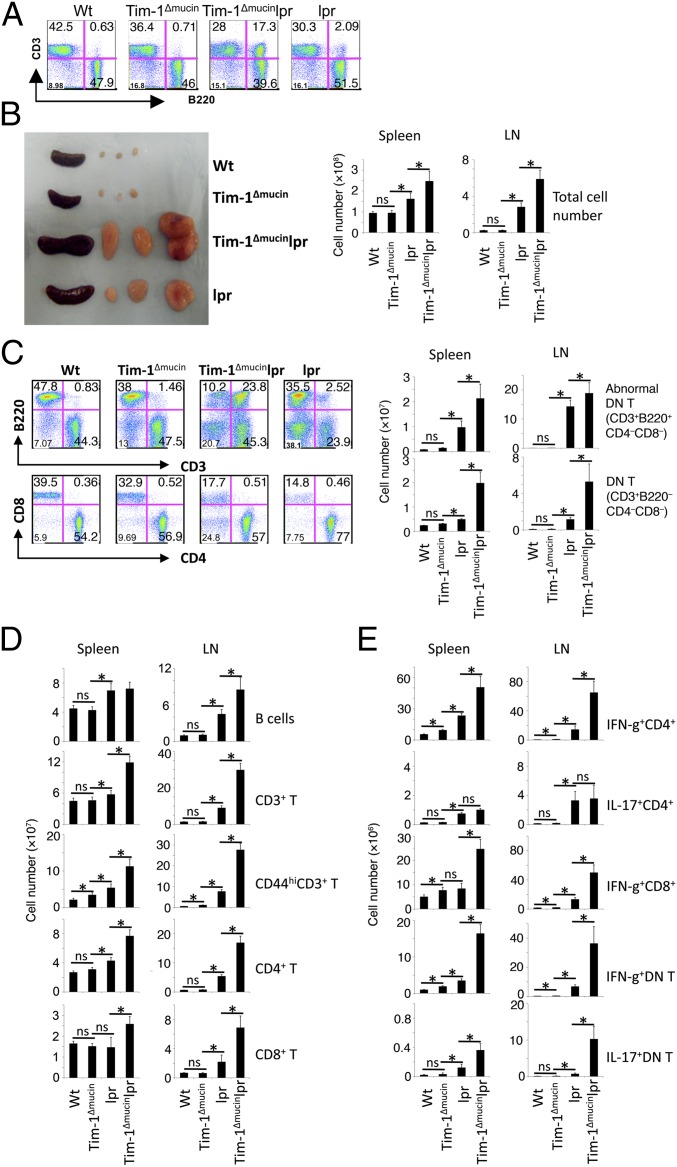
Our data thus far have shown that the major defect in naive Tim-1Δmucin mice at age <6 mo was impaired IL-10 production by Bregs. When Tim-1Δmucin mice were >10 mo old, these Bregs lost their IL-10 production almost completely. Associated with the defect in IL-10–producing Bregs, Tim-1Δmucin mice showed signs of systemic inflammation with age. Because _Fas_-deficient lpr mice on the C57BL/6 background develop only a mild systemic autoimmunity (34), we asked whether introducing Tim-1Δmucin into lpr mice on the C57BL/6 background would promote more severe autoimmunity. By 14 wk of age, Tim-1Δmucinlpr mice already had a much greater accumulation of B220+CD3+ (abnormal) T cells in the peripheral lymphoid compartments than did lpr mice (Fig. 4_A_). By 10+ mo of age they developed much more severe splenomegaly and lymphadenopathy (Fig. 4_B_) with massive accumulation of T cells (both CD3+B220− CD4+ and CD8+ T cells), B cells (CD3−B220+), CD3+B220−CD4−CD8− double-negative T cells, and the abnormal CD3+B220+CD4−CD8− T cells (Fig. 4 C and D). Furthermore, the majority of T cells in these Tim-1Δmucinlpr mice had an activated/memory-like (CD44hi) phenotype, compared with those in lpr, WT, or Tim-1Δmucin mice (Fig. 4_D_). The frequency and number of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in Tim-1Δmucinlpr mice were much higher than in lpr mice, whereas the number of IL-17+CD4+ T cells was similar in the two groups (Fig. 4_E_); IL-17+CD8+ T cells were not detectable. Interestingly, compared with lpr mice, Tim-1Δmucinlpr mice also had a massive accumulation of CD3+B220−CD4−CD8− (normal DN) T cells and many of them produced IFN-γ and/or IL-17 (Fig. 4_E_); the number of normal DN T cells was very low and was similar in 10- to 12-mo-old WT and Tim-1Δmucin mice (Fig. 4_C_). However, the frequency and number of the normal DN T cells increased dramatically in Tim-1Δmucin mice that were >18 mo old (Fig. 2_F_), indicating that this cell population eventually does accumulate in Tim-1Δmucin mice even without the lpr mutation.
