The catalytic sites of 20S proteasomes and their role in subunit maturation: A mutational and crystallographic study (original) (raw)
Abstract
We present a biochemical and crystallographic characterization of active site mutants of the yeast 20S proteasome with the aim to characterize substrate cleavage specificity, subunit intermediate processing, and maturation. β1(Pre3), β2(Pup1), and β5(Pre2) are responsible for the postacidic, tryptic, and chymotryptic activity, respectively. The maturation of active subunits is independent of the presence of other active subunits and occurs by intrasubunit autolysis. The propeptides of β6(Pre7) and β7(Pre4) are intermediately processed to their final forms by β2(Pup1) in the wild-type enzyme and by β5(Pre2) and β1(Pre3) in the β2(Pup1) inactive mutants. A role of the propeptide of β1(Pre3) is to prevent acetylation and thereby inactivation. A gallery of proteasome mutants that contain active site residues in the context of the inactive subunits β3(Pup3), β6(Pre7), and β7(Pre4) show that the presence of Gly-1, Thr1, Asp17, Lys33, Ser129, Asp166, and Ser169 is not sufficient to generate activity.
Proteasomes are essential, ubiquitous intracellular proteases that degrade a broad variety of cytoplasmic, nuclear, and membrane proteins that have been marked for degradation by the attachment of polyubiquitin chains (1–3). Eukaryotic proteasomes are large protein complexes with a molecular mass around 2,000 kDa, with a modular architecture (4, 5). The catalytic core of the molecule is the 20S proteasome, a cylindrical particle that consists of four heptameric rings made from seven different subunits each, which are present in two copies and in unique locations so that the particle has overall 2-fold symmetry (1, 4–7). The yeast 20S proteasome subunits fall into two different classes phylogenetically related to the two subunits α and β of the archaebacterial proteasome (8) and have been named accordingly (7). The α-subunits are not catalytically active and form antechambers to the central cavity of the 20S complex that is built from the β-subunits. In Thermoplasma acidophilum proteasomes, all β-subunits are transcribed and translated from one gene only and are expressed as precursors. In the process of particle maturation, all copies of the β-subunit become active, so that two rings of seven catalytic sites each are formed on the inner walls of the central chamber. The N-terminal threonine residue is exposed by this processing activity as the nucleophile in peptide bond hydrolysis (9, 10). It will subsequently be referred to as Thr1, thus assigning negative integers to residues of the propeptide. Based on the crystal structure of the T. acidophilum 20S proteasome, the distance between active site threonines was suggested as the molecular ruler that determines the length distribution of proteasome generated peptides (9).
A more complex picture for the mechanism of oligopeptide product generation was suggested by the crystal structure of the yeast 20S proteasome (7). It contains seven different α- and β-type subunits arranged in unique locations (Fig.1). Four β-type subunits are inactive because they contain either unprocessed [β3(Pup3) and β4(Pre1)] or intermediately processed propeptides [β6(Pre7) and β7(Pre4)]. The remaining three subunits β1(Pre3), β2(Pup1), and β5(Pre2) have N-terminal threonine residues, are active, and have specificities determined largely by the nature of their S1 pockets (7). Specific mutants of the active β-type subunits have been isolated (11). They allowed the identification of different substrate specificities (11, 12) of the proteasome and led to a hypothesis for an intermolecular processing mechanism of inactive β-subunits.
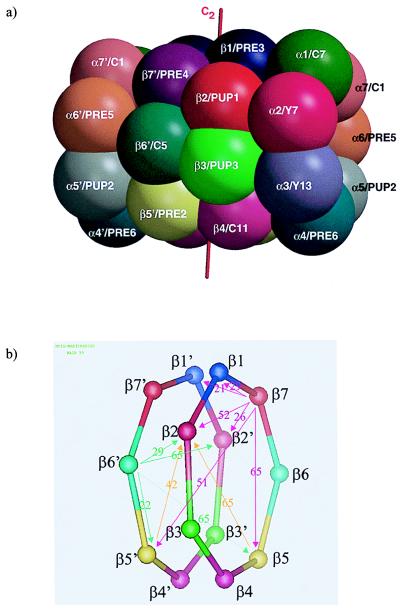
Figure 1.
(a) Topology of the yeast 20S proteasome. The active site threonine 1 residues are located at the inner wall of the cylindrical particle. (b) Scheme of the β-rings with given distances between the active site threonines.
Functional and structural analysis of the mutant proteasomes allows us to investigate substrate specificities, catalytic and autolytic mechanisms, and intermediate processing of propeptides. They also provide hints to the role of propeptides in proteasome maturation and enzymatic activity and help to clarify the mechanism by which peptide product length is controlled. They provide critical tests of possible allosteric interactions in the proteasome. A number of mutants of inactive subunits was generated to define the roles of individual residues for inactivity with the ultimate goal to activate those subunits.
MATERIALS AND METHODS
Protein Preparation and Analysis.
