Cigarette Smoking Predicts Differential Benefit from Naltrexone for Alcohol Dependence (original) (raw)
. Author manuscript; available in PMC: 2013 Nov 15.
Abstract
Background
Identifying factors that modify responsiveness to pharmacotherapies for alcohol dependence is important for treatment planning. Cigarette smoking predicts more severe alcohol dependence and poorer treatment response in general. Nevertheless, there is limited research on cigarette smoking as a potential predictor of differential response to pharmacological treatment of alcoholism.
Methods
We examined the association between cigarette smoking and drinking outcomes in the COMBINE study, a randomized, double-blind placebo-controlled 16-week trial comparing combinations of medications (i.e., acamprosate and naltrexone) and behavioral interventions (i.e., medical management (MM), combined behavioral therapy (CBI)) in 1383 alcohol dependent individuals.
Results
Smokers (i.e., more than half the sample) significantly differed from nonsmokers on several demographic and drinking-related variables at baseline and generally had poorer treatment outcomes than nonsmokers. However, smokers who received naltrexone had better drinking outcomes than smokers who received placebo, whereas alcohol use among nonsmokers did not vary by naltrexone assignment. This pattern of findings occurred independent of whether patients received CBI or MM and remained after controlling for alcoholism typology and baseline demographic differences. Approximately 9% of smokers quit smoking and an additional 10% reduced their cigarette intake during treatment. Reductions in smoking did not vary by treatment assignment.
Conclusions
These results suggest that naltrexone may be particularly beneficial for improving alcohol use outcomes in alcohol dependent smokers.
Trial Registration
The COMBINE Study, NCT000626, http://www.cscc.unc.edu/combine/.
Keywords: Alcohol dependence, cigarette smoking, pharmacotherapy, naltrexone, acamprosate, behavioral treatment
Introduction
Current pharmacotherapies for alcohol dependence have small to moderate effects on drinking(1,2). Consequently, there is growing interest in identifying factors that predict optimal responsiveness to these interventions(3). Some identified predictors such as alcoholism typology and OPRM1 genotype are complex(4-7). Typology is derived from a multidimensional assessment of alcoholism vulnerability, severity, and consequences, but may be an impractical treatment matching strategy in clinical settings(8). Likewise, the functional polymorphism of the OPRM1 genotype may infrequently occur among subgroups of alcoholics(9) and genetic testing may not be readily available to many clinicians. In contrast, cigarette smoking, a robust predictor of alcohol dependence and severity and an important potential moderator, is easy to assess and common among alcohol dependent individuals(10-11). Moreover, cigarette smoking screening is already a recommended practice for healthcare providers(12) and could identify alcoholics who might preferentially benefit from a particular treatment approach.
An estimated 45% of alcohol dependent individuals smoke cigarettes(10). Cigarette smoking and higher nicotine dependence levels at the start of inpatient behavioral treatment for alcoholism are associated with greater urge to drink, an increased risk of relapsing, and a greater number of drinks consumed upon relapse(13-14). Research on the potential moderating role of cigarette smoking on response to alcohol pharmacotherapies, including naltrexone and acamprosate, however, is limited.
Naltrexone reduces heavy drinking among hazardous drinkers receiving naltrexone for smoking cessation treatment(15-16). Nevertheless, it remains to be determined whether naltrexone’s effect on alcohol use varies by smoking status among alcohol dependent individuals seeking to reduce or stop drinking. Results from a secondary analysis of the U.S. Acamprosate trial found that alcohol dependent smokers were less likely to achieve alcohol abstinence than alcohol dependent nonsmokers irrespective of whether they received acamprosate or placebo(17).
Furthermore, identifying pharmacotherapies that could concurrently reduce both cigarette smoking and heavy drinking in this population would be valuable. Prior research suggests that reductions in cigarette smoking during alcohol treatment may promote better drinking outcomes(18-19). There is little evidence that naltrexone reduces smoking among alcoholics(20-21) and the potential effect of acamprosate on smoking has not been investigated.
The COMBINE study, the largest multi-site, randomized placebo-controlled pharmacotherapy trial for alcoholism, tested the efficacy of naltrexone and acamprosate along with 2 levels of behavioral therapy(22). The main results demonstrated moderate efficacy on drinking outcomes for naltrexone in combination with medical management and no efficacy for acamprosate. The study provides a unique opportunity to examine smoking status as a potential moderator of alcohol treatment efficacy across several interventions and the potential efficacy of these interventions on smoking. We hypothesized that smokers would exhibit differential benefit from naltrexone relative to placebo for several reasons. Compared with nonsmokers, smokers are more likely to report strong cravings to drink and are at greater risk of relapsing during treatment with behavioral alcohol interventions(13-14). Naltrexone has been shown to attenuate crucial components of alcohol dependence such as cue-induced craving and the amount of alcohol consumed following a lapse(23). Thus, naltrexone may be particularly helpful for reducing drinking and drinking-related problems among alcohol dependent smokers. Based on existing literature, we did not anticipate that smoking status would moderate acamprosate efficacy(17). Lastly, we explored the potential effect of naltrexone on smoking among this sample of alcohol dependent smokers who were not attempting to quit smoking.