Fig. 4.
Immune phenotypes in Tim-1Δmucinlpr double-mutant mice. (A) Increased accumulation of abnormal CD3+B220+CD4−CD8− cells in Tim-1Δmucinlpr mice. CD3 and B220 expression in splenocytes of 14-wk-old mice was determined by flow cytometry. (B) Increased splenic and lymph node sizes in Tim-1Δmucinlpr mice. (Left) Representative images of spleens and lymph nodes from 10- to 12-mo-old WT, Tim-1Δmucin, lpr, and Tim-1Δmucinlpr mice. (Right) Splenic and lymph node cells from 10- to 12-mo-old WT (n = 8), Tim-1Δmucin (n = 12), lpr (n = 10), and Tim-1Δmucinlpr (n = 15) mice were counted. (C_–_E) Cells from B were examined for indicated surface markers (C and D) or for IFN-γ and IL-17 production (E) by flow cytometry. Different immune cell populations then were enumerated based on the percentage. *P < 0.05.
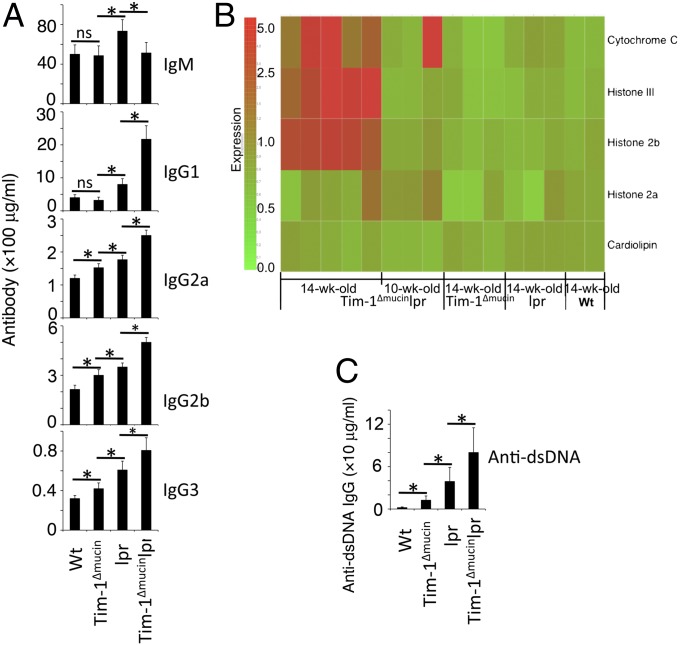
Tim-1Δmucinlpr mice also had a greater accumulation of B cells than lpr mice, whereas lpr mice had more B cells than Tim-1Δmucin mice (Fig. 4_C_). Lpr mice had higher serum levels of IgM, IgG1, IgG2a, and IgG3 than either WT or Tim-1Δmucin mice, whereas Tim-1Δmucinlpr mice produced higher levels of IgG1, IgG2a, IgG2b, and IgG3 but lower levels of IgM than lpr mice (Fig. 5_A_). Using a lupus-associated autoantigen microarray (35), we found that serum levels of autoantibodies to three antigens (cytochrome C, histone III, and histone 2b) out of 39 lupus-associated autoantigens were increased significantly in all Tim-1Δmucinlpr mice at the age of 14 wk but not in any of the age-matched lpr, Tim-1Δmucin, or WT mice (Fig. 5_B_). In fact, some of the Tim-1Δmucinlpr mice at 10 wk of age already showed increased autoantibodies to cytochrome C (Fig. 5_B_). At 10+ months of age, Tim-1Δmucinlpr mice had the highest levels of anti-dsDNA autoantibodies, followed by lpr mice, Tim-1Δmucin mice, and then WT mice (Fig. 5_C_). In addition to increased anti-dsDNA autoantibodies, Tim-1Δmucin mice also developed antibodies against other lupus-associated autoantigens including anti-Sm and anti-histone, among others (Fig. S2).