Yeast strains that express mutant proteasomes were generated as described (11). Cells were grown on a 5l scale, and the modified enzymes were purified as reported for the wild-type (7). 20S proteasomes were separated into subunits by reversed phase HPLC. One-hundred-microgram samples were loaded on a RP60 Supersphere column (Merck). The column was washed with a gradient from 0 to 30% acetonitrile in 0.1% trifluoroacetic acid. Single subunits were eluted in a gradient from 30 to 60% acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 0.3 ml/min and at a back pressure of 140 bar (1 bar = 100 kPa). Peaks were identified and propeptides characterized by N-terminal sequence analysis and mass spectrometry.
Crystals of 20S proteasome mutants from Saccharomyces cerevisiae were grown in hanging drops at 24°C as described (7). The crystals were frozen in a stream of cold nitrogen gas (90 K). Data were collected by using synchrotron radiation with λ=1.1 Å on the BW6 beamline at the Deutschen Elektronen-synchrotron Centre (Hamburg, Germany) (Table 3). The anisotropy of diffraction was corrected by an overall temperature factor by comparing observed and calculated structure amplitudes by using x-plor (13). Electron density was averaged 10 times over the 2-fold noncrystallographic symmetry axis by using main (14). Model building was carried out with frodo (15).
Table 3.
Crystallographic data of data collection and refinement
| β2(Pup1) | β5(Pre2) | β1(Pre3) | β1(Pre3) | β3(Pup3) | β6(Pre7) − 5* | β1(Pre3) without propeptide | |
|---|---|---|---|---|---|---|---|
| Space group | P21 | P21 | P21 | P21 | P21 | P21 | P21 |
| Cell | a = 136.7 | α = 135.6 | α = 135.4 | α = 135.5 | α = 135.5 | α = 135.7 | α = 135.9 |
| constants (Å/°) | b = 300.6 | β = 300.3 | β = 302.5 | β = 300.7 | β = 301.2 | β = 300.3 | β = 301.6 |
| c = 145.2 | γ = 144.0 | γ = 145.5 | γ = 144.4 | γ = 146.5 | γ = 144.6 | γ = 144.5 | |
| β = 113.1 | β = 113.0 | β = 112.6 | β = 112.9 | β = 112.8 | β = 113.2 | β = 112.7 | |
| Resolution, Å | 50 − 2.5 | 50 − 2.5 | 50 − 2.7 | 50 − 1.9 | 50 − 2.9 | 50 − 1.95 | 50 − 2.9 |
| Observation, 2σ | 606959 | 951542 | 702094 | 2181093 | 631736 | 1755406 | 600449 |
| Uniques | 289028 | 343517 | 270036 | 752101 | 225640 | 731544 | 218.345 |
| Completeness | 88.3 | 93.3 | 93.8 | 92.9 | 94.5 | 91.1 | 94.2 |
| Rmerge, % | 14.4 | 12.9 | 12.8 | 11.9 | 12.2 | 12.1 | 13.6 |
| R/Rfree, % | 30.3/36.5 | 26.0/31.2 | 27.5/36.4 | 26.8/33.0 | 21.1/27.3 | 28.5/32.1 | 22.7/297 |
| rms bonds, Å | 0.012 | 0.012 | 0.012 | 0.011 | 0.011 | 0.011 | 0.012 |
| rms angles, ° | 2.0 | 1.9 | 1.84 | 1.8 | 1.85 | 1.933 | 1.89 |
RESULTS AND DISCUSSION
Subunit Processing.
The topology of the yeast 20S proteasome is shown in Fig. 1a with the relevant distances between the active sites given in Fig. 1b. We have purified mutant yeast 20S proteasomes with a reduced number of active subunits carrying exchanges of Thr1 for Ala in β1 and β2. In β5, Lys33 was exchanged for Ala or Arg because β5T1A is not viable. Double mutants of β1 and β2 can be made. Some of these mutants show reduced growth (11), but 20S proteasomes can be isolated. We have characterized the β-subunits chemically by Edman degradation and, in some cases, by mass spectrometry after separation of the individual subunits by HPLC (Tables 1 and2).
Table 1.
