WHY IS DELTA ENDOCYTOSIS REQUIRED FOR EFFECTIVE ACTIVATION OF NOTCH? (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 20.
Published in final edited form as: Dev Dyn. 2006 Apr;235(4):886–894. doi: 10.1002/dvdy.20683
Abstract
A number of recent studies have shown that endocytosis of Notch ligands is required for activation of Notch. There are at least two broad models that account for how Delta endocytosis in one cell might contribute to activation of Notch in the neighboring cell. The first class of models is related to the possibility that Delta endocytosis facilitates S2 cleavage and removal of the Notch extracellular domain, a critical step in Notch activation. A second class of models is related to the possibility that Delta ubiquitylation and endocytosis facilitates interactions between Delta and Notch. In the second set of models, Delta undergoes endocytosis and its subsequent trafficking back to the surface, following modifications or some change in the context in which it is presented, makes Delta a more effective ligand. Though it is still not clear how either or both mechanisms contribute, recent evidence points to the importance of both endocytosis and recycling in Delta signaling.
Introduction
Notch signaling has an evolutionarily conserved role in mediating cell-cell interactions in many different cellular contexts in the developing embryo (Artavanis-Tsakonas et al., 1999; Schweisguth, 2004). In the context of lateral inhibition, Notch signaling facilitates competitive interactions between cells, where a cell that is adopting a particular fate signals neighboring cells to inhibit them from adopting the same fate. In this context it allows adjacent cells with similar potential to adopt different fates, thereby regulating cell diversity in a developing tissue. Notch signaling is also involved in boundary formation and morphogenesis, and in somitogenesis Delta-Notch signaling facilitates synchronized oscillation of gene expression in the presomitic mesoderm (Pourquie, 2001; Lewis, 2003). These examples illustrate just a few of the diverse roles Notch signaling has in the development and maintenance of almost all tissues. Aberrant Notch signaling leads to pathological consequences and contributes to the development of cancers, tumors, and a growing list of diseases like Alagilles syndrome and CADASIL syndrome, which affect multiple organ systems (Hansson et al., 2004; Lasky and Wu, 2005).
Notch signaling (Lewis, 1996; Schweisguth, 2004) is well known for its role in mediating lateral inhibition during neurogenesis. In Drosophila, for example, neural cells are selected from proneural clusters in which all cells initially acquire the potential to adopt a neural fate by expressing “proneural” basic helix-loop-helix (bHLH) transcription factors. The proneural transcription factors promote their own expression while also driving the expression of the Notch ligand Delta. Delta interacts with its receptor Notch on the surface of neighboring cells, and Notch activation drives expression of bHLH Enhancer-of-split (E(spl)) transcription factors that function as repressors. They inhibit neural fate by inhibiting transcription of proneural factors. This simple feedback loop amplifies small differences in potential for neural fate. Eventually cells that acquire high levels of proneural function are selected for a neural fate. The selected cells are more effective at sending a Delta signal to neighboring cells in which Notch activation determines an alternate fate.
The mechanics of Notch receptor activation
Notch receptors are type I transmembrane proteins that typically undergo a Furin based cleavage (S1) and the mature receptor is presented as a heterodimer on the plasma membrane (Figure 1) (Logeat et al., 1998). The Notch heterodimer consists of the Notch Extracellular Domain (NECD), which is bound in a calcium dependent manner to a membrane-tethered fragment that includes an extracellular stub, a transmembrane domain, and an intracellular domain. The NECD consists of a variable number of Epidermal-Growth-Factor-like (EGF-like) repeats that are essential for interaction with DSL family Notch ligands named after Delta and Serrate in Drosophila, and Lag2 in C. elegans. The DSL ligands are also type I transmembrane proteins (Fleming, 1998). They have a conserved DSL motif in the extracellular domain that is required for Notch binding, and as in Notch, a variable number of EGF-like repeats. The intracellular domain is not as conserved though some DSL ligands have a conserved motif at the C-terminal that mediates interactions with PDZ domain containing proteins (Ascano et al., 2003; Pfister et al., 2003; Six et al., 2004; Wright et al., 2004). In addition there are conserved putative trafficking motifs, however, these have not yet been carefully characterized.
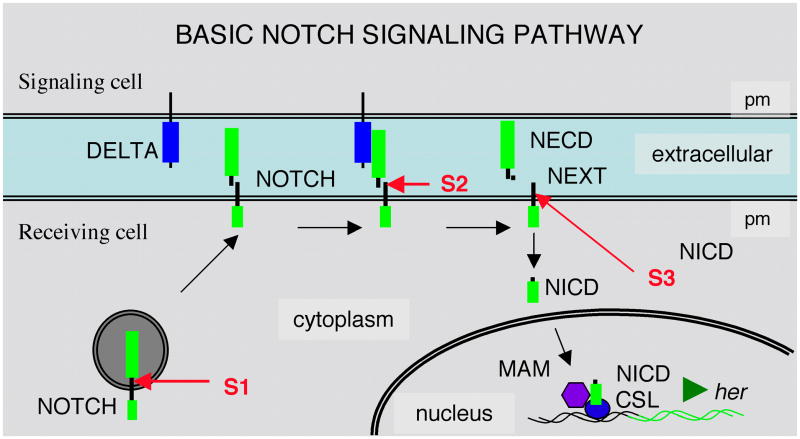
Figure 1. Basic Notch Signaling Pathway.