Fig. 5.
Increased serum levels of Ig and anti-dsDNA autoantibodies in Tim-1Δmucinlpr mice. (A) Levels of different Igs in sera from >10-mo-old WT (n = 8), Tim-1Δmucin (n = 12), lpr (n = 10), and Tim-1Δmucinlpr (n = 15) mice were determined by ELISA. (B) Heat map of lupus-associated autoantigen microarray. Serum IgG reactivity to 5 of 39 lupus-associated autoantigens is shown. Other lupus-associated autoantigens showed no significant difference among the groups of mice. (C) Levels of anti-dsDNA IgG in sera from A were determined by ELISA. *P < 0.05.
Taken together, these data suggested that the combination of Tim-1Δmucin and Fas mutation strongly promoted autoimmunity which was much more severe than that caused by Tim-1Δmucin or Fas mutation alone.
Discussion
In the present study, we generated Tim-1Δmucin mice and studied the effect of the loss of the mucin domain in Tim-1–regulated immune responses. We found that the major defect in Tim-1Δmucin mice was impaired IL-10 production in B cells and that this impairment became more severe with age. Associated with the loss of IL-10 production from B cells, Tim-1Δmucin mice older than 10 mo of age developed autoimmune disease characterized mainly by hyperactivated T cells, increased Th1 responses, activated DCs, and elevated serum Ig and many lupus-associated autoantibodies including anti-dsDNA autoantibodies. When introduced into Fas-mutant lpr mice, Tim-1Δmucin accelerated and worsened systemic autoimmunity as observed in lpr mice.
Although Tim-1 expression has been reported on activated T cells, where it regulates T-cell activation (24), and on DCs, where it affects DC function (36), Tim-1 also is expressed on B cells (16). A recent study has suggested that Tim-1 may be a better marker for IL-10–producing Bregs (32). Indeed, we confirmed that ∼10% of B cells in naive mice expressed Tim-1, and IL-10 was produced almost exclusively by Tim-1+ B cells. CD5+CD1dhi have been shown to be the markers for IL-10–producing Bregs (30), and indeed Tim-1 is coexpressed with these markers on B cells, raising the question of whether Tim-1 is functionally important for Bregs. Our data support this notion, because the expression of mucinless Tim-1 in Tim-1Δmucin mice affected IL-10 production from B cells as the mice aged. Also, we found that Rag1−/− hosts cotransferred with T cells and Tim-1Δmucin B cells developed more severe experimental autoimmune encephalitis (EAE) associated with enhanced pathogenic Th1 and Th17 responses than mice cotransferred with T cells and WT B cells (Fig. S3). This result further supports the notion that Tim-1 is functionally important for Bregs.
B cells generally are considered to act as positive regulators of immune responses by promoting antigen presentation for optimal T-cell activation and by producing antibodies, but now it is clear that Bregs are essential for inducing immune tolerance by negatively regulating immune responses via IL-10 production (30). Lack or loss of Bregs accelerates and exacerbates many autoimmune and inflammatory diseases, including EAE, chronic colitis, arthritis, type 1 diabetes, lupus, and contact hypersensitivity. In many models, IL-10 appears to be critical for Breg activity, although other mechanisms in addition to IL-10 production also might be operational for the regulatory function of Bregs. Impaired IL-10 production in Bregs is the major defect in Tim-1Δmucin mice, and the appearance of systemic autoimmunity correlates with the progressive loss of IL-10 production only in B cells, suggesting that deficient IL-10 production by Bregs is most likely the cause for the development of systemic autoimmune disease in these mice. It is also possible, however, that IL-10 is a functional marker defining the Bregs and that there are additional defects in the Tim-1–mutant Bregs that are regulated by expression of Tim-1 and are responsible for suppressive activity. It will be of interest to determine whether transfer of WT Tim-1+ B cells into Tim-1Δmucin mice reverses the systemic autoimmunity observed in the Tim-1Δmucin mice. In fact, a recent study has shown that transfer of Tim-1+ B cells led to long-term acceptance of islet allografts and inhibited allergic airway disease (32), supporting previous reports that transfer of Bregs inhibited autoimmunity in animal models of many autoimmune diseases, including lupus (30, 37).