Methods and Materials
Design and Procedures
In the COMBINE study, participants across 11 sites were randomly assigned to 1 of 9 groups. Eight groups received medical management (MM) with 16 weeks of naltrexone (100 mg/d) or acamprosate (3 g/d), both, and/or both placebos, with or without a combined behavioral intervention (CBI). A ninth group (cell 9) received CBI alone with no pills.
Participants completed research assessments at baseline and Weeks 1, 2, 4, 6, 8, 10, 12, 16 (end of treatment), 26, 52, and 68. Medications were provided under double-blind conditions: (1) naltrexone and matching placebo as 2 pills daily; (2) acamprosate and matching placebo as 2 pills 3 times daily. Participants assigned to MM attended an initial session and then 8 subsequent sessions over 16 weeks. MM visits with a study physician/nurse practitioner focused on medication side effects, adverse events, medication adherence, drinking, and self-help group attendance. CBI incorporated cognitive-behavioral skills training, motivational enhancement techniques, community reinforcement, and encouragement of self-help attendance. Session number (up to 20 over 16 weeks) and content was flexible and determined by the study therapist in collaboration with the participant.
Participants
Thirteen hundred and eighty three abstinent alcohol dependent volunteers were enrolled. Key eligibility criteria included: (1) DSM-IV alcohol dependence diagnosis; (2) 4-21 days of alcohol abstinence prior to randomization; and (3) ≥ 2 heavy drinking days and an average of ≥ 21 (for men) or ≥ 14 (for women) drinks per week during a 30-day consecutive period within 90 days of intake. Individuals were excluded for psychiatric illness requiring pharmacotherapy, medical contraindications, or a substance use disorder other than nicotine or cannabis.
Treatment conditions were equivalent on 76 pretreatment characteristics(22). Participants who received either naltrexone or CBI without naltrexone demonstrated a shorter time to the first heavy drinking day and a greater percentage of days abstinent compared with participants who received MM + placebo.
More detail about the study, including the CONSORT diagram, is available in the original paper(22).
Measures
Cigarette Smoking
Smoking status, number of days smoked, and number of cigarettes smoked per day were measured at baseline and Weeks 8 and 16 using the Form-90 Interview(24), a semi-structured interview of alcohol and drug use and related behaviors within the past 90 days that integrates the Timeline Followback Interview(25) calendar method to reconstruct daily use, weekly patterns, and atypical episodes of drinking. The following categorical smoking variables were derived from this data: (1) smoking status at baseline and Week 16 (i.e., 0 = non-smoker, 1 = non-daily smoker, 3 = daily smoker); (2) quit smoking by Week 16 (i.e., 1 = yes, 0 = no); (3) initiated smoking by Week 16 (i.e., 1 = yes, 0 = no); and (4) changes in smoking quantity from baseline to Week 16 (i.e., 0 = decreased, 1 = stayed constant, 2 = increased). Consistent with prior research on smoking transitions in the Project MATCH trial(26), smokers whose intake changed by ≤ 5 cigarettes were considered to have stayed constant.
Frequency and Quantity of Drinking
Daily drinking data was measured at baseline and at Weeks 1, 2, 4, 6, 8, 10, 12, and 16 using the Timeline Followback Interview (TLFB)(25). In this analysis we examined percentage of heavy drinking days (PHDD), percentage of days abstinent from drinking (PDA), and number of drinks per drinking day (DPDD) from baseline through the first 8 weeks of treatment (i.e., Week 8) and from Week 8 through the end of treatment (i.e., Week 16) to reflect the assessment periods available for the smoking measures. We also evaluated time to first drinking relapse during the entire 16-week treatment period.
Drinking Consequences
The 50-item Drinker Inventory of Consequences (DrInC) questionnaire was used to assess adverse consequences of drinking at baseline and Weeks 8 and 16. For this investigation, we used the total drinking consequences score(27).
Clinical Outcome
A secondary dichotomous drinking outcome variable at Week 16 was derived in which a good clinical outcome was defined as abstinence or moderate drinking without drinking-related problems. Participants who did not meet this criterion were classified as having a bad clinical outcome (i.e., 1 = good clinical outcome, 0 = bad clinical outcome). Moderate drinking was defined as ≤ 11 (women) or ≤ 14 (men) drinks per week, with ≤ 2 days of consuming 3 drinks (women) or 4 drinks (men). Drinking problems were defined as endorsing ≥ 3 items on the DrInC. This variable was used in the main COMBINE study(22).
Alcohol Dependence
The Structured Clinical Interview for DSM-IV Axis I Disorders(28) assessed history of alcohol abuse, dependence/abuse, and age of onset at baseline. Alcohol dependence severity was also measured using the Alcohol Dependence Scale(29), a self-report scale of alcohol withdrawal symptoms, tolerance, impaired control over drinking, compulsion to drink, and drink-seeking behavior yielding a total severity score.