Yeast 20S proteasome mutants prepared and analyzed by N-terminal sequencing
| β1(Pre3) | β2(Pup1) | β3(Pup3) | β4(Pre1) | β5(Pre2) | β6(Pre7) | β7(Pre4) | |
|---|---|---|---|---|---|---|---|
| Wild type | Gly-1 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| Thr1 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 | |
| β2(Pup1)′ | β2(Pup1)′ | ||||||
| β1(Pre3) | Acetyl | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| without propeptide | (mass*) | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| β2(Pup1)′ | β2(Pup1)′ | ||||||
| β1(Pre3) | Arg-10 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| T1A | Leu-9 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| β2(Pup1) | β2(Pup1)′ | β2(Pup1)′ | |||||
| β2(Pup1) | Gly-1 | Gly-1 | Ala-17 | Val-10 | |||
| T1A | Thr1 | XXX | Met-9 | Met-1 | Thr1 | Ser-16 | Asn-9 |
| β5(Pre2)′ | β1(Pre3)′ | ||||||
| β5(Pre2) | Not viable | Not viable | Not viable | Not viable | Not viable | Not viable | Not viable |
| T1A | |||||||
| β5(Pre2) | Gly-1 | Gly-1 | His-10 | Asn-9 | |||
| K33A | Thr1 | Thr1 | Met-9 | Met-1 | XXX | Gln-9 | Thr-8 |
| (mass*) | β2(Pup1)′ | β2(Pup1)′ | |||||
| β1(Pre3) | Leu-15 | Gly-1 | Ala-17 | Ile-19 | |||
| T1A | Met-19 | Ala-14 | Met-9 | Met-1 | Thr1 | Ser-16 | Ala-18 |
| β2(Pup1) | β5(Pre2); | (mass*) | β5(Pre2); | ||||
| T1A | β5(Pre2)′ | β5(Pre2)′ | β5(Pre2)′ | ||||
| β1(Pre3) | Arg-10 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| T1A | Leu-9 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| β5(Pre2) | β2(Pup1) | β2(Pup1)′ | β2(Pup1)′ | ||||
| K33R | |||||||
| β2(Pup1) | |||||||
| T1A | Not viable | Not viable | Not viable | Not viable | Not viable | Not viable | Not viable |
| β5(Pre2) | |||||||
| K33R | |||||||
| β3(Pup3) | Gly-1 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| G1T | Thr1 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| β2(Pup1)′ | β2(Pup1)′ | ||||||
| β6(Pre7) | Gly-1 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| G1T/ | Thr1 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| A129S/ | β2(Pup1)′ | β2(Pup1)′ | |||||
| A130G/ | (mass*) | ||||||
| H166D/ | |||||||
| V169S | |||||||
| β7(Pre4) | Gly-1 | Gly-1 | Gly-1 | His-10 | Asn-9 | ||
| R33K/ | Thr1 | Thr1 | Met-9 | Met-1 | Thr1 | Gln-9 | Thr-8 |
| F129S | β2(Pup1)′ | β2(Pup1)′ |
Table 2.
Results of mass spectrometry of the subunits in the different yeast 20S proteasome mutants
| β1(Pre3) | β2(Pup1) | β3(Pup3) −MET + Ac | β4(Pre1) + Ac | β5(Pre2) | β6(Pre7) | β7(Pre4) | |
|---|---|---|---|---|---|---|---|
| Wild type | t: 21,494 | t: 25,085 | t: 22,514 | t: 22,558 | t: 23.300 | t: 24,851 | t: 25.919 |
| e: 21,492 | e: XXX | e: 22,504 | e: 22,559 | e: 23,297 | e: 24,850 | e: 25.919 | |
| β1(Pre3) | t: 22,376 | t: 25,085 | t: 22,514 | t: 22,558 | t: 23.300 | t: 24,851 | t: 25.919 |
| T1A | e: 22,374 | e: XXX | e: 22.514 | e: 22,559 | e: 23,296 | e: 24,833 | e: 25.920 |
| (−H2O) | |||||||
| β1(Pre3) | t: 21,536 | t: 25,085 | t: 22,514 | t: 22,516 | t: 23.300 | t: 24,851 | t: 25.919 |
| without | e: 21,539 | e: XXX | e: XXX | e: 22,559 | e: 23,303 | e: 24,854 | e: 25.921 |
| propeptide | |||||||
| β2(Pup1) | t: 21,494 | t: XXX | t: 22,514 | t: 22,558 | t: 23.300 | t: 25,631 | t: 26.033 |
| T1A | e: 21,495 | e: XXX | e: 22.516 | e: 22,559 | e: 23,300 | e: 25,547 | e: 26,045 |
| (+H2O) | |||||||
| β3(Pup3) | t: 21,494 | t: 25,085 | t: 22,559 | t: 22,558 | t: 23.300 | t: 24,851 | t: 25.919 |
| G1T | e: 21,497 | e: XXX | e: 22,548 | e: 22,560 | e: 23,302 | e: 24,856 | e: 25.921 |
| β5(Pre2) | t: 21,494 | t: 25,085 | t: 22,514 | t: 22,558 | e: 23.243 | t: 24,851 | t: 25.919 |
| K33A | e: 21,496 | e: XXX | e: XXX | e: 22,560 | r: 23,246 | e: 24,838 | e: 25.920 |
| (−H2O) | |||||||
| β6(Pre7) | t: 21,494 | t: 25,085 | t: 22,514 | t: 22,558 | t: 23.300 | t: 24,851 | t: 25.919 |
| G1T/A12 | e: 21,496 | e: XXX | e: 22,516 | e: 22,560 | e: 23,301 | e: 24,850 | e: 25.921 |
| 9S/A130G | t′1: 23,870 | ||||||
| /H166D/V | e′1: 23.872 | ||||||
| 169S | |||||||
| β1(Pre3) | t: 23,547 | t: 26,436 | t: 22,514 | t: 22,558 | t: 23.300 | t: 25,327 | t: 26,832 |
| T1A/ | e: 23,562 | e: XXX | e: 22,516 | e: 22,560 | e: 23,301 | e: 25,341 | e: 25.833 |
| β2(Pup1) | t′2: 25,631 | ||||||
| T1A | e′2: 25,629 |
The active subunits β1, β2, and β5 are processed autocatalytically and independently of each other. Inactivating β1 does not affect processing of β2 and vice versa. Similarly, the mutation of β5K33A and β5K33R leads to inactivity of β5 but has no effect on maturation of β1 and β2. This is consistent with earlier findings (16, 17), including pulse–chase experiments, which demonstrate that subunit maturation occurs late in proteasome assembly (11, 16, 18–20) after the formation of 15S-16S proteasome precursor particles. These particles are believed to be half proteasomes. As the active sites in 20S proteasomes are nearly 30 Å apart from each other, it appeared not possible that the Gly-1Thr1 cleavage occurs by a neighboring subunit.