These observations also raise the issue of whether Tim-1 is required for the expansion/maintenance of Bregs or simply regulates IL-10 and other effector functions of Bregs. Our data suggest that impaired IL-10 production in Tim-1Δmucin Bregs is not caused by a decrease in the number or percentage of Bregs, because the total number or frequency of Tim-1+ B cells in Tim-1Δmucin mice did not decrease but rather increased with age. This result suggests that Tim-1 regulates IL-10 production and other effector functions but not their overall frequency and number. Consistent with these data, we observed that addition of one of our monoclonal anti-Tim-1 Ab increased IL-10 production from B cells isolated from naive WT mice but not from the Tim-1Δmucin B cells (Fig. S4). Furthermore, loss of IL-10 production seems to be an inherent defect in Tim-1Δmucin B cells, because Tim-1Δmucin B cells produced significantly less IL-10 than WT B cells in the same Rag−/− hosts reconstituted with 1:1 mixed WT and Tim-1Δmucin bone marrow cells (Fig. S5). These data would suggest that Tim-1 signaling is required for the induction and/or maintenance of IL-10 and possibly for other regulatory functions in Bregs.
Tim-1 has been shown to be a receptor of Tim-4 (18) and phosphatidylserine exposed on apoptotic cells (4). Interestingly, although WT and Tim-1Δmucin B cells had similar levels of binding for Tim-4 (Fig. S6_A_), Tim-1Δmucin–derived cells showed a defect in binding and uptake of apoptotic cells (Fig. S6_B_), suggesting that deletion of the mucin domain affects Tim-1 binding to phosphatidylserine-positive apoptotic cells but does not affect Tim-1 binding to Tim-4. Because binding and engulfment of apoptotic cells during monocyte activation increased their IL-10 production (38), one possible mechanism by which Tim-1 maintains Breg IL-10 production in the hosts may be through the interaction of Tim-1 with apoptotic cells, which may mediate persistent signaling through Tim-1 to maintain or induce IL-10 production. In this regard, apoptotic cells also have been shown to promote Bregs, which inhibited autoimmune inflammation in an IL-10–dependent manner (39). Tim-1 is expressed on IL-10–producing B cells in the naive state but is expressed on T cells only after activation, and this fact correlates with the defect in IL-10 production only in B cells in Tim-1Δmucin mice.
We recently have found that Tim-1 is expressed on DCs and that DCs activated by Tim-1 signaling enhance T-cell responses (36). Consistently, Tim-1Δmucin DCs became more activated in aged mice, and Tim-1Δmucin DCs also promoted effector T-cell responses. These results may suggest that mutant Tim-1 in Tim-1Δmucin DCs may have a cell-intrinsic role in activating DCs, but it also is possible that the DC activation observed in the Tim-1Δmucin mice is secondary to the defect in Bregs.
Tim-1Δmucin mice showed impaired Breg IL-10 production and development of systemic autoimmunity with age; however, no significant defects have been reported in Tim-1−/− mice in which the full-length molecule was deleted (16). Because Tim-2, expressed in mouse, is homologous to Tim-1, we hypothesize that Tim-2 may have compensated for the biological function in the complete absence of Tim-1. Tim-2 also is expressed in both T and B cells and has been shown to regulate Th2 responses (40). However, in Tim-1Δmucin mice, the IgV domain for ligand binding is preserved, and Tim-2 expression is not affected (Fig. S7). This difference may contribute to the dramatic effects observed in the Tim-1Δmucin mice, which are not seen in the Tim-1–knockout mice.