Demographics
A baseline questionnaire, designed for this study, assessed marital status, employment status, education history, age, gender, race, and ethnicity.
Alcoholism Typology
Participants were categorized as either Type A or Type B alcoholics based on prior COMBINE study analyses(5). In that analysis, typology coding was based on composite variables derived from baseline assessments for: (1) standard drinks per drinking day, (2) drinking for withdrawal relief, (3) alcohol-related medical conditions, (4) physical, social, and interpersonal drinking consequences, and (5) onset of alcoholism, family history risk, and comorbid psychopathology. Compared to Type A alcoholics in the COMBINE study, Type B’s were younger, less educated, more likely to be single and male, and reported an earlier onset, more severe dependence, a greater family history risk, and more childhood risk factors. Type A’s had better drinking outcomes with naltrexone than placebo(5). Type B alcoholics, however demonstrated no advantage of naltrexone(5).
Statistical Analyses
The investigation examined the following during the 16-week treatment period: (1) the effect of baseline smoking status on drinking outcomes; (2) the interaction between baseline smoking status and pharmacotherapy treatment assignment (naltrexone and acamprosate) on drinking outcomes; (3) the effect of pharmacotherapy treatment assignment on changes in cigarette smoking. We examined percentage of days abstinent from drinking (PDA) and percentage of heavy drinking days (PHDD) as primary alcohol use outcomes. Secondary alcohol use outcomes included drinking consequences, time to first heavy drinking relapse, drinks per drinking day (DPDD), and the likelihood of achieving a good clinical outcome (GCO) by the end of treatment. Primary outcomes in the COMBINE study included PDA and time to first drinking relapse(22). We prioritized PHDD as an outcome over time to first heavy drinking relapse because the latter ignores all other data after the relapse time point. Analyses were limited to the 16-week treatment period because the primary focus of this investigation was to examine the relationship between smoking and drinking in response to pharmacotherapy treatment for alcoholism. For this same reason, participants assigned to cell 9 (i.e., CBI only, no pills) were excluded from the analyses (n = 157). Analyses are based on the reduced sample of 1226 participants.
T-tests and chi-square analyses evaluated differences between smokers and nonsmokers on alcohol-related measures and demographic variables at baseline and the likelihood of completing treatment. Logistic regression analyses determined the likelihood of quitting smoking, initiating smoking, and changing smoking frequency (i.e., switching from daily to non-daily smoking or vice versa) or smoking quantity by treatment assignment. Repeated measures linear regression analyses fitted using generalized estimating equations (GEE) models examined the independent and combined effects of baseline smoking status and treatment assignment on drinking outcomes over the 16-week treatment period(30). We further probed naltrexone assignment by smoking status interactions in GEE models using two post hoc comparisons: (1) among smokers – naltrexone vs. placebo and (2) among nonsmokers – naltrexone vs. placebo. The alpha level was set to .025 to adjust for these multiple comparisons. Cox regression analysis determined the independent and combined effects of baseline smoking status and treatment assignment on time to first drinking relapse. Logistic regression tested the independent and combined effects of baseline smoking status and treatment assignment on the likelihood of achieving a GCO at Week 16(30).
Additional GEE, Cox regression, and logistic regression models were fitted controlling for significant baseline demographic differences by smoking status. All possible demographic by naltrexone assignment interactions and demographic main effects were considered. Backward elimination was used to drop non-significant interactions and main effects of demographic variables from the models under the restriction that the models were hierarchically well formulated at each step. Separate GEE, Cox regression, and logistic regression models controlling for alcoholism typology and the interaction of typology and naltrexone assignment were also fitted. Typology was not included in the models controlling for demographic variables due to the significant overlap between typology and demographic characteristics. All GEE, Cox, regression, and logistic regression analyses produced the Wald chi square statistic. All analyses were conducted using SPSS version 17.0.
Results
Baseline Smoking Status
Fifty-five percent of participants identified themselves as smokers (n = 673) and reported smoking a mean of 16.99 ± 12.29 cigarettes per day. Daily smokers, (n = 442; 66% of smokers; 36% of total sample) reported smoking 20.44 ± 11.74 cigarettes per day. Non-daily smokers (n= 231; 44% of smokers; 19% of total sample) reported smoking 10.26 ± 10.44 cigarettes per day.
Baseline Differences between Smokers and Nonsmokers
Smokers significantly differed from nonsmokers on several demographic and drinking-related variables at baseline (see Table 1). Compared with nonsmokers, smokers were significantly younger, completed fewer years of education, and were more likely to be male, unmarried, and of a racial/ethnic minority. Smokers were also more severely alcohol dependent and more likely to be diagnosed with an alcohol use disorder at an earlier age. They also reported more drinking consequences and a higher percentage of abstinent days prior to commencing treatment than nonsmokers. A greater proportion of smokers were identified as Type B alcoholics than nonsmokers.