During maturation Notch undergoes Furin-dependent S1 cleavage. Following interaction with Delta, Notch undergoes metalloprotease-dependent S2 cleavage. The Notch Extracellular Domain (NECD) is released and the Notch Extracellular Truncated (NEXT) fragment that remains is a substrate for a gamma-secretase complex that releases the Notch Intracellular Domain (NICD). NICD functions as a transcriptional activator in a complex with CSL proteins and Mastermind (MAM) to activate target hairy E(spl) related (her) genes.
A critical step in the Notch signaling pathway is the interaction of the DSL ligand with Notch, which facilitates extracellular cleavage (S2) of the membrane tethered Notch fragment by transmembrane proteases of the ADAM/TACE family (Mumm et al., 2000). This releases NECD together with a short cleaved fragment of the membrane tethered Notch fragment. The Notch Extracellular Truncated (NEXT) fragment, which remains tethered to the plasma membrane is a constitutive substrate for a final intra-membranous proteolytic cleavage (S3) mediated by a gamma secretase complex (De Strooper et al., 1999). This releases the NICD domain into the cytoplasm. It translocates to the nucleus where it functions as a transcriptional activator in a complex with co-factor Mastermind and a CSL DNA-binding factor (CBF1 in humans, Suppressor-of-Hairless (Su(H)) in Drosophila, and Lag1 in C. elegans). The Notch activator complex destabilizes basal repression of target genes and drives expression of targets like E(spl) factors described above.
Delta endocytosis in Notch signaling- a historical survey
The sequence of events described above defines the basic elements of the Notch signaling pathway. In the context of lateral inhibition it emphasizes negative feedback of Notch activation on proneural or atonal related bHLH genes and on Delta expression. However, it has been known for a long time that the spatiotemporal pattern of Delta expression is not always consistent with some simple predictions of lateral inhibition, which suggests selected cells should have high levels of Delta expression compared to neighbors with relatively high levels of Notch activation. For example in Drosophila, Delta transcripts accumulate in all cells of the neurogenic ectoderm immediately preceding neuroblast segregation, indicating that Delta expression does not differ between presumptive neuroblasts and presumptive epidermoblasts (Kopczynski and Muskavitch, 1989; Kooh et al., 1993). The relatively uniform expression of Delta amongst interacting cells has been interpreted to suggest that Delta mediates mutual inhibition rather than lateral inhibition. However, these observations have also been interpreted to suggest that post-transcriptional and post-translational changes in Delta function may more accurately reflect dynamic changes in a cells ability to deliver an inhibitory signal during lateral inhibition.
It became clear in the 1990s that Delta endocytosis was one of the modifications that has a critical role in regulating Delta function. In 1993 Kooh et al showed that Delta and Notch accumulate in endocytic vesicles (Kooh et al., 1993), and in 1999 Seugnet et al showed that endocytosis is essential for Notch signaling by demonstrating that shibire/dynamin mutants are characterized by an excess of neural cells (Seugnet et al., 1997). They showed that when Notch is activated by Delta, dynamin is required in both signaling and receiving cells for normal singling out of neural precursors. It was also shown by Klueg and Muskavitch that endocytosis of Delta or Serrate in ligand expressing cells is accompanied by “trans-endocytosis” of the bound Notch extracellular fragment (NECD), and reciprocally in Notch-expressing cells, endocytosis of Notch is accompanied by trans-endocytosis of DSL ligands (Klueg and Muskavitch, 1999). Further examination of dynamin mutants led to the suggestion that endocytosis of NECD-bound Delta in Delta-expressing cells is required to achieve processing and dissociation of the Notch protein in the neighboring cell (Parks et al., 2000).
In 2001 Neuralized was identified as a RING E3 or ubiquitin ligase responsible for ubiquitylation and endocytosis of Delta (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001; Yeh et al., 2001). Ubiquitin ligases have a critical role in recognizing substrates and in facilitating covalent conjugation of ubiquitin, a 76 amino acid peptide, to Lysines on substrate proteins. Additional ubiquitins can be conjugated to the initial ubiquitin peptide to form short ubiquitin chains, and poly-ubiquitylation has long been recognized as a tag that targets proteins for degradation (Hershko, 1983). However, mono-ubiquitylation or multi-ubiquitylation, which is the conjugation of a single ubiquitin peptide to one or multiple Lysines in the substrate, has more recently been recognized as a signal that targets transmembrane proteins for endocytosis (Hicke and Dunn, 2003; d'Azzo et al., 2005).
Identification of Delta as a substrate for ubiquitylation led to models that either emphasized a role for Neuralized in Delta endocytosis or degradation, in the signal-sending or signal-receiving cell. Interactions of Delta and Notch within the Golgi of the signal-receiving cell (cis interactions) reduce the ability of Notch to interact with Delta in trans in neighboring cells. In this context it was suggested that Delta endocytosis and degradation in the signal-receiving cell may be critical to limit cis interactions with Notch that would otherwise interfere with its function as a receptor (Deblandre et al., 2001; Lai et al., 2001). Alternatively, it was suggested that the E3 ligase is essential in the signal-sending cell, where endocytosis of Delta would facilitate activation of Notch in the neighboring cell (Pavlopoulos et al., 2001).