When these results are taken together, expression of Tim-1 appears to be crucial in maintaining effector functions in IL-10–producing B cells. Breg functions are compromised in the presence of mutant Tim-1 that does not contain the mucin domain, and this compromise is associated with the development of autoimmunity. Consistent with the genetic linkage data, our data also underscore the value of the mucin domain in Tim-1 function and in regulating immune responses. Manipulation of the Tim-1 mucin domain without altering ligand binding to the IgV domain may provide a valuable target for regulating autoimmunity and transplant rejection.
Materials and Methods
The Tim-1Δmucin mouse was generated on C57BL/6 background. All mouse experiments were performed according to the animal protocol guidelines of Harvard Medical School. Detailed materials and methods can be found in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. Mohamed Oukka for designing the Tim-1 mutant strategy and Deneen Kozoriz for cell sorting. This work was supported by research Grant RG-3996-A-11 from the National Multiple Sclerosis Society (to V.K.K.) and by National Institutes of Health Grants R01NS045937, R01NS035685, R37NS030843, R01AI044880, P01AI039671, P01NS038037, and P01AI073748 (to V.K.K.), K01DK090105 (to S.X.), and R01DK39773 and R01DK072381 (to J.V.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: Emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 4.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol Lett. 2011;141:28–35. doi: 10.1016/j.imlet.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.McIntire JJ, Umetsu DT, DeKruyff RH. TIM-1, a novel allergy and asthma susceptibility gene. Springer Semin Immunopathol. 2004;25:335–348. doi: 10.1007/s00281-003-0141-3. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein E, et al. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin-and mucin-like regions. J Virol. 2003;77:8765–8774. doi: 10.1128/JVI.77.16.8765-8774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson P, Lu J, Kaplan GG. The Cys-rich region of hepatitis A virus cellular receptor 1 is required for binding of hepatitis A virus and protective monoclonal antibody 190/4. J Virol. 1998;72:3751–3761. doi: 10.1128/jvi.72.5.3751-3761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntire JJ, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 11.McIntire JJ, et al. Immunology: Hepatitis A virus link to atopic disease. Nature. 2003;425:576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 12.Umetsu DT, Dekruyff RH. Regulation of tolerance in the respiratory tract: TIM-1, hygiene, and the environment. Ann N Y Acad Sci. 2004;1029:88–93. doi: 10.1196/annals.1309.012. [DOI] [PubMed] [Google Scholar]
- 13.Umetsu DT, Umetsu SE, Freeman GJ, DeKruyff RH. TIM gene family and their role in atopic diseases. Curr Top Microbiol Immunol. 2008;321:201–215. doi: 10.1007/978-3-540-75203-5_10. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, et al. A polymorphism in TIM1 is associated with susceptibility to severe hepatitis A virus infection in humans. J Clin Invest. 2011;121:1111–1118. doi: 10.1172/JCI44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlow JL, Wong SH, Ballantyne SJ, Jolin HE, McKenzie AN. Tim1 and Tim3 are not essential for experimental allergic asthma. Clin Exp Allergy. 2011;41:1012–1021. doi: 10.1111/j.1365-2222.2011.03728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SH, Barlow JL, Nabarro S, Fallon PG, McKenzie AN. Tim-1 is induced on germinal centre B cells through B-cell receptor signalling but is not essential for the germinal centre response. Immunology. 2010;131:77–88. doi: 10.1111/j.1365-2567.2010.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanishi Y, et al. TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med. 2010;207:1501–1511. doi: 10.1084/jem.20090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers JH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Manzanet R, et al. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706–4713. doi: 10.4049/jimmunol.180.7.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 23.Santiago C, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umetsu SE, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 25.Xiao S, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crispín JC, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 31.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 32.Ding Q, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen PL, Eisenberg RA. Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 35.Wu HY, Quintana FJ, Weiner HL. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J Immunol. 2008;181:6038–6050. doi: 10.4049/jimmunol.181.9.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao S, et al. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur J Immunol. 2011;41:1539–1549. doi: 10.1002/eji.201040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe R, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 39.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarti S, et al. Tim-2 regulates T helper type 2 responses and autoimmunity. J Exp Med. 2005;202:437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information