Table 1. Demographic and Clinical Characteristics by Baseline Smoking Status.
| Smoking Status | |||
|---|---|---|---|
| Characteristics | Nonsmokers(n = 548) | Smokers(n = 673) | Test Statistic |
| Age [M ± SD] | 47.89 ± 9.88 | 41.39 ± 9.43 | t (1219) = 11.72p < .001 |
| Gender [n (%)] | |||
| Male | 356 (65) | 489 (73) | χ2 (1) = 8.37 |
| Female | 192 (35) | 184 (27) | p = .004 |
| Marital Status [n (%)] | |||
| Single | 250 (46) | 457 (68) | χ2 (1) = 62.37 |
| Married | 298 (54) | 215 (32) | p < .001 |
| Race/Ethnicity [n (%)] | |||
| Racial/Ethnic Minority | 99 (18) | 186 (28) | χ2 (1) = 15.71 |
| Caucasian | 449 (82) | 487 (72) | p < .001 |
| Years of Education [M ± SD] | 15.44 ± 2.78 | 13.86 ± 2.46 | t (1196) = 10.47p < .001 |
| Alcohol Dependence Scale Score [M ±SD)] | 15.26 ± 7.28 | 17.87 ± 7.20 | t (1215) = -6.25p < .001 |
| Age Diagnosed with Alcohol UseDisorder [M ± SD] | 33.18 ± 11.96 | 28.61 ± 10.43 | t (1051) = 6.59p < .001 |
| Drinking Consequences [M ± SD] | 42.33 ± 19.19 | 52.39 ± 20.29 | t (1218) = -8.83p < .001 |
| % Abstinent Days [M ± SD] | 21.94 ± 24.08 | 28.14 ± 25.45 | t (1219) = -4.43p < .001 |
| Alcoholism Typology [n (%)] | |||
| Type A | 348 (66) | 385 (60) | χ2 (1) = 5.00 |
| Type B | 179 (34) | 260 (40) | p = .03 |
Baseline Smoking as a Predictor of Alcohol Treatment Outcomes
Smoking status was significantly associated with treatment retention and drinking outcomes. Compared with nonsmokers, smokers were more likely to withdraw prematurely from treatment [23.3% vs. 17.4%; χ2 (1) = 6.49, p =.01]. Smokers also reported higher PHDD [17.45 ± 1.02 vs. 14.16 ± 1.04; χ2 (1) = 5.13, p =.02], greater drinking consequences [15.54 ± 0.76 vs. 9.59 ± 0.59; χ2 (1) = 38.10, p <.001], and greater DPDD [5.19 ± .20 vs. 4.01 ± .21; χ2 (1) = 16.09, p <.001] during treatment.
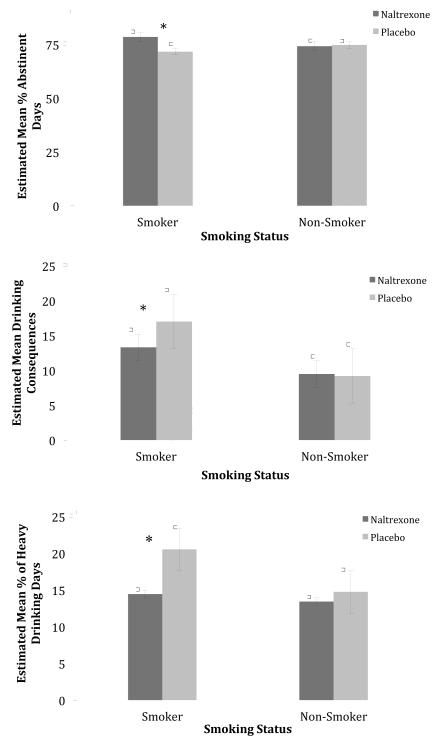
As shown in Table 2, the first column of data, baseline smoking status significantly interacted with naltrexone assignment on PDA and drinking consequences [p’s <.05]. Among smokers, those who received naltrexone reported significantly higher PDA [M = 78.36, SE = .97 vs. M = 71.65, SE = 1.80; Mean Diff = 6.71, SE = 2.32, p =.004] and significantly lower drinking consequences [M =13.57, SE =1.04 vs. M = 17.50, SE = 1.10; Mean Diff = 3.93, SE = 1.52, p =.01] than those who received placebo (see Figure 1). Nonsmokers, however, reported similar PDA [M =74.03, SE = 2.03 vs. M = 74.64, SE 1.80; p =.82] and drinking consequences [M = 9.69, SE = .89 vs. M = 9.49, SE =.78; p =.86] regardless of naltrexone assignment.