The data on β5 maturation are less straightforward to interpret. β5K33R has very low enzymatic activity but is autoprocessed. β5K33A is also inactive, but partially processed. We find clear electron density for the propeptide to residue Cys-8 in this mutant, but we can isolate by HPLC and mass spectrometry also the autoprocessed species (Table 2). An explanation might be an exceptional lability of the Gly-1Thr1 bond under the strongly acidic conditions of sample preparation for mass spectrometry.
The β1 and β2 T1A exchange in both the single and the double mutants leads to a failure in autoprocessing and to the presence of intact or intermediately processed propeptides of these subunits. In the β1T1A β2T1A double mutant, β1 has its full length propeptide attached, and β2 is intermediately processed after Leu-15. β7 is cleaved after Ile-19. In β6, cleavage after Ala-17 and Thr-14 is found. Cleavage occurs after nonpolar residues, consistent with cleavage by β5. Cleavage sites are at a sufficient distance from residue 1 to reach the remaining active centers of β5 in the same ring for β6 and β7 and in the opposite ring for β2 (Fig.1b).
In the single β1T1A-mutant, processing of β6 and β7 is as in the wild type, but β1 is cleaved after Arg-10, obviously by β2, whereas in β2T1A the β6 and β7 propeptides are longer than in the wild type. Here, β2 itself could not be characterized.
The inactive subunits β6 and β7 are intermediately processed by one of the active subunits. β6 is adjacent to β5 on the same ring and to β2 on the opposite ring but further away from β1 on both rings of the 20S proteasome (Fig. 1). The nine amino acid propeptide in the mature wild-type protein is too short to span the distance to either of the β1 subunits. Experimentally, we find that inactivating β1 in the β1T1A-mutant, β5 in the β5K33A-mutant, and β1 and β5 in the β1T1A β5K33R mutant has no effect on the propeptide processing of β6. In contrast, a significantly longer propeptide remains attached to β6 in the β2T1A-mutant. We conclude that β6 is processed by β2. Cleavage occurs after His-10 (Table 1), consistent with the trypsin-like activity of β2. Because β2 in the same ring is too far away to be reached by a nonapeptide Gln-9 to Gly-1, β2 of the opposite ring must be the subunit that processes β6.
In the case of β7, the situation is similar, but the subunits β5 and β1 swap roles. β7 is close to β2 on the opposite ring and to the subunits β1 on both rings. β5 is too far away to be involved in the final maturation step. Experimentally, the wild-type propeptide of β7 is found in the β1T1A, in the β5K33A mutant, and in the β1T1Aβ5K33R double mutant. In the β2T1A mutant, the cleavage that occurs in the wild type is suppressed, identifying β2 as the responsible subunit in the wild type. The cut occurs after Asn-9, a residue for which β2 has some specificity (12). These data substantiate previous biochemical findings on β7 maturation in the β2T1A single and β1T1A β2T1A double mutant, which led to the hypothesis that inactive β-subunits are processed by the closest active neighbor subunit (11).
The intermediately processed propeptides of β6 and β7 had been found in well defined locations in the molecular structure of the wild-type protein such that their N termini lie at the inner annulus of the β-subunit rings far removed from the sites of proteolytic cleavage defined here (7). The same holds for the intermediately processed β1 propeptide in β1T1A and in β1T1Aβ5K33R. It has defined electron density to Leu-9, which also lies at the inner annulus, not far (16 Å) from β6Gln-9 and β7Thr-8. These observations indicate a major rearrangement of the propeptides after intermediate processing and fixation at the final sites seen in the crystal structure. In β1T1A β2T1A, the full length propeptide of β1 and the intermediately processed propeptides of β2, β6, and β7 have well defined electron density up to residues Met-19 (β1), Ala-14(β2), Gln-9(β6), and Thr-8(β7), respectively.
Implications for Cleavage Specificity.
Two β subunits, β3 and β4, have propeptides of eight and one amino acids, respectively, which are too short to reach any catalytic site in the mature particle and are, indeed, not cleaved. The propeptides of all other subunits are longer, and processing intermediates are observed. The discussed mutants are defective in some of the final maturation steps and show changes in the processing pattern. As shown above, most of the subunits responsible for these cleavages are defined and can be related to cleavage specificities.
In the β1T1A-mutant, a nine-residue propeptide cleaved after Arg-10 is found, consistent with cleavage by β2 in the same ring, according to its tryptic specificity and distance. Processing is completely suppressed in the β1T1Aβ2T1A double mutant, and the β1Met-19 N terminus is observed. In the β2T1A-mutant, autoactivation is suppressed, but the subunit could no longer be separated by HPLC. We were able to characterize the cleavage site of the propeptide of β2 in the double mutant β1T1A β2T1A between Leu-15 and Ala-14. As the only active subunit left, β5 must be responsible for this cut, assigning to it branched chain amino acid preferring (BrAAP) specificity, consistent with previous studies (12).