Failure of Notch signaling in the zebrafish mind bomb (mib) mutants results in the production of an excess of early neurons and premature differentiation of neural progenitors (Jiang et al., 1996; Schier et al., 1996; Itoh et al., 2003). In 2003 Mib was identified as another RING ubiquitin ligase that is required for ubiquitylation of Delta (Itoh et al., 2003; Chen and Casey Corliss, 2004). Cell transplantation studies with mind bomb (mib) mutants provided additional evidence that ubiquitylation of Delta is essential in the signal-sending cell for effective activation of Notch in neighboring cells (Itoh et al., 2003). A growing number of studies in Drosophila have now confirmed that ubiquitylation and endocytosis of Delta is required in the signaling-sending cell for effective Notch signaling (Le Borgne and Schweisguth, 2003b; Overstreet et al., 2004; Wang and Struhl, 2004).
Identification of a Drosophila Mib homologue has shown that, though structurally different, Neuralized and Mib have functional similarities and partially complementary roles (Lai et al., 2005; Le Borgne et al., 2005b; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005). While Serrate is typically the substrate for Mib in Drosophila, Delta is typically the substrate for Neuralized. Nevertheless, Mib is required for ubiquitylation of Drosophila DSL ligands in situations where Neuralized is not expressed. Analysis of mouse Mibs has also shown that the Delta and Jagged Notch ligands are substrates for Mib ubiquitylation (Koo et al., 2005a; Koo et al., 2005b).
Epsin, is a critical component of the endocytic machinery with distinct domains that mediate interactions with either clathrin or with ubiquitylated cargo (Chen and De Camilli, 2005). Recent studies have now shown that endocytosis in association with Epsin is critical for effective Delta function (Overstreet et al., 2004; Wang and Struhl, 2004; Wang and Struhl, 2005); while Delta is capable of being internalized by multiple mechanisms, only Serrate and Delta that are internalized by Mib in association with Epsin are able to signal Notch (Wang and Struhl, 2005).
Delta interacts with Neuralized or Mib through its intracellular domain and DeltaΔICD, which lacks its intracellular domain, cannot be ubiquitylated and cannot effectively activate Notch. Replacement of intracellular domain of Delta with the intracellular domain of the Low Density Lipoprotein Receptor (LDLR), a receptor that undergoes rapid endocytosis and recycling, restores DeltaΔICD’s ability to effectively signal to Notch in an ubiquitin-independent manner. This observation by Wang and Struhl shifted the focus in the evolving story of Delta endocytosis to the role of Delta endocytosis and recycling in Notch signaling (Wang and Struhl, 2004). Additional studies have added to the significance of Delta recycling in determination of cell fate in the progeny of Sensory Organ Precursors in Drosophila (Emery et al., 2005; Jafar-Nejad et al., 2005). Despite the explosion of papers on the subject there is still no consensus on how endocytosis of Notch ligands contributes to activation of Notch in neighboring cells (Le Borgne et al., 2005a). This review will discuss different models that potentially account for the role of Delta endocytosis in the efficient activation of the Notch receptor.
Models for the role of Delta endocytosis in Notch signaling
There are at least two broad models that account for how Delta endocytosis in one cell might contribute to activation of Notch in the neighboring cells. The first class of models is related to the possibility that Delta endocytosis facilitates S2 cleavage and NECD removal. A second class of models is related to the possibility that Delta ubiquitylation and endocytosis facilitates interactions between Delta and Notch. In these sets of models, Delta undergoes endocytosis and its subsequent recycling back to the surface, following modifications or some change in the context in which it is presented, makes Delta a more effective ligand.
A. Delta endocytosis promotes S2 cleavage and NECD removal
Removal of the NECD is a critical step in the activation of the Notch receptor. Mutant forms of Notch, that eliminate the NECD or manipulations that release the NECD, like calcium chelation, can result in ligand independent S2 cleavage-dependent activation of Notch. Replacement of the Notch extracellular domain with an alternate CD4 ectodomain, which does not undergo extracellular cleavage, has shown that an extracellular domain can prevent S3 cleavage (Mumm et al., 2000). However, replacement of the CD4 extracellular domain with a truncated shorter CD4 ectodomain permits S3 cleavage, even though there is still an short ectodomain present. This suggests that the conformation of the truncated CD4 ectodomain may permit access of the S3 site to gamma secretases. Together these and other observations have been interpreted to suggest that the conformation of the juxta-membranous Notch extracellular domain, not necessarily its absence, determines access of the gamma secretase complex to the S3 site.
In the context of these observations it is thought that Delta endocytosis could activate Notch by physically removing NECD, leaving the membrane-tethered fragment accessible to metalloproteases responsible for S2 cleavage. Alternatively, mechanical stress associated with initiation of Delta-NECD endocytosis could induce conformational changes in Notch that make the S2 cleavage site accessible to ADAM/TACE metalloproteases (Figure 2A). In some situations it has been demonstrated that the Notch receptors do not undergo Furin based S1 cleavage (Kidd and Lieber, 2002). In this context it would be difficult for the NECD fragment to be removed prior to S2 cleavage and it would be more likely that Delta endocytosis facilitates conformational changes in Notch that make the S2 site available.
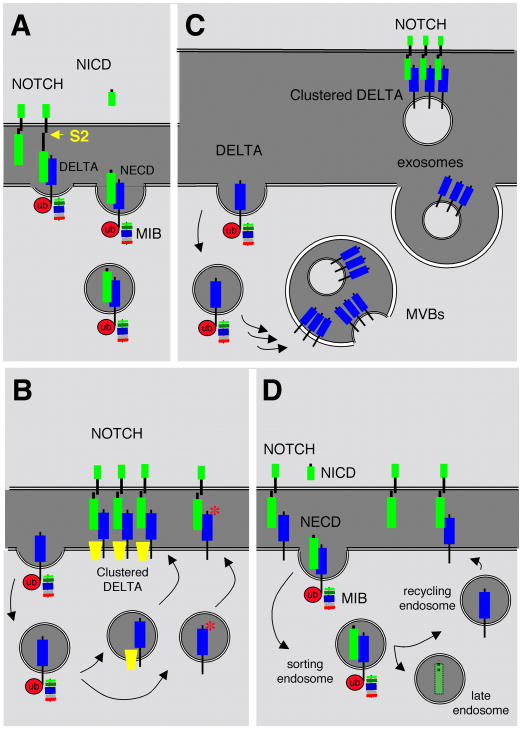
Figure 2. Models for the role of Delta endocytosis.