Table 2. Summary of Smoking Status x Naltrexone Assignment in Models with and without Controlling for Demographics and Alcoholism Typology1.
| Model Adjusted for: | |||
|---|---|---|---|
| Unadjusted | Demographics | Typology | |
| % Days Abstinent | Naltrexone: χ2 (1) =2.92, p =.09 | Naltrexone: χ2 (1) =3.49, p =.06 | Naltrexone: χ2 (1) =2.38, p =.12 |
| Smoking: χ2 (1) =.14, p =.71 | Smoking: χ2 (1) =.37, p =.54 | Smoking: χ2 (1) =.00, p =.99 | |
| Nalt x Smok: χ2 (1) =4.20, p =.04* | Nalt x Smok: χ2 (1) =4.81, p =.03* | Nalt x Smok: χ2 (1) =4.00, p =.045* | |
| % Heavy Drinking Days | Naltrexone: χ2 (1) =5.98, p =.01* | Naltrexone: χ2 (1) =3.00, p =.08 | Naltrexone: χ2 (1) =3.99, p <.05* |
| Smoking: χ2 (1) =5.13, p =.02* | Smoking: χ2 (1) =.86, p =.35 | Smoking: χ2 (1) =5.15, p =.02* | |
| Nalt x Smok: χ2 (1) =2.78 p =.096 | Nalt x Smok: χ2 (1) =5.62, p =.02* | Nalt x Smok: χ2 (1) =3.06, p =.08 | |
| Drinking Consequences | Naltrexone: χ2 (1) =3.74, p =.05 | Naltrexone: χ2 (1) =3.87, p <.05* | Naltrexone: χ2 (1) =1.04, p =.31 |
| Smoking: χ2 (1) =38.10, p <.001*** | Smoking: χ2 (1) =17.50, p <.001*** | Smoking: χ2 (1) =36.37, p <.001*** | |
| Nalt x Smok: χ2 (1) =4.62, p =.03* | Nalt x Smok: χ2 (1)=4.80, p =.03* | Nalt x Smok: χ2 (1)=4.28, p =.04* | |
| Time to Relapse | Naltrexone: χ2 (1) =3.71, p =.05 | Naltrexone: χ2 (1) =3.01, p =.08 | Naltrexone: χ2 (1) =1.69, p =.19 |
| Smoking: χ2 (1) =.01, p =.93 | Smoking: χ2 (1) =.05, p =.83 | Smoking: χ2 (1) =.03, p =.87 | |
| Nalt x Smok: χ2 (1) =2.89, p =.09 | Nalt x Smok: χ2 (1) =1.99, p =.16 | Nalt x Smok: χ2 (1) =2.87, p =.09 | |
| Drinks per Drinking Day | Naltrexone: χ2 (1) =3.08, p =.08 | Naltrexone: χ2 (1) =2.99, p =.08 | Naltrexone: χ2 (1) =2.39, p =.12 |
| Smoking: χ2 (1) =16.09, p <.001*** | Smoking: χ2 (1) =.78, p =.38 | Smoking: χ2 (1) =13.56, p <.001*** | |
| Nalt x Smok: χ2 (1) =1.32, p =.25 | Nalt x Smok: χ2 (1)=2.82, p =.09 | Nalt x Smok: χ2 (1)=1.71, p =.19 | |
| Odds of Good Clinical Outcome | Naltrexone: χ2 (1) =8.53, p =.004** | Naltrexone: χ2 (1) =.07, p =.80 | Naltrexone: χ2 (1) =.83, p =.36 |
| Smoking: χ2 (1) =1.36, p =.24 | Smoking: χ2 (1) =.38, p =.54 | Smoking: χ2 (1) =1.37, p =.24 | |
| Nalt x Smok: χ2 (1) =.65, p =.42 | Nalt x Smok: χ2 (1) =.92, p =.34 | Nalt x Smok: χ2 (1) =.63, p =.43 |
Figure 1.
Interaction of Smoking Status and Naltrexone Assignment on Drinking Outcomes over Weeks 8 to 16 (Smoker + Naltrexone =700, Smoker + Placebo = 646, Nonsmoker + Naltrexone = 522, Nonsmoker + Placebo = 574)2
The interaction for PHDD was not significant at the p <.05 level [p =.096]. However, there was a significant mean difference in PHDD between smokers who received naltrexone and those who received placebo [Mean Diff = 5.98, SE = 2.03, p =.003]. Specifically, smokers on naltrexone reported significantly lower PHDD than smokers on placebo [M =14.46, SE =1.23 vs. M = 20.44, SE =1.62] (see Figure 1).
There was no significant interaction of baseline smoking status and naltrexone assignment on,time to relapse, number of drinks per drinking day, or the likelihood of achieving a GCO. Likewise, there were no significant interactions of smoking status and acamprosate assignment or smoking status and therapy assignment on drinking outcomes [p’s >.05].