In the case of β5, we have mutated Lys 33 to Ala and to Arg, abolishing activity. In the β5K33A mutant, the resulting propeptide of β5 is heterogeneous and could not be analyzed by Edman degradation. A fraction is found that is autolysed and has a Thr1 N terminus. In the x-ray structure, however, there is defined density to Cys-8, indicating that the major proportion is not autolysed. However, β5K33R is fully autolysed.
β6 and β7 are processed to their final forms by β2 of the opposite ring. Therefore, we have analyzed the β2T1A-mutant for changes in the cleavage pattern of β6 and β7 propeptide. In β6, the cut occurs between Ala-17 and Ser-16, as analyzed by Edman degradation of an HPLC fraction. In the β1T1A β2T1A double mutant, a component with cleavage between Thr-14 and Pro-13 is found by mass spectrometry. This bond must be hydrolyzed by β5, assigning small neutral amino acid preferring (SNAAP) specificity to β5.
In the β2T1A-mutant, β7 has one extra amino acid at the N terminus compared with the wild type. Inactivating β1 in addition to β2 shifts the cleavage further upstream to Ile-19 Ala-18. We conclude that β1 and β5 cleave after Val-10 and Ile-19, respectively, demonstrating BrAAP activity for both subunits, consistent with the apolar character of the P1 pocket of β5. In the case of β1, we assume that the positive charge of Arg 45 at the base of its P1 pocket is compensated by a bound bicarbonate anion to allow binding of neutral ligands, as had been observed before in the Leu-Leu-Norleucinal complex of the wild-type protein (7, 21).
The Role of the β1 Propeptide.
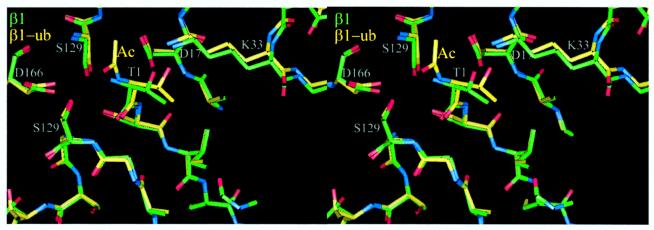
The propeptide of β5 has been shown to be essential for cell viability but is functional when expressed in trans, suggesting a chaperone-like role in proteasome biogenesis (20). To investigate the role of the propeptide of β1, we have replaced its propeptide with ubiquitin. As in other linear ubiquitin fusions (22, 23), ubiquitin is cleaved by ubiquitin C-terminal hydrolases (24) to liberate the N-terminal threonine. The mutant proteasomes were inactive when assayed for postacidic cleavage (PGPH) activity. Their β1 subunit could be isolated by HPLC but was blocked for N-terminal sequencing. Structural analysis of the mutant proteasomes showed no significant differences to the wild-type structure except for extra density at the amino group of Thr1 that was interpreted as an acetyl group (Fig.2) and confirmed by mass spectroscopy (Table 2). We conclude that the propeptide of β1 has a role in preventing co- or posttranslational acetylation and inactivation of this subunit. The lack of enzymatic activity of the_N-_acetyl-β1 mutant supports the proposed mechanism of catalysis (9) assigning to the amino group of Thr1 the role of the proton acceptor, but steric hindrance of substrate docking by the acetyl group also may contribute to inactivity. It is noted that the acetyl group is not cleaved via autolysis, probably for steric and electronic reasons. The role of the conserved Lys33 is in maintaining the appropriate structure and electrostatic potential in the vicinity of the active site (7, 21).
Figure 2.
Stereodiagram of the superposition of β1 (green) and _N-_acetyl-β1 (yellow) around the Thr1 site. The structures match closely.
The Role of Lys33 in the Enzymatic Mechanism.