A, , Delta endocytosis facilitates Notch S2 cleavage. B-D, Delta endocytosis and recycling returns a Delta that is more effective at interacting with Notch. B, Recycling returns Delta to the surface in a microdomain where it is clustered, associated with co-factors (yellow trapezoids), or with modifications (red asterisk) of the extracellular domain that make Delta interactions with Notch more effective. C, Following endocytosis Delta is internalized in Multi Vesicular Bodies (MVBs) from which packets of clustered Delta are released as exosomes. D, Delta re-sensitization. Following endocytosis, the Delta-NECD complex dissociates, and unbound Delta returns to the surface via the recycling endosome for another round of interaction with Notch. NECD traffics to the late endosome and is eventually degraded in the lysosome.
NECD internalization with Delta in Delta-expressing cells (Klueg and Muskavitch, 1999; Parks et al., 2000) is consistent with NECD “trans-endocytosis” being linked to S2 cleavage, however, this observation is also consistent with an alternate model, discussed below, where endocytosis and recycling clears the bound NECD-Delta complex from the surface, it is dissociated in an internal compartment, and unbound Delta is re-presented on the surface.
B. Endocytosis makes Delta a more effective ligand for Notch
A second group of models describes how Delta endocytosis and subsequent trafficking back to the surface could make Delta a more attractive ligand. Briefly, Delta could be returned to the surface in a clustered state, with modifications of its extracellular domain, or in association with cofactors, that allow it to bind more easily to Notch (Figure 2B-D). In addition, Delta internalized from the apical surface of polarized epithelial cells could be recycled back to the basolateral membrane, where it could interact with Notch on the basolateral surface of adjacent cells.
(i) Endocytosis and Recycling promotes Delta Clustering?
a) Clustering could regulate signaling efficiency of Delta expressing cells
Use of a secreted form of Delta has shown that it is much more effective at binding and activating Notch on the cell surface when it is pre-clustered (Hicks et al., 2002). This observation suggests that clustering of Delta makes it a more effective ligand for Notch. One possibility is that ubiquitylation, directly or following endocytosis and recycling, promotes clustering of Delta (Figure 2B). In this context, ubiquitin could promote interactions with ubiquitin recognition domain containing membrane proteins that promote clustering, or unique post-endocytic trafficking of Delta, following ubiquitin-mediated endocytosis, might ensure entry into microdomains where Delta is clustered. Clustering of Delta proteins could promote better interactions with Notch if individual Delta-Notch interactions are weak and the association of multiple weak bonds is a strategy to achieve significant molecular interaction. Clustered Delta could form multivalent bonds with Notch receptors on the adjacent membrane, especially if Notch is also clustered. Indeed, just as a clustered form of secreted Delta binds membrane-tethered Notch more effectively, pre-clustering of a secreted form of Notch is also essential for effective interaction with Delta on the cell surface (our unpublished data). These observations suggest that in vivo clustering of both Delta and Notch could enhance interactions.
Receptor clustering has been suggested to dynamically regulate interactions mediated by cadherins (Iino et al., 2001) and integrin receptors (van Kooyk and Figdor, 2000). For example, while the integrin complement receptor is inactive on resting phagocytes, neutrophil exposure to suitable stimuli such as phorbol ester promotes clustering of receptors into small aggregates of 2–6 molecules and this change is associated with a concomitant increase in cell capacity to bind complement-coated particles (Detmers et al., 1987). These and other studies have suggested that “inside-out” signaling alters the degree of integrin receptor clustering on the cell surface, which thereby regulates efficiency of adhesive interactions based on the physiological status of a cell. If Delta clustering enhanced interactions with Notch, and if the level of Notch activation regulated clustering, then this would provide a novel mechanism for dynamically regulating the efficiency of signaling during lateral inhibition.
b) Exosomes
Delta endocytosis could also promote clustering by contributing to the formation of “exosomes” (Denzer et al., 2000; Fevrier and Raposo, 2004). The endocytic cargo on the limiting membrane of an endosome is internalized by inward budding to form intra-luminal vesicles in multi-vesicular endosomes or multi-vesicular bodies (MVE/MVB) (Figure 2C). Ubiquitylation serves as a signal for internalization of cargo on the endocytic limiting membrane, as it does at the cell surface. In most cases the MVBs fuse with pre-existing lysososmes to degrade the internalized cargo. However, in a number of contexts it has been shown that, instead, the MVBs fuse with the plasma membrane releasing 50–100nm vesicles with associated proteins into the extracellular space. These vesicles are called “exosomes”. Release of exosomes was originally described in reticulocytes as a strategy by which the cells shed transferrin receptor during the process of maturation. It has also been described in the context of Antigen Presenting cells (APCs) that present clustered antigen on exosomes to activate adjacent T-lymphocytes. If Delta were internalized from the limiting membrane of the endocytic vesicle to form such vesicles, each could contain multiple copies of the Delta with their extracellular domain facing outward into the lumen of an MVE. Release of Delta-bearing vesicles in this context would expose the neighboring cells to little packets of clustered Delta, a form in which they could more effectively interact with Notch (Le Borgne and Schweisguth, 2003a) (Figure 2C). Though there is no direct evidence for Delta-bearing exosomes, it is interesting to note that 5X concentrated conditioned medium from Delta-expressing S2 cells has been demonstrated to contain a soluble full-length Delta with an ability induce target gene expression in Notch-expressing S2 cells (Mishra-Gorur et al., 2002). The relevance of this observation has not been examined.