As shown in Table 2, the second and third columns of data, a similar pattern of findings was obtained for models controlling for baseline demographic differences (i.e., gender, marital status, race, age, and years of education) and models controlling for alcoholism typology. Of note, the interaction of smoking status and naltrexone assignment on PDA and drinking consequences remained significant across all models [p’s <.05]. Other interactions between naltrexone and smoking status varied slightly in the models controlling for demographics or alcoholism typology. The interaction for PHDD became significant at an alpha level of .05 in the demographic model [p =.02] and was very similar in the typology model [p =.08]. For DPDD, the interaction between naltrexone and smoking status became stronger but remained nonsignificant [p = .08] when controlling for demographics. There were no significant interactions of smoking status and naltrexone assignment on the likelihood of a GCO in all models [p’s >.10].
Smoking as an Outcome of Alcohol Treatment
Nine percent of smokers quit smoking by the end of treatment (n = 60). In addition, roughly 16% of smokers (n = 111) reduced their cigarette intake on smoking occasions and approximately 13% of daily smokers (n = 50) reduced to non-daily smoking. Six percent of nonsmokers initiated smoking by the end of treatment (n = 33). Approximately 6% of smokers (n = 41) increased their cigarette intake on smoking occasions and roughly 31% of non-daily smokers (n = 60) increased to daily smoking during this period.
Rates of smoking cessation and initiation and changes in smoking quantity and frequency did not vary by treatment assignment [p’s >.10].
Discussion
This investigation examined cigarette smoking as a predictor and outcome of treatment in the COMBINE study, the largest multi-site, randomized placebo-controlled pharmacotherapy trial for alcoholism. Consistent with recent epidemiological studies(10), approximately 55% of the participants reported current smoking. These smokers had more severe alcohol dependence and alcohol related problems at baseline. In line with prior reports(13-14), smoking status was associated with poor response to alcohol treatment. Smokers drank more heavily and on more occasions and reported greater alcohol consequences than nonsmokers. Among smokers treated with naltrexone, however, these effects were attenuated. In fact, naltrexone was more efficacious in reducing alcohol consumption and drinking consequences among smokers than nonsmokers. Moreover, this benefit occurred whether or not participants received a specialized behavioral intervention or a primary model of care suggesting that smoking status can be used to identify candidates for naltrexone treatment for alcohol dependence in both settings.
The finding that smoking status moderates naltrexone response adds to the growing literature on individual characteristics that may predict naltrexone efficacy such as family history of alcoholism(6-7), age of onset of alcoholism(5-7), mu-opioid receptor gene variants(4,7), and alcoholism typology(5). In the present study, smoking status as a moderator of naltrexone response persisted even after controlling for demographic differences between smokers and nonsmokers and alcoholism typology, a factor previously shown to moderate naltrexone response in the COMBINE Study.
In clinical settings, assessing smoking status may be a more efficient method for matching alcohol dependent individuals to effective pharmacotherapy than more costly and time-consuming phenotyping and genotyping strategies. For instance, alcoholism typology is determined by administering, scoring, and interpreting hours worth of biopsychosocial assessments(5,8). In addition to providing an efficient, useful screen for potential alcohol misuse in general(11), assessing smoking status may be an important indicator of alcoholism severity and treatment responsiveness(31). Our data confirm prior findings that alcohol dependent smokers have a poorer prognosis than alcohol dependent nonsmokers(13-14). For the first time, however, we also show that naltrexone ameliorates this negative prognosis and decreases the frequency of drinking and drinking related consequences.
Smokers did not benefit differentially from acamprosate in this secondary analysis of the COMBINE Study, consistent with the results of a secondary analysis of the U.S. Acamprosate study(17).
Neither naltrexone nor acamprosate had an effect on smoking behavior. Moreover, only a small percentage of smokers successfully quit smoking or reduced their cigarette intake in response to either treatment. Given that smoking reductions may promote better alcohol treatment outcomes(26,32), more active interventions to promote smoking cessation will be needed in conjunction with these treatments. Evidence-based interventions to consider include telephone quit-lines or brief, motivational enhancement counseling and smoking cessation pharmacotherapies such as nicotine replacement therapies or varenicline(12,18). Of particular interest, combined nicotine patch and nicotine gum has evidence of efficacy among alcohol dependent patients(33) and varenicline may also reduce alcohol craving and drinking in addition to promoting smoking cessation(34-35). Topiramate, another medication with efficacy for reducing alcohol drinking, may also promote smoking reductions(20,36).
Several possible mechanisms may account for the finding that naltrexone was particularly beneficial for alcohol dependent smokers. First, smoking status may be a proxy for greater alcohol dependence and drinking-related problems(11). Though the research is somewhat mixed, some studies have shown better naltrexone response among alcoholics with greater severity or risk factors(6, 37-39). Second, smokers may expose themselves more to alcohol-related cues than nonsmokers and, in turn, nicotine may enhance the reinforcement efficacy of these cues(40-42). There is both pre-clinical and clinical evidence that naltrexone reduces cue-induced alcohol craving and alcohol-seeking behavior in response to cues(23). Third, adaptations of the endogenous opioid system play a role in both nicotine and alcohol addiction(43) and nicotine’s effects on other reinforcers may work through this mechanism(44). Naltrexone is an opioid antagonist(23).