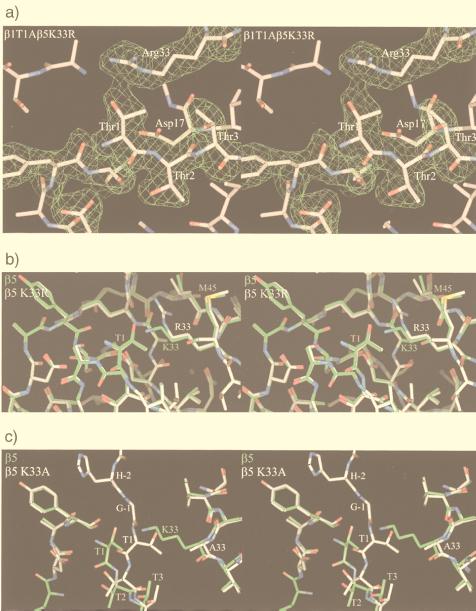
The conservative exchange of Lys33 to arginine abolishes both autolysis and proteolysis in T. acidophilum proteasomes (25). We were particularly interested in this mutation because arginine in position 33 occurs naturally in the subunit β7 of the yeast proteasome, where it displaces the Thr1 side chain, leading to incompetence in autolysis and to enzymatic inactivity (7). We exchanged Lys33 in β5 of the yeast proteasome with arginine. Crystals that diffract to 1.9-Å resolution (Table 3) could be obtained with a double mutant, which additionally has Ala exchanged for Thr1 in subunit β1. In contrast to wild-type β7 and the quintuple mutant of β6 (see below), all residues in the vicinity of the active site of β5, including Thr1, remain in their wild-type positions. The arginine residue has its side chain in the same orientation as the lysine residue, but its guanidino group is tilted with respect to the position of the amino group in the lysine residue to avoid a clash with Thr1 (Fig. 3 a and_b_). As in T. acidophilum proteasomes, the chymotryptic activity of this mutant against chromogenic substrates is abolished. However, in contrast to results obtained for the T. acidophilum proteasome, the propeptide in the yeast mutant is cleaved. We attribute this observation to a weak residual activity that suffices for autolysis during particle maturation. The mutant grows slowly at 30°C but not at 37°C (11), and it overexpresses 20S proteasomes. The phenotype could be attributable either to the lack of chymotryptic activity or to delayed or impaired proteasome maturation. Genetic studies favor the latter explanation (11, 20). Because autolysis still occurs in the β5K33R mutant, we analyzed the β5 mutant carrying the Lys33Ala mutation. The mutant strain was viable, although again it grew slowly and contained unusually high amounts of 20S proteasome. As expected, both autolysis and proteolysis did no longer occur. The 2.5-Å crystal structure of this mutant shows defined density for the propeptide and a major rearrangement of the position of Thr1 that fills the cavity created by the loss of the lysine residue and displaces Met45 (Fig. 3c). Mass spectrometry of a fraction separated by HPLC, however, showed also the presence of some correctly processed species (Table 2).
Figure 3.
(a) Stereodiagram of the β1T1A β5K33R double mutant in the vicinity of residue Thr1 in β5. The electron density is calculated with phases from the wild-type β5 model. (b) Stereodiagram of wild-type (green) and β5K33R (white) mutant around Thr1. They superimpose closely except for the site of mutation. β5K33R autolyses and has a free Thr1. (c) Comparison of the wild-type (green) and β5K33A (white) mutant. Loss of the Lys33 side chain leads to a large movement of the backbone of Thr1. The mutant is unable to autolyse and has the propeptide attached.
Some Structural and Functional Comparisons.
A comparison of the refined molecular models of the mutants β1T1A, β2T1A, and β1T1A β2T1A showed no significant variation of subunit positions or backbone structures. The activity against chromogenic substrates of a particular subunit is insignificantly altered by the presence or absence of intact sites of other subunits. We had previously shown that the covalent binding of a specific bound irreversible inhibitor of β2 has no significant influence on the PGPH and chymotryptic activity associated with β1 and β5 and does not show noticeable structural changes (26). Similarly, there is no measurable change in the activity and structure of β1 and β5 by strong binding of bifunctional reversible inhibitors to β2 (27). Also, yeast 20S proteasome with lactacystin bound to β5 shows no structural change compared with the unligated species (7). These results do not support the existence of allosteric interactions between the active sites in general and argue against interactions mediated by conformational equilibria in particular. We are aware, however, that crystal lattice forces may oppose ligand-induced conformational changes occurring in solution.
Reactivation Studies.
All proteasomal β-subunits are members of a family of proteins having diverged from a single ancestor possibly similar to the archaebacterial β subunit. Nevertheless, only three subunits, β1, β2, and β5, are proteolytically active in yeast and higher eukaryotes The other β-subunits, β3, β4, β6, and β7, are inactive and unable to autolyse. β3, β4, and β6 lack the nucleophilic threonine in position 1, and β7 has Arg33 and Phe129 instead of Lys33 and Ser129, respectively, as the most conspicuous changes.
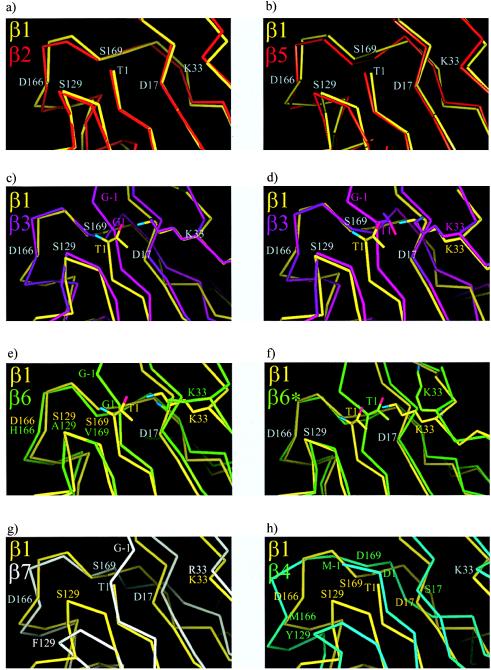
The conservation of backbone geometry and of the majority of residues making up the active site also in inactive proteasome β-subunits has prompted us to investigate the possibility of reactivating inactive subunits. We first chose the inactive subunits β3 and β6 as promising targets for subunit activation experiments because of the close similarity of their backbone fold with the active subunits β1, β2, and β5 (Fig. 4 a_–_c and e).
Figure 4.