(ii) Recycling returns Delta to microdomains where it is associated with cofactors essential for effective interaction with Notch?
Endocytosis and recycling might also/instead promote association with other cofactors in microdomains that alter the manner in which it is presented to Notch (Figure 2B). Lipid rafts are an example of a microdomain that have been suggested to function as signaling platforms, in which clustering of receptors and associated proteins at the cell surface facilitates signaling (Simons and Toomre, 2000). Lipid rafts are cholesterol and sphingolipid rich lipid ordered domain that are typically recognized by their resistance to detergent extraction with cold triton-X100 and other reagents. Recent studies, however, have called into question the physiological significance of lipid rafts (Munro, 2003). Nevertheless, it appears that in the context of T-cell receptor signaling, where lipids rafts were previously thought to play a critical role, transiently formed protein networks at the cell surface dynamically regulate many aspects of signaling (Douglass and Vale, 2005).
Tetraspanin-enriched microdomains, distinct from lipid raft microdomains, have also recently been suggested to help serve in the assembly and modulation of signaling platforms at the cell surface (Vogt et al., 2002; Levy and Shoham, 2005). For example, the tetraspanin CD9 associates with and modulates the functions of the membrane-bound agonists for the EGF receptor. CD9 association increases the potency of these ligands during juxtacrine signaling (Higashiyama et al., 1995; Inui et al., 1997; Shi et al., 2000) by an unspecified mechanism that might involve prevention of membrane ligand cleavage, concentration of the ligand in CD9 microdomains, and by increasing the amount of ligand available at the cell surface (Shi et al., 2000).
(iii) Endocytosis and recycling returns Delta to the appropriate membrane compartment?
Delta-Notch signaling often takes place in the context of polarized epithelial cells where interactions occur between cells on the basolateral surface of the cells. For these interactions it is critical that cells are appropriately apposed with each other and that Delta and Notch are expressed on surfaces where interactions are possible. Proteins are either directly targeted to the appropriate membrane compartment following synthesis in the Trans-Golgi Network or indirectly following entry into the endocytic recycling system. Analysis of E-cadherin trafficking, for example, has shown that endocytosis and recycling is critical for targeting E-cadherin to the basolateral surface (Lock and Stow, 2005). In the absence of recycling via the Rab11 endocytic recycling compartment basolateral expression is reduced. Similarly, endocytosis and recycling could contribute to availability of Delta on the basolateral surface of the cell where it could interact with Notch.
(iv) Endocytosis and recycling returns unbound Delta to the cell surface?
Endocytosis and recycling may serve as a mechanism for returning unbound Delta to the cell surface, a common strategy for re-sensitizing receptors following interaction with agonists. Following the interaction of Delta with Notch, Delta-NECD complexes could be internalized in endocytic vesicles, Delta and NECD dissociated, and unbound Delta returned to the surface via recycling vesicles, while NECD is retained and eventually destroyed (Figure 2D). Factors that influence the decision to return unbound Delta to the surface following its endocytosis, instead of targeting it for destruction, could have a critical role in determining the strength and duration of Notch signaling.
The mechanisms that determine post-endocytic trafficking of DSL Notch ligands remain poorly characterized, however, the G-protein coupled receptors (GPCRs) serve as examples of receptors that undergo desensitization or resensitization following interaction with their ligands based on whether the receptors are internalized and degraded or recycled (Grady et al., 1997; Oakley et al., 1999; Trapaidze et al., 2000; Rosenfeld et al., 2002; Whistler et al., 2002; Qiu et al., 2003; Fan et al., 2004; Bomberger et al., 2005). In this context association with ß arrestin appear to be an important determinant of receptor recycling and re-sensitization. For example, exposure of the bradykinin type-2 receptor (B2R) to bradykinin (BK) leads to association of B2R with beta arrestin. Upon agonist removal, beta-arrestin rapidly dissociates from B2R into endosomes, and the receptors return back to the plasma membrane, fully competent for reactivating B2R signaling. In contrast, persistent association with beta arrestin targets the receptor for degradation (Simaan et al., 2005). Other studies indicate that the C-terminal of a number of GPCRs contain motifs that mediate interactions with PDZ domain containing proteins that are critical for promoting post-endocytic recycling of the receptors (Bomberger et al., 2005; Paasche et al., 2005; Trejo, 2005). Whether C-terminal PDZ-domain binding motifs in DSL ligands have similar roles will be an important question for future studies.