It is worth noting that this study utilized a dose of naltrexone that is higher than the standard approved dose for alcohol dependence (i.e., 100 mg daily vs. 50 mg daily). Moreover, naltrexone is currently being used or investigated for a number of indications using lower doses (45-48). Whether smoking status modifies response to naltrexone for alcohol drinking at other doses deserves examination. In this regard, a prior placebo-controlled trial of naltrexone for smoking cessation found reductions in heavy drinking with 25 mg of naltrexone among smokers not seeking alcohol treatment(16).
There are limitations to the study. Differences in drinking outcomes in response to naltrexone by smoking status, though significant, were modest. Smoking behavior assessment was confined to self-report and there was no biochemical verification of smoking using carbon monoxide or cotinine levels. Similarly, smoking topography methods, which provide an objective measure of smoking behavior (i.e., puff volume, puff number, puff duration, average flow, interpuff interval)(49), were not employed to assess smoking exposure. Smokers may adjust their smoking by making subtle changes in their puffing behavior(50).
Combined alcohol and nicotine dependence represents a serious public health burden(51). Our data and that of others show that alcohol dependent smokers have more severe alcohol dependence and a poorer treatment prognosis than alcohol dependent nonsmokers(13-14,17). Therefore, smoking status may be an important prognostic indicator that can be used for treatment planning. Specifically, our findings suggest that alcohol dependent smokers are a patient group that is likely to benefit from naltrexone therapy for alcohol dependence. Interventions that could concurrently reduce smoking and drinking in this population warrant further investigation.
Acknowledgements
We acknowledge the COMBINE Study Research Group and Ran Wu, MS for her assistance with data management and analysis.
This research was supported in part by grants from the National Institute on Alcohol Abuse and Alcoholism: K05-AA014715 (SSO), R01-AA016621 (SSO, RG), K23-AA02000 (LMF), and T32-AA015496 (LMF).
The author(s) acknowledge(s) that the reported results are, in whole or in part, based on analyses of the COMBINE Data Set. These data were collected as part of a multisite clinical trial of alcoholism treatments supported by a series of grants from the National Institute on Alcohol Abuse and Alcoholism, NIH, DHHS. This paper has not been reviewed or endorsed by NIAAA or the COMBINE Research Group and does not necessarily represent the opinions of its members or NIAAA, who are not responsible for the contents.
Footnotes
2
Means are estimated means. Error bars are standard errors. P values reflect significant differences by naltrexone assignment among smokers: * p < .05. Maximum possible value for drinking consequences = 150.
Financial Disclosures
Dr. Stephanie O’Malley is a member American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative, sponsored by Eli Lilly, Janssen, Johnson & Johnson, Schering Plough, Lundbeck, Glaxo Smith Kline and Alkermes; partner, Applied Behavioral Research; contract, Nabi Biopharmaceuticals; medication supplies, Pfizer Inc; Advisory Board, Gilead Pharmaceuticals; consultant, GlaxoSmithKline, Lundbeck, Brown University; Scientific Panel of Advisors, Hazelden. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data from this project was presented at the 34th Annual Scientific Meeting of the Research Society on Alcoholism in Atlanta, GA on June 26, 2011.
References
- 1.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- 2.Pettinati HM, Rabinowitz AR. Choosing the right medication for the treatment of alcoholism. Curr Psychiatry Rep. 2006;8(5):383–388. doi: 10.1007/s11920-006-0040-0. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 4.Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogenschutz MP, Scott Tonigan J. Effects of alcoholism typology on response to naltrexone in the COMBINE study. Alcohol Clin Exp Res. 2009;33(1):10–18. doi: 10.1111/j.1530-0277.2008.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubio G, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J, Palomo T. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40(3):227–233. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- 7.Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr., McGeary JE, et al. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32(1):58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball S. Type A and type B alcoholism applicability across subpopulations and treatment settings. Alc Res Health. 1996;20(1):30–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4:476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 10.Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 11.McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG. Smoking status is a clinical indicator for alcohol misuse in US adults. Arch Intern Med. 2007;167(7):716–721. doi: 10.1001/archinte.167.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence, 2008 update. Clinical Practice Guideline. Public Health Service; USDHHS. Rockville, MD: 2008. [Google Scholar]
- 13.Abrams DB, Rohsenow DJ, Niaura RS, Pedrazac M, Longabaugh R, Beattiee MC, et al. Smoking and treatment outcome for alcoholics: effects on coping skills, urge to drink, and drinking rates. Behav Ther. 1992;23:283–297. [Google Scholar]