A gallery of superposition of main chain traces around Thr 1. a and b show the three active subunits β1, β2 and β5. In c and_d_, β1 is compared with wild-type β3 and β3G1T, respectively, in e and f, β1 is superimposed with wild-type β6 and the 5-fold β6 mutant (β6*), and, in g and h, β1 is compared with β7 and β4.
β3.
Gly1 replaces the canonical threonine in β3 as the most conspicuous exchange. It was mutated to threonine. The resultant yeast strain is viable and does not show a growth phenotype. Purified proteasomes from this strain show a blocked N terminus as the wild type. An antibody was raised against β3, and the migration of the mutant and of the wild-type subunit on denaturing SDS gels was compared. No difference could be observed, implying that the propeptide was not cleaved and the subunit remains inactive. Mass spectrometry confirms these results (Table 2). Additionally, we determined the crystal structure of this mutant, which, when compared with the wild-type β3-subunit, does not show major rearrangements and confirms that the propeptide is attached (Fig. 4 c and d).
β6.
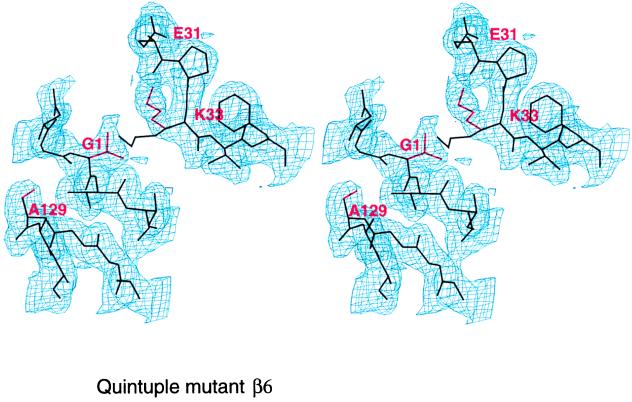
We repeated the experiment in an analogous manner with β6. Although this subunit has a severely impaired catalytic machinery with Gly1, Ala129, His166, and Val169 instead of the cannonical Thr1, Ser129, Asp166, and Ser169, its backbone superimposes well with those of the active subunits, and the position of Lys33 is identical (Fig.4e). Gly1 is shifted slightly toward Lys33 compared with the active subunits. We have replaced Gly1, Ala129, His166, and Val169 by their equivalents in active subunits. In addition, we exchanged Ala130 with glycine because this residue is conserved in all three active subunits, although its role in catalysis is not obvious. The 5-fold mutant is again viable, but it has a severe growth defect. In comparison with the wild type, cells are up to 10× larger and express severalfold more proteasome, which could be purified and crystallized. The crystal structure analysis at 1.95-Å resolution shows defined electron density at β6 for all nine residues of the partially processed propeptide, but it is substantially lower than in the wild type and particularly blurred at residues Asn-2 and Gly-1. Temperature factors of the propeptide are very high. Also, the mass spectrum of the corresponding HPLC fraction showed the molecular weight of the Gln-9 species, but a component with the molecular weight, corresponding to the autolysed species, also occurs. We conclude that the mutant protein is partially autolysed. Residues Asp17, Ser129, Asp166, and Ser169 of the mutant subunit β6 are positioned as the corresponding residues in active wild-type subunit, but Thr1 remains where Gly1 in the wild-type β6 subunit is. A close contact between the Thr1 and Lys33 side chains displaces the lysine side chain into an outwardly oriented position, where it is stabilized by hydrogen bonds to Glu31 and Asp53 (Figs.4f and 5). The distortion of Thr1 with respect to its position in active subunits prevents the binding of a water molecule in the vicinity of Thr1, as seen in active subunits (7). As the phenotype of the mutant is unlikely be accounted for by an extra proteasomal activity, we have looked for other explanations. The major activities of wild-type proteasomes are present but somewhat reduced. Therefore, we suspect a decreased stability of the quintuple mutant. β6 is in contact with β5 and β7 in the same ring and β2 and β3 of the opposite ring. His166 and Val169 contact β5, β2, and β3 whereas Ala129 and Ala130 contact β7. As seen from the lack of a phenotype of the triple mutant, β6G1T A129S A130G, which presumably has a displaced lysine residue and impaired contacts with β7, and from the lack of a phenotype of the quadruple mutant β6A129S A130G H166D V169S, individual residue effects count to be weak. Only in the quintuple mutant, where contacts of β6 with all neighboring β-subunits are disturbed, is a notable phenotype seen.
Figure 5.
Stereo diagram of the 5-fold β6 mutant in the vicinity of residue 1. The electron density is calculated with phases from the wild-type β6 model (black bonds). The mutations A129S, G1T, and the rearrangement of K33 are clearly visible (red bonds).
β7.
Based on our observation that the displacement of the mutationally introduced Thr1 with respect to its position in active subunits could explain the failure to activate β3 and β6, and based on the perfect match of the polypeptide backbone around Thr1 of β7 and of the active subunits (Fig. 4g), we then attempted to activate β7. Two residues have to be replaced, Arg33 and Phe129. The resulting yeast strain was viable and indistinguishable from the wild-type. N-terminal sequencing of β7 revealed the presence of the wild-type propeptide. In the absence of a crystal structure, we can only suspect that the distortion in the backbone of wild-type β7 in the region around Phe129, which we attribute mainly to unfavorable interactions with Asp166 (Fig. 4g), is still present in the mutant and responsible for the inactivity and inability to autolyse. We did not try to activate β4 because major differences between the Cα-traces of this subunit and of the active subunits exist (Fig.4h).