Clues from chimaeric DSL Notch ligands
Analysis of chimaeric DSL ligands provides some clues to how ubiquitylation makes Notch DSL ligands more effective. Deletion of the entire Delta intracellular domain (DeltaΔICD) or specific removal of all intracellular Lysines that serve as substrates for conjugation of Ubiquitin, prevents Delta from being ubiquitylated and from delivering an effective signal (Itoh et al., 2003; Wang and Struhl, 2004). Interestingly, fusion of DeltaΔICD with the 76-amino acid ubiquitin peptide creates a DeltaΔICD-Ub chimera with restored ability to activate Notch (Itoh et al., 2003; Wang and Struhl, 2004). As discussed earlier, replacement of the Delta intracellular domain with the intracellular domain of the Low Density Lipoprotein receptor (LDLR) also restores function of Delta (Wang and Struhl, 2004). In this context the function is independent of ubiquitin and E3 ligases, and trafficking directed by motifs in the LDLR intracellular domain can functionally substitute for trafficking normally mediated by ubiquitylation. The LDLR undergoes rapid recycling and these observations have been interpreted to suggest that endocytosis and recycling of Delta, whether by a ubiquitin-dependent pathway or a ubiquitin-independent pathway, as in the case of the Delta-LDLR chimera, allows Delta to be presented in a more effective manner to Notch. It is interesting to note that targeting of the LDLR to the basolateral surface in epithelial cells is dependent on endocytosis from the apical surface followed by recycling in association with AP1B (Gan et al., 2002), however there is no direct evidence for the Delta-LDLR chimera following this route. Further analysis of specific changes in the organization, trafficking, and surface availability of these chimaeric forms of Delta will likely provide clues to the role of ubiquitylation.
In addition to interaction with E3 ligases, interaction with Epsin, a Ubiquitin-Interaction Motif (UIM) containing protein, is an absolute requirement for effective Delta signaling. Though Epsin is often associated with clathrin mediated endocytosis, recent studies exploring the role of the E3 ligase CBL and Espin in EGF receptor (EGFR) endocytosis provide some novel insights (Sigismund et al., 2005). These studies indicate that when the EGFR is exposed to low levels of EGF it is internalized by a clathrin dependent mechanism. In this context CBL does not ubiquitylate EGFR, instead it is essential for ubiquitylation of associated proteins involved in endocytosis. In contrast, when the EGFR is exposed to high levels of EGF, it is ubiquitylated by CBL; it associates with Epsin, Eps15 and Eps15R, and is then also internalized by a non-clathrin mediated pathway, where it also associates with caveolin. Interestingly, an EGFR/ubiquitin chimera, in which a poly-ubiquitylation deficient Ubiquitin replaces the intracellular domain, also associates with Epsin and Eps15, and is exclusively internalized by a non-clathrin dependent pathway. It will be important to determine if association of Epsin with Delta also promotes internalization by the non-clathrin mediated internalization route and whether entry by this route also ultimately facilitates recycling and association with critical co-factors that make DSL ligands more effective.
Since the EGFR/ubiquitin chimera recapitulates many aspects EGFR trafficking following ubiquitylation, it serves as an extremely useful tool for exploring specific changes in post-endocytic sorting that are regulated by ubiquitylation of the EGFR. Furthermore, since a transferrin receptor/ubiquitin chimera is also forced into a non-clathrin mediated pathway, a path not taken by the wild-type transferrin receptor, it appears that the ubiquitin chimeras acquire common behaviors, and their analysis may provide general lessons about the role of ubiquitylation in transmembrane protein trafficking. However, some differences in trafficking between naturally ubiquitylated substrates and membrane protein/ubiquitin chimeras should be expected since de-ubiquitylation is not possible in the chimeras.
Delta recycling in the progeny of Sensory Organ Precursors
Sensory organ precursors (SOP) generate the Drosophila external sensory organs through a series of stereotypic asymmetric divisions. The basis for the distinct fate of the sibling cells produced from each of these divisions is that Notch signaling is activated in only one of the daughters. Numb and Neuralized proteins asymmetrically localize to the anterior cortex of dividing SOPs and segregate exclusively into the anterior pIIb daughter. Numb inhibits Notch activation and Neuralized promotes endocytosis of Delta in pIIb cells. Asymmetric inheritance of Numb and Neuralized in the pIIb cell ensures that it has no Notch activation and it becomes more effective at sending a Delta signal. In contrast, the pIIa cell is more effective at receiving the Delta signal and activation of Notch in this cell helps to specify its fate.
Now a third independent player has been identified that ensures the pIIb cell is more effective at sending a Delta signal (Emery et al., 2005; Jafar-Nejad et al., 2005). Nuclear Fallout (Nuf), a homologue of vertebrate arfophilin that binds Rab11 (Hickson et al., 2003), associates with the centrosome and helps establish a pericentrosomal recycling center in the pIIb cell immediately after SOP mitosis (Emery et al., 2005). In contrast, association of Nuf with the centrosome is prevented by an, as yet, unidentified mechanism in the pIIa cell, and consequently the recycling endosome is not as rapidly established in this sibling. As a result Delta that was internalized prior to division in the SOP cell is recycled in the pIIb cell and this contributes to the rapid establishment of the pIIb cell as the better signal-sending cell. When mechanisms that ensure asymmetric segregation of Neuralized and Numb are disrupted, the Rab 11-associated pericentrosomal recycling endosome is still established asymmetrically in the pIIb cell and this is sufficient to specify asymmetric fates based on differential activation of Notch in the pIIa and pIIb cells. These observations illustrate how redundant mechanisms ensure asymmetric fates in the SOP progeny and they underscore the importance of Delta recycling in establishing the pIIb cell as a more effective signal-sending cell.
Analysis of Drosophila sec15 mutants provides further evidence for the role of recycling in effective Delta signaling (Jafar-Nejad et al., 2005). Sec15, originally identified as a component of the exocyst complex, has more recently also been identified as an effector for Rab11 (Zhang et al., 2004) required for delivery of cargo in recycling endosomes to the plasma membrane. In the context of this role, sec15 mutants are characterized by defects in external sensory organs consistent with pIIa to pIIb transformations resulting from reduced Notch signaling.