- 14.Hintz T, Mann K. Long-term behavior in treated alcoholism: Evidence for beneficial carry-over effects of abstinence from smoking on alcohol use and vice versa. Addict Behav. 2007;32(12):3093–3100. doi: 10.1016/j.addbeh.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 15.King A, Cao D, Vanier C, Wilcox T. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol Clin Exp Res. 2009;33(6):1044–1050. doi: 10.1111/j.1530-0277.2009.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, et al. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2009;12(5):589–597. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason BJ, Lehert P. Effects of nicotine and illicit substance use on alcoholism treatment outcomes and acamprosate efficacy. J Addict Med. 2009;3(3):164–171. doi: 10.1097/ADM.0b013e3181917d53. [DOI] [PubMed] [Google Scholar]
- 18.Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30(1):12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 20.Baltieri DA, Daro FR, Ribeiro PL, Andrade AG. Effects of topiramate or naltrexone on tobacco use among male alcohol-dependent outpatients. Drug Alcohol Depend. 2009;105(1-2):33–41. doi: 10.1016/j.drugalcdep.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Swift RM, Abrams DB. Naltrexone treatment for alcoholics: effect on cigarette smoking rates. Nicotine Tob Res. 2003;5(2):231–236. doi: 10.1080/1462220031000073298. [DOI] [PubMed] [Google Scholar]
- 22.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 23.O’Malley SS, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev Alc. 2003;16:217–245. [PubMed] [Google Scholar]
- 24.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58(4):358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB. Alcohol consumption measures. 2nd ed Bethesda, MD: 2003. [Google Scholar]
- 26.Friend KB, Pagano ME. Changes in cigarette consumption and drinking outcomes: findings from Project Match. J Subst Abuse Treat. 2005;29:221–229. doi: 10.1016/j.jsat.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller WR, Tonigan JS, Longabaugh R, editors. The drinker inventory of consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse. National Institutes of Health; Rockville, MD: 1995. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders Research Version. State Psychiatric Institute; New York: 2002. [Google Scholar]
- 29.Skinner H, Horn J. Alcohol Dependence Scale: User’s Guide. Addiction Research Foundation; Toronto: 1984. [Google Scholar]
- 30.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- 31.Schmidt LG, Smolka MN. Results from two pharmacotherapy trials show alcoholic smokers were more severely alcohol dependent but less prone to relapse than alcoholic non-smokers. Alcohol Alcohol. 2007;42(3):241–246. doi: 10.1093/alcalc/agm027. [DOI] [PubMed] [Google Scholar]
- 32.Friend KB, Pagano ME. Smoking cessation and alcohol consumption in individuals in treatment for alcohol use disorders. J Addict Dis. 2005;24(2):61–75. doi: 10.1300/J069v24n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, et al. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009;104(9):1588–1596. doi: 10.1111/j.1360-0443.2009.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215(4):655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch Intern Med. 2005;165(14):1600–1605. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- 37.Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, et al. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- 38.Rohsenow DJ, Miranda R, Jr, McGeary JE, Monti PM. Family history and antisocial traits moderate naltrexone’s effects on heavy drinking in alcoholics. Exp Clin Psychopharmacol. 2007;15:272–281. doi: 10.1037/1064-1297.15.3.272. [DOI] [PubMed] [Google Scholar]
- 39.Kiefer F, Helwig H, Tarnaske T, Otte C, Jahn H, Wiedemann K. Pharmacological relapse prevention of alcoholism: clinical predictors of outcome. Eur Addict Res. 2005;11:83–91. doi: 10.1159/000083037. [DOI] [PubMed] [Google Scholar]
- 40.Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating, and weight. Physiol Behav. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Löf E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, et al. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology. 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- 42.Chiamulera C. Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Rev. 2005;48:74–97. doi: 10.1016/j.brainresrev.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Trigo JM, García EM, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D, et al. Nicotine decreases food intake through activation of POMC neurons. Science. 2011;332(6035):1330–1332. doi: 10.1126/science.1201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frech T, Novak K, Revelo MP, Murtaugh M, Markewitz B, Hatton N, et al. Low-dose naltrexone for pruritus in systemic sclerosis. Int J Rheumatol. 2011;2011:804296. doi: 10.1155/2011/804296. Epub 2011 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72(3):333–337. doi: 10.1016/j.mehy.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 47.Mannelli P, Peindl K, Patkar AA, Wu LT, Tharwani HM, Gorelick DA. Problem drinking and low-dose naltrexone-assisted opioid detoxification. J Stud Alcohol Drugs. 2011;72(3):507–513. doi: 10.15288/jsad.2011.72.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith JP, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS. Low-dose naltrexone therapy improves active Crohn’s disease. Am J Gastroenterol. 2007;102(4):820–828. doi: 10.1111/j.1572-0241.2007.01045.x. Epub 2007 Jan 11. [DOI] [PubMed] [Google Scholar]
- 49.Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. doi: 10.1080/1462220031000158645. [DOI] [PubMed] [Google Scholar]
- 50.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology. 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 51.Pelucchi C, Gallus S, Garavello W, Bosetti C, La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alc Res Health. 2006;29(3):193–198. [PMC free article] [PubMed] [Google Scholar]