Acknowledgments
We thank Silvia Körner and Frank Siedler (Max-Planck-Institut für Biochemie, Martinsried, Germany) for help with mass spectrometry, Karlheinz Mann (Max-Planck-Institut für Biochemie, Martinsried, Germany) for help with N-terminal sequence analysis, and G. B. Bourenkow and H. Bartunik (DESY, Hamburg, Germany) for assistance with the x-ray experiments. The Sonderforschungsbereich 469 provided financial support. The work was furthermore supported by a grant from the Deutsche Forschungsgemeinschaft (Bonn) and the Fonds der Chemischen Industrie (Frankfurt).
Note Added in Proof
While this paper was in press, a publication by Arendt and Hochstrasser (28) appeared suggesting acetylation of β1, β2, and β5 subunits by genetic methods in mutants lacking the respective propeptides. These results are in agreement with our findings in β1 by analytical methods.
Footnotes
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1RYP).
References
- 1.Hilt W, Wolf D H. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 3.Hochstrasser M. Annu Rev Genet. 1996;30:405–409. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 4.Baumeister W, Walz J, Zühl F, Seemüller E. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 5.Peters J M, Cejka Z, Harris R J, Kleinschmidt J A, Baumeister W. J Mol Biol. 1993;234:932–937. doi: 10.1006/jmbi.1993.1646. [DOI] [PubMed] [Google Scholar]
- 6.Coux O, Tanaka K, Goldberg A L. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 7.Groll M, Ditzel L, Löwe J, Stock D, Bochtler M, Bartunik H D, Huber R. Nature (London) 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 8.Dahlmann B, Kopp F, Kuehn L, Niedel B, Pfeifer G, Hegerl R, Baumeister W. FEBS Lett. 1989;251:125–131. doi: 10.1016/0014-5793(89)81441-3. [DOI] [PubMed] [Google Scholar]
- 9.Löwe J, Stock D, Jap B, Zwickl P, Baumeister W, Huber R. Science. 1995;268:533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- 10.Seemüller E, Lupas A, Stock D, Löwe J, Huber R, Baumeister W. Science. 1995;268:579–581. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 11.Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf D H. J Biol Chem. 1997;272:25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- 12.Nussbaum A K, Dick T P, Keilholz W, Schirle M, Stevanovic S, Dietz K, Heinemeyer W, Groll M, Wolf D H, Huber R, et al. Proc Natl Acad Sci USA. 1998;95:12504–12509. doi: 10.1073/pnas.95.21.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunger A. x-plorVersion 3.1; A System for X-Ray Crystallography and NMR. New Haven, CT: Yale Univ. Press; 1992. [Google Scholar]
- 14.Turk D. Ph.D. thesis. Munich: Technical Univ.; 1992. [Google Scholar]
- 15.Jones T A. J Appl Crystallogr. 1978;15:24–31. [Google Scholar]
- 16.Schmidtke G, Kraft R, Kostka S, Henklein P, Frömmel C, Löwe J, Huber R, Kloetzel P M, Schmidt M. EMBO J. 1996;15:6887–6898. [PMC free article] [PubMed] [Google Scholar]
- 17.Ditzel L, Stock D, Löwe J. Biol Chem. 1997;378:239–247. doi: 10.1515/bchm.1997.378.3-4.239. [DOI] [PubMed] [Google Scholar]
- 18.Frentzel S, Pesold-Hurt B, Seelig A, Kloetzel P M. J Mol Biol. 1994;236:975–981. doi: 10.1016/0022-2836(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Nandi D, Woodward E, Ginsburg D B, Monaco J J. EMBO J. 1997;16:5363–5375. doi: 10.1093/emboj/16.17.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen P, Hochstrasser M. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 21.Ditzel L, Huber R, Mann K, Heinemeyer W, Wolf D H, Groll M. J Mol Biol. 1998;279:1187–1191. doi: 10.1006/jmbi.1998.1818. [DOI] [PubMed] [Google Scholar]
- 22.Bachmair A, Finley D, Varshavsky A. Science. 1986;234:179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- 23.Arfin S M, Bradshaw R A. Biochemistry. 1988;27:7979–7984. doi: 10.1021/bi00421a001. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson K D. FASEB J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 25.Seemüller E, Lupas A, Baumeister W. Nature (London) 1996;382:468–470. doi: 10.1038/382468a0. [DOI] [PubMed] [Google Scholar]
- 26.Loidl G, Groll M, Musiol H-J, Ditzel L, Huber R, Moroder L. Chem Biol. 1999;6:197–204. doi: 10.1016/S1074-5521(99)80036-2. [DOI] [PubMed] [Google Scholar]
- 27.Loidl G, Groll M, Musiol H-J, Huber R, Moroder L. Proc Natl Acad Sci USA. 1999;96:5418–5422. doi: 10.1073/pnas.96.10.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendt C S, Hochstrasser M. EMBO J. 1999;18:3575–3585. doi: 10.1093/emboj/18.13.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]