CONCLUSIONS
In this review I have speculated about potential mechanisms by which Delta endocytosis facilitates activation of the Notch receptor and used examples from other signaling pathways to illustrate how each of the mechanisms is relevant in specific contexts. Though the precise mechanism by which Delta ubiquitylation and endocytosis contributes to Notch activation remains unclear, there is mounting evidence for endocytosis and recycling ultimately playing a critical role. The explosion of recent papers on this subject raise a number of questions that pose an exciting challenge for future research in this area. Is Delta ubiquitylation and endocytosis stimulated by interaction with Notch and/or does constitutive endocytosis and recycling generate a more effective ligand prior to interaction. Does endocytosis and recycling affect clustering of Delta, promote association with specific proteins critical for its function, or permit entry into specific microdomains that facilitate interaction Notch? Is Delta-Notch binding weaker in the absence of endocytic recycling and recycling. Is Delta endocytosis and recycling an absolute requirement for interaction and activation of Notch in specific contexts or is it more important for making these events more efficient in specific cells. Is Delta endocytosis also directly required to facilitate S2 cleavage?
Interestingly, formalin-fixed Delta-expressing S2 cells can induce expression of target genes when they are mixed with Notch-expressing S2 cells, showing that presentation of membrane bound Delta can, in principal, activate Notch without active Delta internalization (Mishra-Gorur et al., 2002). Furthermore, compared to non-fixed cells, fixed Delta-expressing S2 cells produce more sustained target gene expression in Notch expressing cells. These observations argue against an absolute requirement for membrane-tethered Delta actively pulling on Notch to facilitate S2 cleavage. Though it remains possible that this mechanism makes activation more efficient. The unusually sustained effect of fixed Delta-expressing also argues against endocytosis and recycling being essential for sustained presentation of active Delta. Since endocytosis and recycling could have contributed to making a more effective Delta ligand prior to fixation, it would be interesting to examine if interference with Delta recycling prior to fixation reduces the ability of Delta expressing cells to deliver a signal. It should be kept in mind that in experiments like this, over-expression of Delta could obscure the relevance of trafficking mechanisms that are essential in vivo, where there might be limiting amounts of Delta available. Indeed, a better understanding of the specific contexts in which Delta endocytosis is required for signaling will provide clues about how it contributes to signaling.
This review started with a description of Delta-Notch signaling in lateral inhibition emphasizing a role for negative feedback of Notch activation on proneural gene and Delta transcription. However, as noted earlier, Delta expression levels are often not obviously different in cells selected by Delta-Notch signaling. In this context it will be important to investigate if the level of Notch activation or proneural function in a cell directly regulates endocytosis and recycling of Notch ligands and if this provides a dynamic post-translational mechanism for regulating the outcome of Notch signaling.
Acknowledgments
The author thanks laboratory members for critical comments on the manuscript. Ajay Chitnis is supported by the Intramural Research Program of the NIH, NICHD.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ascano JM, Beverly LJ, Capobianco AJ. The C-terminal PDZ-ligand of JAGGED1 is essential for cellular transformation. J Biol Chem. 2003;278:8771–8779. doi: 10.1074/jbc.M211427200. [DOI] [PubMed] [Google Scholar]
- Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS. Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem. 2005;280:9297–9307. doi: 10.1074/jbc.M413786200. [DOI] [PubMed] [Google Scholar]
- Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci U S A. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Casey Corliss D. Three modules of zebrafish Mind bomb work cooperatively to promote Delta ubiquitination and endocytosis. Dev Biol. 2004;267:361–373. doi: 10.1016/j.ydbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- d'Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev Cell. 2001;1:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Detmers PA, Wright SD, Olsen E, Kimball B, Cohn ZA. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987;105:1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric rab11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Sai J, Richmond A. Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis. Mol Biol Cell. 2004;15:2456–2469. doi: 10.1091/mbc.E03-09-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fleming RJ. Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol. 1998;9:599–607. doi: 10.1006/scdb.1998.0260. [DOI] [PubMed] [Google Scholar]
- Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- Grady EF, Bohm SK, Bunnett NW. Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am J Physiol. 1997;273:G586–601. doi: 10.1152/ajpgi.1997.273.3.G586. [DOI] [PubMed] [Google Scholar]
- Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14:320–328. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ubiquitin: roles in protein modification and breakdown. Cell. 1983;34:11–12. doi: 10.1016/0092-8674(83)90131-9. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hicks C, Ladi E, Lindsell C, Hsieh JJ, Hayward SD, Collazo A, Weinmaster G. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J Neurosci Res. 2002;68:655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- Hickson GR, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol Biol Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S, Iwamoto R, Goishi K, Raab G, Taniguchi N, Klagsbrun M, Mekada E. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino R, Koyama I, Kusumi A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys J. 2001;80:2667–2677. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S, Higashiyama S, Hashimoto K, Higashiyama M, Yoshikawa K, Taniguchi N. Possible role of coexpression of CD9 with membrane-anchored heparin-binding EGF-like growth factor and amphiregulin in cultured human keratinocyte growth. J Cell Physiol. 1997;171:291–298. doi: 10.1002/(SICI)1097-4652(199706)171:3<291::AID-JCP7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Kidd S, Lieber T. Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev. 2002;115:41–51. doi: 10.1016/s0925-4773(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Klueg KM, Muskavitch MA. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila. J Cell Sci. 1999;112 ( Pt 19):3289–3297. doi: 10.1242/jcs.112.19.3289. [DOI] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, Lee J, Chitnis AB, Kim CH, Kong YY. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005a;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Koo BK, Yoon KJ, Yoo KW, Lim HS, Song R, So JH, Kim CH, Kong YY. Mind bomb-2 is an E3 ligase for Notch ligand. J Biol Chem. 2005b;280:22335–22342. doi: 10.1074/jbc.M501631200. [DOI] [PubMed] [Google Scholar]
- Kooh PJ, Fehon RG, Muskavitch MA. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophila development. Development. 1993;117:493–507. doi: 10.1242/dev.117.2.493. [DOI] [PubMed] [Google Scholar]
- Kopczynski CC, Muskavitch MA. Complex spatio-temporal accumulation of alternative transcripts from the neurogenic gene Delta during Drosophila embryogenesis. Development. 1989;107:623–636. doi: 10.1242/dev.107.3.623. [DOI] [PubMed] [Google Scholar]
- Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132:2319–2332. doi: 10.1242/dev.01825. [DOI] [PubMed] [Google Scholar]
- Lasky JL, Wu H. Notch signaling, brain development, and human disease. Pediatr Res. 2005;57:104R–109R. doi: 10.1203/01.PDR.0000159632.70510.3D. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005a;132:1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 2005b;3:e96. doi: 10.1371/journal.pbio.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Notch signaling: endocytosis makes delta signal better. Curr Biol. 2003a;13:R273–275. doi: 10.1016/s0960-9822(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev Cell. 2003b;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- Lewis J. Neurogenic genes and vertebrate neurogenesis. Curr Opin Neurobiol. 1996;6:3–10. doi: 10.1016/s0959-4388(96)80002-x. [DOI] [PubMed] [Google Scholar]
- Lewis J. Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr Biol. 2003;13:1398–1408. doi: 10.1016/s0960-9822(03)00534-7. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra-Gorur K, Rand MD, Perez-Villamil B, Artavanis-Tsakonas S. Down-regulation of Delta by proteolytic processing. J Cell Biol. 2002;159:313–324. doi: 10.1083/jcb.200203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol. 2005;67:1581–1590. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch MA, Moschonas NK, Delidakis C. neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev Cell. 2001;1:807–816. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- Pfister S, Przemeck GK, Gerber JK, Beckers J, Adamski J, Hrabe de Angelis M. Interaction of the MAGUK family member Acvrinp1 and the cytoplasmic domain of the Notch ligand Delta1. J Mol Biol. 2003;333:229–235. doi: 10.1016/j.jmb.2003.08.043. [DOI] [PubMed] [Google Scholar]
- Pitsouli C, Delidakis C. The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development. 2005;132:4041–4050. doi: 10.1242/dev.01979. [DOI] [PubMed] [Google Scholar]
- Pourquie O. The vertebrate segmentation clock. J Anat. 2001;199:169–175. doi: 10.1046/j.1469-7580.2001.19910169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Law PY, Loh HH. Mu-opioid receptor desensitization: role of receptor phosphorylation, internalization, and representation. J Biol Chem. 2003;278:36733–36739. doi: 10.1074/jbc.M305857200. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JL, Knoll BJ, Moore RH. Regulation of G–protein-coupled receptor activity by rab GTPases. Receptors Channels. 2002;8:87–97. [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–178. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–138. [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- Shi W, Fan H, Shum L, Derynck R. The tetraspanin CD9 associates with transmembrane TGF-alpha and regulates TGF-alpha-induced EGF receptor activation and cell proliferation. J Cell Biol. 2000;148:591–602. doi: 10.1083/jcb.148.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simaan M, Bedard-Goulet S, Fessart D, Gratton JP, Laporte SA. Dissociation of beta-arrestin from internalized bradykinin B2 receptor is necessary for receptor recycling and resensitization. Cell Signal. 2005;17:1074–1083. doi: 10.1016/j.cellsig.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Six EM, Ndiaye D, Sauer G, Laabi Y, Athman R, Cumano A, Brou C, Israel A, Logeat F. The notch ligand Delta1 recruits Dlg1 at cell-cell contacts and regulates cell migration. J Biol Chem. 2004;279:55818–55826. doi: 10.1074/jbc.M408022200. [DOI] [PubMed] [Google Scholar]
- Trapaidze N, Gomes I, Bansinath M, Devi LA. Recycling and resensitization of delta opioid receptors. DNA Cell Biol. 2000;19:195–204. doi: 10.1089/104454900314465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J. Internal PDZ ligands: novel endocytic recycling motifs for G protein-coupled receptors. Mol Pharmacol. 2005;67:1388–1390. doi: 10.1124/mol.105.011288. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr Opin Cell Biol. 2000;12:542–547. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC-peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev. 2002;189:136–151. doi: 10.1034/j.1600-065x.2002.18912.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Leslie JD, Ariza-McNaughton L, Lewis J. Delta proteins and MAGI proteins: an interaction of Notch ligands with intracellular scaffolding molecules and its significance for zebrafish development. Development. 2004;131:5659–5669. doi: 10.1242/dev.01417. [DOI] [PubMed] [Google Scholar]
- Yeh E, Dermer M, Commisso C, Zhou L, McGlade CJ, Boulianne GL. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr Biol. 2001;11:1675–1679. doi: 10.1016/s0960-9822(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]